Summary
Substantial variability exists in the serum 25(OH)D increase observed in response to vitamin D supplementation. Measurement of circulating cholecalciferol and 24,25(OH)2D, as indicators of vitamin D absorption and degradation, respectively, account for approximately half of the variation in serum 25(OH)D observed following supplementation.
Introduction
Vitamin D supplementation produces a variable response in serum 25(OH)D. This variability likely reflects, in part, differences in vitamin D absorption and/or degradation. Despite this variation in response, virtually all expert recommendations endorse a fixed vitamin D supplementation dose, an approach also used in most prospective studies. Such utilization of a single vitamin D dose does not assure attaining any pre-specified target 25(OH)D level, thereby compromising clinical care and prospective supplementation trials. This study begins addressing this weakness by exploring the feasibility of vitamin D metabolite measurements to predict serum 25(OH)D level attained following supplementation.
Methods
Ninety-one community-dwelling postmenopausal women with baseline 25(OH)D of 10–30 ng/mL received oral vitamin D3, 2300 or 2500 IU, daily for 4–6 months. Serum 25(OH)D, cholecalciferol (D3), and 24,25(OH)2D were measured before and at the end of supplementation to determine if metabolite concentrations allow prediction of the 25(OH)D level attained.
Results
From baseline and follow-up data, we derived a multiple linear regression model predicting posttreatment 25(OH)D as follows: final 25(OH)D=8.3+(1.05*initial 25(OH)D) − (7.7*initial 24,25(OH)2D) + (0.53*final D3)+ (4.2*final 24,25(OH)2D). This model has an adjusted R2= 0.55, thus accounting for approximately half of the observed variance in the final 25(OH)D level.
Conclusions
The contributions of circulating cholecalciferol and 24,25(OH)2D to this predictive model can be considered as indicators of intestinal absorption and clearance, respectively. This paradigm requires further study; it may allow efficient “treat-to-25(OH)D-target” strategies useful in optimizing prospective studies and clinical practice.
Keywords: 24,25-Dihydroxyvitamin D; 25-Hydroxyvitamin D; Cholecalciferol
Introduction
Substantial differences exist in the serum 25(OH)D response observed among individuals following vitamin D supplementation [1–3]. It is logical that such differences are due, at least in part, to between-individual variation in vitamin D absorption, degradation, and distribution. However, vitamin D absorption has not been widely studied, as assays for cholecalciferol have historically been challenging [4]. Nonetheless, existing, albeit limited, data do document substantial between-individual variation in vitamin D absorption [5–8], which could contribute to variability in 25(OH)D observed following supplementation. Additionally, some reports suggest that enhanced 24-hydroxylation, the first step in vitamin D degradation, predicts a less robust 25(OH)D increase after supplementation [9, 10]. Thus, poorer absorption, manifesting as a less robust cholecalciferol increase, and/or greater degradation, manifesting as a higher 24,25(OH)2D or 24, 25(OH)2D/25(OH)D ratio, might result in a less robust 25(OH)D increase following supplementation in a given individual. As such, knowledge of these vitamin D metabolite levels may allow personalization of the vitamin D dose needed to achieve a target 25(OH)D level. Fortunately, advances in liquid chromatography tandem mass spectroscopy (LC-MS/MS) methodology, recognized as the gold standard for 25(OH)D measurement [11], have recently made cholecalciferol and 24,25(OH)2D measurements available [12–15].
Despite the known variability of 25(OH)D response to supplementation, virtually all expert recommendations endorse, and most randomized prospective studies have utilized, a single fixed vitamin D dose for all individuals, an approach that does not assure attainment of any pre-defined, “target” 25(OH)D level. Such fixed dose approaches, and subsequent meta-analytic evaluation of dose administered, have served to fuel the controversy surrounding how to define vitamin D inadequacy, since some people experience little or no serum 25(OH)D increase following what many would consider high-dose vitamin D supplementation. Individuals with no, or minimal, change in 25(OH)D would be expected to have no physiologic effect; however, such subjects are included in the “supplemented” group for meta-analysis. Therefore, it is not surprising that meta-analyses that have focused on dose administered, rather than response obtained, have failed to clearly define vitamin D inadequacy.[16]
Understanding the causes of variation in 25(OH)D level attained following supplementation, and consideration of this variable response in future clinical trials, will allow a biologically meaningful definition of vitamin D inadequacy to be established. To this end, the purpose of this study was to explore the utility of cholecalciferol and 24,25(OH)2D measurements to predict serum 25(OH)D level attained following vitamin D supplementation.
