Abstract
Progranulin (PGRN) is a growth factor that has been implicated in wound healing, inflammation, infection, tumorigenesis, and is most known for its neuroprotective and proliferative properties in neurodegenerative disease. This pleiotropic growth factor has been found to be a key player and regulator of a diverse spectrum of multi-systemic functions. Its critical anti-inflammatory role in rheumatoid arthritis and other inflammatory disease models has allowed for the propulsion of research to establish its significance in musculoskeletal diseases, including inflammatory conditions involving bone and cartilage pathology. In this review, we aim to elaborate on the emerging role of PGRN in the musculoskeletal system, reviewing its particular mechanisms described in various musculoskeletal diseases, with special focus on osteoarthritis and inflammatory joint disease patho-mechanisms and potential therapeutic applications of PGRN and its derivatives in these and other musculoskeletal diseases.
Keywords: Progranulin, PGRN, Growth factor, Disintegrin, Review
2. INTRODUCTION
The “isolation and characterization of a novel class of leukocyte peptides with possible cytokine-like activities called granulins” were first researched in 1990 (1). “Granulins” were initially found in inflammatory cells and bone marrow. Within the following two years the intact structure was coming into a clearer view by presenting the structural composition of its domains (2). Progranulin (PGRN), a 593-amino-acid autocrine growth factor, also known as GP88 (3), granulin epithelin precursor (GEP) (4), PC-cell-derived growth factor (PCDGF) (5), proepithelin (6), and acrogranin (7), contains seven-and-a-half repeats of a cysteine-rich motif (CX5–6CX5CCX8CCX6CCXDX2HCCPX4CX5–6C) in the order P–G–F–B–A–C–D–E, where A–G are full repeats and P is the half-motif.
PGRN is heavily glycosylated and appears as a ~90-kDa protein, and when secreted undergoes proteolysis, leading to the release of its constituent peptides, the granulins (8). It consists of a subdomain shared by small toxins, protease inhibitors as well as the EGF-like protein modules (9). PGRN is digested into 6-kDa GRN peptides by many proteinases, including matrix metalloproteinase 9, 12, and 14, elastase, and proteinase 3, and ADAMTS-7 (10).
It has been known to interact with ADAMTS7, ADAMTS 12 (11), COMP, Perlecan (12), HDL/apo A–I (13), TLR 9 (14), Sortilin (15), and its most significant anti-inflammatory functions can be attributed to its direct inhibition of TNFa through interaction with TNFR1 and especially TNFR2 (16). (Reference protein interaction chart from “cubic of I review” (17)) (Figure 1).
Figure 1.
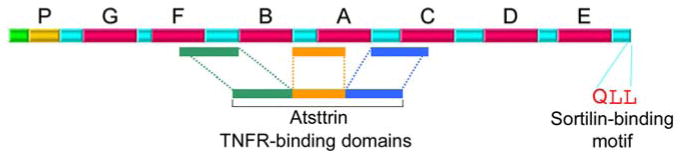
A Diagram depicting the structure of PGRN. TNFR binding domains and Sortilin binding motif are indicated.
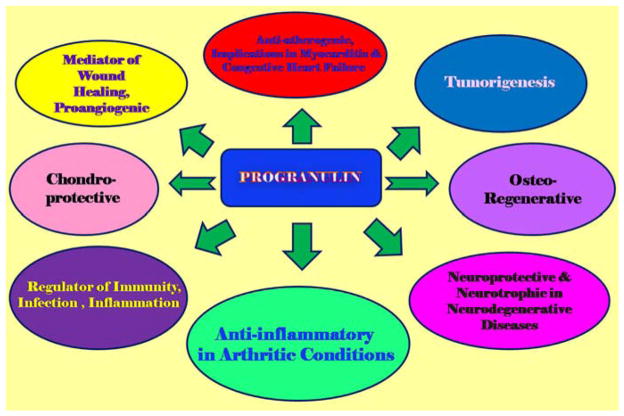
Due to the pleiotropic nature of PRGN, it is highly expressed in a broad range of cells including, epithelial cells (18), neurons (19), and macrophages (20), immune cells (21), chondrocytes, adipose tissue (22), hematopoietic cells, including neural stem cells (23), skeletal muscle (24), endothelial cells (25), as well as lung parenchyma, where it has been known to counter the pneumo-toxic effects of LPS induced ARDS (26). PGRN has also been implicated in a wide variety of biological processes, including wound healing (27), embryo development (28), morphogenesis (29), and cancer (30). PRGN overexpression has been found to be associated with cholangiocarcinoma (31), sarcoma (32), glioblastoma (33) and both ovarian and breast cancer (19). PGRN knockout models however, have presented with rheumatoid arthritis, osteoarthritis, and frontotemporal lobar degeneration (FTLD) (34), implicating its intricate protective role in various diseases of inflammatory etiology (35).
Progranulin’s role and function have been indeed, widely studied throughout the systems, with implications of its anti-inflammatory properties in rheumatoid arthritis (36), cardiovascular pathology, mainly atherosclerosis (20), autoimmune disorders, and it has also been found that PGRN may act as a prognostic marker in breast cancer (37). It influences the prevention of muscle-atrophy (38), and its neurotrophic and neuro-protective characteristics have been vastly researched in neurodegenerative diseases including frontotemporal dementia (39) (Figure 2). However, its protective, growth-promoting characteristics are of particular interest and significance in osteoarthritis and articular disease models (40).
Figure 2.
A schematic representation of PGRN’s multiple functions
Recently, it has been identified as a factor stimulating chondrogenesis, and is considered an important regulator of cartilage formation and function (41). PGRN is known to selectively interact with the COMP epidermal growth factor repeat domain. Progranulin overexpression can stimulate chondrocyte proliferation, which is then enhanced by COMP (42). Progranulin’s ability to influence chondrocyte differentiation is mediated through the extracellular regulation of the kinase (Erk) 1/2 signaling pathway and Jun B transcription factor (43). Its chondro-protective nature manifests in its ability to directly bind to ADAMTS-7 and ADAMTS-12 and inhibit their degradation of COMP (44).
