SUMMARY
Human papillomavirus (HPV) is strongly associated with squamous esophageal cancer. The potential role of HPV in Barrett's esophagus (BE) has been examined but remains unclear. The aim of the study was to determine the prevalence of HPV in esophageal and gastric tissues obtained from patients with and without BE. We designed a cross-sectional study was conducted with prospective enrollment of eligible patients scheduled for esophagogastroduodenoscopy (EGD). All participants had biopsies of endoscopic BE, squamous-lined esophagus, and stomach. Immunohistochemistry (IHC) on formalin-fixed and paraffin-embedded tissue was conducted using monoclonal antibodies. Polymerase chain reaction (PCR) for HPV was performed on DNA extracted from esophageal biopsies snapped frozen within 30 minutes after endoscopic capture. The Roche HPV Linear Array Assay with PGMY primers that has high sensitivity for detecting 37 types of HPV was used. A total of 127 subjects were included: 39 with definitive BE had IHC done on samples from non-dysplastic BE, squamous esophagus, gastric cardia, and gastric body; and 88 control patients without BE had IHC done on squamous esophageal samples, gastric cardia, and gastric body. HPV was not detected in any of the samples in either group. For confirmation, HPV DNA PCR was performed on randomly selected samples from 66 patients (both esophagus and BE from 13 patients with BE, and 53 esophagus from patients without BE); no sample had HPV DNA detected via PCR in the presence of adequate quality control. HPV infection does not play a role in the formation of non-dysplastic Barrett's esophagus in men in the United States.
Keywords: Barrett's esophagus, esophageal adenocarcinoma, HPV, risk factor
INTRODUCTION
Human papillomavirus (HPV) is an oncogenic virus that is strongly associated with squamous cell dysplasia of female uterine cervix and its progression to cervical carcinoma. Cervical transitional cells in the squamocolumnar junction are well-known targets for HPV infection and potential cancerous transformation.1 It has also been suggested that the progenitor cells in esophageal squamous cell carcinoma (ESCC) and possibly Barrett's esophagus (BE) resemble those of cervical cancer.2,3 Given these similarities and the possibility of HPV exposure to the upper gastrointestinal (GI) tract through oral transmission, HPV has been examined as a possible risk factor for esophageal cancer as well as BE.
ESCC has been associated with HPV-induced transformation.4 For example, association studies from Iran and some regions of China reported HPV infection in 45% to 64% of ESCC specimens as compared with 0% to 34.7% in control samples.5–8 On the other hand, studies from other regions of the world such as western China, Australia, and Italy did not find a significant association between HPV and ESCC.9–11
The potential role of HPV and BE and esophageal adenocarcinoma (EA) has been less investigated than ESCC, and the cumulative findings of association studies have been inconclusive. A study from Mexico revealed a strong association between HPV and BE; Acevedo-Nuño et al. reported that HPV DNA was detected in 88% of 17 esophageal cancers of unspecified type and 96% of 28 BE samples.12 On the other hand, investigators from the United Kingdom did a similar study, but HPV DNA was found in only 1.4% of 73 BE samples.13 Intermediate estimates have been reported from patients in the United States; a multinational study reported presence of HPV DNA in 18 of 34 EA samples obtained from US patients.14 However, the only study to examined patients as well as controls from North America reported HPV DNA in 27.4% BE, 31% of EA, and 20.7% normal esophagus with no significant difference in HPV prevalence among the three groups.15
Given the remarkable variations in HPV prevalence in esophageal samples, additional studies are required in US populations. In addition, because HPV primarily infects squamous and transitional epithelium, it may also be important to examine esophageal squamous epithelium, in addition to BE tissue, when examining the association between HPV and BE. Therefore, in this study, we performed a comprehensive examination using immunohistochemistry (IHC) for HPV in esophageal and gastric samples of patients with and without BE and further confirmed the findings in a representative sample using polymerase chain reaction (PCR).
MATERIALS AND METHODS
Study population
This is a case–control study of patients who are eligible to receive their health care from Michael E. DeBakey Veterans Administration Medical Center (MEDVAMC) in Houston, TX. The institutional review boards at both the MEDVAMC and Baylor College of Medicine approved this study. Study subjects were recruited from eligible patients who were scheduled from a nonurgent upper endoscopy at MEDVAMC and from a randomly selected group of patients presenting primary care clinics at the same hospital who were eligible for screening colonoscopy. We excluded patients with contraindications to obtaining mucosal biopsies, those with any history of gastroesophageal surgery or malignancy and those with active non skin cancer.
