SUMMARY
Missense mutations in the nucleotide-binding oligomerization domain (NOD)-like receptor pyrin domain containing family of gene 12 (Nlrp12) are associated with periodic fever syndromes and atopic dermatitis in humans. Here, we have demonstrated a crucial role for NLRP12 in negatively regulating pathogenic T cell responses. Nlrp12−/− mice responded to antigen immunization with hyperinflammatory T cell responses. Furthermore, transfer of CD4+CD45RBhi Nlrp12−/− T cells into immunodeficient mice led to more severe colitis and atopic dermatitis. NLRP12-deficiency did not, however, cause exacerbated ascending paralysis during experimental autoimmune encephalomyelitis (EAE), instead Nlrp12−/− mice developed atypical neuroinflammatory symptoms that were characterized by ataxia and loss of balance. Enhanced T cell-mediated interleukin-4 (IL-4) production promotes the development of atypical EAE disease in Nlrp12−/− mice. These results define an unexpected role for NLRP12 as an intrinsic negative regulator of T cell-mediated immunity, and identify altered NF-κB regulation and IL-4 production as key mediators of NLRP12-associated disease.
Keywords: NLR, NLRP12, T cells, multiple sclerosis, autoinflammation, colitis, atopic dermatitis
INTRODUCTION
Nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are a family of intracellular sensor molecules that are involved in the regulation of inflammatory signaling in response to infection and cellular stress(Kanneganti, 2010; Kanneganti et al., 2007; Lukens et al., 2012). Recent studies have revealed pivotal roles for NLR-mediated inflammation in a spectrum of diverse human autoimmune and inflammatory disorders(Kanneganti and Dixit, 2012; Lamkanfi et al., 2011; Lukens et al., 2011). The vast majority of NLRs to date have been defined as activators of inflammatory signaling in innate immune cells. For instance, the prototypical NLR members, NOD1 and NOD2, initiate proinflammatory NF-κB and mitogen activated protein kinase (MAPK) signaling in response to their direct recognition of bacterial peptidoglycan fragments(Strober et al., 2006). Multiple NLRs have also been described to promote the activation and secretion of interleukin-1β (IL-1β) and IL-18 by coordinating the assembly of the inflammasome complex(Shaw et al., 2011; Strowig et al., 2012). Negative regulation of inflammatory signaling has also recently been ascribed to NLRP6, NLRP12 and NLRX1(Anand et al., 2012; Chen et al., 2011; Elinav et al., 2011; Lei et al., 2012; Normand et al., 2011; Xia et al., 2011; Zaki et al., 2011). However, the cellular and molecular mechanisms that direct the suppression of inflammation by this new class of inhibitory NLRs remain to be formally elucidated.
NLRP12 is a recently identified member of this class of inhibitory NLR proteins. Missense mutations in Nlrp12 lead to periodic fever syndromes and atopic dermatitis in humans(Borghini et al., 2011; Jeru et al., 2008; Jeru et al., 2011; Macaluso et al., 2007), although little is known about how defective NLRP12-mediated signaling contributes to these autoinflammatory disorders. In vitro studies with human and mouse macrophages suggest that NLRP12 is a negative regulator of toll-like receptor (TLR)-induced cytokine production(Lich et al., 2007; Williams et al., 2005; Zaki et al., 2014; Zaki et al., 2011). Moreover, NLRP12-mediated suppression of proinflammatory signaling was recently shown to play a central role in the attenuation of colon inflammation and tumorigenesis in mice(Allen et al., 2012; Zaki et al., 2011). These studies establish NLRP12 as a critical regulator of inflammatory responses in innate immune cells. However, the putative role and importance of NLRP12 in regulating T cell responses during inflammatory disease progression has not been characterized. T cells are centrally involved in the pathogenesis of numerous autoinflammatory diseases, to which they participate in both tissue destruction and the sustained recruitment of inflammatory cells through their release of effector cytokines and chemokines(Goverman, 2009).
In this study, we focused on investigating the pathophysiological role of NLRP12 in shaping autoinflammatory T cell responses. Deficiency in NLRP12 promoted the generation of hyperinflammatory T cells in response to antigen immunization. Nlrp12−/− mice did not, however, exhibit exacerbated demyelinating disease in the T cell-driven experimental autoimmune encephalomyelitis (EAE) mouse model. Rather, the absence of NLRP12 provoked an alternative form of neuroinflammation that was characterized by atypical EAE symptoms that included loss of balance and ataxia. This development of atypical demyelinating neuroinflammation in Nlrp12−/− mice was found to be dependent on dysregulated IL-4 production and we further identify NLRP12 as an intrinsic negative regulator of T cells.
RESULTS
NLRP12 negatively regulates in vivo T cell responses
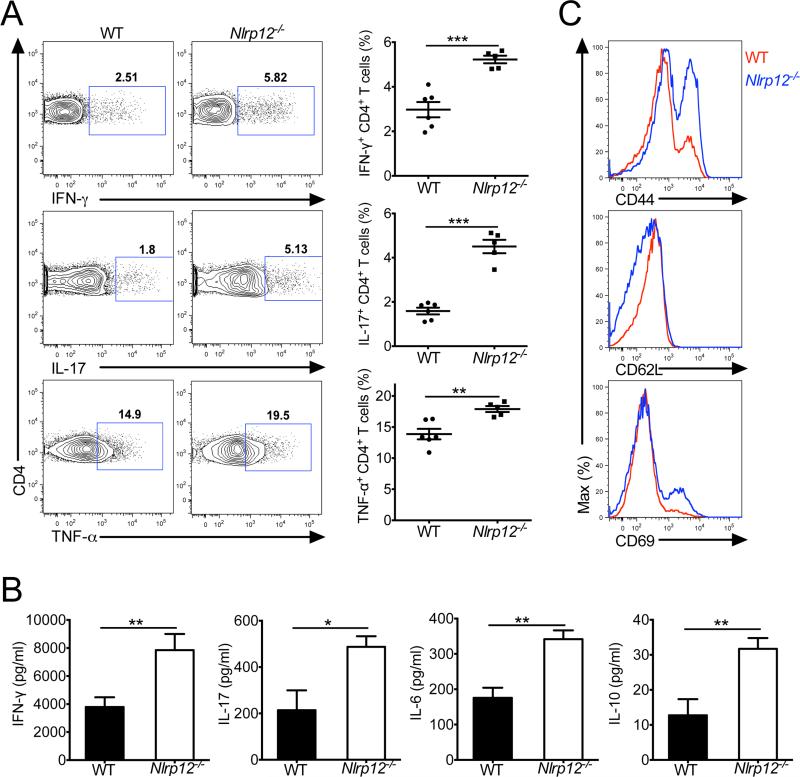
NLRs have emerged as central regulators of inflammatory signaling, and multiple NLRs have been identified to be critically involved in the regulation of proinflammatory cytokine production by antigen-presenting cells. In contrast, the ability of NLRs to modulate T cell responses and how NLR-dependent control of T cells influences autoinflammatory disease progression remains poorly defined. Thus, to elucidate the role of NLRP12 in shaping T cells responses, wild-type (WT) and NLRP12-deficient mice were immunized with MOG peptide in CFA adjuvant. T cells isolated from the spleens of Nlrp12−/− mice at day 27 post-immunization displayed a hyperinflammatory phenotype and produced higher amounts of interferon-γ (IFN-γ), IL-17, and tumor necrosis factor-α (TNF-α) following restimulation (Figure 1A). Likewise, Nlrp12−/− splenocytes were also found to secrete increased amounts of cytokines following stimulation with MOG peptide (Figure 1B). Surface marker expression analysis revealed that Nlrp12−/− T cells expressed higher amounts of CD44 and CD69, and markedly downregulated CD62L relative to WT cells (Figure 1C), which suggests that T cells are in a hyperactivated state in NLRP12-deficient mice. To further characterize the ability of NLRP12 to modulate T cell responses in a separate model, we evaluated T cell activation status and effector cytokine production following DSS-induced inflammation. In agreement with what was observed in the antigen immunization model, Nlrp12−/− T cells isolated from dextran sodium sulfate (DSS)-challenged mice also exhibited a more activated phenotype (CD44hiCD62Llo) and produced greater amounts of inflammatory cytokines (Figure S1). Collectively, these results establish NLRP12 as a negative regulator of T cell activation in models of antigen immunization and DSS-induced colitis.
