Summary
Aims
To examine the association between relative muscle mass (RMM) and nine risk factors for cardiovascular disease and diabetes (CVD/DM) in U.S. youth.
Methods
We used a sample representative of the U.S. population of youth, aged 8–20 years (NHANES 1999–2004). We compared the prevalence of adverse levels of nine CVD/DM risk factors between youths in the lowest quartile of RMM and their peers in the remaining quartiles, controlling for age, sex, and race/ethnicity. We also examined variations in the adjusted prevalence of these risk factors along the entire range of RMM.
Results
The adjusted prevalence of adverse levels of risk factors among youths in the lowest quartile of RMM was significantly higher for seven of the nine risk factors examined compared with their peers in the other quartiles. Over the entire range of RMM, the adjusted prevalence of adverse levels of each of these seven risk factors decreased gradually with increasing RMM values (all p for trend <0.001).
Conclusions
RMM and prevalence of adverse risk factors for CVD/DM are highly and inversely associated in U.S. youth. Among youth with low RMM, the risk of these chronic diseases could be significantly high later in life.
Keywords: Muscle mass, Fat mass, CVD/DM risk factors, NHANES
Introduction
An elevated prevalence of overweight among children and young adults is of great concern to the medical and public health communities because it presages an elevated prevalence of obesity-associated chronic conditions later in the population [1,2]. Numerous studies have identified typical risk factors for diabetes, atherosclerosis, heart disease and even sub-clinical signs of these chronic conditions in obese youth. Most of these studies have used the body mass index (BMI) as indicator of adiposity [3–8]. The use of BMI is widespread and there are age- and sex-specific BMI charts to assess overweight and obesity among children and youths in several populations [9]. BMI, however, reflects overall adiposity. It does not reflect body fat distribution or the relative contributions of fat mass and muscle mass to bodyweight, all of which could affect the health risks attributed to obesity in general [10,11]. Several studies have investigated the association between body fat distribution or body composition and disease risks in children and youth [12–15]. These studies have confirmed that central obesity and a preponderance of fat mass over muscle mass increase the risk of disease in this age group.
The National Health and Nutrition Examination Survey (NHANES, 1999–2004) offers the opportunity to examine in detail the association between body composition and health risks in children and adults [16]. This survey includes DXA whole body measurements for a wide age range of ages (8 and older) in a sample representative of the U.S. population. NHANES data have been used to generate reference values for body composition and a previous study with these data found that children and adolescents (aged 8–19 years) with a high percent body fat were more likely to have an adverse lipid profile than their peers with a low percent body fat [13].
The effect of low muscle mass enhancing the risk for insulin resistance and diabetes among adults has been recognized [17–19]. This is a dose-response effect: even a modest increase in muscle mass can diminish the risk [20,21]. However, it is not known whether this relation would be consistently found outside the adult population. We designed this study to test the association between low relative muscle mass (RMM) and a panel of nine measurements related to the risk for cardiovascular disease (CVD) and diabetes mellitus (DM) in U.S. youth, aged 8–20 years. We also examined the association between RMM and these risk factors along the entire range of RMM.
Methods
Survey
The sample was obtained from the NHANES, conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC) [16]. NHANES is a complex, multistage probability sample of the U.S. civilian non-institutionalized population. This survey includes data from interviews and physical and laboratory examinations. The period included in the study was 1999–2004, during which Non-Hispanic blacks, Mexican Americans, adolescents (aged 12–19 years), and people aged ≥60 years were oversampled. The survey was approved by NCHS Institutional Review Board. Written consent was obtained for participants aged ≥18 years, and parental consent was obtained for youths aged 7–17 years.
