Abstract
Background
Non-nucleoside reverse transcriptase (NNRTI) inhibitor-based antiretroviral therapy is not suitable for all treatment-naïve HIV-infected persons.
Objective
Perform a rigorous evaluation of three NNRTI-sparing initial antiretroviral regimens to demonstrate equivalence for virologic efficacy and tolerability.
Design
Phase-III, 1:1:1 randomized, open label, >96 week study.
Setting
Fifty-seven sites in United States and Puerto Rico.
Patients
Treatment naïve, ≥18 years, HIV-1 RNA >1000 copies/mL, no nucleoside reverse transcriptase or protease inhibitor resistance.
Intervention
Atazanavir 300 mg with ritonavir 100 mg, daily; or raltegravir 400 mg twice daily; or darunavir 800 mg with ritonavir 100 mg, daily; plus emtricitabine 200 mg + tenofovir disoproxil fumarate 300 mg daily.
Measurements
Virologic failure defined as confirmed HIV-1 RNA >1000 copies/mL between 16 and 24 weeks, or >200 copies/mL at or after 24 weeks; tolerability failure defined as discontinuation of atazanavir, raltegravir or darunavir for toxicity. A secondary endpoint was a combination of virologic efficacy and tolerability.
Results
Among 1,809 participants all pairwise comparisons of incidence of virologic failure over 96-weeks demonstrated equivalence within ±10%. Raltegravir and ritonavir-boosted darunavir were equivalent for tolerability, whereas ritonavir-boosted atazanavir resulted in a 12.7% and a 9.2% higher incidence of tolerability discontinuation than raltegravir and ritonavir-boosted darunavir respectively, primarily due to hyperbilirubinemia. For combined virologic efficacy and tolerability ritonavir-boosted darunavir was superior to ritonavir-boosted atazanavir, and raltegravir was superior to both protease inhibitors. Antiretroviral resistance at time of virologic failure was rare but more likely with raltegravir.
Limitations
Open label; ritonavir not provided
Conclusions
Over 2 years all three regimens attain high and equivalent rates of virologic control. Regimens containing raltegravir or ritonavir-boosted darunavir have superior tolerability compared to the ritonavir-boosted atazanavir regimen.
Primary Funding Source
National Institute of Allergy and Infectious Diseases
Introduction
The 2014 United States (US) Department of Health and Human Services antiretroviral therapy guidelines recommend a combination of two reverse transcriptase inhibitors plus either a non-nucleoside reverse transcriptase inhibitor (NNRTI), a ritonavir-boosted protease inhibitor (PI), or an integrase inhibitor for the initial treatment of HIV-1 infected adults and adolescents. (1) The recommended NNRTI is efavirenz, which when co-formulated with emtricitabine and tenofovir disoproxyl fumarate (tenofovir DF) allows one pill, once daily dosing. Globally, efavirenz-based combinations are recommended as first-line therapy by the World Health Organization. (2) However, women who are contemplating becoming pregnant, patients with pre-existing NNRTI resistance and those with severe psychiatric disorders are not considered good candidates for efavirenz-based therapy when other options are available. Ritonavir-boosted protease inhibitor-containing therapy may be limited by hepatic, gastrointestinal, and metabolic side effects; cardiovascular and cerebrovascular morbidity may also be increased. (3–5) Integrase inhibitors are virologically potent first-line agents with a favorable toxicity profile, but have more limited long-term safety data and are less widely available in resource-constrained settings. To understand better the long-term efficacy and tolerability of alternatives to efavirenz, we undertook a randomized study of tenofovir DF-emtricitabine with ritonavir-boosted atazanavir, raltegravir, or ritonavir-boosted darunavir.
Methods
Study Patients
The AIDS Clinical Trials Group (ACTG) Study A5257 included HIV-1–infected adults in the US and Puerto Rico with plasma HIV-1 RNA >1000 copies per milliliter (copies/mL) who had received no more than 10 days of prior antiretroviral therapy. Participants had documented absence of genotypic resistance to reverse transcriptase and protease inhibitors; integrase genotyping was not required since transmitted integrase resistance remains rare. (6, 7) There were no limitations on CD4 cell count at entry. This study was approved by the ethics committee at each site, and all participants gave written informed consent before study enrollment.
Study Design
Study A5257 was a Phase 3, randomized, open label trial. Participants were followed, regardless of meeting an endpoint, for 96 weeks after enrollment of the final volunteer. Participants were randomly assigned 1:1:1 to receive one of three regimens: 300 mg of atazanavir (Reyataz, Bristol-Myers Squibb) with 100 mg of ritonavir (Norvir, Abbott Laboratories) both once daily (ritonavir-boosted atazanavir), 800 mg of darunavir (Prezista, Janssen Therapeutics) with 100 mg of ritonavir both once daily (ritonavir-boosted darunavir), or 400 mg of raltegravir (Isentress, Merck Inc.) twice daily – each with a fixed-dose combination of 300 mg of tenofovir DF plus 200 mg of emtricitabine (Truvada, Gilead Sciences). Randomization used permuted blocks stratified according to the HIV-1 RNA level (≥100,000 vs. <100,000 copies/mL) with balancing by institution. To ensure treatment balance by cardiovascular risk for an embedded cardiovascular substudy (8), randomization was stratified by intent to participate in the substudy and Framingham 10-year risk of myocardial infarction or coronary death (<6% vs. ≥6%). Screening HIV-1 RNA levels were performed at Clinical Laboratory Improvement Amendments compliant laboratories, subsequent levels were measured using the Abbott RealTime HIV-1 assay at Johns Hopkins University. Study evaluations were completed before entry, at entry, at weeks 4, 8, 16, 24, 32 and every 16 weeks thereafter.