During endoscopy, standardized mucosal biopsies were obtained from suspected BE, the esophagus 2–3 centimeters above suspected BE or the normal squamocolumnar line in controls, and several gastric biopsies. Based on the histopathological interpretation of the biopsies by a GI pathologist, subjects were categorized into cases with confirmed BE in the presence of specialized intestinal epithelium in targeted BE samples and controls without BE.
IHC
Sections of formalin-fixed and paraffin-embedded tissue were immunostained for HPV utilizing a monoclonal antibody and the immunoperoxidase method. Following deparaffinization in xylenes and rehydration in decreasing concentrations of ethanol ending in phosphate buffered saline (PBS), sections were subjected to steam heat antigen retrieval in 10 mmol/L citrate buffer pH 6. Sections were then incubated in ready-to-use (pre-diluted) monoclonal anti-HPV antibody (CM177AA, Biocare Medical, Concord, CA, USA) for 30 minutes at room temperature, and the bound antibody was detected utilizing MACH 3 Mouse Probe HRP polymer detection kit (Biocare Medical) with diaminobenzidine (DAB) as chromogen, and the procedure was performed utilizing an automated immunostainer from Biocare Medical. The monoclonal antibodies used in this study were specific for HPV-1, -6, -11, -16, -18, and -31. The tissue sections were then counterstained in hematoxylin, dehydrated, mounted, and coverslipped. Positive control consisted of section of formalin-fixed and paraffin-embedded human cervix with previously documented HPV infection, and negative controls were sections incubated without antibody or with irrelevant monoclonal antibody. Controls were included in every staining batch. Sections were scored ‘positive for HPV’ if there were one or more positive nuclei.
PCR
Cryo-frozen esophageal samples were processed using the ALLPrep Kit (Qiagen #80204, Valencia, CA, USA) to obtain simultaneous purification of genomic DNA and total RNA from the tissues. Per manufacturer's instructions, frozen tissue weighing less than 30 mg was placed into a precooled 1.5 mL tube for disruption using the mortar and pestle technique while keeping the tubes submerged in liquid nitrogen. The sample was homogenized by passing lysate (tissue and RLT Plus) at least 5 times through a 20-gauge needle fitted to a ribonuclease (RNase)-free syringe. The lysate was centrifuged for 3 minutes at max speed. The supernatant was removed and transferred to the ALLPrep DNA spin column which was centrifuged for 30 seconds at 8000 × g. The column was removed and placed in a fresh collection tube and stored at +4 C for subsequent DNA purification. The flow-through was used for immediate RNA purification and was mixed with one volume of 70% ethanol. This was transferred to an RNeasy spin column and centrifuged for 15 seconds at 8000 × g. The flow-through was discarded and the membrane washed three times with Buffer RPE. To prevent any ethanol carryover, the column was carefully removed and added to a fresh collection tube and centrifuged for 1 minute at max speed. The column was transferred to a 1.5-mL tube and 30 uL of RNase-free water was added directly to the column membrane followed by centrifugation for 1 minute at 8000 × g to elute the RNA. The concentration of the RNA was determined using the Nano-Drop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). The RNA was stored frozen at −80 C. For purification of the DNA, the DNA remaining on the column from earlier in the protocol was resuspended in Buffer AW1 and centrifuged for 15 seconds at 8000 × g. The supernatant was discarded and the column was washed once with Buffer AW1. The column was removed carefully from the collection tube so as not to carry over any ethanol and placed in a 1.5-mL tube. DNA was resuspended in 100 uL of Buffer EB and incubated at room temperature for 1 minute. The tube and column were centrifuged for 1 minute at 8000 × g to elute the DNA. The DNA concentration was measured by spectrophotometry using the NanoDrop 1000. The DNA was stored at −30 C. The presence of genomic DNA was confirmed by real-time PCR quantitation of the human endogenous retrovirus group 3 as previously described16 and further tested for presence of 37 HPV genotypes/subtypes by the HPV Linear Array test (Roche Diagnostics, Indianapolis, IN, USA) using consensus primer PCR.17
Statistical methods
The demographic features of cases and controls with and without BE were compared. The proportions of patients with HPV detected with IHC, PCR, or both (if any) were also compared between the two groups. Chi-square tests were planned for the comparisons of dichotomous variables and t-tests for continuous variables.
