Abstract
Prenatal exposure of the ovine fetus to excess testosterone (T) leads to neuroendocrine disruptions in adulthood, evidenced by defects in responsiveness to the ability of gonadal steroids to regulate GnRH secretion. In the ewe, neurones of the arcuate nucleus (ARC), which co-expresses kisspeptin, neurokinin B (NKB) and dynorphin (termed KNDy cells), play a key role in steroid feedback control of GnRH and show altered peptide expression after prenatal T-treatment. KNDy cells also colocalise NKB receptors (NK3R), and it has been proposed that NKB may act as an autoregulatory transmitter in KNDy cells where it participates in the mechanisms underlying steroid negative feedback. In addition, recent evidence suggests that NKB/NK3R signaling may be involved in the positive feedback actions of oestradiol leading to the GnRH/LH surge in the ewe. Thus we hypothesise that decreased expression of NK3R in KNDy cells may be present in the brains of prenatal T-treated animals, potentially contributing to reproductive defects. Using single- and dual-label immunocytochemistry we found that NK3R-positive cells in diverse areas of the hypothalamus; however, after prenatal T-treatment, decreased numbers of NK3R immunoreactive (IR) cells were seen only in the ARC. Moreover, dual-label confocal analyses revealed a significant decrease in the percentage of KNDy cells (using kisspeptin as a marker) that colocalised NK3R. To investigate how NKB ultimately affects GnRH secretion in the ewe, we examined GnRH neurones in the POA and mediobasal hypothalamus (MBH) for the presence of NK3R. Although, consistent with earlier findings, we found no instances of NK3R colocalization in GnRH neurones in either the POA or MBH, >70% GnRH neurones in both areas were contacted by NK3R-IR presynaptic terminals suggesting that, in addition to its role at KNDy cell bodies, NKB may regulate GnRH neurones by presynaptic actions. In summary, decreased NK3R within KNDy cells in prenatal T-treated sheep complement previous observations of decreased NKB and dynorphin in the same population, and may contribute to deficits in the feedback control of GnRH/LH secretion in this animal model. The possibility that NKB agonists may be able to ameliorate the severity of neuroendocrine deficits in prenatal T-treated animals remains to be explored.
Keywords: Neuroendocrine, Developmental programming, Reproduction, Neurokinin B, Kisspeptin, GnRH
Introduction
Gonadotrophin-releasing hormone (GnRH) cells represent the final common pathway controlling pituitary luteinizing hormone (LH) release in mammals, and are under regulatory control by a number of exogenous and endogenous signals, including the ovarian sex steroids, oestradiol and progesterone. However, because GnRH cells do not express the appropriate gonadal hormone receptors responsible for feedback control of GnRH secretion (1–3), regulation of GnRH, and hence LH, release by oestradiol and progesterone is thought to result from the activation of upstream, steroid-responsive neurones. A candidate afferent signaling system that has received much recent attention in the central control of reproduction is that comprised of tachykinin neurokinin B (NKB) and its high affinity receptor neurokinin-3 (NK3R) (4, 5). Although NKB/NK3R signaling is implicated in diverse physiological functions (6), its importance in modulating gonadotrophin release was established when human genetic studies revealed that patients bearing inactivating mutations in the gene encoding NKB (TAC3) or its receptor NK3R, (encoded by TAC3R), displayed hypogonadotrophic hypogonadism and infertility (7, 8). Since then, a growing number of animal studies have established a close association between NKB/NK3R signaling and GnRH/LH secretion in species including the sheep (9), goat (10), primate (11) and rodent (12). However, the precise neuronal pathway(s), via which NKB stimulates GnRH secretion, are not yet fully determined. While a subset of GnRH neurones in the rat have been shown to colocalise NK3R (13), similar studies in sheep (14) and mice (15) have failed to reveal NK3R in GnRH neurones, suggesting that NKB action upon GnRH secretion is likely exerted via inputs from other neurones, either directly or indirectly.
The neuroanatomical location of NK3R has been previously described in the ewe and includes NK3R-immunoreactive (IR) cells in a variety of preoptic and hypothalamic nuclei, including the preoptic area (POA), retrochiasmatic area (RCh), and arcuate nucleus (ARC) (14). In the ARC, NKB is co-localised with two other neuropeptides, kisspeptin and dynorphin in a population that are termed KNDy (Kisspeptin, Neurokinin B and Dynorphin) cells (16). KNDy cells are present in the ARC of all species studied to date (17), although their presence in the male human brain remains controversial (18). KNDy cells are major targets for the influence of gonadal steroid hormones (16), and are thought to play a key role in the negative feedback effects of oestradiol and progesterone upon GnRH in sheep and other species (17). In addition, KNDy cells are thought to comprise a critical component of the circuitry responsible for the generation of GnRH/LH pulses (10, 12, 19). Accumulating evidence suggests that NKB acts as an auto-regulatory signal within the network of reciprocally interconnected KNDY cells, a signal that is responsible for the initiation of each GnRH pulse (19). Supporting this role is evidence that NK3R agonists and antagonists, microinjected into the ARC of ovariectomised ewes, are able to stimulate and inhibit LH pulse frequency, respectively (19). Because of the high degree of colocalization of KNDy peptides with oestrogen receptor alpha (ERα) and progesterone receptor (PR) (16), gonadal steroids are thought to act directly on KNDy cells to modulate the frequency of GnRH/LH pulses, but the effects of oestradiol and progesterone on NKB and NK3R gene expression in the sheep ARC have yet to be examined and other sites of action may also participate in negative feedback control.
In addition to its role in negative feedback, in the sheep, NKB/NK3R signaling may also be important in the generation of the preovulatory GnRH/LH surge (16, 20–22). Intracerebroventricular (icv) microinjections of senktide, a NK3R specific agonist (5), results in a surge-like elevation of LH during the follicular but not the luteal phase of the ovine oestrous cycle (9). Bilateral senktide microinjections into the RCh (9) and POA (23) are each able to produce a similar surge-like elevation of LH suggesting that these two areas, each of which contain NK3R-positive cells may participate in the control of the LH surge. In addition, there is evidence that KNDy cells may participate in the GnRH/LH surge in the ewe. In the sheep (20), unlike rodents (24), oestradiol implants in the mediobasal hypothalamus, close to vicinity of KNDy cells, are sufficient to induce a GnRH/LH surge. ARC kisspeptin gene and peptide expression is increased during the late follicular phase in the ewe (25, 26), and i.c.v. delivery of a Kiss1r antagonist [p271; (27)] significantly reduces the amplitude of the LH surge (28). In addition, a majority of KNDy cells express Fos during the time of the GnRH/LH surge in the ewe (29, 30) and a surge-inducing dose of oestradiol induced Fos expression in KNDy neurones (26). Thus NKB/NK3R signaling could potentially play a role in both negative and positive feedback effects of gonadal steroids in the sheep, acting at potential target sites that include the POA, RCh, and KNDy cells of the ARC.