Progranulin with its specific binding and key regulation of the TNFa signaling pathway, through binding to TNFR receptors, competitively inhibits TNFα-induced ADAMTS-7 and ADAMTS-12 expression, and prevents COMP degradation by ADAMTS-7 and ADAMTS-12 through direct protein–protein interactions. Also, it has been well established that PGRN activates ERK and PI3K/AKT pathways in several types of cells, such as chondrocytes (43).
In this review, we will present an updated review of the functions of PGRN in the musculoskeletal system by looking at its effects in osteo-degenerative and inflammatory pathology and other select musculoskeletal diseases presented in the literature.
3. FUNCTION OF PGRN IN THE MUSCULOSKELETAL SYSTEM
3.1. PGRN in chondrocyte and cartilage metabolism
PGRN plays a definitive role in regulating the equilibrium of the ECM by protecting functionally intact cartilage and preventing the degradation of its integral components (45). This function is considered to be vital in determining articular disease progression of both inflammatory and degenerative etiology, and constitutes a “balancing mechanism”, the curtailing of which provides a preventative method for the progression of chondro-degradative and osteoarthritic changes of articular surfaces. PGRN is a key signaling molecule at the onset of the pro-inflammatory cascade which prevents the degradation of COMP by inhibiting TNFα-induced ADAMTS-7 and ADAMTS-12 expression and cleavage of COMP (11), acting thereof, to entirely disrupt the breakdown of the pathology-stricken cartilage matrix. Also, PGRN directly binds to the cartilage oligomeric matrix protein (COMP), thereby exuding its salvaging role of this essential collagen-binding, structurally stabilizing component of the ECM (42, 46), and simultaneously prevents its degradation which otherwise would have led to the morphological and clinical exacerbation of arthrosis (47).
In a previous study, it has been established that ADAMTS-7 overexpression leads to increased expression of TNF and metalloproteinases which promotes the breakdown of the ECM and consequent OA. Through signaling by NFκB, this patho-mechanism is further enhanced by TNF’s direct induction of ADAMTS-7 expression, which constitutes a positive feedback loop contributing to accelerated degeneration of cartilage and osteoarthritic transformation of the joint (48, 49). PGRN has the ability to intervene in this process as the dominant and rate limiting mediator by acting directly on TNF receptor signaling to inhibit this biofeedback mechanism altogether.
The significant chondroprotective role of PGRN is further substantiated by evidence showing that the overexpression of PGRN stimulates the proliferation of chondrocytes and this stimulation is enhanced in turn by the effects of COMP (42). A preliminary in vitro study of cartilage explant cultures of human chondrocytes has shown that both PGRN and the PGRN engineered derivative, Attstrin (50), were effective in inhibiting the release of TNFα-induced inflammatory and catabolic mediators, including MMP13, RUNX2, COX-2, and iNOS, in human osteoarthritis chondrocytes, and in exuding its chondro-proliferative effects and stimulating the anabolic metabolism, including the synthesis of ECM integrating components, such as Collagen 2 and Aggrecan (Zhao and Richbourgh et al. unpublished data).
PGRN exhibits its chondro-regenerative characteristics by acting as a downstream molecule of bone morphogenetic protein 2 (BMP2) in chondrogenesis as well as in cartilage repair (47). Also, PGRN activates chondrocyte differentiation through Erk1/2 signaling and JunB transcription factor is one of key downstream molecules of PGRN in chondrocyte differentiation (43). The intricate interplay of these stimulatory and promoting mechanisms of PGRN as well as its anti-inflammatory properties inarguably makes it an essential regulator of cartilage metabolism, the presence of which maintains the integrity of the structural matrix and at the onset of osteo-degenerative processes, could act as a preventative agent for the progression of disease.
3.2. PGRN in bone formation and bone remodeling
PGRN’s role in the process of bone development and healing by means of endochondral ossification indisputably lies in its regulatory function of chondrogenesis, however, its most noteworthy properties can be found in its ability to induce osteoblastogenesis. PGRN assists in the initiation of the process of bone healing and development by, at least in part, triggering the proliferation of osteoblasts, creating the trabecullar matrix for structurally integral bone (1).
Progranulin is induced by bone morphogenic protein 2 (BMP-2) and is required for BMP-2 mediated ectopic bone formation (51). BMP-2 is a growth factor that has been shown to induce bone formation, and has been used clinically to treat bone fractures (51). In the presence of high levels of TNF-α induced by a local or systemic inflammatory process, it has been established that bone formation and regeneration are decelerated and impaired by inhibiting BMP-2 signaling (52, 53). It is also known that the process of bone resorption is signaled via TNFR1 (54). Hence, PGRN’s known interaction with both TNFR 1/2 may serve as an important regulator in bone metabolism, allowing for stimulation of osteoblastogenesis and bone formation as a downstream mediator of BMP-2 signaling, and the prevention of osteoclastogenesis and bone resorption through inhibiting TNF/TNFR signalin (51). PGRN is also important for endochondral ossification in bone regeneration and repair. A recent study has shown that PGRN increased cartilaginous callus formation in bone de novo synthesis by means of TNF signaling (51). In addition to the already mentioned anti-inflammatory properties of the PGRN-TNFR interaction, and its known regulation of rheumatoid arthritis (50), PGRN binds to TNFR2 to stimulate PGRN-mediated endochondral ossification (51).
PGRN has also been studied in the process of bone remodeling and has been found to prevent osteoclastogenesis, dramatically influencing the regeneration of bone in osteolysis models. Its significant effects on the regeneration of iatrogenically induced osteolytic lesions, presents its novel regeneration enhancing role by stimulating osteoblastic synthesis of new bone trabecullae (Zhao et al. unpublished data). The focus of these findings provides a promising potential therapeutic target in arthroplasty implant failure and aseptic loosening caused by implant-induced osteolysis and can be an eventual therapeutic target in complex cases of osteosynthesis including high-energy comminuted fractures with bone fragment loss.
