Abstract
Background
Aspirin for the primary prevention of coronary heart disease (CHD) is only recommended for individuals at high risk for CHD although the majority of CHD events occur in individuals who are low to intermediate risk.
Methods and Results
To estimate the potential of coronary artery calcium (CAC) scoring to guide aspirin use for primary prevention of CHD, we studied 4229 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) who were not on aspirin at baseline and were free of diabetes. Using data from median 7.6-year follow-up, five-year number-needed-to-treat (NNT5) estimations were calculated by applying an 18% relative CHD reduction to the observed event rates. This was contrasted to 5-year number-needed-to-harm (NNH5) estimations based on the risk of major bleeding reported in an aspirin meta-analysis. Results were stratified by a 10% 10-year CHD Framingham Risk Score (FRS). Individuals with CAC ≥ 100 had an estimated net benefit with aspirin regardless of their traditional risk status (estimated NNT5 of 173 for individuals <10% FRS and 92 for individuals ≥ 10% FRS, estimated NNH5 of 442 for a major bleed). Conversely, individuals with zero CAC had unfavorable estimations (estimated NNT5 of 2,036 for individuals <10% FRS and 808 for individuals ≥ 10% FRS, estimated NNH5 of 442 for a major bleed). Gender specific and age-stratified analyses showed similar results.
Conclusion
For the primary prevention of CHD, MESA participants with CAC ≥ 100 had favorable risk/benefit estimations for aspirin use while participants with zero CAC were estimated to receive net harm from aspirin.
Keywords: Aspirin, imaging, prevention, coronary disease
Introduction
The current role of aspirin in the primary prevention of cardiovascular disease (CVD) is limited to use only in individuals at elevated risk for a cardiovascular event, thus withholding aspirin from lower risk patients who represent the majority of the primary prevention population and in whom a very large proportion of cardiovascular events occur (1). When tested for primary prevention in clinical trials of predominantly very low risk individuals, aspirin has been shown to decrease the rate of CVD events but at a near-equivalent risk of increased bleeding (2–5). For primary prevention, more liberal use of aspirin would include treatment of individuals at low risk for CVD, resulting in a small absolute benefit that is likely to be outweighed by the increase in bleeding associated with aspirin use. Conversely, limiting aspirin use to only high-risk individuals negates the opportunity to prevent a significant number of cardiovascular events, many of which present as unheralded myocardial infarction or sudden cardiac death (6,7). Therefore, there is much interest in improving assessment of CVD risk to identify individuals with the most favorable risk/benefit profiles.
Coronary artery calcium (CAC) score is a highly specific marker of the atherosclerotic plaque burden in the coronary arteries. There is a nearly 10-fold higher risk of coronary heart disease (CHD) events in patients with substantially elevated CAC (8). In addition, a CAC score of zero has been shown to be a powerful predictor of a favorable prognosis, even in the presence of traditional risk factors (9,10). These strong associations give CAC the ability to improve discrimination and provide a significant improvement in net risk reclassification (8,11,12).
The goal of this analysis, using data from the Multi-Ethnic Study of Atherosclerosis (MESA), is to evaluate if risk stratification with CAC could guide the use of aspirin therapy, potentially focusing treatment on more individuals at high risk and therefore more likely to prevent a CVD event while avoiding aspirin in individuals who are truly low risk in whom aspirin risk exceeds benefit.
Methods
Study Design and Participants
MESA is a longitudinal epidemiologic study of 6,814 multi-ethnic men and women 45 to 84 years old initiated in July of 2000 to evaluate the prevalence, progression, and clinical significance of subclinical atherosclerosis. Complete details of the design and recruitment strategy of MESA have been previously published (13). In summary, between July 2000 and September 2002, MESA enrolled participants at six US field centers (New York, Baltimore, St. Paul, Chicago, Los Angeles, and Forsyth County, North Carolina). Communities with significant ethnic diversity were targeted for recruitment, and participants who identified themselves as white, African-American, Hispanic, or Chinese and were free of known clinical CVD at baseline were enrolled. The study protocol was reviewed and approved by the institutional review board at the participating institutions. Each participant gave informed consent for the study.
Of the 6,814 MESA participants included in the baseline exam, we excluded participants with diabetes at the time of baseline examination (n=880), defined as a fasting glucose level of ≥ 126 mg/dL or use of hypoglycemic medications. We excluded individuals with diabetes due to the consideration of diabetes as a CHD risk equivalent as well as the two recent randomized trials in individuals with diabetes that did not show a reduction in CVD events with aspirin use (14, 15). Participants using aspirin (n=978) or with missing aspirin data (n=227) at the time of the baseline examination were also excluded. Aspirin use was defined as any aspirin dose taken three or more times per week. Additionally, five MESA participants were missing outcomes data, and 495 were missing covariates, thus 4,229 participants were included in our sample. A flow chart of participants included in the study is shown in Figure 1.
Figure 1.
A flow chart of MESA participants included in the study.