Methods
Subjects
Data from 91 postmenopausal women whose baseline 25(OH)D was between 10.0–29.9 ng/mL and who received either 2300 or 2500 IU of vitamin D3 daily in one of two placebo-controlled oral vitamin D supplementation trials were combined for use in this study. The serum 25(OH)D range was selected as various expert groups consider such individuals to have suboptimal vitamin D status. Inclusion criteria for these two studies were virtually identical; subjects were generally healthy community-dwelling women without known conditions contraindicating vitamin D supplementation or interfering with vitamin D absorption. The duration of vitamin D supplementation was either 4 or 6 months. The time of study supplement dosing was not specified; all serum specimens were obtained in the morning from 0800 to 1100. Supplement compliance was assessed by returned pill count. Vitamin D3 study preparation content was independently validated to contain the stated cholecalciferol amount. Baseline serum cholecalciferol and 25(OH)D did not differ between these two cohorts. The University of Wisconsin Health Sciences Institutional Review Board approved both studies.
Vitamin D metabolite measurements
Vitamin D metabolites (25(OH)D, cholecalciferol (D3), and 24,25(OH)2D) were measured at baseline and study end using liquid chromatography tandem mass spectroscopy (LC-MS/MS) at the Mayo Endocrinology Laboratory. In this laboratory, there is excellent agreement of 25(OH)D measurements with the NIST reference materials (SRM 972a). The intra/inter-assay variability, respectively, for the vitamin D metabolites measured over a physiological range of values varies as follows: cholecalciferol, 3.5–4.5/6.9–8.9 %; 25(OH)D3, 4.6–8.0/5.9–12.9 %; and 24,25(OH)2D, 3.1–6.2/4.0–5.9 %.
Statistical analysis/prediction model development
Study cohort differences were evaluated by factorial ANOVA; vitamin D metabolite relationships at baseline and at 4–6 months were evaluated using multiple regression following data transformation as necessary to satisfy parametric assumptions. All analyses were performed using SigmaStat (San Jose, CA).
Results
Vitamin D metabolite concentrations
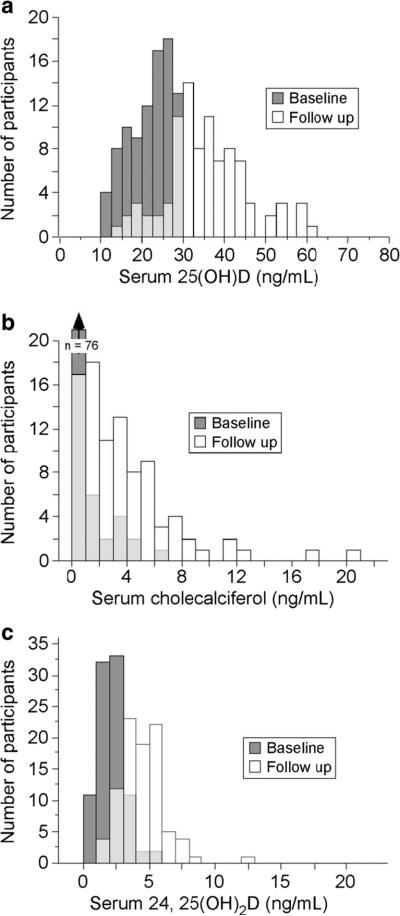
Study participants were 62.3±8.9 (mean ± SD) years of age with a BMI of 29.4±6.1 kg/m2. At baseline, their mean 25(OH)D, cholecalciferol, and 24,25(OH)2D concentrations were 21.8±5.2, 0.6±1.2, and 2.1±1.0 ng/mL, respectively. No between-cohort differences in serum cholecalciferol or 25(OH)D were present; 24,25(OH)2D was statistically lower (p<0.05) in cohort 2 (Table 1). Mean supplement compliance was 94.5±10.4 % leading to an average 25(OH)D increase following supplementation of 13.7±10.3 ng/mL. Similarly, serum cholecalciferol increased by 3.1±3.3 and mean 24, 25(OH)2D increased by 2.3±1.5 ng/mL. The distribution of serum 25(OH)D, cholecalciferol, and 24,25(OH)2D in these 91 women at baseline and study end is depicted in Fig. 1. Notably, serum cholecalciferol was unmeasurable in 64/91 of these women at baseline but only 11/91 at study end.
Table 1.