Responsiveness of the adult GnRH system to hormonal feedback controls is programmed during development by events that include fetal exposure to androgens (31, 32). While normal sexual differentiation depends on appropriate timing of exposure of fetuses to androgens, exposure to excess androgens in animal models can result in long-term deficits in reproductive functions at multiple levels including the GnRH system (33). For example, exposure of female ovine fetuses to excess testosterone (T) during days 30–90 of the 147 day gestation, leads to neuroendocrine defects in the responsiveness of the GnRH system to both negative and positive steroid feedback (34–37). KNDy neurones have been implicated as critical mediators of the detrimental effects of prenatal T (38), and prenatal T treatment results in dramatic alterations in KNDy peptides in the adult ARC, with NKB and dynorphin being markedly reduced but kisspeptin remaining unaltered. This peptide imbalance within a single neuronal population has been hypothesised to underlie some of the defects in responsiveness of the GnRH system to oestradiol and progesterone seen in adult female sheep exposed prenatally to excess T (38).
Whether postsynaptic receptors for any of the KNDy peptides are similarly altered in prenatal T animals has not yet been examined, and given the evidence for participation of NKB/NK3R signaling in both pulsatile and surge modes of GnRH/LH secretion, we hypothesised that changes in NK3R expression in either the ARC or in other regions where it has been shown to alter LH secretion (e.g., RCh, POA), may be present in the brains of prenatal T female sheep. To test this hypothesis, we first compared the overall number of NK3R-IR cells in the ARC, RCh, POA and other hypothalamic nuclei between prenatal T-treated and control animals. Second, we used dual-label immunofluorescence and confocal microscopy to determine whether NK3R might be specifically altered within the KNDy cell subpopulation of the ARC. Finally, since NK3R-IR is seen in fiber and terminals as well as cell bodies, we explored the possibility that NKB might act presynaptically to influence GnRH secretion by determining whether NK3R-IR terminals in the preoptic area (POA) and medial basal hypothalamus (MBH) are in direct synaptic contact with GnRH cell bodies in those regions. To control for the possible influence of differences in circulating steroids between the experimental and control groups, animals were ovariectomised prior to sacrifice and implanted with hormonal regimens designed to produce late follicular phase levels of oestradiol.
Materials and Methods
Animal Care and Treatment
All procedures involving animals were approved by the University of Michigan Animal Care and Use Committee and are consistent with National Research Council’s Guide for the Care and Use of Laboratory Animals. Experiments were conducted in 2-year old control and prenatal T-treated Suffolk ewes during the breeding season. Breeding, lambing and maintenance took place at the Sheep Research Facility at the University of Michigan (Ann Arbor, MI, 42°18’ north latitude).
Pregnant ewes were administered intramuscular (im) injections of T propionate (100mg/injection catalog item T1875; Sigma-Aldrich, St. Louis, MO; n=8) twice weekly, suspended in cottonseed oil (catalog item C7767; Sigma-Aldrich, St.Louis, MO, USA) in the hind leg from days 30–90 of pregnancy (term=147 days). The dose of T propionate administered results in levels of T in the female fetus comparable to those in fetal males (39). Control ewes received an equal volume of vehicle (2 ml cottonseed oil; n=9) in the same regimen as T. Lambs were born in March/April. After weaning, they were maintained outdoors under natural photoperiods with a daily maintenance feeding and free access to water until the age of 2 years. Oestrus cycle and behavioural profiles of 2 year-old ewes treated with an identical T regimen have been previously described (33, 39–42).
Before sacrifice, at approximately 2 years of age, all ewes were ovariectomised (OVX). To normalise the hormonal milieu between all animals, a 1-cm-long SILASTIC capsule (inner diametre, 3.35 mm and outer diametre, 4.65 mm; Dow Corning Corp., Midland, MI) filled with 17β oestradiol (oestradiol; Sigma-Aldrich, St. Louis, MO, USA) in addition to two controlled internal drug release (CIDR) progesterone implants (InterAG, Hamilton, Waikato, New Zealand) was inserted subcutaneously in to each animal. CIDRS were removed 14 days later and sequentially all animals received an additional four 3-cm-long oestradiol implants (see before) to stimulate ovarian steroid levels equivalent to a normal follicular phase as well as generate a GnRH/LH surge (43). Animals were sacrificed ~20 hours after insertion of the ooestradiol implants at the time of the expected GnRH/LH surge.
Tissue collection and preparation
Ewes were injected intravenously (iv) twice at 10-minute intervals with 25,000 U of heparin (catalog item 402588B; Abraxiz pharmaceutical Products, Schaumburg IL, USA) and then deeply anaesthetised with iv sodium pentobarbital (2–3g; catalog item P3761; Sigma-Aldrich, St. Louis, MO, USA). Animals were rapidly decapitated, and the heads perfused via both internal carotids with 6 litres of 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.3) mixed with 0.1 % sodium nitrite and administered with 10 U/ml heparin. After perfusion, the brain was removed and a tissue block containing the septal region, POA, and hypothalamus dissected out. Blocks were incubated in 4% paraformaldehyede at 4 °C overnight for post-fixation and then transferred into 30% sucrose in 0.1 M PB for cryoprotection until infiltration took place. A sliding freezing microtome (Leica Biosystems, SM 200R, Walldorf, Germany) was used to section frozen blocks of tissue containing POA and hypothalamus into 6 series of coronal 45 µm slices. Free-floating sections were stored in cryoprotectant solution (30% ethylene glycol, 1% polyvinylpyrrolidone, 30% sucrose in sodium phosphate buffer; (44) at −°C until processed for immunocytochemistry. Within each experiment, tissue sections from all experimental groups were processed simultaneously as described below. All immunocytochemical procedures were carried out at room temperature under gentle agitation. Unless otherwise stated, tissue sections were washed with 0.1 M phosphate buffer saline (PBS; pH 7.2) between steps. Antibodies were diluted with blocking solution, comprised of 0.4% Triton X-100 (catalog item BP151–500, Sigma-Aldrich, St.Louis, MO, USA) and 4% normal goat serum (NGS; catalog item H005-000-121, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in 0.1M PBS.
Experiment 1: Effects of prenatal T-treatment on NK3R-IR in the POA and hypothalamus
Single-label immunocytochemistry for NK3R
The distribution and quantification of NK3R-IR cells was determined in a series of every 6th section (270 µm apart). Free-floating sections were washed thoroughly in 0.1 M PBS for several hours to remove excess cryoprotectant followed by a 10 minute incubation with PBS containing 1% hydrogen peroxide (H2O2; catalog item H325, Fishers Scientific, Pittsburgh, PA, USA) to eliminate endogenous peroxidase activity. Next, sections were incubated in blocking solution for 1 hour followed by an overnight (17 hour) incubation with polyclonal rabbit anti-NK3R (1:10,000; catalog item NB300-102, Novus Biological, Littleton, CO, USA). After incubation with the primary antiserum, sections were incubated with biotinylated goat anti-rabbit IgG (1:500; catalog item BA-1000; Vector laboratories, Burlingame, CA, USA) for 1 hour followed by incubation with ABC reagent (1:500 diluted in 0.1 M PBS; avidin and biotinylated horseradish peroxidase macromolecular complex, catalog item PK-6100; Vector Laboratories) for 1 hour. NK3R labelling was visualised using 3,3’-diaminobenzidine tetrahydrochloride (0.2 mg/ml) (DAB; Catalogue # D5905, Sigma-Aldrich, St.Louis, MO, USA) with 0.00004% hydrogen peroxide in PB as substrate. Finally, they were mounted onto Superfrost/Plus Microscope Slides (Fisher), air dried, and coverslipped with DPX Mountant. Omission of NK3R antibody from the immunohistochemical protocol resulted in complete absence of staining. Furthermore, preabsorption controls with purified antigen have been previously performed and published (see below) (14).
