Abstract
BACKGROUND
Arterial calcium as measured by 64-slice computed tomography coronary angiography (64-CT) is a reliable predictor of cardiovascular disease risk. Lipid-rich plaques with lower degrees of calcification may pose greater risk for adverse coronary events than more stabilized calcified plaques as a result of the increased risk of plaque rupture, migration, and subsequent acute coronary syndrome. We sought to examine coronary artery calcium scores as measured via 64-CT to assess the extent of calcification and plaque distribution in women compared to men.
METHODS
A total of 138 patients referred for 64-CT were evaluated. Computerized tomographic angiography was performed using the GE LightSpeed VCT. Subgroup analysis comparing male and female data (including demographic data) was performed. All major coronary arteries were analyzed for coronary stenosis/plaque characterization as well as total vessel calcium (Agatston) score quantification. Patient demographics and coronary risk factors were recorded.
RESULTS
A total of 552 coronary arteries were evaluated in 138 patients (85 men, 53 women). The average age for females was 64.4 ± 10.8 years and for males 60.0 ± 12.8 years. The only demographic/cardiovascular risk factor in which the difference between men and women was significant was smoking history, where 23.5% of men had a history of smoking while only 9.6% of females endorsed having a smoking history (P < 0.044). On comparison of all total vessel calcium scores, males had a higher total mean calcium score than females in each individual vessel. The results were as follows for males versus females, respectively: left main total vessel calcium score 46.49 versus 16.71 (P = 0.167); left anterior descending 265.21 versus 109.6 (P < 0.003); left circumflex 130.5 versus 39.7 (P < 0.004); and right coronary 213.5 versus 73.8 (P < 0.01). The odds of having a total calcium score >100 (versus not) was 3.62 times greater in males relative to females, given that all the other cardiovascular risk factors are adjusted for (95% confidence interval: 1.37–9.54). On average, men had an average of 2.1 ± 1.5 epicardial vessels with a calcium score ≥11 compared to 1.3 ± 1.4 for women (P < 0.005).
CONCLUSION
There are clear differences between males and females regarding total vessel calcium scores and therefore risk of future adverse coronary events. Males tended to have higher average calcium scores in each coronary artery than females with a greater tendency to have multiple vessel involvement. Using this information, more large-scale, randomized controlled studies should be performed to correlate differences in the extent of coronary calcification with the observed variance in clinical presentation during coronary events between males and females as a means to potentially establish gender-specific therapeutic regimens.
Keywords: coronary artery disease, coronary computed tomography, gender
Introduction
The etiology of and pathophysiology surrounding the long-accepted and studied female gender advantage in coronary heart disease (CHD) remains poorly understood. Numerous studies have characterized the significant gender differences with regards to the incidence and rate of development of atherosclerosis and the resulting morbidity and mortality associated with almost any degree of vascular compromise. Despite numerous studies quantifying and attempting to explain the female advantage in the development of CHD, the explanation for this advantage remains elusive. Differences between men and women in the presentation, diagnosis, assessment, as well as prognosis of cardiovascular disease are evident and have prompted a worldwide campaign to redefine cardiovascular disease as a female as well as male disease and to structure treatment guidelines specifically for women.
Recent population data in the United States indicates that despite an aging population and the obesity epidemic currently plaguing the country, the mortality associated with CHD has decreased in women from one in three to one in four.1 This is likely at least partially a result of an increasing focus on cardiac disease as not only a male disease. In fact, while often underappreciated, cardiovascular disease is the leading cause of death in women, far exceeding cancer and infectious causes. Along with the increasing focus on cardiovascular disease as a cause of significant morbidity and mortality in women have come increasing observational studies and case series delineating the significant differences in almost every aspect of cardiac disease between males and females. While large, randomized controlled studies in the past focused on cardiovascular disease primarily in male subjects, recent large-scale cardiac trials in women have prompted the development of evidence-based guidelines for CHD diagnosis and management in women.
