Abstract
The female reproductive tract undergoes remarkable functional and structural changes associated with cycling, conception and pregnancy, and it is likely advantageous to both individual and species to alter relationships between reproductive tissues and innervation. For several decades, it has been appreciated that the mammalian uterus undergoes massive sympathetic axon depletion in late pregnancy, possibly representing an adaptation to promote smooth muscle quiescence and sustained blood flow. Innervation to other structures such as cervix and vagina also undergo pregnancy-related changes in innervation that may facilitate parturition. These tissues provide highly tractable models for examining cellular and molecular mechanisms underlying peripheral nervous system plasticity. Studies show that estrogen elicits rapid degeneration of sympathetic terminal axons in myometrium, which regenerate under low-estrogen conditions. Degeneration is mediated by the target tissue: under estrogen's influence, the myometrium produces proteins repulsive to sympathetic axons including BDNF, neurotrimin, semaphorins, and pro-NGF, and extracellular matrix components are remodeled. Interestingly, nerve depletion does not involve diminished levels of classical sympathetic neurotrophins that promote axon growth. Estrogen also affects sympathetic neuron neurotrophin receptor expression in ways that appear to favor pro-degenerative effects of the target tissue. In contrast to the uterus, estrogen depletes vaginal autonomic and nociceptive axons, with the latter driven in part by estrogen-induced suppression BMP4 synthesis. These findings illustrate that hormonally mediated physiological plasticity is a highly complex phenomenon involving multiple, predominantly repulsive target-derived factors acting in concert to achieve rapid and selective reductions in innervation.
1. Introduction
The ability to reproduce is essential to the survival of all species. Even in the simplest of organisms, reproduction is a highly complex phenomenon. As we advance along the evolutionary spectrum, the challenges and complexity become greater. As organismal development in utero becomes more advanced, increasingly sophisticated homeostatic mechanisms presumably are required. In the case of humans, the highly developed nervous system creates special challenges in terms of how precisely the uterine environment must be maintained and the degree to which the system must rapidly adapt in order to shift from maintaining pregnancy to completing parturition. In the case of the female reproductive tract, maintaining the environment requires the dynamic interplay of nerves and hormones.
The female reproductive tract is imbued with a rich ground plexus of autonomic nerves that regulate vascular and nonvascular smooth muscle contractile activity, glandular secretions, immune cell interactions, and convey information to the central nervous system (CNS) regarding the internal environment and potential noxious stimuli. In transitioning from nonpregnant to gravid states, it is advantageous for reproductive tract innervation to alter its properties in ways that accommodate reproductive needs. For example, while activation of excitatory uterine sympathetic pathways during a fight or flight response may be beneficial in the nonpregnant female, doing so in an advanced state of pregnancy could result in deprivation of blood flow to the placenta or premature delivery. How does innervation of the female reproductive tract adapt to meet the needs of dramatically changing target tissues?
This review covers some 40 years of research beginning with reports by Owman and colleagues of changes in uterine sympathetic innervation during pregnancy (Owman et al., 1976). In this period, our knowledge of the extent to which autonomic innervation changes as a function of reproductive status has grown significantly. Moreover, we now have a richer understanding of the hormonal, cellular and molecular mechanisms through which reproductive tract neural plasticity takes place. In addition to the importance of this process to the survival of the species, the female reproductive tract provides a unique model for understanding processes underlying physiological neural plasticity. While it is now generally well accepted that remodeling is ongoing within both the central and peripheral nervous systems of adult mammals, these processes typically occur asynchronously and randomly over long periods. The ability to trigger synchronous nerve degeneration or regeneration simply by altering the hormonal milieu provides a highly tractable model system in which to study how target innervation is regulated.
In this review, we first provide an overview of the innervation of the female reproductive tract. We then examine how this innervation is modulated under normal physiological conditions such as puberty, the menstrual or estrous cycle, and pregnancy, as well as following hormonal manipulations. We conclude by summarizing what is known regarding molecular mechanisms mediating changes in target innervation. While the picture remains incomplete, results point to surprisingly complex multifactorial processes by which reproductive tract innervation is regulated.
2. Innervation of the female reproductive tract: an overview (Fig. 1)
Fig. 1.
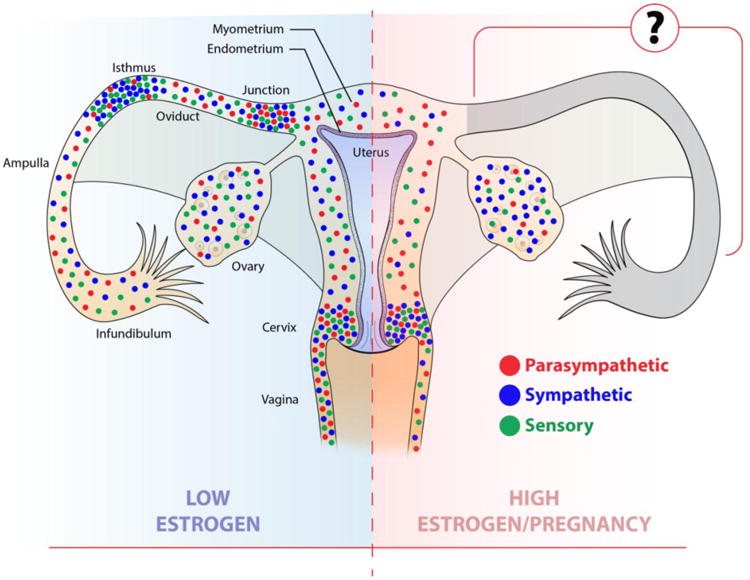
Schematic diagram major reproductive tract structures and showing a ‘consensus’ depiction of associated innervation. For the sake of simplicity, the simplex uterus (e.g., human) is used for illustrative purposes. Distributions of nerves are based on observations from various mammals including pig, guinea pig, rat and human. The low estrogen state is depicted on the left, and shows the distributions and relative abundance of sympathetic axons (blue), parasympathetic axons (red), and sensory peptidergic nociceptor fibers (purple). Axon distributions under high-estrogen conditions or at term pregnancy are shown on the right. See text for citations.
2.1 Ovary
The ovary receives sympathetic innervation from the upper lumbar spinal segments via splanchnic nerve fibers and parasympathetic innervation via the vagus nerves. Autonomic axons travel to the ovary via the ovarian nerve plexus and the superior ovarian nerve (Papka and Traurig, 1993; Traurig and Papka, 1993). Sympathetic innervation appears to play an important role in the prepubertal development of ovarian function (Albuquerque-Araujo et al., 1995; Lara et al., 1990). The ovaries are also well imbued with sensory axons containing calcitonin gene-related peptide (CGRP) as well as other neuropeptides (Ghatei et al., 1985) and may be involved in vasomotor regulation. The organization and functions of ovarian innervation is addressed in more detail by Uchida (this issue).
2.2 Oviduct
Many oviductal functions are under the control of autonomic and sensory nerves, which innervate the muscularis, vasculature and to a lesser extent the oviductal mucosa (Owman et al., 1986a; Owman and Stjernquist, 1988; Papka and Traurig, 1993; Traurig and Papka, 1993). Sympathetic nerves arising from the thoraco-lumbar sympathetic chain and prevertebral ganglia densely innervate the oviduct. In the pig, the paracervical ganglia also supply sympathetic innervation to the oviducts (Czaja et al., 2001a, b), while in the rat, this plexus does not innervate the upper genital tract (Hondeau et. al., 1998a). Oviductal sympathetic innervation shows regional variations; most nerves associate with blood vessels in the ampulla, whereas in the isthmus fibers mainly innervate the prominent circular muscle layer and are involved in neural control of the sphincter. Interestingly, in women with hydrosalpinx a loss of adrenergic nerves occurs at the isthmus and leads to a distal fluid-filled dilation of the Fallopian tube causing infertility (Zhu et al., 2013).
Parasympathetic nerves are relatively scarce in the oviduct and mainly confined to the vasculature and muscularis (Wrobel and Kujat, 1993; Jankovic et al., 2004). These nerves are particularly well developed in the tubo-uterine junction, suggesting a role in sphincter control (Moustafa et al., 1987). Oviductal efferent nerves express a range of neuropeptides including neuropeptide Y (NPY) and vasoactive intestinal polypeptide (VIP) and peptide histidine isoleucine (PHI), (Helm et al., 1982; Papka et al., 1985; Kannisto et al., 1986; Owman et al., 1986b; Czaja et al., 1996).
Afferent innervation to the oviduct contributes to the regulation of oviductal blood flow, non-vascular muscle tone and epithelial secretion. In the rat, afferent sensory nerves arise from T13 to L2 dorsal root ganglia (DRG) and follow the superior ovarian and ovarian plexus nerves (Nance et al., 1988). In the pig, sensory nerves arise from T10 to L3 (Czaja et al., 2001b). Afferent neurons to the oviduct are immunoreactive for substance P (SP) and CGRP, while a small population displays a combination of VIP and nitric oxide synthase (NOS, Czaja, 2000). In addition, some neurons show immunoreactivity (ir) for both SP and NOS, solely SP or solely NOS. Co-labeling for NOS/VIP and NOS/SP is also seen in the swine oviduct (Majewski et al., 1995).
2.3 Uterus: myometrium
The uterus is supplied by sympathetic, parasympathetic and sensory nerves which mainly innervate blood vessels and myometrial smooth muscle (Owman et al., 1967; Marshall, 1970; Haase et al., 1997; Zoubina et al., 1998; Bae et al., 2001; Gnanamanickam and Llewellyn-Smith, 2011). In the rat, 90% of sympathetic nerve fibers innervating the upper part of the uterine horn originate from neurons in the suprarenal and T10-L3 sympathetic chain ganglia while innervation of the lower uterus and the cervix originates in paravertebral ganglia, principally at the L2-L4 level. The paracervical ganglia have a very limited contribution to the innervation of the lower part of rat the uterus (Houdeau et al., 1995; 1998a). In contrast, in guinea pigs and humans a considerable portion of the sympathetic innervation of the uterine body and cervix arises from neurons in the pelvic plexus located at the utero-vaginal junction (Owman, 1981). The overall density of innervation varies substantially among different species. In addition, innervation density is heavier in the tubal end of the uterine horn and cervix than in the main parts of the uterine horn/body (Owman et al., 1967; Adham and Schenk, 1969; Melo and Machado, 1993).
Cholinergic nerves to the uterus mainly originate from paracervical ganglia and enter the uterus via the pelvic plexus (Papka et al., 1999). There is considerable interspecies variation in the density of uterine cholinergic nerves, being well-develop in mice and humans but almost absent in the guinea pig (Moustafa et al., 1987). NPY co-localizes with acetylcholine (Ach) in neurons of the paracervical ganglia innervating the cervix (Traurig and Papka, 1993). Most parasympathetic nerves to the rat uterus and cervix are NOS-positive (Papka and McNeill, 1992; Papka et al., 1995 a, b).
The myometrium is innervated by afferent fibers immunoreactive for a range of co-transmitters including SP, CGRP, VIP, NOS, neurokinin A, galanin (GAL) and secretoneurin (Papka and McNeil, 1992; Majewski et al., 1995; Collins et al., 2000). In the rat, fibers innervating the cranial region of the uterine horn arise from T13-L1 DRG whereas neurons in the L6 and S1 ganglia innervate the caudal region of the uterine horn and cervix (Nance et al., 1988). The nodose ganglia via the vagus nerve contribute to the afferent innervation of the rat uterus (Ortega-Villalobos et al., 1990).
2.4 Uterus: endometrium and endometriosis
In most mammalian species, the endometrium is poorly innervated by autonomic and afferent nerve fibers, and those present are mainly associated with blood vessels. In the rat (Gnanamanickam and Llewellyn-Smith, 2011), sympathetic nerves arising from perivascular myometrial blood vessels penetrate into the endometrium but only occasionally reach the epithelium. Sympathetic fibers containing NPY approach uterine glands, suggesting a role in endometrial secretion. Parasympathetic nerves rarely penetrate deeply in the endometrium and do not associate with endometrial glands. In the guinea pig, in contrast, both the basal and functional endometrial layers contain acetylcholinesterase-positive nerves that may be involved in the control of endometrial secretion (Hammarström and Sjöstrand, 1980). Many CGRP-ir nerve fibers travel deep in the rat endometrium close to uterine glands. The density of afferent nerve fibers is apparently greater than that of sympathetic or parasympathetic innervation.
In the normal human endometrium, nerve fibers are restricted to the basal layer while the functional layer is devoid of innervation (Tokushige et al., 2006). In recent years, the presence of an aberrant innervation in the functional layer of the endometrium has been described in patients with endometriosis. This innervation is composed of sympathetic, parasympathetic and afferent nerve fibers (Tokushige et al., 2006; 2007). The abnormal presence of nerve fibers in the functional layer of the endometrium has been proposed as a semi-invasive method for diagnosis of this pathology (Al-Jefout et al., 2007, 2009; Bokor et al., 2009).
2.5 Cervix
The cervix receives prominent sympathetic innervation from the hypogastric nerve and parasympathetic projections through the pelvic and vagus nerves. Hypogastric and pelvic nerve pathways to the cervix connect with sensory and autonomic areas in the thoraco-lumbar spinal cord, which have ascending connections with integrative centers in the brainstem and hypothalamus (Kirby et al., 2005; Yellon et al., 2010). Moreover, sensory fibers containing CGRP also innervate cholinergic uterine- and cervical-projecting parasympathetic neurons in the rat paracervical ganglia, accessory ganglia and hypogastric plexus (Houdeau et al., 2002).
Cervical innervation plays a crucial role in cervical ripening. In rodents, the innervation of the cervix increases markedly the day before delivery (Kirby et al., 2005; Boyd et al., 2009), and the synthesis and release of both SP and CGRP are markedly elevated (Mowa et al., 2003a, b). Transection of the sensory branch of the rat pelvic nerve delays labor (Mackay et al., 2009), while transection of sympathetic hypogastric nerves has no effect on cervical ripening or birth (Kirby et al., 2005; Boyd et al., 2009). Transection of parasympathetic pathways (pelvic and vagus nerves) delays birth, affects local inflammatory processes including macrophage immigration, and prevents the normal withdrawal of progesterone from circulation (Clyde et al., 2011). In contrast, pelvic and vagal parasympathetic neurectomy is not involved in the final remodeling of the cervix at term.
2.6 Vagina and external genitalia
In the rat, vaginal innervation is composed of sympathetic nerves, cholinergic parasympathetic nerves immunoreactive for vesicular acetylcholine transporter (VAChT), NOS-ir axons, and NPY-, VIP- and PACAP-ir terminals (Polak et al., 1984; Huang et al., 1984; Giraldi et al., 2002; Ting et al., 2004). In pigs and cows, NOS frequently co-localizes with SP and VIP (Majewski et al., 1995). In the central region of the rat vagina, nerves immunoreactive for tyrosine hydroxylase (TH), CGRP and VAChT exist in similar proportions (Ting et al., 2004). These fibers associate intimately with the prominent vaginal smooth muscle layer and with blood vessels. CGRP-ir fibers derive from pelvic and hypogastic nerves (Ghatei et al., 1985), with sympathetics traveling in the L2-L4 lumbar splanchnic nerves and the hypogastric nerve, and parasympathetics coursing within the pelvic nerve (Giuliano et al., 2002). Whereas overall vaginal innervation density in rats is reported to increase toward the distal vaginal introitus (Giraldi et al., 2002), studies in humans report consistent density throughout the vagina (Pauls et al., 2006).
Functional studies provide evidence that sympathetic nerves are excitatory to vaginal nonvascular smooth muscle (Gunn et al., 1922) while NOS-ir nerves appear to mediate muscle relaxation and vasodilation in the rat (Calka et al., 1988; Giraldi et al., 2001, 2002; Giuliano et al., 2002; Munarriz et al., 2003). CGRP-ir sensory nerves are also inhibitory to vaginal smooth muscle (Giraldi et al., 2001), as well as presumably mediating sensations of discomfort associated with vaginal irritation or distension (Berkley et al., 1995). While some intraepithelial nerve fibers are present at the vaginal orifice, the vaginal epithelium appears largely devoid of any innervation proximal to the introitus in both rats and humans (Hilliges et al., 1995; Ting et al., 2004).
The clitoris is also innervated by sensory, sympathetic and parasympathetic fibers traveling through the dorsal clitoral nerve and clitoral plexus. Sympathetic nerves are believed to diminish clitoral erectile tissue tumescence by way of vasoconstriction, while parasympathetic nitrergic innervation increases blood flow and tumescence (Giuliano et al., 2002; Munarriz et al., 2003; Vachon et al., 2000). Clitoral fibers also contain an array of neuropeptides including NPY, VIP and SP (Hauser-Kronberger et al., 1999). The clitoris exhibits a specialized arrangement of mechanoreceptors in complex with CGRP-ir presumptive nociceptor fibers (Vilimas et al., 2011). Perivaginal vulvar tissue of the vaginal vestibule in humans also contains abundant innervation including fibers immunoreactive for CGRP, SP, VIP and galanin within the epithelium, while the subepithelium additionally contains NPY-ir fibers (Bohm-Starke et al., 1999; Tympanidis et al., 2003).
