Abstract
Objectives
Pancreatic cancer has a five year survival rate of less than 5%, partly due to limited chemotherapeutic options, thereby highlighting the need for novel therapies. Triptolide, a diterpene triepoxide derived from a Chinese herb has shown great promise in preclinical testing against pancreatic cancer using immune compromised animals.
Results
In this study, we tested the ability of triptolide to induce cell death in cell lines derived from a primary tumor and adjacent liver metastases of immuno-competent animals (KRasG12D; Trp52R172H; Pdx-1 Cre (KPC)). Both cell lines were more aggressive in their ability to form tumors when compared to other pancreatic cancer cell lines, and showed constitutive activation of the NFkB pathway. Triptolide induced apoptotic cell death in both cell lines, as evidenced by decreased cell viability and increased caspase 3/7 activity, Annexin V positivity, and increased TUNEL positivity in tumors from KPC animals treated with Minnelide. Additionally, triptolide decreased levels of HSP70, its transcription factor HSF1, and the anti-apoptotic proteins Bcl-xL, Bcl-2 and Mcl-1, known to be up-regulated in pancreatic cancer.
Conclusion
The ability of triptolide to cause cell death in cell lines derived from immune-competent animals further validates its potential as a novel agent against pancreatic cancer.
Keywords: Pancreatic Cancer, Genetically engineered mouse model, Triptolide, Cell death
Introduction
Pancreatic cancer is the fourth leading cause of cancer related deaths in the United States, with over 45,000 cases expected and over 38,000 succumbing to the disease in 2013. Survival five years after diagnosis is less than 5%, with only 15% of the patients eligible for surgical resection at presentation.1 Current chemotherapies, such as gemcitabine and erlotinib, have failed to have impact survival statistics, keeping the prognosis stable over the past 30.2,3 Novel therapies are therefore urgently needed against this deadly disease. We have identified triptolide, a diterpene triepoxide derived from the Chinese herb Triptoleum wilfordii, as an effective agent against pancreatic cancer using pancreatic cancer cell lines of varying aggressiveness.4,5 The clinical usefulness of triptolide is restricted by its low solubility in water. We have therefore designed a water-soluble prodrug of triptolide, named Minnelide, that has shown great promise in preclinical studies using immortalized pancreatic cancer cell lines in immunocompromised mouse models.4 In an immunocompetent environment, the genetically engineered mouse bearing the KRasG12D;Trp53R172H mutations expressed under the control of the Pdx-1 Cre promoter (KPC) mimics the progression of human disease, making it a relevant mouse model to study novel therapies.6 Recent studies have shown that gemcitabine monotherapy is ineffective in these animals. Additionally, desmoplastic stroma, present in both human and KPC tumors is believed to play an important role in chemoresistance.7 We have previously shown that Minnelide is able to retard tumor formation in these animals.4 However, the efficacy of triptolide has not been tested in tumor-bearing immunocompetent KPC animals. As a first step towards assessing the efficacy of triptolide in KPC animals, we have derived non-immortalized cell lines from the primary tumor and adjacent liver metastases of a KPC animal and compared them to other known pancreatic cancer cell lines. Triptolide causes apoptotic cell death in both cell lines tested and decreases levels of HSP70 and HSF1, as well as several anti-apoptotic proteins associated with cell survival and known to be over-expressed in pancreatic cancer.
Materials and Methods
Cell Lines
KRasG12D; Trp53R172H; Pdx-1 Cre animals were sacrificed and single cell suspensions of tumor were isolated by digestion with collagenase B and dispase II. Cells were plated in growth medium containing growth factors (EGF= 5ng/ml; Insulin = s5 µg/ml) and 2% serum for 48h, after which medium was replaced with serum-free medium. Cells were maintained for 2–3 weeks in the absence of serum until all fibroblasts were absent. Cells were then grown in DMEM with 10% serum for all experiments. One animal with a primary tumor and adjacent liver metastases was used to derive the KPC1 and Liver Metastasis (KPC1-LM) cell lines, and another animal bearing only a primary tumor was used to derive the KPC023 cell line. Triptolide and Minnelide were dissolved in DMSO and saline, respectively.
Cell viability assay
Cells were treated with 0–200 nM triptolide and cell viability determined using a WST-8 based assay (Dojindo Labs) at times indicated. Briefly, 10µL of tetrazolium substrate was added to each well and incubated for 1h at 37°C, after which absorbance at 450 nm measured. All treatments were done in triplicate and the data presented includes results from at least three independent replicates in each case.
Caspase assay
Caspase-3/7activity was analyzed using the Caspase-Glo luminescent-based assays (Promega) according to the manufacturer’s instructions. Briefly, cell were treated with triptolide at the times and concentrations indicated and appropriate Caspase-Glo reagent added to each well. Luminiscence was measured 45 mins after substrate addition. Caspase activity detected was normalized to the number of live cells present detected using the Dojindo cell viability kit.
Annexin V assay
Cells were seeded in a 6-well plate and treated with triptolide and Phosphatidylserine externalization was analyzed using the Guava Nexin Kit by flow cytometry, according to the manufacturer’s instructions.
Subcutaneous model
Cell lines were trypsinized, resuspended in PBS:Matrigel in a 1:1 ratio and injected into the flanks of BalbC nu/nu animals (NCI). KPC1, KPC023 or KPC1-LM (5× 104), AsPC-1, S2-013, S2-VP10 or MIA PaCa-2 (5×105) cells were used to form tumors. Animals were sacrificed on Day 21 post-injection and tumor weight and volume measured.