Figure 1. NLRP12 deficiency promotes the generation of hyperinflammatory T cell responses.
WT and Nlrp12−/− mice were immunized with MOG peptide in CFA adjuvant and mice were harvested on day 27. (A) Splenocytes were restimulated directly ex vivo and the intracellular production of IFN-γ, IL-17, and TNF-α by CD4+ T cells was determined. Pooled data is presented in the right panel. (B) Splenocytes were stimulated for 48 hrs with MOG peptide and cytokine production was measured by ELISA. The bar graphs show mean ± s.e.m. (C) Expression of activation markers by splenic CD4+ T cells. Data are representative of at least four independent experiments with 3-6 mice per group. All graphs depict mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001; Student's t-test. See also Figure S1
NLRP12-deficient mice develop atypical EAE disease
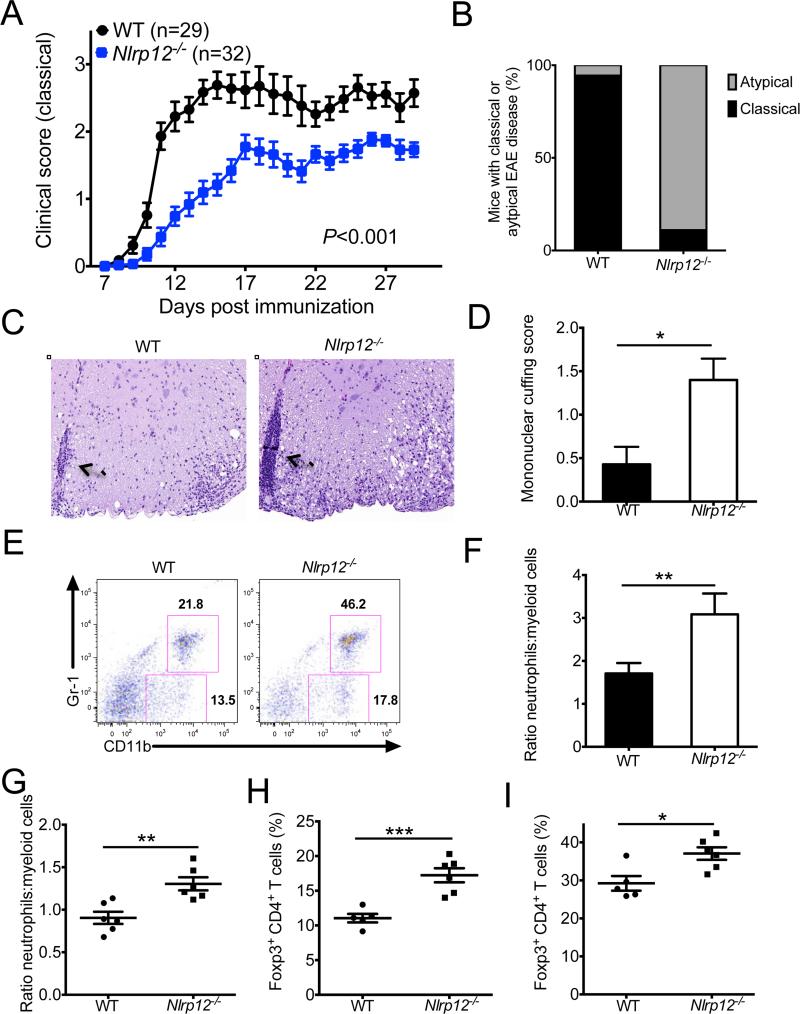
To delineate the role of NLRP12 in the regulation of T cell-driven autoimmune disease progression we evaluated demyelinating neuroinflammatory disease in the experimental autoimmune encephalomyelitis (EAE) model. Given our initial observation that Nlrp12−/− T cells were hyperinflammatory following antigen immunization (Figure 1), we hypothesized that NLRP12-deficient mice would develop exacerbated neuroinflammatory disease. In contrast to our expectations, classical clinical disease scores that are based on ascending paralysis were consistently lower in Nlrp12−/− mice (Figure 2A). Instead, Nlrp12−/− mice presented with atypical symptoms of neuroinflammatory disease that included loss of balance and ataxia (Figure 2B and Movies S1 and S2). Histological analysis revealed that atypical neuroinflammatory disease in Nlrp12−/− mice was associated with enhanced mononuclear cuffing in the brain (Figure 2C-D). Likewise, protection against paralyzing demyelination in NLRP12-deficient mice was further confirmed by Luxol fast blue staining (LFB) of spinal cord sections (Figure S2). Consistent with previous studies that linked atypical EAE disease with extensive infiltration of neutrophils into the central nervous system (CNS) (Kroenke et al., 2010; Stromnes et al., 2008), neutrophils also represented an increased fraction of the infiltrating myeloid cell population in Nlrp12−/− mice (Figure 2E, F). Furthermore, the proportions of neutrophils to other myeloid cells were increased in the periphery of diseased Nlrp12−/− mice (Figure 2G). In addition, non-classical neuroinflammatory disease in Nlrp12−/− mice was associated with increased frequencies of Foxp3-expressing regulatory T (Treg) cells in both the periphery (Figure 2H) and the central nervous system (CNS) (Figure 2I). Although multiple NLRs have been identified to play important roles in the orchestration of inflammasome-mediated cytokine production (Vladimer et al., 2012), atypical EAE disease in Nlrp12−/− mice was not associated with altered generation of IL-1β or IL-18 (Figure S3).
Figure 2. Nlrp12−/− mice develop atypical EAE disease.
WT and Nlrp12−/− mice were immunized with MOG+CFA and pertussis toxin to induce EAE. (A) Classical clinical scores that are based on ascending paralysis. (B) Percentages of mice that developed classical (ascending paralysis) or atypical (ataxia and impaired balance) EAE disease symptoms. (C) H&E staining of brain lesions. The arrows highlight areas of extensive mononuclear cuffing (original magnification ×20). (D) Mononuclear cuffing histology scores from WT (n = 7) and Nlrp12−/− (n = 5) brain sections. (E) Frequencies of CNS infiltrating cells that are neutrophils (CD11b+Gr-1+) or other myeloid cells (CD11b+Gr-1−) on day 28. (F) Ratios of neutrophils to other myeloid cells in the CNS. (G) Ratios of neutrophils to other myeloid cells in the spleen. (H,I) Frequencies of CD4+ T cells that express Foxp3 in the spleen (H) and CNS (I). Each point represents an individual mouse. Data are representative of four independent experiments with 3-6 mice per group. Statistical significance in (D, F-I) was determined by Student's t-test. Statistical significance in (A) was determined by two-way ANOVA. All graphs depict mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. See also Figure S2 and Movies S1 and S2.
Dysregulated IL-4 production promotes atypical neuroinflammatory disease in Nlrp12−/− mice
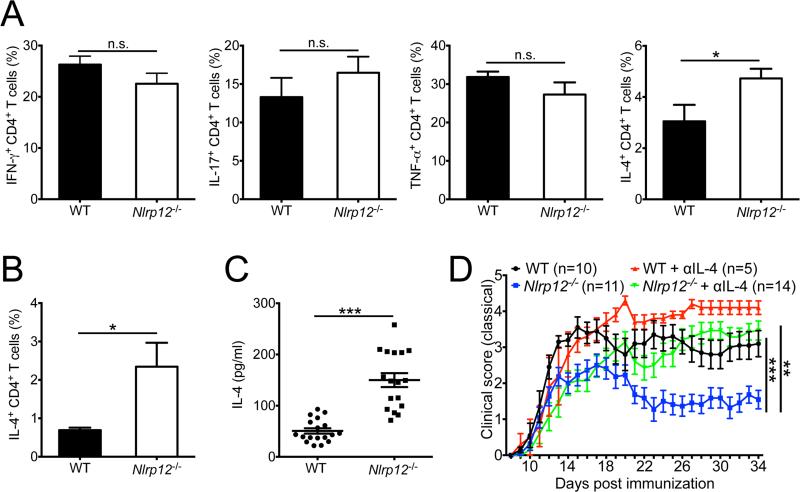
To investigate the immunological factors that contribute to the development of non-classical neuroinflammatory disease symptoms in Nlrp12−/− mice, we next explored whether differences in clinical disease parameters were associated with differential regulation of T cell-mediated cytokine production in the CNS. Deficiency in NLRP12 was not, however, found to affect the production of IFN-γ, IL-17 or TNF-α by CD4+ T cells in the CNS (Figure 3A). Our knowledge of the cellular and molecular mediators that potentiate atypical EAE currently remains limited, however, a few studies have pointed to important roles for IL-4 in the induction of atypical neuroinflammation(Delgoffe et al., 2011; Wensky et al., 2001). Thus, we next assessed whether dysregulated IL-4 production promoted atypical neuroinflammatory disease in Nlrp12−/− mice. We observed greater production of IL-4 by both CNS and splenic CD4+ T cells in Nlrp12−/− mice (Figure 3A, B). Furthermore, restimulation of NLRP12-deficient splenocytes with myelin-specific antigen provoked markedly enhanced secretion of IL-4 (Figure 3C).
