Abstract
Rationale
Allopregnanolone (ALLO) is an endogenous neuroactive steroid thought to alter the reinforcement value of alcohol (ethanol) due to its actions as a positive modulator of the GABAA receptor (GABAAR). Extrasynaptic GABAARs may be a particularly sensitive target of ethanol and neuroactive steroids. Previous work showed that systemic injections of an ALLO analog, ganaxolone (GAN), or an extrasynaptic GABAAR agonist (gaboxadol; THIP), decreased ethanol intake in male mice with limited access to ethanol.
Objectives
The present studies tested whether activation of GABAARs in the nucleus accumbens (NAc) shell by GAN or THIP was sufficient to reduce ethanol intake. C57BL/6J male mice had 2-h access to 10% ethanol (10E) and water, and 10E intake was measured following site-specific infusions of GAN or THIP.
Results
Decreases in limited-access 10E consumption were observed following site-specific bilateral infusions of either drug into the NAc shell. Significant changes in intake were absent when the drugs were infused in a region dorsal to the target site (GAN) or into the lateral ventricle (THIP). Locomotor data confirmed that the decreases in intake were not due to a sedative effect of the drugs.
Conclusions
These data demonstrate the sufficiency of GABAAR activation by a positive allosteric modulator or an agonist with selectivity for extrasynaptic GABAARs to decrease ethanol consumption in mice. Importantly, more refined GABAAR-active targets that decrease ethanol intake may enhance our understanding and ability to treat alcohol use disorders.
Keywords: alcohol, allopregnanolone, extrasynaptic, GABAA receptor, locomotor, neurosteroids, THIP, tonic
INTRODUCTION
Alcohol use disorders (AUDs) affect approximately 18 million Americans, yet underlying mechanisms remain poorly understood, and treatment options are limited. One neuroanatomical region that has been postulated to be important in the regulation of alcohol (ethanol) intake is the nucleus accumbens (NAc) (Koob, 1992; Hodge et al., 1995), and evidence suggests that simple states of reward and aversion are encoded by the activity of NAc medium spiny γ-aminobutyric acid (GABA)ergic neurons (see Carlezon and Thomas, 2009). Additionally, infusion of GABAA receptor (GABAAR) agonists or antagonists into the shell subregion of the NAc decreases ethanol intake in rats (Stratford and Wirtshafter, 2011; Hyytiä and Koob, 1995; Eiler and June, 2007), suggesting that GABAARs in the NAc shell may contribute to the regulation of ethanol consumption.
Allopregnanolone (ALLO) is an endogenous neuroactive steroid that acts as a potent positive modulator at GABAARs. ALLO levels may be increased in the brain or plasma following ethanol injection or oral administration in rodents (e.g. VanDoren et al., 2000; Finn et al., 2004) and in the plasma of humans following ethanol intake (Torres and Ortega, 2003, 2004; but see Porcu et al., 2010). Systemic or intracerebroventricular (ICV) infusions of ALLO produced dose-dependent biphasic changes in ethanol intake in a variety of procedures in rodents (Ford et al., 2005, 2007; Sinnott et al., 2002; Janak et al., 1998; Janak and Gill, 2003). Similar biphasic effects on ethanol intake were documented following systemic injections of ganaxolone (GAN), the 3β-methylated analog of ALLO (Besheer et al., 2010; Ramaker et al., 2011, 2012) that has a similar pharmacological property as ALLO, but a half-life about 3–4 times longer when given systemically (Carter et al., 1997). The first aim of the present study was to test the hypothesis that positive modulation of GABAARs in the NAc shell with GAN is sufficient to decrease limited-access ethanol intake in mice.
GABAARs are pentameric chloride channels comprised from a pool of at least 16 possible subunits. GABAARs containing the δ-subunit are thought to be located exclusively in the extrasynaptic space, where they regulate tonic inhibition (Farrant and Nusser, 2005). In vitro work suggests that δ-subunit-containing GABAARs may show enhanced sensitivity to neuroactive steroids (Belelli et al., 2002) and ethanol (Mody et al., 2007; Olsen et al. 2007; but see Borghese and Harris, 2007). Similarly, δ subunit knockout mice showed reduced sensitivity to neuroactive steroids and ethanol, and these mice consumed less ethanol than their wild-type counterparts (Mihalek et al., 1999, 2001).
Administration of a systemic GABAAR agonist with preference for the δ-subunit, gaboxadol (THIP), decreased ethanol intake in mice (Moore et al., 2007; Ramaker et al., 2011, 2012). The brain areas underlying the effects of systemic THIP are unknown, but knockdown of the δ-or α4–subunit (generally paired with δ) in the NAc shell, but not the core, decreased ethanol intake in rats (Rewal et al., 2009, 2011; Nie et al., 2011). Therefore, the second aim tested the hypothesis that preferentially activating extrasynaptic GABAARs in the NAc shell with THIP is sufficient to decrease limited-access ethanol intake in mice. The use of lickometer circuits allowed for additional analysis of underlying bout characteristics contributing to alterations in intake, and locomotor studies examined whether changes to general locomotor activity accounted for the changes in intake following GAN or THIP treatment.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice of approximately 8 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, ME). For drinking studies, mice were singly housed in lickometer chambers on a 12-h dark:light cycle (lights off at 0600). For locomotor studies, mice were pair housed on a 12-h light:dark cycle (lights on at 0600). Mice had at least a week to acclimate to conditions prior to any experiments and had ad libitum access to rodent chow and water throughout experiments. Efforts were made to minimize animal suffering and to reduce the number of mice used. All procedures complied with the United States Public Health Service Institutes of Health guidelines in accordance with the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research,” were approved by the local Institutional Animal Care and Use Committee, and complied with NIH guidelines.
Apparatus
Lickometer chambers (described in Ford et al., 2005) were each composed of a 4-walled plexiglas insert (7 × 4 × 7 inches) positioned on an elevated wire grid floor inside a shoebox cage with bedding. The insert contained a hinged top and two small holes where the sippers of the drinking bottles protruded into the cage. A lickometer device (MED Associates Inc., St. Albans, VT), which was interfaced to a computer running MED-PC IV software (MED Associates Inc.), recorded time-stamped licks for each mouse and each fluid presented.
Automated locomotor chambers (40 × 40 × 30 cm; AccuScan Instruments Inc., Columbus, OH) were used (described in Gubner et al., 2013). The chambers contained 8 pairs of photocell beams positioned 2 cm above the floor. Beam interruptions were converted to horizontal distance traveled (cm) by VERSADAT software (AccuScan Instruments Inc.).
Drugs
Ethanol (200 proof; Pharmco Products, Brookfield, CT) was diluted in tap water to yield a 10% (v/v) ethanol solution (10E). GAN was purchased from Dr. Robert Purdy (VA Research Foundation, San Diego, CA) and mixed with 20% (w/v) 2-hydroxypropyl-β-cyclodextrin (β-CD; Cargill Inc., Cedar Rapids) and 3% (v/v) dimethyl sulfoxide (DMSO, Mallinckrodt Baker, Inc., Paris, KY) in high-purity water. THIP (4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol hydrochloride) was purchased from Tocris Bioscience (Ellisville, MO) and dissolved in artificial cerebral spinal fluid. Control (0 ng) infusions used the vehicle appropriate for each drug.