Study population
The study population was restricted to youths aged 8–20 years for the 6-year period selected. Of interest for this study was that the data for this period included body composition measurements performed with dual-energy X-ray absorptiometry (DXA) among individuals aged 8 years and older. Approximately 21% of NHANES participants for the period of this study had one or more DXA values missing. People with valid missing values included pregnant women, subjects who weighted over 300 pounds or were taller than 6 ft 5 in. The selected age group included 9751 individuals. The sample for analysis was reduced to 7321 individuals after excluding individuals other than non-Hispanic white, non-Hispanic black, or Mexican American (n = 807); and individuals with no measurement of DXA (n = 1623). Fasting values for LDL-C, triglycerides, glucose, and insulin were available in a subsample aged ≥12 years and fasted between8.5 and 23 h overnight. Thus, the sample size for measurements that required overnight fasting (morning sample) was reduced even further (range: 2273–2643).
DXA measurements
The elements of body composition used in this study were lean tissue mass without bone (muscle mass) and fat mass. The missing DXA data of eligible participants were imputed and five sets of DXA values were generated for analysis [16]. We performed each analysis five times, one for each set of imputed DXA values, to obtain the mean estimate for RMM and its adjusted standard error (SE) in the analysis as recommended [16].
Definition of adverse levels of risk factors and relative muscle mass
The panel of nine variables selected for this study, which were considered risk factors for CVD/DM, includes C-reactive protein (CRP), diastolic blood pressure (DBP), systolic blood pressure (SBP), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), serum triglycerides, plasma glucose, and insulin. LDL-C was derived from Friedewald’s equation LDL-C = (TC) − (HDL-C) − (Triglycerides/5) [22]. To define an adverse level of a risk factor, we divided our sample into three age groups (8–11, 12–15, and 16–20 years), and within each age group and sex we divided the distribution of each of the nine risk-defining variables into quartiles. We considered the individuals at the top quartile of each variable (bottom quartile for HDL-C) as being in the adverse risk category. Unlike the case of adults, among youths there are no thresholds for risk factors that reliably predict CVD/DM later in life. Cutoffs for metabolic and blood pressure risk in children are commonly based on their location along the percentile distribution of the variable of interest. This is done not much for clinical reasons but for epidemiological reasons: children tend to maintain their percentile ranking as they age [23,24].
We defined RMM as the percentage of muscle mass relative to the sum of muscle and fat mass (i.e., 100 × muscle mass (kg)/(muscle mass (kg) + fat mass (kg))), a measure of the contribution of relative muscle mass to body composition. This is a variation of a measure introduced recently [25]. To rank the subjects according to RMM we distributed them into quartiles. The cutoffs for these quartiles were, from lowest to highest, ≤64.2%, 64.3–70.9%, 71.0–77.4%, and ≥77.5%.
Statistical analyses
To obtain unbiased national estimates and proper standard errors (SE) of estimates due to the complex probability sample of NHANES, sample weights and the cluster design were considered in all analyses [16]. For analyses that required fasting values of risk factors (LDL-C, triglycerides, glucose, and insulin) we used the morning sample weights. For analyses involving the other risk factors, we used the examination sample weights. To compare the prevalence of individuals with adverse levels of each risk factor between the lowest quartile and the remaining quartiles of RMM, we used multiple logistic regressions controlling for age group, sex, and race/ethnicity. To further investigate the association between adverse levels of risk factors and RMM along the entire range of RMM, we also used multiple logistic regressions treating the four quartiles of RMM as an independent, continuous variable in the model. All data analyses were done in SAS version 9.3 using complex survey analysis procedures [26].
Results
To test for possible bias in our sample, we compared included and excluded individuals. The excluded group included more women than the included group (p < 0.001). This difference is probably because DXA measurements were not performed on women whose pregnancy status was positive or uncertain. Regarding the nine risk factors included in this study, there were statistically significant differences in the mean values of SBP, HDL-C, triglycerides, and fasting insulin between included and excluded (all p < 0.01); but the absolute differences were minor and probably have not clinical relevance.