At the time of protocol-defined virologic failure, genotyping of the HIV-1 reverse transcriptase and protease regions was performed at both Brigham and Women’s Hospital and at the University of Alabama, Birmingham; samples obtained at study entry were also assayed concurrently. Genotyping of the HIV-1 integrase region was performed in batch at the end of the study at Brigham and Women’s Hospital for subjects with virologic failure on raltegravir and for a small number of randomly selected participants from each PI-containing arm. In the event of treatment changes for virologic or tolerability failure, within-class substitutions for protease inhibitor-regimens were recommended but not mandated. Adverse events were graded using the 2004 Division of AIDS toxicity scale. (9)
Statistical Analysis
The primary objective was to evaluate regimen equivalence with regard to virologic efficacy and tolerability over 96 weeks. Virologic failure was defined as the time from randomization to a confirmed HIV-1 RNA level >1000 copies/mL at or after 16 weeks and before 24 weeks, or >200 copies/mL at or after 24 weeks. (10) The primary tolerability endpoint was the time from randomization to discontinuation of the randomized regimen component for toxicity (per site attribution); treatment discontinuations for other reasons were considered competing events. Substitution of any component of the fixed-dose combination of tenofovir DF plus emtricitabine was not considered tolerability failure. A pre-planned composite endpoint was defined as the earlier occurrence of either virologic failure or tolerability failure. The target sample size of 600 participants per arm would provide 90% power to demonstrate equivalence in pairwise regimen comparisons, assuming virologic failure, tolerability failure, and lost-to-follow-up rates of 25%, 10%, and 12% per arm, respectively. Equivalence was predefined as a 2-sided 97.5% confidence interval on the pairwise difference in 96-week cumulative incidence of each individual or composite endpoint falling entirely within −10% and +10%. For comparisons in which equivalence was not demonstrated, superiority was defined as exclusion of 0 from the 97.5% confidence interval. The equivalence bound was determined by the protocol team consistent with Food and Drug Administration (FDA) guidelines and contemporary trials. Inference was based on the 97.5% confidence level to control type I error at 5% with evaluation of three pairwise comparisons. (11)
Unless otherwise noted, all analyses followed the intention-to-treat (ITT) principle; sensitivity as-treated analyses of virologic failure included treatment discontinuation as a competing event. Event cumulative incidence was estimated over time accounting for competing events as appropriate (for tolerability failure and as-treated virologic failure outcomes). Inference regarding equivalence was made based on the pairwise differences in 96 week cumulative incidence of the event of interest with 97.5% confidence interval stratified by HIV-1 RNA level at screening; in each case, the inverse of the stratum-specific variance was used for the stratum weights. (12) Modification of treatment group differences across pre-specified subgroups of screening HIV-1 RNA, sex, and race/ethnicity were evaluated by Wald chi-squared tests of interaction constructed from the weighted sum of the squared differences of the subgroup specific treatment effects from a pooled treatment effect; weighting was by the inverse of the variances of the subgroup specific effects. Pairwise comparisons of the distributions of changes in continuous outcomes by treatment group used Student’s t-tests.
The key toxicity secondary endpoint was defined as the time from the initiation of treatment to the first grade 2, 3 or 4 sign or symptom (grade 3 or 4 if after week 48), or any grade 3 or 4 laboratory abnormality while the patient was receiving the randomized treatment (as-treated). A pre-specified sensitivity analysis was performed which excluded hyperbilirubinemia and elevations in the creatine phosphokinase level from the endpoint definition. After review of the data and a concern about underestimation of rates of adverse events because of treatment switching, a further sensitivity analysis was performed that included all qualifying adverse events regardless of status on randomized treatment (ITT). An independent data safety monitoring board reviewed the data on 3 occasions according to pre-specified monitoring guidelines; no concerns with the conduct, safety, or efficacy of the study were noted. Reported P values are two-sided. Analyses were performed with the use of SAS Version 9.2 (Cary, NC).
Role of the funder
The study was funded by the Division of AIDS/National Institute of Allergy and Infectious Disease which provided input into protocol design, conduct, and manuscript development. Bristol-Myers Squibb, Merck Inc., Janssen Therapeutics, and Gilead Sciences provided the study medications (atazanavir, raltegravir, darunavir, and tenofovir-emtricitabine, respectively) and had input into protocol development and review of the manuscript. Ritonavir was not provided by the study, however participant copayments were reimbursed for participants randomized to ritonavir-containing regimens where allowed by law. Final review and approval of the manuscript was the sole responsibility of the ACTG.
Results
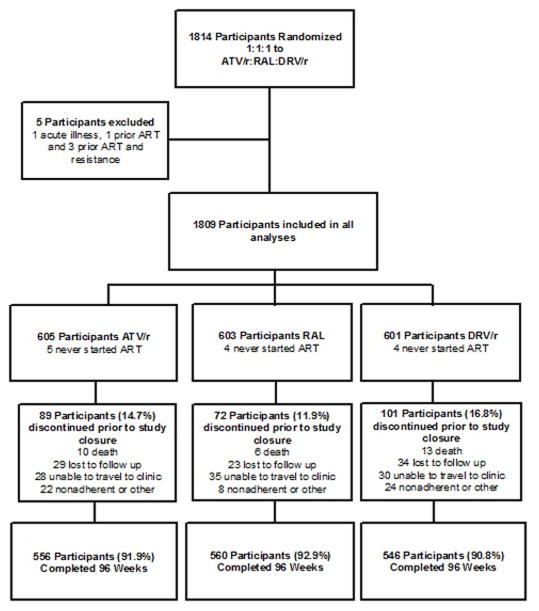
Between May 22, 2009 and June 9, 2011 1,814 participants from 57 sites enrolled in the study. Subsequent to randomization five participants were found to be ineligible due to an acute illness, presence of an exclusionary resistance mutation, or previous use of antiretroviral therapy; per protocol, these participants were excluded from all analyses. The final population for analysis therefore included 1,809 participants (Figure 1). Demographic characteristics of the population were well balanced between the three arms (Table 1). Women comprised 24.0% of the study population, 34.0% of the participants were non-Hispanic white, median (Q1–Q3) CD4+ cell count was 0.308 (0.170–0.425) × 109 cells/L, median (Q1–Q3) HIV-1 RNA level at entry was 4.6 (4.1–5.1) log10 copies/mL, and 69.4% of the population had a baseline HIV-1 RNA level less than 100,000 copies/mL. Discontinuation of follow-up prior to the completion of the study in June 2013 occurred in 262 participants (14.5%).. Among these, 162 (61.8%) discontinued after reaching a virologic failure endpoint and thus contributed events to the primary virologic failure analysis. There was no difference in the cumulative probability of study discontinuation prior to virologic failure over time between the three arms (p=0.134, log-rank test). There were 10 deaths in the ritonavir boosted atazanavir arm, 6 in the raltegravir arm and 13 in the ritonavir boosted darunavir arm; the most common categories were sudden death of unknown cause and malignancy. (Table 2, supplemental appendix)
Figure 1.
