Abstract
Background
The Met158 allele of catechol-O-methyl transferase (COMT) gene is associated with increased levels of catecholamines in the prefrontal cortex and may increase the likelihood of aggressiveness. We conducted a meta-analysis to test the hypothesis that the Met158 allele of the COMT gene is associated with aggressive and violent behavior in schizophrenia.
Methods
MEDLINE search (12/31/11) yielded 14 studies examining the association of the COMT gene polymorphism (rs4680) and aggression in schizophrenia (total n=2219). Three separate analyses were conducted using a random effects model for Met allele carriers vs. Val/Val homozygotes, Met/Met homozygotes vs. Val allele carriers, and Met allele vs. Val allele, respectively. Primary outcome was frequency of patients with aggressive behavior and odds ratio (OR) was the effect size measure.
Results
The frequency of violent patients in the sample ranged from 20% to 75%. The pooled effect sizes for the Met homozygotes vs. Val allele carriers, Met allele carriers vs. Val homozygotes and the Met allele vs. Val allele comparisons were 1.74, 1.65 and 1.35, ps<.05, respectively, suggesting that the Met 158 allele of the COMT gene is associated with higher risk for violence in schizophrenia. Results remained significant after examining heterogeneity among samples and potential publication biases.
Conclusions
TheMet158 allele of the COMT gene confers a significantly increased risk for aggressive and violent behavior in schizophrenia. These data may provide basis for developing informative strategies for reducing violence in patients with schizophrenia.
Keywords: Aggression, Genetics, Polymorphism, Psychosis, Dopamine, rs4680
1. Introduction
The recent news articles about tragic and high profile killings by people with mental illness (Web link), suggest an urgent need to understand how to predict and prevent violent behaviors and how to manage patients after the occurrence of violent behaviors. Some patients with schizophrenia may display violent behavior (Nestor, 2000), and the risk for such behavior is two to ten times higher than in the general population (Wessely, 1997). A meta-analysis (Large and Nielssen, 2011), found that about a third of patients with schizophrenia exhibited some violent behavior and approximately 1 in 6 committed an act of more serious violence involving the assault of another person.
The causes for violent behavior are complex and multi-factorial. Some common causes include young age, lack of education, and prior history of violence, hallucinations, delusions, treatment non-adherence and substance abuse (Fazel et al., 2009). However, these factors are variable and not specific to schizophrenia, thus have a low predictive value. Accumulating evidence suggests that aggressive or violent behavior in schizophrenia is partially determined by genetic factors (Cadoret et al., 1995; Eley et al., 1999). Several candidate genes have been shown to be associated with violence in schizophrenia, including the catechol-O-methyl transferase (COMT) gene; however, there have been no genome wide association studies examining violence in schizophrenia to date.
The catecholamine system, including dopamine and norepinephrine, in the prefrontal cortex (PFC) is known to regulate the appropriate expression of aggressive behavior (Gerra et al., 1997; Nelson and Trainor, 2007). The COMT gene, located on the long arm of chromosome 22, encodes for a key catecholamine catabolic enzyme (COMT enzyme) that is involved in regulating dopamine transmission within the PFC and therefore has been linked to aggressive behavior. Thus a genetic variant that alters COMT enzyme activity and increases the concentration of dopamine in synapses of the PFC may lead to diminished prefrontal function, which can result in an increased propensity for impulsive violent behavior (Nolan et al., 2004). A common functional single nucleotide polymorphism (SNP) in the COMT gene at codon 108/158, Val158Met (rs4680), generates a valine (Val)-to-methionine(Met) substitution and results in a fourfold reduction in COMT enzyme activity in the Met/Met homozygotes (Lotta et al., 1995), and increased dopamine levels in the prefrontal cortex.
Several studies have shown a strong association between the Met allele and risk for violence in schizophrenia (Han et al., 2004, 2006; Hong et al., 2008; Kotler et al., 1999; Lachman et al., 1998; Strous et al., 1997; Strous et al., 2003; Tosato et al., 2011). In contrast, Jones et al. (2001) reported that Val/Val genotype was associated with greater risk for violence in patients with schizophrenia; others have found the association to be inconsistent (Liou et al., 2001; Koen et al., 2004; Zammit et al., 2004; Kim et al., 2008; Gu et al., 2009).
To systematically synthesize these disparate studies, a potentially useful methodology is to use meta-analytic techniques that incorporate results from multiple studies in an unbiased fashion (Munafo and Flint, 2004). Therefore, in the present study, we performed a meta-analysis of studies examining the association between the COMT gene polymorphism and risk for violence in patients with schizophrenia and hypothesized that the Met158 allele of the COMT gene is associated with increased risk for violence and aggressive behavior in schizophrenia.