Study participant demographic and baseline vitamin D metabolite data
| Age (years) | BMI (kg/m2) | 25(OH)D (ng/mL) | Cholecalciferol (ng/mL) | 24,25(OH)2D (ng/mL) | |
|---|---|---|---|---|---|
| Study 1 (n=56) | 64.1* (9 33) | 27.2* (4.69) | 22.4 (4.61) | 0.73 (1.26) | 2.28* (0.98) |
| Study 2 (n=35) | 59.3 (7.45) | 33.0 (6.49) | 20.9 (6.06) | 0.41 (0.95) | 1.77 (1.04) |
| Total (n=91) | 62.3 (8.94) | 29.4 (6.12) | 21.8 (5.23) | 0.6 (1.16) | 2.09 (1.03) |
Data as mean (SD)
=different between cohorts, p<0.05
Fig. 1.
Vitamin D metabolite distribution at baseline and study end. This figure depicts the frequency distribution for 25(OH)D (a), cholecalciferol (b), and 24,25(OH)2D (c) at baseline and following 4–6 months of daily vitamin D3 supplementation. An increase in all vitamin D metabolites is evident. Note: The light gray color indicates overlap of the baseline and follow-up results
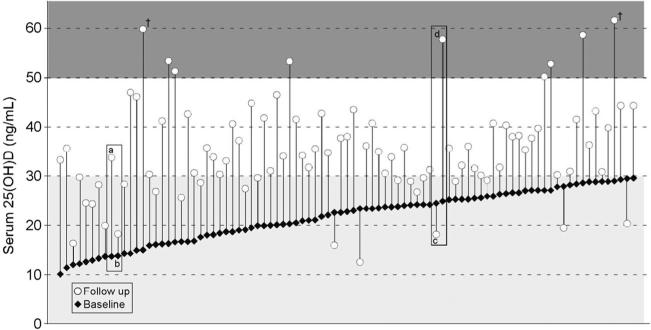
While the mean 25(OH)D increased by ~14 ng/mL, on an individual basis, the 25(OH)D change observed following daily vitamin D3 supplementation varied dramatically, from −11 to +45 ng/mL, as noted in Fig. 2. Indeed, 24 women (26 %) in this group did not achieve a 25(OH)D level above 30 ng/mL and the final value for 6 was below 20 ng/mL. In contrast, nine attained a 25(OH)D value >50 ng/mL. An obvious potential cause of a low 25(OH)D increase is poor supplement compliance; however, this does not appear to explain the observed variability. Indeed, reported compliance for the five individuals in whom 25(OH)D declined was 75, 92, 93, 95, and 111 %.
Fig. 2.
Individual 25(OH)D concentration prior to and following daily vitamin D3 supplementation. It is apparent that major between-individual differences in the serum 25(OH)D change exists. This is exemplified by the individuals noted in the rectangles; these selected subjects (a/b and c/d) had similar baseline 25(OH)D levels but markedly differing 25(OH)D response to supplementation. As a consequence of this variability, many individuals (~26 %; n=24 noted in light gray shaded region) did not achieve a potential “target” 25(OH)D of 30 ng/mL, while 9 (~10 %) achieved values above 50 ng/mL (noted in the dark gray shaded region) and 2 reached levels of ~60 ng/mL (indicated by cross)
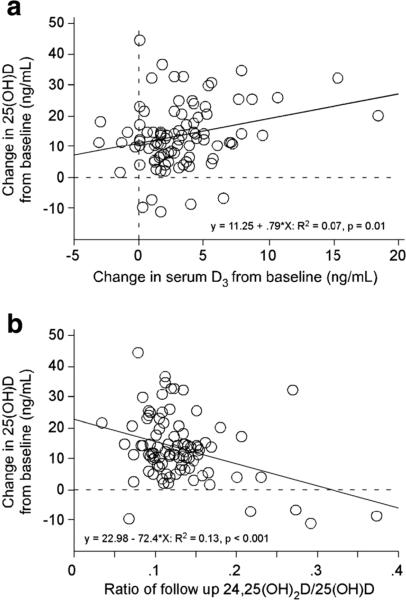
Serum 25(OH)D (both at baseline [data not shown] and study end [Fig. 3]) was highly correlated (p<0.01) with the respective cholecalciferol and 24,25(OH)2D values. Additionally, the 25(OH)D increase following supplementation was positively correlated (p<0.01) with the increase in serum cholecalciferol and negatively correlated (p<0.001) with the ratio of 24,25(OH)2D/25(OH)D (Fig. 4).