Experiment 2: Effects of prenatal T-treatment on NK3R-IR within the POA kisspeptin and ARC KNDy cell population
Dual-label immunofluorescent detection of NK3R and kisspeptin
In order to determine if changes in NK3R-IR occurred specifically within the POA kisspeptin or ARC KNDy cell population an alternate series of every 6th section (270 µm apart) containing the POA or ARC was processed for dual-label immunofluorescence and confocal microscopic analysis. A modification of the protocol used by Hunyady et al was carried out to eliminate possible cross-linking between kisspeptin and NK3R antibodies (both raised in rabbits) and false colocalization between antigens (45). Initially, free-floating tissue sections were washed several hours in PBS for cryoprotectant removal. Thereafter, they were incubated in PBS containing 1% H2O2 for 10 min followed by a 1 hour incubation in blocking solution (with 20% NGS). Next, rabbit polyclonal anti-NK3R (1:10,000, for 17 hours) was applied. Sections were then incubated sequentially in biotinylated goat anti-rabbit (1:500 for 1 hour) and ABC-elite solution (1:500 diluted in 0.1 M PBS, for 1 hour). Following amplification with TSA™ Biotin system Biotinyl Tyramide agent (1:250 diluted in 0.1 M PBS with 3% H2O2; catalogue item NEL700A001KT, PerkinElmer Life Sciences, Waltham, MA, USA), NK3R was visualised with Alexa 488 conjugated streptavidin (1:100 diluted in 0.1 M PBS, for 30 min; catalogue item S-32354 Invitrogen/Molecular Probe, Eugene, OR). Sections were then processed for detection of kisspeptin. First, they were incubated for 17 hours with primary antibody rabbit anti-Kisspeptin (gift from A. Caraty, Universite Tours, Nouzilly, France, lot number 564) at a dilution of 1:2,000 (for POA sections) or 1:10,000 (for ARC sections) and visualised with goat anti-rabbit Alexa 555 (1:100 in 0.1 M PBS, for 30 min; catalogue item A-21428, Invitrogen/Molecular Probe, Eugene, OR). Finally, sections were mounted on glass slides, dried and coverslipped with mount medium gelvatol. Control sections for the dual immunofluorescent procedure included omission of each of the primary antibodies from the immunostaining protocol, which resulted in a complete absence of staining for the corresponding antigen. In addition, pre-absorption controls have been performed for each of the antibodies in previous studies (14) in each case pre-incubation of the diluted antiserum with nanomolar concentrations of purified antigen was shown to be sufficient to eliminate all specific staining in ewe hypothalamic sections. Finally, the kisspeptin antibody used has been shown to be specific for kisspeptin cells of the ovine brain and not to cross-react with other RFamide peptides (46).
Experiment 3: Identification of pre-synaptic NK3R terminals onto GnRH neurones in the POA and MBH
Triple-label immunofluorescent detection of GnRH, NK3R and Synaptophysin
A series of every 12th section (540 µm apart) through the POA and MBH were used for GnRH/NK3R/Synaptophysin triple labelling. Similar to the protocols described above, free-floating sections were washed in 0.1 M PBS for several hours in order to remove cryoprotectant. Next, they were incubated in 1% H2O2 diluted in PBS for 10 minutes, followed by a 1 hour incubation in blocking solution (with 20% NGS). Thereafter, sections were incubated sequentially in rabbit polyclonal anti-NK3R, biotinylated goat anti-rabbit, ABC-elite solution and TSA™ Biotin system Biotinyl Tyramide agent, as described above. NK3R was visualised with Alexa 488 conjugated streptavidin (1:100 in 0.1 M PBS, for 30 min). The second primary antibody, rabbit anti-GnRH (1:1,000; LR-5, gift from R. Benoit, Montréal General Hospital, Montréal, Canada), was visualised using indirect detection with goat anti-rabbit Alexa 555 (1:100 in 0.1 M PBS; catalogue item S-32354, Invitrogen/Molecular Probe, Eugene, OR). During the GnRH antibody incubation period, mouse anti-synaptophysin (1:200; catalogue item S5768; Sigma-Aldrich, St. Louis, MO, USA) was also co-incubated and visualised with Donkey anti-Mouse Cy5 (1:100 in 0.1 M PBS, for 30 min; Catalogue item 715175151, Jackson Immunoresearch West Grove, PA). Controls omitting one, two or all three primary antisera from the protocol completely eliminated all specific staining for the corresponding antigen(s).
Data analysis
For single-label NK3R, the distribution of IR cells and fibers was examined in sections through the POA and hypothalamus of each ewe. Three representative sections of the rostral, middle, and caudal divisions of the ARC, retrochiasmatic area (Rch), ventral portion premammillary nucleus (PMv), preoptic area (POA), lateral hypothalamic area (LHA), paraventricular nucleus (PVN) were quantitatively analyzed per animal in each group. Areas chosen for analysis were based on the regional distribution of NK3R-IR cells previously described in the ewe (14). The ARC, which contains prominent NK3R-IR, was divided in to 3 rostral-caudal divisions for more detailed analysis in this study as previously (14, 47). Our preliminary observations revealed that the rostral ARC contained very few NK3R-IR cells and fibers compared to the middle and the caudal ARC. Moreover, given that a large majority of KNDy cells are found in the middle and caudal divisions (47, 48), we selected these sub-regions for detailed comparison between control and prenatal T-treated animals.
For single-label analyses (Experiment 1), NK3R-IR cells were examined and quantified with a Leica DMRD microscope (Leica Microsystems GmbH, Wetzlar, Germany) and identified by the presence of dense reaction product that labelled their somas and dendrites. Images were captured using a digital camera (Magnafire; Optronics, Goleta, CA, USA) attached to the microscope and imported in to Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA, USA). Photomicrographs were not altered in any way except for minor adjustments of brightness and contrast.
Sections processed for dual and triple immunofluorescence were analyzed using a Zeiss LSM-510 laser-scanning confocal microscope system (Zeiss, Thronwood, NY). Alexa 488 fluorescence was visualised and imaged with a 505–530 nm emission filter and Argon laser whereas Alexa 555 and CY5 fluorescence with a 560-nm and 680-nm emission filter and a HeNe laser. Confocal Z-stacks of optical sections (1 µm at 63× magnification) were captured through NK3R, Kisspeptin and GnRH-IR neurones. Three Z stacks from the middle and caudal ARC of each animal were used for analysis of NK3R/Kisspeptin colocalization. A total of 700 kisspeptin-IR cells from the mARC and 692 cells from cARC (between 38–42 kisspeptin-IR cells per treatment group and ARC subdivision) were analyzed. For examination of possible colocalization in the POA, a total of 42 kisspeptin-IR cells from 3 control animals (between 12–16 kisspeptin-IR cells per animal) were analyzed.
For analysis of GnRH/NK3R/Synaptophysin material, 6–10 Z-stacks were captured from the POA and ARC to gather sufficient number of GnRH-IR neurones for analysis. Putative contacts between NK3R/synaptophysin-positive terminals and GnRH-IR somas were defined as a direct apposition without any intervening (black) pixels. A total 49 POA GnRH neurones and 34 MBH GnRH neurones were analyzed from 5 control animals (between 7–12 POA and 6–7 MBH GnRH neurones per animal). First, the somal perimetre was calculated by tracing the neurone. Thereafter, in each z-stack of 1 µm optical section, the number of NK3R-positive terminals in direct contact with the GnRH neurone was determined. The percentage of GnRH neurones in the POA and MBH having one or more NK3R-positive contact was calculated, as was the mean number of NK3R-positive contacts onto GnRH somas per animal, and the mean number of contacts per 10 µm of GnRH somal perimetre.