Surgery
Mice were maintained under isoflurane anesthesia and implanted with either 2 independent guide cannulae (26 Ga. Stainless steel, 12.0 mm long) aimed at the NAc shell [A/P: +1.34, L/M: ± 0.5, and D/V −2.75 (−4.75 mm with injectors; 33 Ga.)] or a unilateral guide cannula aimed at the lateral ventricle [A/P: −0.46, L/M: ± 1.2, D/V −2.0 (−2.5 mm with injectors)]. Mice were allowed a minimum of one week to recover from surgery before being used in any experiment.
General Drinking Experiment
Drinking studies were performed during the dark phase of the light:dark cycle to maximize ethanol drinking. Beginning two hours into the dark cycle, mice had 2-h access to 10E and water; bottle positions were counterbalanced between the left or right side across lickometer chambers. This concentration of ethanol was chosen in order to match studies testing the effects of systemic GAN and THIP on ethanol intake (Ramaker et al., 2012). At the conclusion of the 2-h period, fluid consumed was measured (to the nearest 0.05 ml), and bottles were replaced with two 50-ml water bottles. To control for leakage, fluid lost from bottles on control cages were subtracted from the amount consumed from each bottle. Mice had a minimum of 4 weeks of ethanol access before any drug infusions to allow the establishment of stable drinking. Drug infusions were given immediately prior to the session in 200 nL of fluid delivered per side over 60 seconds, with an additional 30 seconds allowed for diffusion. Infusions were given weekly and baseline intake was re-established between each drug infusion. Baseline 10E intakes (the average of all non-infusion days that immediately preceded a drug day) are provided in Table 1 and did not statistically differ between studies.
Table 1.
Baseline 10% ethanol (10E) intakes
| NAc shell GAN |
Dorsal GAN | 1 shell + 1 core GAN |
ICV GAN | NAc shell THIP |
ICV THIP |
|---|---|---|---|---|---|
| 2.44 ± 0.15 | 2.34 ± 0.29 | 2.40 ± 0.19 | 2.81 ± 0.17 | 2.45 ± 0.23 | 2.68 ± 0.18 |
Baseline 10E intakes for each study are given in g/kg and are defined as the average intake on all days that immediately preceded a drug or vehicle day. Values represent mean ± SEM for each study. Baseline intakes did not differ statistically between studies. NAc = nucleus accumbens. GAN = ganaxolone. ICV = intracerebroventricular.
NAc shell GAN: 10E intake
A total of 15 mice with confirmed bilateral NAc shell placements were used to test the effect of intra-NAc shell GAN on 10E intake. GAN was tested across 4 experimental passes using separate groups of mice, with doses given in either ascending or descending order to control for potential order effects. In 3 of the passes (n = 11), mice received weekly GAN infusions in ascending dose order: 0 ng (pre), 50 ng, 100 ng, 500 ng, 0 ng (post). In the 4th pass, mice (n = 4) received weekly GAN infusions in descending order: 0 ng (pre), 500 ng, 100 ng, 50 ng, 0 ng (post). Pre and post vehicle infusions were used to determine whether mechanical or psychological stress of the infusions altered ethanol intake differently after multiple infusions. Each mouse received 5 microinfusions; pilot data from our lab examined the effect of 5 microinfusions of 3% DMSO/20%β-CD, and using thionin staining, determined this number of infusions did not lead to scarring outside of the injector tracks (Ramaker and Finn, unpublished). The concentrations of GAN were chosen to be consistent with ICV doses of ALLO examined in a previous study (Ford et al., 2007).
NAc shell THIP: 10E intake
A total of 9 mice with confirmed bilateral NAc shell placements were used to test the effect of intra-NAc shell THIP on 10E intake. THIP was tested across 2 experimental passes, using separate groups of mice. In pass 1 (n = 5), drug was infused weekly in ascending order: 0 ng (pre), 50 ng, 100 ng, 500 ng, 0 ng (post). In order to assess order effects, drug was given in descending order in the second group of mice (n = 4): 0 ng (pre), 500 ng, 100 ng, 0 ng (post). Because 50 ng was not repeated in the second cohort, only 0, 100, and 500 ng THIP doses were analyzed. Concentrations of THIP were chosen based on a study testing the effect of NAc core infusions of THIP in α4 knockout and wildtype mice (Maguire et al., 2014).
ICV GAN or THIP: 10E intake
Mice (n = 10 confirmed ICV placements) received weekly GAN infusions in the following order: 0 ng, 500 ng, 0 ng. After a week of no infusions, the same mice received weekly infusions of THIP: 0 ng, 500 ng, 1000 ng, 0 ng.
NAc shell GAN or THIP: locomotor activity
Naïve mice (n = 11 confirmed bilateral NAc shell placements) were used to test the locomotor effects of 500 ng GAN or THIP. Mice were tested during the light phase of the light:dark cycle and at the same time each day. Locomotor studies were performed during the light phase in order to allow the detection of both drug-induced increases and decreases in activity. On days 1 (acclimation) and 2 (baseline), injectors were lowered but no drug was infused, and mice were immediately placed in the chambers where locomotor activity was recorded for 2 hours. On day 3, separate groups of mice received a bilateral infusion of either 0 (n = 6) or 500 ng (n = 5) THIP and were immediately placed in the activity chambers. A week later (day 4), mice received either 0 (n = 5) or 500 ng (n = 6) GAN, with groups balanced for previous drug infusion. Drug-induced locomotor activity was determined by subtracting baseline horizontal locomotor (cm) activity on day 2 from activity on day 3 (THIP) or on day 4 (GAN).
Blood ethanol concentrations (BECs)
At the conclusion of a 2-hour access period on a non-infusion day, blood samples (20 µL) were collected from the orbital sinus in a subset of mice and analyzed using head-space gas chromatography, as described previously (Finn et al., 2007). Concentrations of samples were interpolated from a standard curve with 6 pairs of external standards with known ethanol concentrations (from 0.5 to 3.0 mg/ml).
Histological Confirmation
At the conclusion of studies, mice were euthanized and infused with 17 mg/ml methylene blue dye in 20% β-CD. Intact whole brains were removed and flash frozen in isopentane and stored at −80°C. Brains were sliced (35 µm sections) using a Leica cryostat, mounted on glass slides, and photographed with an IM50 imaging system (Leica Microsystems Imaging Solutions Ltd, Buffalo Grove, IL). Only placements confirmed by two independent investigators as bilateral hits for the NAc shell or unilateral ICV were used in analyses.
For the drinking study, bilateral cannulation of the NAc shell was attempted in 41 mice for use in the GAN experiment. Correct placements were confirmed for 15 mice. For 9 mice, placements were too dorsal, and for another 9 mice, placements consisted of one injector terminating in the shell and one terminating in the core. These misses were therefore used as control regions to examine the brain region specificity of the GAN effect (see below), and importantly, average g/kg 10E intake following vehicle infusion did not statistically differ for those used in the control studies versus the NAc shell study. There were 3 placements that could not be verified, and the remaining 5 mice had placements with 1 injector in shell but 1 dorsal to shell (n = 2), in the shell but also in the ventricles (n = 1), or too anterior (n = 2).
