Abstract
OBJECTIVES
Outcomes of catheter ablation of atrial fibrillation (AF) vary widely. We used late-gadolinium enhancement MRI (LGE-MRI) to examine the relationship of ablation-induced scarring in the pulmonary vein (PV) region and overall atrium to evaluate the role of PV encirclement and substrate modification in predicting outcome of catheter ablation in AF.
METHODS AND RESULTS
LGE-MRI was performed to quantify baseline atrial fibrosis, which was classified into four stages (stage I with fibrosis <10%, stage II with fibrosis 10–20%, stage III with fibrosis 20–30%, and stage IV with fibrosis ≥30%). Patients then underwent ablation and repeat LGE-MRI at three months to assess for ablation-induced scarring. PVs were studied to evaluate for complete encirclement with scar. Image processing was used to overlay the scar onto baseline fibrosis to assess the overlap and calculate residual fibrosis. A total of 172 patients were included with an average baseline fibrosis of 14.6 ± 8.4%. The average number of PVs encircled with scar at three months was 1.2 ± 1.3 with only 9% of patients having all four PVs completely encircled. The average residual fibrosis was 11.9 ± 7.3%. High residual fibrosis was defined as >10%. Recurrent AF was found in 60 patients (34.9%) over a follow-up of 346 ± 82 days. Baseline and high residual fibrosis were significant predictors of recurrence (hazard ratio [HR] of 2.2; P < 0.01 and HR of 3.2; P < 0.01, respectively). The number of PV encircled was not a significant predictor of recurrence.
CONCLUSION
LGE-MRI of ablation-induced scarring demonstrates that chronic PV encirclement is rarely achieved. Procedural outcomes are better predicted by baseline atrial fibrosis and ablation-induced substrate modification.
Keywords: atrial fibrillation, catheter ablation, cardiac MRI
Introduction
Atrial fibrillation (AF) is estimated to currently affect 5 million Americans and 6 million Europeans, and its prevalence is expected to increase over the next three decades.1,2 The mechanisms underlying the initiation and maintenance of this arrhythmia have been studied in humans as well as in animal models.3 Most experts agree that triggers identified in the pulmonary veins (PVs) play an important role in arrhythmia initiation,4 while maintenance of the arrhythmia requires electrical as well as structural atrial tissue remodeling to be present.5
Catheter ablation of the left atrium is increasingly performed to treat patients with AF. Several approaches to ablation are in use at different centers. Most operators aim to achieve electrical isolation of PV triggers, and some advocate ablation of additional targets.6
Late-gadolinium enhancement MRI (LGE-MRI) has been shown to identify areas of fibrosis7,8 and scarring8–10 in the left atrium before and after ablation. The predictive value of atrial tissue fibrosis prior to ablation in predicting arrhythmia recurrence following ablation has been demonstrated.8,9 The amount of ablation-induced scarring has been suggested to be an important predictor of arrhythmia recurrence.8,9
In this study, we evaluated the effect of radiofrequency ablation on the atrial substrate in two ways: first, by studying its effectiveness in achieving encircling scar around the PV antra. Second, we studied the relationship of ablation-induced scarring with pre-ablation atrial fibrosis and quantified the overlap of scar with pre-existing atrial fibrosis, estimating the residual unablated fibrotic atrial tissue. We hypothesized that the larger the amount of residual fibrotic atrial tissue following ablation, the higher the risk of arrhythmia recurrence.
Methods
Patient population
A total of 172 patients presenting for the first time catheter ablation of AF were included in this retrospective study. All patient information used was drawn from the University of Utah AF database. Baseline patient demographics, comorbidities, prior medical histories, medications, and pertinent laboratory and imaging findings were recorded and protected according to Health Insurance Portability and Accountability Act (HIPAA) regulations. The University of Utah institutional review board approved the database protocol. This study did not require a separate IRB protocol. The research protocol was in accordance to the Declaration of Helsinki.