Figure 3. Dysregulated IL-4 production promotes atypical neuroinflammatory disease in Nlrp12−/− mice.
WT and Nlrp12−/− mice were immunized with MOG+CFA and pertussis toxin to induce EAE and mice were harvested either on day 27 (A,B) or day 29 (C). (A) Frequencies of CD4+ T cells that express IFN-γ, IL-17, TNF-α or IL-4 in the CNS. (B) Frequencies of IL-4 producing CD4+ T cells in the spleen. (C) Splenocytes were stimulated with MOG peptide for 48 hrs and IL-4 production was measured by ELISA. Data in (A-C) are representative of three independent experiments with 3-6 mice per group. (D) Classical ascending paralysis clinical scores for WT and Nlrp12−/− EAE mice that were treated with either vehicle control or 500 μg of anti-IL-4 blocking antibody on days -1, 0, 2, 4, 6, 8, and 13. Data are representative of two independent experiments with 5-14 mice per group. Statistical significance in (A-C) was determined by Student's t-test. Statistical significance in (D) was determined by two-way ANOVA. All graphs depict mean ± s.e.m. *P < 0.05, **P < 0.01, ***P < 0.001. See also Figure S3.
To confirm that dysregulated IL-4 production is indeed involved in dampening ascending paralysis while conversely spurring the development of atypical EAE disease symptoms in Nlrp12−/− mice, we treated mice with anti-IL-4 neutralizing antibody to disrupt IL-4-mediated signaling. In agreement with published reports(Bettelli et al., 1998; Falcone et al., 1998; Furlan et al., 1998; Shaw et al., 1997), blockade of IL-4 during EAE caused exacerbated classical disease symptoms in WT mice (Figure 3D). Furthermore, ablation of IL-4 during EAE caused NLRP12-deficient mice to develop classical symptoms of demyelinating neuroinflammatory disease that included severe ascending paralysis and morbidity (Figure 3D). Collectively these results suggest a causal role for hyperinflammatory IL-4 production in driving atypical EAE disease progression in Nlrp12−/− mice.
NLRP12 is dispensable for thymic development
To elucidate the level at which NLRP12 influences T cell activation, we first evaluated the influence of NLRP12 deficiency on T cell development. NLRP12 deficiency was not found to promote major perturbations in positive T cell selection under homeostatic conditions, and standard thymic development flow cytometry gating strategies revealed normal distribution of thymocytes in Nlrp12−/− mice (Figure S4A, B). Moreover, genetic deletion of Nlrp12 failed to alter the accumulation of CD4−CD8−, CD4+CD8+, CD4+CD8−, or CD4−CD8+ thymocytes (Figure S4C). Analysis of the double negative (DN) population using CD44 and CD25 discrimination also ruled out a homeostatic role for NLRP12 in negative selection (Figure S4D, E). These results indicate that NLRP12 is dispensable for normal thymic development, and suggest a role for NLRP12 in the regulation of mature T cell responses.
NLRP12 shapes peripheral T cell development in naïve mice
To formally address whether the absence of NLRP12 causes alterations in peripheral T cell development and activation under homeostatic conditions, we next evaluated T cell numbers, cytokine production and Treg cell frequencies in naïve mice. We detected enhanced numbers of peripheral CD4+ and CD8+ T cells in Nlrp12−/− mice (Figure S4F). NLRP12-deficient T cells also displayed a more inflammatory phenotype and produced greater amounts of IFN-γ and IL-17 following brief restimulation (Figure S4G). Consistent with a role for NLRP12 in shaping T cell development in the periphery, we also observed greater frequencies of Foxp3-expressing Treg cells in naive Nlrp12−/− mice (Figure S4H). These results indicate that NLRP12 is involved in the regulation of mature T cell development and activation. However, NLRP12 appeared to exert a more prominent effect on T cells responses following overt stimulation, as was observed in the antigen immunization model (Figure 1).
NLRP12 is an intrinsic negative regulator of T cell activation
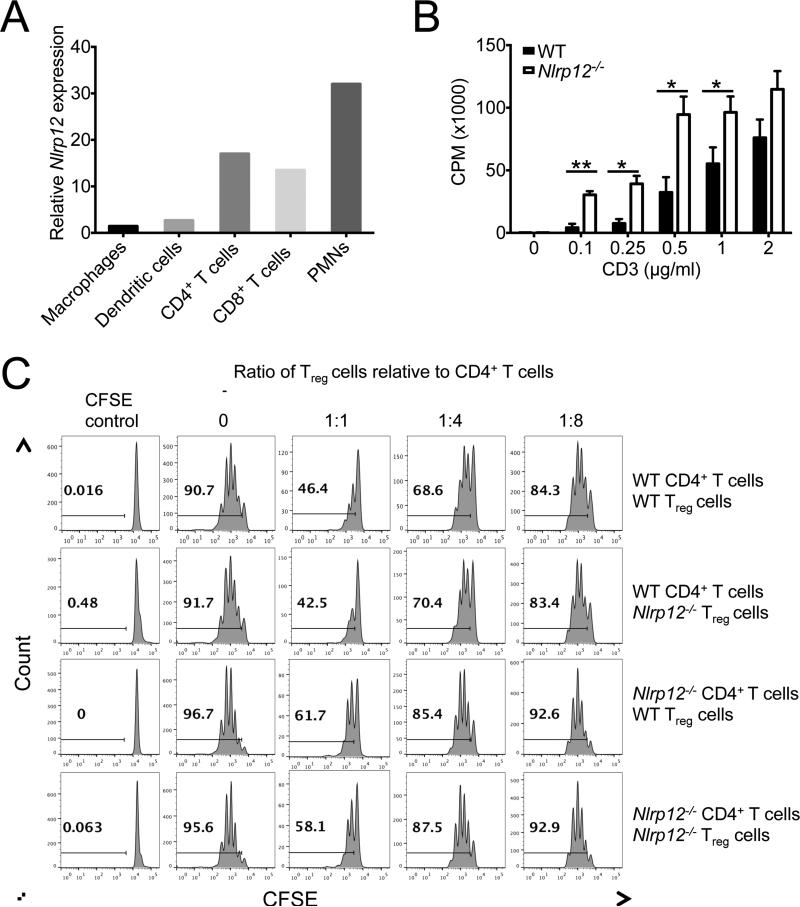
We were next interested in determining whether NLRP12 modulates T cell activation through an extrinsic role in antigen-presenting cells (APCs) or through an intrinsic function in T cells. As a first approach we evaluated the expression of Nlrp12 mRNA transcripts in various immune cell populations in an effort to help identify the cell types that NLRP12 could potentially function in to shape T cell responses. We found that Nlrp12 mRNA was most highly expressed in polymorphonuclear leukocytes (PMNs), CD4+ T cells and CD8+ T cells (Figure 4A). Nlrp12 mRNA expression was also detected in resting dendritic cells and macrophages, however its expression in these antigen presenting cells was markedly less in comparison to T cells and PMNs. To ascertain if the observed expression of NLRP12 in T cells influences functional responses, in vitro T cell thymidine incorporation assays were conducted. Purified Nlrp12−/− T cells displayed more extensive thymidine incorporation than WT T cells in response to anti-CD3 stimulation (Figure 4B), which implicates NLRP12 in the attenuation of T cell expansion.
Figure 4. NLRP12 negatively regulates T cell responses.
