Abstract
Acute antibody-mediated rejection (AMR) occurs in a minority of sensitized liver transplant recipients. Although histopathologic characteristics have been described, a generalizable scoring system used to trigger a more in-depth analysis is needed to screen for this rare but important finding. Toward this goal, we created a training and validation cohort from 3 high volume liver transplant programs of putative acute AMR and control cases that were evaluated blindly by 4 independent transplant pathologists. The evaluations were performed on H&E sections alone without knowledge of serum DSA results nor C4d stains. Characteristics strongly correlated with acute AMR included portal eosinophilia (OR=4.37, p<0.001), portal vein endothelial cell hypertrophy (OR=2.88, p<0.001), and eosinophilic central venulitis (OR=2.48, p=0.003). These and other characteristics were incorporated into models created from the training cohort alone. The final Acute-AMR (aAMR) score exhibited a strong correlation with acute AMR in the training (OR=2.86, p<0.001) and validation cohort (OR=2.49, p<0.001). SPSS tree classification was used to select 2 cutoffs, one that optimized specificity at a score >1.75 (sensitivity = 34%, specificity = 87%) and a second that optimized sensitivity at a score >1.0 (sensitivity = 81%, specificity = 71%). In conclusion, routine histopathological features of the aAMR score can be used to screen for acute AMR on routine H&E in liver transplant biopsies, a diagnosis that requires substantiation by donor-specific HLA alloantibody testing, C4d staining, and exclusion of other insults.
Keywords: Acute-AMR, Microvasculitis, Antibody-Mediated Rejection, liver transplantation, donor-specific antibodies
Introduction
The first evidence that antibodies can cause acute injury/rejection (antibody-mediated rejection; AMR) in human liver allografts was observed in ABO-incompatible cadaveric, brain-dead whole organ donors (1, 2). Antibody and complement deposition, platelet-fibrin thrombi, micro-vasculitis, and arteritis were typical and expected histopathological findings (1), based on previous observations in ABO-incompatible renal allografts (3) and in ABO-compatible renal allografts harboring allo-antibodies (4, 5).
It was recognized early on, however, that human liver allografts were highly resistant to acute AMR from preformed HLA alloantibodies compared to kidney allografts (6). This relative resistance was attributed to: the liver's inherent “tolerogenic” properties, the difficultly detecting antibody and complement tissue deposits, the paucity of typical histopathological findings (6) and, even when damage was present, the noticeably diminished severity of injury compared to ABO-incompatible liver transplants (7, 8). Relative hepatic resistance to AMR has been attributed to: a) secretion of soluble HLA class I molecules that form immune complexes with alloantibodies, which are then cleared by Kupffer cells; b) Kupffer cell phagocytosis of platelet aggregates, immune complexes, and activated complement components (9); c) limited distribution of HLA class II expression in the microvasculature; d) large liver size and dual hepatic vasculature; and e) marked hepatocyte regenerative capacity after injury [reviewed in (7, 8)]. In addition, the imperfect sensitivity and specificity of cytotoxic cell-based antibody detection methods impaired prior investigators abilities to find associations between HLA antibodies and adverse patient and graft outcomes (1, 7, 8).
Nevertheless, in the late 1980's and early to mid-1990's HLA class I and II antibodies, as measured in cytotoxic cell-based assays, were suspected to cause or substantially contribute to acute and chronic liver allograft rejection (7, 10-13). In addition, experimental rat studies clearly showed that extreme sensitization (14, 15) could override the liver's natural resistance and defense mechanisms. Similar observations were made in humans and risk factors for acute liver allograft AMR included high-titer pretransplant sensitization with persistence of serum alloantibodies after transplantation. When acute liver allograft AMR ensued refractory thrombocytopenia, circulating immune complexes, and severe liver injury were then seen (7, 11).
Recent studies using more sophisticated and sensitive (16) solid phase donor-specific HLA alloantibody (DSA) detection methods have confirmed and extended earlier studies with cytotoxic cell-based assays, even though the two tests have been documented to sometimes produce substantially different results on the same serum samples (17, 18). These confirmed findings include: 1) the liver allograft's relative resistance to AMR (18) associated with the rapid disappearance of the vast majority of low to moderate MFI class I and II alloantibodies (11, 17, 18); and 2) an association of acute AMR with high-titer alloantibodies that most-often persist after transplantation and result in refractory thrombocytopenia and acute liver injury that can evolve into combined acute antibody-mediated and T-cell-mediated rejection. Inadequately treated, the end result can be chronic or ductopenic rejection (17, 19-23). Solid phase DSA analyses have also shown an association between multiple IgG subclasses, especially when alloantibodies of the IgG3 subclass are present, and chronic rejection and diminished allograft survival (24). These newer serum assays have also facilitated a closer correlation between histopathological findings and serum DSA characteristics (18, 22, 23, 25).
Similar to AMR in other solid organ allografts, histopathological patterns of injury associated with liver allograft AMR include portal edema, marked portal microvascular endothelial cell hypertrophy and eosinophilia; monocytic/histiocytic, eosinophilic, and neutrophilic portal microvasculitis, ductular reaction, hepatocyte swelling and hepatocanalicular cholestasis. During the early stages the constellation of findings can resemble preservation/reperfusion injury or biliary stricturing, but often quickly progress to acute “cellular” or T-cell-mediated and finally chronic rejection (8, 11, 21-23, 25, 26). Detection of microvascular complement deposition with C4d staining has been a valuable adjunct to the histopathological evaluation for acute AMR in all solid organ allografts, but C4d staining should not be used in isolation to establish an AMR diagnosis in liver allografts [reviewed in (26-28)].
Finally, although severe acute AMR is rare, unrecognized it can lead to allograft failure (22, 29, 30), as evidenced by its substantial contribution to ∼ 10-20% of previously idiopathic early allograft failures (<90 days post-transplant) in sensitized patients (23). Early recognition of acute AMR can prompt plasmapheresis (31) and plasma cell-specific therapy in rare patients and may result in improved outcomes (22, 29). Toward the goal of facilitating earlier diagnosis of acute liver allograft AMR, this study was designed to identify and validate a limited constellation of routine histopathological features in the form of a generalizable scoring system on liver biopsy H&E analysis that can be easily used to trigger a more thorough clinicopathological evaluation needed to establish the diagnosis with certainty.