LGE-MRI imaging
All patients underwent LGE-MRI prior to catheter ablation as well as three months following ablation. All scans were performed using a 3-T Verio clinical scanner (Siemens Medical Solutions). MRI acquisition sequences have been described previously.7–9 Briefly, high-resolution LGE images of the left atrium were acquired about 15 minutes following contrast injection (0.1 mmol/kg, Multihance [Bracco Diagnostics Inc.]) using a three-dimensional (3D) inversion recovery prepared, respiration-navigated, electrocardiogram (ECG)-gated, gradient echo pulse sequence. Typical acquisition parameters were free breathing using respiratory navigation, a transverse imaging volume with voxel size = 1.25 × 1.25 × 2.5 mm (reconstructed to 0.625 × 0.625 × 1.25 mm), repetition time/echo time (TR/TE) = 3.1/1.4 mseconds, flip angle = 14°, inversion time (TI) = 280–330 mseconds, and Generalized Autocalibrating Partially Parallel Acquisition (GRAPPA) with reduction factor of 2. Inversion pulse was applied every heart beat, and fat saturation was applied immediately before data acquisition. Data acquisition was limited to 15% of cardiac cycle and was performed during the diastolic phase of the atrial cardiac cycle. The TE of the scan (1.4 mseconds) was chosen such that fat and water were almost out of phase and the signal intensity of partial volume fat-tissue voxels was reduced allowing improved delineation of the atrial wall boundaries. The TI value for the LGE-MRI scan was identified using a TI scout scan. Typical scan time for the LGE-MRI study was four to nine minutes depending on subject respiration.
Catheter ablation
Ablation was performed under conscious sedation, with a double trans-septal puncture approach and using a circular mapping catheter and an ablation catheter. Ablation lesions were placed using a 3.5-mm open irrigation catheter (Thermocool; Biosense Webster). Ablation parameters were set at 35 W while ablating posteriorly and 50 W for septal and anterior ablation. The PV antral regions were targeted with ablation with a primary goal of electrical isolation verified with pacing to test for entrance and exit block. Isoproterenol was infused following PV isolation to assess for reconnection and extra-PV focal firing. The antral regions were retargeted with ablation if reconnection was demonstrated. Extra-pulmonary focal firing was also targeted when found with and without isoproterenol. For patients who remained in AF, additional ablation lesions were placed in the posterior left atrial wall, inter-atrial septum, and mitral isthmus. Direct current cardioversion was performed to restore sinus rhythm when AF persisted following ablation. All ablations were performed on therapeutic anti-coagulation with warfarin and intra-procedural intravenous heparin dosed to maintain an activated clotting time of 350–400 seconds.
Quantification of atrial tissue fibrosis and post- ablation scarring
Left atrial wall volumes were manually segmented from the LGE-MRI images by expert observers using the Corview image processing software (Marrek Inc.). The protocol for segmentation was as follows: First, the endocardial border of the LA was defined, including an extent of PV sleeves, by manually tracing the LA-PV blood pool in each slice of the LGE-MRI volume. Next, the endocardial segmentation was morphologically dilated and then manually adjusted to create an assessment of the boundary of the epicardial LA surface. Finally, the endocardial segmentation was subtracted from the epicardial segmentation to define a wall segmentation, which was manually edited to exclude the mitral valve and PVs. Thus, the resulting LA wall segmentation included the 3D extent of both the LA wall and the antral regions of the PVs.
To delineate regions of fibrosis in LGE-MRI images, we defined enhancement through an intensity threshold that was determined by expert inspection. To assist this process, initial visualization used a volume-rendering tool in Corview that allowed the operator to visualize the distribution of enhancement in 3D. A custom transfer function allowed the operator to define gradations of enhancements, while suppressing blood and normal tissue with a transfer function. Fibrosis and scarring were reported as a percentage of the atrial wall. Patients were divided according to their baseline fibrosis into four stages described previously8: stage I when the baseline tissue fibrosis was <10%, stage II when the fibrosis was 10–20%, stage III when the fibrosis was 20–30%, and stage IV when the fibrosis was ≥30% (Fig. 1).
Figure 1.