(A) Relative expression of Nlrp12 in purified macrophages (Ly6G−CD11b+), dendritic cells (CD11c+MHCIIhi), CD4+ T cells (Thy1.2+CD4+), CD8+ T cells (Thy1.2+CD8+) and polymorphonuclear leukocytes (PMNs) (Ly6G+CD11b+). (B) Purified naïve T cells isolated from WT and Nlrp12−/− mice were stimulated with plate bound anti-CD3 for 48 hrs and the incorporation of thymidine was measured during the final 8 hrs. (C) Purified conventional CD4+ T cells (CD4+CD62Lhi) and Treg cells (CD4+CD25+) were isolated from WT and Nlrp12−/− mice by flow cytometry sorting. Conventional CD4+ T cells were labeled with CFSE and stimulated with anti-CD3 and irradiated splenocytes. Treg cell suppression assays were then conducted by mixing conventional CD4+ T cells and Treg cells in various combinations and at different ratios. All graphs depict mean ± s.e.m. *P < 0.05, **P < 0.01; Student's t-test. See also Figure S4 & S5.
It is possible that defects in Treg cell-mediated suppressive functions are responsible for the hyperactive T cell responses that are observed in Nlrp12−/− mice. Thus, to formally test this possibility, in vitro Treg cell suppression assays were conducted. In agreement with our anti-CD3 thymidine incorporation results, we also observed enhanced proliferation by CFSE dye dilution of Nlrp12−/− T cells in the absence of Treg cells. Furthermore, the suppressive capacity exhibited by Treg cells that lacked NLRP12 was equal to that of WT Treg cells in classical in vitro suppression assays (Figure 4C), and Nlrp12−/− Treg cells did not display any impairment in IL-10 production (Figure S4I). Conversely, NLRP12-deficient T cells were less responsive to the suppression exerted by increasing concentrations of WT Treg cells, and conventional Nlrp12−/− T cells were more likely to undergo division even when WT Treg cells were present at high numbers (Figure 4C). These findings indicate that the altered hyperinflammatory T cell responses that were observed in Nlrp12−/− mice are most likely not the result of dysfunctional Nlrp12−/− Treg cell responses. Rather they indicate that dysregulated Nlrp12−/− effector T cells are responsible for the observed T cell-mediated effects in NLRP12-deficient mice.
NLRP12 is an intrinsic negative regulator of in vivo T cell responses
To confirm an intrinsic role for NLRP12 in the regulation of T cell responses in vivo, 1:1 competitive adoptive T cell transfer assays were carried out. For these experiments, congenically mismatched WT and NLRP12-deficient T cells were adoptively transferred into Rag1−/− recipient mice. Following two weeks of homeostatic expansion, CD45.2+ Nlrp12−/− T cells markedly outcompeted CD45.1+ WT T cells in both the lymph nodes (LNs) and spleens of recipient mice (Figure S5A). Nlrp12−/− T cells also expressed greater amounts of proinflammatory cytokines on a per cell basis (Figure S5B). To ascertain whether NLRP12 also intrinsically controls T cells responses under pathophysiological conditions, we generated 1:1 chimera mice with CD45.2+ Nlrp12−/− and CD45.1+ WT bone marrow cells. Following reconstitution, mice were immunized with MOG peptide plus CFA adjuvant and T cell responses were monitored in the spleen at day 21. Intriguingly, NLRP12-deficient T cells outcompeted WT T cells in the periphery following stimulation (Figure S5C). Furthermore, Nlrp12−/− T cells produced greater amounts of IFN-γ and IL-17 on a per-cell basis in the 1:1chimeric mice (Figure S5C). These results establish an unexpected, intrinsic role for NLRP12 in the negative regulation of T cell responses under pathophysiological conditions.
NLRP12 can regulate inflammatory disease through T cell-intrinsic functions
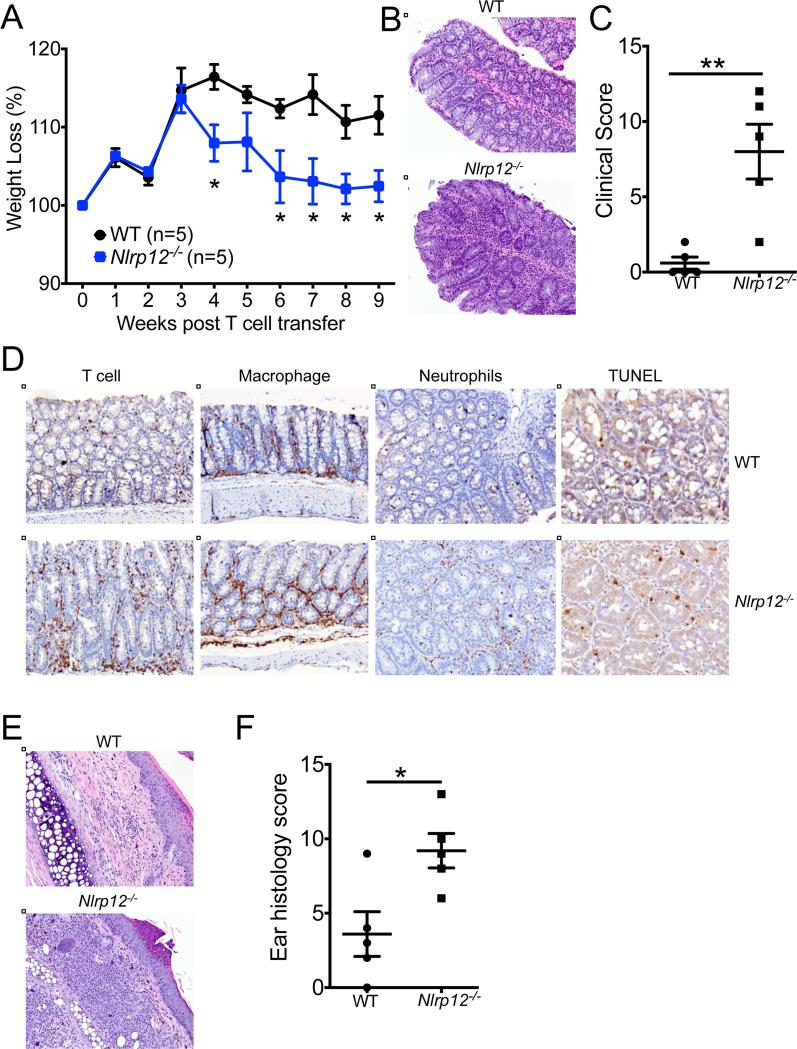
Defective NLRP12 expression was shown to promote exacerbated inflammatory disease in models of colon damage and tumorigenesis(Zaki et al., 2011). However, the cellular mechanisms responsible for enhanced inflammation in Nlrp12−/− mice have not been formally elucidated. To ascertain whether Nlrp12−/− T cells directly promote hyperinflammation and disease pathology, we transferred CD4+ CD45RBhi T cells from WT or Nlrp12−/− mice into Rag1−/− mice and tracked the development of colitis. In comparison to the injection of WT T cells, adoptive transfer of Nlrp12−/− T cells provoked more severe weight loss (Figure 5A) and intestinal inflammation (Figure 5B-C). The development of aggravated disease in Nlrp12−/− T cell transfer mice was associated with enhanced recruitment of inflammatory cells and cell death in the afflicted mucosa (Figure 5D and Figure S6). Adoptive transfer of Nlrp12−/− CD4+CD45RBhi T cells into Rag1−/− mice also provoked the development of atopic dermatitis in recipient mice (Figure 5E), and this was associated with more severe skin pathology (Figure 5F). Cutaneous inflammation in this model is typically associated with dysregulated T helper-2 (Th2) cell-associated inflammation(Davenport et al., 2002; Leon et al., 2006; Schon et al., 1997), suggesting that altered IL-4 production may also be contributing to disease pathology.
Figure 5. Nlrp12−/− T cells are pathogenic.
. Purified CD4+CD45RBhi WT or Nlrp12−/− T cells were adoptively transferred into Rag1−/− mice. (A) Percent weight change over time. (B) Representative large intestine histology at 9 weeks post adoptive transfer of CD4+CD45RBhi T cells (original magnification ×20). (C) Combined colon histology scores. (D) Representative immunohistochemistry staining of T cells (anti-CD3), macrophages (anti-F4/80), neutrophils (anti-Gr-1), and cell death (TUNEL) in inflamed colon tissue (original magnification ×20). (E) Representative H&E ear sections (original magnification ×20). (F) Combined ear histology scores. Each point represents an individual mouse and the line represents the mean ± s.e.m. Data are representative of four independent experiments with 3-7 mice per genotype. *P < 0.05, **P < 0.01; Student's t-test. See also Figure S6.