Table 1 shows the percent distribution by age group, sex, and race/ethnicity and the mean value (±SE) of each variable of our panel of risk factors across quartiles of RMM in our study population. About 61% of this population was aged 15 years or younger. This percentage peaks at about 70% in the third quartile and reaches a minimum of about 53%in the upper quartile of RMM. Approximately 58%of our study population was male but this percentage varies from 31% in the first quartile to 95.2% in the top quartile of RMM. Regarding race/ethnicity, 70.2% of the study population was non-Hispanic white, 16.8% was non-Hispanic black, and the rest was Mexican American. The total sample size having DXA values was 7321 but the sample size used for the analyses of the nine risk factors ranged from4158 to 6674 for the non-fasting samples and from 2273 to 2643 for the fasting subsample. Table 1 shows that as RMM increases, the mean for most risk factors decreases (increases for HDL-C). The means for DBP and glucose change little across quartiles of RMM.
Table 1.
Percentage or mean (±SE) of basic demographic, haemodynamic and metabolic characteristics of U.S. youth aged 8–20 years by quartile of relative muscle mass (RMM), NHANES 1999–2004.
| Sample sizea | Overall | RMM (by quartiles) | ||||
|---|---|---|---|---|---|---|
| 1 ≤64.2 |
2 64.3–70.9 |
3 71.0–77.4 |
4 ≥77.5 |
|||
| Age group (col %) | ||||||
| 8–11 years | 1657 | 29.5 ± 1.0 | 31.2 ± 1.2 | 28.1 ± 1.4 | 41.0 ± 2.3 | 17.6 ± 1.4 |
| 12–15 years | 2696 | 31.2 ± 0.8 | 30.0 ± 1.6 | 31.8 ± 1.4 | 29.3 ± 1.5 | 35.6 ± 1.5 |
| 16–20 years | 2968 | 39.4 ± 1.3 | 40.9 ± 2.1 | 40.1 ± 2.0 | 29.7 ± 2.1 | 46.8 ± 1.8 |
| Sex (col %) | ||||||
| Male | 4316 | 57.9 ± 0.9 | 31.4 ± 2.0 | 41.5 ± 2.1 | 63.6 ± 1.7 | 95.2 ± 0.7 |
| Female | 3005 | 42.1 ± 0.9 | 68.6 ± 2.0 | 58.5 ± 2.1 | 36.4 ± 1.7 | 4.8 ± 0.7 |
| Race/Ethnicity (col %) | ||||||
| Whiteb | 1931 | 70.2 ± 2.1 | 67.6 ± 2.9 | 74.0 ± 2.4 | 73.3 ± 1.8 | 65.7 ± 2.3 |
| Blackb | 1685 | 16.8 ± 1.6 | 15.2 ± 1.6 | 13.1 ± 1.6 | 14.4 ± 1.5 | 24.4 ± 2.2 |
| Mexican-American | 2009 | 13.1 ± 1.6 | 17.2 ± 2.3 | 12.8 ± 1.8 | 12.3 ± 1.4 | 9.8 ± 1.4 |
| DBP (mmHg) | 6182 | 59.5 ± 0.3 | 60.0 ± 0.6 | 59.9 ± 0.6 | 58.1 ± 0.5 | 59.9 ± 0.5 |
| SBP (mmHg) | 6182 | 108.3 ± 0.3 | 109.5 ± 0.4 | 107.9 ± 0.5 | 106.3 ± 0.5 | 109.5 ± 0.5 |
| CRP (mg/dl) | 6674 | 0.17 ± 0.01 | 0.34 ± 0.02 | 0.14 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 |
| TC (mg/dl) | 6613 | 164.4 ± 0.8 | 171.9 ± 1.2 | 166.7 ± 1.3 | 162.4 ± 0.9 | 156.6 ± 1.1 |
| HDL-C (mg/dl) | 4158 | 48.9 ± 0.3 | 46.3 ± 0.6 | 48.1 ± 0.6 | 50.9 ± 0.5 | 50.1 ± 0.5 |
| LDL-C (mg/dl)c | 2643 | 94.8 ± 1.0 | 101.1 ± 1.9 | 96.5 ± 1.7 | 92.5 ± 1.5 | 89.1 ± 1.8 |
| Triglycerides (mg/dl)c | 2643 | 89.9 ± 1.9 | 104.8 ± 3.5 | 95.5 ± 3.2 | 82.1 ± 2.6 | 77.1 ± 1.8 |
| Glucose (mg/dl)c | 2282 | 91.5 ± 0.3 | 91.4 ± 0.6 | 90.0 ± 0.5 | 93.1 ± 1.0 | 92.0 ± 0.4 |
| Insulin (µU/ml)c | 2273 | 11.8 ± 0.3 | 17.4 ± 0.6 | 12.1 ± 0.4 | 10.2 ± 0.4 | 8.3 ± 0.2 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure; CRP, C-reactive protein; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Mean values and percentages were all weighted.