Procedures
The scanning and interpretation methods for cardiac computed tomography (CT) in MESA have been previously reported (16). CAC scores were determined with chest CT utilizing either a cardiac-gated electron-beam CT scanner (Los Angeles, Baltimore, and New York) or a multi-detector CT system (Chicago, St. Paul, and Forsyth County). All patients were scanned twice, and CAC (Agatston) scores were averaged. A cardiologist or radiologist interpreted all scans at the MESA CT reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center). Agreement for the presence of CAC was high (kappa statistic 0.92) and the intra-class correlation coefficient for the Agatston score between readers was 0.99.
Clinical teams at each of the six centers performed a baseline examination including assessment of standard CVD risk factors. Blood for basic laboratory assessment was obtained and processed at each of the six centers and analyzed at the central MESA laboratory (University of Vermont, Burlington, VT). New occurrences of CVD and CHD events were documented over a median follow-up of 7.6 years. Participants or their family members were contacted via telephone interview every 9–12 months and questioned about interim admissions to the hospital, outpatient diagnoses of CHD or CVD, and deaths. Medical records were successfully obtained in 98% of hospital admissions and 95% of outpatient cardiovascular diagnoses. Two physicians from the MESA mortality and morbidity review committee independently reviewed and classified each event. The full committee adjudicated if there was disagreement between the two physicians.
Outcomes
The cardiovascular benefits of aspirin in clinical trials have mostly been limited to a reduction in myocardial infarction and stroke (17–22). Therefore, the potential benefit of aspirin therapy was applied to only hard CHD and CVD events. Hard CHD events included non-fatal myocardial infarction, resuscitated cardiac arrest, and CHD death. Hard CVD events included hard CHD events plus non-fatal and fatal stroke. Transient ischemic attack (TIA) was not included.
Statistical Analysis
Baseline characteristics of participants included in the study were analyzed after stratification for baseline aspirin use. Frequencies and proportions were calculated for categorical variables. For continuous variables, means with standard deviations (SD) are presented. We used Kaplan Meier estimates of cumulative event-free survival to describe the occurrence of hard CHD and CVD events over time. Absolute event rates for both CHD and CVD were analyzed in patients stratified by baseline CAC score (0, 1–99, ≥ 100), and Cox multivariable hazard ratios were determined for each CAC stratum. Models were adjusted for age, gender, race/ethnicity, MESA site, cigarette smoking status, cigarette pack-years, body mass index (BMI), LDL cholesterol, HDL cholesterol, lipid-lowering medication, hypertension, anti-hypertensive medication, family history of myocardial infarction, education level, and Framingham risk score (FRS). The proportional hazards assumption of the Cox model was confirmed by the inspection of log-negative log survival curves and an interaction term between the CAC score groups and time. Given the exclusion of approximately 10% of participants due to missing variables, we performed an additional Cox model with imputation for participants with missing variables. These results did not significantly differ from the results of the primary analysis (results not shown).
To determine the estimated risk/benefit profiles of aspirin therapy, we performed two separate analyses, one based on the total sample as well as a gender specific analysis. A prior meta-analysis found an 18% reduction in CHD events with aspirin use independent of gender (1). This risk reduction has been used in other studies analyzing the utility of aspirin (23). We applied this relative risk reduction in CHD to both genders stratified by CAC scores and a 10% 10-year CHD risk threshold calculated using the FRS. For the gender specific analysis, we calculated absolute hard CHD event rates in men and hard CVD event rates in women after stratification by baseline CAC score. An estimated aspirin benefit of a 32% reduction in CHD for men and a 17% reduction in CVD for women, as stated in the United State Preventive Service Task Force (USPSTF) guidelines (24), was applied to the absolute MESA event rate in each CAC stratum. Using the reciprocal of the absolute risk reduction, a number needed to treat at a median follow up of 7.6 years was calculated. To contrast the potential cardiovascular benefit with the potential bleeding risk, the direct NNT was adjusted to a 5-year number needed to treat (NNT5), using the method of Altman-Anderson (25) and contrasted with the 5-year number needed to harm (NNH5).
The NNH5 was calculated using the reciprocal of the absolute risk increase on aspirin based on the absolute increase in the rate of major bleeding seen in a gender specific aspirin meta-analysis (2). The major bleeding rate for both genders combined was increased by a rate of 0.23% at 5-years; therefore, the estimated NNH5 was 442 for a major bleed. The gender specific major bleeding rate with aspirin was increased by a rate of 0.26% at 5-years in men and by a rate of 0.20% in women; therefore, the estimated NNH5 was 388 for a major bleed in men and 512 for a major bleed for women.
For the gender analysis, men and women were stratified based on the threshold for qualification for aspirin therapy by current American Heart Association (AHA) guidelines, including a greater than 10-year 10% CHD risk for men and a greater than 10-year 10% CVD risk for women (26,27). Framingham risk scores were used for CHD and CVD risk estimations (28,29).