RESULTS
A total of 127 subjects were analyzed including 39 cases with BE and 88 controls of whom 27 were primary care controls. The demographic features of the study groups are displayed in Table 1. The mean age was 62 years and most (94%) were men. Except for the expected larger proportion of Caucasians among BE cases, there were no significant differences in age or sex distribution between cases and controls.
Table 1.
Demographic features of cases and controls that were tested for human papillomavirus
| BE cases (n = 39) | Non-BE controls (n = 88) | P-value | |
|---|---|---|---|
| Mean age (standard deviation) | 62.7 (7.5) | 62.7 (7.2) | 0.99 |
| Men | 39 (100%) | 81 (92%) | 0.07 |
| Race | 0.12 | ||
| White | 32 (82%) | 58 (66%) | |
| African-Americans | 5 (13%) | 26 (30%) | |
| Other | 2 (5%) | 4 (4%) |
BE, Barrett's esophagus.
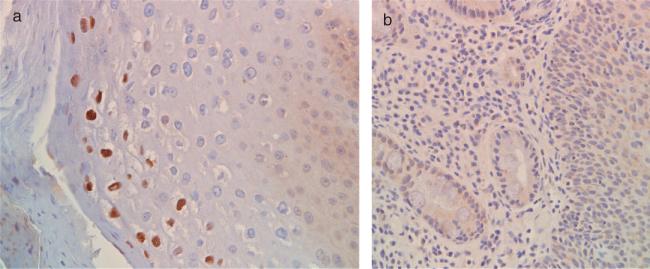
For the 39 cases with BE, IHC for HPV was performed on samples obtained from BE, squamous esophagus, gastric cardia, and gastric body. For the 88 controls without BE, IHC for HPV was performed on samples obtained from squamous esophagus, gastric cardia, and gastric body. HPV products were not detected by IHC in any of the esophageal or gastric samples in the non-BE control or the esophageal, BE, and gastric samples in the BE groups (Fig. 1).
Fig. 1.
(a) Positive control: immunohistochemical staining for human papillomavirus (HPV) in cervical biopsy. Note the strong brown nuclear staining in the superficial squamous epithelial cells. (b) Example of HPV immunohistochemical staining in esophageal biopsy of a patient with Barrett's esophagus. Note the complete absence of brown nuclear staining from both Barrett's epithelium and the adjoining squamous epithelium.
A random convenience sample was selected for HPV DNA testing. Samples from a total of 66 subjects including 13 with BE and 53 without BE were PCR tested. For the 13 patients with BE, both BE and squamous esophageal samples were examined, while for the 53 cases without BE, only squamous esophageal samples were examined. None of these samples had HPV DNA detected via PCR in the presence of adequate quality control demonstrated during testing.
DISCUSSION
Our study has demonstrated the absence of HPV infection in esophageal and gastric tissue samples taken from patients with and without non-dysplastic BE using both IHC and PCR methods. These findings argue strongly against a causal relationship between HPV and BE in the United States.
Previous studies demonstrated varying regional prevalence of HPV in BE, EA, as well as control (Table 2). Our study results are comparable with a recent UK study where virtually no HPV was detected in their samples.11 The previous North American study detected the presence of HPV in some cases of BE as well as EA, but the HPV prevalence was not statistically significant from their control group.12 This leaves the one study from Mexico as the clear outlier both in terms of very high proportion of BE and EA cases with esophageal HPV infection as well as infected as the disproportionate increase in HPV compared with controls. Regional variations in the overall HPV may partly account for the observed differences among the studies BE and EA. For example, studies from China, Egypt, and Iran, where HPV infection is more common in the population, HPV was also found in large proportions of high-grade dysplasia as well as ESCC cases.7,9,18–21
Table 2.