PGRN’s osteoregenerative properties can also be applied to osteoporosis and osteopenia models constituting PGRN’s influence on both qualitative and quantitative structural bone loss. There is substantial evidence confirming the correlation of PGRN with estrogen and progesterone. Serum concentration levels of PGRN were noted to be augmented with increased circulating levels of estrogen and progesterone (55). Confirmatory findings have been presented in a study clearly depicting the up-regulation of PGRN by exogenously administered estrogen in the hypothalamus of newborn mice (56). Estrogen is a key regulator of bone mineral density and the direct positive feedback loop that it participates in with PGRN could provide a postulation to identify the mechanism by which PGRN maintains and enhances bone integrity. There has been a recent study providing substantial evidence that there is a direct and positive correlation between endogenous PGRN levels and the qualitative integrity of bone. Recent investigations have allowed for the visualization of PGRN’s role in the development of osteoporosis. A PGRN knockout ovariectomized mouse model has presented with increased osteoclastic activity, trabecular bone frailty and regions of demineralization confirming the osteoporotic phenotype (Tang et al. unpublished data).
PGRN’s influence on Vitamin D 25-OH metabolism is also worthy of note and has been studied in an obesity patient model (BMI>30). It has been found that osteopenic obese patients presented with higher circulating levels of IL-17, IL-6, TNFα and IL-4 and lower concentrations of IL-13, IL-10, and PGRN. Through a direct positive correlation, measured concentrations of IL-13, IL-10 and 25- (OH) vitamin D were increased and the concentration of TNFα and IL-17 were decreased, by raising the concentration of PGRN (57). The patients presented with a marked improvement of bone status by steadily increasing PGRN levels, hence further confirming PGRN’s vital function in osteoblastogenesis.
3.3. PGRN in skeletal muscle pathology
PGRN’s implications in the musculoskeletal system, also include its role as a critical mediator in skeletal muscle differentiation. PGRN is part of a regulatory feedback loop along with MyoD and JunB, where MyoD causes PGRN expression which is able to inhibit myotube formation, suppressing the process of myogenesis and in turn repressing JunB. By influencing myogenic differentiation, PGRN poses itself as a critical factor in regulating disease processes affecting functional muscular capacity (58). A PGRN mutation has also been found to correlate with several neuromuscular diseases, including amyotrophic lateral sclerosis and spinal muscular atrophy, in which denervation produces atrophy of myocytes with an abnormal quantity and functional ability of myogenic progenitor cells (MPC) (59).
It has been shown that PRGN is detectable within skeletal muscle tissue and is differentially expressed during the fusion of myoblasts to myotubes. MyoD, a muscle-specific transcription factor, regulates this differential expression of PGRN. Current research has shown that regulation of myogenesis is, at least partially, mediated by the transcription factor JunB (58). Murine studies have shown that PGRN promotes myotube hypertrophy by way of the PI3K/Akt/mTOR pathway (38).
Significantly, it was discovered that PGRN, JunB, and MyoD transcription factor form a regulatory loop, which acts in concert in the course of myogenesis. This regulatory feedback loop, in which MyoD induces PGRN expression, inducing JunB, appears to inhibit MyoD. MyoD induces PGRN, by binding to the specific sites within the 50-flanking regulatory region of the gene encoding PGRN. The action of PGRN, then, partially depends on JunB, since the silencing of JunB reverses the inhibitory effect of PGRN. Thus, PGRN as a MyoD- inducible growth factor acts as a novel regulator of myogenic differentiation. PGRN has the ability to inhibit myotube formation in vitro and has modulatory effects on muscle tissue development in vivo. The elucidation of PGRN’s role and molecular events involved in myogenic differentiation will better our understanding of normal muscle development and the pathogenesis of muscular diseases (58).
PGRN also acts in concert with IGF signaling exhibiting its myotrophic properties. Numerous in vivo and in vitro studies have demonstrated the critical role Insulin-like growth factor 1 (IGF-1), a signaling factor, plays in the regulation of postnatal muscle growth (60). In skeletal muscle, IGF-1 serves in a plethora of functions. Most notably, it stimulates myoblastic proliferation, regulating myoblastic differentiation, and promoting protein synthesis (61). The liver is the principal source of circulating IGF-1, however, several reports have also demonstrated the importance of locally produced IGF-1 in muscle growth. PGRN has the ability to circumvent IGF-1 signaling to stimulate muscle growth through myoblastic hypertrophy. PGRN can be used to mediate an adaptive strategy for sustaining partial muscle growth via the PI3K/Akt/mTOR pathway in the absence of IGF-1 signaling (38).
4. PGRN POTENTIAL THERAPEUTIC ROLE IN OSTEOARTHRITIS AND OTHER MUSCULOSKELETAL DISEASES
Progranulin’s main therapeutic properties are entailed in its role as a TNFR ligand and competitive inhibitor of one of the most critical immunological cascades which is responsible for the inductive patho-mechanism of a broad range of inflammatory diseases, including rheumatoid arthritis, ulcerative colitis and other inflammatory bowel diseases, ankylosing spondylitis, psoriasis, including pathologies spanning the cardiovascular system such as ischaemia- reperfusion injury (62), myocarditis, with the resultant progression of congestive heart failure (63) and can even be proposed as a potential target therapy in graft versus host disease, which has been shown to be triggered by TNFR1 mediated induction of TNFa (64).
Through its binding to TNFR1/2, via F-A-C GRN domains, as opposed to sole targeting of TNFa, as effectuated by current therapeutic inhibitors, such as adalimumab (Humira) (65, 66), Etanercept (Enbrel) (67, 68), infliximab (Remicade) (69, 70), it can intervene through selective receptor affinity, especially TNFR2 (71), and may simultaneously regulate and effectuate a broader range of therapeutic functions. The discernment of this binding specificity was recently established, by in turn, identifying the TNFR1 and TNFR2 binding domains required for interaction with PGRN. TNFR1 has been shown to bind with PGRN through its CDR2 and CRD3 domain, whereas, TNFR2 similarly strongly exhibits affinity for both of these binding domains (Jian et al, 2013). It has been shown that TNFa binding occurs through interaction with the CDR2 and CDR3 domains of TNFR (16). However, established binding specificity of PGRN through specific targeting of TNFR1 and 2, regulates a broader spectrum of functions especially imperative for targeting inflammatory processes in for instance, rheumatoid and osteoarthritis, and can specifically, as presented, target the induction of chondrocyte proliferation and bone regeneration presenting a potential for the enactment of complex therapeutic functions and may serve as a preventative measure for the progression of osteoarthritis at the onset of disease.