NF-κB activity assay
Activity of the p50 subunit of NF-κb was assessed using the Transcription Factor Assay kit (ThermoScientific) according to manufacturer’s instructions. Briefly, protein lysates from tumor samples were extracted in RIPA buffer. Protein concentration was measured using a BCA kit (Pierce). Luminiscence values obtained were normalized to protein concentration to obtain RLU/µg protein.
RNA analysis
Subcutaneous tumors were isolated from animals and RNA isolated using Trizol (invitrogen) following manufacturers’ instructions and mRNA expression analyzed by qPCR using the Applied Biosystems AB7300 real time PCR machine and SYBR (Qiagen). 18s expression was used to normalize results obtained.
Protein Analysis
Proteins were extracted in a 1% triton X-100 lysis buffer containing protease and phosphatase inhibitors. Anti-HSP70 (Abcam), Anti-HSF1 (Cell Signaling), Anti-Mcl-1, Bcl-2, Bcl-XL (Cell Signalling) were used to assess the effect of triptolide on protein levels. Anti-Actin (I-9, Santa Cruz) was used as a loading control.
Terminal Deoxynucleotidyl transferase-Mediated dUTP Nick End Labeling (TUNEL) Assays
KPC animals were treated with saline or Minnelide (0.42mg/kg) for seven days and tumors harvested. Tumors were fixed in formalin and embedded in paraffin. Sections from tissues were, treated with 3% H2O2 in PBS, and then permeabilized with 0.1% Triton X-100 in PBS for 2 min on ice. The TUNEL assay (Roche Molecular Biochemicals) was carried out following the manufacturer’s instruction.
Statistical Analysis
All in vitro experiments were performed a minimum of three times, and all in vivo experiments were performed on a minimum of n=3 animals for each group. Statistical significance was calculated using the Students t-test, and results were considered significant if p ≤ 0.05.
Results
KPC tumor-derived cell lines are more aggressive than human pancreatic cancer cell lines
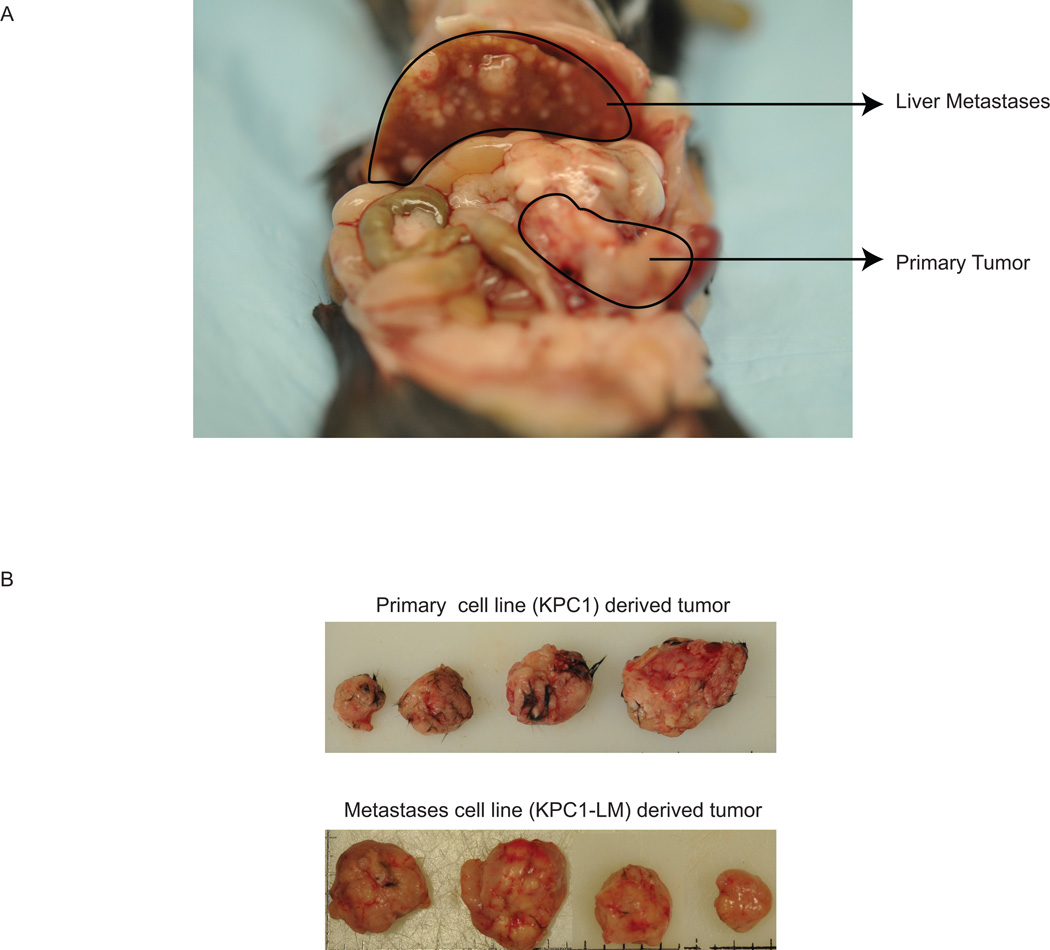
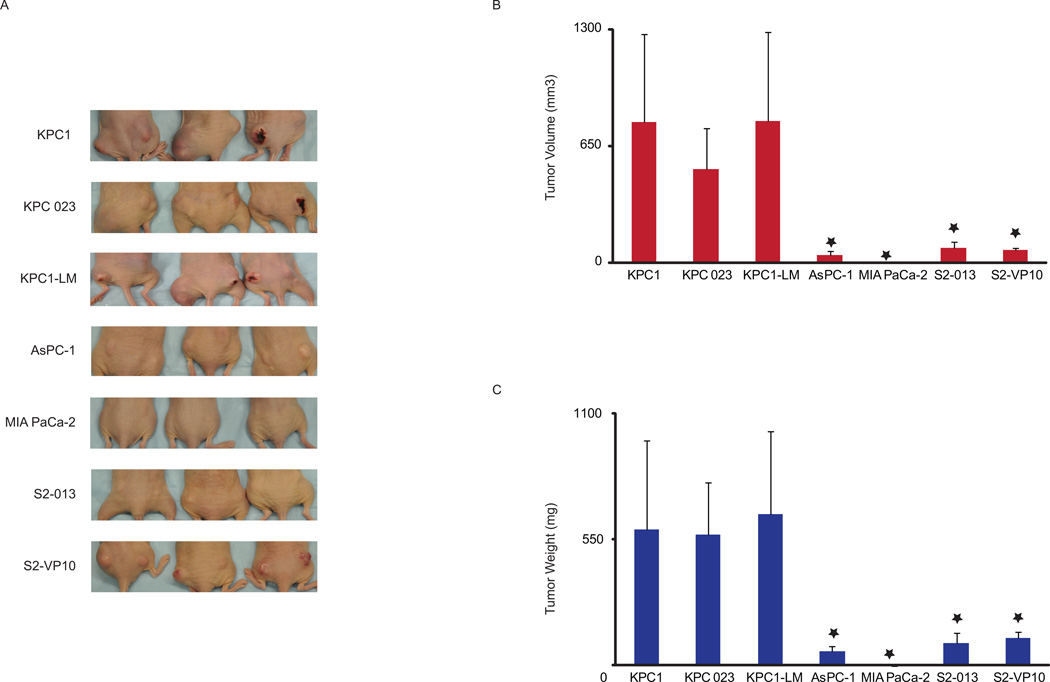