For the drinking study, bilateral NAc shell cannulation was attempted in 19 mice for use in the THIP experiment. Correct placements were confirmed for 9 mice. For the remaining 10 mice, the placements consisted of: dorsal to the NAc shell (n = 2); one injector in the core and one in the shell (n = 2); in the shell, but also in the ventricles (n = 2); too ventral (n = 2); and one side in the shell and one side too dorsal (n = 2). Because there were not enough placements in one particular brain region, the misses were not used to examine the site-specificity of THIP treatment.
For the locomotor study, bilateral NAc shell cannulation was attempted in 20 mice. Correct placements were confirmed in 11 mice. There were 3 mice in which placements could not be verified. For the remaining 6 mice, placements consisted of: dorsal to the NAc shell (n = 2), one injector in the core and one in the shell (n = 1), or one injector in the core and one too medial (n = 3).
For the ICV study, unilateral cannulation was attempted and confirmed in 10 mice.
Statistical Analysis
For drinking studies, data were removed if intake was more than 2 standard deviations above or below the group mean. Data removed as statistical outliers or a missed infusion due to a clogged cannula were: [GAN NAc shell: 1 each at 0 ng (pre), 50 ng, 100 ng, 500 ng; dorsal control: 1 at 500 ng; unilateral shell + unilateral core: 1 each at 0 ng (pre), 50 ng, and 100 ng; THIP NAc shell: 1 each at 100 ng and 500 ng)]. For all studies, there were no pass by dose interactions and no differences between 0 ng (pre) and 0 ng (post) for g/kg 10E consumed, so data were collapsed on pass and pre/post vehicle values. For all studies, 2-h water intake was below the level of detection (< 0.05 ml), preventing statistical analysis.
Patterns of 10E licks were recorded using MED-PC IV software, and SoftCR version 4 (MED Associates, Inc.) was used to access the time-stamped data. A bout was defined as a minimum of 20 licks with no more than a 60-s pause between successive licks (Ford et al., 2005, 2007; Ramaker et al., 2011, 2012). There were 3 instances of lost data for 10E licks [GAN bilateral NAc shell: 2 data files that did not save (50 ng) and 1 where the lickometer was disconnected (100 ng)]. For the remaining data, g/kg intake correlated with 10E licks recorded on each vehicle or drug infusion day (range: r = 0.66 to 0.97; p = 0.02 to < 0.001).
For drinking experiments, the effect of drug was analyzed using repeated-measures analyses of variance (ANOVAs) factored on dose. Licks were “binned” in 20-minute intervals to assess patterns of drinking; a two-way ANOVA was conducted with both time (20-minute bins) and dose analyzed as repeated measures, and following a significant interaction, one-way ANOVAs were performed. In the event of a significant main effect, pair-wise differences against vehicle were determined by the Fisher’s Least Significance Difference multiple comparisons test. For locomotor studies, dose was analyzed as a between-subjects measure and time (20-minute bins) was analyzed as a repeated-measure. All statistical analyses were performed using SYSTAT 11. Graphs were made using GraphPad Prism 4 for Windows. For all analyses, statistical significance was set at p ≤ 0.050.
RESULTS
NAc GAN: 10E intake
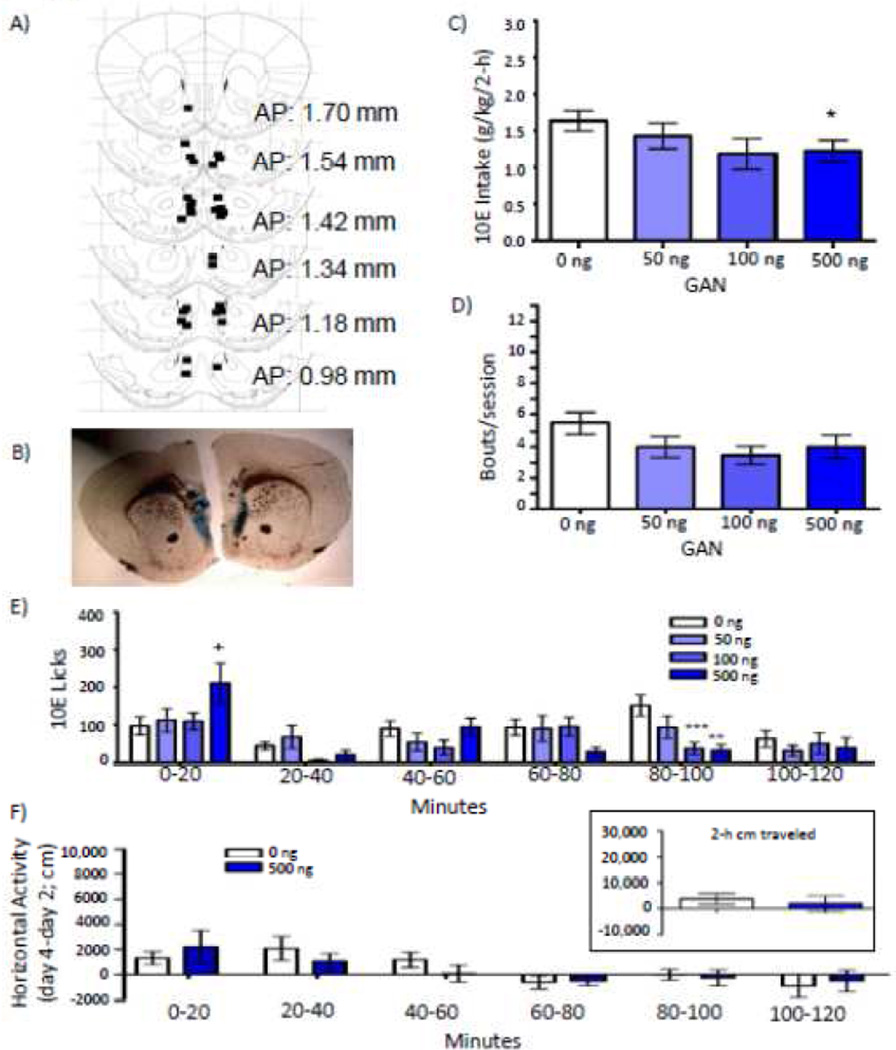
Placements for mice that received GAN (n = 15) into the NAc shell for the drinking study are shown in Figure 1A, with a representative micrograph shown in Figure 1B. BECs on a non-infusion day were 61 ± 18 mg/dl (consumption = 2.51 ± 0.25 g/kg; n = 6; data not shown). Over the entire 2-h time period, GAN decreased g/kg ethanol intake [F(3,33) = 2.944; p = 0.047; Fig. 1C], with a significant decrease of 25% following 500 ng (p = 0.025). GAN led to early termination of the session [F(3,27) = 3.871; p = 0.020], measured by the time at which each mouse initiated its last bout, and this was significant following 500 ng (p = 0.002; Table 2). Despite a 27% decrease in bout frequency with 500 ng GAN, there was no significant dose effect on this (Fig. 1D) or any other bout parameter measured (Table 2).
Figure 1.