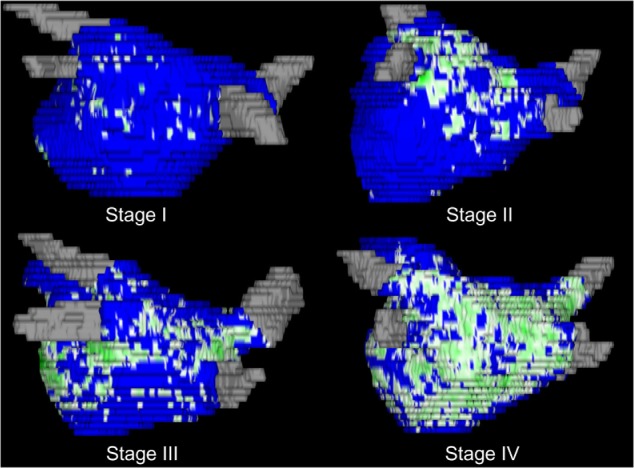
3D left atrial reconstructions from four different patients representing examples of the four-stage system. Green areas represent areas of atrial fibrosis.
Evaluation of the PV antra for encirclement
Using the three months LGE-MRI scans (Fig. 2), we studied the PV antral regions in all 172 patients for the purpose of evaluating whether ablation resulted in an uninterrupted line of scar representing complete encirclement (Fig. 3). When gaps were found, making the scarred areas discontinuous, the antrum was considered not completely encircled.
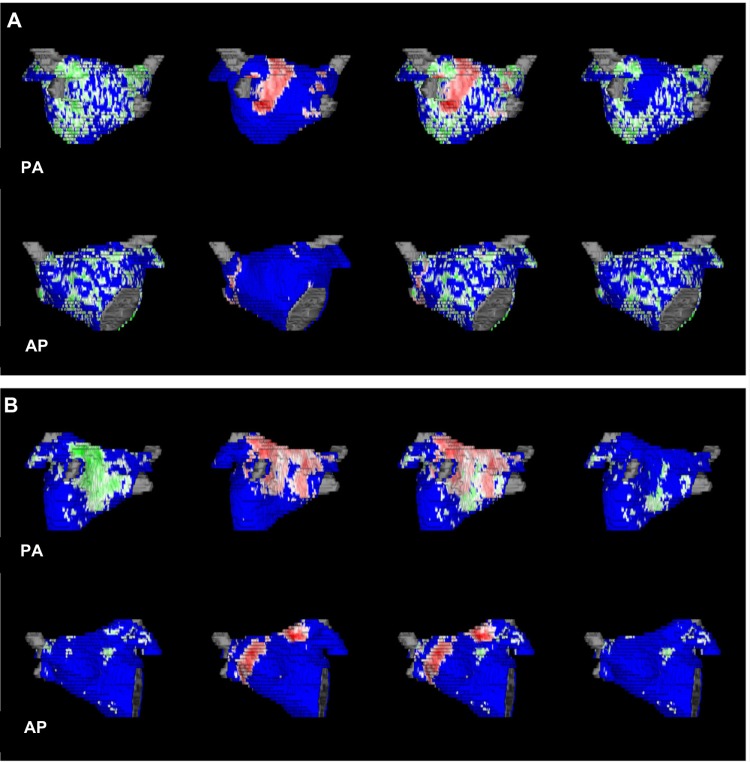
Figure 2.
3D left atrial reconstructions obtained at three months following catheter ablation. Red areas represent ablation-induced scar.
Figure 3.
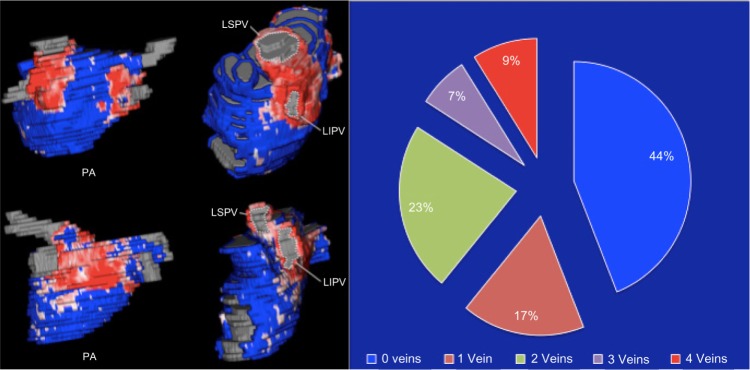
Representative examples of ablation-induced scarring around the PVs and pie chart representing the percentage of patients in groups of PV antra encircled with ablation scar.