The development of new means of noninvasive cardiac imaging, particularly cardiac computerized tomographic angiography (CTA; 64-CT) allows more extensive analysis of the presence and nature of intra-arterial plaques. The presence and degree of intracoronary calcification has been shown to correlate with the degree of atherosclerosis.1 The utility of CTA in predicting risk of adverse cardiovascular disease events has been demonstrated in numerous studies. Few studies, however, have utilized this relatively new technology to shed light upon the significant gender differences in the long-term development of intra-arterial plaques. CTA allows for the description, both quantitatively and qualitatively, of a lesion’s area, peak density, and mean density, enabling commentary regarding the composition (calcified as opposed to lipid-laden, for example) of each intra-arterial lesion. Given the long-acknowledged disparity between males and females when it comes to cardiac disease, more studies are acknowledging these differences and attempting to explain the observed variations. This has led to more stringent recommendations regarding the approach to treatment of cardiac disease, particularly in postmenopausal women where there has been significant debate regarding the utility (or lack thereof) of hormone replacement therapy and the use of vitamins and antioxidants. In order to attempt to further investigate the observed differences between genders, we sought to use cardiac CT (Agatston method of quantification),2 which has revolutionized the noninvasive visualization and quantification of intracoronary plaque burden over the past decade, to compare the pattern and incidence of disease in males and females. A more extensive understanding of differences in the development, severity, and distribution of coronary disease in males and females may shed light on a still elusive subject.
Methods
Data collected from 138 consecutive patients referred for 64-CT at our institution was evaluated. CTA was performed using the General Electric LightSpeed VCT (GE Healthcare). Data were analyzed in a retrospective fashion after the institutional review board granted an exemption from the requirement for approval. The exemption was granted as the research involved study of existing, de-identified data. Subgroup analysis comparing male and female data was performed. The four major coronary arteries were analyzed for the presence of calcified coronary stenosis/plaque as according to the total vessel calcium (Agatston) score quantification. Vessels analyzed were the left main (LM) coronary artery, the left anterior descending (LAD), the left circumflex (LCX), and the right coronary artery (RCA). Patient demographics and coronary risk factors including diabetes mellitus, hypertension, smoking history, and dyslipidemia were recorded.
In order to be included in the study, patients must have been at least 18 years of age. Patients underwent 64-slice CT coronary angiography using the GE LightSpeed VCT. Patients on whom no background information (demographic data, medical history/risk factors) was recorded in the CTA database were excluded from data analysis.
Statistical analysis
Based on the scores on calcium scoring of the LM, LAD, LCX, and RCA, the number of vessels with a calcium score >11 and the number of vessels with lesions 101–400 were categorized. These two variables were also analyzed to determine if they were associated with gender using the Fisher’s exact test. A result was considered to be statistically significant when two-tailed P was <0.05. Patients were classified into two groups: with “significant” disease or without “significant” disease. A subject was classified as having significant disease if his/her total calcium score exceeds 100 HU (as described above). Univariable comparisons of continuous variables were carried out using either the t-test or Mann–Whitney test, as appropriate; for categorical variables, either the chi-square test or Fisher’s exact test was used, as appropriate.
Logistic regression analysis was carried out with “significant disease” as the “event” of interest using the following explanatory variables in the model: gender, age, body mass index (BMI), smoking status, hypertension, hypercholesterolemia and diabetes, as well as the interaction effects of gender and each of diabetes and smoking (gender × diabetes and gender × smoking interaction).
In order to arrive at a more parsimonious model, a backward selection algorithm was applied in order to determine which factors were most significantly associated with significant disease. Adjusted odds ratios are presented along with the corresponding 95% confidence intervals. All analyses were generated using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 552 coronary arteries were evaluated in 138 patients (85 men, 53 women). Baseline demographic and risk factor data is presented in Table 1. The average age for males was 60.0 ± 12.8 years and for the females, 64.4 ± 10.8 years. Males had an average BMI of 29.5 ± 5.2 kg/m2 while for females it was 28.5 ± 5.3 kg/m2. The only demographic/cardiovascular risk factor in which the difference between men and women was significant was smoking history, where 23.5% of men had a history of smoking while only 9.6% of females endorsed having a smoking history (P = 0.04). The differences between men and women in terms of the other demographic data were not significant (Table 1).
Table 1.