Materials and Methods
Case Selection & Study Design
Previous University of Pittsburgh Medical Center (UPMC) studies examined the effect of a conventional lymphocytotoxic crossmatch on patient and allograft survival (7) and the utility of C4d staining in primary liver allograft recipients (26). As part of these prior studies a constellation of routine histopathological findings associated with acute AMR was described (26). These findings included microvascular (portal vein and portal capillary) endothelial cell hypertrophy; variable histiocytic, eosinophilic, and neutrophilic portal inflammation with microvasculitis; portal/periportal edema; cholangiolitis; centrilobular hepatocyte swelling; and hepatocanalicular cholestasis. The goals of this study were: 1) to determine whether four pathologists from 3 different liver transplant centers in two continents could blindly recognize specific routine histopathological findings on H&E staining alone associated with diffusely C4d-positive putative AMR episodes, and 2) to develop and validate a simple, generalizable scoring system that would facilitate recognition and an earlier diagnosis of acute AMR in liver allografts.
The training set consisted of UPMC for cause biopsies (n = 26) obtained within 21 days of primary liver transplant and divided into two groups: 1) those showing evidence of putative acute AMR based on the expected findings described above (i.e. microvascular endothelial cell hypertrophy and microvasculitis) along with strong and diffuse microvascular C4d positivity with or without co-existent “cellular” rejection (n=13); and 2) an equally-sized group of control biopsies matched for Banff rejection grade (n = 13; indeterminate = 4; mild = 6; moderate = 3), but with negative C4d staining.
A single blinded pathologist (AJD) re-reviewed the H&E-stained slides without knowledge of the C4d results and evaluated 27 different histologic features. After the initial appraisal, several histopathological categories were combined and those with a p-value <0.3 or those with a strong pathophysiological basis for inclusion remained part of the final list of 9 variables (Table 3). Following selection of the histopathological variables, 3 additional pathologists (SMS, CB, and MAN) evaluated the training material without knowledge of the number of C4d-positive or C4d-negative cases in each group that originated outside their own institution or C4d staining results for all cases. In addition, each pathologist was asked to predict the C4d stain result (diffusely positive or not) based on the H&E slide alone on all cases that they did not help select. Variables positively associated with putative AMR or mixed AMR and cellular rejection in the training set were considered for inclusion in the numerator of the model based on a p-value <0.2. The denominator variables were selected for their negative association with AMR.
Table 3. ORs From All 4 Pathologists (Blinded to C4d Results) for the 9 Variables With the Strongest Correlations With C4d-Positive Rejection in the UPMC Training Cohort and the Edinburgh University/BUMC Validation Cohorts.
| Training Cohort | OR (CI) | P Value | Coefficient of Concordance* |
|---|---|---|---|
| Eosinophilic central venulitis | 1.93 (1.25-2.96) | 0.003 | 0.49 |
| Portal vein endothelial cell hypertrophy | 1.89 (1.19-2.99) | 0.007 | 0.42 |
| Eosinophilic portal venulitis | 2.48 (1.24-4.96) | 0.01 | 0.30 |
| Central venulitis severity | 2.26 (1.20-4.25) | 0.02 | 0.33 |
| L ymphocytic portal inflammation | 0.59 (0.34-1.03) | 0.06 | 0.40 |
| Portal eosinophilia | 1.43 (0.92-2.21) | 0.11 | 0.40 |
| L ymphocytic venulitis | 0.78 (0.49-1.24) | 0.30 | 0.32 |
| Hepatocyte ballooning | 1.14 (0.76-1.72) | 0.53 | 0.44 |
| Cholestasis | 1.00 (0.71-1.42) | 1.00 | 0.29 |
| Validation Cohort | OR (CI) | P Value | Coefficient of Concordance* |
|---|---|---|---|
| Eosinophilic central venulitis | 2.48 (1.37-4.49) | 0.003 | 0.63 |
| Portal vein endothelial cell hypertrophy | 2.88 (1.83-4.55) | <0.001 | 0.62 |
| Eosinophilic portal venulitis | 3.05 (1.96-4.69) | <0.001 | 0.38 |
| Central venulitis severity | 2.44 (1.47-4.06) | <0.001 | 0.63 |
| L ymphocytic portal inflammation | 1.33 (0.79-2.22) | 0.30 | 0.58 |
| Portal eosinophilia | 4.37 (2.54-7.51) | <0.001 | 0.61 |
| L ymphocytic venulitis | 1.65 (1.05-2.58) | 0.03 | 0.42 |
| Hepatocyte ballooning | 2.00 (1.35-2.95) | <0.001 | 0.63 |
| Cholestasis | 2.09 (1.35-2.95) | <0.001 | 0.65 |
Kendall's coefficient of concordance displays the interobserver variability.
Multiple models were made from the training cohort variables based on the following guiding principles: 1) a scientific understanding of AMR, 2) simplicity, 3) the least inter-observer variability, and 4) the best correlation with C4d staining. The final model was selected for its lowest p-value from the training cohort data only.
Following evaluation of the UPMC training set, a separate validation cohort was created from 2 different centers: 1) Edinburgh University and 2) Baylor University Medical Center (BUMC). The Edinburgh University cases (between 2007 and 2013) were selected in a similar fashion to UPMC cases: a diagnosis of rejection within 21 days of transplant with histopathological evidence of rejection-related injury, strong and diffuse microvascular C4d staining, and a pretransplant positive cytotoxic or flow crossmatch or single antigen bead assay (n=5) and matched to a control group based on the Banff grade of cellular rejection with negative C4d staining and negative pre-transplant DSA testing (n=5).
The second portion of the validation cohort included all 29 HCV RNA-negative cases of biopsy-proven steroid-resistant rejection from BUMC within 60 days of liver transplantation with single antigen bead testing performed pre-transplant (from 1/1/00 to 5/31/09) (20). This approach was based on the unrealized expectation that the cohort would be enriched for recipients suffering from acute AMR (21), but only 4 stained diffusely positive for C4d and 1 showed focal positivity. Three showing diffuse C4d positivity were also DSA positive and included in the final group; the remaining two cases: one originally interpreted as diffuse had high background staining and one with focal C4d positivity were excluded because of equivocal C4d staining and negative DSA testing leaving a total of 27 cases. None of the DSA-negative cases had definitive diffuse C4d-positive staining.