Effect of bilateral intra-nucleus accumbens (NAc) shell infusions of ganaxolone (GAN) on 10% ethanol (10E) intake. A) Squares depict injector tip locations for mice (n = 15) used in the drinking studies for GAN infused into the NAc shell. B) A sample photomicrograph of a mouse that received a bilateral NAc shell infusion of GAN. C) Effect of NAc shell GAN on total 2-hour 10E intake, D) average bout frequency, and E) 10E licks split into 20-minute bins. F) Horizontal locomotor activity (cm) following a NAc shell infusion of 0 or 500 ng GAN across 20-minute bins, subtracting out cm moved on a baseline day. The inset depicts total 2-hour activity. Values represent mean ± SEM. + p ≤ 0.10, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 relative to 0 ng.
Table 2.
Effect of bilateral intra-nucleus accumbens shell ganaxolone (GAN) infusions on bout parameters for limited-access consumption of 10% ethanol (10E)
| Dose GAN | 0 ng | 50 ng | 100 ng | 500 ng |
|---|---|---|---|---|
| 2-hr 10E licks | 541 ± 74 | 467 ± 67 | 334 ± 38 | 425 ± 72 |
| 22-hr water intake (ml) | 3.3 ± 0.4 | 3.2 ± 0.4 | 3.0 ± 0.3 | 2.6 ± 0.5 |
| Total fluid intake (ml) | 3.9 ± 0.4 | 3.7 ± 0.5 | 3.4 ± 0.3 | 3.1 ± 0.5 |
| Average bout size (licks) | 98 ± 10 | 111 ± 11 | 103 ± 10 | 115 ± 14 |
| Latency to first lick (min) | 14.3 ± 4 | 19.2 ± 7 | 19.7 ± 9 | 9.1 ± 3 |
| Latency to first bout (min) | 20.8 ± 6 | 22.3 ± 7 | 20.6 ± 9 | 9.4 ± 3 |
| First bout size (licks) | 118 ± 20 | 120 ± 18 | 97 ± 10 | 116 ± 21 |
| Termination of last bout (minutes) | 104 ± 10 | 86 ± 10 | 84 ± 10 | 58 ± 12** |
Values represent mean ± SEM for each dose. 0 ng represents the average of the pre and post vehicle infusion.
p ≤ 0.01 relative to 0 ng.
Consistent with the g/kg consumption data, there was a trend for GAN to reduce overall 10E licks [F(3,33) = 2.672; p = 0.063; Table 2]. To better understand the temporal distribution of drinking, 10E licks were parsed into 20-minute bins. GAN differentially altered 10E licks across the 20-minute intervals revealed by a significant time by dose interaction [F(15,135) = 3.395; p = 0.001; Fig. 1E]; specifically, GAN increased 10E licks during minutes 0 – 20 [F(3,27) = 5.635; p = 0.004] and decreased 10E licks during minutes 80 – 100 [F(3,27) = 3.875; p = 0.020].
NAc GAN: locomotor activity
There was no difference in day 2 baseline activity between mice that subsequently received an infusion of 0 versus 500 ng GAN. Locomotor activity over the 2-h session did not differ following a NAc shell infusion of 0 ng (n = 5) versus 500 ng GAN (n = 6) (Fig. 1F inset), and there was no interaction between dose and time (20-minute interval; Fig. 1F).
Dorsal control GAN: 10E Intake
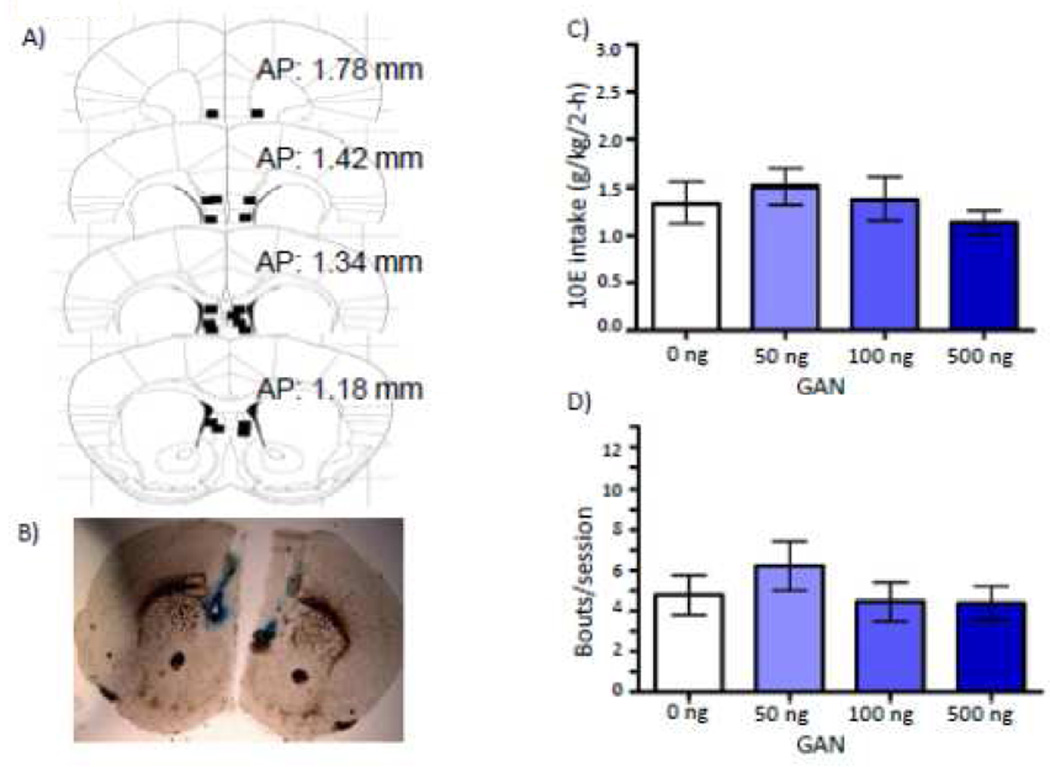
Histological analysis revealed that nine mice that had received GAN prior to 10E access had bilateral injector placements that were located dorsal to the NAc shell (Fig. 2A & B). Data from these mice were analyzed in order to assess the specificity of the NAc shell GAN effects on 10E intake. There was no effect of GAN on g/kg 10E intake [F(3,21) = 1.74; p = 0.540; Fig 2C), bout frequency (Fig. 2D) or any other bout parameter measured (data not shown). There was an interaction between GAN dose and 20-minute interval [F(15,105)= 2.329; p = 0.007] on 10E licks, but post-hoc ANOVAs revealed no time point at which there was a significant effect of dose (data not shown).
Figure 2.
Effect of dorsal control infusions of ganaxolone (GAN) on 10% ethanol (10E) intake. A) Squares depict injector tip locations for mice (n = 9) used in the drinking studies for dorsal controls infused with GAN. B) A sample photomicrograph of a mouse that received a bilateral infusion of GAN dorsal to the NAc shell. C) Effect of GAN into regions dorsal to the nucleus accumbens shell (NAc) on total 2-hour 10E intake and D) average bout frequency.