Baseline characteristics and cardiovascular disease risk factors.
| MALES (n = 85) |
FEMALES (n = 53) |
P VALUE | |
|---|---|---|---|
| Age (years) | 60.0 ± 12.8 | 64.4 ± 10.8 | 0.05 (ns) |
| BMI (kg/m2) | 29.5 ± 5.2 | 28.5 ± 5.3 | 0.26 (ns) |
| Hypertension | 49 (57.6%) | 33 (63.5%) | 0.59 (ns) |
| Dyslipidemia | 53 (62.4%) | 33 (63.5%) | 1.00 (ns) |
| Smoking history | 20 (23.5) | 5 (9.6) | 0.04 (s) |
On comparison of all total vessel calcium scores (Table 2), males had a higher total mean calcium score than females in each individual vessel. The results were as follows for males versus females, respectively: LM total vessel calcium score 46.49 versus 16.71 (P = 0.167); left anterior descending 265.21 versus 109.6 (P < 0.003); LCX 130.5 versus 39.7 (P < 0.004); right coronary 213.5 versus 73.8 (P < 0.01). The odds of having a total calcium score >100 (versus not) were 3.62 times greater in males relative to females, given that all the other cardiovascular risk factors are adjusted for (95% confidence interval [CI]: 1.37–9.54). On average, men had a mean of 2.1 ± 1.5 epicardial vessels with a calcium score ≥11 compared to 1.3 ± 1.4 for women (P < 0.005). Using a calcium score ≥101, men had an average of 1.2 ± 1.4 epicardial vessels involved while women had 0.67 ± 1.0 (P < 0.036).
Table 2.
Comparison of mean coronary artery calcium score based upon gender.
| VESSEL | MALES (n = 85) |
FEMALES (n = 53) |
P VALUE |
|---|---|---|---|
| LM | 46.5 ± 142.9; (0; 0–1,094) | 16.7 ± 56.5; (0; 0–358) | <0.167 (ns) |
| LAD | 265.2 ± 395.2; (88; 0–2,203) | 109.56 ± 187.8; (12; 0–989) | <0.003 (s) |
| LCX | 130.5 ± 305.9; (14; 0–1,993) | 39.7 ± 115.6; (0; 0–667) | <0.004 (s) |
| RCA | 213.5 ± 364.9; (14; 0–1,455) | 73.79 ± 166.8; (0; 0–806) | <0.010 (s) |
| Total vessel calcium score | 655.7 ± 1,037.5; (154; 0–6,001) | 239.71 ± 476.9; (47.50; 0–2,820) | <0.003 (s) |
| Number of vessels ≥11 | 2.1 ± 1.5; (2; 0–4) | 1.3 ± 1.4; (1; 0–4) | <0.005 (s) |
| Number of vessels >101 | 1.2 ± 1.4; (1; 0–4) | 0.67 ± 1.0; (0; 0–4) | <0.036 (s) |
Abbreviations: M, left main coronary artery; ns, nonsignificant; s, significant.
Discussion
The marked gender differences in the presentation, diagnosis, and management of cardiovascular disease have been established by numerous studies over the past half-century.3–6 Premenopausal women in particular have significantly lower rates of cardiovascular disease compared to age-matched males. The explanation behind acknowledged differences between men and women when it comes to cardiovascular disease remains elusive, however. Numerous theories attempting to explain these differences have been posited over the past 50 years. Nevertheless, no single unifying explanation can account for the gender gap. The cause of these observed differences is likely an amalgam of numerous variables, which have each been shown to individually contribute to observed variations including differences in vascular beds, hormonal factors, as well as lifestyle issues. Our study indicates that in addition to the differences already described, there are stark dissimilarities between men and women in the composition, distribution, and location of intracoronary lesions which may explain some of the observed variations in clinical presentation, sequelae, and outcomes.