To achieve adequate statistical power we combined the Edinburgh and BUMC cohorts into one validation cohort. The appraisal performed by all pathologists on this validation cohort was on the H&E material alone without knowledge of the C4d staining results.
Pretransplant DSA Evaluation
All UPMC patients had a pretransplant T-cell cytotoxic crossmatch preformed prior to liver transplantation. In crossmatch-positive patients a steroid recycle was routinely given regardless of laboratory parameters, followed by standard per protocol immunosuppression. Neither pretransplant nor post-transplant serum was available for single antigen bead DSA analysis.
All Edinburgh University patients had pretransplant DSA testing performed since 2007; however the protocol has evolved: from 2007 to 2010 a cytotoxic T- and B-cell crossmatch, from 2011 to 6/2012 a flow cytometric crossmatch, and since 7/2012 all patients are screened for anti-HLA antibodies with multi-antigen beads to class I and class II antigens, with single antigen bead testing for DSA specificities in all positive patients.
All BUMC patients had prospectively collected pretransplant serum available for retrospective analysis of preformed DSA by single antigen bead technology, where mean fluorescence intensity (MFI) >5000 was considered positive, although data was acquired and reported on all DSA with MFI >1000. All patients and donors were typed for HLA-A, -B, -DRB1, -DRB345 and -DQ using commercially available serologic typing trays or by molecular methods (Terasaki HLA tissue typing trays and Micro SSPTM or LabType® SSO, respectively; One Lambda Inc., Canoga Park, CA). All sera were blindly analyzed at the Terasaki Foundation Laboratory for HLA IgG antibodies using LABScreen single antigen class I (lot 6) and II (lot 8) beads (One Lambda Inc., Canoga Park, CA) according to the manufacturer's protocol. No serum was available to perform additional testing at the time of liver biopsy.
Statistical Analyses
Patient characteristics for the 3 cohorts are reported with median values and interquartile ranges of continuous data and percentages of categorical data where appropriate. Chi squared analyses of categorical variables and two-sample t-tests of continuous variables were performed. Univariate logistic regression was utilized to evaluate individual variables and the model's ability to predict association with C4d positive rejection.
Although our final model produced a linear score, the output was not thought to be linearly associated with the ability to predict AMR. Therefore, we employed SPSS 16.0 to determine predictive cutoffs using tree classification. This was performed on the training cohort data from all 4 blinded pathologists before the validation cohort data was available for analysis and not modified after its completion.
Inter-observer variability was assessed with the Kendall's coefficient of concordance (32). Coefficient of concordance analyses were performed for each individual variable. This measure, unlike the Kappa statistic, is for ordinal values and takes into consideration the magnitude of disagreement between evaluators. For the final model the coefficient of concordance measured their agreement on the Acute-AMR (aAMR) category (≤1, >1 but ≤1.75 and >1.75).
Significance was always defined as a P<0.05. SAS 9.1 was used for all statistical analyses except SPSS 16.0 was utilized for tree classification.
Results
Patient characteristics for the 3 cohorts are presented in Table 1. The cohorts were chosen differently because of local care standards, therefore, intergroup differences existed, but because of the blinded nature of analysis, none were felt to substantially influence the results.
Table 1. Patient Characteristics of the Training Cohort From University of Pittsburgh Medical Center (UPMC) and the Validation Cohort From Edinburgh University and Baylor University Medical Center (BUMC).
| UPMC (n 5 26)* | Edinburgh (n 5 10)* | BUMC (n 5 27)† | |
|---|---|---|---|
| C4d-positive [n (%)] | 13 (50) | 5 (50) | 3 (11) |
| Male sex (%) | 46 | 10 | 59 |
| Age (years)‡ | 55 (42-59) | 55.5 (50-59) | 53 (38-57) |
| Model for End-Stage Liver Disease score‡ | 18 (14-23) | 15.5 (11-16) | 18 (14-24) |
| Cold ischemia time (hours)‡ | 10 (8-12) | 10 (8-13) | 9 (6-11) |
| Hepatocellular carcinoma (%) | 8 | 30 | 14 |
| HCV RNA–positive (%) | 31 | 10 | 0 |
| Recipient race (%) | |||
| Caucasian | 96 | 100 | 73 |
| African American | 4 | 0 | 10 |
| Other | 0 | 0 | 17 |
| Donor race (%) | |||
| Caucasian | 69 | 100 | 62 |
| African American | 27 | 0 | 14 |
| Other | 4 | 0 | 24 |
| Donor age (years)‡ | 53 (41-74) | 56 (49-67) | 54 (39-61) |
| Induction (%) | 0 | 0 | 24 |
| Calcineurin inhibitor (%)§ | 100 | 100 | 81 |
| Steroids (%)§ | 100 | 100 | 52 |
| Sirolimus (%)§ | 0 | 0 | 26 |
| Mycophenolate (%)§ | 38 | 0 | 44 |
C4d-positive cases of rejection within 21 days of transplantation were matched by the Banff grade to C4d-negative cases of rejection.
All HCV RNA–negative patients with steroid-resistant rejection within 60 days of transplantation between January 1, 2000 and April 28, 2009 who had a pretransplant sample tested for DSAs underwent staining for C4d.
The data are presented as medians and interquartile ranges.
Immunosuppression at the time of rejection.
Table 2A shows pretransplant T-cell cytotoxic crossmatch data from UPMC cases according to C4d staining; of the 13 diffusely C4d positive cases 38% were crossmatch positive, whereas the remainder were T-cell crossmatch negative. All C4d-negative cases/biopsies had a negative T-cell cytotoxic crossmatch except one. Table 2B shows the pretransplant DSA correlation with C4d staining in the Edinburgh cases. All C4d positive cases had evidence of pre-transplant DSA by either single antigen beads [class I MFISUM of 28,500 and class II MFISUM of 27,300] (n=1), T-cell flow crossmatch (n=1), or T-cell cytotoxic crossmatch (n=3). All C4d negative controls were also DSA negative by either single antigen bead analyses (n=4) or flow crossmatch (n=1). Table 2C shows the pretransplant single antigen bead data from the BUMC cases: only 3 cases were C4d positive with DSA in serum, and each one had at least one DSA with MFI >5000. The first had a single class I DSA with MFI of 11,353, the second had 2 class I DSAs with MFISUM of 13,620 and 4 low MFI (all between 1000 and 5000) class II DSAs with MFISUM of 9,066, and the third had one class I DSA with MFI 1,868 and 5 class II DSAs with MFISUM of 62,375. None of the C4d negative cases had any single DSA with a MFI >5000, but nine had low MFI DSAs (between 1000 and 5000); 2 with class I only, 4 with class II only and 3 with class I and II.