1 Shell + 1 Core GAN: 10E Intake
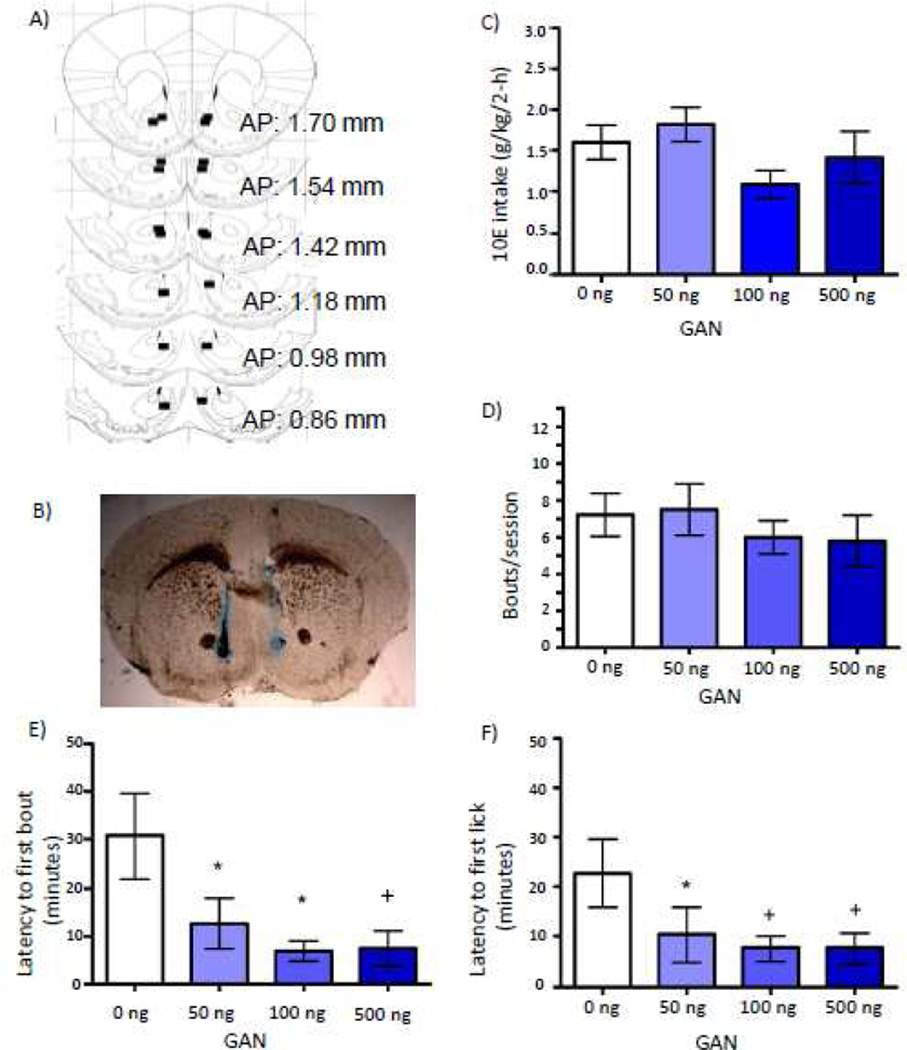
In a subset of mice that received GAN (n = 9) prior to 10E access, there were placements consisting of one injector terminating in the shell and one in the core (Fig. 3 A & B). Data were analyzed from this cohort in order to gain insight into the extent of potential diffusion of the drug. There was no significant effect of GAN infusion on 10E intake [F(3,18)= 1.627; p = 0.218; Fig. 3C), bout frequency (Fig. 3D), average bout size, first bout size, or termination of final bout (data not shown). GAN significantly decreased latency to first bout [F(3,15)= 4.808; p = 0.015; Fig. 3E] and latency to first lick [F(3,18)= 3.991; p = 0.024; Fig 3F]. There was no significant interaction between dose and time (20-minute bins) on 10E licks (data not shown).
Figure 3.
Effect of intra-NAc GAN, with one injector placed in the NAc shell and one in the NAc core on 10% ethanol (10E) intake. A) Squares depict injector tip locations for mice (n = 9) that received GAN prior to the drinking studies, where one injector terminated in the NAc shell and one in the NAc core. B) A sample photomicrograph of a mouse that received a bilateral infusion of GAN with one injector located in the core and one injector located in the shell. C) Effect of GAN into the NAc core + shell on total 2-hour 10E intake, D) average bout frequency, E) latency to first bout, and F) latency to first lick. Values represent mean ± SEM. + p ≤ 0.10, * p ≤ 0.05 relative to 0 ng.
ICV GAN: 10E Intake
An ICV infusion of 500 ng GAN (n = 10) significantly decreased g/kg 10E intake, with a 22% decrease from vehicle (t(9) = 2.758; p = 0.022; 0 ng: 2.7 ± 0.2 g/kg; 500 ng: 2.1 ± 0.2 g/kg). GAN also significantly decreased bout frequency (t(9) = 3.126; p = 0.012; 0 ng: 10.5 ± 0.9 bouts/session; 500 ng: 8.2 ± 0.7 bouts/session) but did not alter any other bout parameter (data not shown). There was no significant interaction between dose and time when examining 10E licks split into 20-minute intervals (data not shown).
NAc THIP: 10E intake
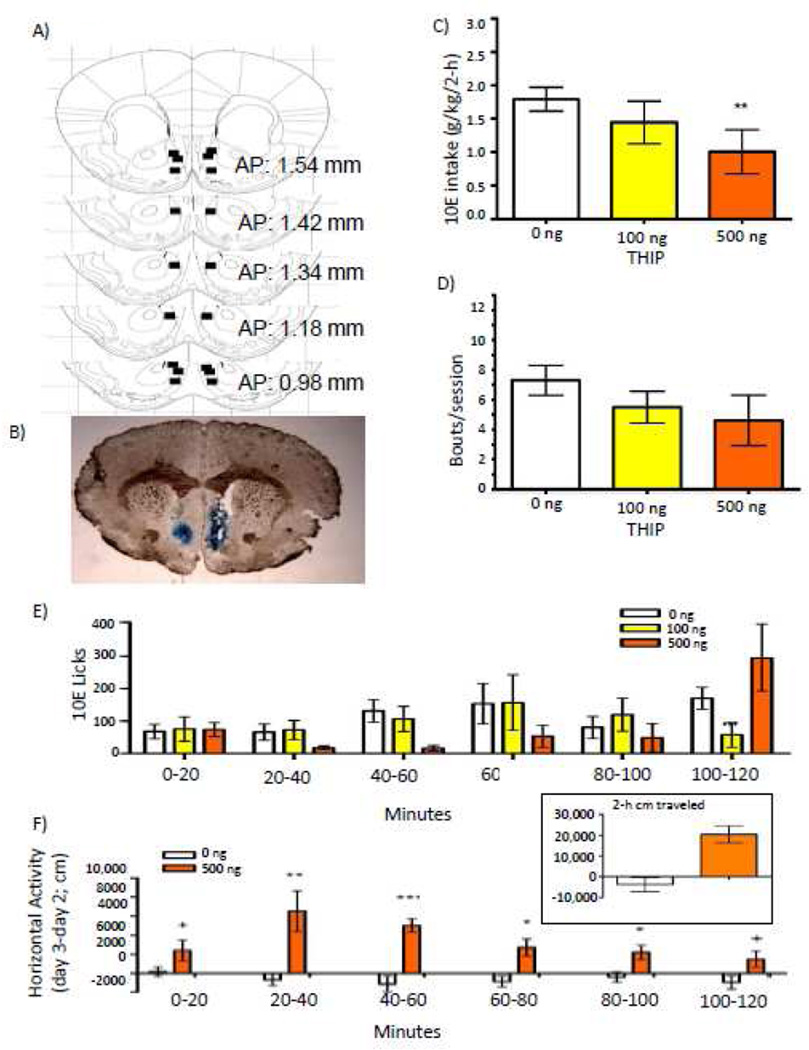
Placements for mice that received THIP (n = 9) into the NAc shell for the drinking study are shown in Figure 4A, with a representative micrograph shown in Figure 4B. BECs on a non-infusion day were 91 ± 21 mg/dl (consumption = 3.01 ± 0.41 g/kg; n = 5; data not shown). Over the entire 2-h time period, THIP decreased g/kg ethanol intake [F(2,12) = 8.75; p = 0.036; Fig. 4C], with 500 ng THIP significantly reducing intake by 46% (p = 0.008). There was no significant effect of THIP on bout frequency (Fig. 4D) or any other bout parameter measured (Table 3). THIP dose differentially altered 10E licks across 20-minute bins [F(10,60) = 2.419; p = 0.017; Fig. 4E], but post-hocs revealed no bin in which there was a main effect of dose.