2. Methods
2.1. Literature search
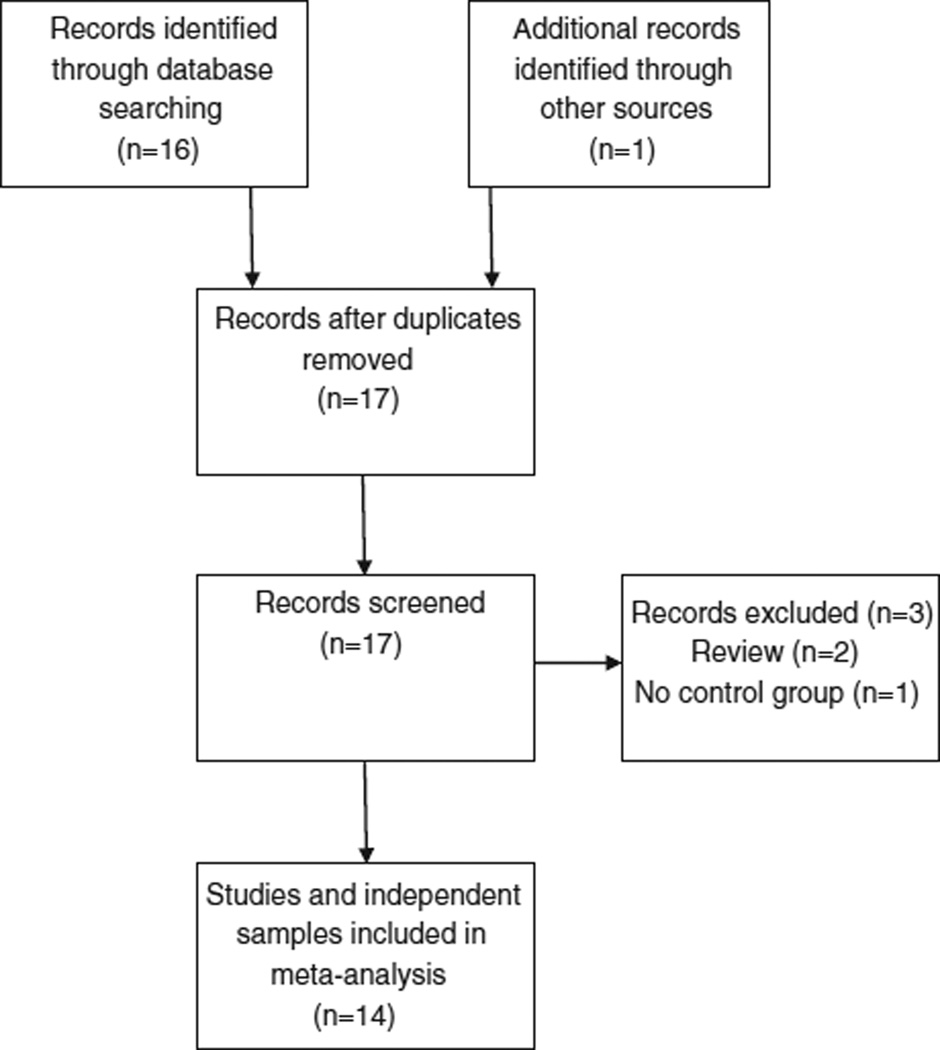
To identify studies eligible for this meta-analysis, we searched MEDLINE for all publications available up to December 31, 2011, that examined the association between the COMT gene and violence in patients with schizophrenia. The following key words were used in the literature search: aggression, violence, schizophrenia, polymorphism, gene, and COMT. We also used the reference lists from identified articles and recent literature review articles to identify additional relevant studies. Each article included in the meta-analysis met the following inclusion criteria: 1) reported the association between the COMT gene polymorphisms (rs4680) and violence in human subjects; 2) included patients with schizophrenia; 3) included both violent (case) and non violent (control) schizophrenia patients and 4) used either a structured or an unstructured method to assess violence. Using this approach, 14 articles were identified, with 14 independent cohorts and were included in the meta-analysis. Fig. 1 shows the flow chart of the literature search process. Table 1 lists the characteristics of the 14 samples.
Fig. 1.
Flow chart of literature search.
Table 1.
Characteristics of articles on the association between the COMT gene polymorphism and violence in schizophrenia patient population.