Statistical Analysis
All data are expressed as the mean ± standard error of mean (SEM). Statistical comparison between control and prenatal T-treated ewes (Experiments 1 and 2), and between brain regions (Experiment 3) were assessed with a Student t-test. All statistics were done using Sigma Stat for windows (SPSS Inc., Chicago, Illinois, USA) and a P value of less than 0.05 was considered significant in all analyses.
Results
Experiment 1: Effects of prenatal T-treatment on NK3R-IR cell number in the POA and hypothalamus
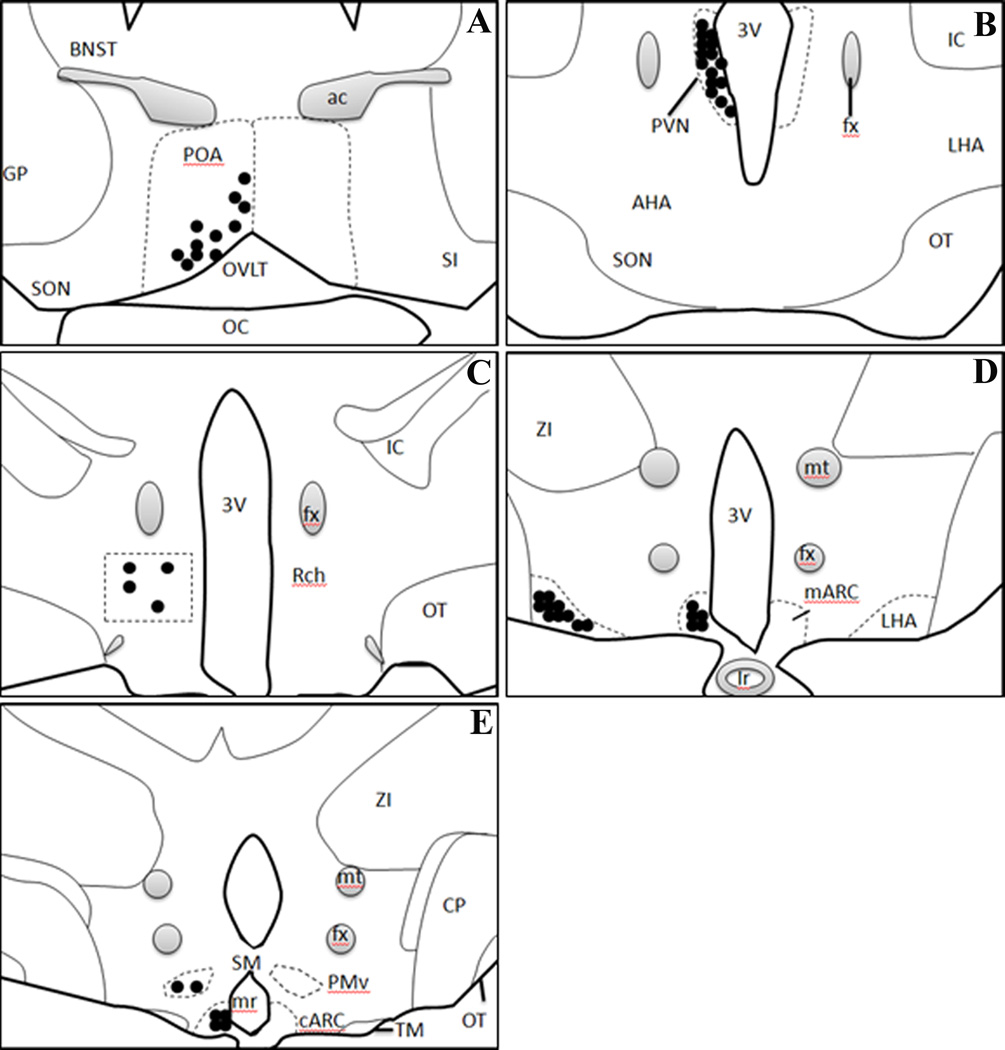
NK3R-IR cells were present in a number of areas of the hypothalamus in addition to the ARC, as depicted in Fig. 1. The most prominent and dense populations of NK3R-IR neurones, other than the ARC, were observed in the following regions (in descending order of overall cell number): the hypothalamic paraventricular nucleus (PVN), lateral hypothalamic area (LHA), ventral premammillary nucleus (PMv), Rch, and POA. In the ARC, where KNDY cells reside, we confirmed a large number of NK3R-IR cells, specifically in the middle and caudal divisions of this nucleus (Fig. 1).
Figure 1.
Schematic drawings of coronal sections through the ovine POA and hypothalamus, depicting the distribution of NK3R-IR cells. Each solid circle represents approximately 10 NK3R-IR cells. Abbreviations; (A) BNST: Bed nucleus of stria terminalis; GP: globus pallidus; ac: Anterior commissure; POA: preoptic areal; SON: superior optic nucleus; OVLT: organum vasculosum of lamina terminalis; SI: substantia innominata; OC: optic chiasm; (B) fx: fornix; PVN: paraventricular nucleus; 3V: 3rd ventricle; IC: internal capsule; AHA: anterior hypothalamic area; OT: optic tract; LHA: lateral hypothalamic area; (C) RCh: retrochiasmatic area; (D) ZI: zona incerta ; mt: mammillary tract; mARC: middle arcuate; (E) CP: cerebral peduncle; PMv: premammillary ventricle; cARC: caudal Arcuate; mr: mammillary recess.
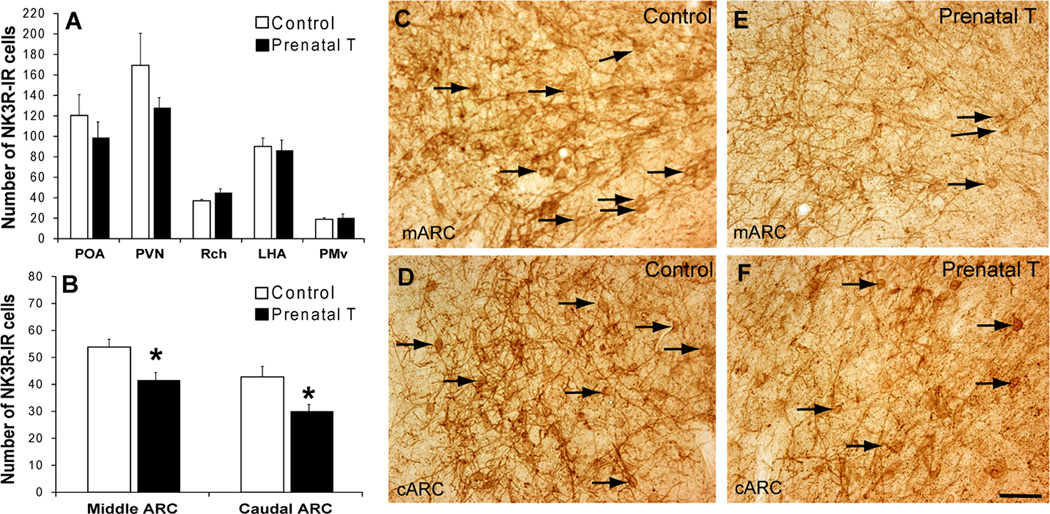
Quantitative cell counts revealed that the mean number of NK3R-IR cells observed in the ARC of control ewes was significantly greater than that of prenatal T-treated animals in both the middle (control: 53.8 ± 2.9 vs. prenatal T: 41.6 ± 2.8; P=0.009) and caudal portions (control: 42.7 ± 4.0 vs. prenatal T: 30.0 ± 2.5; P= 0.019; Fig. 2) of this nucleus. No significant differences in NK3R-IR cell number between control and prenatal T-treated animals were observed in any of the other nuclei or areas analyzed (Fig. 2).