The KRasG12D mutation is sufficient to cause pancreatic tumors in animals. In the presence of a Trp53R172H mutation, tumors progress rapidly, as a result of which 80% of these animals develop micrometastasis.6 Cell lines were derived from KPC animals bearing either a primary tumor alone (KPC023) or a primary tumor with visible liver metastases (KPC1, KPC1-LM) (Fig. 1A). In order to test the ability of these cell lines to form tumors, 5 × 104 cells were injected into the flanks of C57BL/6 animals. All animals injected formed tumors within two weeks, suggesting that the cells derived from the animals maintain their tumorogenic potential. The experiment was terminated on day 28 due to the accelerated rate of growth of tumors (Fig 1B). Since tumor formation with the KPC1 and KPC1-LM lines was very rapid, we compared the aggressiveness of these cell lines with other known human cell lines routinely used to assess efficacy of novel chemotherapies. Ten-fold more of immortalized human pancreatic cancer cells (5 × 105) were injected into BalbC nu/nu animals to compare aggressiveness of these cells to KPC-derived cells (5× 104). The experiment was terminated 21 days after implanting the cells into the animals since KPC-derived tumors reached the permissible parameters (Fig. 2A). Intriguingly, whether the human cell lines were derived from a primary tumor (MIA PaCa-2), from a liver metastases (S2-VP10 and S2-013), or from ascites (AsPC-1), average tumor weight of the KPC cell lines was 569.2 +/− 228.65 mg to 661.93 +/− 362.27 mg compared to 117.16+/− 27.0 and 0 mg for the human cell lines (Fig. 2B). Correspondingly, tumor volume of the KPC lines was between 783.87+/− 490.5 mm3 to 522.65 +/− 225.61 mm3 whereas that from the human cell lines was 1.14 +/− 1.14 to 85.78 +/− 30.46 mm3 (Fig. 2C). If the ten-fold lower cell number for the KPC1 and KPC1-LM lines is taken into account, average tumor volume with these cell lines is 7838.7 to 5226.5 mm3. Our data therefore clearly show that KPC cell lines posses the ability to form tumors at a more rapid rate than other immortalized human cell lines.
Figure 1. Cell lines derived from both primary and liver metastasis form tumors in syngenic animals.
A. Pictorial representation of animal used to obtain tissue from which both the KPC1 and KPC1-LM cell lines were derived. B. KPC1 (top panel) and KPC1-LM (bottom panel) cells are able to form tumors in syngenic immune-competent animals. 5 × 104 cells were injected into the flank of C57 BLK6 animals and animals sacrificed 28 days post-injection.
Figure 2. Tumors derived from KPC1 and KPC1-LM cells show greater tumorigenicity compared to other pancreatic cancer cell lines.