As a sensitivity analysis, we performed an age-stratified analysis, analyzing the sample in three separate age categories including both genders and assuming an 18% reduction in CHD. The absolute increase in bleeding for the three age categories was based on the major bleeding rate for both genders combined (0.23% at 5-years in a study population with a mean age of 56 [8] years) and USPSTF guidelines that assume, compared to 45–59 year-old adults, a 3-fold and 4.5-fold increase in bleeding in individuals age 60–69 and 70–79 years old respectively. Finally, we calculated the relative risk reduction with aspirin that would be required for aspirin to have a net benefit (NNT5 > NNH5) in individuals with zero CAC in our sample. We also calculated the absolute increase in bleeding that would have to be present for individuals with CAC ≥ 100 to have an estimated net harm (NNH5 < NNH5) with aspirin use. All statistical analyses were performed with SAS v9.2 (SAS Inc, Cary, NC).
Results
Compared to MESA participants taking aspirin at baseline, the 4,229 participants not on aspirin were younger (mean age 60.6 ± 10.2 years versus 66.2 ± 9.2), more often non-white, and had fewer cardiovascular risk factors with a mean 10-year FRS of 7.4% compared to a FRS of 10.3% in those participants using aspirin at baseline (Table 1).
Table 1.
Baseline characteristics of MESA participants included in the study compared to those patients excluded due to baseline aspirin use.
| Characteristic | No Aspirin Use at Baseline (n=4229) | Aspirin Use at Baseline (n=978) | P-Value |
|---|---|---|---|
| Age (years) | 60.6 (10.2) | 66.2 (9.2) | <0.0001 |
| Female (%) | 56.0 | 45.4 | <0.0001 |
| Race (%) | <0.0001 | ||
| White | 36.7 | 58.6 | |
| Chinese | 13.1 | 7.2 | |
| African-American | 27.4 | 22.1 | |
| Hispanic | 22.8 | 12.1 | |
| Education Level (%) | <0.0001 | ||
| High School | 46.9 | 43.7 | |
| College or Above | 35.7 | 45.3 | |
| BMI (kg/m2) | 28.0 (5.5) | 28.1 (4.9) | 0.77 |
| Hypertension (%) | 38.3 | 55.6 | <0.0001 |
| Anti-Hypertensive Medication Use (%) | 29.8 | 49.9 | <0.0001 |
| Former smoker (%) | 34.2 | 44.2 | <0.0001 |
| Current smoker (%) | 13.6 | 9.4 | <0.0001 |
| Pack-years of Smoking | 10.4 (21.9) | 13.8 (23.2) | <0.0001 |
| HDL Cholesterol (mg/dL) | 51.7 (14.8) | 51.4 (15.1) | 0.47 |
| LDL Cholesterol (mg/dL) | 118.9 (31.4) | 113.8 (29.1) | <0.0001 |
| Lipid-Lowering Medications (%) | 11.6 | 27.7 | <0.0001 |
| Family Hx of CHD (%) | 40.8 | 52.0 | <0.0001 |
| CAC distribution | <0.0001 | ||
| CAC = 0 | 55.8 | 37.5 | |
| CAC 1–99 | 25.9 | 27.4 | |
| CAC ≥100 | 18.3 | 35.1 | |
| 10-Year Framingham CHD Risk Score (%) | 7.4 (7.0) | 10.3 (7.2) | <0.0001 |
Abbreviations: MESA – Multi-Ethnic Study of Atherosclerosis, Hx – History, CHD – Coronary Heart Disease, BMI – Body Mass Index, CAC – Coronary Artery Calcification
Of the participants included in this analysis, 2,361 (55.8%) had a CAC score of zero, 1,093 (25.8%) had a score of 1–99, and 775 (18.3%) had a CAC ≥ 100. The frequency of CHD and CVD events, event rates per 1,000 person-years, and hazard ratios for MESA participants stratified by CAC score are shown in Table 2. Compared to participants with a CAC score of zero, those with CAC ≥ 100 had over a 9-fold higher risk for a CHD event and over a 6-fold higher risk for a CVD event. After adjusting for traditional risk factors, CAC scores were still significantly associated with CHD and CVD events (Hard CHD HR = 4.19 [2.36–7.43] and Hard CVD HR = 2.85 [1.81–4.50] for participants with CAC ≥ 100 compared to those with CAC=0).
Table 2.
CHD and CVD event rates per 1,000 patient-years and hazard ratios by CAC burden in the MESA population not using aspirin.
| CAC Score | N (%) | CHD Event Rate | CHD H.R. (95% CI)* | CHD H.R.(95% CI)** | CVD Event Rate | CVD H.R.(95% CI)* | CVD H.R.(95% CI)** |
|---|---|---|---|---|---|---|---|
| 0 | 2,361 (55.8%) | 1.30 | 1.00 (ref) | 1.00 (ref) | 2.30 | 1.00 (ref) | 1.00 (ref) |
| 1–99 | 1,093 (25.8%) | 4.05 | 3.13 (1.82–5.41) | 2.09 (1.18–3.70) | 6.71 | 2.92 (1.93–4.43) | 1.88 (1.21–2.92) |
| ≥ 100 | 775 (18.3%) | 11.56 | 9.03 (5.54–14.72) | 4.19 (2.36–7.43) | 15.02 | 6.57 (4.47–9.66) | 2.85 (1.81–4.50) |
Abbreviations: CHD – Coronary Heart Disease, CVD – Cardiovascular Disease, CAC – Coronary Artery Calcification, MESA – Multi-Ethnic Study of Atherosclerosis, H.R. – Hazard Ratio
Unadjusted Model
Model adjusted for age, gender, race/ethnicity, MESA site, cigarette status, cigarette pack-years, body mass index, LDL cholesterol, HDL cholesterol, lipid-lowering medication use, hypertension, anti-hypertensive medication use, family history of myocardial infarction, education level, and Framingham risk score.