Figure 2.
(A) Mean (± SEM) number of NK3R immunoreactive cells/hemisection in POA, PVN, Rch, LHA and PMv of control (n=9) and prenatal T-treated (n=8) groups. There were no statistically significant differences between control and prenatal T-treated ewes in these areas. (B) Mean (± SEM) number of NK3R immunoreactive cells/hemisection in the middle and caudal ARC from control (n=9) and prenatal T-treated (n=8) groups. * indicates statistically significant differences within each subdivision compared to controls (P<0.05). (C–F) Representative images showing examples of NK3R-IR cells (arrows) in the ARC of control (C, mARC; D, cARC) and prenatal T-treated ewes (E; mARC; F, cARC). Scale bar = 50 µm.
Experiment 2: Effect of prenatal T-treatment on NK3R-IR colocalization within the ARC KNDy cell population
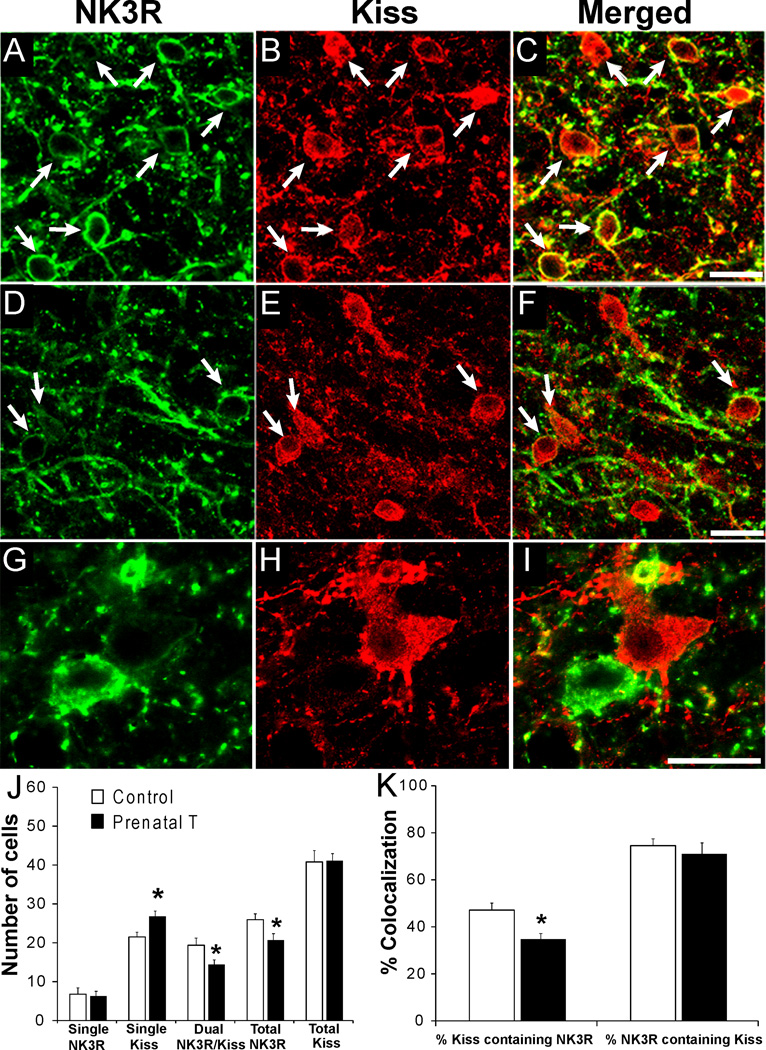
To determine whether changes in NK3R-IR cell number observed in the ARC, reflect a change in NK3R specifically in the KNDy cell population, we analyzed sections processed for dual-immunofluorescent localization of NK3R and kisspeptin (Kiss). Prenatal T-treated animals showed a decrease in the number of dual-labelled NK3R/Kiss cells (control: 19.4 ± 1.7 vs. prenatal T: 14.4 ± 1.2; P=0.049; Fig. 3G) as well as the total number (single-labelled + dual-labelled) of ARC NK3R-IR cells (control: 25.9 ± 2.1 vs. prenatal T: 20.7 ± 1.7; P=0.021 Fig. 3G). As in previous studies (38), we saw no difference between control and prenatal T animals in the total number of Kiss cells (Fig. 3G), and consistent with the decrease in number of dual NK3R/Kiss cells, the number of single-labelled Kiss cells was significantly higher in prenatal T ewes (control: 21.5 ± 1.6 vs. prenatal T: 26.7 ± 1.4; P=0.038; Fig. 3G).
Figure 3.
(A–F): Confocal images (1 µm optical sections) showing dual-label immunofluorescent detection of NK3R-IR and kisspeptin-IR in the middle ARC of control (A–C) and prenatal T-treated ewes (D–F). Arrows indicate examples of dual-labelled neurones. Scale bar = 20 µm (63 ×). (G) Mean (± SEM) number of single-labelled NK3R, single-labelled kisspeptin (Kiss), dual-labelled NK3R and kisspeptin (NK3R/Kiss), and total kisspeptin and NK3R-IR neurones in the ARC of control (n=9) and prenatal T-treated (n=8) ewes. (H) Mean (± SEM) percentage of kisspeptin cells co-localizing NK3R (%Kiss/NK3R; left) and percentage of NK3R cells co-localizing kisspeptin (%NK3R/Kiss; right) in the ARC of control (n=9) and prenatal T-treated (n=8) groups. * indicates statistically significant difference compared to controls (P<0.05).
We used the numbers of dual-labelled and total cells in individual animals to calculate the percentage of ARC Kiss-IR cells co-localizing NK3R, and, conversely, the percentage of NK3R-IR neurones co-localizing Kiss. The mean percentage of Kiss-IR neurones co-localizing NK3R was significantly decreased in prenatal T animals compared to controls (control: 47.1 ± 3.0% vs. prenatal T: 34.7 ± 2.4%; P=0.005; Fig. 3H). By contrast, there was no significant difference between control and prenatal T-treated animals in the percentage of NK3R-IR neurones co-localizing Kiss (Fig. 3H).
Since NK3R-IR cells are present in the POA (Figs. 1 and 2), we also examined kisspeptin cells in the ovine POA for colocalization of NK3R. However, the kisspeptin/NK3R colocalization in the POA was infrequent and variable (5.3 ± 5.3%, mean ± S.E.M.) so that further comparison with prenatal T-treated animals was not pursued.