Table 1.
Baseline characteristics
| Treatment Group | ||||
|---|---|---|---|---|
|
| ||||
| Characteristic | Total (N=1809) | ATV/r (N=605) | RAL (N=603) | DRV/r (N=601) |
| Sex – N (%) | ||||
| Female | 435 (24.0%) | 144 (23.8%) | 148 (24.5%) | 143 (23.8%) |
| Age (years) | ||||
| Median | 37 | 37 | 36 | 37 |
| Race/Ethnicity – N (%) | ||||
| White Non-Hispanic | 615 (34.0%) | 212 (35.0%) | 212 (35.2%) | 191 (32%) |
| Black Non-Hispanic | 757 (41.8%) | 252 (41.6%) | 254 (42.1%) | 251 (42%) |
| Hispanic (Regardless of Race) | 390 (21.6%) | 125 (20.7%) | 117 (19.4%) | 148 (25%) |
| Asian, Pacific Islander | 30 (1.7%) | 11 (1.8%) | 13 (2.2%) | 6 (1%) |
| American Indian, Alaskan Native | 6 (<1%) | 2 (<1%) | 2 (<1%) | 2 (<1%) |
| More than one race | 7 (<1%) | 2 (<1%) | 3 (<1%) | 2 (<1%) |
| Baseline CD4+ cell count (x 109/L) | ||||
| Median | 0.308 | 0.309 | 0.304 | 0.310 |
| <0.050 | 216 (11.9%) | 71 (11.7%) | 76 (12.6%) | 69 (11.5%) |
| 0.050–0.199 | 320 (17.7%) | 104 (17.2%) | 111 (18.4%) | 105 (17.5%) |
| 0.200–0.349 | 528 (29.2%) | 186 (30.7%) | 172 (28.5%) | 170 (28.3%) |
| 0.350–0.500 | 504 (27.9%) | 157 (26.0%) | 169 (28.0%) | 178 (29.6%) |
| >0.500 | 241 (13.3%) | 87 (14.4%) | 75 (11.9%) | 79 (13.1%) |
| Baseline HIV-1 RNA (log10 copies/mL) | ||||
| Median | 4.62 | 4.60 | 4.66 | 4.61 |
| Baseline HIV-1 RNA (copies/mL) – N (%) | ||||
| <100,000 | 1,256 (69.4%) | 412 (68.1%) | 410 (68.0%) | 434 (72.2%) |
| 100,000–500,000 | 425 (23.5%) | 151 (25.0%) | 143 (23.7%) | 131 (21.8%) |
| >500,000 | 128 (7.1%) | 42 (6.9%) | 50 (8.3%) | 36 (6.0%) |
| Mode of Transmission – N (%) | ||||
| Homosexual Contact | 977 (54.0%) | 330 (54.5%) | 324 (53.7%) | 323 (53.7%) |
| Heterosexual Contact | 575 (31.8%) | 186 (30.7%) | 190 (31.5%) | 199 (33.1%) |
| Injection Drug Use | 37 (2.0%) | 15 (2.5%) | 15 (2.5%) | 7 (1.2%) |
| Transfusion/Occupational Exposure | 18 (<1%) | 3 (<1%) | 9 (1.5%) | 6 (1.0%) |
| Unknown/Unreported/Other | 202(11.2%) | 71 (11.7%) | 65 (10.8%) | 66 (11.0%) |
| IV drug history – N (%) | ||||
| Never | 1,673 (92.5%) | 558 (92.2%) | 563 (93.4%) | 552 (91.8%) |
| Currently | 4 (<1%) | 1 (<1%) | 0 | 3 (<1%) |
| Previously | 132 (7.3%) | 46 (7.6%) | 40 (6.6%) | 46 (7.6%) |
| Hepatitis B surface antigen* – N (%) | ||||
| Positive | 49 (2.7%) | 15 (2.5%) | 16 (2.7%) | 18 (3.0%) |
| Negative | 1,752 (96.8%) | 587 (97.0%) | 582 (96.53) | 583 (97.0%) |
| Indeterminate | 1 (<1%) | 0 | 1 (<1%) | 0 |
| Not done | 7 (<1%) | 3 (<1%) | 4 (<1%) | 0 |
| Hepatitis C+– N (%) | ||||
| Positive | 141 (7.8%) | 47 (7.8%) | 49 (8.1%) | 45 (7.5%) |
| Negative | 1,660 (92.2%) | 553 (91.4%) | 551 (91.4%) | 556 (92.5%) |
| Indeterminate | 5 (<1%) | 3 (<1%) | 2 (<1%) | 0 |
| Not done | 3 (<1%) | 2 (<1%) | 1 (<1%) | 0 |
| Calculated creatinine clearance (mL/min)≠ | ||||
| Median | 120 | 120.9 | 122.1 | 117.7 |
| <60 mL/min | 23 (1%) | 10 (2%) | 5 (1%) | 8 (1%) |
| 60–90 mL/min | 253 (14%) | 93 (15%) | 75 (12%) | 85 (14%) |
| >90 mL/min | 1,533 (85%) | 502 (83%) | 523 (87%) | 508 (85%) |
| EFV appropriate for participants?§ – N (%) | ||||
| No | 440 (24.3%) | 153 (25.3%) | 153 (25.4%) | 134 (22.3%) |
| Reason EFV not appropriate§ – N (%) | ||||
| Woman of childbearing potential | 91 (20.7%) | 28 (18.3%) | 26 (17.0%) | 37 (27.6%) |
| Psychiatric illness | 188 (42.7%) | 64 (41.8%) | 69 (45.1%) | 55 (41.0%) |
| Methadone withdrawal risk | 5 (1.1%) | 1 (<1%) | 2 (1.3%) | 2 (1.5%) |
| NNRTI Resistance | 112 (25.4%) | 43 (28.1%) | 45 (29.4%) | 24 (17.9%) |
| Prior NNRTI Intolerance | 7 (1.6%) | 4 (2.6%) | 1 (<1%) | 2 (1.5%) |
| Other condition | 37 (8.4%) | 13 (8.5%) | 10 (6.5%) | 14 (10.4%) |
ATV/r= atazanavir plus ritonavir; RAL= raltegravir; DRV/r= darunavir plus ritonavir; EFV= efavirenz; NNRTI= non-nucleoside reverse transcriptase inhibitor