The hormonal differences between males and females, particularly premenopausal females who appear to have a significant protective factor, have always been at the root of comparisons in the rate of cardiovascular disease between the genders. In the past five years, however, reports of the Women’s Health Initiative and the Heart and Estrogen/Progestin Replacement Study have indicated that combination hormone therapy is actually associated with an increased risk of CVD events in postmenopausal women, which seemed somewhat counterintuitive considering the theories that the female hormones estrogen and progesterone were cardioprotective.7–10 These studies underscore the complicated nature of gender difference and cardiovascular disease. Epidemiological data indicate that compared with age-matched males, premenopausal females have a decreased incidence of virtually all types of cardiovascular diseases, including coronary artery disease (CAD), hypertension, and potentially harmful cardiac structural remodeling such as left ventricular hypertrophy. These differences translate into a significant female advantage in the rates of mortality from cardiovascular disease, with male death rates from CHD ranging from 2.5 to 4.5 times that of females across populations in different countries, with different lifestyles, and with variable population cardiovascular disease rates.11 The incidence of CHD in postmenopausal females does increase significantly and in fact surpasses that of men as demonstrated by the Framingham data.12,13
Gender dimorphism has been observed in nearly all aspects of cardiovascular disease, from hypertension to cardiac remodeling to outcomes following percutaneous coronary intervention for acute myocardial infarction. Animal models have implicated a significant role for various estrogen receptors in the development of CHD and cardiac remodeling.14 In addition, these hormones have even been implicated in rates of hypertension. While sex hormones have been hypothesized to contribute to the gender difference in blood pressure regulation,15 it is more likely that androgens and not estrogens are primarily responsible since androgens are known to increase blood pressure, whereas studies on hormone replacement therapy show essentially no effect on blood pressure in postmenopausal women.16 Studies indicate that the increase in serum testosterone in postmenopausal women may result in increased risk of hypertension and cardiovascular disease in these women.16 Physiological changes associated with menopause affect several biochemical pathways, including lipid metabolism, resulting in a tendency toward elevated total cholesterol, triglycerides, and low-density lipoprotein cholesterol.17,18 Postmenopausal changes also result in enhanced activation of the coagulation pathway with elevated serum levels of plasminogen activator inhibitor-1 as well as fibrinogen.19,20 Further enhancing the risk of cardiovascular disease in postmenopausal women is impaired coronary artery vasoelasticity as well as increased endothelial dysfunction.21 Thus, postmenopausal women have numerous prothrombotic, atherogenic stimuli for the development of cardiovascular disease.
Prior to the recent development of noninvasive cardiac imaging, it was difficult to characterize the nature of intracoronary arterial plaques. Using 64-CT coronary angiography allows characterization of the degree of calcification of observed lesions and perhaps the likelihood of atherosclerotic plaque rupture and vascular stenosis potentially leading to myocardial ischemia or infarction. The ability of CTA to characterize atherosclerotic plaque burden has been shown to be nearly as accurate as intravascular ultrasound.22,23 While the main goal of CTA is not to identify potentially vulnerable plaques, lipid-laden plaques with the potential to rupture can be a clinical guide as seen on CTA.24 CTA is capable of detecting calcified or mixed plaques with sensitivities and specificities of approximately 90%. The ability to detect lipid-laden, noncalcified plaques is not as good, however, with sensitivities/specificities between 60% and 80%.22,25
Our data indicate that men have a significantly higher average calcium score across all major coronary vessels, despite similarities in the age range and rate of the various major independent cardiovascular disease risk factors. Even after adjusting for smoking (the only variable in which there was a statistically significant difference), males were still 3.62 times more likely to have a total vessel calcium score greater than 100 (95% CI: 1.37–9.54). In addition to higher overall calcium scores, men also tended to have a greater number of significant calcium scores (101–400), indicating a high likelihood of a stenotic lesion. This may shed light on the inconsistency that despite markedly higher calcium scores in most coronary arteries, the rate of CHD between postmenopausal females and males of similar age are comparable and even higher in women according to some data.