The 5-year hard CHD event rates, estimated NNT5, and estimated NNH5 with aspirin use in MESA participants assuming an 18% relative reduction in CHD and an absolute increase in bleeding rate of 0.23% at 5-years with aspirin for both genders is shown in Table 3. Individuals with CAC ≥ 100 had an estimated net benefit with aspirin regardless of their traditional CHD risk status (estimated NNT5 of 173 for individuals <10% FRS and 92 for individuals ≥ 10% FRS, estimated NNH5 of 442 for a major bleed). Conversely, individuals with zero CAC had unfavorable estimations (estimated NNT5 of 2,036 for individuals <10% FRS and 808 for individuals ≥ 10% FRS, estimated NNH5 of 442 for a major bleed).
Table 3.
Estimated number-needed to treat and number-needed to harm with use of aspirin in MESA participants stratified by 10-year CHD risk and baseline CAC assuming an 18% reduction in CHD in both genders.
| CHD risk < 10% | No. of participants | 5-yr CHD event rate | Estimated5-yr NNT | 5-yr estimated absolute increase in bleeding rate | Estimated5-yr NNH |
|---|---|---|---|---|---|
| CAC = 0 | 1907 | 0.27% | 2036 | 0.23% | 442 |
| CAC 1–99 | 633 | 0.97% | 571 | ||
| CAC ≥ 100 | 289 | 3.22% | 173 | ||
|
| |||||
| CHD risk ≥ 10% | No. of participants | 5-yr CHD event rate | Estimated5-yr NNT | 5-yr estimated absolute increase in bleeding rate | Estimated5-yr NNH |
|
| |||||
| CAC = 0 | 454 | 0.69% | 808 | 0.23% | 442 |
| CAC 1–99 | 460 | 3.82% | 146 | ||
| CAC ≥ 100 | 486 | 6.07% | 92 | ||
Abbreviations: MESA – Multi-Ethnic Study of Atherosclerosis, CHD – Coronary Heart Disease, CAC – Coronary Artery Calcification, NNT – Number needed to treat, NNH – number needed to harm
The results of the gender specific analysis are shown in Table 4. MESA men with a CAC score of ≥ 100 had favorable risk/benefit profiles for aspirin regardless of qualification by AHA guidelines (>10% 10-year CHD risk), with an estimated NNT5 of 49 and 56 to prevent a CHD event for aspirin qualifiers and non-qualifiers respectively, compared to an estimated NNH5 of 388 for a major bleed. Conversely, men with a CAC score of zero had unfavorable estimated risk/benefit profiles, with a NNT5 to prevent a hard CHD event of 1,389 for aspirin non-qualifying men and 571 for aspirin qualifying men (estimated NNH5 388). Based on CVD risk, MESA women with CAC ≥ 100 had favorable risk/benefit profiles with aspirin therapy regardless of aspirin qualification (>10% 10-year CVD risk), with an estimated NNT5of 126 and 122 for aspirin qualifiers and non-qualifiers women respectively, compared to an estimated NNH5 of 512. However, the risk/benefit profile for women with zero CAC varied based on aspirin qualification (estimated NNT5 253 and 1,322 for aspirin qualifying and non-qualifying women respectively, estimated NNH5 512). Figure 2 displays the estimated NNT5 values for men and women included in the analysis in reference to the estimated NNH5 values.
Table 4.
Estimated number-needed to treat to prevent a CHD or CVD event and number-needed to harm to cause a major bleed with aspirin in men and women in MESA stratified by qualification for aspirin by AHA guidelines and CAC.