3. Remodeling of reproductive tract innervation by sex hormones (Fig. 1)
3.1 Ovarian innervation
The sympathetic innervation to the ovary of the developing rat undergoes a rapid advancement, becoming functionally mature at about the time of puberty (Ricu et al., 2008). In young adult female swine, long-term 17β-estradiol administration at supra-physiological levels for 16 days reduces the number sympathetic neurons in the caudal mesenteric and paravertebral ganglia (Koszykowska et al., 2011a, b). Similarly, this regimen reduces the numbers and altered phenotypes of ovary-projecting sensory neurons in the L2 and L3 DRG (Jana et al., 2012) and it also reduces numbers of labeled paracervical ganglion neurons (Jana et al., 2013). In contrast, numbers of ovarian terminal fibers immunostained for the pan-neural marker protein gene product 9.5 (PGP9.5), dopamine-β-hydroxylase (DβH), NPY and GAL are all increased by chronic estrogen treatment (Koszykowska et al., 2013). In a similar vein, newborn rats receiving estradiol valerate show increased ovarian NA content as adults, suggesting that abnormal exposure to estrogen early in life can ‘imprint’ ovarian innervation in such a way as to permanently alter its relation to the ovarian target (Sotomayor-Zarate et al., 2008). While it is unclear whether physiological fluctuations in estrogen normally influence ovarian innervation, clearly ovary-projecting neurons are hormonally responsive under some conditions, raising the possibility that feedback mechanisms exists where autonomic nerves influence ovarian estrogen secretion (see Uchida, this issue), which in turn influences ovarian innervation.
3.2 Uterine innervation
3.2.1 Puberty
Sympathetic nerves enter the developing uterus at early stages of postnatal life and grow progressively between the infantile and prepubertal periods. Following the first estrus at puberty, there is a marked reduction in the density of myometrial sympathetic nerves, which is reflected in a decrease in the concentration of NA (Brauer et al., 1992). No change occurs in total NA content, suggesting that sympathetic nerves fail to grow in parallel with myometrial hypertrophy; however, the alternative possibility that partial axonal degeneration occurs during the pubertal transition cannot be disregarded. Puberty has no effect on the density of sympathetic nerves supplying the uterine artery and its intrauterine branches (Corbacho et al., 1997).
3.2.2 The menstrual and estrous reproductive cycles
Cyclic variations in circulating levels of ovarian sex hormones strongly influence uterine innervation, with sympathetic nerves being most susceptible (Fig. 2). Early studies showed that NA levels fluctuate along the sex cycle (e.g., Adham and Schenk, 1969; Sjöberg, 1968; Falk et al.,1975; Owman et al., 1976; Thorbert et al., 1978; Van Orden et al., 1980). One potential mechanism explaining this fluctuation is that NA content depletes and repletes in a fixed number of otherwise intact sympathetic axons. An alternative possibility is that actual reductions in numbers of nerve fibers occur, involving either the retraction or degeneration of axon terminal branches. In 1978, Sporrong et al. speculated that axon degeneration may occur in the non-gravid uterus, stating that “Scattered degenerative changes were also observed in myometrial specimens from virgin animals, but probably reflect the normal continuous turnover of axons” (Sporrong et al., 1978). However, evidence for structural remodeling of during the rat estrous cycle was not demonstrated for another 20 years (Zoubina et al., 1998; Latini et al., 2008). Using quantitative light microscopic morphometric approaches, the authors showed that density of myometrial nerves immunoreactive for both PGP9.5 and DβH decrease during proestrus and estrus, and return to a relatively high density over the next 2-3 days during metestrus and diestrus. Changes in the density of innervation persist even after correction for accompanying alterations in myometrial size, indicating that a genuine loss of sympathetic nerve fibers occurs.
Fig. 2.
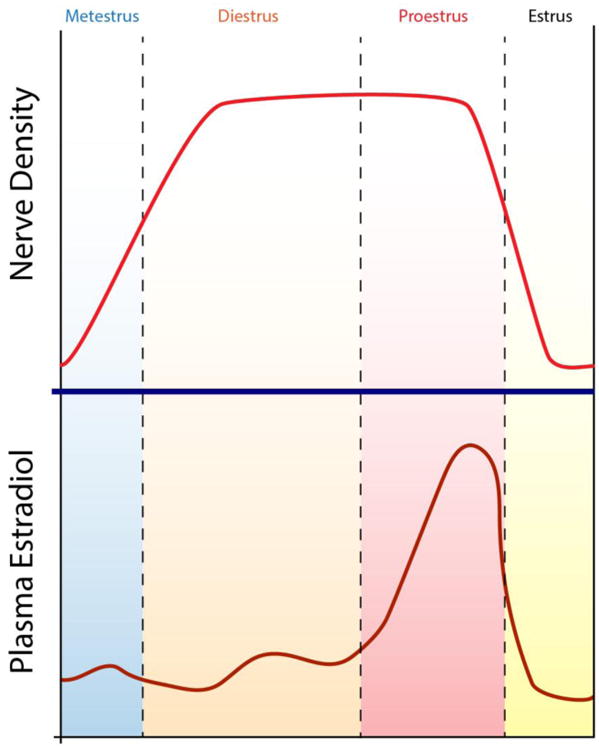
Depiction of changes in axon density in the uterus as a function of fluctuations in estrogen levels occurring during the estrous cycle in the rat.
Evidence that the reduction in DBH-ir innervation represents axonal degeneration rather than retraction was obtained at the electron microscopic level where many intact axons were observed in diestrus, while at estrus, numbers of intact axons were reduced in association with a marked increase in numbers of degenerate axon remnants (Zoubina et al., 2000). In contrast to myometrial innervation, vascular innervation remains relatively unaffected both at the light and electron microscopic levels of analysis. The appearance of axonal structures with features of growth cones in metestrus provided evidence that axonal regeneration represents the mechanism by which myometrial sympathetic innervation is restored. These results strongly suggest that changes in the density of myometrial sympathetic innervation account for the reduction in NA levels and turnover rate previously described in the estrogen-dominant phases of the cycle.
Cyclic changes in the density of myometrial sympathetic nerves also occur in humans (Barcena de Arellano et al., 2013). Interestingly, women with adenomyosis present a reduced density of myometrial sympathetic nerves and they do not show differences between the proliferative and secretory phases as observed in healthy women. In contrast to sympathetic nerves, no significant cyclic alterations in density of parasympathetic and sensory nerves occur in rats (Zoubina et al., 1998; Houdeau et al., 2003) and humans (Barcena de Arellano et al., 2013).
3.2.3 Role of estrogen
Several lines of evidence indicate that changes in circulating estrogen are the primary determinant of variations in uterine sympathetic innervation during the estrous cycle and puberty. In the estrous cycle, the increased estrogen levels that begin at late diestrus precede the disappearance of uterine sympathetic nerves at proestrus (Zoubina et al., 1998; Zoubina et al., 2000) (Fig. 2). The responses of sympathetic nerves to variations in levels of estrogen are mediated through the estrogen receptor alpha (ERα) (Zoubina et al., 2001b). Uteri of mice in which ERα function has been disrupted through homologous recombination display sympathetic hyperinnervation with respect to the wild type mouse at both diestrus and estrus; this difference becomes more evident when the cycling wild type mice are in the estrus phase. Moreover, while estrogen supplementation to ovariectomized wild type mice significantly reduces total uterine innervation, neither ovariectomy (OVX) nor acute estrogen supplementation affect uterine nerve density in mutant mice. Parasympathetic and sensory nerves show no significant changes in ERα knockout animals.
Both the uterus and postganglionic sympathetic neurons express ERα, suggesting that estrogen may regulate plasticity in uterine sympathetic nerves via direct effects on neurons or by changes in neuron-target interactions. ERα is the primary receptor in uterine tissues (Wang et al., 1999). In postganglionic sympathetic neurons, 92% express ERp protein, while ERα is expressed only in 29%. Interestingly, the proportion of neurons expressing ERα is nearly threefold greater in uterine-projecting neurons than in sympathetic neurons at large (Zoubina et al., 2001b), suggesting that uterine-related sympathetic neurons may be selectively susceptible to estrogen‟s effects. In patients with adenomyosis, aromatase expression is elevated in myometrial smooth muscle cells and ERα expression predominates in the innervating sympathetic neurons. These results suggest that estrogen synthesis is increased locally in the adenomyotic myometrium and this elevation may be responsible in part for the reduced myometrial sympathetic innervation observed in these patients (Barcena de Arellano et al., 2013).
3.2.3.1 Effects of ovariectomy on sympathetic innervation
In adult rats, the density of sympathetic nerves observed one and three weeks following OVX is identical to that seen in intact rats at diestrus, suggesting that reductions in estrogen levels at diestrus are sufficient to achieve maximal myometrial innervation (Zoubina et al., 2001a). In contrast, OVX of prepubertal rats (4 weeks old) provokes a generalized increase in the density of uterine innervation and increases the total content of NA (Chávez-Genaro et al., 2002). These results suggest that in immature rats, sympathetic neurons innervating the uterus retain a robust capacity for axonal growth that subsequently decreases as the animal matures.
3.2.3.2 Effects of acute and chronic estrogen treatment on sympathetic innervation
Acute administration of a single dose of 10mg/k of 17β-estradiol to adult OVX rats provokes an 85% reduction in the density of myometrial sympathetic nerves at 24h. The reduction persists after normalizing for changes in the muscle size (69%), indicating that the total number of nerves is reduced (Zoubina et al., 2001a). In prepubertal rats, acute treatment with 40 μg 17β–estradiol cypionate largely mimics the effects of natural puberty in that it reduces the density of myometrial sympathetic nerves in the uterine horn after 24h. Reductions in nerve density are paralleled by a fall in NA concentration (Brauer et al., 1995). These results indicate that an acute rise in estrogen is sufficient to reduce uterine sympathetic innervation with a timeframe similar to that occurring naturally during puberty and the estrous cycle.
In adult OVX rats, implantation of estrogen-releasing pellets that produce estrogen elevations approximating term pregnancy (high-physiological range, Zoubina et al., 2001a) decreases the total number of myometrial sympathetic nerves (Zoubina et al., 2000). The degree of reduction is similar to that observed after acute treatment. In intact infant rats, chronic exposure to supra-physiological levels of estrogen along the infantile prepubertal period provokes a complete loss of myometrial sympathetic nerve fibers (Fig. 3), suggesting that immature nerve fibers are more vulnerable to increases in estrogen levels. In the rat uterine artery, the density of innervation and the total content and concentration of NA remain unchanged (Corbacho et al., 1997).
Fig. 3.
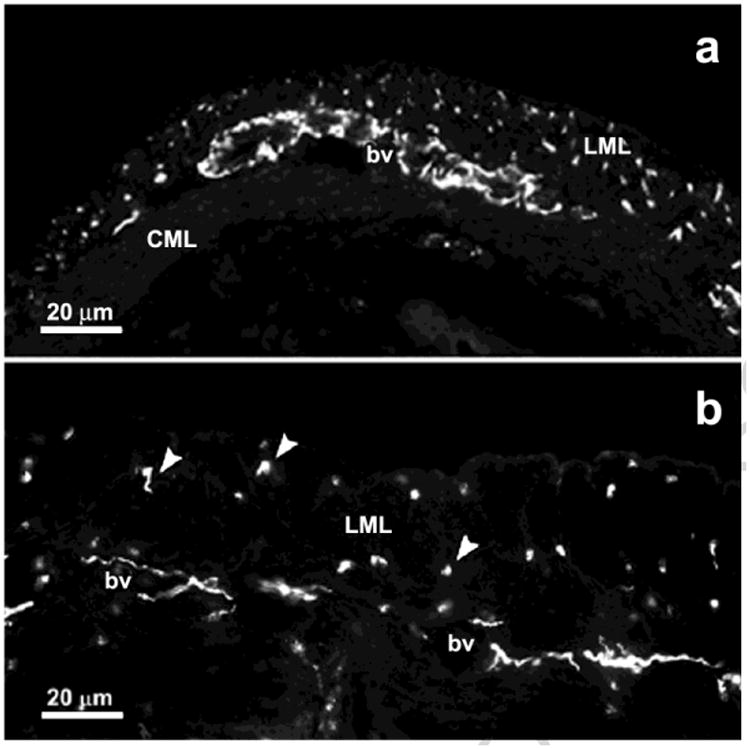
Transverse cryostat tissue sections of the cephalic region of the rat uterine horn showing tyrosine hydroxylase-immunoreactive sympathetic nerves in a prepubertal animal (a) and following acute treatment with estrogen (b). bv, blood vessels; CML, circular myometrial layer; LML, longitudinal myometrial layer. Modified from Brauer, 2000. Auton. Neurosci. 140, 1–16.
3.2.3.3 Effects of chronic estrogen treatment of immature rats on uterine cholinergic nerves
Following chronic estrogen treatment of immature rats, a robust cholinergic perivascular and myometrial plexus remains within the rat uterus (Richeri et al., 2002), thus confirming observations in the estrous cycle (Zoubina et al., 1998). A marked increase in the diameter of cholinergic bundles and nerve fibers is apparent at the end of treatment, and morphometric studies showed a rise in the density of large and medium-size cholinergic bundles. Interestingly, this increase is not reflected in finer myometrial nerve fibers whose density is reduced in the hypertrophied myometrium. Uterine-projecting preganglionic and postganglionic parasympathetic neurons located in rat paracervical ganglia express immunoreactivity for estrogen receptors α and β (Williams and Papka 1996; Papka et al. 1997, 2001; Williams et al. 1997; Papka and Mowa, 2003). In addition, immature and adult cholinergic neurons are receptive to several trophic and axon guidance factors (i.e., Laurikainen et al., 2000; Nangle and Keast, 2001; Hasan, 2013; Simpson et al., 2013). It is therefore possible, that estrogen regulates plasticity in immature cholinergic nerves via direct effects on neurons as well as through alterations in neuron-target interactions.
3.2.3.4 Effects of chronic estrogen treatment of immature rats on uterine afferent nerves
After infantile/prepubertal chronic estrogen treatment, the density of myometrial sensory nerves is reduced (Chalar et el., 2003). This decrease, however, does not persist after correction for changes in myometrium size, suggesting that early exposure to estrogen prevents sensory nerves from matching an increasing target volume as the uterus enlarges. Similarly, the density of CGRP-ir sensory nerves is unaffected by the estrous cycle (Zoubina et al. 1998) or by inactivation of ERα (Zoubina et al., 2001b). Uterine-related DRG neurons express ERα and ERβ (Papka et al., 1997, 2001; Papka and Mowa, 2003), and dorsal horn spinal cord neurons also express ER (Williams and Papka, 1996). Sensory neurons are receptive to a variety of target-derived signals (i.e., Sohrabji et al., 1994; Reza et al., 1999; Chalar et al., 2003) suggesting that estrogen-induced alteration in the production of these molecules could also regulate plasticity in uterine sensory nerves.
3.2.4 Effects of pregnancy
In most species including humans, pregnancy provokes an essentially complete loss of intrauterine myometrial and perivascular nerves. Although initially thought to be limited to sympathetic nerves (Owman et al., 1976; Sporrong et al., 1978, 1981; Marshall, 1981; Owman, 1981; Owman and Stjernquist, 1988), pregnancy also affects cholinergic and afferent nerves to the uterus (Stjerquist et al., 1985; Traurig and Papka, 1993; Haase et al., 1997).
The neurodegeneration elicited by pregnancy is likely attributable to hormonal alterations, mechanical strain and hypertrophy of myometrium, as well as the local influence of the placenta (Marshall, 1981). Local elevation in progesterone contributes to degeneration of uterine nerves since implantation of progesterone pellets in the lumen of virgin guinea pigs largely mimics the effect of pregnancy while systemic administration has no effect (Bell and Malcolm, 1978, 1988). Mechanical stretching and hypertrophy of the smooth muscle also affects myometrial innervation (Chávez-Genaro et al., 2006). Observations in guinea pig unilateral pregnancies revealed that in the non-fetus bearing uterine horn, reductions in NA levels result from a functional impairment of intact nerves and not from axonal degeneration (Owman and Stjernquist, 1988). The relevance of myometrial mechanical stretching is reinforced by the observation that implantation of porcelain pellets in the lumen of the uterine horn of virgin guinea pigs causes a partial nerve degeneration, which is further increased by the hormonal environment of pregnancy (Lundberg et al., 1989). Innervation is restored after delivery, requiring several weeks in the guinea pig but only a few days in rat (Moustafa, 1988; Haase et al., 1997; Klukovits et al., 2002). This difference appears to correlate with the degree of degeneration of the extrinsic innervation in the mesometrium (Alm et al., 1988; Bianchimano et al., 2007) as well as of intrauterine blood vessels (Haase et al., 1997; Bianchimano et al., 2007).