Experiment 3: Colocalization of NK3R-IR in presynaptic terminals contacting GnRH neurones
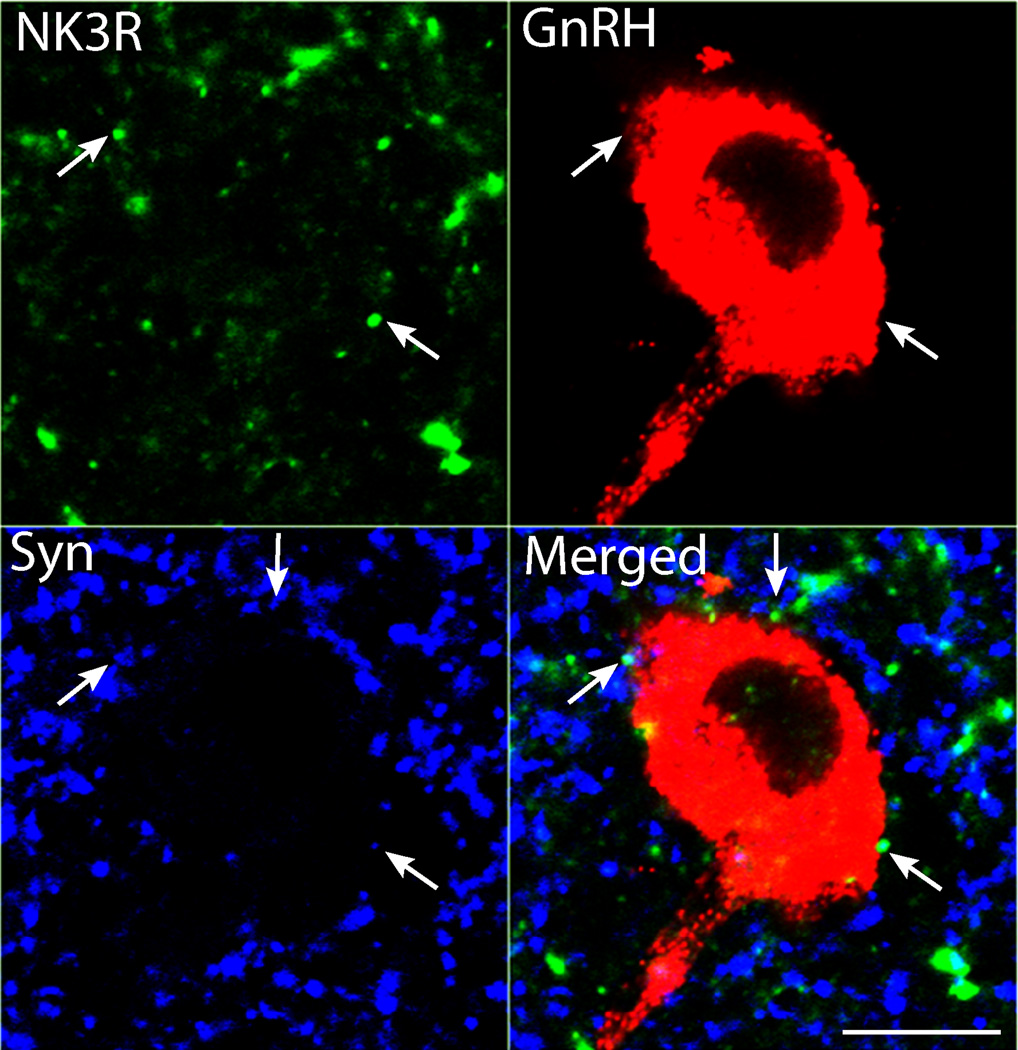
In addition to detecting NK3R-IR in cell bodies (Experiment 1), we also noted NK3R localization in fibers and terminals throughout a number of hypothalamic regions including the POA and mediobasal hypothalamus (MBH). Consequently we processed section for triple-label detection of NK3R, GnRH, and synaptophysin to determine whether any of these NK3R-positive terminals were directly presynaptic to GnRH cell bodies in either the POA or MBH. Examination of triple-labelled sections showed that from a total of 83 GnRH cells analyzed (49 in the POA, and 34 in the MBH), none contained NK3R, confirming our earlier results showing the lack of colocalization of NK3R in ovine GnRH cells (14). However, NK3R-positive fibers were observed adjacent to, and intermixed with, GnRH cells and dendrites in both the POA and MBH. We found that >70 % of GnRH neurones examined were contacted by one or more NK3R-IR presynaptic bouton (defined by the colocalization of synaptophysin; Fig. 4, Table 1). Neither the percentage of GnRH cells receiving inputs, nor the mean number of inputs, varied regionally between the POA and MBH (Table 1). Similarly, the mean number of contacts per 10µm GnRH somal perimetre did not differ between the POA and MBH (Table 1).
Figure 4.
Triple-label detection of NK3R, GnRH and synaptophysin (Syn) in a 1µm confocal optical section, demonstrating the presence of presynaptic NK3R-IR terminals in contact with GnRH neurones in the MBH. Scale bar = 10 µm.
Table 1. Presynaptic NK3R terminals in contact with GnRH somas.
Mean (± SEM) percentage of GnRH neurones receiving one or more NK3R-IR contact, mean number of NK3R-IR contacts per GnRH soma, and mean number of NK3R-IR contacts per 10 µm cell surface, for GnRH cells in the POA and MBH of control ewes (n=5).
| GnRH cell bodies | POA | MBH | P value |
|---|---|---|---|
| % somas receiving one or more NK3R contacts | 71.3 ± 8.1 | 79.1 ± 7.6 | 0.508 |
| Total number of NK3R contacts/soma/animal | 3.0 ± 0.6 | 4.8 ± 1.0 | 0.158 |
| Mean number of NK3R contacts/10 µm somal perimeter | 0.5 ± 0.2 | 0.8 ± 0.1 | 0.189 |
Discussion
Our results indicate that prenatal T-treated ewes show significantly diminished numbers of NK3R-IR neurones in the ARC compared to control animals. Furthermore, the decrease in NK3R was primarily due to changes within the KNDy cell population and not in other ARC cells as the number of single-labelled NK3R-IR cells in this region showed no difference between control and prenatal T animals. The reduced number of NK3R-IR cells observed in prenatal T female sheep parallels the decrease in numbers of NKB cells previously observed in this animal model (38) and suggests that the combined decrease in both ligand and receptor may contribute to defects in the control of GnRH/LH secretion.
Two possible functional consequences of this change may be envisioned. One rests upon the proposed role of NKB/NK3R signaling in the generation of GnRH pulses (16). In the current model of GnRH pulse generation in ruminants (49), NKB serves as a “start signal” that is responsible for initiation of each GnRH pulse, and by way of reciprocal connections, activates other NK3R-containing KNDy cells and ultimately GnRH neurones. Conceivably, decreased NKB and NK3R would lead to a diminished ability to initiate GnRH pulses and hence a decrease in GnRH/LH pulse frequency. However, prenatal T animals show the opposite, an increase in LH pulse frequency in gonadal-intact ewes during anoestrous and the luteal phase of the oestrous cycle due to decreased responsiveness to the negative feedback influence of oestradiol and progesterone, respectively (36, 38, 42). However, in addition to NKB, dynorphin peptide expression is also reduced in KNDy cells of prenatal T sheep (38). Evidence in ruminants supports the role of dynorphin in KNDy cells as a “stop” signal, terminating each GnRH/LH pulse (19). Hence it is possible that reductions in dynorphin signaling compensate for that of NKB and NK3R, rendering the KNDy network less responsive to the negative feedback influence of oestradiol and progesterone in prenatal T animals. Nonetheless, although KNDy cells are known to be potential targets for direct actions of oestradiol and progesterone (16), we do not know whether these gonadal hormones inhibit GnRH/LH pulse frequency by acting directly on KNDy cells or indirectly via afferents from other cells. Evidence in from KNDy cell-ablated rats suggests that while KNDy cells participate in the negative feedback influence of oestradiol on LH, other non-KNDy cells and pathways may also play a role (50).