| Men | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 10-year CHD risk < 10% | No. of participants | 5-yr CHD event rate | Relative CHD risk reduction with ASA | Estimated5-yr NNT | 5-yr estimated absolute increase in bleeding rate | Estimated5-yr NNH |
| CAC = 0 | 461 | 0.23% | 1389 | 0.26% | 388 | |
| CAC 1–99 | 178 | 1.74% | 32% | 180 | ||
| CAC ≥ 100 | 71 | 5.68% | 56 | |||
|
| ||||||
| 10-year CHD risk ≥ 10% | No. of participants | 5-yr CHD event rate | Relative CHD risk reduction with ASA | Estimated5-yr NNT | 5-yr estimated absolute increase in bleeding rate | Estimated5-yr NNH |
|
| ||||||
| CAC = 0 | 373 | 0.55% | 571 | 0.26% | 388 | |
| CAC 1–99 | 386 | 3.72% | 32% | 85 | ||
| CAC ≥ 100 | 392 | 6.42% | 49 | |||
| Women | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Global CVD Risk < 10% | No. of participants | 5-yr CVD event rate | Relative CVD risk reduction with ASA | Estimated 5-yr NNT | 5-yr estimated absolute increase in major bleeding | Estimated5-yr NNH |
| CAC = 0 | 1167 | 0.45% | 1322 | 0.20% | 512 | |
| CAC 1–99 | 299 | 1.37% | 17% | 430 | ||
| CAC ≥ 100 | 107 | 4.84% | 122 | |||
|
| ||||||
| Global CVD Risk ≥ 10% | No. of participants | 5-yr CVD event rate | Relative CVD risk reduction with ASA | Estimated 5-yr NNT | 5-yr estimated absolute increase in major bleeding | Estimated5-yr NNH |
|
| ||||||
| CAC = 0 | 360 | 2.33% | 253 | 0.20% | 512 | |
| CAC 1–99 | 230 | 4.95% | 17% | 119 | ||
| CAC ≥ 100 | 205 | 4.68% | 126 | |||
Abbreviations: CHD Coronary Heart Disease, CVD – Cardiovascular Disease, CAC – Coronary Artery Calcification, MESA – Multi-Ethnic Study of Atherosclerosis, NNT – Number needed to treat, NNH – number needed to harm
Figure 2.
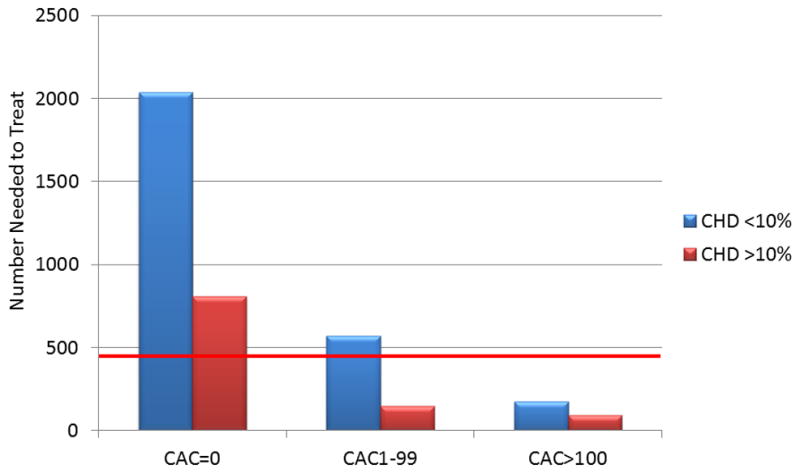
Estimated risk/benefit of aspirin in primary prevention by coronary artery calcium score in MESA participants.
* CHD and CVD risk based on the Framingham Risk Score.
**Red lines represents estimated 5-year number needed to harm estimations based on a 0.23% increase in major bleeding over 5 years.
*** Five-year number needed to treat estimations based on a 32% relative reduction in CHD events for men and a 17% relative reduction in CVD events for women.
An age stratified analysis showed similar results across three age categories though, for MESA participants with CAC ≥ 100, younger individuals had more favorable estimations due to a lower bleeding risk (Table 5). Finally, a sensitivity analysis looking at the effect of different CHD risk reductions and varying bleeding rates on NNT5 and NNH5 estimations in shown in Table 6. Assuming the rate of major bleeding used in the primary analysis (0.23% over 5-years), for individuals with CAC zero in this sample to have an estimated net benefit with aspirin (NNT5 > NNH5), aspirin would have to produce a 64% relative risk reduction for hard CHD events. Assuming an 18% reduction in CHD, for individuals with CAC ≥ 100 in this sample to have a net harm with aspirin (NNT5 < NNH5), the absolute bleeding rate would have to increase by a rate of 0.9% over 5 years with aspirin use, a 3.5-fold higher rate of major bleeding compared to rate seen in the meta-analysis used for this study.
Table 5.
Estimated number-needed to treat and number-needed to harm with use of aspirin in MESA stratified by age and baseline CAC assuming an 18% reduction in CHD in both genders and
| Age 45–59 | No. of participants | 5-yr CHD event rate | Estimated 5-yr NNT | 5-yr estimated absolute increase in bleeding | Estimated 5-yr NNH |
|---|---|---|---|---|---|
| CAC = 0 | 1528 | 0.41% | 1,355 | 0.13% | 770 |
| CAC 1–99 | 413 | 1.76% | 316 | ||
| CAC ≥ 100 | 141 | 7.18% | 78 | ||
|
| |||||
| Age 60–69 | No. of participants | 5-yr CHD event rate | Estimated 5-yr NNT | 5-yr estimated absolute increase in bleeding | Estimated 5-yr NNH |
|
| |||||
| CAC = 0 | 584 | 0.17% | 3,268 | 0.46% | 218 |
| CAC 1–99 | 375 | 1.14% | 488 | ||
| CAC ≥ 100 | 239 | 3.94% | 141 | ||
|
| |||||
| Age 70–84 | No. of participants | 5-yr CHD event rate | Estimated 5-yr NNT | 5-yr estimated absolute increase in bleeding | Estimated 5-yr NNH |
|
| |||||
| CAC = 0 | 249 | 0.43% | 1,292 | 0.56% | 179 |
| CAC 1–99 | 305 | 3.89% | 143 | ||
| CAC ≥ 100 | 395 | 4.83% | 115 | ||
Abbreviations: MESA – Multi-Ethnic Study of Atherosclerosis, CAC – Coronary Artery Calcification, CHD – Coronary Heart Disease, NNT – Number needed to treart, NNH – number needed to harm
Table 6.