Participants with prior documentation of positive Hepatitis B virus (HBV) surface antibody and/or HBV core antibody with negative HBV surface antigen were not required to be tested, and are categorized into the ‘negative’ group
Participants with chronic hepatitis C and prior documentation of positive hepatitis C antibody were not required to be tested, and are categorized into the ‘positive’ group
Calculated creatine clearance used Cockcroft-Gault equation
As determined by study investigator. Other specified conditions are grouped according to a search for specific terms
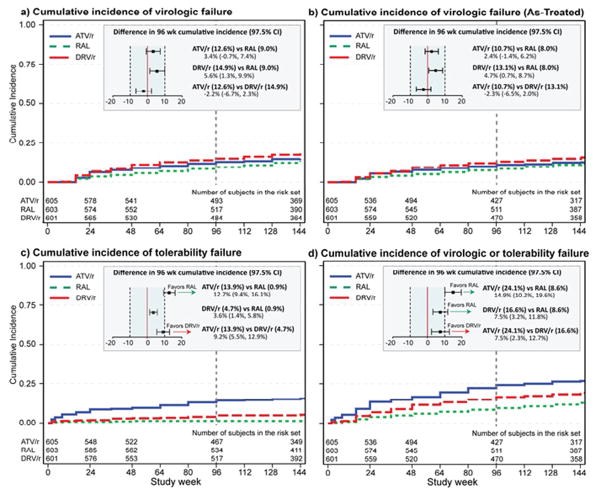
A total of 295 participants (16.3%) experienced confirmed virologic failure. Of these, 54 (18.3%) occurred before 24 weeks, 67 (22.7%) between weeks 24 and 48, and 174 (59.0%) after week 48. The cumulative incidence of virologic failure by treatment group and difference by 96 weeks is presented in Figure 2a. The cumulative probability of virologic failure by 96 weeks was 12.6% in the ritonavir-boosted atazanavir arm, 9.0% in the raltegravir arm and 14.9% in the ritonavir-boosted darunavir arm. For all pairwise treatment comparisons the 97.5% confidence intervals were within the pre-specified equivalence bound of ±10%, demonstrating equivalence of the three regimens with respect to this endpoint; results for as-treated analyses were consistent (Figure 2b). For all comparisons, with the exception of some evidence of a differential benefit of raltegravir over ritonavir-boosted darunavir for non-Hispanic blacks and Hispanics (p=0.050), differential treatment effects by screening HIV-1 RNA level (p for interaction >0.32) and sex (p>0.167) were not apparent (see Supplemental Appendix, Figure 1).
Figure 2.
The primary tolerability endpoint of toxicity-associated discontinuation of the randomized treatment was equivalent between the raltegravir arm and the ritonavir-boosted darunavir arm at 96 weeks, whereas ritonavir-boosted atazanavir resulted in a 12.7% (97.5% CI 9.4%, 16.1%) higher incidence of tolerability discontinuation than raltegravir and a 9.2% (97.5% CI 5.5%, 12.9%) higher incidence of discontinuation than ritonavir-boosted darunavir (Figure 2c). Of the 95 discontinuations of ritonavir-boosted atazanavir due to toxicity, 46 (48%) were attributed to either jaundice or an increased blood bilirubin and 25 (26%) were due to nausea or other gastrointestinal toxicities (Supplemental Appendix, Table 1). In the ritonavir-boosted darunavir group 14 of the 32 (44%) tolerability endpoints were attributed to gastrointestinal symptoms; only 2 of 8 (25%) tolerability endpoints in the raltegravir group were for this reason. Some evidence of differential treatment effects for tolerability by sex was apparent for ritonavir-boosted atazanavir versus raltegravir by screening HIV-1 RNA level (P=0.036) and ritonavir-boosted darunavir versus raltegravir (P=0.047). A greater tolerability benefit of raltegravir compared to ritonavir-boosted atazanavir was observed among participants with a baseline HIV-1 RNA <100,000 copies/mL; similarly, a greater tolerability benefit of raltegravir over ritonavir-boosted darunavir was observed in women, (see Supplemental Appendix, Figure 2). No other differential treatment effects were apparent (P>0.128).
In pairwise comparisons of the cumulative incidence of a pre-specified secondary composite endpoint of time to first of either virologic or tolerability failure, ritonavir-boosted atazanavir was inferior to both raltegravir by 14.9% (97.5% CI 10.2%, 19.6%) and to ritonavir-boosted darunavir by 7.5% (97.5% CI 2.3%, 12.7%). (Figure 2d) Ritonavir-boosted darunavir was inferior to raltegravir by 7.5% (97.5% CI 3.2%, 11.8%). No differential treatment effects by viral load, race/ethnicity or sex were apparent (P>0.09). A time to loss of virologic response (TLOVR) endpoint analysis using a virologic failure threshold of >200 copies/mL was consistent with the ITT composite endpoint results.