The differences demonstrated by our CT data indicate that males have a higher incidence of significant plaque calcification possibly resulting in some degree of flow limitation while females have significantly lower scores in each coronary vessel except for the LM coronary artery (Figure 1). In the LAD, LCX, and RCA, the males had a statistically significant higher measured calcium score in each vessel. The fact that postmenopausal women still have similar rates of CHD indicates that the plaques seen on CTA in women may in fact, be more lipid-laden and therefore less stable than those observed in males. As a result, the mechanisms resulting in the variety of cardiovascular disease events such as stable/unstable angina and myocardial infarction may vary somewhat between males and females. Acute coronary syndromes most often result from disruption of intra-arterial coronary plaques resulting in thrombus formation, migration, and vessel occlusion. Large plaques that limit coronary artery flow (particularly under stress) as well as unstable, less calcified plaques have a greater likelihood of rupture and thrombus formation. The implications of this are significant for the utility of coronary CTA in predicting the risk of cardiovascular disease events based solely upon calcium scoring. The published data from the Multi-Ethnic Study of Atherosclerosis trial demonstrated the significantly higher risk of CAD events among patients with a calcium score between 1 and 10 compared to those with a calcium score of 0 (hazard ration 3.23, 95% CI 1.17–8.95).26 Data from this large-scale study also indicated that approximately 16% of coronary arteries with significant stenosis on conventional invasive angiography had no calcification on 64-CTA.27 The trend seen in this study cohort of 6,110 individuals showed that men had higher calcium scores than women, and the amount and prevalence of calcium increased steadily with age. Almost two-thirds of women (62%) had calcium scores of zero in this sample, as opposed to 40% of men.26,27 While these findings show that calcification differences exist, further studies have demonstrated that microvascular dysfunction gender variations exist28 and may explain differences in outcome in stable patients and in patients who present with acute coronary syndromes.29
Figure 1.
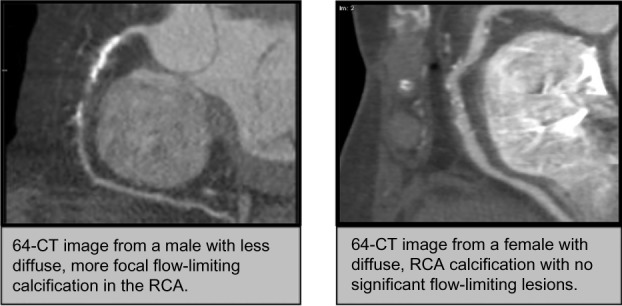
Comparison images of coronary plaque distribution between males and females.
According to our data, males have significantly larger, more calcified plaques than females, who had smaller, significantly less calcified lesions. The differences in plaque characteristics may be a clue as to variations in the presentation of acute coronary syndrome (ACS) in females compared to males. In addition, the previously described factors used to explain gender differences in cardiovascular disease such as hormonal variations, differences in vascular beds, and lifestyle issues likely contribute to the differences observed in plaque characteristics. Given the differences in observed plaque characteristics, more studies should be performed to analyze weather a different approach to medical therapy (such as more aggressive lipid-lowering therapy) in women with suspected or proven cardiovascular disease is warranted.
Study Limitations
A drawback of our analysis is the selection bias of referring only low-risk patients to undergo coronary CTA and referring higher risk patients directly to coronary angiography. Although this may be a prudent means of evaluating and treating patients, it limits the assessment of the accuracy CTA for the detection of CAD and introduces a select set of patients for analysis which may lead to confounding factors. The present study was also limited to small numbers of patients in a single center who underwent CTA and while the age of these patients was known and can be surmised in many cases, the menopausal status of the women was not available in this study. Finally, all the baseline medications that each patient was taking were not readily available, however, all these were patients closely followed by their referring physicians and presumably taking their required medication regimen.
Conclusion
The observed differences between the sexes in cardiovascular disease are likely the result of a variety of biologic/genetic, environmental, and behavioral factors. Noninvasive cardiac imaging can potentially shed light on these differences by describing intra-arterial lesions both quantitatively and qualitatively. Our study indicates that among males and females with comparable age and presence of cardiovascular disease risk factors, males tend to have significantly higher intra-arterial coronary calcium scores than females in most coronary arteries. Since postmenopausal females have an equal if not higher risk of developing cardiovascular disease as males, the mechanisms underlying the development of angina and myocardial infarction, for example, may, at least partially, be different according to gender. Differences in the composition of intra-arterial coronary lesions may explain why men and women present with cardiovascular disease sequelae and why outcomes may vary according to gender. More prospective studies correlating coronary plaque characteristics and long-term outcomes in patients deemed to be at risk for coronary disease should be performed to optimize therapy based upon all patient characteristics, including gender.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: ANM, JNM. Analyzed the data: ANM, CS, MK, JNM. Wrote the first draft of the manuscript: ANM, JNM. Contributed to the writing of the manuscript: ANM, MK, JNM. Agree with manuscript results and conclusions: ANM, CS, MK, JNM. Jointly developed the structure and arguments for the paper: ANM, MK, JNM. Made critical revisions and approved final version: ANM, CS, MK, JNM. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Mehta PK, Wenger NK. Coronary heart disease in women: battle is won, but the war remains. Minerva Med. 2007;98(5):459–78. [PubMed] [Google Scholar]
