Abstract
Wolcott-Rallison syndrome (WRS) is an autosomal recessive disease, characterized by neonatal or early-onset non-autoimmune insulin-dependent diabetes. WRS, although rare, is recognized to be the most frequent cause of neonatal-onset diabetes. The majority of reported patients are from consanguineous families. Several mutations with variable expression of the syndrome are reported. Here we describe a six-year-old boy with WRS who was evaluated at Sultan Qaboos University Hospital and was found to have a novel homozygous nonsense mutation in the EIF2AK3 gene. His younger sister also had WRS but with milder expression. The mutation exhibited different clinical characteristics in the siblings proving that WRS patients phenotypic variability correlates poorly to genotype. This is the first case report of two Omani children with WRS and a report of a novel mutation.
Keywords: Wolcott-Rallison syndrome, Permanent Neonatal Diabetes Mellitus, Osteochondrodysplasia
Introduction
Wolcott-Rallison syndrome (WRS) is a rare autosomal recessive disease, characterized by non-autoimmune permanent neonatal or early-onset diabetes mellitus (PNDM).1 WRS, although rare, is the most frequent cause of PNDM2 especially in consanguineous families and Arab populations.1-6 WRS is associated with frequent acute liver failure episodes of variable severity.1,6 Some episodes may regress spontaneously, but others can result in death.1 Recurrent infections, renal insufficiency, neutropenia, growth and developmental retardation, osteopenia, multiple epiphyseo-metaphyseal dysplasia, and exocrine pancreas insufficiency are common.1,7-9 WRS is caused by mutations in the gene encoding eukaryotic translation initiation factor 2-alpha kinase 3 (EIF2AK3), also known as pancreatic EIF2a kinase (PEK) and PEK-like endoplasmic reticulum kinase (PERK).10-12 The absence of PERK activity reduces the ability of the endoplasmic reticulum to deal with stress, leading to apoptosis in many tissues.11,12 Diabetes and skeletal dysplasia are due to absence of PERK activity in pancreatic beta cells13 and osteoblasts.14 Many mutations have been reported in the EIF2AK3 gene.1,2,5,7 Radiological, biochemical, and histological features usually support clinical diagnosis and molecular genetic testing is used for confirmation.1 WRS should be differentiated from other forms of permanent syndromic and isolated neonatal or early-onset insulin-dependent diabetes, transient neonatal diabetes, and type 1 diabetes mellitus.1,2,15 The disease has a poor prognosis mainly due to multi-organ involvement and liver and renal failure.1,2,5,10,16
Case Reports
Case one
A six-year-old male Omani child presented to Sultan Qaboos University Hospital (SQUH). He was born at term to healthy consanguineous parents, with no perinatal complications and an unremarkable family history. He developed PNDM at the age of four weeks, which was managed accordingly. He had frequent episodes of febrile neutropenia and acute liver failure. He had a total of eight such episodes: the first one was at the age of 10 months and the last was at the age of six years, which was fatal. His developmental age at six years was determined to be three years and six months. He also had a history of hyperactivity. Physical examination showed growth failure and dysmorphic features including triangular face, microcephaly, outward protruding ears, short upper and lower limbs compared to the trunk with short stout fingers and toes, square hands, flat feet with rocker bottom deformity, a short broad chest, and a protuberant abdomen. His liver ranged from normal size to hepatomegaly at different times of his disease course, with no splenomegaly. Eye and fundus examination were unremarkable.