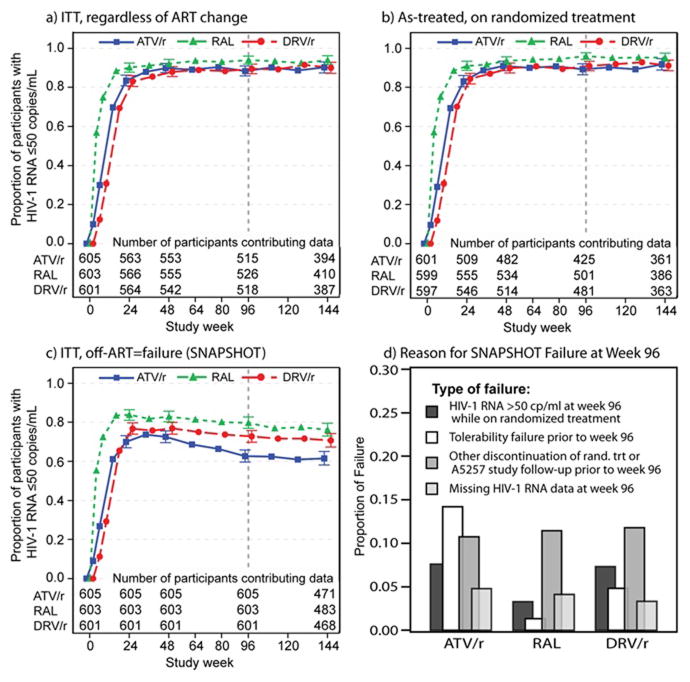
The proportion of participants with HIV-1 RNA ≤ 50 copies/mL at 96 weeks by ITT analysis (regardless of treatment status) was 88.3% for ritonavir-boosted atazanavir, 93.9% for raltegravir and 89.4% for ritonavir-boosted darunavir (Figure 3a). As treated analysis yielded similar proportions of viral suppression as observed by intent to treat (Figure 3b). In a snapshot approach the proportion with ≤50 copies/mL and on randomized treatment at week 96 was 62.6% for ritonavir-boosted atazanavir, 79.8% for raltegravir and 72.7% for ritonavir-boosted darunavir (Figure 3c).
Figure 3.
The mean change in CD4 count from baseline to week 96 was 0.28 × 109 cells/L for ritonavir-boosted atazanavir, 0.288 × 109 cells/L for raltegravir, and 0.256 × 109 cells/L for ritonavir-boosted darunavir. The difference in mean CD4 count change from baseline was greater for raltegravir (0.032 × 109 cells/L, 95% CI 6, 57, p=0.005) and for ritonavir boosted atazanavir (0.028 × 109 cells/L, 95% CI 3, 54, p=0.011) than for ritonavir-boosted darunavir.
Of 295 participants who met criteria for virologic failure, HIV-1 sequence data were available for 294 at baseline and 239 (81.0%) at virologic failure; sequencing failure due to low-level viremia was the primary reason for lack of sequence data (Table 2). Overall, virologic failure with resistance occurred in 3.0% of study participants randomized to raltegravir, of whom two participants developed intermediate-level resistance to dolutegravir, and in ≤1.5% of those in either boosted protease inhibitor arm. Twenty-seven participants randomized to a ritonavir boosted-protease inhibitor regimen who experienced virologic failure had integrase genotyping performed. Two participants had evidence of treatment emergent raltegravir resistance despite absence of known exposure to an integrase inhibitor.
Table 2.
Genotypic Analysis for Resistance to Randomized Medications at Virologic Failure
| Treatment Group | |||
|---|---|---|---|
| ATV/r | RAL | DRV/r | |
| Virologic Failure | 95 | 85 | 115 |
| Genotype Testing Complete | 75 | 65 | 99 |
| Any Resistance Detected | 9 | 18 | 4 |
| PI Resistance Detected | 0 | 0 | 0 |
| NRTI Only Resistance Detected | 8 | 7 | 3 |
| - emtricitabine | 5 | 7 | 3 |
| - tenofovir | 2 | ||
| - emtricitabine and tenofovir | 1 | ||
| INI Only Resistance Detected* | 1 | 1 | 1 |
| NRTI and INI Resistance Detected | 0 | 10 | 0 |
| - emtricitabine and raltegravir | 7 | ||
| - emtricitabine, tenofovir and raltegravir | 3 | ||
ATV/r= atazanavir/ritonavir, RAL= raltegravir, DRV/r = darunavir/ritonavir, PI= protease inhibitor, NRTI= nucleoside reverse transcriptase inhibitor, INI= integrase inhibitor
Emtricitabine resistance= M184V (21 subjects), M184I (4 subjects), or M184V/I (1 subject)
Tenofovir resistance= K65R (3 subjects), K70N (2 subjects), T215A (1 subject), or T215I (1 subject)
15/75 subjects in the Atazanavir/ritonavir arm and 12/99 subjects in the darunavir/ritonavir arm had INI resistance testing performed. Raltegravir resistance= N155H (6 subjects) plus one or more of E92Q (4 subjects), Q138K (2 subject), V151I (3 subjects), E157Q (2 subjects), G163R (1 subject); or single mutations at E92Q (1 subject), T97A (1 subject on ATV/r), N155H (2 subjects), E157Q (2 subjects), and G163K (1 subject).
Adverse Events
96-week cumulative incidence of first clinical or laboratory adverse event was 80.8% in the ritonavir-boosted atazanavir arm, 59.5% in the raltegravir arm, and 64.9% in the ritonavir-boosted darunavir arm; when bilirubin and creatine phosphokinase abnormalities were excluded, these cumulative incidences were 62.3%, 59.3% and 64.9% respectively. Table 3 lists clinical adverse events and laboratory abnormalities (grade 2 or higher) occurring in at least 5% of the participants in any arm. The most frequently reported abnormality was elevated bilirubin, for which 286 of 295 individuals were in the ritonavir-boosted atazanavir arm. In pre-specified safety analyses excluding elevated bilirubin events, all three study regimens were equivalent with respect to the cumulative incidence of first adverse event over 96 weeks; ITT analyses yielded consistent findings. Fasting LDL cholesterol and triglyceride levels increased in the ritonavir-boosted protease inhibitor-containing arms to a similar degree; and each had greater increases in lipids than the raltegravir arm (all p ≤0.001). A grade 3 or 4 elevation in serum creatinine was experienced by seven, four and twelve participants treated with ritonavir-boosted atazanavir, raltegravir, and ritonavir-boosted darunavir respectively. Substitutions of any component of the fixed dose combination of tenofovir DF plus emtricitabine while on randomized therapy occurred in 20, 9 and 23 of the participants in the ritonavir-boosted protease atazanavir, raltegravir and ritonavir-boosted darunavir arms respectively.