Sensitivity Analysis: Estimated number-needed to treat and number-needed to harm with use of aspirin according to varying relative CHD risk reductions and varying absolute bleeding risk in MESA participants stratified by 10-year CHD risk and baseline CAC assuming.
| MESA Participants | Estimated 5-yr NNT | Estimated 5-yr NNH | |||||
|---|---|---|---|---|---|---|---|
| CHD risk < 10% | 10% CHD risk reduction | 20% CHD risk reduction | 30% CHD risk reduction | 0.5-fold bleeding risk | Standard bleeding risk | 2-fold bleeding risk | 4-fold bleeding risk |
| CAC=0 (n=1,907) | 3,664 | 1,832 | 1,222 | 883 | 442 | 221 | 148 |
| CAC ≥ 100 (n=289) | 311 | 156 | 104 | ||||
|
| |||||||
| CHD risk ≥ 10% | 10% CHD risk reduction | 20% CHD risk reduction | 30% CHD risk reduction | 0.5-fold bleeding risk | Standard bleeding risk | 2-fold bleeding risk | 4-fold bleeding risk |
|
| |||||||
| CAC=0 (n=454) | 1454 | 727 | 485 | 883 | 442 | 221 | 148 |
| CAC ≥ 100(n=486) | 165 | 83 | 55 | ||||
Abbreviations: MESA – Multi-Ethnic Study of Atherosclerosis, CHD – Coronary Heart Disease, CAC – Coronary Artery Calcification, NNT – Number needed to treat, NNH – number needed to harm
Discussion
The results of our study demonstrate that, for the primary prevention of cardiovascular disease, MESA participants with CAC ≥ 100 have an estimated net treatment benefit on aspirin while participants with a CAC score of zero have unfavorable risk/benefit profiles with aspirin. Both of these findings are independent of CHD risk based on traditional risk factors.
In a gender specific analysis, we estimated that both men and women with CAC ≥ 100 would benefit from aspirin regardless of qualification for aspirin by AHA guidelines. For MESA men with zero CAC, we estimated a net harm with aspirin use, including in men who qualify for aspirin by AHA guidelines. Results for women varied according to baseline CVD risk as low risk MESA women with zero CAC were estimated to have a net harm with aspirin use while women at elevated global CVD risk were estimated to receive a net benefit from aspirin regardless of the presence of CAC.
In our study sample, over 10% of men and women who would not qualify for aspirin by AHA guidelines had CAC ≥ 100. Additionally, over 30% of MESA participants who would qualify for aspirin by AHA guidelines have zero CAC. The latter patients would have an estimated net harm with aspirin use, as the risk of an aspirin-induced major bleed was estimated to be 2-fold higher than the likelihood of aspirin preventing a CHD event. An age stratified analysis showed similar results across three age strata though older individuals with CAC ≥ 100 did not have as favorable of estimations due to their increased risk of bleeding.
Our study is not the first to raise the question of the utility of CAC to estimate benefit from preventive cardiovascular therapy. Screening for CAC may be useful in determining the need for statin therapy as well. A recent analysis of MESA participants with an elevated hsCRP who could have qualified for the JUPITER trial (30), showed that CAC provided excellent risk stratification in this statin-eligible population (31). JUPITER-eligible MESA participants with a CAC score ≥100 had markedly higher event rates compared to JUPITER-eligible MESA participants with a CAC score of zero. Applying the risk reduction seen with rosuvastatin in the JUPITER trial to the JUPITER-eligible MESA participants produced vastly different estimated absolute benefits depending on the baseline CAC score, with an estimated NNT5 to prevent a CHD event of 549 for participants with zero CAC compared to a NNT5 of 24 for patients with CAC ≥100 (31). These findings, combined with the results of our analysis, suggest that CAC may be useful in determining the potential benefit of both aspirin and statin therapy, thus increasing the utility of CAC as a tool for improved clinical decision-making.
The cost of a CAC score is approximately $100 and is currently not covered by most insurance companies. Concern has been raised that CAC scoring may be used to generate motivation for additional testing (stress testing and angiography) and as well as further imaging for incidental findings though prior research has suggested that there is potential cost savings downstream for those identified with zero CAC, as they are less likely to undergo additional testing (32). CAC is associated with radiation exposure. The measured dose of radiation in MESA was equivalent to bilateral mammography (0.89 mSv) though modern scanners frequently perform scans with a delivered dose of approximately 0.5mSv. Concern has also been raised that CAC testing may be associated with unfavorable psychological or behavioral effects such as increased anxiety in those with elevated CAC or less motivation to follow healthy lifestyle behaviors in those with zero CAC. These concerns lend further support to repeated calls for randomized data on the effect of CAC scoring on patient and physician behavior as well as hard CVD outcomes (33–35).