| Author, year |
Strous RD, 1997 | Lachman HM, 1998 | Kotler M, 1999 | Jones G, 2001 | Liou, YJ, 2001 | Strous RD, 2003 | Zammit S, 2004 | Koen L, 2004 | Han DH, 2004 | Han DH, 2006 | Hong JP, 2008 | Kim YR, 2008 | Yan G, 2009 | Tosato S, 2011 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race | 32% Caucasian |
96% Caucasian | 100% European Ancestry |
100% Caucasian |
100% Asian | 100% European ancestry |
100% Caucasian |
10% Caucasian | 100% Asian |
100% Asian |
100% Asian | 100% Asian |
100% Asian |
100% Caucasian |
| Population | Chronic scz, inpatient |
Chronic scz, inpatient, community care facilities |
Chronic scz, inpatient and homicidal scz, maximum security |
Scz, inpatient+ outpatient |
Chronic scz | Scz, inpatient |
Scz, inpatient+ outpatient |
Scz, inpatient | Chronic Scz, inpatient |
First onset scz |
Homicidal and non homicidal Scz, outpatient |
Scz, inpatient |
Scz, inpatient |
Scz, outpatient |
| SCZ (n) | 37 | 65 | 92 | 180 | 198 | 122 | 150 | 63 | 168 | 132 | 193 | 165 | 584 | 80 |
| Sex (% male) |
86.48 | 62 | 63 | 75.6 | 47.4 | 77 | 70.75 | 100 | 100 | 100 | 100 | 58 | 100 | 51 |
| Age (mean) |
40.6 | 42.8 | 41.91 | Not reported |
37.8 | Not reported |
45.39 | Not reported | 39.52 | 26.93 | Not reported | 38.4 | 34.86 | 42.1 |
| Violence measure |
RAD | History of violence chart review |
History of violence chart review |
OAS | Chart review, history of violence 2 weeks prior to admission |
LHA | OAS | Corrigan Agitated Behavior Scale—aggression component, PANSS-excitedvfactor |
OAS | OAS | OAS, LHA | MOAS | MOAS | OAS |
RAD—Risk Assessment for Dangerousness; OAS—Overt Aggression Scale; LHA—Life History of Aggression; PANSS—Positive and Negative Symptom Scale; MOAS—Modified Overt Aggression Scale.
2.2. COMT gene (SNP rs4680)
The human COMT gene SNP rs4680 is a commonly-studied functional polymorphism. The frequency of the low-activity allele that encodes Met 108/158 ranges from approximately 50% in Caucasian subjects to 20–30% in East Asians, with some populations having even lower allele frequencies, for example, 6% in Ghana (Palmatier et al., 1999). The frequency of the Met and the Val alleles across all the studies included in the meta-analysis was in keeping with the Hardy–Weinberg equilibrium. The SNP rs4680 was analyzed across all studies included in the meta-analysis.
2.3. Definition of phenotype
A challenge for meta-analysis of this literature is the use of differing clinical definitions of violence/aggression and use of different clinical tools to assess violence or aggression such as use of life time history of violence which is an unstructured measure to assess violence as opposed to using a more structured measure like the overt aggression scale. Because the specific measures used in each study differ, individual tests or domains cannot be directly compared across multiple studies. Therefore, in the present meta-analysis, we chose to use violence vs non violence, defined by each individual study, as the phenotype.
The schizophrenia patients in each sample were categorized into violent or non-violent group based on the life time history or an aggression scale. They were further stratified based on the genotype, for example Met/Met homozygotes violent vs Met/Met homozygotes non violent. For studies where the categorical data was not available for individual genotypes, authors were contacted to request additional data. To provide a uniform metric for meta-analysis, odds ratio was computed as a measure of effect size and was generated for each analyses within each study, as described in the next section.
2.4. Statistical analysis
Three separate analyses were conducted for Met allele carriers vs. Val/Val homozygotes (dominant model), Met/Met homozygotes vs. Val allele carriers (recessive model), and Met allele vs. Val allele, respectively. Data for each analysis were entered into and analyzed separately by the Comprehensive Meta-Analysis software version 2 (Biostat, Eaglewood, New Jersey). Odds ratio (OR) was used as the effect size measure, representing the risk for violence in one group compared to another group (i.e., Met allele carriers vs Val homozygotes, Met homozygotes vs Val allele carriers, and Met allele vs Val allele, respectively). Pooled effect sizes across studies were computed with a random effects model, which estimates the likely effect size across different populations and takes heterogeneity across studies into account (Borenstein et al., 2009). This model is different from a fixed effects model, which estimates the most likely effect size from multiple studies assuming that they are sampled from a single population, but it can be biased by high heterogeneity across studies (Munafo and Flint, 2004).
Heterogeneity between studies was assessed by the Q and I2 statistics. The Q statistic is a chi-square test for heterogeneity. The I2 is the proportion of observed variance in effect sizes across studies attributable to true differences among studies. A conventional interpretation of I2 is that it defines bounds for low (<25%), moderate (~50%), and high (>75%) heterogeneity (Higgins et al., 2003).
Publication bias was assessed with the funnel plot, Egger's regression test (Egger et al., 1997), and the trim and fill method (Duval and Tweedie, 2000). The trim and fill method is an iterative procedure to assess whether small extreme included studies and/or potentially not included studies can bias the estimate of the true effect size. To examine the influence of potential moderators, meta-regression analysis was conducted with the following predictors: percentage of male subjects in the sample, Asians vs Non Asians, and mean age of the sample.
Sensitivity analysis was conducted to assess potential influences of any one single study on the pooled effect size. Within each meta-analysis, included studies were removed one at a time to check for significant alterations to pooled effect sizes and associated p values. This method also explicitly tests for a potential “winner's curse,” in which the first study has the largest effect size and tends to bias the meta-analysis (Kraft, 2008).