Quantification of residual fibrosis
The software pipeline used to quantify residual fibrosis involved a series of image operations and registration algorithms. We started by resampling the volume data to make it isotropic (so that it has the same spacing in all directions). Next, we automatically cropped the image volumes to an area surrounding the left atrium. Registration began first with an affine registration to give a rough alignment of the images. In order to establish the best possible correspondence between the two wall segmentations, we then performed a deformable registration11 to map the post-ablation scan onto the pre-ablation scan.
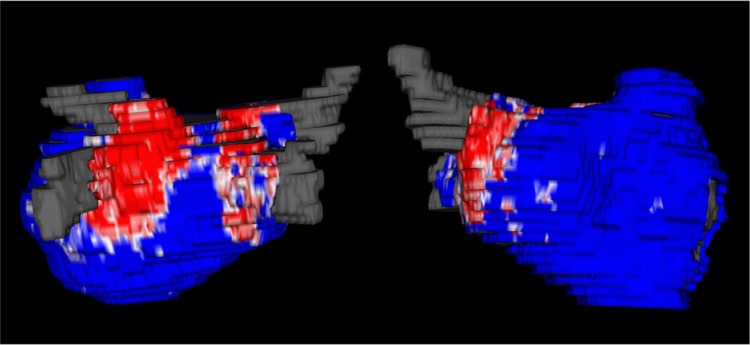
After we established correspondence via registration, we mapped the scarred areas of the post-ablation scan through the transformation and marked them on the pre-ablation scan as an area that has been scarred. The pre-ablation wall segmentation becomes marked such that each pixel inside the wall is fibrosis, scar, both, or neither. From this, we subtracted out areas of fibrosis that were scarred over and computed the residual fibrosis by counting the number of remaining fibrosis pixels in the wall and reported their percentage of wall volume (Fig. 4).
Figure 4.
Two examples of residual fibrosis: the first column represents the pre-ablation fibrosis distribution, the second column represents ablation-induced scarring, the third column represents scarring overlaid on areas of fibrosis, and the fourth column represents the residual fibrosis obtained after subtracting areas of scarring. Panel A represents a patient with diffuse baseline fibrosis; PVAI was performed based on the scar generated. The residual fibrosis is high, and large areas of fibrotic tissue are untouched with ablation. Panel B represents a patient with fibrosis involving the posterior wall; PVAI was also performed based on the scar generated. The degree of overlap is high, and residual fibrosis is low.
Inter-observer variability
Three blinded observers were asked to quantify fibrosis and scarring on randomly selected subjects from this study. The correlation coefficients between observers ranged from 0.82 to 0.97 for fibrosis, 0.98 to 0.99 for scarring, and 0.95 to 0.97 for PV encirclement. This indicates significant reproducibility of the quantified parameters, which reflects the expertise in our laboratory in acquisition, segmentation, and processing of LGE-MRI images.
Statistical analysis
Continuous variables were reported as means and standard deviations. Categorical variables were reported as a percentage of the overall cohort. Comparison of means was done using a student t-test, while a chi-square test was used for comparison of proportions.
Recurrence was defined according to the HRS consensus document on catheter and surgical treatment of AF.12 Recurrence was evaluated using scheduled 7 day ambulatory monitoring performed at 3, 6, and 12 months, in addition to 12-lead ECGs obtained if patients reported symptoms suggestive of arrhythmia.
A Cox proportional hazards model was used to evaluate arrhythmia recurrence. Since baseline and post-ablation residual fibrosis are naturally correlated, separate regression models were built, each including one of these two variables. Up to six variables were included in each of the multivariate models (60 recurrences). A log-rank test was used to compare recurrence between the two groups of residual fibrosis. P values of <0.05 were considered statistically significant for all statistical tests.
Results
Patient characteristics
A total of 172 patients were included in this study. The average baseline atrial fibrosis was 14.7 ± 8.5%. Analysis of baseline patient characteristics showed that with increasing atrial fibrosis (stage classification), a significant increase was noted in the prevalence of persistent AF. Other baseline characteristics were not significantly different across the stage groups. A summary of baseline characteristics is provided in Table 1.
Table 1.