Current screening guidelines do not recommend CAC testing in low risk patients (36). However, a recent analysis of 44,052 asymptomatic people showed that individuals with no cardiovascular risk factors but elevated CAC had higher mortality rates than individuals with multiple risk factors but zero CAC, suggesting that exclusive use of traditional risk factors to determine preventive therapy may not be the optimal approach to CVD prevention (37). The relatively low prevalence of CAC scores ≥ 100 in low-risk participants raises questions about cost-effectiveness. However, the importance of a finding of zero CAC, and possibly avoiding costs of bleeding that may result from aspirin therapy, must also be considered. The absence of CAC is associated with a very low risk of CHD, CVD, and all-cause mortality (10,38). Patients with zero CAC could potentially be reassured that they are making the correct clinical choice in deferring preventive pharmacotherapy.
In our study, 74% of participants had either a CAC score of 0 or CAC ≥ 100, suggesting the majority of individuals could obtain useful information from the test. The remainder of individuals had CAC scores in the range of 1–99. These individuals, while at greater CHD risk than those with zero CAC, did not have as definitive risk/benefit profiles. To determine the utility of aspirin in these individuals, patient preference may play a larger role, and consideration must also be given to clinical equivalence, as many patients may be more willing to experience a bleeding event as opposed to suffering a heart attack.
There are several limitations to this study. The necessary exclusion of patients on aspirin at baseline created a lower risk study population compared to the overall MESA cohort and thus the cardiovascular event rates may be underestimated compared to a typical middle-aged population. The ideal approach to address this study’s hypothesis would be a randomized controlled trial. However, conducting such a trial for a diagnostic screening test in the setting of primary prevention requires a large sample size, long duration, and high cost (39). The 18% CHD reduction for the total sample, the 32% reduction in CHD for men, and 17% reduction in CVD for women are larger benefits than what the recent meta-analyses of aspirin in primary prevention have shown (4,5). However, we chose to use the treatment benefits for aspirin estimated by the USPSTF guidelines due to the fact that the MESA sample is more similar to the samples of the first six randomized aspirin trials (17–22) as opposed to the three recent trials (14, 15, 40) that focused on patients with diabetes and PAD (we excluded individuals with diabetes and < 1% of our population had PAD). Though we attempted to account for differences in bleeding rates in each gender and across separate age groups, the estimated increased risk of bleeding on aspirin applied to each of these strata was fixed despite that bleeding risk is known to vary by other risk factors (2). A recent large prospective cohort suggested that the incidence of major bleeding in the general population might be higher than the rates seen in randomized trials (41), and a recent meta-analysis used “non-trivial” bleeding as an outcome and found a significantly higher rate of increased rate of bleeding (0.76% at 5-years) on aspirin (5). Therefore, our estimated NNT and NNH calculations should be regarded as hypothesis generating. Nevertheless, it would take significant variations in the cardiovascular event rates, the estimated treatment benefits, or the estimated bleeding risks to negate the concept that individuals with zero CAC have an unfavorable risk/benefit profile on aspirin while those CAC score ≥ 100 should have a net benefit.
In conclusion, for individuals who could be treated with aspirin for the primary prevention of CVD, MESA participants with CAC ≥ 100 had favorable risk/benefit estimations for aspirin use while participants with zero CAC were estimated to receive net harm from aspirin. These results were independent of CHD risk calculated by traditional methods.
Supplementary Material
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. A group of individual investigators proposed and undertook this specific project. The proposal, abstract, and manuscript were reviewed and approved by NIH sponsored MESA committees. All authors had full access to the data.
Funding Source
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-RR-024156 and UL1-RR-025005 from the National Center for Research Resources.