3. Results
Fourteen articles with 14 independent samples (total n=2219) met the inclusion criteria and were included in the meta-analysis (Fig. 1). As shown in Table 1 the 14 samples consist of mostly men (80%), with an average age of 38 years old. The majority of subjects were Asians (65%). Seven samples used the overt aggression scale (OAS) (Yudofsky et al., 1986) to classify patients with schizophrenia as violent or non-violent, whereas five samples used the history of violence either lifetime or two weeks prior to admission. Strous et al. (1997) used the risk assessment for dangerousness scale (Volavka, 1995) and Koen et al. (2004) used the Corrigan agitated behavior scale and the PANSS (Kay et al., 1989) excited factor. The frequency of violent schizophrenia patients in each sample ranged from 23% to 77%.
3.1. Main analyses
3.1.1. Dominant model: Met allele carriers vs Val/Val homozygotes
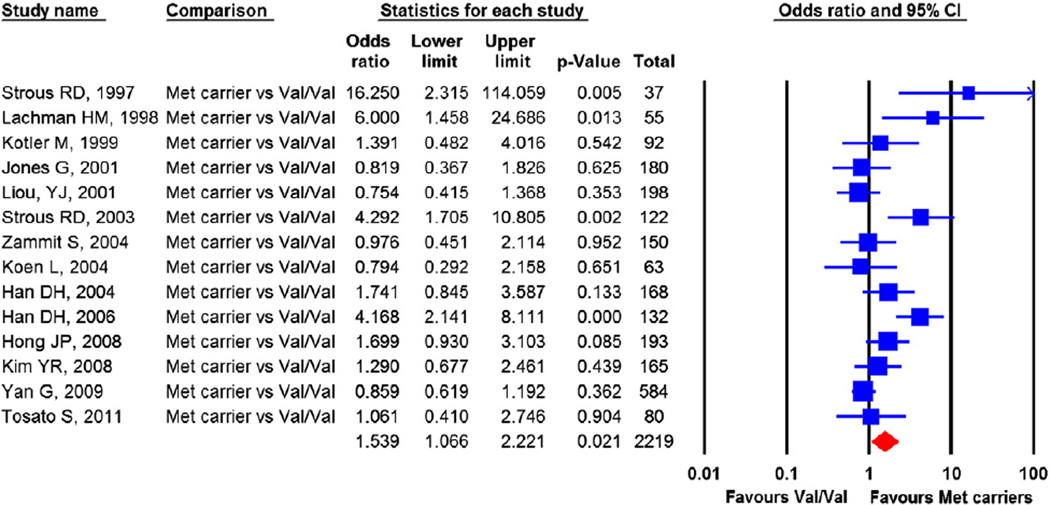
Fourteen independent samples with 2219 schizophrenia patients contributed to the meta-analysis. The pooled OR was 1.539 (95% confidence interval (CI)=1.066 to 2.221), p=0.021, indicating that in patients with schizophrenia, carriers of the low activity Met 158 allele were at significantly higher risk for violence than the high activity Val homozygotes (Fig. 2). Examination of heterogeneity across studies was significant, Q-statistic=42.78, p<0.001, I2=69.61%.
Fig. 2.
Comparing the risk for violence in the Met carrier vs Val homozygotes. The effect sizes indicate that the Met carriers are at greater risk for violence in schizophrenia patients. CI, confidence interval. Heterogeneity: Q=42.78, df=13, p<0.001, I2=69.61. Publication bias: Egger's regression test: p=0.04. Trim and fill method: no missing study identified.
3.1.2. Recessive model: Met/Met homozygotes vs Val allele carriers
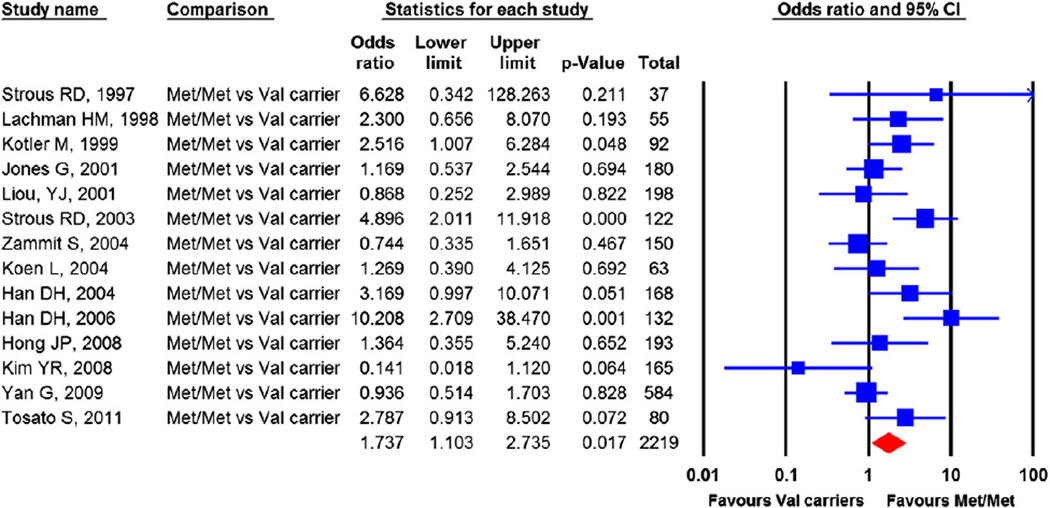
Fourteen independent samples with 2219 schizophrenia patients contributed to the meta-analysis. The pooled OR was 1.737 (CI= 1.103 to 2.735), p=0.017, indicating that homozygotes for the Met158 allele were at significantly greater risk for violence in schizophrenia than the Val allele carriers (Fig. 3). Tests of heterogeneity among studies were significant, Q-statistic=31.67, p=0.003, I2= 58.96%.