Figure 4.
Effect of intra-NAc shell infusions of THIP on 10% ethanol (10E) intake. A) Squares depict injector tip locations for mice (n = 9) used in the drinking studies for nucleus accumbens (NAc) shell infusions with THIP. B) A sample photomicrograph of a mouse that received a bilateral NAc shell infusion of THIP. C) Effect of NAc shell THIP on total 2-hour 10E intake, D) average bout frequency, and E) 10E licks split into 20-minute bins. F) Horizontal locomotor activity (cm) following a NAc shell infusion of 0 or 500 ng THIP, subtracting out cm moved on a baseline day. The inset depicts total 2-hour activity. Values represent mean ± SEM for each dose. + p ≤ 0.10, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 relative to 0 ng.
Table 3.
Effect of bilateral intra-nucleus accumbens shell THIP infusions on bout parameters for limited-access consumption of 10% ethanol (10E)
| Dose THIP | 0 ng | 100 ng | 500 ng |
|---|---|---|---|
| 2-hr 10E licks | 668 ± 70 | 587 ± 139 | 502 ± 161 |
| 22-hr water intake (ml) | 3.2 ± 0.4 | 2.6 ± 0.5 | 3.8 ± 0.2 |
| Total fluid intake (ml) | 3.9 ± 0.3 | 3.1 ± 0.6 | 4.1 ± 0.3 |
| Average bout size (licks) | 98 ± 7 | 104 ± 15 | 105 ± 22 |
| Latency to first lick (minutes) | 24.8 ± 6 | 19.3 ± 8 | 8.7 ± 4 |
| Latency to first bout (minutes) | 25.0 ± 7 | 20.7 ± 8 | 11.5 ± 5 |
| First bout size (licks) | 93 ± 16 | 101 ± 23 | 71 ± 13 |
| Termination of last bout (minutes) | 115 ± 1 | 83 ± 7 | 102 ± 14 |
Values represent mean ± SEM for each dose. 0 ng represents the average of the pre and post vehicle infusion.
NAc THIP: locomotor activity
There was no difference in day 2 baseline activity between mice that subsequently received an infusion of 0 versus 500 ng THIP. There was a significant increase in 2-h locomotor activity following NAc shell THIP [F(1,9) = 20.864; p < 0.001; Fig. 4F inset). THIP differentially altered locomotor activity over time, indicated by a significant dose by 20-minute bin interaction [F(5,45) = 3.183; p = 0.015; Fig. 4F]. Specifically, THIP significantly increased activity during minutes 20 – 40 (p = 0.010), minutes 40 – 60 (p = 0.001), minutes 60 – 80 (p = 0.013), and minutes 80 – 100 (p = 0.049).
ICV THIP: 10E Intake
Although there was a 30% decrease in 10E intake following an ICV infusion of 1000 ng THIP (n = 10), the effect of dose was not statistically significant (0 ng: 2.1 ± 0.2 g/kg; 500 ng: 1.9 ± 0.3 g/kg; 1000 ng: 1.4 ± 0.3 g/kg). Despite a 31% decrease in bout frequency with 1000 ng, there was no significant effect of ICV THIP on the number of bouts in the session (0 ng: 8.8 ±1.4 bouts/session; 500 ng: 7.0 ±1.3 bouts/session; 1000 ng: 6.1 ±1.3 bouts/session). THIP had no significant effect on any other bout parameter measured (data not shown), and there was no significant interaction between dose and time when analyzing 10E licks across 20-minute bins (data not shown).
DISCUSSION
The present study demonstrates that a microinfusion of GAN or THIP into the NAc shell is sufficient to account for decreases in limited-access ethanol consumption in mice as had been observed following systemic injections of those drugs (Ramaker et al., 2012). This is the first study showing that a NAc shell infusion of a GABAAR positive modulator or an agonist with preference for extrasynaptic GABAARs decreases ethanol intake in mice, which expands on data demonstrating that a general GABAA agonist (muscimol) infused into the NAc shell decreased ethanol intake in rats (Stratford and Wirtshafter, 2011).
Despite an overall decrease in 10E intake following either GAN or THIP, analysis of time-dependent effects revealed important differences between the drugs. Whereas GAN led to an initial increase in intake followed by a suppression, THIP decreased intake throughout the session. The differences in the drinking profiles are likely related to both the differences in pharmacology and the differences in GABAAR targets between GAN and THIP. GAN can act at both synaptic and extrasynaptic GABAARs (Belelli and Herd, 2003) and due to its properties as a positive allosteric modulator of GABAARs, GAN potentiates the GABA-evoked response (Carter et al., 1997). On the other hand, THIP is a direct agonist that is effective in the absence of GABA, and has a much higher selectivity for extrasynaptic versus synaptic GABAARs (Stórustovu and Ebert, 2006). An initial increase in ethanol intake following GAN treatment is consistent with previous work using systemic ALLO or GAN, whereas an initial increase in intake was absent following THIP injection (Ford et al., 2005, 2007; Ramaker et al., 2011). Although it is not clear if the initial increase in ethanol intake following intra-NAc GAN reflects ethanol seeking, previous data have shown an increase in ethanol seeking following systemic ALLO or GAN, but not THIP injection (Janak and Gill, 2003; Finn et al., 2008; Ramaker et al., 2012, 2014). Together these studies suggest that differences in receptor pharmacology or GABAAR targets between the synthetic neuroactive steroid and direct extrasynaptic agonist may differentially alter ethanol seeking or initial ethanol intake.