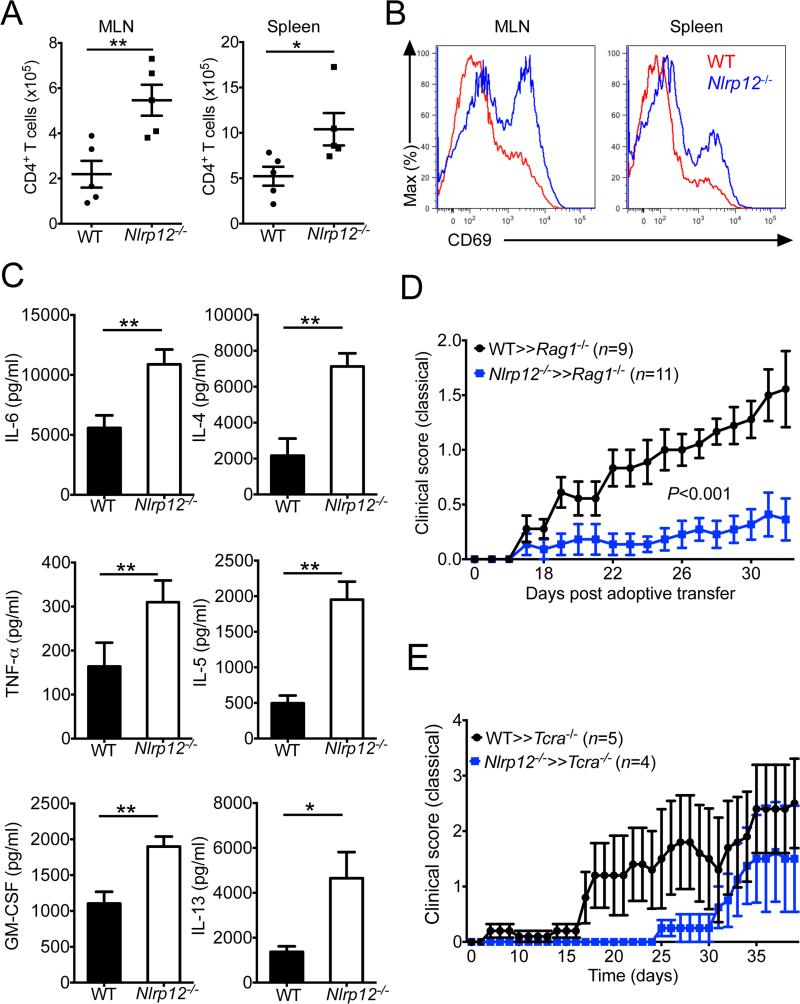
We next determined the mechanisms by which Nlrp12−/− T cells induce intestinal inflammation and atopic dermatitis. We found that Nlrp12−/− T cells accumulated in greater numbers (Figure 6A) and exhibited a more activated phenotype (Figure 6B) in the T cell transfer colitis model. To investigate the contributions of altered inflammatory cytokine production in exacerbated disease progression, we evaluated cytokine production following overnight (16 hrs) anti-CD3 stimulation. T cell receptor (TCR)-mediated activation of cells isolated from Nlrp12−/− CD4+CD45RBhi T cell transfer mice resulted in more robust secretion of proinflammatory cytokines including IL-6, TNF-α, and GM-CSF (Figure 6C left panel). Furthermore, Nlrp12−/− T cells produced potent amounts of Th2 cell-associated cytokines including IL-4, IL-5, and IL-13 (Figure 6C right panel), which helps to explain the development of atopic dermatitis in these mice. Consistent with the more pronounced production of Th2 cell-derived cytokines in Nlrp12−/− T cell transfer mice, the expression of YM1 and MBP, which are markers that have been associated with Th2 cell-mediated inflammation, were also markedly increased in the colons of Nlrp12−/− CD4+CD45RBhi T cell transfer mice (Figure S7)(Ponomarev et al., 2007). These results identify NLRP12 as a negative regulator of T cell-induced inflammatory disease and suggest that unchecked T cell-mediated cytokine production centrally contributes to disease pathology.
Figure 6. Dysregulated T cell activation and cytokine production contribute to the pathogenicity of Nlrp12−/− T cells.
Purified CD4+CD45RBhi WT or Nlrp12−/− T cells were adoptively transferred into Rag1−/− mice. (A) Total numbers (mean ± s.e.m.) of CD4+ T cells in the mesenteric LNs (MLN) and spleen at 9 weeks post-adoptive transfer. (B) Expression of CD69 by adoptively transferred CD4+ T cells in the MLNs and spleen. (C) WT (n = 5) and Nlrp12−/− (n = 5) splenocytes were stimulated overnight with anti-CD3 and cytokine production was measured by ELISA. (D) Passive transfer EAE was induced in Rag1−/− recipient mice by transferring enriched myelin-specific CD4+ T cells that were generated and expanded from WT and Nlrp12−/− mice. Classical signs of EAE disease (ascending paralysis) were assessed over time. (E) T cell deficient mice (Tcra−/− mice) were reconstituted with WT or Nlrp12−/− thymocytes. Mice were then immunized with MOG plus CFA to induce EAE and the development of classical signs of clinical disease (ascending paralysis) were assessed over time. All graphs depict mean ± s.e.m. *P < 0.05, **P < 0.01; Student's t-test. See also Figure S7.
Since our findings in the CD4+CD45RBhi T cell transfer model of colitis indicated that NLRP12 can influence autoinflammatory disease pathogenesis through specific actions in T cells, we were next interested in elucidating whether NLRP12-dependent regulation of T cells responses contributed to the altered EAE disease phenotype that was observed in Nlrp12−/− mice. To this end we evaluated the development of demyelinating neuroinflammatory disease in the passive transfer model of EAE disease. Consistent with our previous data showing abrogated classical EAE disease in germline NLRP12 deficient mice (Figure 2), the transfer of enriched myelin-specific Nlrp12−/− T cells into Rag1−/− recipient mice resulted in less severe paralyzing neuroinflammatory disease (Figure 6D). As an independent approach, WT or Nlrp12−/− thymocytes were also adoptively transferred into T cell deficient mice (Tcra−/− mice) and EAE was induced. In agreement with our previous results, Tcra−/− mice that were reconstituted with Nlrp12−/− thymocytes were also protected from severe paralyzing EAE disease (Figure 6E). These studies in T cell transfer models of disease thus implicate critical T cell intrinsic roles for NLRP12 in the regulation of autoinflammation and autoimmune disease pathogenesis.