Fig. 3.
Relationship of various vitamin D metabolites following daily vitamin D3 supplementation. Serum 25(OH)D was positively correlated (p<0.001) with serum cholecalciferol (a) and serum 24,25(OH)2D (b). Additionally, serum cholecalciferol was positively correlated with serum 24,25(OH)2D (c)
Fig. 4.
Relationship of serum D3 and the 24,25(OH)2D/25(OH)D ratio with change in 25(OH)D following supplementation. The change in serum cholecalciferol from baseline to study end (a surrogate measure of vitamin D absorption) was positively correlated with the 25(OH)D change from baseline (a). Additionally, the ratio of 24,25(OH)2D to 25(OH)D at study follow-up (a potential surrogate of vitamin D degradation) was negatively correlated with the 25(OH)D change from baseline (b)
Relationship of vitamin D metabolites with achieved 25(OH) D level
Regression analyses were performed to determine predictors of 25(OH)D achieved after supplementation. This analysis revealed the following: (1) Baseline vitamin D3, 24,25(OH)2D, and 25(OH)D were all robust (p<0.001) predictors of the 25(OH)D achieved; (2) age, weight, BMI, and dose/duration were not predictive of the 25(OH)D achieved; and (3) a multiple linear regression model, including baseline 25(OH)D, baseline and follow-up 24,25(OH)2D, and the increment in vitamin D3, accounts for ~50 % of the change in 25(OH)D. Baseline vitamin D3 values could not be used in this model as ~70 % of these women had no measureable vitamin D3 at baseline; thus, the data were skewed and could not be transformed to satisfy parametric statistics assumptions. At follow up, ~10 % had no measurable vitamin D3 allowing this value to be included in our model. The multiple regression model for 25(OH)D achieved is determined by the following equation: achieved 25(OH)D= 8.3 + (1.05*baseline 25(OH)D) − (7.7*baseline 24, 25(OH)2D + (0.53*follow-up D3)+(4.2*follow-up 24, 25(OH)2D). This has an adjusted R2=0.55, p<0.001, for each of the parameters, thus accounting for approximately half of the variance in the final 25(OH)D level.
Discussion
Vitamin D supplementation trials generally use fixed doses and fail to consider between-individual variability of the 25(OH)D response [1, 2, 17]. In this report, we demonstrate that a substantial amount of the between-individual variation in 25(OH)D observed following supplementation can be attributed to differences in absorption and degradation as assessed by serum cholecalciferol and 24,25(OH)2D, respectively.
While it is widely accepted that vitamin D supplementation produces a greater 25(OH)D increase in those whose baseline level is lower [18], this is not always true. Indeed, two people with identical 25(OH)D levels can experience markedly different 25(OH)D increments following supplementation (examples denoted by rectangles in Fig. 2). In these examples, the baseline cholecalciferol level for subjects a and b were both not detectable (i.e., <0.1 ng/mL) whereas at follow up, the cholecalciferol concentration of subject a increased to 4.6 ng/mL, but subject b remained undetectable despite 99 % compliance with supplementation. Additionally, subjects c and d started with virtually identical 25(OH)D levels, but subject c declined, perhaps due to this individual having one of the highest 24,25(OH)2D/25(OH)D ratios at follow up. Importantly, her supplementation compliance was 92 %. Given such variable response, using a “one-size-fits-all” dose will not assure attaining a 25(OH)D level above any arbitrary cutpoint, e.g., 30 ng/mL, unless the selected dose is very large. However, conducting clinical trials using huge doses would be unwise, as some people attain 25(OH)D values of ~60 ng/mL with a dose as low as 2500 IU (e.g., cross in Fig. 2), levels associated with increases in cancer risk and overall mortality [19, 20]. Rather than using single fixed doses, or alternatively, using huge doses to assure a 25(OH)D value above a given target level, in this report, we demonstrate that measuring cholecalciferol and 24,25(OH)2D can account for approximately half the observed inter-individual variation in response to vitamin D supplementation. Having the capability to individually tailor vitamin D supplementation dose using readily obtainable, patient-specific data will allow improved study design for future vitamin D trials. It is reasonable to expect that the model developed here can subsequently be improved based upon additional clinical trials. Assuming this to be the case, it is reasonable to assume that a model can be developed to facilitate attaining a target level of 25(OH)D in clinical practice, i.e., a “treat-to-target” dosing strategy, while simultaneously avoiding supplementation to potentially toxic levels. Future work to define such an approach is indicated.