In the rat uterine artery, signs of degeneration of sympathetic nerves also occur at 15 days of pregnancy and nerves are almost completely absent at term (Klukovits et al., 2002). In the guinea pig, the apparent density of innervation and NA levels are reduced in the uterine artery, but this reduction is not related to axonal degeneration (Mione et al., 1990). Indeed, electron microscopy showed that in this species, numbers of sympathetic, parasympathetic and afferent nerves increase during pregnancy thus matching the hypertrophy of the artery (Mione and Gabella, 1991; Mione et al., 1993).
3.3 Vaginal axon remodeling
Unlike the uterus, vaginal innervation is not affected appreciably by short-term changes in serum estrogen levels. Hence, cyclical variations in serum estrogen do not result in detectable changes in any population of vaginal axons in the adult nulliparous rat (Liao et al., 2011), and a single acute injection of 17β-estradiol sufficient to deplete uterine innervation does not alter vaginal innervation in adult OVX rats (unpublished). Accordingly, relative to the uterus, vaginal innervation is apparently more resistant to short-term effects of elevated estrogen.
As with the uterus, however, prolonged estrogen elevations result in depletion of sympathetic nerves. Thus, implantation of OVX rats with estrogen-releasing pellets that produce estrogen elevations approximating term pregnancy (Zoubina et al., 2001a) results in significant reductions in numbers of axons immunoreactive for TH and PGP9.5-ir relative to OVX rats. These decreases persist after correcting for changes in target size, indicating a true loss of vaginal axons. However, unlike the uterus, chronic exposure to estrogen is also accompanied by depletion of vaginal VAChT-ir cholinergic axons and CGRP-ir nociceptive afferents (Ting et al., 2004). A similar depletion was observed in vaginal innervation of rats at term pregnancy (d21) where serum estrogen has been elevated for several days when compared to pregnant rats at 10 d post-coitus when estrogen levels are low (Liao et al., 2011). Accordingly, it appears that vaginal innervation shows distinct differences from uterine innervation in its time course to respond to estrogen, and in the selectivity of the axonal populations affected.
Effects of altered estrogen levels on vaginal innervation can be demonstrated not only in experimental models but also in humans. In postmenopausal women, hormone replacement therapy (HRT) results in decreased overall vaginal innervation density. Affected populations include TH-ir sympathetic, VIP-ir presumptive parasympathetic axons, and non-adrenergic, non-cholinergic (NANC) fibers that presumably represent both peptidergic (i.e., CGRP-ir) and non-peptidergic afferent fibers. Interestingly, topical HRT is significantly more effective than systemic HRT in reducing vaginal innervation (Griebling et al., 2012), which is consistent with the idea that estrogen's effects are due to direct actions on the target tissue rather than being secondary to effects. It should be noted that some indices of vaginal innervation do change as a function of cyclic changes in reproductive hormone levels including nociceptive threshold (Cason et al., 2003) and neuropeptide receptor transcripts (Dangoor et al., 2005).
Other hormonal systems may also contribute to vaginal neuroplasticity. Testosterone is reported to increase numbers of small NA nerve fibers (Pessina et al., 2006), while progesterone administration to juvenile rats for 7 days results in increased PGP- and CGRP-ir vaginal innervation density which persist into young adulthood (Liao and Smith, in review). Clearly, vaginal innervation can be strongly influenced by the hormonal milieu, and it seems likely that some post-menopausal symptoms result not only from loss of hormonal trophic support of target tissues, but also as a result of increased sympathetic, parasympathetic and visceral afferent vaginal innervation due to diminished levels of estrogen.
4. Molecular mechanisms of reproductive tract neuroplasticity
The prior discussion illustrates the remarkable dynamism of innervation patterns of the female reproductive tract, owing largely to the effects of estrogen. Accordingly, this system has served as a very tractable model in helping us understand how the body can initiate quite dramatic changes in innervation (‘physiological plasticity’) associated with normal functions essential to the survival of the organism and the species. The following section addresses central questions regarding mechanisms mediating physiological plasticity, including what is the site of action of the initiating hormones, and what are the signaling molecules that mediate these dramatic effects?
4.1 Role of the target
Pregnancy- and cycle-induced effects on uterine sympathetic innervation was classically explained by a selective effect of sex hormones on the system of ‘short’ adrenergic neurons supplying the uterus (Falk et al., 1969; Owman and Stjernquist, 1988). However, subsequent studies provided evidence that plasticity may not result from sex hormones acting directly on short adrenergic neurons, but instead through changes in neuron-target interactions. The anterior eye chamber transplantation method has proven to be an excellent method for determining whether pregnancy affects sympathetic innervation patterns via target-mediated actions (Brauer et al., 1998; Brauer, 2008). In these experiments, explants of myometrium from nulliparous and early postpartum guinea pigs were transplanted to the anterior eye chamber of intact cyclic guinea pigs. Three weeks following transplantation, myometrium from nulliparous donors was shown to be organotypically reinnervated by irideal sympathetic nerve fibers from the superior cervical ganglion (SCG). In contrast, sympathetic nerves failed to reinnervate myometrial transplants from postpartum donors. These results suggest that the degeneration of uterine sympathetic nerves at late pregnancy as well as their slow regeneration after delivery is initiated by changes in the target myometrium, rather than a particular sensitivity of ‘short’ adrenergic neurons to pregnancy-induced hormonal changes.
The anterior eye chamber transplantation method was further exploited to assess the role of estrogen in neuronal-target interactions that lead to uterine sympathetic denervation (Brauer et. al., 2000a; Brauer, 2008). In these studies, explants of myometrium from prepubertal rats were transplanted into the anterior eye chamber of adult OVX host rats and the effect of systemic chronic estrogen treatment on transplant reinnervation analyzed. Myometrium from prepubertal donors displayed an organotypic pattern of innervation (Fig. 4a), while transplants from estrogen-treated donors were almost completely devoid of innervation (Fig. 4b). Since the density of innervation and NA levels of the host irises were unaffected by estrogen, the data are consistent with the conclusion that the effects of estrogen are neither selective nor direct for the system of short adrenergic neurons, but instead are the result of the interaction between sympathetic nerves and the estrogen-stimulated myometrium.
Fig. 4.
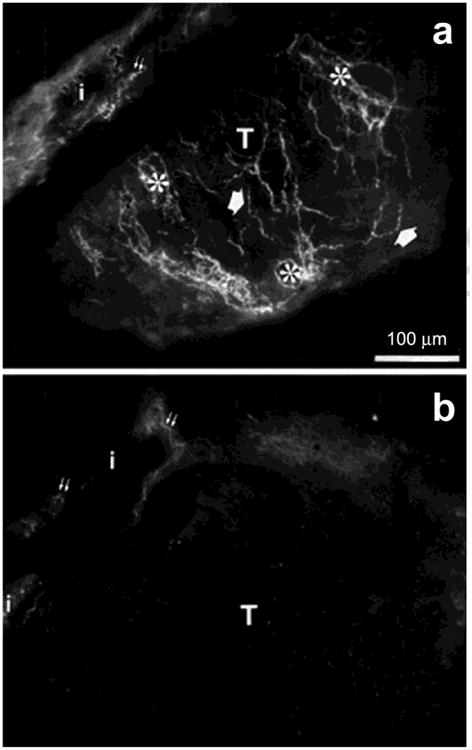
An example of sympathetic reinnervation (glyoxylic acid technique) of myometrial transplants (T) from prepubertal rats. (a) Myometrial transplant after 5 weeks in the anterior eye chamber of an adult ovariectomized rat hosts. Several preterminal and terminal fibers are seen in association with the smooth muscle and blood vessels. (b) Myometrial transplant in an ovariectomized rat hosts after chronic estrogen treatment. Note the absence of nerve fibers. i, host iris; double small arrows, irideal sympathetic nerve fibers. Modified from Brauer et al., 2000. J. Anat. 196, 347-355.
Co-cultures of myometrium with SCG explants confirmed the importance of the target in estrogen-induced uterine sympathetic neuroplasticity (Krizsan-Agbas and Smith, 2002). Ganglion explants from adult OVX donors extended neurites when cultured alone, and this growth was unaffected by the addition of estrogen to the culture medium. Neurite outgrowth increased significantly when ganglion explants were co-cultured with myometrial tissues from ovariectomized adult rats. The ability of myometrium to induce neurite growth was abolished by administration of 17β-estradiol to the myometrial tissue donors as well as by the addition 10-8 M estradiol to the culture medium. These results indicate that estrogen acts directly on myometrium to abrogate its neurite-promoting effects, probably by affecting the release of outgrowth-regulating diffusible factors from the myometrium in vitro. Of note, these studies also showed that estrogen can modulate neurite outgrowth by acting directly on the ganglion in vivo. Hence, while ganglia obtained from untreated OVX donors were unresponsive to estrogen in vitro, those obtained from OVX females treated with estrogen in vivo showed diminished neurite outgrowth in vitro; and addition of estrogen to the culture medium enhanced this inhibition. These results suggest that factors present in the whole animal other than estrogen are also involved. Indeed, removal of the pituitary gland rendered the SCG sensitive to neurite-suppressing effects of estrogen, and in vitro studies showed that prolactin was the most likely candidate.
4.2 Role of neurotrophins and their neuronal receptors
4.2.1 Neurotrophic factors in myometrial sympathetic neuroplasticity
The earliest studies designed to explain the reduced ability of pregnant or estrogen-primed myometrium to support sympathetic innervation focused in the role of nerve growth factor (NGF). NGF is essential for the development and survival of adrenergic and sensory neurons both in the central and peripheral nervous systems (Skaper, 2012). A close correlation between levels of NGF and the density of innervation of peripheral targets during postnatal development has been reported (Korsching and Thoenen, 1993; Shelton and Reichard, 1984). In the adult, sympathetic neurons lose their dependence on NGF for survival; however, other aspects of neuronal physiology, such as axonal growth, remain under the influence of NGF (Orike et al. 2001; Cowen, 2002). Accordingly, it was predicted that decreases in NGF synthesis and content would occur in pregnancy and after estrogen administration if withdrawal of this neurotrophic factor is responsible for reductions in uterine sympathetic innervation. Studies in the pregnant uterus provide some evidence in support of this hypothesis since the concentrations of NGF protein and mRNA decrease at middle and term pregnancy in the rat (Varol et al., 2000). Similarly, a decline in NGF protein was observed in the guinea pig at term pregnancy (Brauer et al., 2000b).
Contradicting the NGF hypothesis, however, are findings from several studies in conditions other than pregnancy where NGF levels do not correlate with innervation density. For example, estrogen administration to prepubertal rats is more effective in suppressing sympathetic axon growth than it is in mature rats; however, this does not correlate with uterine NGF levels (Chávez-Genaro et al., 2002). Moreover, since estrogen depletes sympathetic nerves, it would be expected that it should reduce myometrial NGF. In fact, all available evidence indicates that in rodents, administration of exogenous estrogen increases NGF protein levels. In adult mice, low estrogen levels following OVX increases mouse uterine sympathetic innervation (Zoubina and Smith, 2001b); this is associated with decreased NGF protein content, which can be reversed by estrogen and progesterone administration (Bjorling et al., 2002). Similarly, in situ hybridization and protein quantitation by ELISA showed that rat uterine NGF gene expression and protein levels are increased in the high-estrogen, hyperinnervated state (Krizsan-Agbas et al., 2003). Concordant NGF mRNA findings were obtained following estrogen administration to the prepubertal rat (Chalar et al., 2003).
Clearly, assumptions that estrogen-induced uterine sympathetic axon depletion is due to NGF withdrawal (and that sympathetic target density strictly correlates with NGF levels) are incorrect. Similarly, the possibility that reductions in another sympathetic neuritogenic protein, neurotrophin-3 (NT-3) might explain sympathetic depletion can be excluded as levels of this neurotrophic factor remain unchanged 24 h following estrogen administration to adult OVX rats (Krizsan-Agbas et al., 2003). Taken together, these results indicate that reductions in levels of the sympathetic pro-neuritogenic proteins NGF and NT-3 do not explain the denervation elicited by estrogen in the uterus.
It must be noted, however, that multiple NGF isoforms exist. While the ‘mature’ 13.5 kD form is known to promote outgrowth, pro-NGF has less affinity for the NGF trkA receptor than for the pro-degenerative p75 NTR (see below). Hence, while mature NGF promotes sympathetic neuronal survival and sprouting, pro-NGF has been suggested to induce apoptosis and sympathetic axon pruning (Bamji et al., 1998; Kaplan et al., 2000; Majdan et al., 2001; Al-Shawi et al., 2008). Interestingly, in the pregnant rat, there is an imbalance between NGF isoforms such that the ratio of pro- to mature NGF is increased (Lobos et al., 2005), raising the as-yet untested possibility that elevated proNGF may contribute to uterine sympathetic nerve depletion. It is also noteworthy that myometrial expression of the mature, pro-axonogenic form of NGF, and the ratio of mature to pro-NGF, is increased postpartum, which is likely to contribute to the massive sympathetic reinnervation that occurs in this period (Varol et al., 2000; Brauer et al., 2000b; Lobos et al., 2005).
While withdrawal of NGF's pro-neuritogenic effects appears unable to explain uterine sympathetic axon loss, there is direct evidence that activation of p75NTR is involved. The neurotrophic protein, brain-derived neurotrophic factor (BDNF) does not interact appreciably with the trkA receptor, but does stimulate p75NTR. Studies examining BDNF protein and mRNA show that estrogen is a potent stimulator of BDNF synthesis in the myometrium (Krizsan-Agbas et al., 2003). Further, functional studies using co-cultures revealed that BDNF added to the medium abolishes SCG neurite outgrowth that is normally promoted by myometrium of adult OVX rats. To show that BDNF is synthesized by the estrogen-primed myometrium, BDNF function-blocking antibodies were used. Estrogen treatment renders uterine explants relatively incapable of inducing ganglion sprouting, but BDNF neutralization largely restores this ability (Fig. 5). Complementary studies using BDNF knockout mice showed that reductions in BDNF synthesis in homo- and heterozygous knockouts eliminated the ability of estrogen to reduce sympathetic axon sprouting toward the uterine explant. These studies were interpreted to indicate that estrogen influences neuritogenic properties of the rodent uterus, in part, by regulating synthesis of BDNF which exerts a repulsive effect on sympathetic neurites via p75NTR. This conclusion is bolstered by findings that myometrial sympathetic innervation fails to undergo normal estrogen-induced remodeling in mice lacking functional p75NTR receptors (Selfridge et al., 2010)
Fig. 5.
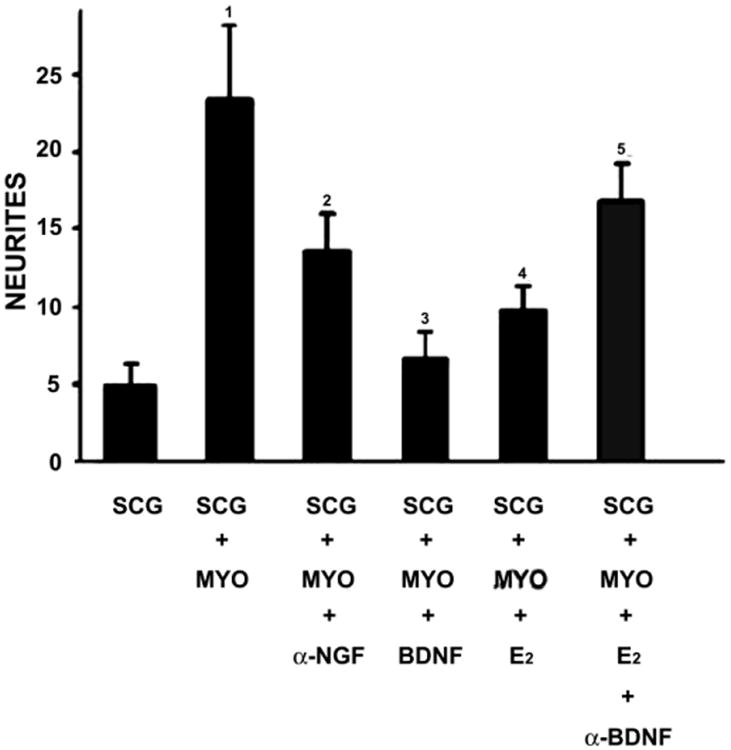
Quantitative analysis of neurites produced by superior cervical ganglion (SCG) explants under different culture conditions. SCG from ovariectomized (OVX) rats cultured alone in defined media (n=14). SCG+MYO, superior cervical ganglia from OVX rats cultured with myometrium explants from OVX rats (n=20). SCG+MYO-NGF, superior cervical ganglia from OVX rats in the presence of a function blocking antibody to NGF (n=14). SCG+MYO+BDNF, superior cervical ganglia from OVX rats cultured with myometrium explants from OVX rats in the presence of 50ng/mL BDNF (n=20). SCG+MYO+E2, superior cervical ganglia from OVX rats cultured with myometrium explants from OVX rats with 17β-estradiol added to the medium. SCG+MYO+ E2+αBDNF, superior cervical ganglia from OVX rats cultured with myometrium explants from OVX rats with 17β-estradiol and a BDNF function-blocking antibody added to the medium (n=15). 1, P=0.03 vs. SCG. 2, P =0.006 vs. SCG. 3, P =0.002 vs. SCG+MYO. 4, P=0.042 vs. SCG+MYO. 5, P =0.023 vs. SCG+MYO+ E2. Modified from Krizsan-Agbas et.al., 2003. Eur. J. Neurosci. 18, 2760-2768.