NLRP12 negatively regulates NF-κB signaling in T cells
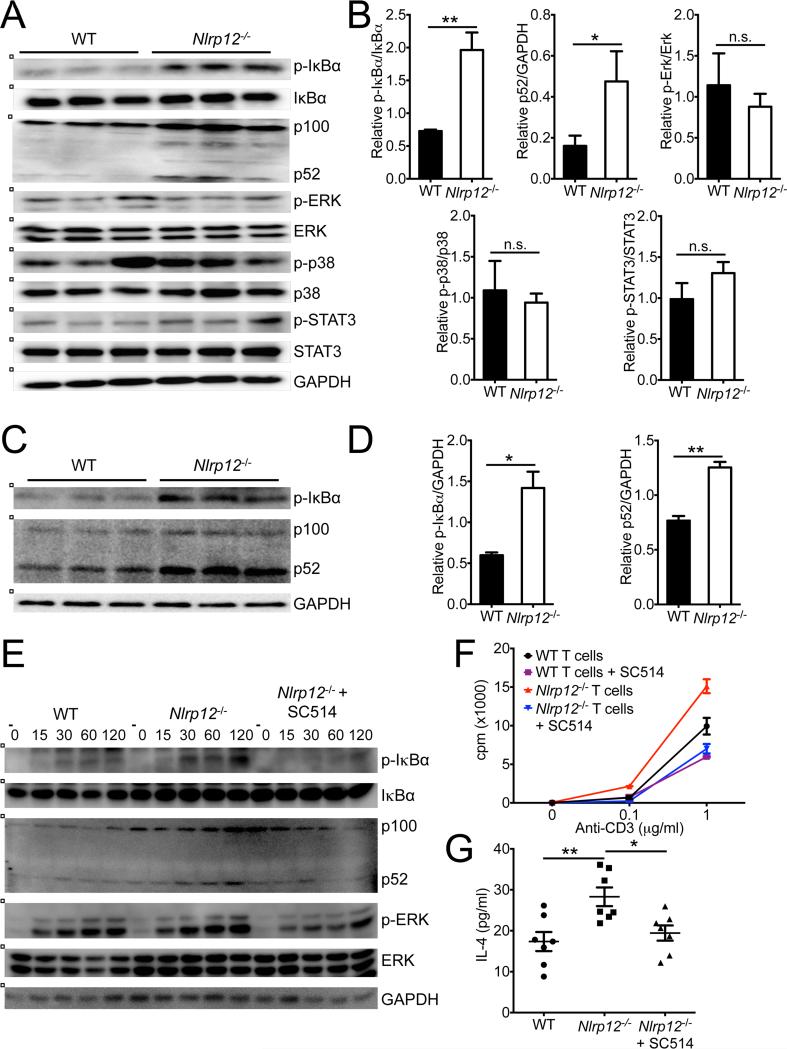
Previous studies suggest that NLRP12 dampens TLR-induced NF-κB activation in macrophages and dendritic cells(Lich et al., 2007; Williams et al., 2005; Zaki et al., 2011), however, the specific molecular pathways that are controlled by NLRP12 in T cells remain unknown. Many of the cytokines that were found to be enhanced in Nlrp12−/− T cell transfer colitis mice are known to be regulated by NF-κB-dependent signaling (Figure 6C), thus we were interested in determining whether NLRP12 limits T cell-induced inflammatory disease through its regulation of NF-κB activation. Indeed, transfer of Nlrp12−/− CD4+CD45RBhi T cells resulted in enhanced phosphorylation of IκBα (Figure 7A, B). Moreover, we also detected greater NF-κB2 processing (conversion of p100 to p52) in Nlrp12−/− CD4+CD45RBhi T cell transfer colon lysates, which indicates enhanced non-canonical NF-κB signaling in the absence of NLRP12 (Figure 7A, B). To elucidate whether NLRP12 is involved in the global dampening of proinflammatory signaling or if it is specifically involved in the NF-κB pathway, we also evaluated the regulation of other important signaling pathways that are known to drive disease pathology in the T cell transfer colitis model. Exacerbated inflammatory disease in CD4+CD45RBhi Nlrp12−/− T cell transfer mice was not, however, associated with altered ERK, p38 or STAT3 signaling (Figure 7A, B). Importantly, we also observed enhanced canonical and non-canonical NF-κB signaling in the brains of NLRP12-deficient mice during EAE (Figure 7C, D).
Figure 7. NLRP12 negatively regulates both canonical and non-canonical NF-κB signaling.
(A,B) Purified CD4+CD45RBhi WT or Nlrp12−/− T cells were adoptively transferred into Rag1−/− mice. Colons were collected from WT and Nlrp12−/− CD4+CD45RBhi transfer mice at 9 weeks post-adoptive transfer. (A) Western blot analysis of colon lysates. Each lane represents an individual mouse. (B) Densitometry quantification of band intensity. Data are representative of four independent experiments with 3-7 mice per genotype. (C,D) WT and Nlrp12−/− mice were immunized with MOG+CFA and pertussis toxin to induce EAE and mice were harvested on day 29. Western blot analysis of brain lysates. (D) Densitometry quantification of band intensity. Data are representative of three independent experiments with 3-5 mice per genotype. (E-G) Purified WT and Nlrp12−/− T cells were stimulated with anti-CD3 (1 μg/ml) plus anti-CD28 (1 μg/ml) in the presence or absence of the NF-κB inhibitor SC514 (3 μg/ml). (E) Lysates were probed for total and phosphorylated IκBα, ERK p100/p52 and GAPDH. (F) Counts per minute (CPM) of T cells at 72 hrs post-stimulation with anti-CD3 and anti-CD28. (G) Secretion of IL-4 at 72 hrs post-stimulation with anti-CD3 and anti-CD28. The bar graphs show mean ± s.e.m. *P < 0.05, **P < 0.01; Student's t-test.
To determine if NLRP12-mediated regulation of NF-κB activation in T cells potentially contributes to the altered inflammatory signaling that was observed in these two models of autoimmunity, we evaluated signaling in purified T cells. We found that stimulation of Nlrp12−/− T cells with anti-CD3 plus anti-CD28 led to enhanced phosphorylation of IκBα and greater amounts of processing of p100 to p52 (Figure 7E). Furthermore, pharmacological inhibition of NF-κB activation with SC514 treatment rescued the hyperproliferation exhibited by anti-CD3 plus anti-CD28 stimulated Nlrp12−/− T cells (Figure 7F). Inhibition of NF-κB signaling with SC514 was also found to return IL-4 secretion by Nlrp12−/− T cells to wild-type amounts (Figure 7G). Collectively, these findings identify NLRP12 as a negative regulator of both canonical and non-canonical NF-κB signaling in T cells.
DISCUSSION
Mutations in Nlrp12 have been described to cause autoinflammatory disorders in humans (Borghini et al., 2011; Jeru et al., 2008; Jeru et al., 2011; Macaluso et al., 2007), however the cellular and molecular mechanisms involved in NLRP12-mediated regulation of immune responses remain poorly defined. In this study, we focused on elucidating the roles of NLRP12 in shaping inflammatory T cell responses and T cell-mediated disease. We discovered that the induction of experimental autoimmune encephalomyelitis (EAE) in NLRP12-deficient mice provoked the generation of hyperinflammatory myelin-specific T cell responses. Nlrp12−/− T cells did not, however, cause exacerbated demyelinating disease in the T cell-driven EAE model. In contrast, Nlrp12−/− mice developed atypical neuroinflammatory disease symptoms that included loss of balance and ataxia. Moreover, the development of non-classical EAE disease in mice that lack NLRP12 was characterized by altered brain pathology and a myeloid cell CNS infiltrate that was dominated by neutrophils. We demonstrated that NLRP12 is an intrinsic negative regulator of T cells, and that NLRP12 deficiency in T cells was sufficient to induce colitis and atopic dermatitis in the CD4+CD45RBhi T cell transfer model. Mechanistically, dysregulated NF-κB activation and enhanced IL-4 production were found to contribute to T cell-induced inflammation in Nlrp12−/− mice. Our findings define a previously unknown role for NLRs in the negative regulation of T cell activation, and unravel a T cell-intrinsic function for NLRP12 in the suppression of inflammatory cytokine production. Furthermore, our discovery that NLRP12 deficiency leads to unchecked IL-4 production helps to explain how missense mutations in Nlrp12 contribute to the development of atopic dermatitis in humans.
Utilization of the EAE model system has helped to define the inflammatory factors that contribute to demyelination and paralysis in multiple sclerosis (MS) pathogenesis. However, considerably less is known about the conditions and pathways that induce many of the other disease symptoms that are common in MS patients; including ataxia, impaired balance and loss of sensation. Our findings presented here, suggest that investigation of EAE in Nlrp12−/− mice could provide a much needed model system to formally characterize the immune factors that trigger non-paralyzing neuroinflammatory disease symptoms in MS patients. Importantly, excessive production of both classical inflammatory factors (IFN-γ, IL-6, GM-CSF) and Th2 cell-associated cytokines (IL-4 and IL-5) have been observed in patients with severe MS(Hedegaard et al., 2008; Hohnoki et al., 1998; Link et al., 1994). However, the physiological role of Th2 cell cytokines in MS disease pathogenesis has remained elusive. In the EAE mouse model, IL-4 has been described to provide protection against classical ascending paralysis(Bettelli et al., 1998; Falcone et al., 1998; Furlan et al., 1998; Shaw et al., 1997). In agreement with these findings, we observed that enhanced IL-4 production limited the development of paralysis in NLRP12-deficient mice. Unchecked IL-4 production during EAE has also been shown to induce atypical neuroinflammatory disease symptoms (ataxia and issues with balance), which is consistent with the IL-4-associated atypical neuroinflammatory disease phenotype that was observed in Nlrp12−/− mice(Delgoffe et al., 2011; Wensky et al., 2001).