Worthy of note is PGRN’s interaction at the neutrophilic level which reduces the synthesis of ROS, and regulates the oxidative burst response to the release of TNFa (62). The high expression of PGRN in the cytoplasm of neutrophils converted into GRNs by neutrophil- released elastase (10), accounts for PGRN’s significant role in the regulatory mechanism of acute inflammation processes. The later activated granulins promote the release of inflammatory cytokines, including IL-8 expression in epithelial cells to summon further recruitment of neutrophils and other mediatory cells to the inflammation site (72). The counter-regulatory mechanism of this promotion of inflammation can be found at the level of SLPI binding with PGRN thus inactivating PGRN conversion to GRNs by elastase (6).
It has been also established that PGRN plays a key regulatory role in acute (73) and chronic inflammatory processes involving the musculoskeletal system (17), and could play a role in the treatment of auto-immune and inflammatory conditions presenting with musculoskeletal manifestations. For instance, through its recently established expression in the endothelial cell and its induction of endothelial cell migration and proliferation mediating angiogenesis (25), and the coincidental presence of anti-PGRN antibodies found in autoimmune diseases such as granulomatosis and polyangiitis (45), it can be postulated that the administration of PGRN in such cases, may exhibit anti-inflammatory effects and prevent the exacerbation of clinical musculoskeletal and systemic symptoms of these and other auto-immune disorders.
Recently, the potentiation of the efficacy of PGRN’s therapeutic effects has been obtained by creating the engineered construct Attstrin, the antagonist of TNFa signaling, which selectively interacts with TNFR1/TNFR2, and consequently antagonizes TNFa and TNF/TNFR signaling (41). The application of this PGRN-derivative can act in a myriad of clinical conditions whose patho-mechanism involves upregulation and a disproportion of TNFa signaling and its resultant induction of inflammatory processes, including but not limited to neuro-degenerative and inflammatory diseases, Crohn’s disease (74), IBD (75), and ulcerative colitis (76), asthma (77), dermatitis and psoriasis (78), and rheumatoid arthritis (79). Attstrin’s effects have been studied in rheumatoid arthritis (50) and dermatitis models (27), and its clear signaling interception and competitive inhibition of TNFa via TNFR1/2 binding proposes it as a highly promising therapeutic target for the prevention of inflammatory diseases affecting the musculoskeletal system. In addition, the presented chondro-proliferative and osteo-regenerative effects of PGRN, present a clear therapeutic implication for the use of Attstrin and other effective PGRN recombinant derivatives, in OA and other conditions requiring the induction of bone remodeling and repair.
5. CONCLUSION
The application of PGRN’s functions in the processes of chondrocyte regeneration and bone de- novo synthesis propose a relevant potential clinical therapeutic alternative. Considering its broad range of biological functions as a multi-factorial progenitor growth hormone, the precise identification of its binding domains and the therapeutic implementation of a biologically specific and effective engineered molecule could provide a pharmaceutical alternative to the current available treatment options in rheumatoid arthritis, osteoarthritis, osteomyelitis, and may possibly constitute an alternative to proposed therapies accelerating fracture healing, such as parathyroid hormone–related peptide (PTHrP) (80), and also the recently proposed deferoxamine (81).
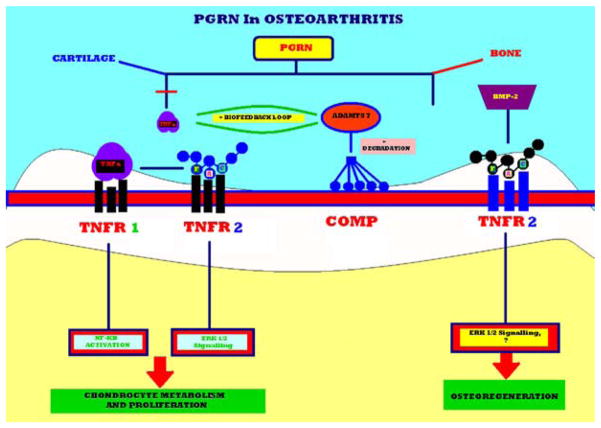
PGRN targets both cartilage and bone metabolism and its chondroprotective role in the cartilage degradative cascade, stimulation of chondrocyte proliferation, via binding to TNFR2 receptors, along with induction of osteoblastogenesis and bone regeneration constitute key therapeutic goals in OA treatment, prevention, and prophylaxis. In cartilage, these functions are executed by PGRN’s particular affinity for the TNFR2 receptor, and the binding that occurs via the F-A-C domain activates ERK 1/2 signaling thus promoting chondrocyte metabolism and proliferation. TNFR 1 binding to PGRN’s F-A-C domain prevents the activation of the NF-KB pathway by TNFα and the resultant chondroprotective inhibition of the degradation of COMP by ADAMTS-7. PGRN directly regulates the positive biofeedback loop which exists between TNFa and ADAMTS 7. The PGRN/TNFR2, in turn, induces the process of osteo-regeneration, where PGRN is a downstream molecule of BMP-2. Targeting of the F-A-C domain is fundamental in the treatment of OA- and rheumatoid arthritis-related arthroses and chondral pathology, which allows for an effectuation of the highly specific functions without influencing the occurrence of potential side-effects, related to its properties as a pleiotrophic growth hormone (Figure 3).
Figure 3.
A proposed model for explaining the role of PGRN in the musculoskeletal system. PGRN plays a chondroprotective role in the metabolic cascade of cartilage degradation, promotes chondrocyte proliferation, via binding to TNFR2 receptor binding which acts as a downstream mediator of BMP-2 signaling and triggers osteoblastogenesis and bone regeneration. In cartilage, PGRN exhibits a particular affinity for the TNFR2 receptor, binds via the F-A-C domain and activates ERK 1/2 signaling stimulating chondrocyte function and proliferation. TNFR 1 binding to PGRN’s F-A-C domain prevents the activation of the NF-KB pathway by TNFα assisting in the chondro-protective process, thus, inhibiting the degradation of COMP by ADAMTS-7. The positive biofeedback loop between TNFa and ADAMTS 7 is indicated in the figure, and PGRN directly tampers with this biofeedback mechanism. In bone metabolism, TNFR2 receptors are of particular importance, where PGRN/TNFR interaction may mediate BMP-2 induction of de-novo osteogenesis.