Summary of patient characteristics.
| OVERALL (n = 172) | STAGE I (FIBROSIS <10%) (n = 68) | STAGE II (FIBROSIS 10–20%) (n = 58) | STAGE III (FIBROSIS 20–30%) (n = 37) | STAGE IV (FIBROSIS ≥30%) (n = 9) | P VALUE | |
|---|---|---|---|---|---|---|
| Age (yrs) | 66 ± 11 | 64 ± 13 | 68 ± 10 | 67 ± 11 | 68 ± 11 | 0.21 |
| Gender (% female) | 35.5% | 25.0% | 44.8% | 37.8% | 44.4% | 0.12 |
| Hypertension (%) | 58.7% | 54.1% | 55.2% | 67.6% | 77.8% | 0.10 |
| Diabetes (%) | 17.4% | 19.1% | 15.5% | 18.9% | 11.1% | 0.70 |
| Coronary Disease (%) | 21.5% | 20.6% | 13.8% | 32.4% | 33.3% | 0.16 |
| Congestive Heart Failure (%) | 10.5% | 10.3% | 12.1% | 5.4% | 22.2% | 0.47 |
| Persistent AF (%) | 46.5% | 38.2% | 46.6% | 51.4% | 88.9% | 0.01 |
| Number of PVAs encircled | 1.2 ± 1.3 | 1.4 ± 1.3 | 1.1 ± 1.3 | 1.1 ± 1.4 | 1.0 ± 1.5 | 0.26 |
| Ablation Scarring (%) | 13.1 ± 7.0 | 13.2 ± 6.2 | 12.5 ± 7.1 | 13.2 ± 8.1 | 15.2 ± 8.1 | 0.64 |
PV encirclement
We found that ablation-induced scarring formed a contiguous line around all four veins in only 15 of 172 patients (8.7%). A large proportion of patients (76/172 or 44%) had none of their vein antra completely encircled with ablation scar. Figure 3 shows two examples of scarring around the PV antra and illustrates the distribution of patients in groups according to the number of pulmonary antra encircled.
Residual fibrosis
After subtracting the ablated areas from the baseline atrial scan, the residual fibrosis was calculated. The average residual fibrosis was found to be 11.9 ± 7.3%. High residual fibrosis was defined as ≥10% of the left atrial wall. In all, 85 of 172 patients (49.4%) had high residual fibrosis compared to 87 patients (50.6%) who did not. Overall ablation-induced scarring was not significantly different in patients with high residual fibrosis compared to those without high residual fibrosis (12.5 ± 7.7 vs 13.6 ± 6.3; P = 0.24). Other baseline comorbidities, including age, gender, prevalence of hypertension, diabetes, congestive heart failure, and persistent AF, were not significantly different in patients with and without high residual fibrosis.
Arrhythmia recurrence
Following an average follow-up of 346 ± 82 days, sustained atrial arrhythmia was observed in 60 patients (34.9%) after observing a 90-day blanking period. The mean time to AF recurrence post blanking was 160 ± 68 days. Recurrence was observed in 9 patients in stage I (13.2%), 23 patients in stage II (39.7%), 22 patients in stage III (59.5%), and 6 patients in stage IV (66.7%). The recurrence rate was significantly different across the stages (P < 0.01).
The average residual fibrosis was 15.9 ± 7.6% in patients with recurrent arrhythmia compared to 9.9 ± 6.2% in patients without recurrence (P < 0.001). In patients with high residual fibrosis (>10%), 44 of 85 patients (51.8%) had recurrent AF compared to 16 of 86 patients without high residual fibrosis (18.4%); P < 0.001.
We then examined the role of baseline and residual fibrosis in predicting arrhythmia recurrence using Cox proportional hazards models. In univariate analysis, fibrosis stage classification was a significant predictor of AF recurrence (hazard ratio [HR] of 1.9; P < 0.001) and so was high residual fibrosis (HR of 3.3; P < 0.001). Persistent AF was nearly a significant predictor of recurrence in univariate analysis with an HR of 1.6; P = 0.055 Table 2. Two separate multivariate models were used to examine the roles of baseline fibrosis stage and high residual. In the first multivariate model assessing the role of baseline fibrosis staging, stage II fibrosis was associated with an HR of 3.6 for AF recurrence compared to stage I (P < 0.01), whereas stage III and stage IV were associated with HRs of 5.2 and 6.2, respectively, compared to stage I (P < 0.01 for both) Table 3A. In the second multivariate model, including residual fibrosis, high residual was found to be associated with an HR of 3.3 for recurrent arrhythmia; P < 0.01. Both models included number of PVA encircled, hypertension, diabetes, congestive heart failure, and persistent AF (Table 3B).