A summary of previous studies that examined human papillomavirus (HPV) infection in Barrett's esophagus (BE) or esophageal adenocarcinoma (EA)
| Study first author (year) | Region | Cases n (BE/EA) | Case tissue examined (design) | Control subjects n | Control tissue examined | Method of HPV testing | % HPV positive |
|---|---|---|---|---|---|---|---|
| Acevedo-Nuno E et al (2004)12 | Mexico | 45 (28/17) | Lesion-targeted biopsy | 23 esophagitis | Gastroesophageal junction | PCR IHC |
96% BE, 26% controls (P < 0.01). increasing correlation with esophagitis, BE, and EA (P = 0.000) |
| Rai N et al (2008)13 | United Kingdom | 73 (73/0) | Biopsy from suspected BE lesion | None | N/A | PCR | 1.4% in BE |
| Wang X et al. (2010)14 | United States | 34 (0/34) | Tumor site | 54 ESCC biopsies | Tumor site | PCR | 52.9% in EA versus 66.7% in ESCC (P = 0.2) |
| Iyer A et al (2011)15 | North America | 116 (80/36) | Lesion-targeted biopsy | 29 normal biopsies | Biopsy at gastroesophageal junction | PCR | 28% BE, 31% EA, 21% control |
ESCC, esophageal squamous cell carcinoma; IHC, immunohistochemistry; N/A, not applicable; PCR, polymerase chain reaction.
Our study demonstrates the absence of gastroesophageal HPV infection in a mostly male veteran population. The generalizability to other populations may be limited. It is also possible if a larger number of subjects were tested, few HPV cases would have been found. However, it is reasonable to conclude that HPV prevalence would be very low. Lastly, our samples did not include dysplasia or EA, and therefore an HPV-related role to BE progression cannot be excluded, although it is unlikely.
However, the study had several strengths including the prospective design, the evaluation of well-characterized control subjects and samples, HPV testing using IHC as well as state-of-the-art PCR methods by a very experienced lab, and the inclusion of both esophageal and gastric tissues. The use of PCR allows the detection of HPV below the threshold of detection for in situ hybridization, which was not used in this study.22 Previous studies have evaluated samples from BE tissue with very few samples from surrounding esophageal tissue and none from the gastric mucosa.
In summary, there was no evidence of HPV infection in the esophagus or stomach of subjects with or without non-dysplastic BE in a US population. The methods used in this study could prove useful as other HPV association studies.
Acknowledgments
Funding: This work is funded in part by NIH grant R01CA116845-01A2, and in part by the Houston VA HSR&D Center of Excellence (HFP90-020) and P30 Center Grant DK56338.
ABBREVIATIONS
- BE
Barrett's esophagus
- EA
esophageal adenocarcinoma
- HPV
human papillomavirus
Footnotes
Contributorship: H. B. El-Serag: Funding, conception, design, analysis, interpretation results, manuscript writing, editing, decision to publish.
J. M. Hollier: Data collection, editing manuscript, decision to publish.
P. Gravitt: Conception, data collection, editing manuscript, decision to publish.
A. Alsarraj: Data collection, editing manuscript, decision to publish.
M. Younes: Conception, data collection, editing manuscript, decision to publish.
Conflict of interest statement: The authors declare no conflict of interest.
References
- 1.Ng WK, Li AS, Cheung FM, Chow J C. Transitional cell metaplasia of the uterine cervix is related to human papilloma-virus: molecular analysis in seven patients with cytohistologic correlation. Cancer. 2002;96:250–8. doi: 10.1002/cncr.10722. [DOI] [PubMed] [Google Scholar]
- 2.Rajendra S, Robertson IK. Similar immunogenetics of Barrett's oesophagus and cervical neoplasia: is HPV the common denominator? J Clin Pathol. 2010;63:1–3. doi: 10.1136/jcp.2009.067447. [DOI] [PubMed] [Google Scholar]
- 3.Shields HMZF, Antonioli DA, Doos WG, Kim S, Spechler SJ. Detection by scanning electron microscopy of a distinctive esophageal surface cell at the junction of squamous and Barrett's epithelium. Dig Dis Sci. 1993;38:97–108. doi: 10.1007/BF01296780. [DOI] [PubMed] [Google Scholar]
- 4.Lyronis ID, Baritaki S, Bizakis I, Krambovitis E, Spandidos D A. K-ras mutation, HPV infection and smoking or alcohol abuse positively correlate with esophageal squamous carcinoma. Pathol Oncol Res. 2008;14:267–73. doi: 10.1007/s12253-008-9032-1. [DOI] [PubMed] [Google Scholar]