Unweighted.
Non-Hispanic.
Fasting conditions.
The cutoffs that we used to define adverse levels of risk factors are presented in Table 2. Overall, these cutoffs increased with age (decreased for HDL-C). Table 3 shows that, independently of age group, sex, and race/ethnicity, the prevalence of adverse levels for all seven risk factors (except for DBP and fasting glucose) is statistically significantly greater among youths in the lowest quartile of RMM than among their peers in the other quartiles. In the lowest quartile of RMM, the risk factor with the lowest prevalence was adverse DBP (31.0%) and the one with the highest prevalence was adverse CRP (51.5%). In contrast, the risk factor with the lowest prevalence in the rest of the quartiles was adverse fasting insulin (15.9%) and the one with the highest prevalence was adverse DBP (29.0%).
Table 2.
Cutoffs used to define abnormal levels of risk factors by age and sex. Each cutoff is the threshold between the third and fourth quartile (first and second quartile for adverse HDL-C levels).
| Sex | Risk factor | Age group | ||
|---|---|---|---|---|
| 8–11 years | 12–15 years | 16–20 years | ||
| Male | DBP (mmHg) | ≥61.8 | ≥65.2 | ≥71.4 |
| SBP (mmHg) | ≥108.1 | ≥115.5 | ≥122.1 | |
| CRP (mg/dl) | ≥0.107 | ≥0.097 | ≥0.137 | |
| TC (mg/dl) | ≥182.2 | ≥178.5 | ≥177.4 | |
| HDL-C (mg/dl) | <42.1 | <40.1 | <36.9 | |
| LDL-C (mg/dl)a | ≥107.9 | ≥110.9 | ||
| Triglycerides (mg/dl)a | ≥108.5 | ≥121.5 | ||
| Glucose (mg/dl)a | ≥97.9 | ≥97.2 | ||
| Insulin (µU/ml)a | ≥14.1 | ≥13.3 | ||
| Female | DBP (mmHg) | ≥62.9 | ≥67.9 | ≥69.6 |
| SBP (mmHg) | ≥107.2 | ≥111.2 | ≥113 | |
| CRP (mg/dl) | ≥0.115 | ≥0.088 | ≥0.313 | |
| TC (mg/dl) | ≥185.7 | ≥177.3 | ≥189.2 | |
| HDL-C (mg/dl) | <42.3 | <42.1 | <42.2 | |
| LDL-C (mg/dl)a | ≥104 | ≥113.5 | ||
| Triglycerides (mg/dl)a | ≥103.9 | ≥112.9 | ||
| Glucose (mg/dl)a | ≥93.8 | ≥92.2 | ||
| Insulin (µU/ml)a | ≥15.8 | ≥14 | ||
See list of abbreviations in footnote to Table 1.
Fasting subsample.
Table 3.
Adjusted prevalence (%) of youth aged 8–20 years with an adverse levela of a CVD/DM risk factor according to quartile of RMM: lowest (≤64.2%) and the rest (>64.2%), adjusted for age group, sex, and race/ethnicity; NHANES 1999–2004.
| Adverse level of CVD/DM risk factor | RMM | p value | |
|---|---|---|---|
| Lowest quartile ≤64.2% |
Other quartiles >64.2% |
||
| DBP | 31.0 | 29.0 | 0.421 |
| SBP | 38.1 | 23.6 | <0.001 |
| CRP | 51.5 | 16.6 | <0.001 |
| TC | 31.6 | 23.0 | <0.001 |
| HDL-C | 45.4 | 23.7 | <0.001 |
| LDL-Cb | 31.3 | 24.4 | 0.002 |
| Triglyceridesb | 43.2 | 24.4 | <0.001 |
| Glucoseb | 34.0 | 27.6 | 0.054 |
| Insulinb | 48.5 | 15.9 | <0.001 |
See list of abbreviations in footnote to Table 1.