A. Cells were injected into flanks of athymic nude animals and their ability to form tumors assessed. Animals were sacrificed 21 days after cell injection. B. Tumor volume, and C. Tumor weight of tumors derived from pancreatic cancer cells (KPC1, KPC1-LM, KPC023, MIA PaCa-2, AsPC-1, S2-013 and S2-VP10). Errors bars represent SEM. *= p ≤ 0.05 vs. KPC1
NFkB is constitutively activated in KPC tumor-derived and other pancreatic cancer cell lines
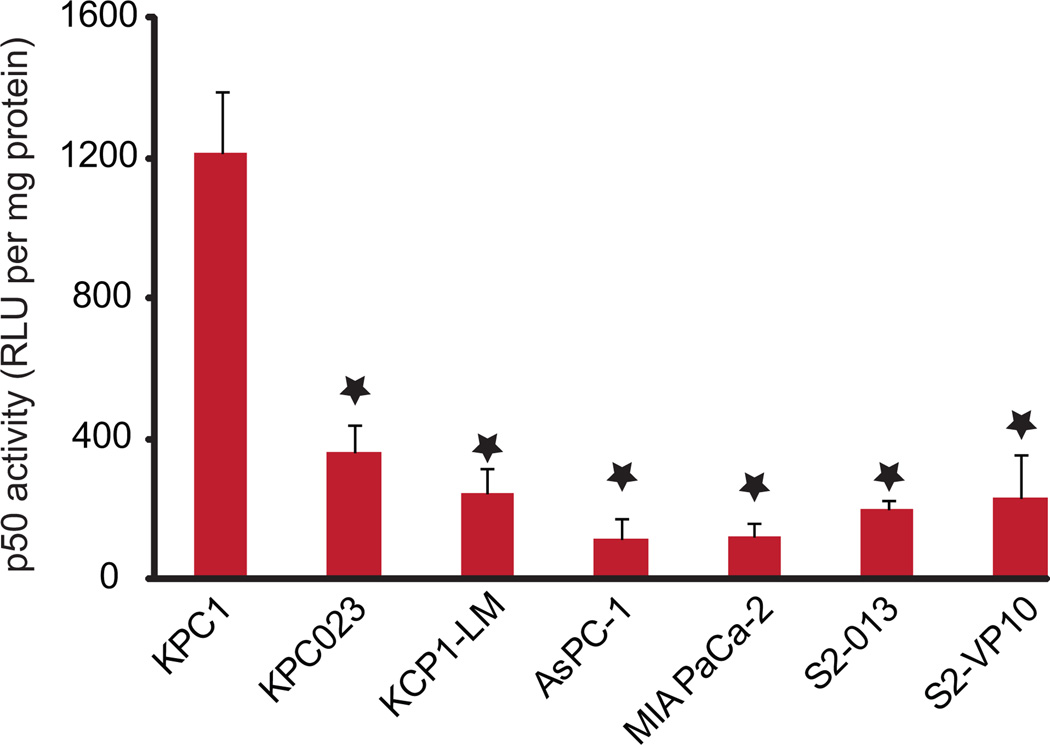
NFkB is the transcription factor for a diverse array of genes involved in normal stress response of a cell. NfkB regulated proteins include cytokines, chemokines and their associated receptors, as well as genes regulating cell survival, proliferation, and microenvironment.8 The pro-survival role of NfkB in cancer is attributed to its ability to regulate the expression of genes associated with anti-apoptotic, cyclins, cell adhesion and angiogenesis.9–11 Previous studies have shown that the NFκB pro-survival pathway is up-regulated in 70% of pancreatic tumors.12 Importantly, inhibition of NFκB activity by a mutant IκBα inhibited pancreatic tumor progression.13 Recently, using a KRas G12D; Ink4a/Arff/f; Ikk2/b F/F; Pdx-1 Cre pancreatic tumor mouse model. Ling et al. have shown that progression of pancreatic tumors requires IKK2 activity.14 To measure the effect NFκB activity in cell line derived tumors, we therefore used the ability of the active p50 NFκB subunit present in the tumor lysate to bind to the NFκB biotinylated-consensus sequence. The p50 activity varied from 104 RLU/µg protein in KPC1 derived tumors to 9.6 RLU/µg protein in AsPC-1 derived tumors. These data show that, in agreement with previously published work, NFκB was active in tumor lysates from all pancreatic cancer cell lines tested (Fig. 3).
Figure 3. NF-κB is constitutively active in pancreatic cancer cell-derived tumors.
Protein lysates from tumors of animals injected with pancreatic cancer cell lines indicated were assessed for the activity of the p50 subunit of NFkB and normalized to protein concentration. *= p ≤ 0.05 vs. KPC1