Table 2. Comparison of Pretransplant Serological and C4d Staining Data for Patients From UPMC, Edinburgh University, and BUMC.
| UPMC | n | |
|---|---|---|
| Total patients/biopsies | 26 | |
| C4d-positive | Positive by T cell cytotoxic cross match | 5 |
| Negative by T cell cytotoxic cross match | 8 | |
| C4d-negative | Positive by T cell cytotoxic cross match | 1 |
| Negative by T cell cytotoxic cross match | 12 |
| Edinburgh University | n | |
|---|---|---|
| Total patients/biopsies | 10 | |
| C4d-positive | None | 0 |
| Positive by class I and II single antigen beads | 1 | |
| Positive by T cell flow cytometry cross match | 1 | |
| Positive by T cell cytotoxic cross match | 3 | |
| C4d-negative | Negative by single-antigen beads | 4 |
| Negative by flow cytometry cross match | 1 |
| BUMC* | n | |
|---|---|---|
| Total patients/biopsies | 27 | |
| C4d-positive | None | 0 |
| Class I* | 2 | |
| Class II* | 1 | |
| Classes I and II* | 0 | |
| C4d-negative | None | 24 |
| Class I* | 0 | |
| Class II* | 0 | |
| Classes I and II* | 0 |
NOTE: See the Patients and Methods section for the case selection and study design. C4d staining was considered positive only when it was diffuse (>50% of PTs).
Only cases with at least 1 individual DSA with an MFI > 5000 were considered positive.
Table 3 highlights the 7 evaluated histologic characteristics associated with C4d positive early rejection or putative acute AMR (Figures 1-4): eosinophilic central venulitis, portal vein endothelial cell hypertrophy, eosinophilic portal venulitis, central venulitis severity, portal eosinophilia, hepatocyte ballooning, and cholestasis; and 2 histologic characteristics inversely associated with acute AMR in the training cohort: lymphocytic portal inflammation and lymphocytic venulitis (Figure 2). Although cholestasis was not associated with DSA injury when all 4 pathologists scores were utilized in the training cohort, when particular attention was refocused to distinguish hepatocanalicular cholestasis from centrilobular hepatocyte lipofuscin deposition, an association was found in the validation cohort. In addition, the coefficients of concordance improved significantly after learning from the training cohort was followed by evaluation of the validation cohort.
Figure 1.
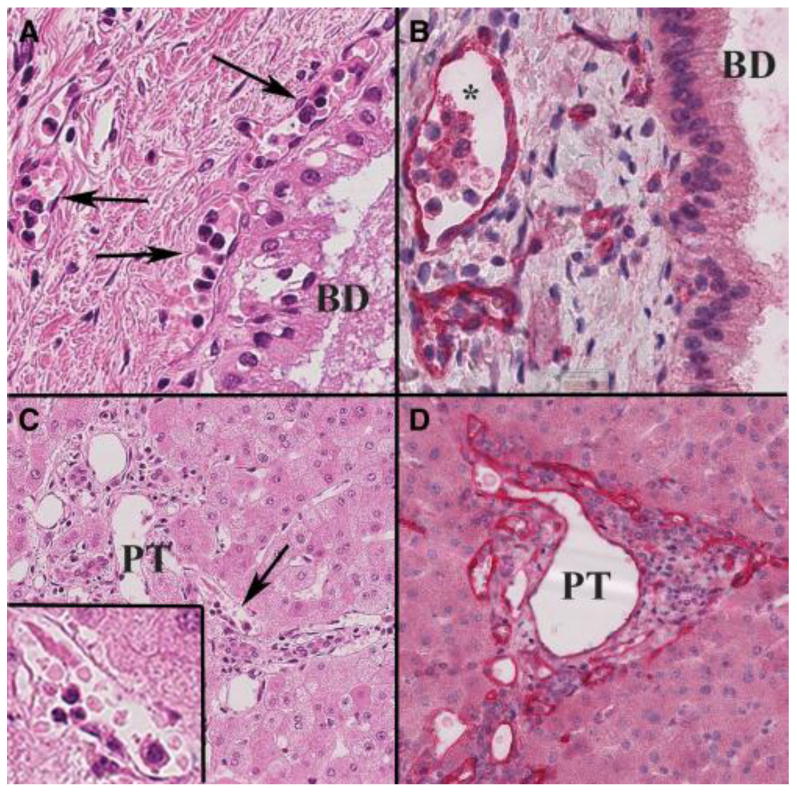
Composite of early acute AMR histopathological changes in an allograft that failed 18 days after transplantation because of hepatic artery thrombosis in a highly sensitized patient. A) Note the monocytic and eosinophilic “capillaritis” in the peribiliary capillary plexus (arrows) surrounding a large segmental bile duct (BD) on H&E stain (40X). B) A C4d stain of the same area and throughout the entire liver showed diffuse endothelial cell C4d positivity (red *; 40X). C) Moncoytic capillaritis was also noted in the smaller portal tracts (PT; 30X) (arrow shows area shown at higher magnification in the inset (80X). D) A C4d stain (red) showed strong and diffuse portal microvascular positivity, typical of severe acute AMR.
Figure 4.