Th2 cell-associated cytokines also play pathogenic roles in intestinal inflammatory disease. In particular, Th2 cell cytokines are highly expressed in combination with Th1 cell-associated cytokines in ulcerative colitis (UC) patients(Fuss et al., 1996), and Th2 cell cytokines are believed to be the major drivers of pathology in UC(Cho, 2008). Likewise, Th2 cell cytokines potentiate UC-like intestinal disease in the oxazolone colitis mouse model (Boirivant et al., 1998; Heller et al., 2002; Mizoguchi et al., 1999). In these studies, it was shown that Th2 cell cytokines cause inflammation to the intestinal epithelial layer and that neutralization of IL-4 with blocking antibody treatment rescues oxazolone-mediated colitis. Similarly, hyperinflammatory IL-4 production has also been described to cause exacerbated disease in the CD4+CD45RBhi T cell transfer colitis model(Fort et al., 2001). In this setting, Th2 cell cytokines promote the upregulation of major hisotocmpatibility complex (MHC) class II expression in the lamina propria of the colon and also stimulate the expression of exorbitant amounts of both Th1 and Th2 cell-associated cytokines in the intestinal mucosa. Our findings demonstrate prominent roles for NLRP12 in the attenuation of colitogenic T cell responses and identify NLRP12 as an attractive candidate for the treatment of Th2 cell-associated intestinal inflammatory disease.
In summary, we identify NLRP12 as a negative regulator of inflammatory T cell responses and T cell-mediated disease. NLRP12 intrinsically regulates T cell activation, and dysregulated NLRP12-mediated signaling in T cells is sufficient to induce inflammatory disease. However, deficiency in NLRP12 did not provoke more severe demyelinating disease and paralysis in the EAE model. In contrast, Nlrp12−/− mice developed atypical neuroinflammatory disease that was characterized by worsened brain pathology and excessive production of both traditional inflammatory cytokines (IL-6, GM-CSF, TNF-α) and IL-4. Furthermore, dysregulated T cell-mediated NF-κB signaling and downstream IL-4 were found to contribute to inflammatory responses in Nlrp12−/− mice. These findings define additional roles for NLRP12 as an intrinsic attenuator of T cell responses and IL-4 associated inflammation. Moreover, they suggest that NLRP12 targeted therapies may provide a novel treatment strategy in T cell-mediated disease.
EXPERIMENTAL PROCEDURES
Mice
Nlrp12−/− mice have been previously described(Zaki et al., 2011). Tcra−/− mice were kindly provided by Dr. Maureen McGargill (St. Jude Children's Research Hospital) and Rag1−/− mice were received from Dr. Terrence L. Geiger (St. Jude Children's Research Hospital). Mice at 6–12 weeks of age were used. All mice were kept in specific pathogen-free conditions within the Animal Resource Center at St. Jude Children's Research Hospital. Animal protocols were approved by the Institutional Animal Care and Use Committee of St. Jude Children's Research Hospital.
Flow cytometry protocols and antibodies
For the flow cytometry cellular analysis, the following monoclonal antibodies were used: CD4 (L3T4), IFN-γ (XMG1.2), IL-17A (eBio17B7), IL-4 (11B11), MHCII (M5/114.15.2), CD11b (M1/70), CD19 (6D5), CD44 (IM7), Ly-6G (1A8), CD25 (3C7), B220 (RA3-6B2), and Gr-1 (RB6-8C5) from eBioscience and TCR-β (H57-597), CD8 (53-6.7), Foxp3 (FJK-16s), CD62L (MEL-14), CD69 (H1.2F3), TNF-α (MP6-XT22), CD11c (N418), CD45.1 (A20), and CD45.2 (104) from Biolegend.
Histopathology and immunohistochemistry
Nlrp12 expression
Macrophages (Ly6G−CD11b+), dendritic cells (CD11c+MHCIIhi), CD4+ T cells (Thy1.2+CD4+), CD8+ T cells (Thy1.2+CD8+) and polymorphonuclear leukocytes (PMNs) (Ly6G+CD11b+) were purified by flow cytometry sorting and total RNA was isolated using Trizol (Invitrogen) according to the manufacturer's instructions. 1 μg of RNA was reverse-transcribed to cDNA with random RNA-specific primers using the high-capacity cDNA reverse transcription kit (Applied Biosystems). Transcript amounts of Nlrp12 and Gapdh were analyzed using SYBR-Green (Applied Biosystems) according to the manufacturers’ recommendations.
Thymidine incorporation assay
Thy1.2+ T cells were isolated from WT and Nlrp12−/− mice using positive magnetic bead enrichment (Miltenyi Biotec). Naïve T cells (CD44loCD62Lhi) were then purified via FACS sorting. Purified naïve T cells were stimulated with increasing concentrations of plate bound anti-CD3 in triplicate wells for 48 hrs. During the last 8 hrs of stimulation T cells were pulsed with [3H] thymidine and the amount of incorporated [3H] thymidine was measured as counts per minute (cpm).
Competitive T cell adoptive transfer
Mixed bone marrow chimeras
Bone marrow from WT (CD45.1+) and Nlrp12−/− (CD45.2+) mice was flushed from the femurs, filtered through a 40 μm filter, and mixed at a 1:1 ratio. Mixed bone marrow cells (total 5-10×106 cells) were transferred by tail vein injection into lethally irradiated (1,000 rad) Rag1−/− mice. 1:1 bone marrow chimera mice were allowed to reconstitute for 2 months before immunization with MOG peptide in CFA. Congenic CD45 markers were utilized to verify chimerism.
CD4+CD45RBhi T cell transfer colitis model
Splenocytes and peripheral LNs were harvested from WT and Nlrp12−/− mice and bulk T cells were positively enriched using anti-Thy1.2 magnetic beads (Miltenyi Biotec). CD4+CD45RBhi T cells were purified from the bulk T cell population by FACs sorting. Rag1−/− recipient mice received 5×105 WT or Nlrp12−/−CD4+CD45RBhi T cells by intraperitoneal injection and mice were weighed once every week to measure percent weight change.
EAE Model
Mice were immunized subcutaneously with 100 μg MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) emulsified in CFA (Difco Laboratories) with 500 μg Mycobacterium tuberculosis on day 0. Mice also received 200 ng of pertussis toxin (List Biological Laboratories, Inc.) by intraperitoneal injection on days 0 and 2. Classical disease severity was assessed daily by assigning clinical scores according to the following ascending paralysis scale: 0, no disease; 1, tail paralysis; 2, weakness of hind limbs; 3, paralysis of hind limbs; 4, paralysis of hind limbs and severe hunched posture; 5, moribund or death. The incidence of atypical disease was recorded based on the development of balance issues and ataxia. To evaluate the role of IL-4 in Nlrp12-mediated atypical neuroinflammatory disease, EAE was induced in WT and Nlrp12−/− mice as described above. Mice received either vehicle control or 500 μg anti-IL-4 blocking antibody (11B11) by intraperitoneal dosage on days -1, 0, 2, 4, 6, 8, and 13, and classical clinical scores were assigned based on ascending paralysis development.