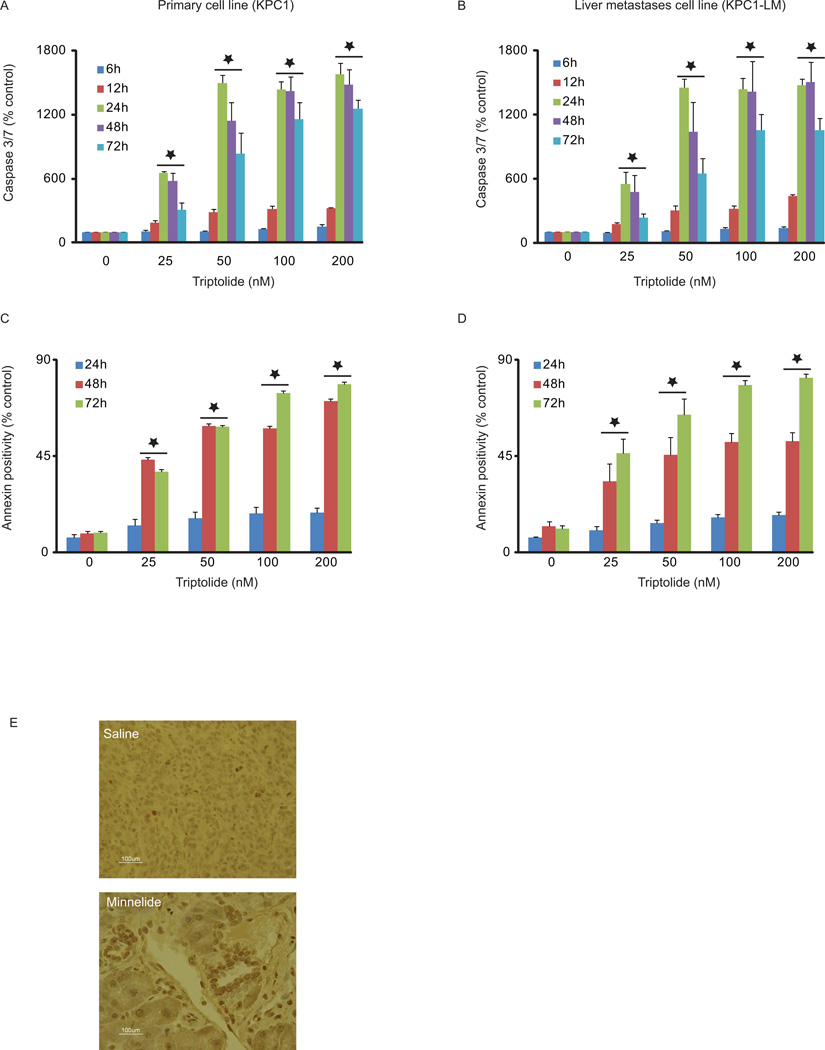
Triptolide causes apoptotic cell death in KPC tumor derived cell lines
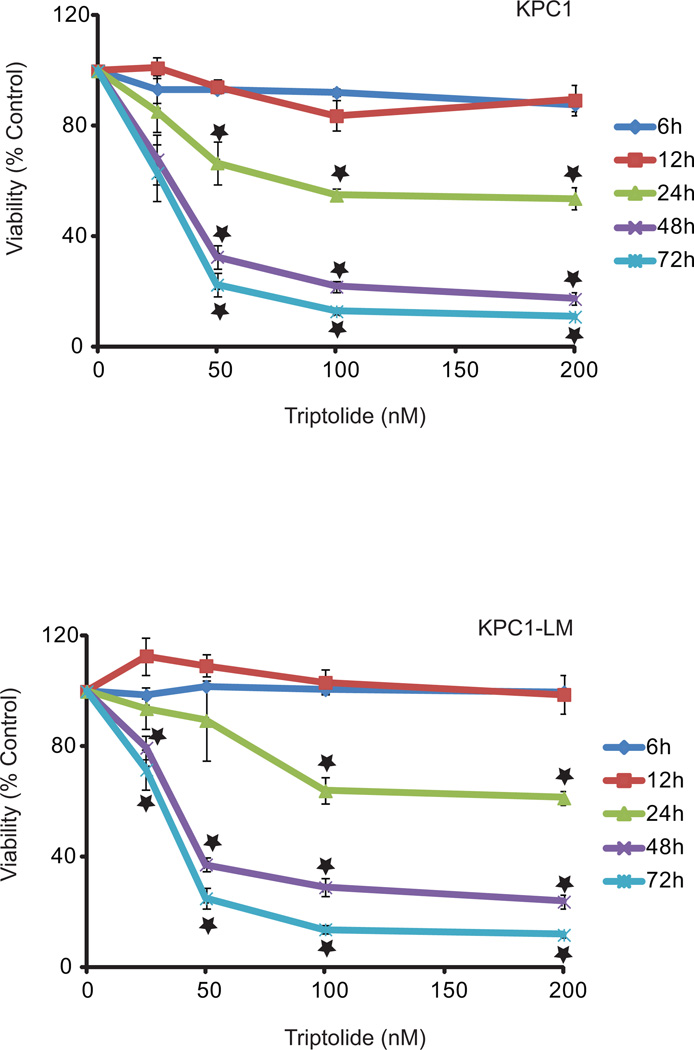
After establishing the ability of KPC cell lines to form tumors, and exhibit NFκB upregulation, we tested the effect of triptolide on these cell lines. Triptolide induced loss of cell viability in both KPC and KPC1-LM cell lines in a time and concentration-dependent manner, with 50 nM triptolide treatment leading to > 75% loss in cell viability 72 hours post-treatment (Fig. 4). We further investigated the mechanism of cell death induced by triptolide treatment. Increase in caspase-3 activation of nearly 1000% and 1100% seen 48 hours post treatment in KPC and KPC1-LM cells, respectively, suggesting that cell death occurs through the apoptotic pathway (Fig. 5A, B). We further investigated apoptotic cell death using Annexin V positivity as a marker for apoptosis. Annexin V positivity increased from 18.2% with 100 nM triptolide at 24 hours to 57.8% at 48 hours post-treatment of KPC cells. Similarly, KPC1-LM cells responded to triptolide by showing an increase from 16.28% 24 hours post triptolide treatment (100 nM) to 51.7% at 48 hours (Fig. 5C–D). In order to further assess the mechanism of cell death, TUNEL staining was performed tumors on tumors from KPC animals treated with either saline or Minnelide for 7 days. Minnelide treatment resulted in an increase in TUNEL positive cells compared to tumors from saline treated animals (Fig. 5E). These data, taken together, show that triptolide mediated cell death occurs via apoptosis.
Figure 4. Triptolide causes cell death in KPC tumor-derived cells.
Cells were treated with triptolide (0–200 nM) and viability assessed at 6, 12, 24, 48 and 72h. Both KPC1 and KPC1-LM cells show decreased cell viability in response to triptolide. *= p ≤ 0.05 vs. untreated controls.
Figure 5. KPC tumor derived cell lines undergo apoptotic cell death in response to triptolide.