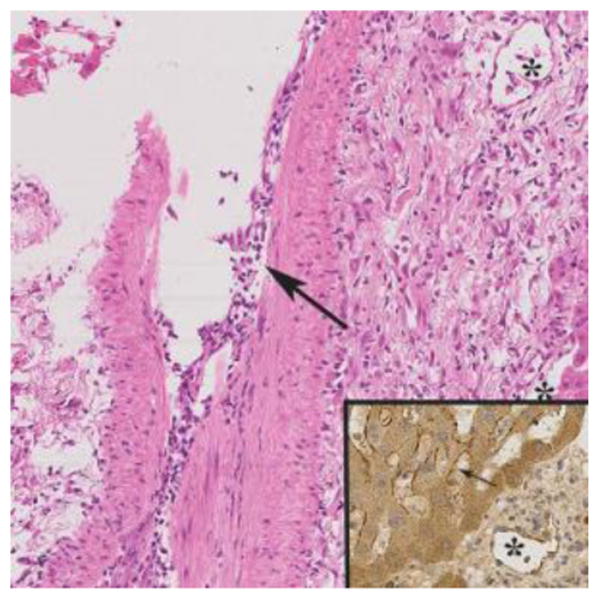
Inflammatory arteritis was present in several of the cases diagnosed as severe acute AMR, as in other solid organ allografts (H&E; 20X). This biopsy was obtained 11 days after transplantation from a 66-year-old female who underwent liver replacement for primary biliary cirrhosis and hepatocellular carcinoma. Solid phase DSA determination revealed several class 1 and 2 DSA at a cumulative MFI >50,000. When arteritis is detected C4d staining (inset) and DSA determinations are recommended. Arteritis, however, was not included in the acute AMR score because it is uncommonly detected in needle biopsies Note the presence of lymphocytes, macrophages and eosinophils within the intima of the affected artery (large arrow), the endothelial hypertrophy in a nearby capillary (*). The inset (40X) shows C4d positivity (brown staining) in a portal capillary (*) and sinusoids (small arrow).
Figure 2.
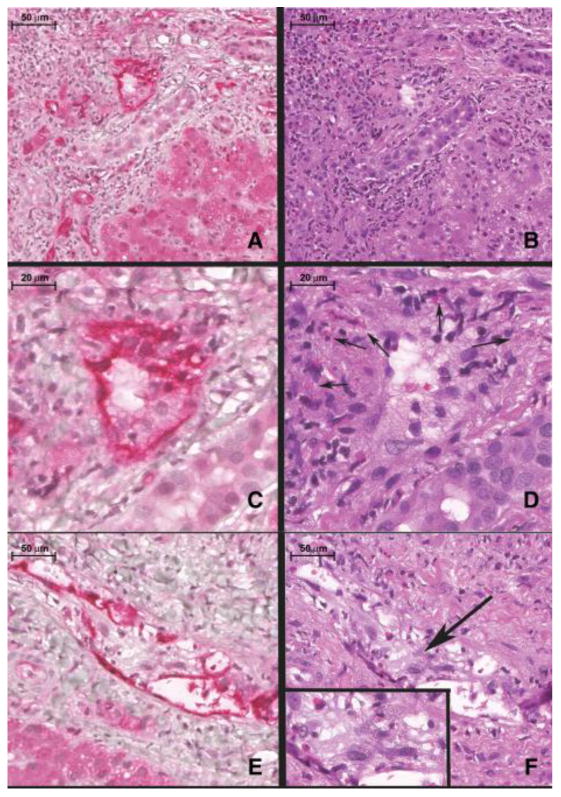
Composite histopathological features of severe, acute, C4d+ antibody mediated rejection (AMR) (A-F). A) Note intense and diffuse C4d staining (red) in the portal vein (PV) and portal capillaries (*). B) Routine H&E appearance of the same portal tract as shown in A). Note the marked endothelial cell hypertrophy of the portal venous endothelium (*). C) Shows a C4d stain (red) of the same portal vein (PV) branch as B) at higher magnification. D) Shows the routine H&E appearance of this vein. Note the marked portal venous endothelial cell hypertrophy intermixed with eosinophils and histiocytes. E) Another C4d staining example of the portal venous changes typical of severe acute AMR with the H&E counterpart shown in figure F). G) In contrast, C4d-negative T-cell mediated rejection (control case) shows predominantly mononuclear portal inflammation with focal subendothelial localization of the lymphocytes in a small portal vein branch (arrow). H) Routine H&E appearance of a control case's portal infiltrate highlights several differences: the substantial decrement in edema, the absence of marked portal microvascular endothelial cell hypertrophy, and the marked diminution in eosinophils. A scale bar is shown at the top left of each image.
Without the use of a formal scoring system, pathologists reported expected C4d staining results to be either diffusely positive or not. From H&E analysis alone, this prediction was 48% sensitive and 84% specific for the diagnosis of acute AMR.
Next, multiple models were created from the training cohort data alone, but Figure 5 shows the final Acute-AMR (aAMR) score. Numerical values are assigned based on the percentage of structures affected (None = 0, <10% = 1, 10-50% = 2, and >50% = 3). For the final model chosen, the OR was not appreciably changed from the training (OR=2.86, P<0.001) to the validation cohort (OR=2.49, P<0.001).
Figure 5.

(A) The Acute-AMR (aAMR) score to predict antibody-mediated rejection was developed from 4 pathologists scores on the training cohort and validated on a separate cohort. (B) The Odds Ratio demonstrates the aAMR model's association with a diagnosis of acute AMR on the training and validation cohorts. (C) SPSS16 tree classification developed diagnostic categories on the training set that were subsequently validated. Sensitivity and specificity of 2 different cutoffs are presented for the training and validation cohorts; the higher cutoff optimizes specificity, while the lower cutoff optimizes sensitivity.
Next tree classification was utilized on the training cohort to optimize the specificity for one cutoff and sensitivity for the other cutoff of the aAMR score (Figure 5). Sensitivity in the validation cohort increased from 34% to 81% when the cutoff used decreased from >1.75 to >1 respectively. Specificity in the validation cohort also decreased from 87% and 71% when the cutoff used decreased from >1.75 and >1 respectively. In addition, the Kendall's coefficient of concordance between pathologists was 0.61 in the training and 0.50 in the validation cohorts.
Discussion
Consensus histopathological criteria exist for the diagnosis of acute AMR in all solid organ transplants with the notable exception of the liver (5, 33-35), mostly because of its relative resistance to AMR and, consequent rarity of recognized cases. Absolute criteria for AMR in extra-hepatic organs invariably include serum DSA, microvascular endothelial cell hypertrophy and micro-vasculitis (36, 37), other tissue-specific injury patterns, and usually diffuse microvascular C4d staining. Kidney and heart allografts (37), however, allow for C4d-negative AMR when convincing microvasculitis is identified in the presence of DSA in serum.