Another possibility is that decreased NKB/NK3R in prenatal T sheep is related to deficits in the amplitude of the LH surge as seen in these animals (33, 41). As noted in the introduction, KNDy cells in the sheep express Fos, a marker of neuronal activation, during the preovulatory LH surge (22, 28, 29); i.c.v. injections of senktide, which elicit a surge-like elevation in LH, also induce Fos in ARC KNDy cells (23). While kisspeptin mRNA and peptide expression in KNDy cells is increased during the late follicular phase in the ewe (25, 26), an oestradiol stimulus that induced an LH surge was unable to increase in mRNA levels for NKB in the ARC (48). In addition, although NKB agonist injections locally into the POA and RCh, like i.c.v. injections result in a prolonged surge-like elevation of LH (see below), senktide injections in the ARC cause only a modest increase in LH (23) consistent with the role of NK3R in pulse generation in this region. Nonetheless, the possibility that NK3R signaling in KNDy cells plays a role in the generation of the LH surge in the ewe needs to be tested directly by NK3R antagonist injections directly into the ARC in follicular phase ewes.
In contrast to the reduction in NK3R-IR cells we observed in the ARC, no changes were seen in the POA or in any other hypothalamic nuclei analyzed in this study. We were particularly surprised by the absence of any changes in the POA and RCh since senktide microinjections into either region is able to elicit a surge-like pattern of LH release (23) similar to that seen after i.c.v. injections of this agonist (9). Microimplants containing NKB antagonist (SB222200) into the RCh but not the POA reduce the amplitude of the LH surge by 40%, suggesting that NKB release in the RCh during the follicular phase is physiologically important to the generation of the LH surge (23). Since i.c.v. injections of kisspeptin antagonists in follicular phase ewes only reduce surge amplitude by 50% (26), it is tempting to speculate that NKB and kisspeptin act synergistically to elevate LH release during the surge. Interestingly, tract tracing data demonstrate that KNDy neurones receive direct input from neurones in the Rch (51) and administration of senktide into the RCh induces c-Fos expression in the ARC population (23). Taken together, these findings suggest that NKB signaling in the RCh plays a role in the preovulatory LH surge, and that the effect of NKB in the RCh is likely mediated, at least in part, by projections to ARC KNDy neurones. We are currently investigating the existence of reciprocal connection from the ARC to the Rch, which could constitute a potential pathway via which NKB/NK3R and kisspeptin/Kiss1r signaling are involved in the GnRH surge mechanism. If NKB/NK3R in KNDy cells contribute to this mechanism, then administration of the NKB agonist, senktide, should, at least partially, reverse the defects in GnRH surge amplitude seen in prenatal T-treated ewes.
Although the above evidence supports a central role for NKB in regulating GnRH secretion, this influence in the ewe has been thought to be largely indirect, based on the complete absence of NK3R-IR colocalization in ovine GnRH cells (14). Instead, the stimulatory influence of NKB in KNDy cells on GnRH secretion is thought to be conveyed by kisspeptin as an output signal, acting upon either GnRH cell bodies or terminals (16). Evidence for this upstream site of action has come from studies in which kisspeptin antagonists have been shown to block the stimulatory effects of NKB or senktide (52), as well as studies in which desensitization of the kisspeptin receptor blocks the stimulatory effect of senktide in monkeys (53), and the absence of the stimulatory effect of senktide in Kiss1r KO mice (54). The current working hypothesis of the mechanisms by which NKB acts as a stimulatory “start” signal in the generation of GnRH pulses in the ewe, posits this action occurring via reciprocal KNDy-KNDy inputs at the level of KNDy cell bodies. Our observation in the present study of NK3R-IR localization in terminals that are presynaptic to GnRH cell bodies suggest another possibility – that NKB release by KNDy terminals acts in an autoregulatory manner upon the same terminals contributing to enhanced release of kisspeptin. However, since we did not co-localise NK3R with KNDy peptides in inputs contacting GnRH neurones, we cannot conclude that the presynaptic NK3R inputs we observed arose from KNDy cells and indeed may have originated from any of the number of other NK3R-IR cell populations. For example, the effects of senktide injections in the POA on LH secretion (23) may be mediated either by actions on NK3R-containing cell bodies in that region, or by presynaptic NK3R in contact with POA GnRH neurones. Since we found very little colocalization (approximately 5%) of NK3R within kisspeptin cells of the POA, it is possible that senktide effects on LH secretion from injections into this area are independent of kisspeptin, and mediated instead by other transmitters/peptides. It is noteworthy that the effects of senktide on GnRH release in tissue slices of the mouse median eminence are also independent of kisspeptin (ref), and since GnRH neurons in the mouse (15) like the sheep (14) lack NK3R, it is possible that the effect of senktide in the median eminence is also mediated by presynaptic actions of NKB, in this case via axo-axonic contacts. The possibility of presynaptic actions of NKB is also supported by evidence in other systems and species, for example, in the rat striatum, in which tachykinins presynaptically stimulate the release of dopamine (55). Finally, we would note that the observations reported here are based on control animals; the possibility that changes in presynaptic localization of NK3R are present in prenatal T female sheep and contribute to reproductive neuroendocrine defects remains to be examined.
In summary, the decreases in NK3R we observed in the ARC of prenatal T-treated ewes complement previous observations of decreases in NKB and dynorphin peptides in KNDy cells (38), and suggest that the combined reduction in ligand and receptor components of NKB/NK3R signaling may contribute to alterations in the control of pulsatile or surge modes of GnRH/LH secretion. The constellation of adult reproductive dysfunction, as well as metabolic defects, in prenatal T-treated ewes is very similar to that observed in women with polycystic ovarian syndrome (PCOS)(34) suggesting that the prenatal T ewe may serve as a model for this disease (33). KNDy cells are present in the human female infundibular nucleus (equivalent to the ARC in ewes) and show morphological changes with loss of steroid feedback regulation of GnRH/LH (56, 57). For example, in the infundibular nucleus of postmenopausal women, NKB gene expression is elevated due to reduced oestrogen negative feedback (58). Thus, we would speculate that alterations in NKB/NK3R signaling may be, at least in part, responsible for the ovulatory defects observed in patients with PCOS. The prenatal T-treated ewe could serve as an important translational model to test this hypothesis, with regard to the feedback control of GnRH/LH pulses as well as the generation of the preovulatory LH surge.
Acknowledgements
This manuscript was supported by a grant from the National Institute of Health (NIH P01 HD044232). We are grateful to Dr. Guanliang Cheng for his assistance.