Adverse level is defined as being in the upper quartile (lowest quartile for HDL-C) of the age group and sex specific distribution of the risk factor.
Fasting subsample.
Table 4 presents the adjusted odds ratio of having an adverse level of each risk factor for each quartile increase of RMM according to the fitted model. In this case, except for DBP and fasting glucose, there is a statistically significant reduction in the odds of having an adverse level for all risk factors for each step increase across quartiles of RMM. The largest reduction in odds occurs for fasting insulin: for each quartile above the bottom quartile of RMM there is a reduction of about 68% in the odds of having an adverse fasting level of this hormone.
Table 4.
Odds ratio (95% confidence interval, CI) of having an adverse levela of a risk factor for each quartile increase of relative muscle mass among youth, aged 8–20 years, controlling for age group, sex, and race/ethnicity; NHANES 1999–2004.
| Adverse level of CVD/DM risk factor | Odds ratio (95% CI) | p value for trend |
|---|---|---|
| DBP | 0.97 (0.89, 1.05) | 0.450 |
| SBP | 0.68 (0.64, 0.74) | <0.001 |
| CRP | 0.39 (0.36, 0.43) | <0.001 |
| TC | 0.74 (0.70, 0.79) | <0.001 |
| HDL-C | 0.55 (0.49, 0.61) | <0.001 |
| LDL-Cb | 0.67 (0.61, 0.75) | <0.001 |
| Triglyceridesb | 0.56 (0.49, 0.64) | <0.001 |
| Glucoseb | 0.93 (0.80, 1.07) | 0.294 |
| Insulinb | 0.32 (0.26, 0.40) | <0.001 |
See list of abbreviations in footnote to Table 1.
Adverse level of a risk factor is defined as being in the upper quartile (lowest quartile for HDL-C) of the age group and sex specific distribution of the risk factor.
Fasting subsample.
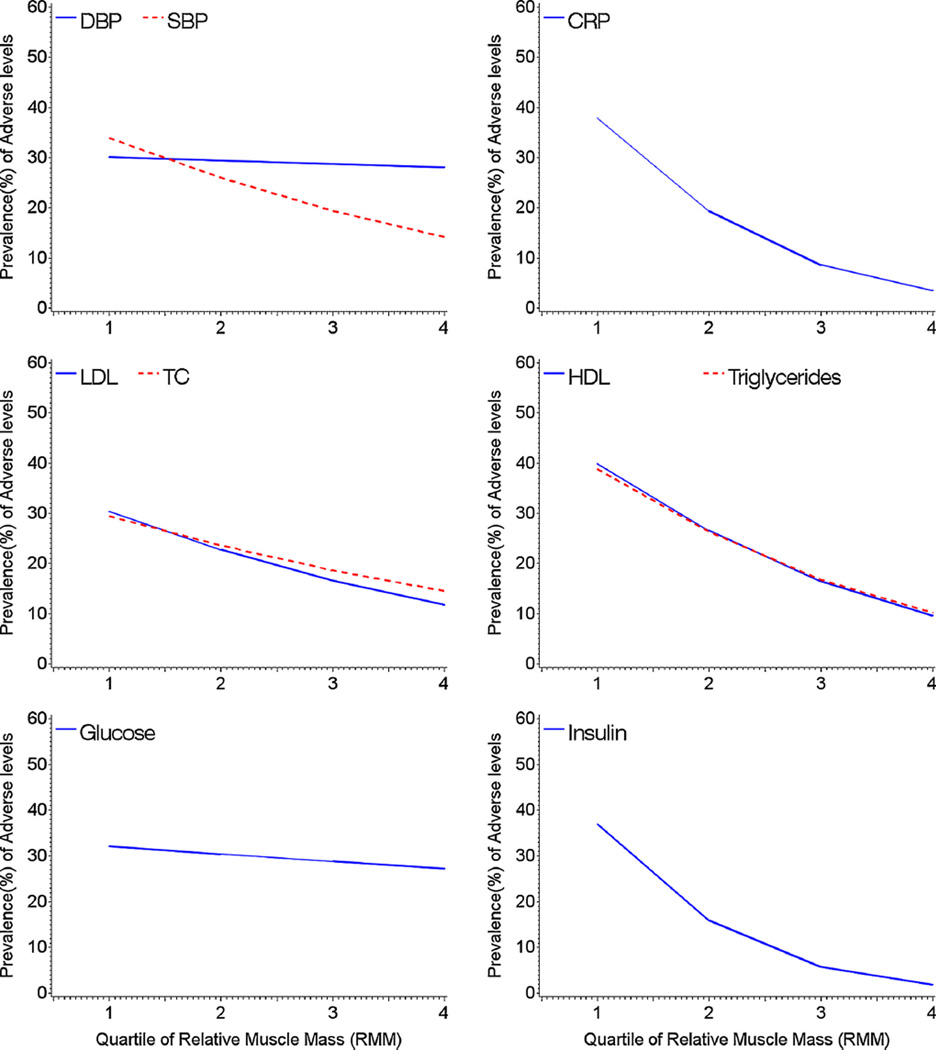
Fig. 1 illustrates the adjusted prevalence of nine adverse risk factors across quartiles of RMM. This figure clearly shows that in most cases the prevalence of an adverse risk factor steadily decreases as RMM increases. This decreasing trend in prevalence was particularly sharp for adverse levels of CRP and fasting insulin and almost non-existent for adverse levels of DBP.
Figure 1.
Adjusted prevalence (%) of nine adverse risk factors across quartiles of relative muscle mass (RMM), adjusting for age group, sex, and race/ethnicity. The quartiles of RMM were treated as a continuous variable with a range from1 to 4. Their cut-off values were 1: ≤64.2%; 2: 64.3–70.9%; 3: 71.0–77.4%; 4: ≥77.5%.
Discussion