Fig. 3.
Comparing the risk for violence in the Met homozygotes vs Val carrier. The effect sizes indicate that the Met homozygotes are at higher risk for violence in schizophrenia patients. Heterogeneity: Q=31.67, df=13, p=0.003, I2=58.96%. No single study biased the pooled effect size. Publication bias: Egger's regression test: p=0.42. Trim and fill method: no missing study identified.
3.1.3. Met allele vs Val allele
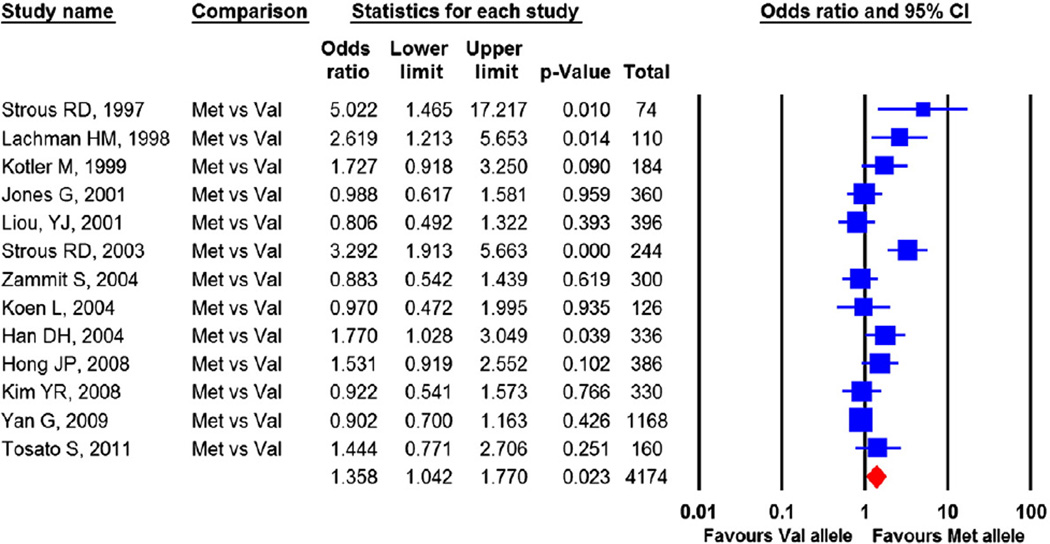
Thirteen independent samples with a total of 4174 alleles contributed to the meta-analysis. One sample (Han et al., 2006)was not included in the analysis because of insufficient data on the allelic distribution. The pooled OR was 1.358, (CI=1.042 to 1.770), p=0.023, indicating that having the Met158 allele significantly increases the risk for violence in schizophrenia than the Val allele (Fig. 4). Heterogeneity across studies was substantial, Q-statistic=37.65, p<0.001, I2=68.13%.
Fig. 4.
Comparing risk for violence for Met allele vs Val allele. The effect sizes indicate that carrying a Met allele increases the risk for violence in schizophrenia patients. Heterogeneity: Q= 37.65, df=12, p<0.001, I2=68.13% Publication bias: Egger's regression test: p=0.02. Trim and fill method: no missing study identified.
3.2. Moderator analysis
Meta-regression analysis was conducted for each of the three separate analyses to assess the effects of the following variables as potential moderators: age, sex and ethnicity. There was no significant effect of percentage of male in the sample, probably due to ceiling effect, as 80% of the sample was men.
A subgroup analysis comparing the Asians vs Non-Asians was not significant; however the non-Asians tend to have larger effect size. This trend could be explained by the high frequency of Met allele in Caucasians (40–60%) as compared to East Asians (10–30%) (Palmatier et al., 1999).
There was a significant effect of mean age in the Met/Met vs Val carrier analysis, such that the older mean age was associated with smaller effect size (b=−0.07, p=0.04), indicating perhaps that as people get older, the effects of genetics on people's aggressive behavior become smaller. However, this analysis included only 10 of 14 studies because 4 studies had missing data on age. Moreover, studies that did not include lifetime aggression measures may have misclassified some individuals as nonviolent if they had not displayed aggressive behavior in the recent past.
3.3. Sensitivity analysis
Sensitivity analysis was conducted for each meta-analysis to assess the influence of any single study. When removing one study at the time from each meta-analysis, there was no significant change in the pooled ORs and their associated p values.
3.4. Publication bias analysis
There was no evidence for publication bias by the trim and fill method (Duval and Tweedie, 2000) for each of the three separate analyses.