Consensus criteria development for acute liver allograft AMR has been hampered by several issues, which, in turn, are related to the well-documented relative hepatic resistance to acute AMR: 1) in contrast to other solid organs, only a small fraction of DSA-positive liver allograft recipients develop overt histopathological evidence of injury (11, 17, 18, 23, 38); consequently 2) few programs routinely tissue type and screen for alloantibodies, or stain for C4d, mostly because they do not find it “cost effective”; and therefore, 3) only a few robust studies correlate histopathological findings, solid phase DSA testing, and C4d staining (18, 22, 23), and even fewer tissue biopsy and serum samples are simultaneously obtained.
Nevertheless, recent liver allograft studies confirmed and extended earlier observations by showing that high-titer DSA, especially if persistent post-transplant, in the presence of refractory thrombocytopenia, and diffuse microvascular C4d staining increase the probability of acute AMR (8, 22, 23, 39). The ability to correlate DSA with impaired outcomes, however, remains suboptimal (17, 19, 20, 40) and more granular and specific histopathological criteria are needed.
Diffuse C4d positivity remains a critical component of an acute liver allograft AMR diagnosis at this time. However, C4d staining should not be interpreted in isolation (22, 23, 25, 26, 29) because C4d staining protocols for formalin-fixed, paraffin-embedded liver allograft tissue are evolving toward more sensitive techniques. In addition, correlation of staining results with liver dysfunction need improvement because even diffuse microvascular endothelial cell C4d deposits can occur with or without histopathological or serological evidence of liver injury [reviewed in (26-28, 41)]. Liver resistance mechanisms (listed above); more restricted hepatic microvasculature class II HLA expression compared to other organs; or the liver's position downstream from the intestine and complement activation by the lectin pathway, by bacterial products, and other factors all contribute to the complexities involved. Even so, most studies show a correlation between cell-based and often a stronger correlation with solid-phase evidence of DSA and tissue C4d staining [reviewed in (26-28, 41)]. A key consideration, therefore, is how to reliably recognize acute microvascular and perhaps stellate cell activation and injury from DSA in liver allografts?
In our opinion, the strong correlation between several histopathological features of microvascular activation (endothelial cell hypertrophy) and injury (microvasculitis) documented in a blinded analysis by 4 independent pathologists, as would be expected with AMR, and diffuse C4d staining and serum DSA in the validation cohort provide compelling evidence that antibodies substantially contribute to this injury pattern. The argument is further substantiated by the relative paucity of similar correlations in more typical lymphocyte-predominant acute T-cell-mediated rejection biopsies matched for Banff grade of severity in controls.
It should be noted, however, that AMR-related microvasculitis is recognized primarily by increased intra-luminal inflammatory cells, some of which might be adherent to or apparently embedded within endothelial cells, and differs from the subendothelial lymphocytic infiltration of portal and central veins seen in otherwise typical T-cell-mediated rejection. Interestingly, some features originally attributed to cell-mediated rejection, such as an emphasis on a “mixed” inflammatory infiltrate consisting of activated and smaller lymphocytes, macrophages, neutrophils, and especially eosinophils (42), likely lumped together mixed T-cell-mediated and antibody-mediated effector mechanisms because of a lack of adequate tools to differentiate the two. Combined AMR and T-cell-mediated rejection is typical of many rejection episodes in all solid organ allografts. Therefore, changes attributable to AMR-related injury might be more difficult to isolate in livers simply because of convention.
We opted, therefore, for high specificity and set a relatively high threshold aAMR score of >1.75 to raise significant concern for an acute AMR diagnosis. This approach is recommended because of potential consequences of AMR therapy and to avoid over-diagnosis, which would inhibit widespread acceptance of a diagnosis that many already view with skepticism. However, to improve sensitivity biopsies with scores >1 should be subjected to C4d staining and serum DSA testing should be carried out to substantiate or refute a putative AMR diagnosis.
This study evaluated acute AMR at a more granular level than prior appraisals in an effort to help recognition of the most severe form of acute AMR. However, there are several shortcomings. One, training and validation cohorts were selected differently because of local standards of care. Two, in the training cohort not all recipients with diffuse C4d-positive putative AMR showed pre-sensitization based on conventional T-cell cytotoxic crossmatches, which: a) miss most class II DSA; and b) are less sensitive (16) and can show substantially different results than solid phase assays when testing the same serum (17). The validity of this training cohort selection is substantiated by our BUMC patients in the validation cohort where a strong correlation between MFI of DSA and C4d staining was found: all patients with steroid resistant rejection and at least one DSA with MFI >5000 stained C4d positive, and all patients with steroid resistant rejection with lower MFI (1000 – 5000) DSA were C4d negative. Three, unavailability of simultaneous serum DSA testing and liver biopsy hindered our ability to make tighter correlations. Four, part of our validation cohort was chosen from all the early (<60 days) steroid resistant rejections that occurred in HCV RNA negative patients with pre-transplant DSA testing; this was done based on prior data showing this approach would enrich (41%) for C4d positive rejection (21), however, only 11% of this group had C4d positive steroid resistant rejection. Finally, the histopathological changes shown in this manuscript represent only the most severe form of acute liver allograft AMR. Qualitatively similar, but more histopathologically subtle, injury characterizes indolent or chronic AMR, which was not addressed in this study.
We attempted to mitigate most of these shortcoming by selecting cases from 3 different institutions, evaluating all material without knowledge of C4d or DSA test results, including 4 different pathologists, creating training and validation cohorts (the latter having solid phase DSA testing for most cases) and, relying on stringent criteria, including: 1) histopathological evidence of diffuse microvascular activation, injury, and microvasculitis; 2) diffuse microvascular C4d staining; 3) serum DSA (usually high MFI); and 4) reasonable exclusion of other causes of a similar type of injury (23). However, over time our understanding of acute AMR and C4d staining protocols will improve and molecular signatures of liver allograft AMR will be developed. As these advances unfold we expect that, like renal transplant pathology, histopathological features of acute and chronic liver AMR will be even more precisely defined, and C4d negative AMR will be described.
In summary, routine histopathological features in the aAMR score can be used to suspect the most severe form of acute AMR, a diagnosis that requires further substantiation by donor-specific HLA alloantibody testing, C4d staining, and exclusion of other insults.
Figure 3.