Table 3.
Adverse Events ≥ Grade 2 Occurring in at Least 5% of Subjects in any Group
| Treatment group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATV/r | RAL | DRVr/r | ||||||||||
|
| ||||||||||||
| Adverse Effect | Grade | Total (%) | Grade | Total (%) | Grade | Total (%) | ||||||
| 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | ||||
| Diarrhea | 35 | 11 | 0 | 46 (7.6) | 26 | 10 | 0 | 36 (6.0) | 46 | 6 | 0 | 52 (8.6) |
| Nausea | 36 | 8 | 1 | 45 (7.4) | 21 | 12 | 0 | 33 (5.5) | 29 | 12 | 0 | 41 (6.8) |
| Vomiting | 22 | 7 | 1 | 30 (5.0) | 15 | 9 | 0 | 24 (4.0) | 21 | 11 | 0 | 32 (5.3) |
| Abdominal pain | 13 | 17 | 1 | 31 (5.1) | 6 | 10 | 1 | 17 (2.8) | 13 | 14 | 2 | 29 (4.8) |
| Headache | 23 | 10 | 2 | 35 (5.8) | 35 | 7 | 0 | 42 (7.0) | 30 | 12 | 2 | 44(7.3) |
| Pain in extremity | 27 | 14 | 1 | 42 (6.9) | 31 | 14 | 0 | 45 (7.5) | 18 | 13 | 1 | 32 (5.3) |
| Arthralgia | 17 | 8 | 0 | 25 (4.1) | 17 | 4 | 1 | 22 (3.6) | 13 | 14 | 1 | 28 (4.7) |
| Back pain | 14 | 4 | 0 | 18 (3.0) | 21 | 10 | 0 | 31 (5.1) | 9 | 12 | 0 | 21 (3.5) |
| Fatigue | 32 | 6 | 1 | 39 (6.4) | 26 | 5 | 0 | 31 (5.1) | 26 | 7 | 0 | 33 (5.5) |
| Cough | 33 | 9 | 0 | 42 (6.9) | 32 | 8 | 0 | 40 (6.6) | 31 | 5 | 0 | 36 (6.0) |
| Dyspnea | 16 | 9 | 1 | 26 (4.3) | 16 | 12 | 0 | 28 (4.6) | 8 | 14 | 1 | 23 (3.8) |
| Pyrexia | 16 | 9 | 1 | 26 (4.3) | 25 | 10 | 0 | 35 (5.8) | 18 | 7 | 2 | 27(4.5) |
| Blood bilirubin increased | 22 | 217 | 47 | 286 (47.3) | 0 | 5 | 0 | 5 (<1) | 0 | 4 | 0 | 4 (<1) |
| Blood phosphorus decreased | 3 | 30 | 1 | 34 (5.6) | 4 | 24 | 1 | 29 (4.8) | 2 | 35 | 0 | 37 (6.2) |
| Blood glucose increased | 11 | 15 | 0 | 26 (4.3) | 15 | 9 | 2 | 26 (4.3) | 15 | 11 | 1 | 27 (4.5) |
ATV/r= atazanavir/ritonavir, RAL= raltegravir, DRV/r = darunavir/ritonavir
For clinical adverse effects, grade 2 = moderate, grade 3 = severe, Grade 4= potentially life-threatening For blood bilirubin increased grade 2 = 1.6–2.5 x upper limit of normal (ULN), grade 3 = 2.6–5.0 x ULN, grade 4 = >5.0 x ULN. For blood phosphorous decreased, grade 2 = 0.65–0.80 mmol/L, grade 3 = 0.32–0.64 mmol/L, grade 4 = <0.32 mmol/L. For blood glucose increased, grade 2 = 8.89–13.88 mmol/L, grade 3 = 13.89–27.75 mmol/L, grade 4 = >27.75 mmol/L
Discussion
In this prospective, randomized, open-label study of the initial treatment of HIV infection, all three study regimens yielded equivalent rates of virologic suppression over 96 weeks. The racial and gender diversity of our study population allowed us to examine the impact of these factors on treatment success. Rates of virologic failure remained equivalent between the three arms when the analysis was stratified by baseline viral load and by sex. Some evidence of a greater virologic benefit of raltegravir compared to ritonavir-boosted darunavir among non-Hispanic Blacks and Hispanics was apparent, although this finding should be interpreted with caution in the setting of multiple comparisons. The ritonavir-boosted atazanavir containing regimen was less well tolerated than regimens containing either ritonavir-boosted darunavir or raltegravir. A composite assessment of virologic efficacy and tolerability found that the raltegravir-based regimen was superior to both protease inhibitor-containing regimens, and that ritonavir-boosted darunavir was superior to ritonavir-boosted atazanavir.
The tolerability result was driven primarily by participant-driven regimen change for jaundice in the ritonavir-boosted atazanavir arm, and also by non-biliary gastrointestinal toxicity for both protease inhibitor-containing regimens compared to the raltegravir regimen. Although ritonavir-boosted atazanavir was less well tolerated than ritonavir-boosted darunavir and raltegravir across all subgroups, for women raltegravir was better tolerated than ritonavir-boosted darunavir. This result is consistent with findings of the GRACE study, which found increased discontinuation of ritonavir-boosted darunavir among women compared to men. (13) Creatine phosphokinase elevations were seen in <1% of raltegravir-treated participants, in contrast to other reports. (14)
The secondary composite endpoint aggregates two major reasons for regimen change in clinical practice: failure to achieve and maintain virologic suppression and lack of tolerability. In this analysis, subtle differences in rates of virologic failure between arms, combined with small differences between raltegravir and ritonavir boosted darunavir tolerability, yielded the observed superiority of the raltegravir regimen over both protease inhibitor-based comparators. Jaundice and other gastrointestinal toxicity leading to discontinuation of randomized therapy drove the composite superiority of ritonavir-boosted darunavir over ritonavir-boosted atazanavir.