4.2.2 Neurotrophic factors in the endometrium and endometriosis
Neurotrophins are synthesized in situ by the human endometrium. Immunohistochemical studies showed that the functional layer of the endometrium of women with endometriosis contains abnormal innervation, which correlates with the presence of immunoreactivity for NGF (Tokushige et al., 2006, 2007; Al-Jefout et al., 2007; Bokor et al., 2009). Other studies claimed that both women with adenomyosis and with adenomyosis plus endometriosis present an unaltered endometrial innervation as well as similar NGF and NT-3 expression levels (Barcena de Arellano et al., 2012). Recent proteomic studies showed that levels of NT-4/5 and BDNF in biopsies from endometriosis cases are greater than in controls, whereas nerve growth factor levels are similar. These results suggest that alterations in levels of NT-4/5 and BDNF may stimulate differential nerve fiber growth and contribute to the pain associated with endometriosis (Browne et al., 2012; Kobayashi et al., 2014). Neurotrophins are also implicated in the development of innervation of ectopic endometrial lesions. High expression of NGF and NT-3 has been shown in peritoneal endometriosis lesions (Anaf et al., 2002; Mechsner et al., 2007; Wang et al., 2009) and endometrioma (Borghese et al., 2010). In addition, an overexpression of NGF but not BDNF occurs in the peritoneal fluid of women with endometriosis (Barcena de Arellano et al., 2011).
4.2.3 Neurotrophin receptors in sympathetic neurons
In addition to changes in neurotrophic factor availability, neurotrophin receptor expression or its regulation might also contribute to neuronal plasticity (Cowen et al. 2002). The neurotrophic effects of NGF are mediated through two cell surface receptors: the high-affinity tropomyosin-related tyrosine kinase receptor TrkA and the 75 kDa low-affinity neurotrophin receptor p75NTR (Skaper, 2012). NGF or NT3 binding to TrkA mediate sympathetic neuron survival and axon growth (Kohn et al., 1999; Brennan et al., 1999). p75NTR, on the other hand, plays a context-dependent role in the response of sympathetic neurons to neurotrophins, either inducing apoptosis or protecting neurons against apoptosis following neurotrophic factor deprivation (Miller and Kaplan, 2001). p75NTR in conjunction with the receptor sortilin mediates the neurotoxic effects of proNGF (Nykjaer et al., 2004; Al-Shawi et al., 2008). BDNF signals through p75NTR to mediate neuronal apoptosis (Kohn et al., 1999). Moreover, BDNF, via p75NTR receptors present on sympathetic axons, activates sphingomyelinase and increases intracellular levels of ceramides, which in turn impede sympathetic outgrowth (Krizsan-Agbas et al., 2003).
Combined retrograde tracing and quantitative measurements of fluorescence intensity (optical density) of uterine-projecting neurons in the thoraco-lumbar sympathetic chain term pregnant rats revealed that decreased levels of p75NTR without changes in TrkA (Richeri et al., 2005). Reductions in levels of p75NTR create an imbalance in the ratio p75NTR to TrkA that could adversely affect TrkA activation and/or NGF retrograde transport. Alternatively, considering that reductions of p75NTR may contribute to the survival of sympathetic neurons deprived of NGF for short periods of time (Zhou and Rush 1996), reduced p75NTR could represent a protective response for uterine-projecting sympathetic neurons deprived of NGF in late pregnancy.
Chronic estrogen treatment of prepubertal rats also influences neurotrophin receptor balance in uterine-projecting sympathetic neurons (Richeri et al., 2005). Indeed, estrogen treatment reduces TrkA levels without affecting levels of p75NTR. Since the growth-promoting effects of NGF and NT3 are mediated by TrkA activation, reductions in levels of TrkA may contribute to the inhibitory effects of estrogen on uterine sympathetic nerves. Moreover, alterations in the ratio p75NTR to TrkA may favor the inhibitory actions of BDNF (Krizsan-Agbas et al., 2003).
In contrast to uterine-projecting sympathetic neurons, prepubertal chronic estrogen treatment does not provoke significant changes in levels of TrkA or p75NTR in neurons of the SCG (Richeri et al., 2005). However, studies in the SCG of adult OVX rats (Hasan et al., 2005) showed that acute estrogen administration reduces TrkA protein significantly without affecting p75NTR expression. Conversely, chronic treatment with estrogen does not alter neuronal TrkA expression in adult SCG neurons but reduces p75NTR mRNA and protein expression significantly. Interestingly, after acute estrogen administration, ERα transcript expression increased by 35% although this was not maintained chronically. These findings were interpreted to suggest that estrogen can influence sympathetic neuronal neurotrophin receptor expression as well as ERα in SCG neurons. Moreover, reductions in TrkA expression after acute estrogen are thought to transiently predispose neurons to degenerative events, while diminished p75NTR expression by chronic estrogen administration may exert long-term effects on survival or axonal outgrowth in sympathetic neurons (Hasan et al., 2005).
4.2.4 Neurotrophin receptors in afferent sensory neurons
Chronic exposure of immature rats to estrogen induces changes in TrkA levels in neurons of the DRG projecting to the uterus (Chalar et al., 2003). In small-diameter neurons, estrogen increased the intensity of TrkA labeling, while it decreased labeling in medium-sized neurons that represent the vast majority of DRG neurons supplying the upper part of the uterine horn. Interestingly, reductions in immunofluorescence intensity were not homogeneous in all medium-sized uterine-projecting neurons but were restricted to a subpopulation. This difference may be explained by the particular pattern of estrogen receptor expression (Papka et al. 2001) as well as by differential interactions with selected regions or tissues in the uterus (i.e., blood vessels, endometrium and myometrium). These results suggest that estrogen may affect physiological aspects of uterine sensory innervation through changes in neuronal responsiveness to neurotrophins.
4.3 Neurotrimin
Neurotrimin (Ntm) is a glycophosphatidylinositol (GPI)–anchored cell-adhesion molecule belonging to the IgLON protein family, which also includes limbic-system associated protein, opioid-binding cell adhesion molecule and kilon (Salzer et al., 1996). Ntm regulates development of neuronal projections via attractive and repulsive mechanisms that are cell-type specific and mediated by homophilic and heterophilic interactions. In DRG neurons, Ntm promotes neurite outgrowth via Ntm forming noncovalent homodimers in the plane of the membrane. Conversely, Ntm inhibits neurite outgrowth in sympathetic neurons via heterophilic interactions because sympathetic neurons do not express Ntm (Rosen et al., 1992; Struyk et al., 1995). In 2008, Krizsan-Agbas and co-authors published an exhaustive study regarding the participation of Ntm in the remodeling of uterine innervation by estrogen, demonstrating that this cell-adhesion molecule is expressed in the rat myometrium and regulated by estrogen (Krizsan-Agbas et al., 2008).
The authors treated adult OVX rats with 10μg/k of β-estradiol benozoate and used microarray and bioinformatics approaches to discover new candidates mediating neuroplasticity. Ntm mRNA was found to be up-regulated in the myometrium at 6 hr and declining at 24 hr after treatment, and this was confirmed by real-time RT-PCR assays. In the myometrium of OVX rats, Ntm immunoblotting showed two distinct bands (58 and 65 kDa), which probably represent glycosylated isoforms. Ntm protein levels increased progressively 6 and 24 hr after estrogen treatment, with the 65-kDa band being up-regulated at 6 hr and both isoforms elevated at 24 hr. Finally, immunohistochemistry revealed Ntm-ir in both the circular and longitudinal layers as well as in perivascular tissue, luminal epithelium and endometrial glands. Ntm staining was low in myometrium at diestrus when estrogen levels are low and sympathetic axon density is high. Conversely, Ntm staining markedly increased at estrus when sympathetic nerve density is at its nadir.
To determine whether altered Ntm levels affect sympathetic innervation, sympathetic neurons were co-cultured on myometrial smooth muscle cell monolayers, which allow assessment of Ntm as both a contact and a secreted signaling agent. Dissociated smooth muscle cells from OVX rats expressed the 65kDa Ntm isoform, and treatment with estrogen for 24 hr increased cellular Ntm protein levels. Interestingly, Ntm was also detected in the conditioned medium of myometrial smooth muscle cultures, and estrogen significantly increased the amount in the medium. This is relevant because both the GPI-anchored and soluble Ntm forms elicit similar effects on neurite outgrowth (Gil et al., 1998; Lodge et al., 2001). In myometrial cell cultures not supplemented with estrogen, sympathetic neurons extend robust neuritic arbors while the addition of estrogen to the culture medium markedly decreased neurite outgrowth (Fig. 6). To assess whether Ntm mediates this effect, its synthesis was down-regulated using RNAi. Under low estrogen conditions, Ntm knockdown had no effect on neurite outgrowth. However in estrogen-treated conditions, neither increased Ntm protein nor the reduction in sympathetic neurite outgrowth was observed. Collectively, these results indicate that estrogen acts directly on myometrial smooth muscle cells to increase Ntm synthesis and secretion, and supports the hypothesis that Ntm contributes to sympathetic myometrial neurodegeneration elicited by estrogen.
Fig. 6.
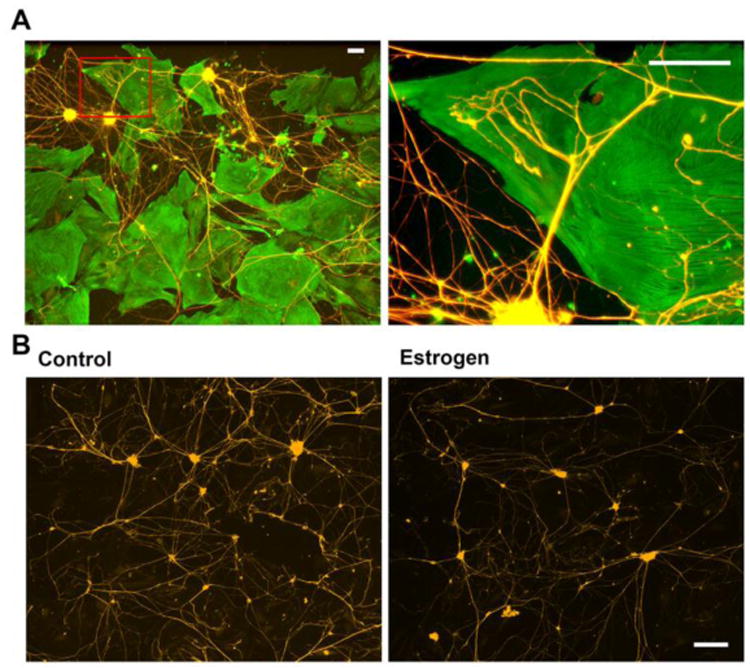
Estrogen decreases neurite outgrowth in smooth muscle-neuron co-cultures. Uterine myometrium smooth muscle cells were grown as a monolayer and sympathetic neurons seeded over the muscle cells and grown for 3 days. Smooth muscle cells were immunostained for α-smooth muscle actin (Cy2) and neurons for peripherin (Cy3). A: Low-magnification image (left) showing sympathetic neurons extending neurites to smooth muscle cells. Box insert corresponds to the region depicted on the right. This higher-magnification image shows sympathetic neurites branching and making terminal contacts with cultured smooth muscle cells. Scale bar = 100 μm. B: Sympathetic neurons were co-cultured with smooth muscle for 3 days and stained for peripherin. In cultures lacking estrogen, neurons elaborated many neurites (left). In the presence of 3 × 10-8 M 17β-estradiol, neurite outgrowth was greatly reduced (right). Scale bar = 200 μm. Modified from Krizsan-Agbas et al., 2008. J. Neurosci. Res. 86, 3086–3095.
4.4 Semaphorins and Neuropilins
The Semaphorins are a large, phylogenetically conserved protein family that includes secreted, transmembrane and membrane-anchored guidance proteins (Raper, 2000; Kumanogoh and Kikutani, 2010). These molecules bind and signal through receptor complexes consisting of members of the neuropilin (NRP) and plexin families (Chen et al., 1998; Grunwald and Klein, 2002). Semaphorins were originally identified as repulsive guidance cues that are important in patterning the nervous system during development (Kolodkin 1998, Raper, 2000; Paterkamp, 2012). Some semaphorins are reused in postnatal and adult life, when they regulate aspects of neuronal plasticity and regeneration (Pasterkamp and Verhaagen, 2001; Pasterkamp and Giger, 2009). Studies have shown the involvement of semaphorins in the regulation of plasticity in autonomic nerves both under physiological and pathological conditions (Brauer and Richeri, 2013).
In 2004, Marzioni and co-authors reported that the non-pregnant human myometrium displays low levels of immunoreactivity for the secreted semaphorin 3A (Sema3A). During middle and term pregnancy Sema3A immunostaining increased markedly, thus correlating with the degeneration of uterine nerves. In addition, this study showed that some nerve fibers present in the non-pregnant uterus are positive for NRP1 and Plexin-A1, thus making these nerves potentially responsive to the neurorepulsive actions of Sema3A (Marzioni et al., 2004).
In 2011, the first evidence indicating a positive correlation between the pattern and kinetics of semaphorin 3F (Sema3F) expression and estrogen-induced degeneration of uterine sympathetic nerves was reported (Richeri et al., 2011). Sema3F is a member of the vertebrate class-3 secreted semaphorins and has been shown to be repellent for sympathetic nerves (Fassold et al., 2009; Ieda and Fukuda, 2009; Fassold and Straub, 2010). RT-PCR assays demonstrated the presence of mRNA encoding Sema3F in the uterus of both control and estrogen-treated prepubertal rats, and estrogen treatment increased Sema3F gene expression 5-fold according to quantitative real-time RT-PCR. In situ hybridization showed that, in controls, Sema3F mRNA is scarcely evident and predominantly confined to the outer myometrial compartment. Following estrogen treatment, increased expression of Sema3F was detected in hypertrophic connective tissue separating myometrial smooth muscle bundles and surrounding intra-myometrial major blood vessels (Fig. 7a, c). Within connective tissue, Sema3F mRNA is expressed selectively in fibroblasts (Fig. 7b) and infiltrating eosinophil leukocytes (Fig. 7d, e). In the endometrial stroma, a subtle but apparent induction was observed in estrogen-treated animals, and labeled cells were recognized as eosinophil leukocytes.
Fig. 7.
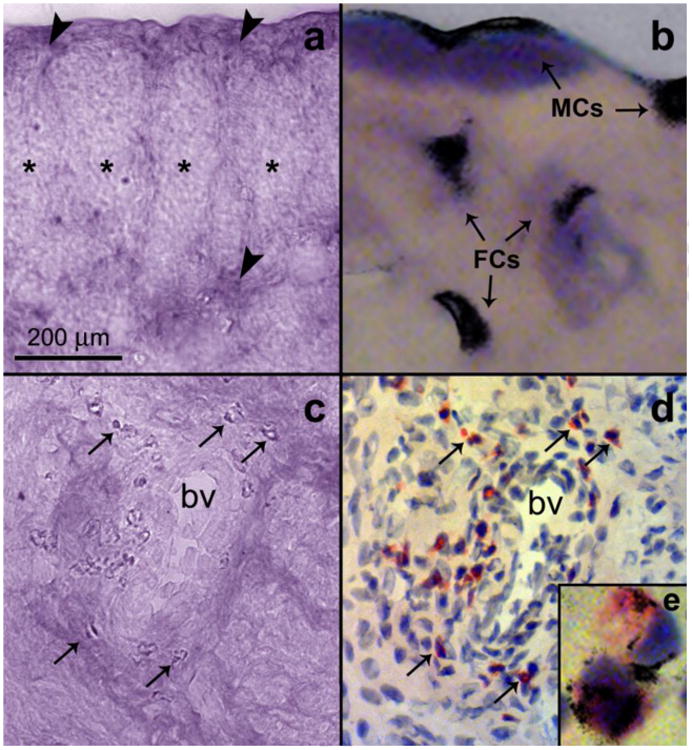
In situ hybridization (false color) showing the localization of Sema3F mRNA in cross sections of the uterine horn from prepubertal rats treated with four doses of 10 μg of β-estradiol 17-cypionate on days 10, 15, 20 and 25 of postnatal development and killed at 28 days. (a) Sema3F hybridization signal in the hypertrophied connective tissue (arrowheads) surrounding myometrial bundles (asterisks). (b) Higher magnification of the area marked in Fig. a, now showing the hybridized section stained with hematoxylin and eosin. An intense label is observed in the cytoplasm of fibroblast-like cells (FCs) and mesenteric cells (MCs). (c) Illustrates Sema3F signal associated to the connective tissue surrounding intramyometrial major blood vessels (bv). Arrows point to intense labeled cells. Fig. d shows the same field than in Fig. c, now stained with Sirius Red (specific for eosinophil leukocytes, arrows). In e, a high-magnification image shows the presence of Sema3F transcripts overlapping the cytoplasm of two eosinophil leukocytes. Modified from Richeri et al., 2011. Auton. Neurosci. 164, 43-50.