A–D. Cells were treated with triptolide (0–200 nM) and caspase 3/7 activity and number of Annexin positive cells assessed at times indicated. A. and B. Activation of caspase 3/7 was assessed using Caspase-Glo and normalized to viability. C and D. Annexin positivity was assessed using flow cytometry. Data is represented as percent untreated cells. Both KPC1 and KPC1-LM cells show an increase in caspase activiation and Annexin positivity. Errors bars represent SEM. E. KPC animals were injected with either saline or Minnelide, the water soluble prodrug of triptolide for 7 days and tumors harvested. TUNEL staining on formalin fixed sections of the tumors show greater positivity in response to Minnelide treatment. *= p ≤ 0.05 vs. untreated control.
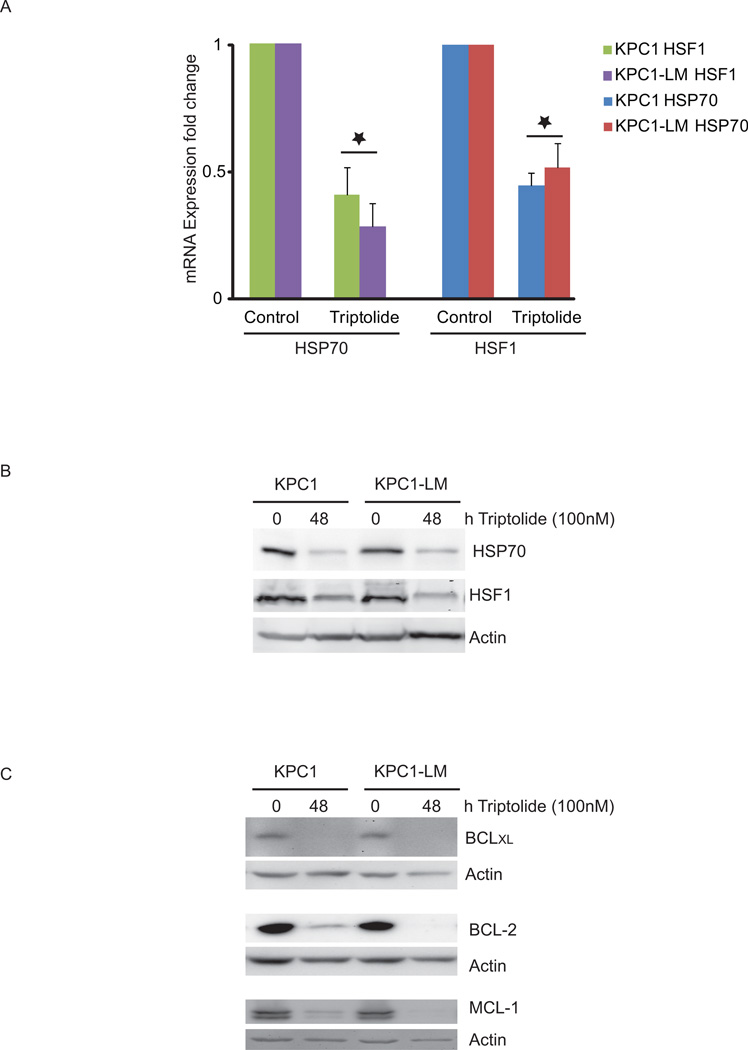
Triptolide treatment decreases anti-apoptotic and pro survival protein levels in KPC tumor-derived cell lines
Escape from apoptosis is a hallmark of cancer cells that is caused by deregulation of several cell death checkpoints found in normal cells. Therefore, therapies that shift the balance between the pro- and anti-apoptotic pathways can cause cancer cell.15 The Bcl-2 family of proteins which includes anti-apoptotic proteins such as Mcl-1, Bcl-XL and Bcl-2 have been shown to be up-regulated in pancreatic cancer. In addition, pancreatic tumors have been associated with higher levels of the chaperone, HSP70, and its transcription factor, HSF1, when compared to adjacent normal tissue.16,17 We have previously shown that triptolide targets HSP70, HSF1 and Mcl-1 in pancreatic cancer cells.16–18 We therefore evaluated the effect of triptolide on these and other anti-apoptotic proteins in KPC primary and liver metastasis-derived cells (KPC 1 and KPC1-LM). Treatment of KPC1 cells with 100 nM of triptolide for 24 hours reduced HSP70 and HSF1 RNA levels to 44% and 40% of untreated controls respectively. KPC1-LM cells responded to triptolide by decreasing HSP70 and HSF1 RNA levels to 51% and 28% respectively (Fig. 6A). This decrease in RNA levels correlated with a decrease in protein levels of both HSP70 and HSF1 in both the KPC and KPC1-LM cell lines. Triptolide treatment also led to a decrease in expression of the members of the Bcl-2 family of anti-apoptotic proteins, namely, Bcl-2, Bcl-XL and Mcl-1, in both KPC1 and KPC1-LM cells (Fig. 6C). These data suggest that triptolide causes cell death by down modulation of several cell survival pathways.
Figure 6. Anti-apoptotic proteins are down regulated in response to triptolide in KPC and KPC1-LM cells.
A. Cells were treated for 24 hours with 100 nM triptolide and gene expression evaluated using real time PCR. 18s was used to normalize expression and data represented as fold change compared to control untreated cells. Errors bars represent SEM. *= p ≤ 0.05 vs. untreated cells. B. Protein lysates from cells treated with triptolide as described in A. were immuno-blotted for HSP70 or HSF1. C. Protein lysates from cells treated with triptolide as described in A. were immuno-blotted for Bcl-2, Bcl-xl and Mcl-1. Actin was used as a loading control for all western blots.