Although THIP led to a greater suppression of intake compared to GAN, a direct comparison between the doses of GAN and THIP should be interpreted with caution. The lipophilic nature of GAN renders it more difficult to get into solution, which may have limited how much of the target concentration of drug was available at the infusion site. A solubility limit was confirmed when we attempted to solubilize 1000 ng GAN in the current vehicle and were unsuccessful, and this possibility is further supported by the fact that 500 ng GAN led to little further decrease in intake than the 100 ng dose. It also is possible that the lack of a further decrease in ethanol intake with 500 ng GAN was due to lower selectivity at GABAARs with this dose, since µM concentrations of ALLO have been reported to exert activity at other receptor systems in addition to GABAARs (e.g., Rupprecht and Holsboer, 1999).
Placements were verified with a dye of a similar molecular weight as GAN. Although we cannot rule out diffusion to other brain areas, the data obtained in the control regions suggest that there was regional specificity to the observed effect. Specifically, data obtained in the dorsal control regions suggest that the observed effects in the bilateral shell group cannot be accounted for by diffusion of the drug up the cannula to more dorsal sites. Since there is no bilateral core comparison, limited interpretations can be made from the mice with a unilateral core plus a unilateral shell placement and do not allow interpretations as to the effect of GAN in the core on ethanol intake. However, data obtained in this cohort provide important information about potential diffusion of the drug since promiscuous diffusion throughout the core and shell would be expected to produce a similar effect in the bilateral shell compared to the core + shell group. In contrast, the significant decrease in intake following the bilateral shell infusion of GAN was absent in mice that received GAN with one placement in each of the shell and the core, suggesting that the bilateral shell infusions resulted in minimal diffusion to the core. Interestingly, GAN decreased latency to first bout and first lick in the mice with a unilateral shell and unilateral core placement, but these measures were not statistically altered in the bilateral shell group; these measures could reflect a motor component to ethanol seeking and are consistent with a greater relative role of the core versus the shell on locomotor functions (Maldonado-Irizarry and Kelley, 1995). Together, use of the data from control regions suggests that the bilateral shell GAN infusions were likely limited to the target region.
With GAN, we have now seen similar effects in a 2-h 2-bottle choice procedure following systemic (Ramaker et al., 2012), ICV, and NAc shell infusions, with a 22%, 22%, and 25% decrease in intake following the highest dose administered, respectively, highlighting the contribution of the NAc shell in these effects. There were also similar quantitative differences in bout frequency across these 3 modes of GAN administration, which was also comparable to what was observed following systemic ALLO (Finn et al., 2010; Ford et al., 2005, 2007). Although the present data suggest that the NAc shell is an important brain region underlying GAN's effects, given a similar effect of the ICV and intra-NAc shell infusion (where available GAN concentrations should be much more diffuse following a unilateral ICV infusion of 500 ng versus a bilateral infusion of 500 ng localized to the NAc), it is likely that the NAc shell is not the only brain area contributing to 10E intake following ICV or systemic injections of GAN and that many interacting brain regions contributed to a net decrease in intake.
Decreases in ethanol intake following activation of extrasynaptic GABAARs with THIP may at first seem counter to work showing that a global knockout of the δ subunit or conditional knockdown of the δ subunit in the shell decreased ethanol consumption in rodents (Nie et al., 2011; Mihalek et al., 2001). Based on these studies, it has been postulated that activation of GABAARs containing the δ subunit, particularly in the NAc shell, might be an important component to the signal of ethanol reward. Consistent with this hypothesis, it is possible that activation of δ subunit containing GABAARs by THIP may have led to a left-ward shift in the dose-response curve for ethanol, which can also present itself as a decrease in ethanol intake. A further consideration is that although THIP is thought to act preferentially at δ subunit containing GABAARs, the current THIP doses may have activated synaptic as well as extrasynaptic receptors. The concentrations in the current study correspond to 2.8 and 14 mM for 100 and 500 ng THIP respectively. A previous study found that 3 mM THIP infused into the NAc core altered cocaine reinforced behavior in wild-type but not α4 subunit knockout mice, suggesting that a 3 mM concentration of THIP was selective for α4δ subunit containing receptors in this brain region (Maguire et al., 2014). However, the selectivity of the larger dose of THIP is unknown, and studies in α4 or δ subunit knockout mice may be necessary to better understand the GABAA receptor targets at this dose.
Given that systemic injections of THIP decreased limited-access ethanol intake (Ramaker et al., 2012), the lack of a significant effect following ICV infusion was unexpected. However, δ-subunit-containing GABAARs make up only about 5% of total GABAARs in the brain (McKernan and Whiting, 1996); expression is low or absent in many brain areas, with expression of the δ-subunit most prominent in the thalamus, dorsal and ventral striatum, hippocampus, cerebellum, and cortex (e.g., Hörtnagl et al., 2013). Thus, it is possible that ICV THIP became too diluted as it diffused and was sub-threshold upon reaching the NAc or other relevant brain regions to significantly decrease 10E intake. In other words, larger ICV doses were likely necessary to attain the same concentrations as those following intra-NAc shell or systemic injections. Interestingly, the smaller effect of THIP to decrease intake following infusion into the ventricles (30% with 1000 ng) versus directly into the NAc (46% with 500 ng) provides confidence that the observed outcomes following intra-NAc shell infusions of THIP were not an artifact of inadvertently hitting the ventricles, a potential concern when targeting the shell.
Although the present data do not allow us to determine whether GAN or THIP decreased10E intake by increasing or decreasing the rewarding value of ethanol, the decreases in ethanol intake following GAN and THIP are consistent with data showing the importance of GABAARs in the NAc shell on ethanol consummatory behaviors. GABAARs, including those containing the δ-subunit, are present on medium spiny neurons of both the direct and indirect pathways, as well as several classes of NAc interneurons (Maguire et al., 2014). The cells of the direct pathway primarily contain the dopamine type 1 (D1) receptor and project directly back to the ventral tegmental area (VTA) (Gerfen et al., 1990). Cells of the indirect pathway primarily contain the dopamine type 2 (D2) receptor and project indirectly to the VTA via the ventral pallidum (Gerfen et al., 1990). GAN or THIP’s ability to decrease the excitability of either or both of these groups of cells may have influenced the rewarding properties of ethanol, either by disinhibiting downstream brain regions or by directly altering the encoding of inputs within the NAc. Future studies are necessary to elucidate the cell type(s) that GAN or THIP act on to ultimately decrease ethanol intake, which could provide clues into the mechanism by which these drugs ultimately decrease ethanol intake.
Although our aim was not to limit placements to the medial portion of the shell or to compare it to other areas of the shell, it is of interest that our placements were primarily centered in this area, as data have shown that knockdown of the δ subunit only decreased ethanol intake when the knockdown was localized to the medial, but not the lateral or intermediate regions of the NAc shell (Nie et al., 2011). Consideration of medial shell projections may therefore be important for the interpretation of our results. Specifically, the medial NAc shell is in part distinguished from the rest of the shell by its reciprocal projections with the lateral hypothalamus (Groenewegen et al., 1999), an area with well-established roles in ingestive behaviors (Wise, 1974). For this reason, future studies examining the specificity of the effects of GAN or THIP on ethanol versus other natural reinforcers, such as sucrose, saccharin, or other drugs of abuse will be important.