- 2.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 3.Patel H, Rosengren A, Ekman I. Symptoms in acute coronary syndromes: does sex make a difference? Am Heart J. 2004;148:27–33. doi: 10.1016/j.ahj.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg R, Goff D, Cooper L, et al. Age and sex differences in presentation of symptoms among patients with acute coronary disease: the REACT Trial. Rapid early action for coronary treatment. Coron Artery Dis. 2000;11:399–407. doi: 10.1097/00019501-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 5.De Von HA Zerwic JJ. Symptoms of acute coronary syndromes: are there gender differences? A review of the literature. Heart Lung. 2002;31:235–45. doi: 10.1067/mhl.2002.126105. [DOI] [PubMed] [Google Scholar]
- 6.Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70:222–6. doi: 10.1253/circj.70.222. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, et al. Writing group for the Women’s Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]; CMAJ. 2002;167:377–8. Summary for patients in. [PMC free article] [PubMed] [Google Scholar]; J Fam Pract. 2002;51:821. and. [PubMed] [Google Scholar]
- 8.Manson JE, Hsia J, Johnson KC, et al. Women’s Health Initiative Investigators Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 9.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. WHI Investigators Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 10.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) research group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 11.Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel keys lecture. Circulation. 1997;95:252–64. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- 12.Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB. The Framingham study: historical insight on the impact of cardiovascular risk factors in men versus women. J Gend Specif Med. 2002;5:27–37. [PubMed] [Google Scholar]
- 14.Skavdahl M, Steenbergen C, Clark J, et al. Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol. 2005;288(2):H469–76. doi: 10.1152/ajpheart.00723.2004. [DOI] [PubMed] [Google Scholar]
- 15.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 16.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo study. J Clin Endocrinol Metab. 2000;85:645–51. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98:83–90. doi: 10.1016/0021-9150(93)90225-j. [DOI] [PubMed] [Google Scholar]
- 18.Peters HW, Westendorp IC, Hak AE, et al. Menopausal status and risk factors for cardiovascular disease. J Intern Med. 1999;246:521–8. doi: 10.1046/j.1365-2796.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- 19.Gebara OC, Mittleman MA, Sutherland P, et al. Association between increased estrogen status and increased fibrinolytic potential in the Framingham offspring study. Circulation. 1995;91:1952–8. doi: 10.1161/01.cir.91.7.1952. [DOI] [PubMed] [Google Scholar]
- 20.Scarabin PY, Plu-Bureau G, Bara L, Bonithon-Kopp C, Guize L, Samama MM. Haemostatic variables in menopausal status: influence on hormone replacement therapy. Thromb Haemost. 1993;70:584–7. [PubMed] [Google Scholar]
- 21.Taddei S, Virdis A, Ghiadoni L, et al. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28:576–82. doi: 10.1161/01.hyp.28.4.576. [DOI] [PubMed] [Google Scholar]
- 22.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–7. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 23.Kopp AF, Schroeder S, Baumbach A, et al. Non-invasive characterisation of coronary lesion morphology and composition by multislice CT: first results in comparison with intracoronary ultrasound. Eur Radiol. 2001;11:1607–11. doi: 10.1007/s003300100850. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann U, Ferencik M, Cury RC, Pena AJ. Coronary CT angiography. J Nucl Med. 2006;47:797–806. [PubMed] [Google Scholar]
- 25.Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 2004;43:1241–7. doi: 10.1016/j.jacc.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 26.Budoff MJ, McClelland RL, Nasir K, et al. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2009;158(4):554–61. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen BD, Fernandes V, McClelland RL, et al. Relationship between baseline coronary calcium score and demonstration of coronary artery stenoses during follow-Up MESA (Multi-Ethnic Study of Atherosclerosis) JACC Cardiovasc Imaging. 2009;2(10):1175–83. doi: 10.1016/j.jcmg.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129(24):2518–27. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lansky AJ, Ng VG, Maehara A, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3 suppl):S62–72. doi: 10.1016/j.jcmg.2012.02.003. [DOI] [PubMed] [Google Scholar]