The highly specific properties of Attstrin, for instance, which encompass the targeting of the F-A-C domains and the implementation of their therapeutic function, may provide the achievement of the curative and preventative effects at the level of chondrocyte metabolism and the process of osteoregeneration through TNFR signaling and the prevention of degradation of cartilage and its ECM structural components, and the mediation of BMP2 signaling pathways, respectively. Clinical trials which correlate and contrast the effects of current available treatment options such as methotrexate – TNFa inhibitor combination therapy (82), and target TNFR inhibitors, i.e. Attstrin, could allow for a differentiation of therapeutic effectiveness on many levels.
Acknowledgments
C. J. Liu is grateful to his gifted collaborators who made the explorations in his laboratory possible. We would also like to thank Daniel Waxman-Lenz for his assistance. This work was supported partly by NIH research grants R01AR062207, R01AR061484, R56AI100901, a Disease Targeted Research Grant from American College of Rheumatology Research and Education Foundation.
Abbreviations
- PGRN
progranulin
- TNF
tumor necrosis factor
- ECM
Extracellular Matrix
- TNFR
tumor necrosis factor receptors
- COMP
cartilage oligomeric matrix protein
- ADAMTS
A Disintegrin And Metalloproteinase with Thrombospondin Motifs
- MMP
matrix metalloproteinase
- PEPI
proepithelin
- PCDGF
GP88/PC-cell derived growth factor
References
- 1.Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Granulins, a novel class of peptide from leukocytes. Biochemical and biophysical research communications. 1990;173(3):1161–8. doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cDNA from human bone marrow reveals tandem cysteine-rich granulin domains. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(5):1715–9. doi: 10.1073/pnas.89.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tangkeangsirisin W, Serrero G. PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis. 2004;25(9):1587–92. doi: 10.1093/carcin/bgh171. [DOI] [PubMed] [Google Scholar]
- 4.Ho JC, Ip YC, Cheung ST, Lee YT, Chan KF, Wong SY, Fan ST. Granulin-epithelin precursor as a therapeutic target for hepatocellular carcinoma. Hepatology. 2008;47(5):1524–32. doi: 10.1002/hep.22191. [DOI] [PubMed] [Google Scholar]
- 5.Xia X, Serrero G. Identification of cell surface binding sites for PC-cell-derived growth factor, PCDGF, (epithelin/granulin precursor) on epithelial cells and fibroblasts. Biochemical and biophysical research communications. 1998;245(2):539–43. doi: 10.1006/bbrc.1998.8498. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, Lacomis L, Erdjument-Bromage H, Tempst P, Wright CD, Ding A. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111(6):867–78. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 7.Cuevas-Antonio R, Cancino C, Arechavaleta-Velasco F, Andrade A, Barron L, Estrada I, Fernandez RL, Olguin V, Ruiz S, Imani F, Zeferino-Toquero M, Ulloa-Aguirre A, Gerton GL, Diaz-Cueto L. Expression of progranulin (Acrogranin/PCDGF/Granulin- Epithelin Precursor) in benign and malignant ovarian tumors and activation of MAPK signaling in ovarian cancer cell line. Cancer investigation. 2010;28(5):452–8. doi: 10.3109/07357900903346455. [DOI] [PubMed] [Google Scholar]
- 8.Zhou J, Gao G, Crabb JW, Serrero G. Purification of an autocrine growth factor homologous with mouse epithelin precursor from a highly tumorigenic cell line. The Journal of biological chemistry. 1993;268(15):10863–9. [PubMed] [Google Scholar]
- 9.Hrabal R, Chen Z, James S, Bennett HP, Ni F. The hairpin stack fold, a novel protein architecture for a new family of protein growth factors. Nature structural biology. 1996;3(9):747–52. doi: 10.1038/nsb0996-747. [DOI] [PubMed] [Google Scholar]
- 10.Kessenbrock K, Fröhlich L, Sixt M, Lämmermann T, Pfister H, Bateman A, Belaaouaj A, Ring J, Ollert M, Fässler R, Jenne DE. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. The Journal of clinical investigation. 2008;118(7):2438–47. doi: 10.1172/JCI34694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo F, Lai Y, Tian Q, Lin EA, Kong L, Liu C. Granulin-epithelin precursor binds directly to ADAMTS-7 and ADAMTS-12 and inhibits their degradation of cartilage oligomeric matrix protein. Arthritis and rheumatism. 2010;62(7):2023–36. doi: 10.1002/art.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez EM, Mongiat M, Slater SJ, Baffa R, Iozzo RV. A novel interaction between perlecan protein core and progranulin: potential effects on tumor growth. The Journal of biological chemistry. 2003;278(40):38113–6. doi: 10.1074/jbc.C300310200. [DOI] [PubMed] [Google Scholar]
- 13.Okura H, Yamashita S, Ohama T, Saga A, Yamamoto-Kakuta A, Hamada Y, Sougawa N, Ohyama R, Sawa Y, Matsuyama A. HDL/apolipoprotein A-I binds to macrophage-derived progranulin and suppresses its conversion into proinflammatory granulins. Journal of atherosclerosis and thrombosis. 2010;17(6):568–77. doi: 10.5551/jat.3921. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka A, Tsukamoto H, Mitoma H, Kiyohara C, Ueda N, Ayano M, Ohta SI, Inoue Y, Arinobu Y, Niiro H, Horiuchi T, Akashi K. Serum progranulin levels are elevated in patients with systemic lupus erythematosus, reflecting disease activity. Arthritis research & therapy. 2012;14(6):R244. doi: 10.1186/ar4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y, Brady OA, Meng PS, Mao Y, Hu F. C-terminus of progranulin interacts with the beta-propeller region of sortilin to regulate progranulin trafficking. PloS one. 2011;6(6):e21023. doi: 10.1371/journal.pone.0021023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sfikakis PP, Tsokos GC. Towards the next generation of anti-TNF drugs. Clinical immunology. 2011;141(3):231–5. doi: 10.1016/j.clim.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Jian J, Konopka J, Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. Journal of leukocyte biology. 2013;93(2):199–208. doi: 10.1189/jlb.0812429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donald CD, Laddu A, Chandham P, Lim SD, Cohen C, Amin M, Gerton GL, Marshall FF, Petros JA. Expression of progranulin and the epithelin/granulin precursor acrogranin correlates with neoplastic state in renal epithelium. Anticancer research. 2001;21(6A):3739–42. [PubMed] [Google Scholar]