Ritonavir-boosted atazanavir and raltegravir both had greater increases in mean CD4 cell counts over 96 weeks of therapy than did ritonavir-boosted darunavir, although the magnitude of the differences may be of limited clinical significance. Increases in triglycerides and fasting LDL-cholesterol observed in both protease inhibitor-based regimens were not different between ritonavir-boosted atazanavir and ritonavir-boosted darunavir, consistent with 48-week results from a prior study, and were greater than those observed for raltegravir. (15) Despite the inclusion of tenofovir DF in each arm a grade 3 or 4 elevation of blood creatinine was observed in only 1.27% of subjects, possibly due to the careful pre-study screening and close on-study monitoring.
DeJesus et al. reported the first study demonstrating that an integrase inhibitor-based regimen (tenofovir DF-emtricitabine-cobicistat-elvitegravir) was non-inferior to a protease inhibitor-based regimen (tenofovir DF-emtricitabine with ritonavir-boosted atazanavir) for the initial treatment of HIV-1 infection. (16) In contrast to our study, the rate of ocular icterus was 14%, with discontinuation of ritonavir-boosted atazanavir for toxicity in only 5.1% of participants (compared to 14% discontinuation for toxicity in the current study). Moreover, there were no significant differences in total cholesterol or low density lipoprotein cholesterol between arms, likely due to the known effects of cobicistat on lipid metabolism. (17, 18) Important differences from our study are that participants who changed study treatment before week 48 were classified as treatment failures, and the rates of medication discontinuation were not different between arms, perhaps due to the placebo controlled nature of their study.
Clotet et al. reported a randomized, open-label study demonstrating superiority of dolutegravir compared to ritonavir-boosted darunavir for initial treatment of HIV-1 infection, driven by tolerability differences and higher rates of virologic suppression for dolutegravir among the subset of participants with baseline HIV-1 RNA > 100,000 copies/mL. (19) Important differences in study population (only 15% female, 28% non-white, non-uninform nucleoside backbone) compared to our study may have contributed to differences in subgroup results.
The observed development of antiretroviral resistance at virologic failure is consistent with other studies, with minimal resistance to protease inhibitors and supporting nucleoside backbones in both protease inhibitor-containing regimens. Those randomized to raltegravir frequently developed integrase mutations and nucleoside backbone resistance when virologic failure occurred. Two of the raltegravir-treated participants also developed intermediate-level dolutegravir resistance, potentially limiting the use of this medication. The apparent emergence of integrase inhibitor resistance in two of twenty-seven protease inhibitor-treated participants with no known exposure to integrase inhibitors is unexplained.
Limitations of the current study include its open-label design. While reducing pill-burden compared to double-blind dummy designs, participants and investigators may have been more prone to switch ritonavir-boosted atazanavir treatment for signs or symptoms of elevated bilirubin. Although the study did not provide ritonavir, drop-outs due to difficulty obtaining ritonavir were limited by participant co-pay reimbursements. Analyses of the primary study endpoints assumed that censoring of participants who prematurely discontinued was non-informative. While statistical methodology exists for alternative approaches to such cases – such as inverse probability of censoring weighting – this was not felt warranted given the low rate of such dropout in the study (4% for virologic failure, 4% for the composite outcome) as well as the consistency of the results of our composite outcome with those of the FDA TLOVR endpoint that treats such outcomes as failures.
Our study allowed participants with intolerance to randomized treatment to change to study-provided options that are convenient, well-tolerated, and potent. Participants’ threshold for discontinuing less tolerable treatments may have been lower than in prior studies in which discontinuation required participants to leave the study or receive suboptimal alternatives.
This first head-to-head comparison of these DHHS-recommended initial treatment regimens provides useful information to guide clinicians about choosing between them. All three regimens achieved equivalent and high rates of virologic suppression, while raltegravir had a more favorable tolerability profile and caused less elevation in lipids. Among protease inhibitor-containing regimens, ritonavir-boosted darunavir was better tolerated than ritonavir-boosted atazanavir, primarily due to differences in hyperbilirubinemia. When tolerability and virologic response are considered together, raltegravir-based therapy was overall superior to both protease inhibitor-based therapies, and ritonavir-boosted darunavir superior to ritonavir-boosted atazanavir. An advantage of the ritonavir-boosted protease inhibitors over the raltegravir-based regimen is the reduced likelihood of developing drug resistance should virologic failure occur.
Supplementary Material
Acknowledgments
The project described was supported by Award Number UM1AI068636 from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.”