Investigations showed leucopenia, neutropenia, and intermittently low hemoglobin due to glucose-6-phosphate dehydrogenase (G6PD) deficiency-related acute hemolysis. Liver enzymes were high during these episodes with liver failure and cholestasis. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were as high as 5000–6500IU/L during acute episodes. The highest total and direct bilirubin levels were 900µmol/L and 600µmol/L, respectively. High alkaline phosphatase (500IU/L) was measured, and he had normal calcium and phosphate levels. The coagulation profile was deranged during episodes with a prothrombin time (PT) of 21.7 seconds, international normalized ratio (INR) of 1.68, and activated partial thromboplastin time (APTT) of 56 seconds. These were unresponsive to intravenous vitamin K injections but normalized after recovery from the acute episode. His renal function was normal. Fecal elastase concentration was <50µg/g indicating severe exocrine pancreatic insufficiency. During episodes of liver dysfunction, he improved with supportive management and broad-spectrum antibiotics for febrile neutropenia. Viral and autoimmune causes of acute hepatitis and liver failure were ruled out and immunodeficiency workup was normal. Investigations for metabolic disorders were unremarkable. Bone marrow aspirate showed reactive marrow with arrest of granulocytes maturation and preponderance of promyelocytes. Skeletal survey showed generalized osteopenia and skeletal dysplasia [Figure 1a-d]. Ultrasound (US) of the abdomen showed hypotrophic pancreas and mild hepatomegaly. Abdominal computed tomography (CT) scan showed the same. Liver biopsy performed between acute episodes showed markedly swollen hepatocytes with rarefied cytoplasm with occasional multinucleate, degenerated, and necrotic hepatocytes. The portal tract showed scanty lymphocytic infiltrates. There was focal perisinusoidal fibrosis. The possibility of WRS as a diagnosis was confirmed by sequencing analysis which identified a novel homozygous nonsense mutation in exon 11, (c.1764T>G, [p.Y588X]) of the EIF2AK3 gene. He had few episodes of hypoglycemia that could have been the cause for episodic liver failure, which improved spontaneously with normalization of liver enzymes and function over days to weeks. The patient was managed in between acute episodes with insulin for PNDM and pancreatic enzymes replacement therapy and daily oral prophylactic antibiotic. He died at the age of seven years with picture of diabetic ketoacidosis (DKA), liver failure, encephalopathy, and possibly sepsis.
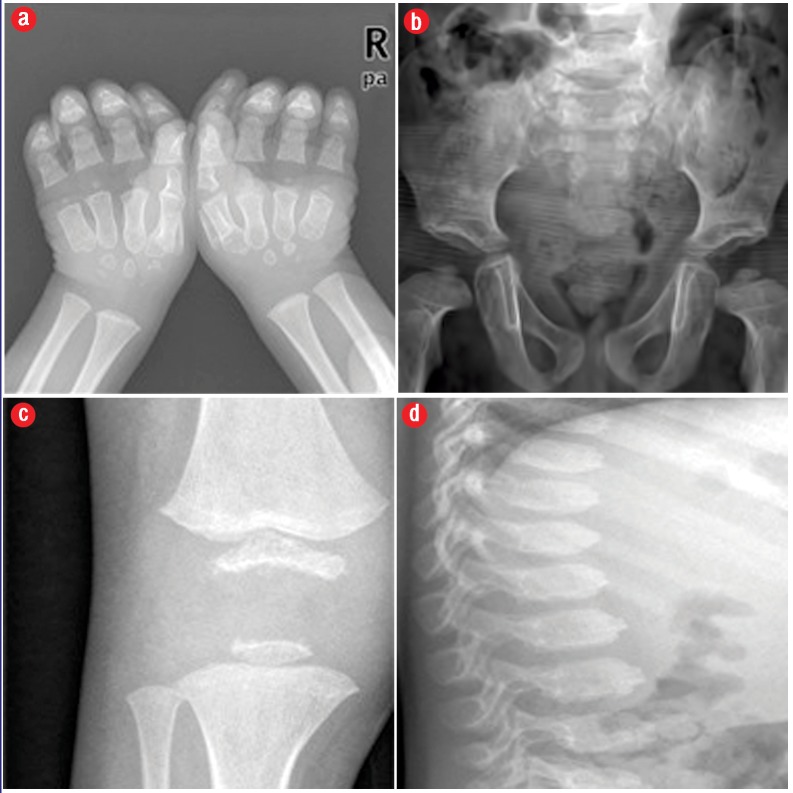
Figure 1.
Radiological features of a six-year-old boy (case 1) with permanent neonatal-onset diabetes mellitus (a) showing small irregular carpal bones, short stubby phalanges with irregular metaphases, and (b) flat femoral epiphyses with irregular acetabular roof and flattened femoral epiphysis. (c) Delayed ossification of the lower tibial epiphyses and (d) irregular spinal anterior vertebral plate was also seen.