Discussion
In the present study, we have evaluated the aggressive potential of two cell lines derived from a primary pancreatic tumor and its adjacent liver metastases in a KPC animal. Tumor progression in KPC animals is associated with the formation of PanINs, and 80% of these animals exhibit metastases.6 Since this model mimics human disease, it is relevant to test novel therapies against pancreatic cancer in this context. KPC animals do not exhibit a significant response to gemcitabine, but various other combinations of compounds are being tested for their ability to decrease tumor volume in these animals.7,19,20 We have previously shown that Minnelide, a water soluble pro-drug of triptolide, is able to act as a chemoprotective agent in KPC animals.4 However, there is no study to date that has evaluated the efficacy of triptolide in this model. As a first step toward an evaluation of the ability of triptolide to cause tumor regression in the KPC model, we assayed the effect of triptolide on KPC tumor derived cell lines. Since 85% of patients diagnosed with pancreatic cancer are ineligible for surgical resection due to metastatic spread of the disease, we also evaluated a cell line derived from a liver metastasis in the KPC animal. Our results presented here show that, compared to immortalized human tumor-derived cell lines, KPC1 and KPC1-LM cell lines are more aggressive in their ability to form tumors in athymic nude mice. Tumors derived from these lines show constitutive activation of the NFkB pathway. Triptolide treatment results in apoptotic cell death and decreased levels of the inducible heat shock protein HSP70 and its transcription factor, HSF1, both at the mRNA and protein levels, as well as decrease in levels of the Bcl-2 family of anti-apoptotic proteins Bcl-2, Bcl-XL and Mcl-1. This is the first report evaluating the aggressiveness of KPC pancreatic tumor-derived cell lines against other known cell lines, and the effect of triptolide on these cells.
Several pancreatic cancer cell lines derived from primary (MIA PaCa-2) or metastatic (S2-VP10, S2-013, AsPC-1) human pancreatic tumors are commonly used to evaluate the efficacy of novel therapies against this disease.4,21,22 The rate at which tumor formation occurs varies greatly between cell lines used, and correlates with the aggressiveness of the cell line used.4 Our results are in agreement with other studies showing MIA PaCa-2 to be the least aggressive cell line tested.4,5,22 Although human cell lines derived from metastatic tumors were more aggressive than those derived from primary tumors, those derived from KPC cell lines (KPC1, KPC023, KPC1-LM) were at least ten-fold more aggressive than the human cell line derived tumors (Fig. 2).
Pancreatic cancer is a disease of inherited and acquired mutations in the oncogenes such as KRAS, tumor suppressor genes such as TP53 and genome maintenance genes such as BRAC2.23 KRAS is activated in more than 90% of all pancreatic cancer patients24, with a vast majority of activating point mutations occurring at codon 12.23 Previous studies have shown that NFkB signaling plays a key role in Ras-driven cancers, and loss of NFkB activity results in delay in tumor formation.14,24 NFκB is a pro survival transcription factor that is constitutively activated in pancreatic cancer. We evaluated the activity of p50, one of the subunits of NFκB, in tumor lysates from tumors derived from various cell lines. In agreement with previously published data, NFκB is activated in all tumors tested. Intriguingly, NFkB activity did not show a direct correlation with aggressiveness of cell lines, suggesting that NFkB is not the only factor responsible for the rapid tumor progression observed in KPC induced tumors.
Triptolide is a diterpene triepoxide which causes cell death in pancreatic, colon and breast cancers as well as cholangiocarcinoma and neuroblastoma cells, among others.25–27 Since all cell lines tested are of human origin, the ability of triptolide to reduce tumor burden in vivo was analyzed in immune depleted animals. We therefore tested triptolide as a therapy against KPC cells derived from KPC tumors, which are derived from immune-competent animals. Since pancreatic cancer patients present in the clinic at an advanced stage of the disease, metastases has occurred in a majority of the patients. We therefore also assessed the ability of triptolide to cause cell death in cells derived from liver metastases in a KPC animal. Our data show that triptolide caused apoptotic cell death in both cell lines tested. We have previously shown that triptolide mediated cell death takes place via apoptosis in cells derived from primary tumors and autophagy in two cell lines derived from metastases.28 It is therefore interesting to note that both cell lines derived from KPC tumors undergo apoptosis in the presence of triptolide, suggesting that triptolide mediated cell death does not correlate with metastasis.
We and others have previously shown that HSP70 and its transcription factor, HSF1 are over-expressed in pancreatic cancer, and their down-regulation, either through chemical or siRNA mediated methods results in cell death.5,16,17,29–34 Other pro-survival proteins known to be upregulated in pancreatic cancer included those of the Bcl-2 family including Bcl-2, BclXL and Mcl-1. An investigation of the effect of triptolide on these proteins in KPC cells show that HSP70 and its transcription factor, HSF1, as well as Bcl-1, BclXL and Mcl-1 are down-regulated in the presence of triptolide. This effect on a multitude of proteins known to be involved in cell survival suggests that triptolide effects multiple pathways, making it more difficult for cancer cells to develop resistance to triptolide.
The ability of triptolide to cause cell death in non-immortalized cells derived from KPC tumors and liver metastases is another step towards assessing the tumor reducing ability of triptolide in KPC animals, and support the use of the water soluble prodrug of triptolide, Minnelide, in humans.
Acknowledgments
Funding: Masonic Cancer Center, the Department of Surgery, Pancreatic Cancer SPORE grant to the University of Minnesota and University of Alabama (P50 CA101955), and the National Institute of Health grants R01CA124723, R01 CA170496 (to AKS), the Katherine and Robert Goodale Foundation and the Hirshberg Foundation to AKS.