4. Discussion
To our knowledge, this is the first meta-analysis testing the effect of the COMT gene SNPrs4680 on violence in patients with schizophrenia. The three separate analyses comparing the low activity Met 158 allele, Met allele carriers and Met/Met homozygotes with the high activity Val allele, Val/Val homozygotes and Val allele carriers respectively, were highly significant, indicating a greater risk for violence in patients with schizophrenia who carry the low activity Met158 allele with the risk being even greater for the Met/Met homozygotes. These results were not influenced by the moderator variables, including age, sex and ethnicity, and the sensitivity analysis. We also found no evidence of significant publication bias.
These data implicating the COMT Met158 allele with violence converge with other lines of evidence. In animal studies, male (but not female) knockout mice deficient in the COMT gene exhibited aggressive behavior suggesting that low activity of COMT enzyme is associated with aggressive behavior (Gogos et al., 1998). The results of our meta-analysis also suggest that males with the low activity Met allele are at risk for violent behavior; however as the sample included was primarily composed of men (80%), we had limited power to detect a relationship in females.
There have been efforts to understand the neurobiology of violence or aggression in schizophrenia patients (Volavka, 1999; Soyka, 2011). On a neurotransmitter level, an increase in the dopaminergic transmission in the prefrontal cortex of the brain caused by the COMT Met 158 allele could possibly explain the risk for aggressive behavior in the carriers of this allele (Egan et al., 2001). Dopamine (DA) transmission is rapidly terminated at most synapses by reuptake through synaptic dopamine transporters. However, the COMT enzyme plays a more significant role in regulating dopamine in areas of the brain where DA transporter concentrations are relatively low, such as the prefrontal cortex. Alterations in inferior frontal white matter microstructure, with resultant frontal lobe dysfunction, have been implicated in the pathophysiology of aggression and impulsivity in patients with schizophrenia (Hoptman et al., 2002). Nolan et al. (2004) proposed that COMT Val158Met may modulate the balance of the tonic and phasic dopamine function in different areas of the brain depending on specific function. According to this hypothesis, the Met allele is associated with increased tonic dopamine activity in the prefrontal cortex, with general benefits for cognitive stability, but costs in the capacity to flexibly alter behavioral programs. The deficits in behavioral inhibition skills needed to cope with the presence of symptoms and other stressful life events in the schizophrenia patients with the Met allele might result in the risk for violence or aggressive behavior (Serper et al., 2008).
Although, the Val158Met polymorphism is not the only variation in the COMT gene, results from studies examining the association of other haplotypes (Gu et al., 2009) and SNP Ala78Ser (Hong et al., 2008) of COMT are too limited to draw any firm conclusions. Other related genes such as MAOA (Strous et al., 2003; Koen et al., 2004; Zammit et al., 2004), MAOB (Zammit et al., 2004), and DRD4 (Kotler et al., 1999), that may influence dopaminergic function have also been examined for association with aggression/violence with no significant results.
Aggressive behavior is likely to result from interaction of several factors. Recently, a meta-analysis (Fazel et al., 2009) examining the relationship between schizophrenia and violence concluded that patients with substance abuse comorbidity have a higher risk of violence. Five of the 14 studies (Strous et al., 1997; Jones et al., 2001; Koen et al., 2004; Zammit et al., 2004; Tosato et al., 2011), included in our meta-analysis had accounted for substance abuse as a confounder and one (Koen et al., 2004) from these 5 studies showed a positive association between risk for violence and cannabis and alcohol abuse in patients with schizophrenia independent of COMT genetic variation. However, in our meta-analysis, a moderator analysis including substance abuse as a variable was not performed because of insufficient data. Moreover, some of the included studies were derived from forensic samples. Due to a small number of studies however we could not assess whether these studies were significantly different from others.
The association between the Met158 allele and violence/aggressive behavior could just have been an epiphenomenon (Soyka, 2011). However, studies examining the association between COMT gene variation and violence in different psychiatric subgroups such as antisocial alcoholics or borderline patients with self injurious behavior have failed to show an association, making the association more specific to the schizophrenia patient population (Hallikainen et al., 2000; Russ et al., 2000). Moreover, the pooled effect sizes in our analysis were moderate, ranging from 1.3 to 1.8, suggesting a strong association between the Met158 allele and risk for violence in schizophrenia patient population. Nevertheless, these moderate effect sizes found in our study are not sufficient to base clinical decision making on COMT genotype.
To summarize, the results of our meta-analysis support the hypothesis that the Met158 allele of the COMT gene confers a significantly increased risk for aggressive and violent behavior in patients with schizophrenia. These data, coupled with other predictors may provide the bases for the development of informative strategies for reducing violence in schizophrenia.
Acknowledgments
We thank the investigators who provided additional data from their studies to make it possible to compute the effect sizes for the meta-analysis.
Role of funding source
This work was supported by the National Alliance for Research on Schizophrenia and Depression (Young Investigator Award to S.G.B.) and the National Institute of Mental Health Grant (5P50MH080173 to A.K.M.).
Dr. Malhotra is a member of the Scientific Advisory Board for Genomind and Shire and has received consulting fees and/or honoraria from Eli Lilly, Schering-Plough/Merck and Sunovion Pharmaceuticals Inc.