Nerve repulsion by Sema3F is mediated by neuropilin-2 (NRP2) and plexin receptors present on the surface of sympathetic axon terminals (Fassold et al., 2009). In the uterus, immunohistochemistry showed the presence of delicate NRP2-ir nerve profiles at the end of chronic estrogen treatment, while staining was absent in prepubertal controls. NRP2-ir fibers were distributed in the myometrial compartment, mainly located at the connective tissue areas where Sema3F transcripts were demonstrated. Simultaneous labeling showed that NRP2-ir nerves do not display TH-ir. This suggests that estrogen downregulates TH expression in uterine sympathetic nerves, thus making them indemonstrable by immunohistochemistry. Degenerating sympathetic nerves are reported to be NRP2-positive but lack TH-ir in synovial tissue affected by rheumatoid arthritis (Fassold et al., 2009). It is therefore possible that during degeneration, NRP2 is enriched in uterine sympathetic nerves at later stages of estrogen-induced degeneration, when TH is no longer present. Although correlative in nature, these results suggest that Sema3F may contribute to the negative regulation of uterine innervation by estrogen.
4.5 Substrate-bound signals
In addition to diffusible signals, axon growth and pathfinding utilize substrate-bound cues. These signals play a crucial role during development of the nervous system, and in postnatal and adult life, they favor or inhibit regeneration and plasticity in a context-dependent manner (Myers et al., 2011; Alberti et al., 2014). The tissue section culture method (cryoculture) has been widely used to assess contributions of substrate-bound signals independent of ongoing synthesis of neurite growth-promoting and growth-inhibiting factors by the target tissue (Crutcher, 1993). Using this approach, the contribution of substrate-bound signals to remodeling of uterine sympathetic nerves by estrogen was analyzed (Richeri et al., 2010).
4.5.1 Myometrium
Cryoculture experiments employing myometrial tissue sections of adult control OVX showed that this substrate supports neurite outgrowth both from neonatal and adult ganglion explants (Fig. 8). Interestingly, in longitudinal sections of myometrium, neurites follow the orientation of the main axis of the longitudinally sectioned muscle cells, and show limited growth on transversally sectioned smooth muscle. The pattern and extent of neurite outgrowth is unaffected by reductions in the number of migrating ganglionic non-neuronal cells (Figs. 8a, b). These studies indicate that adult myometrium continues to provide signals allowing organotypic patterning and growth of sympathetic axons, and that adult sympathetic neurons retain their ability to recognize substrate-bound cues present in the myometrium. Neurite outgrowth from neonatal and adult OVX ganglion donors is markedly reduced on myometrial sections from donor rats treated with estrogen (Figs. 8c, e). These results indicate that estrogen modifies myometrial substrate properties so that it is less supportive of sympathetic neurite growth. Still unknown is whether uterine axon populations other than sympathetics are receptive to myometrial substrate-bond cues and to what extent substrate-bound cues affect their outgrowth.
Fig. 8.
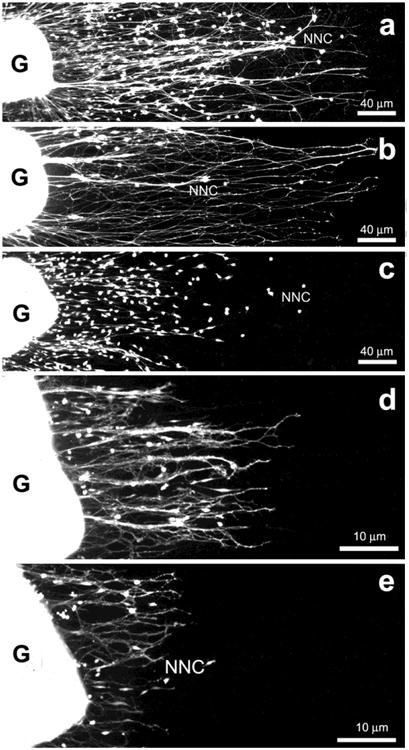
Representative images of fluorescein-labeled cryocultures showing the pattern of neurite outgrowth and non-neuronal cell migration after 3 days in culture from neonatal (a-c) and adult (d, e) sympathetic superior cervical ganglion explants (G) on unfixed tissue sections of the longitudinal myometrial layer of adult ovariectomized rats treated with vehicle (a, b) or 3 subcutaneous injections of 50 μg β-estradiol 17-cypionate (c, d). In b, the effects of reductions in the number of migrating non-neuronal cells (mitomycin-C-treatment) on the pattern and extent of neurite outgrowth is shown. Note that neurite outgrowth from neonatal and adult ganglia is markedly reduced on sections of estrogenized myometrium (c, e). Modified from Richeri et al., 2010. Cell Tissue Res. 340, 287–301.
In the myometrium estrogen elicits a dramatic reorientation of collagen fibrils surrounding individual muscle cells, and it is likely that this change may negatively affect the ability of the myometrial substrate to support neurite outgrowth (Martínez et al., 2009; Richeri et al., 2010). Other ECM signals with inhibitory effects for sympathetic axons may also be involved. For example, chondroitin sulfate proteoglycans have been shown recently to prevent sympathetic reinnervation after cardiac ischemia-reperfusion injury (Gardner and Habecker, 2013). It is also unknown whether other estrogen-regulated inhibitory signals, such as cell adhesion molecules, transmembrane signals and diffusible signals anchored to the ECM remain present in the frozen myometrial tissue sections and whether these signals may contribute to the diminished ability of the myometrial substrate to support sympathetic neurite outgrowth in vitro.
4.5.2 Endometrium
Endometrial frozen tissue from ovariectomized rats supports extensive neurite growth from neonatal SCG neurons (Richeri et al., 2010). This is surprising because, in the rat, the endometrium is largely devoid from sympathetic nerves. This suggests that the growth-promoting effects of endometrial substrates are overridden by inhibitory signals synthesized by the living tissue. Recent studies suggested that endometriotic peritoneal lesions produce Sema3F (Mechsner, 2014), and it is thought that this signal may contribute to the imbalance between sympathetic and sensory nerves observed in these lesions (Arnold et al., 2012). Neurite outgrowth was markedly diminished on endometrial tissue sections from rat donors treated with estrogen. This reduction, however, was restricted to endometrial stroma, while numerous sympathetic nerve fibers remained associated with the endometrial glands. These observations suggest that although normally poorly innervated, the estrogenized endometrial substrate reinforces repulsion of sympathetic nerves.
4.6 Bone Morphogenetic Protein 4
While we now have a detailed understanding of molecular mechanisms governing uterine axon remodeling, the processes by which chronic estrogen depletes vaginal innervation remains largely elusive. One factor that appears to contribute to the estrogen-induced depletion of CGRP-ir afferent vaginal fibers is Bone Morphogenetic Protein 4 (BMP4), a member of the TGFβ superfamily of cytokines and morphogens. While BMPs are extremely important in nervous system development, they have received scant attention as regulators of innervation in the adult nervous system. Bhattacherjee et al. (2013) found that BMP4 is expressed in adult OVX rat vaginal smooth muscle under low-estrogen conditions, but both gene expression and protein levels are markedly reduced in the presence of estrogen. DRG small-diameter neurons projecting to the vagina and elsewhere express BMP receptors, suggesting that they may be targets for the actions of BMP4. In cultures BMP4 facilitated sensory axonal outgrowth, and antibody neutralization or inhibition of BMP4 reduced neurite outgrowth on vaginal smooth muscle cells consistent with its production by these cells. While addition of estrogen to the co-culture reduces axonogenesis, this is overcome by addition of BMP4 protein. Bhattacherjee et al. therefore hypothesized that estrogen may reduce vaginal sensory innervation, in part, by reducing the amount of tissue BMP4 synthesized. To test this, the authors used lentiviral transfection to transduce vaginal smooth muscle cells in vivo to express BMP4 under control of the CMV promoter, which lacks an estrogen response element and therefore is not affected by changing estrogen levels. It was found that, following systemic estrogen administration only regions of the tissue that were successfully transduced with the BMP4 construct (as revealed by a green fluorescent protein marker) showed normal levels of sensory innervation while all other regions are depleted. Sympathetic innervation density did not appear to be affected by BMP4 transduction. These findings suggest that BMP4 is a significant player in regulating vaginal sensory depletion in the presence of estrogen, and is selective in respect to which populations of neurons it affects.
5. Summary and conclusions (Fig. 9)
Fig. 9.
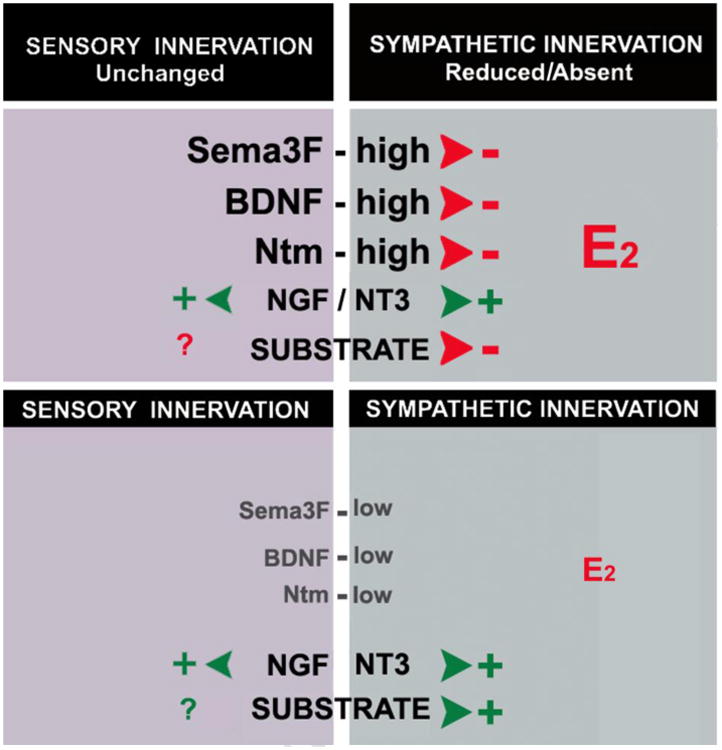
Schematic representation depicting the differential effects of target-derived molecular signals on sympathetic and sensory nerves supplying the myometrium. Under low estrogen levels (bottom), both sympathetic and sensory nerves are present in the uterus, probably supported by neurotrophins and a permissive substrate. When levels of estrogen are high (top), sensory nerves remain largely preserved while sympathetic undergo degenerative events. Although levels of NGF and NT3 are maintained, increases in levels Ntm, BDNF and Sema3F elicit degeneration of sympathetic nerves without affecting (or possibly even stabilizing) sensory nerves. In addition, reductions in substrate permissiveness affect the ability of the estrogenized myometrium to support sympathetic nerve growth.
The innervation of the uterus is remarkably dynamic and shows considerable plasticity during pregnancy and in response to altered levels in systemic estrogen. Current evidence indicates that the target tissues of the uterus play an important role in regulating this plasticity and both estrogen and pregnancy transform the uterus into an inhospitable environment for sympathetic nerves.
In the case of estrogen, this inhospitable environment is not the result of neurotrophic factor deprivation, since uterine levels of pro-neuritogenic factors such as NGF and NT3 remain undiminished. In contrast, estrogen provokes an increase in production of proteins with repulsive effects on sympathetic nerves. Thus far BDNF, Neurotrimin, Semaphorin 3F, substrate-bound signals and possibly pro-NGF appear to contribute to estrogen-induced remodeling of uterine innervation. It is still intriguing why this apparent redundancy of molecular signals is involved and what is the nature of their cross talk for the fine-tuning of this plasticity.
One reason for this redundancy may be that different factors exert their effects at different times in the degenerative and regenerative processes. For instance, substrate-bound signals may be important in the initial patterning of sympathetic and other axons (Richeri et al., 2010). Exposure to estrogen appears to alter properties of the uterine ECM which in turn affects sympathetic nerve ingrowth. Changes in collagen fibrils alignment occur following chronic estrogen exposure to adult OVX rats as well as during peripubertal transition (Martínez and Brauer, unpublished). Substrate changes may also affect the presentation of other axon-interacting proteins by affecting the distribution and/or accessibility of diffusible molecules that in vivo are largely bound to tissues and ECM components, and could serve to maintain sympathetic repulsion through retention of degeneration-promoting proteins. Neurotrimin and BDNF show staggered upregulation following estrogen treatment. Ntm mRNA levels peak 6 hr after estrogen administration to adult OVX rats (Krizsan-Agbas et al., 2008), while BDNF mRNA remains elevated through 24 hr (Krizsan-Agbas et al. 2003). This differential time-course of expression suggests that Ntm may be involved in the initiation of nerve degeneration, while BDNF may sustain this process. Finally, Sema3F receptor NRP2 is expressed in degenerating sympathetic nerves that no longer express tyrosine hydroxylase, suggesting that this signal might act in the final stages of neurodegeneration (Richeri et al., 2011).
The recruitment of multiple molecular signaling pathways may also help to confer selectivity to estrogen actions. Under high estrogen levels, it is likely that Ntm, BDNF and Sema3F may act in concert to induce the degeneration of sympathetic nerves, while other nerve populations, such as sensory nerves, remain preserved. Ntm repels sympathetic axons via heterophilic interactions since sympathetic neurons do not express Ntm. However, sensory neurons do synthesize Ntm, so that target-derived Ntm can promote sensory fiber outgrowth via homophilic interactions (Gil et al., 1998). Hence, Ntm may represent one means by which selective depletion of sympathetic fibers occurs in the uterus.
Semaphorins 3F and 3C are repellent of sympathetic nerves, while semaphorin 3A mainly repels sensory nerve fibers. Indeed, the differential expression of semaphorins contributes to the determination of sensory and sympathetic nerve density in peripheral tissues. Sema3F and 3C are expressed in the synovial tissue of patients affected by rheumatoid arthritis (Miller et al., 2004; Fassold et al., 2009). Similarly, Sema3F appears to contribute to decreased sympathetic nerves in endometriosis lesions (Mechsner, 2014) thus leading to an imbalance that favors sensory nerves, which in turn contributes to the perpetuation of inflammation and increased pain symptoms (Arnold et al., 2012).
In addition to altered levels of target-derived molecular signals, the complement of receptors present in uterine-projecting neurons may participate in the regulation of neuroplasticity. Pregnancy and estrogen elicit alterations in the relative balance of neurotrophin receptors TrkA and p75NTR in sympathetic neurons (Richeri et al., 2005; Hasan et al., 2005). Similarly, alterations in TrkA levels occur in response to estrogen in DRG sensory neurons supplying the uterus (Chalar et al., 2003). Imbalances in levels of TrkA and p75NTR may affect the receptivity of neurons to the pro-neuritogenic effects of NGF and NT3, while favoring the inhibitory actions of BDNF (Krizsan-Agbas et al., 2003) and pro-NGF. Interestingly, Sema3F can antagonize NGF-stimulated TrkA signaling in sympathetic neurons (Atwal et al., 2003). Moreover, p75NTR is required for growth cone collapsing responses of sympathetic neurons to Sema3F; and p75NTR has to be bound to neurotrophins to participate in Sema3-mediated collapse (Naska et al., 2010). This may be relevant because estrogen increases the expression of p75NTR in uterine-projecting sympathetic neurons (Richeri et al., 2005).
Collectively, studies of uterine innervation over more than 40 years has been remarkably instructive in providing an understanding of the complex multifactorial processes by which target organ innervation density is regulated. It is not clear as to the extent to which these processes extend to tissues other than the female reproductive tract; however, in at least some instances, very similar mechanisms pertain such as BDNF-p75NTR-mediated depletion of sympathetic nerves in post-infarcted myocardium (Lorentz et al., 2013). Similarly, while we know that, other regions of the female reproductive tract such as the ovaries, vagina and external genitalia undergo significant remodeling; the mechanisms and outcomes appear to vary. Clearly, additional investigations will be necessary, which ultimately should add to our knowledge of the biological processes underlying peripheral nerve remodeling, but also may lead to discovery of molecular mechanisms and hopefully therapeutic targets associated with a variety of female reproductive tract disorders.
Highlights.
Female reproductive tract innervation undergoes marked plasticity.
This neuroplasticity is associated with hormonal changes driven by changes in reproductive status.
This review summarizes our knowledge of autonomic and sensory innervation to the female reproductive tract.
It describes the effects of estrogen and related hormones on the distribution of nerves.
We also review what is known about the molecular mechanisms responsible for changes in innervation.
Acknowledgments
Studies by PGS were supported by NIH grants R01NS039570, R01HD038670, R01HD049615, and 5P30HD002528. Studies by MMB were supported by PEDECIBA (Uruguay) and Fogarty International Research Collaboration Award (FIRCA, NIH, grant no. TW007213).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
M. Mónica Brauer, Email: brauer2009@gmail.com.
Peter G. Smith, Email: psmith@kumc.edu.