Footnotes
Competing interests: University of Minnesota has filed a patent for Minnelide which has been licensed to Minneamrita Therapeutics, LLC. Inventors on this patent include: RKC, SMV and AKS. SMV and AKS have financial interests in this company. The other authors declare that they have no competing interests. Minnelide synthesis has been filed under patent WO/2010/129918.
References
- 1.Warshaw AL, Gu ZY, Wittenberg J, et al. Preoperative staging and assessment of resectability of pancreatic cancer. Arch Surg. 1990;125:230–233. doi: 10.1001/archsurg.1990.01410140108018. [DOI] [PubMed] [Google Scholar]
- 2.Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33(Suppl 1):S18–S22. doi: 10.1016/s0959-8049(96)00324-3. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 4.Chugh R, Sangwan V, Patil SP, et al. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004334. 156ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips PA, Dudeja V, McCarroll JA, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 6.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 9.Fan Y, Dutta J, Gupta N, et al. Regulation of programmed cell death by NF-kappaB and its role in tumorigenesis and therapy. Adv Exp Med Biol. 2008;615:223–250. doi: 10.1007/978-1-4020-6554-5_11. [DOI] [PubMed] [Google Scholar]
- 10.Perkins ND. The diverse and complex roles of NF-κB subunits in cancer. Nat Rev Cancer. 2012;12:121–132. doi: 10.1038/nrc3204. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–747. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Abbruzzese JL, Evans DB, et al. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 13.Fujioka S, Sclabas GM, Schmidt C, et al. Inhibition of constitutive NF-kappa B activity by I kappa B alpha M suppresses tumorigenesis. Oncogene. 2003;22:1365–1370. doi: 10.1038/sj.onc.1206323. [DOI] [PubMed] [Google Scholar]
- 14.Ling J, Kang Y, Zhao R, et al. KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juin P, Geneste O, Gautier F, et al. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat Rev Cancer. 2013;13:455–465. doi: 10.1038/nrc3538. [DOI] [PubMed] [Google Scholar]
- 16.Aghdassi A, Phillips P, Dudeja V, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 17.Dudeja V, Chugh RK, Sangwan V, et al. Prosurvival role of heat shock factor 1 in the pathogenesis of pancreatobiliary tumors. Am J Physiol Gastrointest Liver Physiol. 2011;300:G948–G955. doi: 10.1152/ajpgi.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Sangwan V, Banerjee S, et al. miR-204 mediated loss of Myeloid cell leukemia-1 results in pancreatic cancer cell death. Mol Cancer. 2013;12:105. doi: 10.1186/1476-4598-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 324:1457–1461. doi: 10.1126/science.1171362. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derosier LC, Vickers SM, Zinn KR, et al. TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth. Mol Cancer Ther. 2007;6:3198–3207. doi: 10.1158/1535-7163.MCT-07-0299. [DOI] [PubMed] [Google Scholar]
- 22.Phillips PA, Sangwan V, Borja--Cacho D, et al. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Lett. 2011;308:181–188. doi: 10.1016/j.canlet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:211–226. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Hruban RH, van Mansfeld AD, Offerhaus GJ, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–554. [PMC free article] [PubMed] [Google Scholar]
- 25.Antonoff MB, Chugh R, Borja-Cacho D, et al. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery. 2009;146:282–290. doi: 10.1016/j.surg.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 26.Clawson KA, Borja-Cacho D, Antonoff MB, et al. Triptolide and TRAIL combination enhances apoptosis in cholangiocarcinoma. J Surg Res. 2010;163:244–249. doi: 10.1016/j.jss.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krosch TC, Sangwan V, Banerjee S, et al. Triptolide-mediated cell death in neuroblastoma occurs by both apoptosis and autophagy pathways and results in inhibition of nuclear factor-kappa B activity. Am J Surg. 2013;205:387–396. doi: 10.1016/j.amjsurg.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mujumdar N, Mackenzie TN, Dudeja V, et al. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology. 2010;139:598–608. doi: 10.1053/j.gastro.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacKenzie TN, Mujumdar N, Banerjee S, et al. Triptolide induces the expression of miR-142-3p: a negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol Cancer Ther. 2013;12:1266–1275. doi: 10.1158/1535-7163.MCT-12-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta SK, Girotra M, Singla M, et al. Serum HSP70: a novel biomarker for early detection of pancreatic cancer. Pancreas. 2012;41:530–534. doi: 10.1097/MPA.0b013e3182374ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dudeja V, Mujumdar N, Phillips P, et al. Heat shock protein 70 inhibits apoptosis in cancer cells through simultaneous and independent mechanisms. Gastroenterology. 2009;136:1772–1782. doi: 10.1053/j.gastro.2009.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saluja A, Dudeja V. Heat shock proteins in pancreatic diseases. J Gastroenterol Hepatol. 2008;23(Suppl 1):S42–S45. doi: 10.1111/j.1440-1746.2007.05272.x. [DOI] [PubMed] [Google Scholar]
- 33.Ehrenfried JA, Herron BE, Townsend CM, Jr, et al. Heat shock proteins are differentially expressed in human gastrointestinal cancers. Surg Oncol. 1995;4:197–203. doi: 10.1016/s0960-7404(10)80036-2. [DOI] [PubMed] [Google Scholar]
- 34.Xia Y, Liu Y, Rocchi P, et al. Targeting heat shock factor 1 with a triazole nucleoside analog to elicit potent anticancer activity on drug-resistant pancreatic cancer. Cancer Lett. 2012;318:145–153. doi: 10.1016/j.canlet.2011.09.043. [DOI] [PubMed] [Google Scholar]