Table 2.
Univariate predictors of AF recurrence following ablation.
| HAZARD RATIO | P VALUE | 95% CI | |
|---|---|---|---|
| Age | 1.02 | 0.15 | 0.99–1.04 |
| Gender | 1.5 | 0.14 | 0.88–2.48 |
| HTN | 1.3 | 0.39 | 0.74–2.18 |
| DM | 1.2 | 0.58 | 0.63–2.26 |
| CAD | 1.4 | 0.29 | 0.77–2.47 |
| CHF | 0.8 | 0.59 | 0.31–1.93 |
| Persistent AF | 1.6 | 0.06 | 0.98–2.77 |
| Fibrosis Stage | 1.9 | <0.001 | 1.47–3.35 |
| Ablation-induced scar | 0.99 | 0.58 | 0.95–1.03 |
| Residual fibrosis >10% | 3.3 | <0.001 | 1.79–5.67 |
Table 3.
Multivariate analyses demonstrating the role of baseline fibrosis stage (A) and high residual fibrosis (B) in arrhythmia recurrence.
| HAZARD RATIO | P VALUE | 95% CI | |
|---|---|---|---|
| A | |||
| Stage I | – | ||
| Stage II | 3.6 | 0.001 | 1.64–7.82 |
| Stage III | 5.2 | <0.001 | 2.23–10.76 |
| Stage IV | 6.2 | 0.001 | 1.70–17.48 |
| # PVA encircled | 1.02 | 0.77 | 0.85–1.27 |
| Persistent AF | 1.38 | 0.26 | 0.79–2.44 |
| Hypertension | 0.98 | 0.96 | 0.59–1.86 |
| Diabetes | 1.15 | 0.69 | 0.59–2.30 |
| Congestive HF | 0.63 | 0.34 | 0.25–1.68 |
| B | |||
| High Residual | 3.3 | <0.001 | 1.75–5.59 |
| # PVA encircled | 1.01 | 0.94 | 0.84–1.25 |
| Persistent AF | 1.54 | 0.11 | 0.90–2.64 |
| Hypertension | 1.04 | 0.90 | 0.62–1.92 |
| Diabetes | 1.12 | 0.73 | 0.58–2.21 |
| Congestive HF | 0.59 | 0.28 | 0.24–1.56 |
Discussion
In this study, we evaluated the atrial substrate at baseline and studied ablation-induced scarring on the PV antral region as well as overall atrial substrate modification. We show that ablation rarely results in encirclement of all antral regions. The atrial substrate, at baseline and that remaining following ablation, is the stronger predictor of procedural outcomes.
PV isolation with antral encirclement
The PV antral region houses drivers that facilitate the initiation and maintenance of AF.13,14 The commonly used approaches to catheter ablation are focused on ablating in these antral regions with an accepted procedural endpoint of electrical isolation.4 This approach has been the most commonly used one over the past decade. The outcomes, however, have been modest, with arrhythmia recurrence rates varying between 30 and 70%.15 This suggests that the PVA isolation approach is not one that is suitable for all AF patients. Moreover, several reports have demonstrated that catheter ablation acutely achieves electrical isolation through a combination of edema, which can be reversible, as well as permanent damage that results in scarring.16 As the acute inflammation resulting from ablation resolves, the end result of the catheter intervention shows the presence of significant gaps and non-contiguous scarring around the PV ostia. This has been shown in examination of ablated atrial tissue where viable tissue was found to be present in patients with17 and without AF recurrence.18 It has also been shown that LGE-MRI can identify these gaps, which correlate with PV reconnection on electrophysiologic evaluation, and targeting of these gaps results in electrical isolation.19
The overall AF recurrence in our study cohort was 34.3% despite the demonstration that only a minority of patients (8.7%) had complete lesion encirclement of all four PVs. In addition, a significant proportion of patients (44%) had no veins circumferentially scarred. The success of the procedure seems, therefore, better than what is expected from PV antral scarring alone, suggesting a larger role for the effect of ablation on the atrial substrate.