- 19.Cenik B, Sephton CF, Kutluk Cenik B, Herz J, Yu G. Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration. The Journal of biological chemistry. 2012;287(39):32298–306. doi: 10.1074/jbc.R112.399170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawase R, Ohama T, Matsuyama A, Matsuwaki T, Okada T, Yamashita T, Yuasa-Kawase M, Nakaoka H, Nakatani K, Inagaki M, Tsubakio-Yamamoto K, Masuda D, Nakagawa-Toyama Y, Nishida M, Ohmoto Y, Nishihara M, Komuro I, Yamashita S. Deletion of progranulin exacerbates atherosclerosis in ApoE knockout mice. Cardiovascular research. 2013 doi: 10.1093/cvr/cvt178. [DOI] [PubMed] [Google Scholar]
- 21.Suh HS, Choi N, Tarassishin L, Lee SC. Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12) PloS one. 2012;7(4):e35115. doi: 10.1371/journal.pone.0035115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsubara T, Mita A, Minami K, Hosooka T, Kitazawa S, Takahashi K, Tamori Y, Yokoi N, Watanabe M, Matsuo E, Nishimura O, Seino S. PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue. Cell metabolism. 2012;15(1):38–50. doi: 10.1016/j.cmet.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Lü L, Luo L, Lu Y, Chen L, Xu J, Guo K. Progranulin expression in neural stem cells and their differentiated cell lineages: An immunocytochemical study. Molecular medicine reports. 2013 doi: 10.3892/mmr.2013.1664. [DOI] [PubMed] [Google Scholar]
- 24.Daniel R, He Z, Carmichael KP, Halper J, Bateman A. Cellular localization of gene expression for progranulin. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2000;48(7):999–1009. doi: 10.1177/002215540004800713. [DOI] [PubMed] [Google Scholar]
- 25.Toh H, Cao M, Daniels E, Bateman A. Expression of the growth factor progranulin in endothelial cells influences growth and development of blood vessels: a novel mouse model. PLoS One. 2013;8(5):e64989. doi: 10.1371/journal.pone.0064989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z, Li Q, Han Y, Liang Y, Xu Z, Ren T. Prevention of LPS-induced acute lung injury in mice by progranulin. Mediators of inflammation. 2012;2012:540794. doi: 10.1155/2012/540794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao YP, Tian QY, Liu CJ. Progranulin deficiency exaggerates, whereas progranulin-derived Atsttrin attenuates, severity of dermatitis in mice. FEBS letters. 2013;587(12):1805–10. doi: 10.1016/j.febslet.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stubert J, Schattenberg F, Richter DU, Dieterich M, Briese V. Trophoblastic progranulin expression is upregulated in cases of fetal growth restriction and preeclampsia. Journal of perinatal medicine. 2012;40(5):475–81. doi: 10.1515/jpm-2011-0277. [DOI] [PubMed] [Google Scholar]
- 29.Li YH, Chen MH, Gong HY, Hu SY, Li YW, Lin GH, Lin CC, Liu W, Wu JL. Progranulin A-mediated MET signaling is essential for liver morphogenesis in zebrafish. The Journal of biological chemistry. 2010;285(52):41001–9. doi: 10.1074/jbc.M110.138743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson AM, Maurer MJ, Goergen KM, Kalli KR, Erskine CL, Behrens MD, Knutson KL, Block MS. Utility of progranulin and serum leukocyte protease inhibitor as diagnostic and prognostic biomarkers in ovarian cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013 doi: 10.1158/1055-9965.EPI-12-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvaro D. Progranulin and cholangiocarcinoma: another bad boy on the block! Gut. 2012;61(2):170–1. doi: 10.1136/gutjnl-2011-301518. [DOI] [PubMed] [Google Scholar]
- 32.Matsumura N, Mandai M, Miyanishi M, Fukuhara K, Baba T, Higuchi T, Kariya M, Takakura K, Fujii S. Oncogenic property of acrogranin in human uterine leiomyosarcoma: direct evidence of genetic contribution in in vivo tumorigenesis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12(5):1402–11. doi: 10.1158/1078-0432.CCR-05-2003. [DOI] [PubMed] [Google Scholar]
- 33.Wang M, Li G, Yin J, Lin T, Zhang J. Progranulin overexpression predicts overall survival in patients with glioblastoma. Medical oncology. 2012;29(4):2423–31. doi: 10.1007/s12032-011-0131-6. [DOI] [PubMed] [Google Scholar]
- 34.Filiano AJ, Martens LH, Young AH, Warmus BA, Zhou P, Diaz-Ramirez G, Jiao J, Zhang Z, Huang EJ, Gao FB, Farese RV, Jr, Roberson ED. Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(12):5352–61. doi: 10.1523/JNEUROSCI.6103-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Siegel RM. Medicine. Progranulin resolves inflammation. Science. 2011;332(6028):427–8. doi: 10.1126/science.1205992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koo DH, Park CY, Lee ES, Ro J, Oh SW. Progranulin as a prognostic biomarker for breast cancer recurrence in patients who had hormone receptor-positive tumors: a cohort study. PloS one. 2012;7(6):e39880. doi: 10.1371/journal.pone.0039880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu SY, Tai CC, Li YH, Wu JL. Progranulin compensates for blocked IGF-1 signaling to promote myotube hypertrophy in C2C12 myoblasts via the PI3K/Akt/mTOR pathway. FEBS letters. 2012;586(19):3485–92. doi: 10.1016/j.febslet.2012.07.077. [DOI] [PubMed] [Google Scholar]