Among U.S. youth, aged 8–20 years, we have examined and quantified the association between RMM and metabolic risk factors. First, our analyses show that for the nine CVD/DM risk factors included in this study, the prevalence of adverse levels among youths in the lowest quartile of RMM is higher than the prevalence among their peers in the other quartiles of RMM. In two cases (CRP and fasting insulin) the prevalence of adverse levels of risk factors is both about 3.1 times higher in the lowest quartile of RMM group than in the remaining quartiles combined. Second, our analysis shows that the inverse association between RMM and the prevalence of adverse levels of CVD and DM risk factors is graded. With the exception of adverse DBP and adverse fasting glucose, the odds of having an adverse level of any of the other seven risk factors gradually diminish as RMM increases. The reduction of the odds of having an adverse level of a risk factor for each full quartile increase of percentage RMM ranged from 32% for adverse SBP to 68% for adverse fasting insulin.
Results similar to ours have been reported in previous studies among adults. A recent study (n = 13,644 adults aged >20 years from the NHANESIII) found that a skeletal muscle index (SMI: muscle mass, measured with bioimpedance, relative to body weight) is inversely related to both insulin resistance and the risk of pre-diabetes [27]. A 10% increase of SMI was associated with an 11% reduction in the indicator of insulin resistance and a12% reduction in the prevalence of pre-diabetes. A separate study [17] with the same population has reported that sarcopenia (SMI more than two standard deviations below the sex specific mean in adults aged 18–39 years) was strongly associated with insulin resistance, particularly among the obese younger than 60 years. A study among Australian men (n = 1195 adults aged 35–81 years) concluded that high levels of fasting insulin, low muscle mass (measured with DXA scans) were independently associated with the presence of the metabolic syndrome [18], a clustering of individual risk factors for CVD/DM with not yet known pathophysiological mechanism [28].
Basically, our study shows that the graded, inverse association between percent muscle mass and metabolic risk observed in adults is also present with noticeable strength among youths aged20 years or younger. Our results, generated with reliable measurements of muscle mass and risk factors, are applicable to the U.S. population in this age range.