Effect of ablation on the atrial substrate
We have previously published on the predictive value of LGE-MRI quantified atrial tissue fibrosis in determining the success of catheter ablation for AF,7,8 and this study consistently shows similar results on a entirely new cohort of patients. In order to reconcile the arrhythmia recurrence results with the PV findings, we used LGE-MRI to study the relationship of ablation-induced scarring to the baseline atrial fibrosis. Our analysis of the scarring that results from the ablation is independent of how extensive the ablation was, the power settings used, and the duration of radiofrequency energy application. Under the assumption that ablation-induced scar tissue plays no further role in the arrhythmia substrate, residual fibrosis remaining following ablation is shown to be a significant predictor of arrhythmia recurrence. Patients with a diffuse pattern of baseline fibrosis, affecting areas such as the anterior left atrium, traditionally not targeted with ablation, are likely to have a high residual fibrosis and, subsequently, are at higher risk of arrhythmia recurrence. By contrast, in patients with fibrosis in the posterior left atrium, in the PV antral region, a typical PV antrum isolation approach for ablation results in significant overlap between ablated regions and baseline fibrosis resulting in a low residual and a lower chance of recurrent AF, independent of PV scar encirclement.
Taken together, our findings suggest that while catheter ablation aims at isolation of the PVs, it is also creating important alterations to the atrial substrate that are key to arrhythmia suppression and procedural success. This is consistent with recent observations that ablation of the PV antral regions may be coincidentally eliminating focal sources and rotor centers.20 The relationship of rotor sites and areas of fibrosis remains to be studied.
Study limitations
The study reflects the experience of a single center with significant expertise in LGE-MRI of the atrium and advanced image processing. Our approach and image processing tools are designed to be widely usable on a large multicenter scale. Our assumption that ablation-induced scar is a homogeneous tissue that does not constitute a part of the arrhythmia substrate requires tissue correlation and animal models to corroborate it.
AF episode duration and overall burden were not adequately ascertained, and therefore, their association with fibrosis could not be reported in this manuscript.
Future directions
While our findings shed an important light on ablation-induced atrial changes, a prospective comparison of outcomes between an MRI-guided PV encirclement approach and an MRI-guided substrate-based (fibrosis) approach is needed to determine the contributions of each of these factors to the outcomes of catheter ablation.
Figure 5.
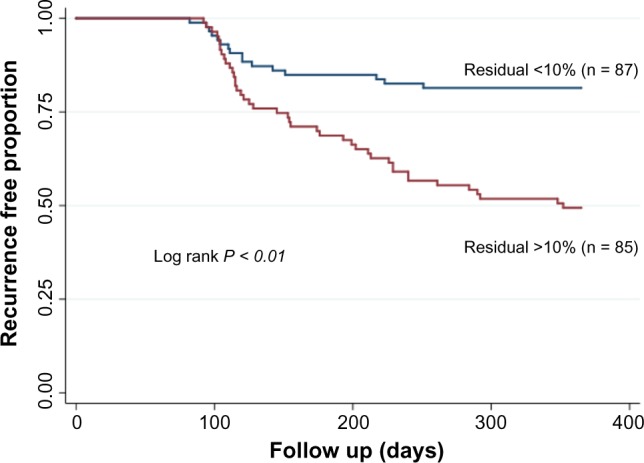
Kaplan–Meier curves showing differences in recurrent atrial arrhythmia in patients with high (>10%) residual fibrosis and patients with low (<10%) residual fibrosis.