References
- Adham N, Schenk EA. Autonomic innervation of the rat vagina, cervix, and uterus and its cyclic variations. Am J Obstet Gynecol. 1969;104:508–516. doi: 10.1016/s0002-9378(16)34239-9. [DOI] [PubMed] [Google Scholar]
- Alberti KA, Hopkins AM, Tang-Schomer MD, Kaplan DL, Xu Q. The behavior of neuronal cells on tendon-derived collagensheets as potential substrates for nerve regeneration. Biomaterials. 2014;35:3551–3557. doi: 10.1016/j.biomaterials.2013.12.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque-Araujo WI, Rosa-e-Silva AA, Antunes-Rodrigues J, Favaretto AL. Is ovarian adrenergic innervation essential to gonadal function in adult rats? Arch Physiol Biochem. 1995;103:109–113. doi: 10.3109/13813459509007572. [DOI] [PubMed] [Google Scholar]
- Al-Jefout M, Andreadis N, Tokushige N, Markham R, Fraser I. A pilot study to evaluate the relative efficacy of endometrial biopsy and full curettage in making a diagnosis of endometriosis by the detection of endometrial nerve fibers. Am J Obstet Gynecol. 2007;197(578):e1–4. doi: 10.1016/j.ajog.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Al-Jefout M, Dezarnaulds G, Cooper M, Tokushige N, Luscombe GM, Markham R, Fraser IS. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: a double blind study. Hum Reprod. 2009;24:3019–3024. doi: 10.1093/humrep/dep275. [DOI] [PubMed] [Google Scholar]
- Al-Shawi R, Hafner A, Olsen J, Chun S, Raza S, Thrasivoulou C, Lovestone S, Killick R, Simons P, Cowen T. Neurotoxic and neurotrophic roles of proNGF and the receptor sortilin in the adult and ageing nervous system. Eur J Neurosci. 2008;27:2103–2114. doi: 10.1111/j.1460-9568.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- Alm P, Lundberg LM. Co-existence and origin of peptidergic and adrenergic nerves in the guinea pig uterus. Retrograde tracing and immunocytochemistry, effects of chemical sympathectomy, capsaicin treatment and pregnancy. Cell Tissue Res. 1988;254:517–530. doi: 10.1007/BF00226501. [DOI] [PubMed] [Google Scholar]
- Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, Buxant F, Noel JC. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17:1895–1900. doi: 10.1093/humrep/17.7.1895. [DOI] [PubMed] [Google Scholar]
- Arnold J, Barcena de Arellano ML, Ruster C, Vercellino GF, Chiantera V, Schneider A, Mechsner S. Imbalance between sympathetic and sensory innervation in peritoneal endometriosis. Brain Behav Immun. 2012;26:132–141. doi: 10.1016/j.bbi.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Atwal JK, Singh KK, Tessier-Lavigne M, Millar FD, Kaplan DR. Semaphorin 3F antagonizes neurotrophin-induced phosphatidylinositol 3-kinase and mitogenactivated protein kinase kinase signaling: a mechanism for growth cone collapse. J Neurosci. 2003;23:7602–7609. doi: 10.1523/JNEUROSCI.23-20-07602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SE, Corcoran BM, Watson ED. Immunohistochemical study of the distribution of adrenergic and peptidergic innervation in the equine uterus and the cervix. Reproduction. 2001;122:275–282. doi: 10.1530/rep.0.1220275. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, Causing CG, Miller FD. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J Cell Biol. 1998;140:911–923. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcena de Arellano ML, Arnold J, Vercellino F, Chiantera V, Schneider A, Mechsner S. Overexpression of nerve growth factor in peritoneal fluid from women with endometriosis may promote neurite outgrowth in endometriotic lesions. Fertil Steril. 2011;95:1123–1126. doi: 10.1016/j.fertnstert.2010.10.023. [DOI] [PubMed] [Google Scholar]
- Barcena de Arellano ML, Arnold J, Sacher F, Blochle M, Staube M, Bartley J, Vercellino GF, Chiantera V, Schneider A, Mechsner S. Eutopic endometrium from women with endometriosis does not exhibit neurotrophic properties. J Neuroimmunol. 2012;249:49–55. doi: 10.1016/j.jneuroim.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Barcena de Arellano ML, Oldeweme J, Arnold J, Schneider A, Mechsner S. Remodeling of estrogen-dependent sympathetic nerve fibers seems to be disturbed in adenomyosis. Fertil Steril. 2013;100:801–809. doi: 10.1016/j.fertnstert.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Bell C, Malcolm SJ. Observations on the loss of catecholamine fluorescence from intrauterine adrenergic nerves during pregnancy in the guinea-pig. J Reprod Fertil. 1978;53:51–58. doi: 10.1530/jrf.0.0530051. [DOI] [PubMed] [Google Scholar]
- Bell C, Malcolm SJ. Neurochemistry of the sympathetic innervation to the uterus. Clin Exp Pharmacol Physiol. 1988;15:667–674. doi: 10.1111/j.1440-1681.1988.tb01126.x. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Wood E, Scofield SL, Little M. Behavioral responses to uterine or vaginal distension in the rat. Pain. 1995;61:121–131. doi: 10.1016/0304-3959(94)00150-D. [DOI] [PubMed] [Google Scholar]
- Bhattacherjee A, Rumi M, Smith HS. PG Bone Morphogenetic Protein 4 Mediates Estrogen-Regulated Sensory Axon Plasticity in the Adult Female Reproductive Tract. J Neurosci. 2013;16:1704–1712. doi: 10.1523/JNEUROSCI.1704-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchimano P, Frías AI, Richeri A, Brauer MM. Effects of dexamethasone on estrogen- and pregnancy-induced plasticity in rat uterine sympathetic nerves. Cell Tissue Res. 2007;330:413–425. doi: 10.1007/s00441-007-0444-0. [DOI] [PubMed] [Google Scholar]
- Bjorling DE, Beckman M, Clayton MK, Wang ZY. Modulation of nerve growth factor in peripheral organs by estrogen and progesterone. Neuroscience. 2002;110:155–167. doi: 10.1016/s0306-4522(01)00568-1. [DOI] [PubMed] [Google Scholar]
- Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecol Obstet Invest. 1999;48:270–275. doi: 10.1159/000010198. [DOI] [PubMed] [Google Scholar]
- Bokor A, Kyama CM, Vercruysse L, Fassbender A, Gevaert O, Vodolazkaia A, De Moor B, Fülöp V, D'Hooghe T. Density of small diameter sensory nerve fibres in endometrium: a semi-invasive diagnostic test for minimal to mild endometriosis. Hum Reprod. 2009;24:3025–3032. doi: 10.1093/humrep/dep283. [DOI] [PubMed] [Google Scholar]
- Borghese B, Vaiman D, Mondon F, Mbaye M, Anaf V, Noel JC, de Ziegler D, Chapron C. Neurotrophins and pain in endometriosis. Gynecol Obstet Fertil. 2010;38:442–446. doi: 10.1016/j.gyobfe.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Boyd JW, Lechuga TJ, Ebner CA, Kirby MA, Yellon SM. Cervix remodeling and parturition in the rat: lack of a role for hypogastric innervation. Reproduction. 2009;137:739–748. doi: 10.1530/REP-08-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer MM, Lincoln J, Blundell D, Corbacho A. Postnatal development of noradrenaline-containing nerves of the rat uterus. J Autonom Nerv Syst. 1992;39:37–50. doi: 10.1016/0165-1838(92)90249-g. [DOI] [PubMed] [Google Scholar]
- Brauer MM, Corbacho A, Burnstock G. Effects of chronic and acute oestrogen treatment on the development of noradrenaline-containing nerves of the rat uterus. Int J Dev Neurosci. 1995;13:791–798. doi: 10.1016/0736-5748(95)00079-8. [DOI] [PubMed] [Google Scholar]
- Brauer MM, Burnstock G, Thrasivoulou C, Cowen T. In oculo transplants of myometrium from postpartum guinea pigs fail to support sympathetic reinnervation. J Anat. 1998;193:509–517. doi: 10.1046/j.1469-7580.1998.19340509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer MM, Chávez R, Llodrá J, Richeri A, Scorza C. Effects of chronic oestrogen treatment are not selective for uterine noradrenaline-containing sympathetic nerves: a transplantation study. J Anat. 2000a;196:347–355. doi: 10.1046/j.1469-7580.2000.19630347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer MM, Klosowsk IK, Chávez R, Richeri A, Cowen T, Crutcher KA. The role of NGF in pregnancy-induced degeneration and regeneration of sympathetic nerves in the guinea pig uterus. J Auton Nerv Syst. 2000b;79:19–27. doi: 10.1016/s0165-1838(99)00094-6. [DOI] [PubMed] [Google Scholar]
- Brauer MM. Cellular and molecular mechanisms underlying plasticity in uterine sympathetic nerves. Auton Neurosci. 2008;140:1–16. doi: 10.1016/j.autneu.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Brauer MM, Richeri A. Highlights in basic autonomic neuroscience: Semaphorins in the remodeling of autonomic innervation. Auton Neurosci. 2013;174:1–4. doi: 10.1016/j.autneu.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influence NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci. 1999;2:699–705. doi: 10.1038/11158. [DOI] [PubMed] [Google Scholar]
- Browne AS, Yu J, Huang RP, Francisco AMC, Sidell N, Robert N, Taylor RN. Proteomic identification of neurotrophins in the eutopic endometrium of women with endometriosis Fertil. Steril. 2012;98:713–719. doi: 10.1016/j.fertnstert.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calka J, McDonald JK, Ojeda SR. The innervation of the immature rat ovary by calcitonin gene-related peptide. Biol Reprod. 1988;39:1215–1223. doi: 10.1095/biolreprod39.5.1215. [DOI] [PubMed] [Google Scholar]
- Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm Behav. 2003;44:123–131. doi: 10.1016/s0018-506x(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Chalar C, Richeri A, Viettro L, Chávez-Genaro R, Bianchimano P, Marmol NM, Crutcher K, Burnstock G, Cowen T, Brauer MM. Plasticity in developing rat uterine sensory nerves: the role of NGF and TrkA. Cell Tissue Res. 2003;314:191–205. doi: 10.1007/s00441-003-0799-9. [DOI] [PubMed] [Google Scholar]
- Chávez-Genaro R, Crutcher K, Viettro L, Richeri A, Coirolo N, Burnstock G, Cowen T, Brauer MM. Differential effects of oestrogen on developing and mature uterine sympathetic nerves. Cell Tissue Res. 2002;308:61–73. doi: 10.1007/s00441-002-0521-3. [DOI] [PubMed] [Google Scholar]
- Chávez-Genaro R, Lombide P, Anesetti G. A quantitative study of rat uterine sympathetic innervation during pregnancy and post partum. Reprod Fertil Dev. 2006;18:525–531. doi: 10.1071/rd05053. [DOI] [PubMed] [Google Scholar]
- Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin–neuropilin interactions underlying sympathetic axon responses to classIII sempahorin. Neuron. 1998;21:1283–1290. doi: 10.1016/s0896-6273(00)80648-0. [DOI] [PubMed] [Google Scholar]
- Clyde LA, Lechuga TJ, Ebner CA, Burns AE, Kirby MA, Yellon SM. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in rats. Biol Reprod. 2011;84:587–594. doi: 10.1095/biolreprod.110.086207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JJ, Wilson K, Fischer-Colbrie R, Papka RE. Distribution and origin of secretoneurin-immunoreactive nerves in the female rat uterus. Neuroscience. 2000;95:255–264. doi: 10.1016/s0306-4522(99)00396-6. [DOI] [PubMed] [Google Scholar]
- Corbacho A, Brauer MM, Pérez T. Development and maturation of noradrenaline-containing nerves of the rat uterine artery. Effects of acute and chronic oestrogn treatment. Int J Dev Neurosci. 1997;15:363–371. doi: 10.1016/s0736-5748(96)00084-6. [DOI] [PubMed] [Google Scholar]
- Cowen T. Selective vulnerability in adult and ageing mammalian neurons. Auton Neurosci. 2002;96:20–24. doi: 10.1016/s1566-0702(01)00376-9. [DOI] [PubMed] [Google Scholar]
- Crutcher KA. Tissue sections as culture substrates: overview and critique. Hippocampus. 1993;3:157–164. [PubMed] [Google Scholar]
- Czaja K, Kaleczyc J, Sienkiewicz W, Majewski M, Łakomy M. Peptidergic innervation of the porcine oviduct studied by double-labelling immunohistochemistry. Folia Histochem Cytobiol. 1996;34:141–50. [PubMed] [Google Scholar]
- Czaja K. Distribution of primary afferent neurons innervating the porcine oviduct and their immunohistochemical characterization. Cells Tissues Organs. 2000;166:275–82. doi: 10.1159/000016741. [DOI] [PubMed] [Google Scholar]
- Czaja K, Kaleczyc J, Pidsudko Z, Franke-Radowiecka A, Łakomy M. Distribution of efferent neurones innervating the oviduct in the pig. Folia Morphol. 2001a;60:243–248. [PubMed] [Google Scholar]
- Czaja K, Krzysztof Wsowicz K, Klimczuk M, Podlasz P, Łakomy M. Distribution and immunohistochemical characterisation of paracervical neurons innervating the oviduct in the pig. Folia Morphol. 2001b;60:205–211. [PubMed] [Google Scholar]
- Dangoor D, Giladi E, Fridkin M, Gozes I. Neuropeptide receptor transcripts are expressed in the rat clitoris and oscillate during the estrus cycle in the rat vagina. Peptides. 2005;26:2579–2584. doi: 10.1016/j.peptides.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Falck B, Owman CH, Rosengren E, Sjöberg E. Reduction by progesterone of the estrogen-induced increase in transmitter level of the short adrenergic neurons innervating the uterus. Endocrinology. 1969;84:958–959. doi: 10.1210/endo-84-4-958. [DOI] [PubMed] [Google Scholar]
- Fassold A, Falk W, Anders S, Hirsch T, Mirsky VM, Straub RH. Soluble neuropilin-2, a nerve repellent receptor, is increased in rheumatoid arthritis synovium and aggravates sympathetic fiber repulsion and arthritis. Arthritis Rheum. 2009;60:2892–2901. doi: 10.1002/art.24860. [DOI] [PubMed] [Google Scholar]
- Fassold A, Straub RH. A new assay for nerve fiber repulsion. Ann N Y Acad Sci. 2010;1193:43–47. doi: 10.1111/j.1749-6632.2009.05295.x. [DOI] [PubMed] [Google Scholar]
- Gardner RT, Habecker BA. Infarct-derived chondroitin sulfate proteoglycans prevent sympathetic reinnervation after cardiac ischemia-reperfusion injury. J Neurosci. 2013;33:7175–7183. doi: 10.1523/JNEUROSCI.5866-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatei MA, Gu J, Mulderry PK, Blank MA, Allen JM, Morrison JF, Polak JM, Bloom SR. Calcitonin gene-related peptide (CGRP) in the female rat urogenital tract. Peptides. 1985;6:809–815. doi: 10.1016/0196-9781(85)90306-7. [DOI] [PubMed] [Google Scholar]
- Gil OD, Zanazzi G, Struyk AF, Salzer JL. Neurotrimin mediates bifunctional effects on neurite outgrowth via homophilic and heterophilic interactions. J Neurosci. 1998;18:9312–9325. doi: 10.1523/JNEUROSCI.18-22-09312.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldi A, Persson K, Werkstrom V, Alm P, Wagner G, Andersson KE. Effects of diabetes on neurotransmission in rat vaginal smooth muscle. Int J Impot Res. 2001;13:58–66. doi: 10.1038/sj.ijir.3900648. [DOI] [PubMed] [Google Scholar]
- Giraldi A, Alm P, Werkstrom V, Myllymaki L, Wagner G, Andersson KE. Morphological and functional characterization of a rat vaginal smooth muscle sphincter. Int J Impot Res. 2002;14:271–282. doi: 10.1038/sj.ijir.3900886. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Rampin O, Allard J. Neurophysiology and pharmacology of female genital sexual response. J Sex Marital Ther. 2002;28(Suppl 1):101–121. doi: 10.1080/00926230252851230. [DOI] [PubMed] [Google Scholar]
- Gnanamanickam GJ, Llewellyn-Smith IJ. Innervation of the rat uterus at estrus: a study in full-thickness, immunoperoxidase-stained whole-mount preparations. J Comp Neurol. 2011;519:621–643. doi: 10.1002/cne.22515. [DOI] [PubMed] [Google Scholar]
- Griebling T, Liao Z, Smith PG. Systemic and topical hormone therapies reduce vaginal innervation density in post-menopausal women. Menopause. 2012;19:630–635. doi: 10.1097/gme.0b013e31823b8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald IC, Klein R. Axon guidance: receptor complexes and signaling mechanisms. Curr Opin Neurobiol. 2002;12:250–259. doi: 10.1016/s0959-4388(02)00323-9. [DOI] [PubMed] [Google Scholar]