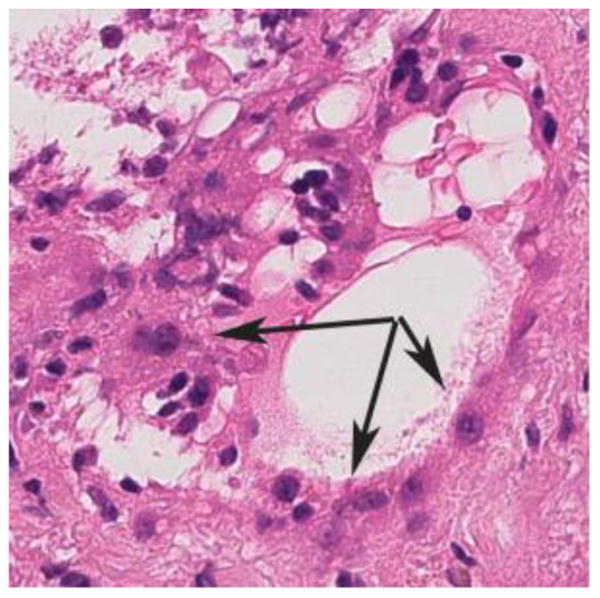
High magnification (60X) H&E stain of the marked microvascular endothelial cell hypertrophy and cytoplasmic eosinophilia (arrows) that is typical of severe acute AMR. Note the cuboidal or “hobnail” appearance of the endothelial cells.
Acknowledgments
Grants: No grant funding was utilized for this research.
Abbreviations
- AMR
antibody-mediated rejection
- aAMR
Acute-AMR score
- BD
bile duct
- BUMC
Baylor University Medical Center
- DSA
donor-specific HLA alloantibody
- MFI
mean fluorescence intensity
- PV
portal vein
- UPMC
University of Pittsburgh Medical Center
Footnotes
Conflicts of Interest: No author has conflicts of interest relevant to this work.
References
- 1.Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am Pathol. 1988 Sep;132(3):489–502. [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon RD, Iwatsuki S, Esquivel CO, Tzakis A, Todo S, Starzl TE. Liver transplantation across ABO blood groups. Surgery. 1986 Aug;100(2):342–8. [PubMed] [Google Scholar]
- 3.Starzl TE, Marchioro TL, Holmes JH, Hermann G, Brittain RS, Stonington OH, et al. Renal Homografts in Patients with Major Donor-Recipient Blood Group Incompatibilities. Surgery. 1964 Feb;55:195–200. [PMC free article] [PubMed] [Google Scholar]
- 4.Terasaki P, Marchioro T, Starzl T. Histocompatibility Testing. Washington, DC: National Academy of Sciences, National Research Council; 1965. [Google Scholar]
- 5.Kissmeyer-Nielsen F, Olsen S, Peterson VP, Fjeldborg O. Hyperacute rejection of kidney allografts associated with pre-existing humoural antibodies against donor cells. Lancet. 1966;2:662–5. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 6.Andres GA, Ansell ID, Halgrimson CG, Hsu KC, Porter KA, Starzl TE, et al. Immunopathological studies of orthotopic human liver allografts. Lancet. 1972 Feb 5;1(7745):275–80. doi: 10.1016/s0140-6736(72)90288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demetris AJ, Nakamura K, Yagihashi A, Iwaki Y, Takaya S, Hartman GG, et al. A clinicopathological study of human liver allograft recipients harboring preformed IgG lymphocytotoxic antibodies. Hepatology. 1992 Sep;16(3):671–81. doi: 10.1002/hep.1840160310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demetris AJ, Murase N, Nakamura K, Iwaki Y, Yagihashi A, Valdivia L, et al. Immunopathology of antibodies as effectors of orthotopic liver allograft rejection. Semin Liver Dis. 1992 Feb;12(1):51–9. doi: 10.1055/s-2007-1007376. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura K, Murase N, Becich MJ, Furuya T, Todo S, Fung JJ, et al. Liver allograft rejection in sensitized recipients. Observations in a clinically relevant small animal model. American Journal of Pathology. 1993;142(5):1383–91. [PMC free article] [PubMed] [Google Scholar]
- 10.Takaya S, Duquesnoy R, Iwaki Y, Demetris J, Yagihashi A, Bronsther O, et al. Positive crossmatch in primary human liver allografts under cyclosporine or FK 506 therapy. Transplan Proc. 1991 Feb;23(1 Pt 1):396–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Manez R, Kelly RH, Kobayashi M, Takaya S, Bronsther O, Kramer D, et al. Immunoglobulin G lymphocytotoxic antibodies in clinical liver transplantation: studies toward further defining their significance. Hepatology. 1995 May;21(5):1345–52. doi: 10.1002/hep.1840210519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaldson PT, Alexander GJ, O'Grady J, Neuberger J, Portmann B, Thick M, et al. Evidence for an immune response to HLA class I antigens in the vanishing-bileduct syndrome after liver transplantation. Lancet. 1987 Apr 25;1(8539):945–51. doi: 10.1016/s0140-6736(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 13.Batts KP, Moore SB, Perkins JD, Wiesner RH, Grambsch PM, Krom RA. Influence of positive lymphocyte crossmatch and HLA mismatching on vanishing bile duct syndrome in human liver allografts. Transplantation. 1988 Feb;45(2):376–9. doi: 10.1097/00007890-198802000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Knechtle SJ, Kolbeck P, Tsuchimoto S, Sanfilippo F, Bollinger RR. Hyperacute rejection of liver transplants in rats. Current Surgery. 1986 Jul-Aug;43(4):303–5. [PubMed] [Google Scholar]
- 15.Knechtle SJ, Kolbeck PC, Tsuchimoto S, Coundouriotis A, Sanfilippo F, Bollinger RR. Humoral rejection of rat hepatic transplants by passive transfer of serum. Transplan Proc. 1987 Feb;19(1 Pt 2):1072–6. [PubMed] [Google Scholar]
- 16.Tait BD, Susal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013 Jan 15;95(1):19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 17.O'Leary JG, Kaneku H, Jennings LW, Banuelos N, Susskind BM, Terasaki PI, et al. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Live Transpl. 2013 Sep;19(9):973–80. doi: 10.1002/lt.23687. [DOI] [PubMed] [Google Scholar]
- 18.Taner T, Gandhi MJ, Sanderson SO, Poterucha CR, De Goey SR, Stegall MD, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012 Jun;12(6):1504–10. doi: 10.1111/j.1600-6143.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Leary JG, Kaneku H, Susskind BM, Jennings LW, Neri MA, Davis GL, et al. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection Postliver transplant. Am J Transplant. 2011 Sep;11(9):1868–76. doi: 10.1111/j.1600-6143.2011.03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Leary JG, Klintmalm GB. Impact of donor-specific antibodies on results of liver transplantation. Curr Opin Organ Transplant. 2013 Jun;18(3):279–84. doi: 10.1097/MOT.0b013e3283614a10. [DOI] [PubMed] [Google Scholar]