Abbreviations
- AF
Atrial fibrillation
- LGE-MRI
Late-gadolinium enhancement magnetic resonance imaging
- PV
Pulmonary vein
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
FUNDING: This work was made possible in part by the NIH/NCI 1KM1CA156723 (Dr Akoum) as well as grants from the National Institute of General Medical Sciences (8 P41 GM103545–14) from the National Institutes of Health through the Center for Integrative Biomedical Computing (CIBC). Dr Cates reports grants from the National Institutes of Health during the conduct of the study. The authors confirm that the funders had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: NM is a partial owner of Marrek, Inc. NB is an employee of Marrek, Inc. EK discloses grants from Marrek, Inc. during the conduct of the study, as well as grants and personal fees from Marrek, Inc. outside the work presented here. In addition, EK has a patent pending for therapeutic success prediction for atrial fibrillation, and a patent pending for evaluation of cardiac structure. Other authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
NA contributed to the concept and design of the study, data analysis, and manuscript drafting. AM, DP, JC, NB, EK, and RM contributed to image acquisition, data analysis, and image processing. NM contributed to the concept and provided critical reviews of the manuscript. All the authors had full access to all the material in the study and take responsibility for the integrity of the data and the accuracy of the analysis. All authors reviewed and approved the final manuscript.
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the european society of cardiology (esc) Eur Heart J. 2010;31:2369–429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 3.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–63. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 4.Haissaguerre M, Jais P, Shah DC, et al. Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation. 2000;101:1409–17. doi: 10.1161/01.cir.101.12.1409. [DOI] [PubMed] [Google Scholar]
- 5.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–46. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 6.Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation. Circulation. 2003;108:2355–60. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 7.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akoum N, Daccarett M, McGann C, et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol. 2011;22(1):16–22. doi: 10.1111/j.1540-8167.2010.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGann CJ, Kholmovski EG, Oakes RS, et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–71. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 10.Peters DC, Wylie JV, Hauser TH, et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience1. Radiology. 2007;243:690–5. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 11.Johnson H, Zhao Y. Brainsdemonwarp: an application to perform demons registration. Insight J. 2009. http://hdl.handle.net/1926/1517.
- 12.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up: a report of the heart rhythm society (HRS) task force on catheter and surgical ablation of atrial fibrillation Developed in partnership with the european heart rhythm association (EHRA) and the european cardiac arrhythmia society (ECAS); in collaboration with the american college of cardiology (ACC), american heart association (AHA), and the society of thoracic surgeons (STS). Endorsed and approved by the governing bodies of the american college of cardiology, the american heart association, the european cardiac arrhythmia society, the european heart rhythm association, the society of thoracic surgeons, and the heart rhythm society. Heart Rhythm. 2007;4:816–61. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Po SS, Li Y, Tang D, et al. Rapid and stable re-entry within the pulmonary vein as a mechanism initiating paroxysmal atrial fibrillation. J Am Coll Cardiol. 2005;45:1871–7. doi: 10.1016/j.jacc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 14.Zhou S, Chang C-M, Wu T-J, et al. Nonreentrant focal activations in pulmonary veins in canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol. 2002;283:H1244–52. doi: 10.1152/ajpheart.01109.2001. [DOI] [PubMed] [Google Scholar]
- 15.Medi C, Sparks PB, Morton JB, et al. Pulmonary vein antral isolation for paroxysmal atrial fibrillation: results from long-term follow-up. J Cardiovasc Electrophysiol. 2011;22:137–41. doi: 10.1111/j.1540-8167.2010.01885.x. [DOI] [PubMed] [Google Scholar]
- 16.Ranjan R, Kato R, Zviman MM, et al. Gaps in the ablation line as a potential cause of recovery from electrical isolation and their visualization using MRI/clinical perspective. Circ Arrhythm Electrophysiol. 2011;4:279–86. doi: 10.1161/CIRCEP.110.960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowalski M, Grimes MM, Perez FJ, et al. Histopathologic characterization of chronic radiofrequency ablation lesions for pulmonary vein isolation. J Am Coll Cardiol. 2012;59:930–8. doi: 10.1016/j.jacc.2011.09.076. [DOI] [PubMed] [Google Scholar]
- 18.Dukkipati SR, Neuzil P, Kautzner J, et al. The durability of pulmonary vein isolation using the visually guided laser balloon catheter: multicenter results of pulmonary vein remapping studies. Heart Rhythm. 2012;9:919–25. doi: 10.1016/j.hrthm.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Badger TJ, Daccarett M, Akoum NW, et al. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ Arrhythm Electrophysiol. 2010;3:249–59. doi: 10.1161/CIRCEP.109.868356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (conventional ablation for AF with or without focal impulse and rotor modulation) J Am Coll Cardiol. 2013;62(2):138–47. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]