Analysis of locomotor behavior suggests that the decreases in 10E intake were not due to a sedative effect resulting from the GAN or THIP infusions, but a competing behavior resulting from THIP treatment (i.e., behavioral stimulation) or a combined pharmacological effect of either drug with ethanol cannot be ruled out. Additionally, since the collection of activity data was not performed simultaneously with the drinking data, several considerations must be taken into account. The locomotor data were obtained in naïve mice during the light phase of their light:dark cycle and in the absence of ethanol access. Therefore, it is possible that locomotor testing during the dark phase or when ethanol was on board could produce locomotor effects different from those shown. Additionally, the locomotor effects in the larger activity apparatus may not necessarily reflect those in the smaller lickometer chamber.
In conclusion, these results demonstrate for the first time that an intra-NAc shell infusion of a synthetic neuroactive steroid contributes to ethanol consumption. These data also provide evidence for the sufficiency of activation of GABAARs in the NAc shell by a positive allosteric modulator or an agonist with preference for extrasynaptic GABAARs to regulate ethanol intake. Because GAN and THIP currently are in clinical trials for other purposes (clinicaltrial.gov), the present findings may shed light on novel therapeutic options and may lead to more refined GABAAR-active targets for the treatment of AUDs.
ACKNOWLEDGEMENTS
We thank Michelle Tanchuck-Nipper, Dr. Debra Cozzoli, and Chris Snelling for assistance. Funding was provided by grants R01 AA16981 and R01 AA12439 and the Department of Veterans Affairs (DAF). MJR was supported by F31 AA020716, MMF was supported by KO1 AA16849 and P51 OD011092, and TJP was supported by P60 AA010760 and the Department of Veterans Affairs.
Footnotes
DISCLOSURE/CONFLICTS OF INTEREST
There are no conflicts of interest or competing financial interests in relation to the work described.
REFERENCES
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Lindsay TG, O’Buckley TK, Hodge CW, Morrow AL. Pregnenolone and ganaxolone reduce operant ethanol self-administration in alcohol-preferring P rats. Alcohol Clin Exp Res. 2010;34:2044–2052. doi: 10.1111/j.1530-0277.2010.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant δ-containing γ-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- ClinicalTrials.gov. [Accessed January 2014]; http://www.clinicaltrial.gov/ct2/results?term=ganaxolone&Search=Search.
- ClinicalTrials.gov. [Accessed January 2014]; http://www.clinicaltrial.gov/ct2/results?term=thip&Search=Search.
- Eiler WA, II, June HL. Blockade of GABAA receptors within the extended amygdala attenuates D2 regulation of alcohol-motivated behaviors in the ventral tegmental area of alcohol-preferring (P) rats. Neuropharmacology. 2007;52:1570–1579. doi: 10.1016/j.neuropharm.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PEM, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6 mice. Neuroscience. 2004;123:813–819. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Mark GP, Fretwell AM, Gililland-Kaufman KR, Strong MN, Ford MM. Reinstatement of ethanol and sucrose seeking by the neurosteroid allopregnanolone in C57BL/6 mice. Psychopharmacology (Berl) 2008;201:423–433. doi: 10.1007/s00213-008-1303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Beckley EH, Kaufman KR, Ford MM. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Horm Behav. 2010;57:12–22. doi: 10.1016/j.yhbeh.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Mark GP, Nickel JD, Phillips TJ, Finn DA. Allopregnanolone influences the consummatory processes that govern ethanol drinking in C57BL/6J mice. Behav Brain Res. 2007;179:265–272. doi: 10.1016/j.bbr.2007.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Gubner NR, McKinnon CS, Reed C, Phillips TJ. Accentuating effects of nicotine on ethanol response in mice with high genetic predisposition to ethanol-induced locomotor stimulation. Drug Alcohol Depend. 2013;127:108–114. doi: 10.1016/j.drugalcdep.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Hörtnagl H, Tasan RO, Wieselthaler A, Kirchmair E, Sieghart W, Sperk G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience. 2013;236:345–372. doi: 10.1016/j.neuroscience.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytiä P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Janak PH, Gill MT. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Maguire EP, Macpherson T, Swinny JD, Dixon CI, Herd MB, Belelli D, Stephens DN, King SL, Lambert JJ. Tonic inhibition of accumbal spiny neurons by extrasynaptic α4βδ GABAA receptors modulates the actions of psychostimulants. J Neurosci. 2014;34:823–838. doi: 10.1523/JNEUROSCI.3232-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE. Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci. 1995;15:6779–6788. doi: 10.1523/JNEUROSCI.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–413. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone L, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, Homanics GE. GABAA receptor δ subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25:1708–1718. [PubMed] [Google Scholar]
- Mody I, Glykys J, Wei W. A new meaning for “Gin & Tonic”: tonic inhibition as the target for ethanol action in the brain. Alcohol. 2007;41:145–153. doi: 10.1016/j.alcohol.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL. GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol. 2007;41:201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic δ-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci. 2011;108:4459–4464. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, O’Buckley TK, Alward SE, Song SC, Grant KA, de Wit H, Morrow AL. Differential effects of ethanol on serum GABAergic 3α,5α/3α,5β neuroactive steroids in mice, rats, cynomolgus monkeys, and humans. Alcohol Clin Exp Res. 2010;34:432–442. doi: 10.1111/j.1530-0277.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Fretwell AM, Finn DA. Alteration of ethanol drinking in mice via modulation of the GABAA receptor with ganaxolone, finasteride, and gaboxadol. Alcohol Clin Exp Res. 2011;35:1994–2007. doi: 10.1111/j.1530-0277.2011.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Strong MN, Ford MM, Finn DA. Effect of ganaxolone and THIP on operant and limited-access ethanol self-administration. Neuropharmacology. 2012;63:555–564. doi: 10.1016/j.neuropharm.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Strong MN, Ford MM, Phillips TJ, Finn DA. Differences in the reinstatement of ethanol seeking with ganaxolone and gaboxadol. Neuroscience. 2014;272:180–187. doi: 10.1016/j.neuroscience.2014.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. α4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci. 2009;29:543–549. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Donahue R, Gill TM, Nie H, Ron D, Janak PH. Alpha4 subunit-containing GABAA receptors in the accumbens shell contribute to the reinforcing effects of alcohol. Addict Biol. 2011;17:309–321. doi: 10.1111/j.1369-1600.2011.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology (Berl) 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Wirtshafter D. Opposite effects on the ingestion of ethanol and sucrose solutions after injections of muscimol into the nucleus accumbens shell. Behav Brain Res. 2011;216:514–518. doi: 10.1016/j.bbr.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stórustovu SI, Ebert B. Pharmacological characterization of agonists at δ-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of γ2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in female adolescent humans. Neuropsychopharmacology. 2003;28:207–1209. doi: 10.1038/sj.npp.1300170. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Alcohol intoxication increases allopregnanolone levels in male adolescent humans. Psychopharmacology (Berl) 2004;172:352–355. doi: 10.1007/s00213-003-1662-0. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Lateral hypothalamic electrical stimulation: does it make animals “hungry”? Brain Res. 1974;67:187–209. doi: 10.1016/0006-8993(74)90272-8. [DOI] [PubMed] [Google Scholar]