There are important limitations to this study, which must be acknowledged. First, it is based on a relatively small number of subjects, all of whom were postmenopausal women. Thus, the generalizability of these results to other populations is unknown. Second, the full regression model requires vitamin D measurement both prior to and following supplementation; an ideal model would include only baseline vitamin D metabolite measurements. Perhaps larger studies and/or improvements in cholecalciferol measurement capabilities that allow detection of lower serum values will permit inclusion of baseline cholecalciferol level into the prediction equation thereby addressing this limitation. Additionally, further study is needed to determine how cholecalciferol dose contributes to the change in 25(OH)D levels. Importantly, the ability to predict 25(OH)D response to vitamin D supplementation does not address the much more fundamental question of what the ideal serum 25(OH)D concentration should be. It seems unlikely that this question will be answered if prospective trials do not address the variation in 25(OH)D response highlighted in this study. Finally, the current model does not include all potential contributors to 25(OH)D status, e.g., season. Indeed, in this study, the mean 25 (OH) D increase postsupplementation was numerically highest when the follow-up blood draw occurred in summer or fall. However, emphasizing the importance of dose individualization, three of the five women who experienced a 25(OH)D decline had their follow-up level obtained in the fall, at which one could have expected their 25(OH)D to have been highest resulting from summer sun. In addition to season, other potential confounders including skin pigmentation, amount/time of sun exposure, and amount of skin exposed were not considered in this work, as how to clinically apply such information in a given patient is unclear; nevertheless, such limitations do confound the ability to predict 25(OH)D response to supplementation. Finally, the time of vitamin D supplement dosing was not specified; clearly defining time of supplement dosing and subsequent blood collection may further improve utility of serum cholecalciferol measurement.
These limitations notwithstanding, our data unequivocally demonstrate that there is a wide individual variation in the 25(OH)D response to daily vitamin D3 supplementation. A single reasonable fixed dose (e.g., 2500 IU daily) of supplemental vitamin D will leave many below a widely accepted 25(OH)D target (30 ng/mL) and even some below the more conservative target of 20 ng/mL, while raising others to a level that may have potential long-term toxicity. Thus, fixed dose studies will not clearly define what constitutes vitamin D inadequacy. In contrast, individualizing supplementation to achieve a biological target, e.g., a pre-specified 25(OH)D level, could accomplish this goal. Our empirically derived regression model leads to a proposed improvement in supplementation strategy, akin to those commonly used in titrating anticoagulants, and is amenable to explicit testing and modification/improvement in future trials. Moreover, by conceptualizing the observed relationship of 25(OH)D and its metabolites as a consequence of differences in surrogates for vitamin D absorption and clearance, we have established a framework for investigating individual variation in vitamin D turnover.
Footnotes
Conflicts of interest None.
Contributor Information
N. Binkley, Osteoporosis Clinical Research Program, University of Wisconsin, 2870 University Avenue, Suite 100, Madison, WI 53705, USA
J. Lappe, Creighton University, Omaha, NE, USA
R. J. Singh, Mayo Clinic, Rochester, MN, USA
S. Khosla, Mayo Clinic, Rochester, MN, USA
D. Krueger, Osteoporosis Clinical Research Program, University of Wisconsin, 2870 University Avenue, Suite 100, Madison, WI 53705, USA
M. K. Drezner, Osteoporosis Clinical Research Program, University of Wisconsin, 2870 University Avenue, Suite 100, Madison, WI 53705, USA