Population-based studies of body composition and risk for chronic conditions in children and young adults are scarce and they have emphasized the fat content over the muscle content as a risk factor for chronic conditions. A recent study involving two large samples of (n = 12,279 U.S. children and youth aged 6–18 years from NHANES) reported that percent body fat is strongly associated with the prevalence of individuals with adverse levels (5th quartile) of seven risk factors in boys and girls [12]. These results, however, are not generalizable to the U.S. population because the authors did not use sampling weights to compensate for the complex sampling scheme of the NHANES.
In summary, we have demonstrated that the association between RMM and the prevalence of risk factors for CVD/DM, proven to be strong among adults, is also strong among U.S. boys. A testable prediction from our study is that boys affected by diseases that compromise muscle mass, such as muscular dystrophy, could be at much higher risk for CVD/DM than their unaffected peers. For example, the average RMM measured by magnetic resonance in a small sample of 9 boys, aged 6–12, with Duchenne Muscular Dystrophy, was 41% (range: 11.5–66.7%) [29]. This average is well below the average for the lowest quartile of our sample, 58.4% (range: 41.3–64.2%).
Our study has several limitations: first, though DXA has been used as a precise tool measuring body composition, it is known than the lean soft tissue mass as measured by DXA is an overestimate of muscle mass [30]; second, the (weighted) T-test revealed that RMM of those who fasted was different (p < 0.01) from the RMM of those who did not. Thus, the results involving fasting values might not be generalizable; third, the cutoff points that we used to define adverse levels of risk factors were arbitrary (quartiles) and other cutoffs might show a different relationship with RMM. However, we tested other cutoffs, such as quintiles or tertiles, and they yielded similar results (data not shown); fourth, the observed relationship between low RMM and adverse risk factors might be mediated by fat mass. With our approach, we cannot separate one effect from the other, but the focus of our study was to examine the metabolic risks associated with low RMM. In our opinion, given the consistency of our study with the results reported in previous studies, these limitations do not invalidate our results.
In conclusion, our study has shown that, in the U.S. population, adverse levels of risk factors related to CVD/DM are more prevalent among youths, aged 8–20 years, with low RMM than among their peers with greater RMM.
Acknowledgement
We thank our colleagues and reviewers at CDC for their assistance and timely review.
Funding
No external funding supported this study.
References
- 1.World Health Organization. World Health Organization; 1998. Obesity, preventing and managing the global epidemic: report of a WHO consultation on obesity. WHO/NUT/NCD/98.1. [PubMed] [Google Scholar]
- 2.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101(3 (Pt2)):518–525. [PubMed] [Google Scholar]
- 3.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents – an IDF consensus report. Pediatr Diabetes. 2007;8(5):299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 4.Skinner AC, Mayer ML, Flower K, Perrin EM, Weinberger M. Using BMI to determine cardiovascular risk in childhood: how do the BMI cutoffs fare? Pediatrics. 2009;124(5):e905–e912. doi: 10.1542/peds.2009-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman DS, Fulton JE, Dietz WH, Pan L, Nihiser AJ, Srinivasan SR, et al. The identification of children with adverse risk factor levels by body mass index cutoffs from 2 classification systems: the Bogalusa Heart Study. Am J Clin Nutr. 2010;92(6):1298–1305. doi: 10.3945/ajcn.2010.29758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai S, Eissa MA, Steffen LM, Fulton JE, Harrist RB, Labarthe DR. Associations of BMI and its fat-free and fat components with blood lipids in children. Project Heart Beat! Clin Lipidol. 2011;6(2):235–244. doi: 10.2217/clp.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels SR. Complications of obesity in children and adolescents. Int J Obes. 2009;33(Suppl. 1):S60–S65. doi: 10.1038/ijo.2009.20. [DOI] [PubMed] [Google Scholar]