- 5.Zhou XB, Guo M, Quan LP, et al. Detection of human papillomavirus in Chinese esophageal squamous cell carcinoma and its adjacent normal epithelium. World J Gastroenterol. 2003;9:1170–3. doi: 10.3748/wjg.v9.i6.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D, Zhang Q, Zhou L, et al. Comparison of prevalence, viral load, physical status and expression of human papillomavirus-16, -18 and -58 in esophageal and cervical cancer: a case-control study. BMC Cancer. 2010;10:650. doi: 10.1186/1471-2407-10-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao PF, Li GC, Li J, et al. Evidence of human papilloma virus infection and its epidemiology in esophageal squamous cell carcinoma. World J Gastroenterol. 2006;12:1352–5. doi: 10.3748/wjg.v12.i9.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farhadi M, Tahmasebi Z, Merat S, Kamangar F, Nasrollahzadeh D, Malekzadeh R. Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. World J Gastroenterol. 2005;11:1200–3. doi: 10.3748/wjg.v11.i8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonsson A, Nancarrow DJ, Brown IS, et al. High-risk human papillomavirus in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:2080–7. doi: 10.1158/1055-9965.EPI-10-0033. [DOI] [PubMed] [Google Scholar]
- 10.Tornesello ML, Monaco R, Nappi O, Buonaguro L, Buonaguro F M. Detection of mucosal and cutaneous human papillomaviruses in oesophagitis, squamous cell carcinoma and adenocarcinoma of the oesophagus. J Clin Virol. 2009;45:28–33. doi: 10.1016/j.jcv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Koshiol J, Wei WQ, Kreimer AR, et al. No role for human papillomavirus in esophageal squamous cell carcinoma in China. Int J Cancer. 2010;127:93–100. doi: 10.1002/ijc.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acevedo-Nuño EG-OA, Vázquez-Camacho G, Balderas-Peña Luz Ma A, Moreno-Villa H, Montoya-Fuentes H. Human papillomavirus DNA and protein in tissue samples of oesophageal cancer, Barrett's oesophagus and oesophagitis. Anticancer Res. 2004;24:1319–23. [PubMed] [Google Scholar]
- 13.Rai N, Jenkins GJ, McAdam E, Hibbitts SJ, Fiander AN, Powell NG. Human papillomavirus infection in Barrett's oesophagus in the UK: an infrequent event. J Clin Virol. 2008;43:250–2. doi: 10.1016/j.jcv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Tian X, Liu F, et al. Detection of HPV DNA in esophageal cancer specimens from different regions and ethnic groups: a descriptive study. BMC Cancer. 2010;10:19. doi: 10.1186/1471-2407-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer A, Rajendran V, Adamson C S, Peng Z, Cooper K, Evans M F. Human papillomavirus is detectable in Barrett's esophagus and esophageal carcinoma but is unlikely to be of any etiologic significance. J Clin Virol. 2011;50:205–8. doi: 10.1016/j.jcv.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Methods. 2001;91:109–17. doi: 10.1016/s0166-0934(00)00244-5. [DOI] [PubMed] [Google Scholar]
- 17.Gravitt PE, Apple RJ, Wheeler CM. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding GC, Ren JL, Chang FB, et al. Human papillomavirus DNA and P16(INK4A) expression in concurrent esophageal and gastric cardia cancers. World J Gastroenterol. 2010;16:5901–6. doi: 10.3748/wjg.v16.i46.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Far AE, Aghakhani A, Hamkar R, et al. Frequency of human papillomavirus infection in oesophageal squamous cell carcinoma in Iranian patients. Scand J Infect Dis. 2007;39:58–62. doi: 10.1080/00365540600740496. [DOI] [PubMed] [Google Scholar]
- 20.Castillo A, Aguayo F, Koriyama C, et al. Human papillomavirus in esophageal squamous cell carcinoma in Colombia and Chile. World J Gastroenterol. 2006;12:6188–92. doi: 10.3748/wjg.v12.i38.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao GF, Wei WQ, Abnet CC, et al. No association between HPV infection and the neoplastic progression of esophageal squamous cell carcinoma: result from a cross-sectional study in a high-risk region of China. Int J Cancer. 2006;119:1354–9. doi: 10.1002/ijc.21980. [DOI] [PubMed] [Google Scholar]
- 22.Matsukura T, Sugase M. Pitfalls in the epidemiologic classification of human papillomavirus types associated with cervical cancer using polymerase chain reaction: driver and passenger. Int J Gynecol Cancer. 2008;18:1042–50. doi: 10.1111/j.1525-1438.2007.01157.x. [DOI] [PubMed] [Google Scholar]