Footnotes
Contributors
AKM proposed the study. SGB and JPZ performed the statistical analysis. SGB collected data and wrote the first draft of the manuscript. All authors contributed substantively to the editing of the manuscript and approved the final version prior to submission.
Conflict of interest
Dr. Zhang and Dr. Bhakta report no biomedical financial interests or potential conflicts of interests.
References
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta analysis. Chichester, West Sussex, UK.: John Wiley & Sons Ltd.; 2009. [Google Scholar]
- Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA. Genetic– environmental interaction in the genesis of aggressivity and conduct disorders. Arch. Gen. Psychiatry. 1995;52:916–924. doi: 10.1001/archpsyc.1995.03950230030006. [DOI] [PubMed] [Google Scholar]
- Duval SJ, Tweedie RL. A non-parametric “trim and fill” method of assessing publication bias in meta-analysis. J. Am. Stat. Assoc. 2000;95:89–98. [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eley TC, Lichtenstein P, Stevenson J. Sex differences in the etiology of aggressive and nonaggressive antisocial behavior: results from two twin studies. Child Dev. 1999;70(1):155–168. doi: 10.1111/1467-8624.00012. [DOI] [PubMed] [Google Scholar]
- Fazel S, Gulati G, Linsell L, Geddes JR, Grann M. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med. 2009;6:e1000120. doi: 10.1371/journal.pmed.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Avanzini P, Chittolini B, Giucastro G, Caccavari R, Palladino M, Maestri D, Monica C, Delsignore R, Brambilla F. Neurotransmitter-neuroendocrine responses to experimentally induced aggression in humans: influence of personality variable. Psychiatry Res. 1997;66(1):33–43. doi: 10.1016/s0165-1781(96)02965-4. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Yun L, Tian Y, Hu Z. Association between COMT gene and Chinese male schizophrenic patients with violent behavior. Med. Sci. Monit. 2009;15:CR484–CR489. [PubMed] [Google Scholar]
- Hallikainen T, Lachman H, Saito T, Volavka J, Kauhanen J, Salonen JT, Ryynänen OP, Koulu M, Karvonen MK, Pohjalainen T, Syvälahti E, Hietala J, Tiihonen J. No association between the functional variant of the catechol-O-methyltransferase (COMT) gene and early onset alcoholism associated with severe antisocial behavior. Am. J. Med. Genet. 2000;96(3):348–352. doi: 10.1002/1096-8628(20000612)96:3<348::aid-ajmg22>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Han DH, Park DB, Na C, Kee BS, Lee YS. Association of aggressive behavior in Korean male schizophrenic patients with polymorphisms in the serotonin transporter promoter and catecholamine-O-methyltransferase genes. Psychiatry Res. 2004;129:29–37. doi: 10.1016/j.psychres.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Han DH, Kee BS, Min KJ, Lee YS, Na C, Park DB, Lyoo IK. Effects of catechol-O-methyltransferase Val158Met polymorphism on the cognitive stability and aggression in the first-onset schizophrenic patients. Neuroreport. 2006;17:95–99. doi: 10.1097/01.wnr.0000192740.38653.91. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JP, Lee JS, Chung S, Jung J, Yoo HK, Chang SM, Kim CY. New functional single nucleotide polymorphism (Ala72Ser) in the COMT gene is associated with aggressive behavior in male schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147:658–660. doi: 10.1002/ajmg.b.30649. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biol. Psychiatry. 2002;52(1):9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Jones G, Zammit S, Norton N, Hamshere ML, Jones SJ, Milham C, Sanders RD, McCarthy GM, Jones LA, Cardno AG, Gray M, Murphy KC, Owen MJ. Aggressive behaviour in patients with schizophrenia is associated with catechol-O-methyltransferase genotype. Br. J. Psychiatry. 2001;179:351–355. doi: 10.1192/bjp.179.4.351. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br. J. Psychiatry—Suppl. 1989:59–67. [PubMed] [Google Scholar]
- Kim YR, Kim JH, Kim SJ, Lee D, Min SK. Catechol-O-methyltransferase Val158Met polymorphism in relation to aggressive schizophrenia in a Korean population. Eur. Neuropsychopharmacol. 2008;18:820–825. doi: 10.1016/j.euroneuro.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Koen L, Kinnear CJ, Corfield VA, Emsley RA, Jordaan E, Keyter N, Moolman-Smook JC, Stein DJ, Niehaus DJ. Violence in male patients with schizophrenia: risk markers in a South African population. Aust. N. Z. J. Psychiatry. 2004;38:254–259. doi: 10.1080/j.1440-1614.2004.01338.x. [DOI] [PubMed] [Google Scholar]
- Kotler M, Barak P, Cohen H, Averbuch IE, Grinshpoon A, Gritsenko I, Nemanov L, Ebstein RP. Homicidal behavior in schizophrenia associated with a genetic polymorphism determining low catechol O-methyltransferase (COMT) activity. Am. J. Med. Genet. 1999;88:628–633. [PubMed] [Google Scholar]