- 39.Miller ZA, Rankin KP, Graff-Radford NR, Takada LT, Sturm VE, Cleveland CM, Criswell LA, Jaeger PA, Stan T, Heggeli KA, Hsu SC, Karydas A, Khan BK, Grinberg LT, Gorno-Tempini ML, Boxer AL, Rosen HJ, Kramer JH, Coppola G, Geschwind DH, Rademakers R, Seeley WW, Wyss-Coray T, Miller BL. TDP-43 frontotemporal lobar degeneration and autoimmune disease. Journal of neurology, neurosurgery, and psychiatry. 2013;84(9):956–62. doi: 10.1136/jnnp-2012-304644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu CJ. Progranulin: a promising therapeutic target for rheumatoid arthritis. FEBS letters. 2011;585(23):3675–80. doi: 10.1016/j.febslet.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu CJ, Bosch X. Progranulin: a growth factor, a novel TNFR ligand and a drug target. Pharmacology & therapeutics. 2012;133(1):124–32. doi: 10.1016/j.pharmthera.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu K, Zhang Y, Ilalov K, Carlson CS, Feng JQ, Di Cesare PE, Liu CJ. Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. The Journal of biological chemistry. 2007;282(15):11347–55. doi: 10.1074/jbc.M608744200. [DOI] [PubMed] [Google Scholar]
- 43.Feng JQ, Guo FJ, Jiang BC, Zhang Y, Frenkel S, Wang DW, Tang W, Xie Y, Liu CJ. Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24(6):1879–92. doi: 10.1096/fj.09-144659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu CJ. The role of ADAMTS-7 and ADAMTS-12 in the pathogenesis of arthritis. Nature clinical practice. Rheumatology. 2009;5(1):38–45. doi: 10.1038/ncprheum0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis research & therapy. 2009;11(3):224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, Mortier GR, Rimoin DL, Lachman RS, Gaines ES. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nature genetics. 1995;10(3):330–6. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- 47.Liu CJ, Kong W, Ilalov K, Yu S, Xu K, Prazak L, Fajardo M, Sehgal B, Di Cesare PE. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20(7):988–90. doi: 10.1096/fj.05-3877fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai Y, Bai X, Zhao Y, Tian Q, Liu B, Lin EA, Chen Y, Lee B, Appleton GCT, Beier F, Yu XP, Liu CJ. ADAMTS-7 forms a positive feedback loop with TNF-alpha in the pathogenesis of osteoarthritis. Annals of the rheumatic diseases. 2013 doi: 10.1136/annrheumdis-2013-203561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckland J. Osteoarthritis: Positive feedback between ADAMTS-7 and TNF in OA. Nature reviews. Rheumatology. 2013 doi: 10.1038/nrrheum.2013.135. [DOI] [PubMed] [Google Scholar]
- 50.Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, Syed NM, Lai Y, Lin EA, Kong L, Su J, Yin F, Ding AH, Zanin-Zhorov A, Dustin ML, Tao J, Craft J, Yin Z, Feng JQ, Abramson SB, Yu XP, Liu CJ. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332(6028):478–84. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao YP, et al. The promotion of bone healing by progranulin, a downstream molecule of BMP-2, through interacting with TNF/TNFR signaling. Biomaterials. 2013;34(27):6412–21. doi: 10.1016/j.biomaterials.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eguchi Y, Wakitani S, Imai Y, Naka Y, Hashimoto Y, Nakamura H, Takaoka K. Antitumor necrotic factor agent promotes BMP-2-induced ectopic bone formation. Journal of bone and mineral metabolism. 2010;28(2):157–64. doi: 10.1007/s00774-009-0127-x. [DOI] [PubMed] [Google Scholar]
- 53.Wahl EC, Aronson J, Liu L, Fowlkes JL, Thrailkill KM, Bunn RC, Skinner RA, Miller MJ, Cockrell GE, Clark LM, Ou Y, Isales CM, Badger TM, Ronis MJ, Sims J, Lumpkin CK., Jr Restoration of regenerative osteoblastogenesis in aged mice: modulation of TNF. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(1):114–23. doi: 10.1359/jbmr.090708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wahl EC, Aronson J, Liu L, Skinner RA, Miller MJ, Cockrell GE, Fowlkes JL, Thrailkill KM, Bunn RC, Ronis MJ, Lumpkin CK., Jr Direct bone formation during distraction osteogenesis does not require TNFalpha receptors and elevated serum TNFalpha fails to inhibit bone formation in TNFR1 deficient mice. Bone. 2010;46(2):410–7. doi: 10.1016/j.bone.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Todoric J, Handisurya A, Perkmann T, Knapp B, Wagner O, Tura A, Pacini G, Esterbauer H, Kautzky-Willer A. Circulating progranulin levels in women with gestational diabetes mellitus and healthy controls during and after pregnancy. European journal of endocrinology/European Federation of Endocrine Societies. 2012;167(4):561–7. doi: 10.1530/EJE-12-0060. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki M, Yonezawa T, Fujioka H, Matuamuro M, Nishihara M. Induction of granulin precursor gene expression by estrogen treatment in neonatal rat hypothalamus. Neuroscience letters. 2001;297(3):199–202. doi: 10.1016/s0304-3940(00)01699-2. [DOI] [PubMed] [Google Scholar]
- 57.Hossein-Nezhad A, Mirzaei K, Ansar H, Khooshechin G, Ahmadivand Z, Keshavarz SA. Mutual role of PGRN/TNF-alpha on osteopenia developing in obesity’s inflammation state. Minerva medica. 2012;103(3):165–75. [PubMed] [Google Scholar]
- 58.Wang D, Bai X, Tian Q, Lai Y, Lin EA, Shi Y, Mu X, Feng JQ, Carlson CS, Liu CJ. GEP constitutes a negative feedback loop with MyoD and acts as a novel mediator in controlling skeletal muscle differentiation. Cellular and molecular life sciences. CMLS. 2012;69(11):1855–73. doi: 10.1007/s00018-011-0901-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li YH, Chen HY, Li YW, Wu SY, Wangta-Liu, Lin GH, Hu SY, Chang ZK, Gong HY, Liao CH, Chiang KY, Huang CW, Wu JL. Progranulin regulates zebrafish muscle growth and regeneration through maintaining the pool of myogenic progenitor cells. Scientific reports. 2013;3:1176. doi: 10.1038/srep01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klover P, Hennighausen L. Postnatal body growth is dependent on the transcription factors signal transducers and activators of transcription 5a/b in muscle: a role for autocrine/paracrine insulin-like growth factor I. Endocrinology. 2007;148(4):1489–97. doi: 10.1210/en.2006-1431. [DOI] [PubMed] [Google Scholar]
