Abstract
Ulceration is a common negative prognostic marker of solid tumors including melanoma. The signaling basis of ulceration is being elucidated. PHIP has been found to be amplified in wild-type melanomas, resulting in Akt activation and aerobic glycolysis (Warburg effect), associated with ulceration. The ulceration phenotype likely represents the genotype of the reactive oxygen driven tumor, in which reactive oxygen drives angiopoietin-2 production, tumor growth, and invasion. This phenotype is amenable to pharmacologic intervention.
Ulceration is a common phenomenon in dermatology and an adverse prognostic factor in melanoma. For many years, ulceration has been described as “a tumor outgrowing its blood supply”. However, if blood supply is insufficient, why is ulceration an adverse prognostic factor? We have shown that the majority of cutaneous ulcers are not due to insufficient vascularity, but due to excess vascularity (Arbiser et al., 2003). High levels of matrix metalloproteinases and unstable vasculature result in a “hostile” environment for epithelialization, leading to ulceration (Munavalli et al., 2003). Ultimately, the factors that promote ulceration are not well understood.
Kashani-Sabet et al. have now identified pleckstrin homology domain–interacting protein (PHIP) copy number, initially as the top-rated gene differentially expressed between metastatic melanoma and primary melanoma (Bezrookove et al. 2014). In this study, they characterized PHIP as being expressed at high levels in ulcerated melanomas. Not only was it present, the investigators also demonstrated functional consequences of PHIP overexpression. Inhibition of PHIP led to a decrease in aerobic glycolysis (Warburg effect). The Warburg effect is commonly associated with defective glucose metabolism, with glucose being metabolized to lactate instead of full metabolism through to respiration. Although the ineffective metabolism of glucose is initially counter-intuitive in terms of tumor growth, more recent studies have established that tumor cells gain certain survival advantages in exchange for switching to aerobic glycolysis, namely NF-κB activation and the ability to survive under extreme hypoxia (Govindarajan et al., 2007). The authors demonstrated the downregulation of biomarkers of the Warburg phenomenon, including LDH5, HIF1a, and VEGF.
Although this study demonstrates PHIP elegantly as a potential therapeutic target, several questions can be asked that might provide elaboration and answers about the role of PHIP in melanoma. First, is amplification the sole mechanism of increased PHIP expression? If this is the case, adjacent genes might provide advantages to melanoma, because coamplification might increase the expression of PHIP and an additional gene in a single step. Second, what are the signaling pathways downstream of PHIP amplification? Glycolysis is associated in melanoma with the reactive oxygen–driven phenotype, in which reactive oxygen drives NF-κB and angiopoietin-2 (Fried and Arbiser, 2008). Angiopoietin-2 is highly associated with ulceration, and it would be of interest to determine whether PHIP overexpression causes increased reactive oxygen generation and angiopoietin-2 expression (Lapidoth et al., 2009). Notably, as with ulceration, angiopoietin-2 is associated with poor prognosis in solid tumors, including melanoma, lymphoid malignancies, and in sepsis (Tanaka et al., 1999; Helfrich et al., 2009; Kranidioti et al., 2009). If this is the case, PHIP-overexpressing melanomas might be susceptible to reactive oxygen inhibition. Finally, could PHIP overexpression be a mechanism of resistance to Braf inhibition, especially as PHIP has been associated with insulin growth factor receptor 1 (IGFR1) signaling (Reuveni et al., 2013)?
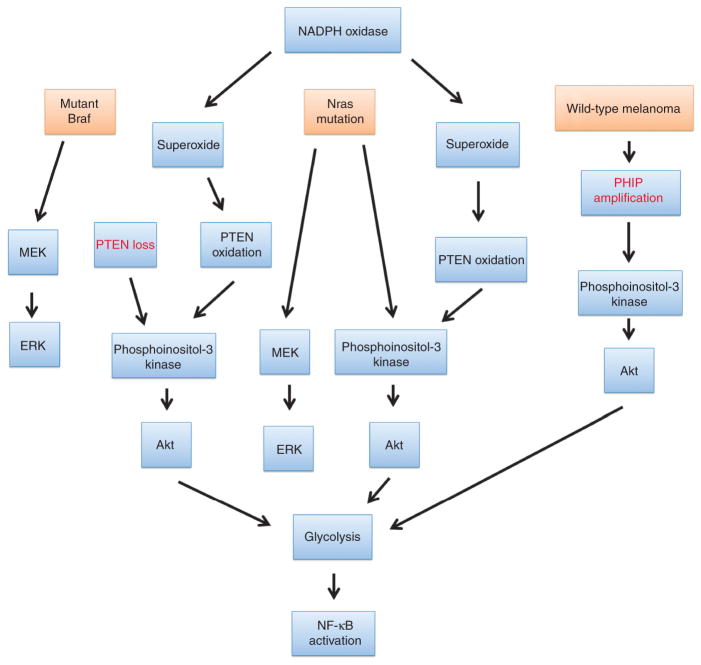
So-called targeted therapies have focused on either Braf inhibition alone or combination treatment with MEK inhibitors. Predictably, resistance has arisen owing to failure to affect the phosphoinositol-3 kinase/Akt/reactive oxygen pathway. The findings of PHIP amplification in ulcerative primary melanoma should provide an impetus for targeting non-MEK/Braf pathways in melanoma, as this has been a relatively overlooked signaling pathway and one that is readily targeted (Figure 1). Combination or sequential targeting of MEK/Braf inhibitors plus phosphoinositol-3 kinase/Akt/reactive oxygen pathway inhibitors could well result in improved long-term survival and even the possibility of cure.