- 21.Musat AI, Agni RM, Wai PY, Pirsch JD, Lorentzen DF, Powell A, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. 2011 Mar;11(3):500–10. doi: 10.1111/j.1600-6143.2010.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozlowski T, Rubinas T, Nickeleit V, Woosley J, Schmitz J, Collins D, et al. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011 Apr;17(4):357–68. doi: 10.1002/lt.22233. [DOI] [PubMed] [Google Scholar]
- 23.O'Leary JG, Kaneku H, Demetris AJ, Marr JD, Shiller SM, Susskind BM, et al. Antibody-mediated rejection as a contributor to previously unexplained early liver allograft loss. Liver Transpl. 2014 Feb;20(2):218–27. doi: 10.1002/lt.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaneku H, O'Leary JG, Taniguchi M, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with chronic rejection and graft loss after liver transplantation. Liver Transpl. 2012 Aug;18(8):984–92. doi: 10.1002/lt.23451. [DOI] [PubMed] [Google Scholar]
- 25.Kozlowski T, Andreoni K, Schmitz J, Hayashi PH, Nickeleit V. Sinusoidal C4d deposits in liver allografts indicate an antibody-mediated response: diagnostic considerations in the evaluation of liver allografts. Liver Transpl. 2012 Jun;18(6):641–58. doi: 10.1002/lt.23403. [DOI] [PubMed] [Google Scholar]
- 26.Lunz J, Ruppert KM, Cajaiba MM, Isse K, Bentlejewski CA, Minervini M, et al. Re-examination of the lymphocytotoxic crossmatch in liver transplantation: can C4d stains help in monitoring? Am J Transplant. 2012 Jan;12(1):171–82. doi: 10.1111/j.1600-6143.2011.03786.x. [DOI] [PubMed] [Google Scholar]
- 27.Bellamy CO. Complement C4d immunohistochemistry in the assessment of liver allograft biopsy samples: applications and pitfalls. Liver Transpl. 2011 Jul;17(7):747–50. doi: 10.1002/lt.22323. [DOI] [PubMed] [Google Scholar]
- 28.Hubscher SG. Antibody-mediated rejection in the liver allograft. Curr Opin Organ Transplant. 2012 Jun;17(3):280–6. doi: 10.1097/MOT.0b013e328353584c. [DOI] [PubMed] [Google Scholar]
- 29.Paterno F, Shiller M, Tillery G, O'Leary JG, Susskind B, Trotter J, et al. Bortezomib for acute antibody-mediated rejection in liver transplantation. Am J Transplant. 2012 Sep;12(9):2526–31. doi: 10.1111/j.1600-6143.2012.04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Demetris AJ, Todo S, Kang Y, Tzakis A, Duquesnoy R, et al. Evidence for hyperacute rejection of human liver grafts: The case of the canary kidneys. Clin Transplant. 1989;3:37–45. [PMC free article] [PubMed] [Google Scholar]
- 31.Martin T, Schwartz J, Demetris A, Comstock J, Lowichik A, Book L. Plasmapheresis treatment of antibody-mediated rejection in an A2 donor to O pediatric liver transplant recipient. Pediatr Transplant. 2011 Feb;15(1):E15–8. doi: 10.1111/j.1399-3046.2009.01224.x. [DOI] [PubMed] [Google Scholar]
- 32.Hallgren KA. Computing Inter-Rater Reliability for Observational Data: An Overview and Tutorial. Tutor Quant Methods Psychol. 2012;8(1):23–34. doi: 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weil R, 3rd, Clarke DR, Iwaki Y, Porter KA, Koep LJ, Paton BC, et al. Hyperacute rejection of a transplanted human heart. Transplantation. 1981 Jul;32(1):71–2. [PMC free article] [PubMed] [Google Scholar]
- 34.Jaramillo A, Smith MA, Phelan D, Sundaresan S, Trulock EP, Lynch JP, et al. Development of ELISA-detected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999 Apr 27;67(8):1155–61. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- 35.de Kort H, Roufosse C, Bajema IM, Drachenberg CB. Pancreas transplantation, antibodies and rejection: where do we stand? Curr Opin Organ Transplant. 2013 Jun;18(3):337–44. doi: 10.1097/MOT.0b013e3283614a5c. [DOI] [PubMed] [Google Scholar]
- 36.Solez K, Racusen LC. The Banff classification revisited. Kidney Int. 2013 Feb;83(2):201–6. doi: 10.1038/ki.2012.395. [DOI] [PubMed] [Google Scholar]
- 37.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Mar;12(3):563–70. doi: 10.1111/j.1600-6143.2011.03926.x. Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012 Jan 8;307(3):283–93. doi: 10.1001/jama.2011.2014. [DOI] [PubMed] [Google Scholar]
- 39.Takaya S, Bronsther O, Iwaki Y, Nakamura K, Abu-Elmagd K, Yagihashi A, et al. The adverse impact on liver transplantation of using positive cytotoxic crossmatch donors. Transplantation. 1992 Feb;53(2):400–6. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaneku H, O'Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, et al. De Novo Donor-Specific HLA Antibodies Decrease Patient and Graft Survival in Liver Transplant Recipients. Am J Transplant. 2013 Jun;13(6):1541–8. doi: 10.1002/ajt.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salah A, Fujimoto M, Yoshizawa A, Yurugi K, Miyagawa-Hayashino A, Sumiyoshi S, et al. Application of complement component 4d immunohistochemistry to ABO-compatible and ABO-incompatible liver transplantation. Liver Transpl. 2014 Feb;20(2):200–9. doi: 10.1002/lt.23789. [DOI] [PubMed] [Google Scholar]
- 42.Datta GS, Hudson M, Burroughs AK, Morris R, Rolles K, Amlot P, et al. Grading of cellular rejection after orthotopic liver transplantation. Hepatology. 1995;21(1):46–57. [PubMed] [Google Scholar]