- 61.Oksbjerg N, Gondret F, Vestergaard M. Basic principles of muscle development and growth in meat-producing mammals as affected by the insulin-like growth factor (IGF) system. Domestic animal endocrinology. 2004;27(3):219–40. doi: 10.1016/j.domaniend.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Egashira Y, Suzuki Y, Azuma Y, Takagi T, Mishiro K, Sugitani S, Tsuruma K, Shimazawa M, Yoshimura S, Kashimata M, Iwama T, Hara H. The growth factor progranulin attenuates neuronal injury induced by cerebral ischemia-reperfusion through the suppression of neutrophil recruitment. Journal of neuroinflammation. 2013;10:105. doi: 10.1186/1742-2094-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrari R. The role of TNF in cardiovascular disease. Pharmacological research: the official journal of the Italian Pharmacological Society. 1999;40(2):97–105. doi: 10.1006/phrs.1998.0463. [DOI] [PubMed] [Google Scholar]
- 64.Levine JE. Implications of TNF-alpha in the pathogenesis and management of GVHD. International journal of hematology. 2011;93(5):571–7. doi: 10.1007/s12185-011-0803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.González Álvarez A, Gómez Barrera M, Borrás Blasco J, Giner Serrer EJ. Adalimumab versus etanercept in the treatment of active rheumatoid arthritis: cost-effectiveness analysis. Farmacia hospitalaria: organo oficial de expresion cientifica de la Sociedad Espanola de Farmacia Hospitalaria. 2013;37(4):286–294. doi: 10.7399/FH.2013.37.4.593. [DOI] [PubMed] [Google Scholar]
- 66.West C, Narahari S, O’Neill J, Davis S, Huynh M, Clark A, Boles A, Feldman SR. Adherence to adalimumab in patients with moderate to severe psoriasis. Dermatology online journal. 2013;19(5):18182. [PubMed] [Google Scholar]
- 67.Chen JS, Makovey J, Lassere M, Buchbinder R, March LM. Comparative effectiveness of anti-tumour necrosis factor (TNF) drugs on health-related quality of life among patients with inflammatory arthritis. Arthritis care & research. 2013 doi: 10.1002/acr.22151. [DOI] [PubMed] [Google Scholar]
- 68.Martínez-Santana V, González-Sarmiento E, Calleja-Hernández M, Sánchez-Sánchez T. Comparison of drug survival rates for tumor necrosis factor antagonists in rheumatoid arthritis. Patient preference and adherence. 2013;7:719–27. doi: 10.2147/PPA.S47453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, Shoda T, Takai S, Makino S, Hanafusa T. Infliximab, a TNF-alpha inhibitor, reduces 24-h ambulatory blood pressure in rheumatoid arthritis patients. Journal of human hypertension. 2013 doi: 10.1038/jhh.2013.80. [DOI] [PubMed] [Google Scholar]
- 70.Papoutsaki M, Osório F, Morais P, Torres T, Magina S, Chimenti S, Costanzo A. Infliximab in psoriasis and psoriatic arthritis. BioDrugs: clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2013;27(Suppl 1):13–23. doi: 10.1007/BF03325638. [DOI] [PubMed] [Google Scholar]
- 71.Tian QY, Zhao YP, Liu CJ. Modified yeast-two-hybrid system to identify proteins interacting with the growth factor progranulin. Journal of visualized experiments: JoVE. 2012;59 doi: 10.3791/3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. Journal of molecular medicine. 2003;81(10):600–12. doi: 10.1007/s00109-003-0474-3. [DOI] [PubMed] [Google Scholar]
- 73.Naphade SB, Kigerl KA, Jakeman LB, Kostyk SK, Popovich PG, Kuret J. Progranulin expression is upregulated after spinal contusion in mice. Acta neuropathologica. 2010;119(1):123–33. doi: 10.1007/s00401-009-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allen PB, Peyrin-Biroulet L. Moving towards disease modification in inflammatory bowel disease therapy. Current opinion in gastroenterology. 2013;29(4):397–404. doi: 10.1097/MOG.0b013e3283622914. [DOI] [PubMed] [Google Scholar]
- 75.Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. The New England journal of medicine. 2013;369(8):754–62. doi: 10.1056/NEJMct1209614. [DOI] [PubMed] [Google Scholar]
- 76.Andrisani G, Guidi L, Papa A, Armuzzi A. Anti-TNF alpha therapy in the management of extraintestinal manifestation of inflammatory bowel disease. European review for medical and pharmacological sciences. 2012;16(7):890–901. [PubMed] [Google Scholar]
- 77.Mathew J, Aronow WS, Chandy D. Therapeutic options for severe asthma. Archives of medical science: AMS. 2012;8(4):589–97. doi: 10.5114/aoms.2012.30280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaur S, Zilmer K, Leping V, Zilmer M. Comparative study of systemic inflammatory responses in psoriasis vulgaris and mild to moderate allergic contact dermatitis. Dermatology. 2012;225(1):54–61. doi: 10.1159/000339866. [DOI] [PubMed] [Google Scholar]
- 79.Yoshida K, Hashiramoto A, Okano T, Yamane T, Shibanuma N, Shiozawa S. TNF-alpha modulates expression of the circadian clock gene Per2 in rheumatoid synovial cells. Scandinavian journal of rheumatology. 2013;42(4):276–80. doi: 10.3109/03009742.2013.765031. [DOI] [PubMed] [Google Scholar]
- 80.Puzas JE, Houck J, Bukata SV. Accelerated fracture healing. The Journal of the American Academy of Orthopaedic Surgeons. 2006;14 (10 Spec No):S145–51. doi: 10.5435/00124635-200600001-00033. [DOI] [PubMed] [Google Scholar]
- 81.Donneys A, Ahsan S, Perosky JE, Deshpande SS, Tchanque-Fossuo CN, Levi B, Kozloff KM, Buchman SR. Deferoxamine restores callus size, mineralization, and mechanical strength in fracture healing after radiotherapy. Plastic and reconstructive surgery. 2013;131(5):711e–9e. doi: 10.1097/PRS.0b013e3182865c57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH, Koncz T, Soma K, Bradley J, Mebus C. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381(9865):451–60. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]