- Kraft P. Curses—winner's and otherwise—in genetic epidemiology. Epidemiology. 2008;19:649–651. doi: 10.1097/EDE.0b013e318181b865. (discussion: 657–658). [DOI] [PubMed] [Google Scholar]
- Lachman HM, Nolan KA, Mohr P, Saito T, Volavka J. Association between catechol O-methyltransferase genotype and violence in schizophrenia and schizoaffective disorder. Am. J. Psychiatry. 1998;155:835–837. doi: 10.1176/ajp.155.6.835. [DOI] [PubMed] [Google Scholar]
- Large MM, Nielssen O. Violence in first-episode psychosis: a systematic review and meta-analysis. Schizophr. Res. 2011;125(2–3):209–220. doi: 10.1016/j.schres.2010.11.026. [DOI] [PubMed] [Google Scholar]
- Liou YJ, Tsai SJ, Hong CJ, Wang YC, Lai IC. Association analysis of a functional catechol-O-methyltransferase gene polymorphism in schizophrenic patients in Taiwan. Neuropsychobiology. 2001;43:11–14. doi: 10.1159/000054858. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase — a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20:439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat. Rev. Neurosci. 2007;8(7):536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Nestor PG. Mental disorder and violence: personality dimensions and clinical features. Am. J. Psychiatry. 2000;159:1973–1978. doi: 10.1176/appi.ajp.159.12.1973. [DOI] [PubMed] [Google Scholar]
- Nolan KA, Bilder RM, Lachman HM, Volavka J. Catechol O-methyltransferase Val158Met polymorphismin schizophrenia: differential effects of Val and Met alleles on cognitive stability and flexibility. Am. J. Psychiatry. 2004;161(2):359–361. doi: 10.1176/appi.ajp.161.2.359. [DOI] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol O-methyltransferase alleles. Biol. Psychiatry. 1999;46:557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Russ MJ, Lachman H, Kashdan T, Saito T, Bajmakov-Kacila S. Analysis of catechol O-methyltransferase and serotonin transporter polymorphisms in patients at risk for suicide. Psychiatry Res. 2000;93(1):73–78. doi: 10.1016/s0165-1781(00)00128-1. [DOI] [PubMed] [Google Scholar]
- Serper M, Beech DR, Harvey PD, Dill C. Neuropsychological and symptom predictors of aggression on the psychiatric inpatient service. J. Clin. Exp. Neuropsychol. 2008;30:700–709. doi: 10.1080/13803390701684554. [DOI] [PubMed] [Google Scholar]
- Soyka M. Neurobiology of aggression and violence in schizophrenia. Schizophr. Bull. 2011;37(5):913–920. doi: 10.1093/schbul/sbr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Bark N, Parsia SS, Volavka J, Lachman HM. Analysis of a functional catechol-O-methyltransferase gene polymorphism in schizophrenia: evidence for association with aggressive and antisocial behavior. Psychiatry Res. 1997;69:71–77. doi: 10.1016/s0165-1781(96)03111-3. [DOI] [PubMed] [Google Scholar]
- Strous RD, Nolan KA, Lapidus R, Diaz L, Saito T, Lachman HM. Aggressive Behavior in schizophrenia is associated with the low enzyme activity COMT polymorphism: a replication study. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2003;120:29–34. doi: 10.1002/ajmg.b.20021. [DOI] [PubMed] [Google Scholar]
- Tosato S, Bonetto C, Di Forti M, Collier D, Cristofalo D, Bertani M, Zanoni M, Marrella G, Lazzarotto L, Lasalvia A, De Gironcoli M, Tansella M, Dazzan P, Murray R, Ruggeri M. Effect of COMT genotype on aggressive behavior in a community cohort of schizophrenic patients. Neurosci. Lett. 2011;495:17–21. doi: 10.1016/j.neulet.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Volavka J. Neurobiology of Violence. Washington, DC: American Psychiatric Press; 1995. pp. 59–61.pp. 144–145. [Google Scholar]
- Volavka J. The neurobiology of violence: an update. J. Neuropsychiatry Clin. Neurosci. 1999;11(3):307–314. doi: 10.1176/jnp.11.3.307. [DOI] [PubMed] [Google Scholar]
- Web link. http://www.huffingtonpost.com/dj-jaffe/gabrielle-gifford-jared-l_b_806279.html.
- Wessely S. The epidemiology of crime, violence and schizophrenia. Br. J. Psychiatry Suppl. 1997:8–11. [PubMed] [Google Scholar]
- Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D. The overt aggression scale for the objective rating of verbal and physical aggression. Am. J. Psychiatry. 1986;143:35–39. doi: 10.1176/ajp.143.1.35. [DOI] [PubMed] [Google Scholar]
- Zammit S, Jones G, Jones SJ, Norton N, Sanders RD, Milham C, McCarthy GM, Jones LA, Cardno AG, Gray M, Murphy KC, O'Donovan MC, Owen MJ. Polymorphisms in the MAOA, MAOB, and COMT genes and aggressive behavior in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;128:19–20. doi: 10.1002/ajmg.b.30021. [DOI] [PubMed] [Google Scholar]