Figure 1. Convergence of pathways in melanoma leads to NF-κB activation and glycolysis.
The major pathways in melanoma, Braf mutation, Nras mutation, and PHIP mutation all potentially involve a superoxide/Akt/NF-κB pathway (reactive oxygen–driven pathway) to result in glycolysis, NF-κB activation, and ulceration through angiopoietin-2 expression.
Clinical Implications.
Ulceration is a known poor prognostic factor for melanoma.
We now are beginning to understand that ulceration is a phenotype of a specific type of signal transduction abnormality, likely that of the reactive oxygen–induced phenotype.
This phenotype can be targeted with inhibitors of superoxide production (Munson et al., 2012), and in fact has already been used in patients with melanoma (Arbiser et al., 2012).
Acknowledgments
JLA was supported by the grant RO1 AR47901and P30 AR42687 Emory Skin Disease Research Core Center Grant from the National Institutes of Health, a Veterans Administration Hospital Merit Award, as well as funds from the Margolis Foundation, Rabinowitch-Davis Foundation for Melanoma Research, and the Betty Minsk Foundation for Melanoma Research.
Footnotes
CONFLICT OF INTEREST
The author states no conflict of interest.
References
- Arbiser JL, Bips M, Seidler A, et al. Combination therapy of imiquimod and gentian violet for cutaneous melanoma metastases. J Am Acad Dermatol. 2012;67:e81–3. doi: 10.1016/j.jaad.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbiser JL, Johnson D, Cohen C, et al. High-level expression of vascular endothelial growth factor and its receptors in an aphthous ulcer. J Cutan Med Surg. 2003;7:225–8. doi: 10.1007/s10227-002-0119-0. [DOI] [PubMed] [Google Scholar]
- Bezrookove V, De Semir D, Nosrati M, et al. Prognostic Impact of PHIP copy number in melanoma: linkage to ulceration. J Invest Dermatol. 2014;134:783–90. doi: 10.1038/jid.2013.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried L, Arbiser JL. The reactive oxygen-driven tumor: relevance to melanoma. Pigment Cell Melanoma Res. 2008;21:117–22. doi: 10.1111/j.1755-148X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Sligh JE, Vincent BJ, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–29. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich I, Edler L, Sucker A, et al. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin Cancer Res. 2009;15:1384–92. doi: 10.1158/1078-0432.CCR-08-1615. [DOI] [PubMed] [Google Scholar]
- Kranidioti H, Orfanos SE, Vaki I, et al. Angiopoietin-2 is increased in septic shock: evidence for the existence of a circulating factor stimulating its release from human monocytes. Immunol Lett. 2009;125:65–71. doi: 10.1016/j.imlet.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lapidoth M, Ben-Amitai D, Bhandarkar S, et al. Efficacy of topical application of eosin for ulcerated hemangiomas. J Am Acad Dermatol. 2009;60:350–1. doi: 10.1016/j.jaad.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Munavalli G, Reisenauer A, Moses M, et al. Weight loss-induced calciphylaxis: potential role of matrix metalloproteinases. J Dermatol. 2003;30:915–9. doi: 10.1111/j.1346-8138.2003.tb00348.x. [DOI] [PubMed] [Google Scholar]
- Munson JM, Fried L, Rowson SA, et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci Transl Med. 2012;4:127ra36. doi: 10.1126/scitranslmed.3003016. [DOI] [PubMed] [Google Scholar]
- Reuveni H, Flashner-Abramson E, Steiner L, et al. Therapeutic destruction of insulin receptor substrates for cancer treatment. Cancer Res. 2013;73:4383–94. doi: 10.1158/0008-5472.CAN-12-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Mori M, Sakamoto Y, et al. Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J Clin Invest. 1999;103:341–5. doi: 10.1172/JCI4891. [DOI] [PMC free article] [PubMed] [Google Scholar]