Case two
The four-year-old sister of the boy described in case one also had WRS. She was born at term with no perinatal complications. She presented with PNDM and cyclic neutropenia at six-weeks of age. She subsequently developed three episodes of acute hepatitis without signs of liver failure. Unlike her brother, she had normal development and growth. Skeletal survey showed mild osteopenia with milder skeletal dysplasia compared to her brother. Her carpal bones and phalanges were normal. She had a wide middle part of the ribs and flat femoral epiphyses. Her spine had irregular anterior vertebral plate at thoracic vertebrae only. Fecal elastase was initially normal but decreased on subsequent testing at the age of three years (<50µg/g) indicating a severe exocrine pancreatic insufficiency. Abdominal CT revealed atrophic pancreas with normal other organs. Abdominal US during episodes of acute hepatitis was normal. She is currently managed with insulin, prophylactic daily oral antibiotics, and pancreatic enzyme replacement therapy. She was alive at the time of writing this report with no episodes of hypoglycemia and very few infections. Her growth is gradually declining with time.
Discussion
WRS is the most common cause of PNDM in areas of high consanguinity.1,3,4,7 Despite the highly consanguineous community, no cases have been reported in Oman. This might indicate that WRS is exceptionally rare in this population, but also that it is unrecognized leading to delay in diagnosis and perhaps early death. We report the case of two siblings with WRS with a novel mutation, the first reported incident in Oman. Our patients were found to be homozygous for a nonsense mutation p.Y588X in EIF2AK3 gene that has not been reported previously. Although the c.1764T>G (p.Y588X) mutation is novel, it is thought to be pathogenic. The EIF2AK3/PERK protein is 1115 amino acids long and the catalytic kinase activity is located at the carboxyl half of the protein starting precisely at amino acid 577. Having a truncating mutation at position 588 abolishes almost completely the kinase catalytic part.
Our first patient presented with typical symptoms and signs of WRS. The second patient had similar features of WRS on initial presentation when compared to her sibling. She differed in the severity of episodes, absence of liver failure, milder skeletal dysplasia, normal weight gain initially and normal development, and the absence of hypoglycemia and severe infections. This further proves the expression variability even within the same sibship that has been described in the literature. Clinical variability between WRS patients, even siblings, is usually intellectual deficit, exocrine pancreatic deficiency, hypothyroidism, neutropenia and recurrent infections, and the presence and frequency of liver failure episodes.1,5,7 Disease variability appears to be independent of EIF2AK3 mutations, with the possible exception of older age at onset and neutropenia.7 This fact is amplified in our case as this truncating mutation is assumed to almost remove the catalytic activity of the protein making variable expression of the EIF2AK3 a less likely factor. Factors causing disease variability may include variable expression of the EIF2AK3 gene, other modified genes, exposure to environmental factors, and disease management differences.2,5,6,16 Pancreatic hypotrophy was observed in both patients and was reported in few cases.1,9
Management of diabetes mellitus in these cases should not be targeted towards a very tight control of blood glucose in order to avoid hypoglycemia, which may trigger acute episodes of liver failure.1 General anesthesia should be avoided when possible because of the risk of acute aggravation. Taking into account the risk of triggering secondary liver and/or kidney failure, drugs or vaccines that are not strictly necessary should be limited.1 Our first patient had few episodes of hypoglycemia, which could be the triggering cause for episodic liver failure. Both patients were started on oral prophylactic antibiotics, which we think was an important step in minimizing the number of the liver dysfunction episodes triggered by infections, especially in the second patient once we recognized the pattern in her older brother. This effect has not been clearly described in the literature and might be of future significance in managing patients with WRS. Genetic counseling was offered to parents who were confirmed to be heterozygote carriers for the same mutation. The couple underwent prenatal genetic testing in their subsequent pregnancy that revealed an unaffected fetus.
Conclusion
WRS should be suspected in cases of early-onset diabetes even if the features are atypical in populations with high consanguinity rate and to be referred to tertiary care centers in order to confirm the diagnosis and optimize their care. WRS is largely underdiagnosed at present because of the early death of patients and/or the higher prevalence of these patients in countries where structures for health care may not be optimal. This report with its novel mutation and phenotypic variability between siblings provides knowledge that furthers our understanding of the genetic mechanism of WRS.
Disclosure
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Dr Sarah Flanagan and Dr Sian Ellard from The Molecular Genetics Laboratory, Royal Devon & Exeter NHS Foundation Trust, UK for performing the molecular genetic testing and supplying the EIF2AK3 sequencing chromatogram.