Footnotes
Disclosures
None
References
- 1.Cooney MT, Dudina A, Whincup P, Capewell S, Menotti A, Jousilahti P, Njølstad I, Oganov R, Thomsen T, Tverdal A, Wedel H, Wilhelmsen L, Graham I SCORE Investigators. Re-evaluating the Rose approach: comparative benefits of the population and high-risk preventive strategies. Eur J Cardiovasc Prev Rehabil. 2009;16:541–9. doi: 10.1097/HJR.0b013e32832b38a1. [DOI] [PubMed] [Google Scholar]
- 2.Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–13. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 4.Berger JS, Lala A, Krantz MJ, Baker GS, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients without clinical cardiovascular disease: a meta-analysis of randomized trials. Am Heart J. 2011;162:115–24. doi: 10.1016/j.ahj.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Seshasai SR, Wijesuria S, Sivakumaran R, Sivakumaran R, Nethercott S, Ergou S, Sattar N, Ray KK. Effect of aspirin on vascular and nonvascular outcomes: meta-analysis of randomized controlled trials. Arch Int Med. 2012;172:209–16. doi: 10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]
- 6.Murabito JM, Evans JC, Larson MG, Levy D. Prognosis after the onset of coronary heart disease. An investigation of differences in outcome between the sexes according to initial coronary disease presentation. Circulation. 1993;88:2548–55. doi: 10.1161/01.cir.88.6.2548. [DOI] [PubMed] [Google Scholar]
- 7.Miedema MD, Cohn JN, Garberich R, Henry J, Graham KJ, Henry TD. Under-use of Cardiovascular Preventive Pharmacotherapy in Patients Presenting with ST-Elevation Myocardial Infarction. American Heart Journal. 2012;164:259–67. doi: 10.1016/j.ahj.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 9.Blaha MJ, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Blankstein R, Budoff MJ, Shaw LJ, Goff DC, Polak JF, Lima J, Blumenthal RS, Nasir K. Predictors of coronary heart disease events among asymptomatic persons with low low-density lipoprotein cholesterol MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2011;58:364–74. doi: 10.1016/j.jacc.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 11.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O’Leary DH, Carr JJ, Goff DC, Greenland P, Herrington DM. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–95. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary DH, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.The prevention of progression of arterial disease diabetes study group. The prevention of progression of arterial disease diabetes (POPADAD) trial: factorial randomized placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–41. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- 16.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (Cardia) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 17.Peto R, Gray R, Collins R, Wheatley K, Hennekens C, Jamrozik K, Warlow C, Hafner B, Thompson E, Norton S. Randomized trial of prophylactic daily aspirin in British male doctors. Br Med J. 1988;296:313–6. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steering Committee of the Physician’s Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 19.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 20.The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomized trial of low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet. 1998;351:233–241. [PubMed] [Google Scholar]
- 21.De Gaetano G Collaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 23.Sussman JB, Vijan S, Choi H, Hayward RA. Individual and Population Benefits of Daily Aspirin Therapy: A Proposal for Personalizing National Guidelines. Circ Cardiovasc Qual Outcomes. 2011;4:268–275. doi: 10.1161/CIRCOUTCOMES.110.959239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aspirin for the Primary Prevention of Cardiovascular Disease: U S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 25.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to event. BMJ. 1999;319:1492–5. doi: 10.1136/bmj.319.7223.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. Circulation. 2002;106:388–91. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 27.Effectiveness-based guidelines for the prevention of CVD in women—2011 update. A guideline from the American Heart Association. Circulation. 2011;123:1243–62. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 29.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Sheperd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 31.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, O’Leary DH, Lima J, Blumenthal RS, Nasir K. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378:684–92. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw LJ, Min JK, Budoff MJ, Gransar H, Rozanski A, Hayes SW, Friedman JD, Miranda R, Wong ND, Berman DS. Induced Cardiovascular Procedural Costs and resource consumption patterns after coronary artery calcium screening: results from the EISNER (Early Identification of Subclinical Atherosclerosis by Non-invasive Imaging Research) study. J Am Coll Cardiol. 2009;54:1258–67. doi: 10.1016/j.jacc.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. The National Academies Press; Washington, D.C: 2009. [Google Scholar]
- 34.Hlatky MA, Douglas PS, Cook NL, Wells B, Benjamin EJ, Dickersin K, Goff DC, Hirsch AT, Hylek EM, Peterson ED, Roger VL, Selby JV, Udelson JE, Lauer MS. Future directions for cardiovascular disease comparative effectiveness research: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2012;60:569–80. doi: 10.1016/j.jacc.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whelton SP, Nasir K, Blaha MJ, Gransar H, Metkus TS, Coresh J, Berman DS, Blumenthal RS. Coronary artery calcium and primary prevention risk assessment: what is the evidence?: an updated meta-analysis on patient and physician behavior. Circ Cardiovasc Qual Outcomes. 2012;5:601–7. doi: 10.1161/CIRCOUTCOMES.112.965566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 37.Nasir K, Rubin J, Blaha MJ, Shaw LJ, Blankstein R, Rivera JJ, Khan AN, Berman D, Raggi P, Calliser T, Rumberger JA, Min J, Jones SR, Blumenthal RS, Budoff MJ. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging. 2012;5:467–73. doi: 10.1161/CIRCIMAGING.111.964528. [DOI] [PubMed] [Google Scholar]
- 38.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffman U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and Prognostic Value of Absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–88. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Ambrosius WT, Polonsky TS, Greenland P, Goff DC, Perdue LH, Fortmann SP, Margolis KL, Pajewski NM. Design of the value of imaging in enhancing the wellness of your heart (VIEW) trial and the impact of uncertainty on power. Clin Trials. 2012;9:232–46. doi: 10.1177/1740774512436882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, Sandercock PA, Fox KA, Lowe GD, Murray GD. Aspirin for Asymptomatic Atherosclerosis Trialists. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–8. doi: 10.1001/jama.2010.221. [DOI] [PubMed] [Google Scholar]
- 41.De Berardis G, Lucisano G, D’Ettorre A, Pellegrini F, Lepore V, Tognoni G, Nicolucci A. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307:2286–94. doi: 10.1001/jama.2012.5034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