Footnotes
The authors have nothing to declare
References
- 1.Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133(2):887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- 2.Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304(5924):345–347. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- 3.Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142(2):573–579. doi: 10.1210/endo.142.2.7956. [DOI] [PubMed] [Google Scholar]
- 4.Mussap CJ, Geraghty DP, Burcher E. Tachykinin receptors: a radioligand binding perspective. J Neurochem. 1993;60(6):1987–2009. doi: 10.1111/j.1471-4159.1993.tb03484.x. [DOI] [PubMed] [Google Scholar]
- 5.Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martin JD, Candenas ML. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11(15):2045–2081. doi: 10.2174/0929867043364748. [DOI] [PubMed] [Google Scholar]
- 6.Chawla MK, Gutierrez GM, Young WS, 3rd, McMullen NT, Rance NE. Localization of neurons expressing substance P and neurokinin B gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1997;384(3):429–442. doi: 10.1002/(sici)1096-9861(19970804)384:3<429::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 Defects Cause Hypothalamic Congenital Hypogonadotropic Hypogonadism in Humans. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2009-2600. [DOI] [PubMed] [Google Scholar]
- 9.Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin B Acts via the Neurokinin-3 Receptor in the Retrochiasmatic Area to Stimulate Luteinizing Hormone Secretion in Sheep. Endocrinology. 2010;151(8):3836–3846. doi: 10.1210/en.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2011;30(8):3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300(1):E202–E210. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- 14.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2009;22(1):1–12. doi: 10.1111/j.1365-2826.2009.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152(11):4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153(11):5105–5118. doi: 10.1210/en.2012-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hrabovszky E, Sipos MT, Molnar CS, Ciofi P, Borsay BA, Gergely P, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012;153(10):4978–4989. doi: 10.1210/en.2012-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. doi: 10.1210/en.2013-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;139(4):1752–1760. doi: 10.1210/endo.139.4.5904. [DOI] [PubMed] [Google Scholar]
- 21.Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (Kisspeptin/Neurokinin B/Dynorphin) Neurons Are Activated during Both Pulsatile and Surge Secretion of LH in the Ewe. Endocrinology. 2012;153(11):5406–5414. doi: 10.1210/en.2012-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fergani C, Routly JE, Jones DN, Pickavance LC, Smith RF, Dobson H. Kisspeptin, c-Fos and CRFR type 2 expression in the preoptic area and mediobasal hypothalamus during the follicular phase of intact ewes, and alteration after LPS. Physiology & Behavior. 2013;110–111(0):158–168. doi: 10.1016/j.physbeh.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 23.Porter KL, Hileman SM, Hardy SL, Goodman RL. Society for Neuroscience. SanDiego, CA, USA: 2013. Neurokinin B signaling in the retrochiasmatic area is essential for the full preovulatory LH surge in ewes. [Google Scholar]
- 24.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/lutenising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol. 2006;18(10):806–809. doi: 10.1111/j.1365-2826.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology. 2009;150(12):5530–5538. doi: 10.1210/en.2009-0712. [DOI] [PubMed] [Google Scholar]
- 27.Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151(2):722–730. doi: 10.1210/en.2009-0803. [DOI] [PubMed] [Google Scholar]
- 28.Smith JT, Li Q, Sing Yap K, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin Is Essential for the Full Preovulatory LH Surge and Stimulates GnRH Release from the Isolated Ovine Median Eminence. Endocrinology. 2011 doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 29.Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153(11):5406–5414. doi: 10.1210/en.2012-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fergani C, Routly JE, Jones DN, Pickavance LC, Smith RF, Dobson H. Kisspeptin, c-Fos and CRFR type 2 expression in the preoptic area and mediobasal hypothalamus during the follicular phase of intact ewes, and alteration after LPS. Physiol Behav. 2013;110–111:158–168. doi: 10.1016/j.physbeh.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Gorski RA. Sexual dimorphisms of the brain. J Anim Sci. 1985;61(Suppl):338–361. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- 32.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 33.Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1–2):8–20. doi: 10.1016/j.mce.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padmanabhan V, Sarma HN, Savabieasfahani M, Steckler TL, Veiga-Lopez A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs. environmental steroid receptor modulators. Int J Androl. 2010;33(2):394–404. doi: 10.1111/j.1365-2605.2009.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod. 2002;66(4):924–933. doi: 10.1095/biolreprod66.4.924. [DOI] [PubMed] [Google Scholar]
- 36.Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod. 2009;80(4):718–725. doi: 10.1095/biolreprod.108.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbosa CG, Dahl GE, Evans NP, Pelt J, Wood RI, Foster DL. Sexual differentiation of the surge mode of gonadotropin secretion: prenatal androgens abolish the gonadotropin-releasing hormone surge in the sheep. J Neuroendocrinol. 1996;8(8):627–633. [PubMed] [Google Scholar]
- 38.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84(1):87–96. doi: 10.1095/biolreprod.110.086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144(4):1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- 41.Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod. 2008;78(4):636–647. doi: 10.1095/biolreprod.107.065904. [DOI] [PubMed] [Google Scholar]
- 42.Robinson JE, Birch RA, Foster DL, Padmanabhan V. Prenatal exposure of the ovine fetus to androgens sexually differentiates the steroid feedback mechanisms that control gonadotropin releasing hormone secretion and disrupts ovarian cycles. Arch Sex Behav. 2002;31(1):35–41. doi: 10.1023/a:1014075016956. [DOI] [PubMed] [Google Scholar]
- 43.Karsch FJ, Foster DL, Legan SJ, Ryan KD, Peter GK. Control of the preovulatory endocrine events in the ewe: interrelationship of estradiol, progesterone, and luteinizing hormone. Endocrinology. 1979;105(2):421–426. doi: 10.1210/endo-105-2-421. [DOI] [PubMed] [Google Scholar]
- 44.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7(1):155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 45.Hunyady B, Krempels K, Harta G, Mezey E. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem. 1996;44(12):1353–1362. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- 46.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 47.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2009;151(1):301–311. doi: 10.1210/en.2009-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141(11):4218–4225. doi: 10.1210/endo.141.11.7743. [DOI] [PubMed] [Google Scholar]
- 49.Goodman RL, Coolen LM, Lehman MN. A Role for Neurokinin B in Pulsatile GnRH Secretion in the Ewe. Neuroendocrinology. 2013 doi: 10.1159/000355285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812. doi: 10.1210/en.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coolen LM, Smith TG, Lehman MN, Hileman SM, Connors JM, Goodman RL. Society for Neuroscience. San Diego, CA, USA: 2013. Arcuate KNDy neurons receive afferent projections from the retrochiasmatic area in the ewe. [Google Scholar]
- 52.Grachev P, Li XF, Lin YS, Hu MH, Elsamani L, Paterson SJ, Millar RP, Lightman SL, O'Byrne KT. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS One. 2012;7(9):e44344. doi: 10.1371/journal.pone.0044344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94(3):237–245. doi: 10.1159/000329045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenrohr M, Tena-Sempere M. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153(1):316–328. doi: 10.1210/en.2011-1260. [DOI] [PubMed] [Google Scholar]
- 55.Glowinski J, Kemel ML, Desban M, Gauchy C, Lavielle S, Chassaing G, Beaujouan JC, Tremblay L. Distinct presynaptic control of dopamine release in striosomal- and matrix-enriched areas of the rat striatum by selective agonists of NK1, NK2 and NK3 tachykinin receptors. Regul Pept. 1993;46(1–2):124–128. doi: 10.1016/0167-0115(93)90022-z. [DOI] [PubMed] [Google Scholar]
- 56.Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30(1):111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- 58.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2010;1364:116–128. doi: 10.1016/j.brainres.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]