References
- 1.Julier C, Nicolino M. Wolcott-Rallison syndrome. Orphanet J Rare Dis 2010;5:29. 10.1186/1750-1172-5-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubio-Cabezas O, Patch AM, Minton JA, Flanagan SE, Edghill EL, Hussain K, et al. Neonatal Diabetes International Collaborative Group . Wolcott-Rallison syndrome is the most common genetic cause of permanent neonatal diabetes in consanguineous families. J Clin Endocrinol Metab 2009. Nov;94(11):4162-4170. 10.1210/jc.2009-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habeb AM, Flanagan SE, Deeb A, Al-Alwan I, Alawneh H, Balafrej AA, et al. Permanent neonatal diabetes: different aetiology in Arabs compared to Europeans. Arch Dis Child 2012. Aug;97(8):721-723. 10.1136/archdischild-2012-301744 [DOI] [PubMed] [Google Scholar]
- 4.Durocher F, Faure R, Labrie Y, Pelletier L, Bouchard I, Laframboise R. A novel mutation in the EIF2AK3 gene with variable expressivity in two patients with Wolcott-Rallison syndrome. Clin Genet 2006. Jul;70(1):34-38. 10.1111/j.1399-0004.2006.00632.x [DOI] [PubMed] [Google Scholar]
- 5.Senée V, Vattem KM, Delépine M, Rainbow LA, Haton C, Lecoq A, et al. Wolcott-Rallison Syndrome: clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes 2004. Jul;53(7):1876-1883. 10.2337/diabetes.53.7.1876 [DOI] [PubMed] [Google Scholar]
- 6.Iyer S, Korada M, Rainbow L, Kirk J, Brown RM, Shaw N, et al. Wolcott-Rallison syndrome: a clinical and genetic study of three children, novel mutation in EIF2AK3 and a review of the literature. Acta Paediatr 2004. Sep;93(9):1195-1201. 10.1111/j.1651-2227.2004.tb02748.x [DOI] [PubMed] [Google Scholar]
- 7.Ozbek MN, Senée V, Aydemir S, Kotan LD, Mungan NO, Yuksel B, et al. Wolcott-Rallison syndrome due to the same mutation (W522X) in EIF2AK3 in two unrelated families and review of the literature. Pediatr Diabetes 2010. Jun;11(4):279-285. 10.1111/j.1399-5448.2009.00591.x [DOI] [PubMed] [Google Scholar]
- 8.al-Gazali LI, Makia S, Azzam A, Hall CM. Wolcott-Rallison syndrome. Clin Dysmorphol 1995. Jul;4(3):227-233. 10.1097/00019605-199507000-00006 [DOI] [PubMed] [Google Scholar]
- 9.Castelnau P, Le Merrer M, Diatloff-Zito C, Marquis E, Tête MJ, Robert JJ. Wolcott-Rallison syndrome: a case with endocrine and exocrine pancreatic deficiency and pancreatic hypotrophy. Eur J Pediatr 2000. Aug;159(8):631-633. 10.1007/PL00008394 [DOI] [PubMed] [Google Scholar]
- 10.Delépine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 2000. Aug;25(4):406-409. 10.1038/78085 [DOI] [PubMed] [Google Scholar]
- 11.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell 2001. Jun;7(6):1153-1163. 10.1016/S1097-2765(01)00264-7 [DOI] [PubMed] [Google Scholar]
- 12.Zhang P, McGrath B, Li S, Frank A, Zambito F, Reinert J, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 2002. Jun;22(11):3864-3874. 10.1128/MCB.22.11.3864-3874.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iida K, Li Y, McGrath BC, Frank A, Cavener DR. PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol 2007;8:38. 10.1186/1471-2121-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J, Sheng X, Feng D, McGrath B, Cavener DR. PERK is essential for neonatal skeletal development to regulate osteoblast proliferation and differentiation. J Cell Physiol 2008. Dec;217(3):693-707. 10.1002/jcp.21543 [DOI] [PubMed] [Google Scholar]
- 15.Nicolino M, Claiborn KC, Senée V, Boland A, Stoffers DA, Julier C. A novel hypomorphic PDX1 mutation responsible for permanent neonatal diabetes with subclinical exocrine deficiency. Diabetes 2010. Mar;59(3):733-740. 10.2337/db09-1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habeb AM. Frequency and spectrum of Wolcott-Rallison syndrome in Saudi Arabia: a systematic review. Libyan J Med 2013;8:21137. 10.3402/ljm.v8i0.21137 [DOI] [PMC free article] [PubMed] [Google Scholar]