- Gunn JA, Franklin KJ. The sympathetic innervation of the vagina. Proceediings of the Royal Society of London Series B. 1922;94:197–203. [Google Scholar]
- Haase EB, Buchman J, Tietz AE, Chramm LP. Pregnancy-induced uterine neuronal degeneration in the rat. Cell Tissue Res. 1997;288:293–306. doi: 10.1007/s004410050815. [DOI] [PubMed] [Google Scholar]
- Hammarström M, Sjöstrand NO. Acetylcholinesterase positive nerves in the guinea-pig endometrium. Acta Physiol Scand. 1980;108:429–431. doi: 10.1111/j.1748-1716.1980.tb06555.x. [DOI] [PubMed] [Google Scholar]
- Hasan W, Smith PG. Nerve growth factor expression in parasympathetic neurons: regulation by sympathetic innervation. Eur J Neurosci. 2000;12:4391–4397. doi: 10.1046/j.0953-816x.2000.01353.x. [DOI] [PubMed] [Google Scholar]
- Hasan W, Smith HJ, Ting AY, Smith PG. Estrogen alters TrkA and p75 neurotrophin receptor expression within sympathetic neurons. J Neurobiol. 2005;65:192–204. doi: 10.1002/neu.20183. [DOI] [PubMed] [Google Scholar]
- Hasan W. Autonomic cardiac innervation: development and adult plasticity. Organogenesis. 2013;9:176–193. doi: 10.4161/org.24892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser-Kronberger C, Cheung A, Hacker GW, Graf AH, Dietze O, Frick J. Peptidergic innervation of the human clitoris. Peptides. 1999;20:539–543. doi: 10.1016/s0196-9781(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Helm GH, Hakanson R, Leander S, Owman C, Sjöberg NO, Sporrong B. Neurogenic relaxation mediated by Vasoactive intestinal polypeptide (VIP) in the isthmus of the human fallopian tube. Regul Pept. 1982;3:145–153. doi: 10.1016/0167-0115(82)90091-x. [DOI] [PubMed] [Google Scholar]
- Hilliges M, Falconer C, Ekman-Ordeberg G, Johansson O. Innervation of the human vaginal mucosa as revealed by PGP 9.5 immunohistochemistry. Acta Anat (Basel) 1995;153:119–126. doi: 10.1159/000147722. [DOI] [PubMed] [Google Scholar]
- Houdeau E, Prud'homme MJ, Rousseau A, Rousseau JP. Distribution of noradrenergic neurons in the female rat pelvic plexus and involvement in the genital tract innervation. J Auton Nerv Syst. 1995;54:113–125. doi: 10.1016/0165-1838(95)00014-o. [DOI] [PubMed] [Google Scholar]
- Houdeau E, Rousseau A, Meusnier C, Prud'Homme MJ, Rousseau JP. Sympathetic of the upper and lower regions of the uterus and cervix in the rat have different origins and routes. J Comp Neurol. 1998a;399:403–412. [PubMed] [Google Scholar]
- Houdeau E, Prudhomme MJ, Rousseau JP. Regional difference in the distribution of vasoactive intestinal polypeptide-immunoreactive nerve fibres along the uterus and between myometrial muscle layers in the rat. Histochem J. 1998b;30:525–529. doi: 10.1023/a:1003299621127. [DOI] [PubMed] [Google Scholar]
- Houdeau E, Barranger E, Rossano B. Do sensory calcitonin gene-related peptide nerve fibres in the rat pelvic plexus supply autonomic neurons projecting to the uterus and cervix? Neurosci Lett. 2002;332:29–32. doi: 10.1016/s0304-3940(02)00907-2. [DOI] [PubMed] [Google Scholar]
- Houdeau E, Rossano B, Prud'homme MJ. Regional and muscle layer variations in cholinergic nerve control of the rat myometrium during the oestrous cycle. Auton Neurosci. 2003;104:1–9. doi: 10.1016/s1566-0702(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Huang WM, Gu J, Blank MA, Allen JM, Bloom SR, Polak JM. Peptide-immunoreactive nerves in the mammalian female genital tract. Histochem J. 1984;16:1297–1310. doi: 10.1007/BF01003727. [DOI] [PubMed] [Google Scholar]
- Ieda M, Fukuda K. New aspects for the treatment of cardiac diseases based in the diversity of functional controls on cardiac muscles: the regulatory mechanisms of cardiac innervation and their critical roles in cardiac performance. J Pharmacol Sci. 2009;109:348–353. doi: 10.1254/jphs.08r25fm. [DOI] [PubMed] [Google Scholar]
- Jana B, Lata M, Bulc M, Calka J. Long term estradiol-17β administration changes population of the dorsal root ganglia neurons innervating the ovary in the sexually mature gilts. Neuropeptides. 2012;46:157–65. doi: 10.1016/j.npep.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Jana B, Palus, Czarzasta J, Calka J. Long-term estradiol-17β administration changes the population of paracervical ganglion neurons supplying theovary in adult gilts. J Mol Neurosci. 2013;50:423–433. doi: 10.1007/s12031-012-9950-y. [DOI] [PubMed] [Google Scholar]
- Jankovik SM, Protic BA, Jankovic SV. Contractile effects of acetylcholine on isolated ampullar segment of the fallopian tubes. Pharmacol Res. 2004;49:31–35. doi: 10.1016/s1043-6618(03)00244-5. [DOI] [PubMed] [Google Scholar]
- Kannisto P, Ekblad E, Helm G, Sjöber NO, Stjenquist M, Sundler F, Walles B. Existence and coexistence of peptides in nerves of the mammalian ovary and oviduct demonstrated by immunocytochemistry. Histochemistry. 1986;86:25–34. doi: 10.1007/BF00492342. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kirby LS, Kirby MA, Warren JW, Tran LT, Yellon SM. Increased innervation and ripening of the prepartum murine cervix. J Soc Gynecol Investig. 2005;12:578–585. doi: 10.1016/j.jsgi.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Klukovits A, Gáspár R, Sántha P, Jancsó G, Falkay G. Functional and histochemical characterization of a uterine adrenergic denervation process in pregnant rats. Biol Reprod. 2002;67:1013–1017. doi: 10.1095/biolreprod.101.002287. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Yamada Y, Morioka S, Niiro E, Shigemitsu A, Ito F. Mechanism of pain generation for endometriosis-associated pelvic pain. Arch Gynecol Obstet. 2014;289:13–21. doi: 10.1007/s00404-013-3049-8. [DOI] [PubMed] [Google Scholar]
- Kohn J, Aloyz RS, Toma JG, Haal-Frendsch M, Miller FD. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci. 1999;19:5393–5408. doi: 10.1523/JNEUROSCI.19-13-05393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL. Semaphorin-mediated neuronal growth cone guidance. Prog Brain Res. 1998;117:115–132. doi: 10.1016/s0079-6123(08)64012-1. [DOI] [PubMed] [Google Scholar]
- Korsching S, Thoenen H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat, correlation with density of sympathetic innervation. Proc Natl Acad Sci (USA) 1983;80:3513–3616. doi: 10.1073/pnas.80.11.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszykowska M, Calka J, Ganko M, Jana B. Long-term estradiol-17beta administration reduces population of neurons in the sympathetic chain ganglia supplying the ovary in adult gilts. Exp Mol Pathol. 2011a;91:353–361. doi: 10.1016/j.yexmp.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Koszykowska M, Calka J, Szwajca P, Jana B. Long-term estradiol-17beta administration decreases the number of neurons in the caudal mesenteric ganglion innervating the ovary in sexually mature gilts. J Reprod Develop. 2011b;57:62–71. doi: 10.1262/jrd.10-061s. [DOI] [PubMed] [Google Scholar]
- Koszykowska M, Calka J, Nidzgorska A, Jana B. Exogenous long-term treatment with 17beta-oestradiol alters the innervation pattern in pig ovary. Reprod Fertil Develop. 2013;25:661–673. doi: 10.1071/RD11271. [DOI] [PubMed] [Google Scholar]
- Krizsan-Agbas D, Smith PG. Estrogen modulates myometrium-induced sympathetic neurite formation through actions on target and ganglion. Neuroscience. 2002;114:339–347. doi: 10.1016/s0306-4522(02)00262-2. [DOI] [PubMed] [Google Scholar]
- Krizsan-Agbas D, Pedchenko T, Hassan W, Smith PG. Oestrogen regulates sympathetic neurite outgrowth by modulating brain-derived neurotrophic factor synthesis and release by the rodent uterus. Eur J Neurosci. 2003;18:2760–2768. doi: 10.1111/j.1460-9568.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- Krizsan-Agbas D, Pedchenko T, Smith PG. Neurotrimin is an estrogen-regulated determinant of peripheral sympathetic innervation. J Neurosci Res. 2008;86:3086–3095. doi: 10.1002/jnr.21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanogoh A, Kikutani H. Semaphorins and their receptors: novel features of neural guidance molecules. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:611–620. doi: 10.2183/pjab.86.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara HE, McDonald JK, Ahmed CE, Ojeda SR. Guanethidine-mediated destruction of ovarian sympathetic nerves disrupts ovarian development and function in rats. Endocrinology. 1990;127:2199–2209. doi: 10.1210/endo-127-5-2199. [DOI] [PubMed] [Google Scholar]
- Latini C, Frontini A, Morroni M, Marzioni D, Castellucci M, Smith PG. Remodeling of uterine innervation. Cell Tissue Res. 2008;334:1–6. doi: 10.1007/s00441-008-0657-x. [DOI] [PubMed] [Google Scholar]
- Laurikainen A, Hiltunen JO, Thomas-Crusells J, Vanhatalo S, Arumäe U, Airaksinen MS, Klinge E, Saarma M. Neurturin is a neurotrophic factor for penile parasympathetic neurons in adult rat. J Neurobiol. 2000;43:198–205. doi: 10.1002/(sici)1097-4695(200005)43:2<198::aid-neu9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Liao Z, Smith PG. Adaptive Plasticity of Vaginal Innervation in Term Pregnant. Rats Reprod Sci. 2011;18:1237–1245. doi: 10.1177/1933719111410706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobos E, Gebhardt C, Kluge A, Spanel-Borowski K. Expression of nerve growth factor (NGF) isoforms in the rat uterus during pregnancy: accumulation of precursor proNGF. Endocrinology. 2005;146:1922–1929. doi: 10.1210/en.2004-0925. [DOI] [PubMed] [Google Scholar]
- Lodge AP, McNamee CJ, Howard MR, Reed JE, Moss DJ. Identification and characterization of CEPU-Se-A secreted isoform of the IgLON family protein, CEPU-1. Mol Cell Neurosci. 2001;17:746–760. doi: 10.1006/mcne.2001.0964. [DOI] [PubMed] [Google Scholar]
- Lorentz CU, Parrish DC, Alston EN, Pellegrino MJ, Woodward WR, Hempstead BL, Habecker BA. Sympathetic denervation of peri-infarct myocardium requires the p75 neurotrophin receptor. Exp Neurol. 2013;249:111–119. doi: 10.1016/j.expneurol.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg LM, Alm P, Thorbert G. Local mechanical effects and humoral factors evoke degeneration of guinea pig uterine innervation. Acta Obstet Gynecol Scand. 1989;68:487–496. [PubMed] [Google Scholar]
- Mackay LB, Shi SQ, Garfield RE, Maner WL. The effect of bilateral pelvic neurectomy on uterine and abdominal electrical and pressure activity, as measured by telemetry in conscious, unrestrained pregnant rats. J Perinat Med. 2009;37:313–319. doi: 10.1515/JPM.2009.075. [DOI] [PubMed] [Google Scholar]
- Majdan M, Walsh GS, Aloyz R, Miller FD. TrkA mediates developmental sympathetic neuron survival in vivo by silencing an ongoing p75NTR-mediated death signal. J Cell Biol. 2001;155:1275–1285. doi: 10.1083/jcb.200110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski M, Sienkiewicz W, Kaleczyc J, Mayer B, Czaja K, Łakomy M. The distribution and co-localization of immunoreactivity to nitric oxide synthase, vasoactive intestinal polypeptide and substance P within nerve fibres supplying bovine and porcine femalegenital organs. Cell Tissue Res. 1995;28:445–464. doi: 10.1007/BF00417862. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Adrenergic innervation the female reproductive tract. Adrenergic innervation of the female reproductive tract: anatomy, physiology and pharmacology. Rev Physiol Biochem Pharmacol. 1970;62:5–67. doi: 10.1007/BFb0111421. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Effects of ovarian steroids and pregnancy on adrenergic nerves of uterus and oviduct. Am J Physiol. 1981;240:C165–174. doi: 10.1152/ajpcell.1981.240.5.C165. [DOI] [PubMed] [Google Scholar]
- Martínez G, Silveira P, Bianchimano P, Richeri A, Brauer MM. VI Jornadas de la Sociedad de Bioquímica y Biología Molecular. Montevideo, Uruguay: 2009. El estrógeno reduce la capacidad neuritogénica del sustrato uterino. Papel de la geometría del colágeno; p. 75. Abstract. [Google Scholar]
- Marzioni D, Tamagnone L, Capparuccia L, Marchini C, Amici A, Todros T, Bischof P, Neidhart S, Grenningloh G, Castellucci M. Restricted innervation of uterus and placenta during pregnancy: evidence for a role of the repelling signal semaphorin 3a. Dev Dyn. 2004;231:839–848. doi: 10.1002/dvdy.20178. [DOI] [PubMed] [Google Scholar]
- Mechsner S, Schwarz J, Thode J, Loddenkemper C, Salomon DS, Ebert AD. Growth-associated protein 43-positive sensory nerve fibers accompanied by immature vessels are located in or near peritoneal endometriotic lesions. Fertil Steril. 2007;88:581–587. doi: 10.1016/j.fertnstert.2006.12.087. [DOI] [PubMed] [Google Scholar]
- Mechsner S. Nerve repellent factors affect the inflammatory condition of endometriosis; 12th World Congress on Endometriosis; Sao Paulo. 2014. pp. S6–1. Abstract. [Google Scholar]
- Melo RC, Machado CR. Noradrenergic and acetylcholinesterase-positive nerve fibres of the uterus in sexually immature and cycling rats. Histochem J. 1993;25:213–218. doi: 10.1007/BF00163817. [DOI] [PubMed] [Google Scholar]
- Miller LE, Weidler C, Falk W, Angele P, Schaumburger J, Schölmerich J, Straub R. Increased prevalence of semaphorin 3 C, a repellent of sympathetic nerve fibers, in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:1156–1163. doi: 10.1002/art.20110. [DOI] [PubMed] [Google Scholar]
- Mione MC, Cavanagh JF, Lincoln J, Milner P, Burnstock G. Pregnancy reduces noradrenaline but not neuropeptide levels in the uterine artery of the guinea-pig. Cell Tissue Res. 1990;259:503–509. doi: 10.1007/BF01740777. [DOI] [PubMed] [Google Scholar]
- Mione MC, Gabella G. Nerve fibres in the uterine artery increase in number in pregnant guinea-pigs. Neuroreport. 1991;2:537–540. doi: 10.1097/00001756-199109000-00010. [DOI] [PubMed] [Google Scholar]
- Mione MC, Cavanagh JF, Burnstock G. Uptake of 5-hydroxydopamine into non-sympathetic nerves of guinea-pig uterine artery in late pregnancy. J Neurocytol. 1993;22:164–175. doi: 10.1007/BF01246355. [DOI] [PubMed] [Google Scholar]
- Moustafa F, Fatani JA, El-Eishi H, El-Badawi MG. Intrinsic innervation of the uterus in the guinea pig and rat. Acta Anat. 1987;129:53–58. doi: 10.1159/000146377. [DOI] [PubMed] [Google Scholar]
- Mowa CN, Usip S, Collins J, Storey-Workley M, Hargreaves KM, Papka RE. The effects of pregnancy and estrogen on the expression of calcitonin gene-related peptide (CGRP) in the uterine cervix, dorsal root ganglia and spinal cord. Peptides. 2003a;24:1163–1174. doi: 10.1016/j.peptides.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Mowa CN, Usip S, Storey-Workley M, Amann R, Papka R. Substance P in the uterine cervix, dorsal root ganglia and spinal cord during pregnancy and the effect of estrogen on SP synthesis. Peptides. 2003b;24:761–771. doi: 10.1016/s0196-9781(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Munarriz R, Kim SW, Kim NN, Traish A, Goldstein I. A review of the physiology and pharmacology of peripheral (vaginal and clitoral) female genital arousal in the animal model. J Urol. 2003;170:S40–44. doi: 10.1097/01.ju.0000075352.03144.15. [DOI] [PubMed] [Google Scholar]
- Myers JP, Santiago-Medina M, Gomez TM. Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions. Dev Neurobiol. 2011;71:901–923. doi: 10.1002/dneu.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance DM, Burns J, Klein C, Burden HW. Afferent fibers in the reproductive system and pelvic viscera of female rats; Anterograde tracing and immunohystochemical studies. Brain Res Bull. 1988;21:701–709. doi: 10.1016/0361-9230(88)90211-0. [DOI] [PubMed] [Google Scholar]