Chronic DSS model
Regulatory T cell (Treg) suppression assay
Passive EAE transfer model
Thymocyte transfer EAE model
CFSE T cell proliferation Assay
Statistics
All results are presented as mean ± s.e.m. Two-way ANOVA analysis was utilized to evaluate differences in clinical EAE scores over time. All other P-values were calculated by two-tailed unpaired Student's t tests. P-values <0.05 were considered significant. P values are denoted by *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Material
ACKNOWLEDGMENTS
ML is supported by European Union Marie-Curie grant 256432, ERC Grant 281600, and grants G030212N, 1.2.201.10.N.00, and 1.5.122.11.N.00 from the Fund for Scientific Research-Flanders. PG is a postdoctoral fellow supported by Paul Barrett Endowed Fellowship from St. Jude Children's Research Hospital. This work was supported by: the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR056296 (T.-D.K); the National Cancer Institute, part of the National Institutes of Health, under Award Number CA163507 (T.-D.K); the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, under Award Number AI101935 (T.-D.K); and ALSAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.R.L., P.G., and T.-D.K. designed the study. J.R.L., P.G., P.J.S., M.H.Z., and M.J.B. performed experiments. P.V. performed and analyzed the histopathology data. S.B. made IL-4 neutralizing antibodies. H.C. provided essential reagents and scientific insights. J.R.L., P.G., and T.-D.K. analyzed data and wrote the manuscript.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth HH, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. Journal of immunology. 1998;161:3299–3306. [PubMed] [Google Scholar]
- Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. The Journal of experimental medicine. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghini S, Tassi S, Chiesa S, Caroli F, Carta S, Caorsi R, Fiore M, Delfino L, Lasiglie D, Ferraris C, et al. Clinical presentation and pathogenesis of cold-induced autoinflammatory disease in a family with recurrence of an NLRP12 mutation. Arthritis and rheumatism. 2011;63:830–839. doi: 10.1002/art.30170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GY, Liu M, Wang F, Bertin J, Nunez G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. Journal of immunology. 2011;186:7187–7194. doi: 10.4049/jimmunol.1100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nature reviews. Immunology. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- Davenport CM, McAdams HA, Kou J, Mascioli K, Eichman C, Healy L, Peterson J, Murphy S, Coppola D, Truneh A. Inhibition of pro-inflammatory cytokine generation by CTLA4-Ig in the skin and colon of mice adoptively transplanted with CD45RBhi CD4+ T cells correlates with suppression of psoriasis and colitis. International immunopharmacology. 2002;2:653–672. doi: 10.1016/s1567-5769(01)00201-6. [DOI] [PubMed] [Google Scholar]
- Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature immunology. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone M, Rajan AJ, Bloom BR, Brosnan CF. A critical role for IL-4 in regulating disease severity in experimental allergic encephalomyelitis as demonstrated in IL-4-deficient C57BL/6 mice and BALB/c mice. Journal of immunology. 1998;160:4822–4830. [PubMed] [Google Scholar]
- Fort M, Lesley R, Davidson N, Menon S, Brombacher F, Leach M, Rennick D. IL-4 exacerbates disease in a Th1 cell transfer model of colitis. Journal of immunology. 2001;166:2793–2800. doi: 10.4049/jimmunol.166.4.2793. [DOI] [PubMed] [Google Scholar]
- Furlan R, Poliani PL, Galbiati F, Bergami A, Grimaldi LM, Comi G, Adorini L, Martino G. Central nervous system delivery of interleukin 4 by a nonreplicative herpes simplex type 1 viral vector ameliorates autoimmune demyelination. Human gene therapy. 1998;9:2605–2617. doi: 10.1089/hum.1998.9.17-2605. [DOI] [PubMed] [Google Scholar]
- Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. Journal of immunology. 1996;157:1261–1270. [PubMed] [Google Scholar]
- Goverman J. Autoimmune T cell responses in the central nervous system. Nature reviews. Immunology. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125:161–169. doi: 10.1111/j.1365-2567.2008.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- Hohnoki K, Inoue A, Koh CS. Elevated serum levels of IFN-gamma, IL-4 and TNF-alpha/unelevated serum levels of IL-10 in patients with demyelinating diseases during the acute stage. Journal of neuroimmunology. 1998;87:27–32. doi: 10.1016/s0165-5728(98)00053-8. [DOI] [PubMed] [Google Scholar]
- Jeru I, Duquesnoy P, Fernandes-Alnemri T, Cochet E, Yu JW, Lackmy-Port-Lis M, Grimprel E, Landman-Parker J, Hentgen V, Marlin S, et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1614–1619. doi: 10.1073/pnas.0708616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeru I, Le Borgne G, Cochet E, Hayrapetyan H, Duquesnoy P, Grateau G, Morali A, Sarkisian T, Amselem S. Identification and functional consequences of a recurrent NLRP12 missense mutation in periodic fever syndromes. Arthritis and rheumatism. 2011;63:1459–1464. doi: 10.1002/art.30241. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nature reviews. Immunology. 2010;10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti TD, Dixit VD. Immunological complications of obesity. Nature immunology. 2012;13:707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Kroenke MA, Chensue SW, Segal BM. EAE mediated by a non-IFN-gamma/non-IL-17 pathway. European journal of immunology. 2010;40:2340–2348. doi: 10.1002/eji.201040489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Walle LV, Kanneganti TD. Deregulated inflammasome signaling in disease. Immunological reviews. 2011;243:163–173. doi: 10.1111/j.1600-065X.2011.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y, Wen H, Yu Y, Taxman DJ, Zhang L, Widman DG, Swanson KV, Wen KW, Damania B, Moore CB, et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity. 2012;36:933–946. doi: 10.1016/j.immuni.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon F, Contractor N, Fuss I, Marth T, Lahey E, Iwaki S, la Sala A, Hoffmann V, Strober W, Kelsall BL. Antibodies to complement receptor 3 treat established inflammation in murine models of colitis and a novel model of psoriasiform dermatitis. Journal of immunology. 2006;177:6974–6982. doi: 10.4049/jimmunol.177.10.6974. [DOI] [PubMed] [Google Scholar]
- Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, Taxman DJ, Ting JP. Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. Journal of immunology. 2007;178:1256–1260. doi: 10.4049/jimmunol.178.3.1256. [DOI] [PubMed] [Google Scholar]
- Link J, Soderstrom M, Olsson T, Hojeberg B, Ljungdahl A, Link H. Increased transforming growth factor-beta, interleukin-4, and interferon-gamma in multiple sclerosis. Annals of neurology. 1994;36:379–386. doi: 10.1002/ana.410360309. [DOI] [PubMed] [Google Scholar]
- Lukens JR, Dixit VD, Kanneganti TD. Inflammasome activation in obesity-related inflammatory diseases and autoimmunity. Discovery medicine1. 2011;2:65–74. [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Gross JM, Kanneganti TD. IL-1 family cytokines trigger sterile inflammatory disease. Frontiers in immunology. 2012;3:315. doi: 10.3389/fimmu.2012.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso F, Nothnagel M, Parwez Q, Petrasch-Parwez E, Bechara FG, Epplen JT, Hoffjan S. Polymorphisms in NACHT-LRR (NLR) genes in atopic dermatitis. Experimental. 2007;16:692–698. doi: 10.1111/j.1600-0625.2007.00589.x. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Mizoguchi E, Bhan AK. The critical role of interleukin 4 but not interferon gamma in the pathogenesis of colitis in T-cell receptor alpha mutant mice. Gastroenterology. 1999;116:320–326. doi: 10.1016/s0016-5085(99)70128-9. [DOI] [PubMed] [Google Scholar]
- Normand S, Delanoye-Crespin A, Bressenot A, Huot L, Grandjean T, Peyrin-Biroulet L, Lemoine Y, Hot D, Chamaillard M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9601–9606. doi: 10.1073/pnas.1100981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon MP, Detmar M, Parker CM. Murine psoriasis-like disorder induced by naive CD4+ T cells. Nature medicine. 1997;3:183–188. doi: 10.1038/nm0297-183. [DOI] [PubMed] [Google Scholar]
- Shaw MK, Lorens JB, Dhawan A, DalCanto R, Tse HY, Tran AB, Bonpane C, Eswaran SL, Brocke S, Sarvetnick N, et al. Local delivery of interleukin 4 by retrovirus-transduced T lymphocytes ameliorates experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 1997;185:1711–1714. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, McDermott MF, Kanneganti TD. Inflammasomes and autoimmunity. Trends in molecular medicine. 2011;17:57–64. doi: 10.1016/j.molmed.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nature reviews. Immunology. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nature medicine. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK, Liu Q, et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity. 2012;37:96–107. doi: 10.1016/j.immuni.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensky A, Marcondes MC, Lafaille JJ. The role of IFN-gamma in the production of Th2 subpopulations: implications for variable Th2-mediated pathologies in autoimmunity. Journal of immunology. 2001;167:3074–3081. doi: 10.4049/jimmunol.167.6.3074. [DOI] [PubMed] [Google Scholar]
- Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA, Su L, et al. The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. The Journal of biological chemistry. 2005;280:39914–39924. doi: 10.1074/jbc.M502820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Cui J, Wang HY, Zhu L, Matsueda S, Wang Q, Yang X, Hong J, Songyang Z, Chen ZJ, Wang RF. NLRX1 negatively regulates TLR-induced NF-kappaB signaling by targeting TRAF6 and IKK. Immunity. 2011;34:843–853. doi: 10.1016/j.immuni.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MH, Man SM, Vogel P, Lamkanfi M, Kanneganti TD. Salmonella exploits NLRP12-dependent innate immune signaling to suppress host defenses during infection. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:385–390. doi: 10.1073/pnas.1317643111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M, Kanneganti TD. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer cell. 2011;20:649–660. doi: 10.1016/j.ccr.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.