R. D. Blank, Clement J Zablocki VAMC, Milwaukee, WI, USA Medical College of Wisconsin, Milwaukee, WI, USA.
References
- 1.Aloia JF, Patel M, DiMaano R, Li-Ng M, Talwar SA, Mikhail M, Pllack S, Yeh JK. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87:1952–1958. doi: 10.1093/ajcn/87.6.1952. [DOI] [PubMed] [Google Scholar]
- 2.Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, Drezner MK. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab. 2011;96:981–988. doi: 10.1210/jc.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguirre Castaneda R, Nader N, Weaver A, Singh R, Kumar S. Response to vitamin D3 supplementation in obese and non-obese Caucasian adolescents. Horm Res Paediatr. 2012;78:226–231. doi: 10.1159/000343446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollis BW. Detection of vitamin D and its major metabolites. In: Feldman D, Pike JW, Glorieus FH, editors. Vitamin D. 2nd edn. Elsevier; Burlington: 2005. pp. 931–950. [Google Scholar]
- 5.Thompson GR, Lewis B, Booth CC. Absorption of vitamin D3-3H in control subjects and patients with intestinal malabsorption. J Clin Invest. 1966;45:94–101. doi: 10.1172/JCI105327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leichtmann GA, Bengoa JM, Bolt MJG, Sitrin MD. Intestinal absorption of cholecalciferol and 25-hydroxycholecalciferol in pathients with both Crohn's disease and intestinal resection. Am J Clin Nutr. 1991;54:548–552. doi: 10.1093/ajcn/54.3.548. [DOI] [PubMed] [Google Scholar]
- 7.Sitrin MD, Bengoa JM. Intestinal absorption of cholecalciferol and 25-hydroxycholecalciferol in chronic cholestatic liveer disease. Am J Clin Nutr. 1987;46:1011–1015. doi: 10.1093/ajcn/46.6.1011. [DOI] [PubMed] [Google Scholar]
- 8.Davies M, Mawer EB, Krawitt EL. Comparative absorption of vitamin D3 and 25-hydroxyvitamin D3 in intestinal disease. Gut. 1980;21:287–292. doi: 10.1136/gut.21.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, Sidhom G, Rousseau D, Cole DEC, Vieth R. The ratio of serum 24, 25-dihydroxyvitamin D3 to serum 25-hydroxyvitamin D3 is predictive of 25-hydroxyvitamin D3 response to vitamin D3 supplementation. J Steroid Biochem Mol Biol. 2011;126:72–77. doi: 10.1016/j.jsbmb.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 10.de Boer IH, Sachs MC, Chonchol M, et al. Estimated GFR and circulating 24,25-dihydroxyvitamin D concentration: a participant-level analysis of 5 cohort studies and clinical trials. Am J Kidney Dis. 2014 doi: 10.1053/j.ajkd.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Hunty A, Wallace AM, Gibson S, Viljakainen H, Lamberg-Allardt C, Ashwell M. UK foods standards agency workship consensus report: the choice of method for measuring 25-hydroxyvitamin D to estimate vitamin D status for the UK National Diet and Nutrition Survey. Br J Nutr. 2010;104:612–619. doi: 10.1017/S000711451000214X. [DOI] [PubMed] [Google Scholar]
- 12.Cipriani C, Romagnoli E, Pepe J, Russo S, Carlucci L, Piemonte S, Nieddu L, McMahon DJ, Singh R, Minisola S. Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: implications for treatment and prophylaxis. J Clin Endocrinol Metab. 2013;98:2709–2715. doi: 10.1210/jc.2013-1586. [DOI] [PubMed] [Google Scholar]
- 13.Thacher TD, Fischer PR, Obadofin MO, Levine MA, Singh RJ, Pettifor JM. Comparison of metabolism of vitamins D2 and D3 in children with nutritional rickets. J Bone Miner Res. 2010;25:1988–1995. doi: 10.1002/jbmr.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burild A, Frandsen HL, Jakobsen J. Simultaneous quantification of vitamin D, 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D in human serum by LC-MS/MS. Scand J Clin Lab Invest. 2014 doi: 10.3109/00365513.2014.900694. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann M, Gallagher C, Peacock M, et al. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D & 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab. 2014:jc20134388. doi: 10.1210/jc.2013-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaney RP. Vitamin D—baseline status and effective dose. N Engl J Med. 2012;367:77–78. doi: 10.1056/NEJMe1206858. [DOI] [PubMed] [Google Scholar]
- 17.Talwar SA, Aloia JF, Pollack S, Yeh JK. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr. 2007;86:1657–1662. doi: 10.1093/ajcn/86.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaney RP. Vitamin D—baseline status and effective dose. N Engl J Med. 2012;367:77–78. doi: 10.1056/NEJMe1206858. [DOI] [PubMed] [Google Scholar]
- 19.Sempos CT, Durazo-Arvizu RA, Dawson-Hughes B, et al. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the U.S. nationally representative NHANES. J Clin Endocrinol Metab. 2013;98:3001–3009. doi: 10.1210/jc.2013-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stolzenberg-Solomon RZ, Jacobs EJ, Arslan AA, et al. Circulating 25-hydroxyvitamin D and risk of pancreatic cancer: cohort consortium vitamin D pooling project of rarer cancers. Am J Epidemiol. 2010;172:81–93. doi: 10.1093/aje/kwq120. [DOI] [PMC free article] [PubMed] [Google Scholar]