- Nangle MR, Keast JR. Semaphorin 3A inhibits growth of adult sympathetic and parasympathetic neurons via distinct cyclic nucleotide signalling pathways. Br J Pharmacol. 2011;162:1083–1095. doi: 10.1111/j.1476-5381.2010.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naska S, Lin DC, Miller FD, Kaplan DR. p75NTR is an obligate signalling receptor required for cues that cause sympathetic neuron growth cone collapse. Mol Cell Neurosci. 2010;45:108–120. doi: 10.1016/j.mcn.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Ortega-Villalobos M, García-Bazán M, Solano-Flores LP, Ninomiya-Alarcón JG, Guevara-Guzmán R, Wayner MJ. Vagus nerve afferent and efferent innervation of the rat uterus: an electrophysiological and HRP study. Brain Res Bull. 1990;25:365–371. doi: 10.1016/0361-9230(90)90221-k. [DOI] [PubMed] [Google Scholar]
- Owman C, Rosengren E, Sjöberg NO. Adrenergic innervation of the human female reproductive organs: a histochemical and chemical investigation. Obstet Gynecol. 1967;30:763–773. [PubMed] [Google Scholar]
- Owman C, Alm P, Rosengren E, Sjöberg N, Thorbert G. Variations in the level of uterine norepinephrine during pregnancy in the guinea pig. Am J Obstet Gynecol. 1976;122:961–964. doi: 10.1016/0002-9378(75)90356-7. [DOI] [PubMed] [Google Scholar]
- Owman C. Pregnancy induces degenerative and regenerative changes in the autonomic innervation of the female reproductive tract. Ciba Found Symp. 1981;83:252–279. doi: 10.1002/9780470720653.ch13. [DOI] [PubMed] [Google Scholar]
- Owman C, Helm G, Sjöberg NO, Walles B. Autonomic neuromuscular mechanisms in the Fallopian tube. In: Siegler AM, Anasari AH, editors. The Fallopian Tube: Basic Studies and Clinical Contributions. Mount Kisko, New York: Futura Publishing Company; 1986a. pp. 37–53. [Google Scholar]
- Owman C, Stjernquist M, Helm G, Kannisto P, Sjöberg NO, Sundler F. Comparative histochemical distribution of nerve fibres storing noradrenaline and neuropeptide Y (NPY) in human ovary, fallopian tube and uterus. Med Biol. 1986b;64:57–65. [PubMed] [Google Scholar]
- Owman C, Stjernquist M. Origin, distribution and functional aspects of aminergic and peptidergic nerves in the male and female genital tracts. In: Björklund A, Hökfelt T, Owman C, editors. Handbook of Chemical Neuroanatomy. Elsevier Science Publishers; 1988. pp. 445–544. [Google Scholar]
- Papka RE, Cotton JP, Traurig HH. Comparative distribution of neuropeptide tyrosine-, vasoactive intestinal polypeptide-, substance P-immunoreactive, acetylcholinesterase-positive and noradrenergic nerves in the reproductive tract of the female rat. Cell Tissue Res. 1985;242:475–490. doi: 10.1007/BF00225412. [DOI] [PubMed] [Google Scholar]
- Papka RE, McNeill DL. Distribution of NADPH-diaphorase-positive nerves in the uterine cervix and neurons in dorsal root and paracervical ganglia of the female rat. Neurosci Lett. 1992;147:224–228. doi: 10.1016/0304-3940(92)90601-3. [DOI] [PubMed] [Google Scholar]
- Papka RE, Traurig HH. Autonomic and visceral sensory innervation of the female reproductive system: special reference to neurochemical markers in nerves and ganglionic connections. In: Magi CA, editor. Nervous Control of the Urogenital System. Harwood Academic Publishers; Switzerland: 1993. pp. 423–436. [Google Scholar]
- Papka RE, McCurdy JR, Williams SJ, Mayer B, Marson L, Platt KB. Parasympathetic preganglionic neurons in the spinal cord involved in uterine innervation are cholinergic and nitric oxide-containing. Anat Rec. 1995a;241:554–562. doi: 10.1002/ar.1092410413. [DOI] [PubMed] [Google Scholar]
- Papka RE, McNeill DL, Thompson D, Schmidt HH. Nitric oxide nerves in the uterus are parasympathetic, sensory, and contain neuropeptides. Cell Tissue Res. 1995b;279:339–349. doi: 10.1007/BF00318490. [DOI] [PubMed] [Google Scholar]
- Papka RE, Srinivasan B, Miller KE, Hayashi S. Localization of estrogen receptor protein and estrogen receptor messenger RNA in peripheral autonomic and sensory neurons. Neuroscience. 1997;79:1153–1163. doi: 10.1016/s0306-4522(97)00076-6. [DOI] [PubMed] [Google Scholar]
- Papka RE, Traurig HH, Schemann M, Collins J, Copelin T, Wilson K. Cholinergic neurons of the pelvic autonomic ganglia and uterus of the female rat: distribution of axons and presence of muscarinic receptors. Cell Tissue Res. 1999;296:293–305. doi: 10.1007/s004410051290. [DOI] [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304:193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- Papka RE, Mowa CN. Estrogen receptors in the spinal cord, sensory ganglia, and pelvic autonomic ganglia. Int Rev Cytol. 2003;231:91–127. doi: 10.1016/s0074-7696(03)31003-4. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Verhaagen J. Emerging roles for semaphorins in neural regeneration. Brain Res Brain Res Rev. 2001;35:36–54. doi: 10.1016/s0165-0173(00)00050-3. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ. Getting neural circuits into shape with semaphorins. Nat Rev Neurosci. 2012;13:605–618. doi: 10.1038/nrn3302. [DOI] [PubMed] [Google Scholar]
- Pauls R, Mutema G, Segal J, Silva WA, Kleeman S, Dryfhout Ma V, Karram M. A prospective study examining the anatomic distribution of nerve density in the human vagina. J Sex Med. 2006;3:979–987. doi: 10.1111/j.1743-6109.2006.00325.x. [DOI] [PubMed] [Google Scholar]
- Pessina MA, Hoyt RF, Jr, Goldstein I, Traish AM. Differential Effects of Estradiol, Progesterone, and Testosterone on Vaginal Structural Integrity. Endocrinology. 2006;147:61–69. doi: 10.1210/en.2005-0870. [DOI] [PubMed] [Google Scholar]
- Polak JM, Bloom SR. Localisation and measurement of VIP in the genitourinary system of man and animals. Peptides. 1984;5:225–230. doi: 10.1016/0196-9781(84)90211-0. [DOI] [PubMed] [Google Scholar]
- Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10:88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Richeri A, Viettro L, Chávez-Genaro R, Burnstock G, Cowen T, Brauer MM. Effects of infantile/prepubertal chronic estrogen treatment and chemical sympathectomy with guanethidine on developing cholinergic nerves of the rat uterus. J Histochem Cytochem. 2002;50:839–850. doi: 10.1177/002215540205000610. [DOI] [PubMed] [Google Scholar]
- Richeri A, Bianchimano P, Mármol NM, Viettro L, Cowen T, Brauer MM. Plasticity in rat uterine sympathetic nerves: the role of TrkA and p75 nerve growth factor receptors. J Anat. 2005;207:125–134. doi: 10.1111/j.1469-7580.2005.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeri A, Bianchimano P, Crutcher KA, Brauer MM. Reduced sympatheticneurite outgrowth on uterine tissue sections from rats treated with estrogen. Cell Tissue Res. 2010;340:287–301. doi: 10.1007/s00441-010-0956-x. [DOI] [PubMed] [Google Scholar]
- Richeri A, Chalar C, Martínez G, Grief G, Bianchimano P, Brauer MM. Estrogen up-regulation of semaphorin 3F correlates with sympathetic denervation ofthe rat uterus. Auton Neurosci. 2011;164:43–50. doi: 10.1016/j.autneu.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Ricu M, Paredes A, Greiner M, Ojeda SR, Lara HE. Functional development of the ovarian noradrenergic innervation. Endocrinology. 2008;149:50–56. doi: 10.1210/en.2007-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CL, Lisanti MP, Salzer JL. Expression of a unique sets of GPI-linked proteins by different primary neurons in vitro. J Cell Biol. 1992;117:617–627. doi: 10.1083/jcb.117.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL, Rosen CL, Struyk AF. GPI anchored proteins in neural cell adhesion. In: Colman DR, editor. Cell adhesion, Vol 16, Advances in molecular and cellular biology. Greenwich, CT: Jai; 1996. pp. 193–222. [Google Scholar]
- Selfridge J, Krizsan-Agbas D, Smith PG. p75NTR is necessary for estrogen-induced remodeling of sympathetic nerves in the uterus. Society for Neuroscience 2010 [Google Scholar]
- Shelton DL, Reichard LF. Expression of the beta-nerve growth factor gene correlates with the density of sympathetic innervation in effector organs. Proc Natl Acad Sci. 1984;81:7951–7955. doi: 10.1073/pnas.81.24.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Keefe J, Nishi R. Differential effects of RET and TRKB on axonal branching and survival of parasympathetic neurons. Dev Neurobiol. 2013;73:45–59. doi: 10.1002/dneu.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg NO. Increase in transmitter content of adrenergic nerves in the reproductive tract of female rabbits after oestrogen treatment. Acta Endocrinol (Copenh) 1968;57:405–413. [PubMed] [Google Scholar]
- Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol. 2012;846:1–12. doi: 10.1007/978-1-61779-536-7_1. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor-Zarate R, Dorfman M, Paredes A, Lara HE. Neonatal exposure to estradiol valerate programs ovarian sympathetic innervation and follicular development in the adult rat. Biol Reprod. 2008;78:673–680. doi: 10.1095/biolreprod.107.063974. [DOI] [PubMed] [Google Scholar]
- Sporrong B, Alm P, Owman C, Sjoberg NO, Thorbert G. Ultrastructural evidence for adrenergic nerve degeneration in the guinea pig uterus during pregnancy. Cell Tissue Res. 1978;195:189. doi: 10.1007/BF00233686. [DOI] [PubMed] [Google Scholar]
- Sporrong B, Alm P, Owman C, Sjöberg NO, Thorbert G. Pregnancy is associated with extensive adrenergic nerve degeneration in the uterus. An electronmicroscopic study in the guinea-pig. Neuroscience. 1981;6:1119–1126. doi: 10.1016/0306-4522(81)90076-2. [DOI] [PubMed] [Google Scholar]
- Stjernquist M, Alm P, Ekman R, Owman C, Sjöberg NO, Sundler F. Levels of neural vasoactive intestinal polypeptide in rat uterus are markedly changed in association with pregnancy as shown by immunocytochemistry and radioimmunoassay. Biol Reprod. 1985;33:157–163. doi: 10.1095/biolreprod33.1.157. [DOI] [PubMed] [Google Scholar]
- Struyk AF, Canoll PD, Wolfgang MJ, Rosen CL, D'Eustachio P, Salzer JL. Cloning of neurotrimin defines a new subfamily of differentially expressed neural cell adhesion molecules. J Neurosci. 1995;15:2141–2156. doi: 10.1523/JNEUROSCI.15-03-02141.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorbert G, Alm P, Owman C, Sjöberg NO. Regional distribution of autonomic nerves in guinea pig uterus. Am J Physiol. 1978;233:C25–34. doi: 10.1152/ajpcell.1977.233.1.C25. [DOI] [PubMed] [Google Scholar]
- Ting AY, Blacklock AD, Smith PG. Estrogen regulates vaginal sensory and autonomic nerve density in the rat. Biol Reprod. 2004;71:1397–1404. doi: 10.1095/biolreprod.104.030023. [DOI] [PubMed] [Google Scholar]
- Tokushige N, Markham R, Russell P, Fraser IS. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Hum Reprod. 2006;21:782–787. doi: 10.1093/humrep/dei368. [DOI] [PubMed] [Google Scholar]
- Tokushige N, Markham R, Russell P, Fraser IS. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertil Steril. 2007;88:795–803. doi: 10.1016/j.fertnstert.2006.12.078. [DOI] [PubMed] [Google Scholar]
- Traurig HH, Papka RE. Autonomic efferent and visceral sensory innervation of the female reproductive system: special reference to functional roles of nerves in reproductive organs. In: Magi CA, editor. Nervous Control of the Urogenital System. Harwood Academic Publishers; Switzerland: 1993. pp. 103–141. [Google Scholar]
- Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. Br J Dermatol. 2003;148:1021–1027. doi: 10.1046/j.1365-2133.2003.05308.x. [DOI] [PubMed] [Google Scholar]
- Vachon P, Simmerman N, Zahran AR, Carrier S. Increases in clitoral and vaginal blood flow following clitoral and pelvic plexus nerve stimulations in the female rat. Int J Impot Res. 2000;12:53–57. doi: 10.1038/sj.ijir.3900480. [DOI] [PubMed] [Google Scholar]
- Van Orden DE, Goodale DB, Baker HA, Farley DB, Bhatnagar RK. Uterine catecholamines and prostaglandins during the estrous cycle of the rat. Endocrinology. 1980;106:1650–1654. doi: 10.1210/endo-106-5-1650. [DOI] [PubMed] [Google Scholar]
- Varol FG, Duchemin AM, Neff HN, Hadjiconstantinou M. Nerve growth factor (NGF) and NGF mRNA change in rat uterus during pregnancy. Neurosci Lett. 2000;294:58–62. doi: 10.1016/s0304-3940(00)01533-0. [DOI] [PubMed] [Google Scholar]
- Vilimas PI, Yuan SY, Haberberger RV, Gibbins IL. Sensory innervation of the external genital tract of female guinea pigs and mice. J Sex Med. 2011;8:1985–1995. doi: 10.1111/j.1743-6109.2011.02258.x. [DOI] [PubMed] [Google Scholar]
- Wang G, Tokushige N, Markham R, Fraser IS. Rich innervation of deep infiltrating endometriosis. Hum Reprod. 2009;24:827–834. doi: 10.1093/humrep/den464. [DOI] [PubMed] [Google Scholar]
- Wang H, Masironi B, Eriksson H, Sahlin L. A comparative study of estrogen receptors alpha and beta in the rat uterus. Biol Reprod. 1999;61:955–964. doi: 10.1095/biolreprod61.4.955. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Papka RE. Estrogen receptor-immunoreactive neurons are present in the female rat lumbosacral spinal cord. J Neurosci Res. 1996;46:492–501. doi: 10.1002/(SICI)1097-4547(19961115)46:4<492::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Chung K, Om AS, Papka RE. Cytosolic estrogen receptor concentrations in the lumbosacral spinal cord fluctuate during the estrous cycle. Life Sci. 1997;61:2551–2559. doi: 10.1016/s0024-3205(97)01009-6. [DOI] [PubMed] [Google Scholar]
- Wrobel KH, Kujat R. The bovine tubouterine junction: general innervation pattern and distribution of adrenergic, cholinergic, and peptidergic nerve fibers. Cell Tissue Res. 1993;274:493–501. doi: 10.1007/BF00314546. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Grisham LA, Rambau GM, Lechuga TJ, Kirby MA. Pregnancy-related changes in connections from the cervix to forebrain and hypothalamus in mice. Reproduction. 2010;140:155–164. doi: 10.1530/REP-10-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XF, Rush RA. Endogenous nerve growth factor is required for regulation of the low affinity neurotrophin receptor (p75) in sympathetic but not sensory ganglia. J Comp Neurol. 1996;372:37–48. doi: 10.1002/(SICI)1096-9861(19960812)372:1<37::AID-CNE4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Zhu L, Chen J, Sun Y, Huang X, Xu H, Zhang X. Loss of nerve fibers in the oviduct isthmus in women with hydrosalpinx. Acta Histochem. 2013;115:609–615. doi: 10.1016/j.acthis.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Zoubina EV, Fan Q, Smith PG. Variation in uterine innervation during the estrous cycle. J Comp Neurol. 1998;397:561–571. [PubMed] [Google Scholar]
- Zoubina EV, Smith PG. Axonal degeneration and regeneration in rat uterus during the estrous cycle. Autonom Neurosci. 2000;84:176–185. doi: 10.1016/S1566-0702(00)00209-5. [DOI] [PubMed] [Google Scholar]
- Zoubina EV, Mize AL, Alper RH, Smith PG. Acute and chronic estrogen supplementation decreases uterine sympathetic innervation in ovariectomized adult virgin rats. Histol Histopathol. 2001a;16:989–996. doi: 10.14670/HH-16.989. [DOI] [PubMed] [Google Scholar]
- Zoubina EV, Smith PG. Uterine sympathetic hyperinnervation in the estrogen receptor a knock-out mouse. Neuroscience. 2001b;103:237–244. doi: 10.1016/s0306-4522(00)00549-2. [DOI] [PubMed] [Google Scholar]
- Zoubina EV, Smith PG. Distributions of estrogen receptors alpha and beta in sympathetic neurons of female rats: enriched expression by uterine innervation. J Neurobiol. 2002;52:14–23. doi: 10.1002/neu.10064. [DOI] [PubMed] [Google Scholar]