- 8.Cali AM, Caprio S. Obesity in children and adolescents. J Clin Endocrinol Metab. 2008;93(11) Suppl. 1:S31–S36. doi: 10.1210/jc.2008-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 10.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl. 4):S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 11.Freedman DS, Sherry B. The validity of BMI as an indicator of body fatness and risk among children. Pediatrics. 2009;124(Suppl. 1):S23–S34. doi: 10.1542/peds.2008-3586E. [DOI] [PubMed] [Google Scholar]
- 12.Going SB, Lohman TG, Cussler EC, Williams DP, Morrison JA, Horn PS. Percent body fat and chronic disease risk factors in U.S. children and youth. Am J Prev Med. 2011;41(4) Suppl. 2:S77–S86. doi: 10.1016/j.amepre.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Lamb MM, Ogden CL, Carroll MD, Lacher DA, Flegal KM. Association of body fat percentage with lipid concentrations in children and adolescents: United States, 1999–2004. Am J Clin Nutr. 2011;94(3):877–883. doi: 10.3945/ajcn.111.015776. [DOI] [PubMed] [Google Scholar]
- 14.Freedman DS, Kahn HS, Mei Z, Grummer-Strawn LM, Dietz WH, Srinivasan SR, et al. Relation of body mass index and waist-to-height ratio to cardiovascular disease risk factors in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr. 2007;86(1):33–40. doi: 10.1093/ajcn/86.1.33. [DOI] [PubMed] [Google Scholar]
- 15.Freedman DS, Katzmarzyk PT, Dietz WH, Srinivasan SR, Berenson GS. Relation of body mass index and skin-fold thicknesses to cardiovascular disease risk factors in children: the Bogalusa Heart Study. Am J Clin Nutr. 2009;90(1):210–216. doi: 10.3945/ajcn.2009.27525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; 2009. National Health and Nutrition Examination Survey. Available at: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. [Google Scholar]
- 17.Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS ONE. 2010;5(5):e10805. doi: 10.1371/journal.pone.0010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Members of the Florey Adelaide Male Ageing Study. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009;58(7):1013–1022. doi: 10.1016/j.metabol.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 19.Dominguez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J Cardiometab Syndr. 2007;2(3):183–189. doi: 10.1111/j.1559-4564.2007.06673.x. [DOI] [PubMed] [Google Scholar]
- 20.Walsh K. Adipokines, myokines and cardiovascular disease. Circ J. 2009;73:13–18. doi: 10.1253/circj.cj-08-0961. [DOI] [PubMed] [Google Scholar]
- 21.Izumiya Y, Hopkins T, Morris C, Sato K, Zeng L, et al. Fast/Glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 2008;7:159–172. doi: 10.1016/j.cmet.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 23.Daniels SR, Greer FR. Committee on nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 24.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl. 5):S213–S256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park BS, Yoon JS. Relative skeletal muscle mass is associated with development of metabolic syndrome. Diabetes Metab J. 2013;37(6):458–464. doi: 10.4093/dmj.2013.37.6.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Institute Inc SAS. SAS/STAT 9.3 User’s guide. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 27.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96(9):2898–2903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 28.Simmons RK, Alberti KG, Gale EA, Colagiuri S, Tuomilehto J, Qiao Q, et al. The metabolic syndrome: useful conceptor clinical tool? Report of a WHO Expert Consultation. Diabetologia. 2010;53(4):600–605. doi: 10.1007/s00125-009-1620-4. [DOI] [PubMed] [Google Scholar]
- 29.Pichiecchio A, Uggetti C, Egitto MG, Berardinelli A, Orcesi S, Gorni KO, et al. Quantitative MR evaluation of body composition in patients with Duchenne muscular dystrophy. Eur Radiol. 2002;12(11):2704–2709. doi: 10.1007/s00330-002-1392-4. [DOI] [PubMed] [Google Scholar]
- 30.Skalsky AJ, Han JJ, Abresch RT, Shin CS, McDonald CM. Assessment of regional body composition with dual-energy X-ray absorptiometry in Duchenne muscular dystrophy: correlation of regional lean mass and quantitative strength. Muscle Nerve. 2009;39(5):647–651. doi: 10.1002/mus.21212. [DOI] [PubMed] [Google Scholar]