The protocol received support from the AIDS Clinical Trials Group, the Site Data Management Center grant of UM1AI68634, the ACTG specialty laboratories listed in the manuscript, and the thirty-seven clinical research sites. From the sites we acknowledge the following personnel and AIDS Clinical Trials Unit grants:
Michelle Saemann, RN and Jennifer Baer, RN- Cincinnati CRS (Site 2401) Grant AI069439; Dr. Susan Koletar, Mark Hite RN- Ohio State University CRS (Site 2301) Grant UM1AI069494; Linda Meixner, RN and Edward Seefried, RN- UCSD Antiviral Research Center CRS (Site 701) Grant AI69432; Vicki Bailey, RN and Rebecca Basham, CCRP- Vanderbilt Therapeutics CRS (Site 3652) Grant 2UM1AI069439-08, CTSA Grant UL1 TR000445; David Currin RN and Miriam Chicurel-Bayard RN- Chapel Hill CRS (Site 3201) Grant UM1 AI069423-08, CTSA Grant 1UL1TR001111, CFAR Grant P30 AI50410; Teresa Spitz and Judy Frain- Washington University Therapeutics CRS (Site 2101) Grant UM1AI069439; Elizabeth Lindsey, RN and Tamara James - Alabama Therapeutics CRS (Site 5801) Grant 2UM1AI069452-08; Beverly Putnam and Cathi Basler- University of Colorado Hospital CRS (Site 6101) Grant 2UM1AI069432, CTSA Grant UL1 TR001082; Michael P. Dube, MD and Bartolo Santos, RN- University of Southern California CRS (Site 1201) Grant AI069432; Eric Daar and Sadia Shaik - Harbor UCLA CRS (Site 603) Grant AI069424, CTSA Grant UL1TR000124; Pablo Tebas MD and Aleshia Thomas RN, BSN- Penn Therapeutics CRS (Site 6201) Grant UM1-AI069534-08, CFAR Grant 5-P30-AI-045008-15; Roger Bedimo, MD and Michelle Mba, MPH- Trinity Health and Wellness Center (Site 31443) Grant U01 AI069471; David Cohn MD and Fran Moran RN- Denver Public Health CRS (Site 31470) Grant UM1 AI069503; Jorge L. Santana Bagur, MD and Ileana Boneta Dueño, RN- Puerto Rico AIDS Clinical Trials Unit CRS (Site 5401) Grant 2UM1AI069415-09; Babafemi Taiwo, MBBS, Baiba Berzins, MPH- Northwestern University CRS (Site 2701) Grant 5U01 AI069471; Dr. Emery Chang and Maria Palmer- UCLA CARE Center CRS (Site 601) Grant A1069424; Mary Adams, RN and Christine Hurley, RN - Univ. of Rochester ACTG CRS/AIDS CARE CRS/Trillium Health (Site 1101/Site 1108) Grant 2UM1 AI069511-08, CTSA Grant UL1 TR024160; Timothy Lane and Cornelius Van Dam- Greensboro CRS (Site 3203) Grant A1069423-08; Karen Tashima MD and Helen Patterson LPN - The Miriam Hospital (TMH) CRS (Site 2951) Grant 2UM1A1069412-08; Carlos del Rio, MD & Ericka Patrick, RN- The Ponce de Leon Ctr. CRS (Site 5802) Grant 2UM1 AI069418-08, CFAR Grant P30 AI050409, CTSA Grant UL1 RR025008; Norman Markowitz and Indira Brar- Henry Ford Hosp. CRS (Site 31472) Grant UM 1 A1069503; Roberto C. Arduino, MD, and Maria Laura Martinez- Houston AIDS Research Team CRS (Site 31473) Grant 2 UM1 AI069503-08, 2 UM1 AI068636-08; Rose Kim, MD and Yolanda Smith, BA- Cooper Univ. Hosp. CRS (Site 31476) Grant UM1 AI069503; Hector Bolivar, MD, Margaret A. Fischl, MD - Univ. of Miami AIDS Clinical Research Unit (ACRU) CRS (Site 901) Grant AI069477; Edward Telzak, MD and Richard Cindrich, MD- Bronx-Lebanon Hosp. Ctr. CRS (Site 31469) Grant UM1 AI069503; Paul Sax MD and Cheryl Keenan RN BC- Brigham and Women’s Hospital Therapeutics CRS (Site 107) Grant UM1AI069472; CFAR grant P30 AI060354, CTSA UL1 TR000170; Kim Whitely, RN and Traci Davis, RN- MetroHealth CRS (Site 2503) Grant AI 69501; CTSA Grant UL1TR000439; Dr. Rodger D. MacArthur and Marti Farrough, RN, BSN - Wayne State Univ. CRS (Site 31478) Grant 2UM1AI069503-08; Judith A. Aberg, MD and Michelle S Cespedes, MPH, MD - NY Univ. HIV/AIDS CRS (Site 401) Grant UM1 AI069532; Shelia Dunaway, MD and Sheryl Storey, PA-C- University of Washington AIDS CRS (Site 1401) Grant UM AI069481; Joel Gallant, MD, and Ilene Wiggins, RN - Johns Hopkins University CRS (Site 201) Grant 2UM1 AI069465, CTSA Grant UL1TR001079; Beverly Sha, MD and Veronica Navarro, RN - Rush University CRS (Site 2702) Grant AI-069471; Vicky Watson RN and Daniel Nixon DO, PhD - Virginia Commonwealth Univ. Medical Ctr. CRS (Site 31475) CPCRA CTU award UM1 AI069503, CTSA UL1TR000058; Annie Luetkemeyer, MD and Jay Dwyer, RN- UCSF HIV/AIDS CRS (Site 801) Grant UM1 AI069496, UCSF-CTSA Grant UL1 TR000004; Kristen Allen RN and Patricia Walton RN- Case CRS (Site 2501) Grant AI069501; Dr. Princy Kumar and Dr. Joseph Timpone- Georgetown University CRS (Site 1008) Grant 1U01AI069494; Mehri McKellar, MD and Jacquelin Granholm, RN- Duke Univ. Med. Ctr. Adult CRS (Site 1601) Grant 5UM1 AI069484-07; Michael T Yin, MD MS and Madeline Torres, RN- Columbia Physicians and Surgeons CRS (Site 30329) Grant 2UM1-AI069470-08, CTSA 5UL1 RR024156; Sandra Valle, PA-C and Debbie Slamowitz, RN- Stanford CRS (Site 501) Grant AI069556; Charles E. Davis Jr., M.D. and William A. Blattner, M.D. - IHV Baltimore Treatment CRS (Site 4651) Grant U01AI069447; BMC ACTG CRS (Site 104) Benjamin Linus, MD, UM1 AI069472; Beth Israel Deaconess Med. Ctr., ACTG CRS (Site 103) Mary Albrecht, MD, UM1 AI069472; CFAR grant P30 AI060354; Christina Megill, PA-C and Valery Hughes, NP- Cornell Chelsea CRS (Site 7804) Grant UM1AI069419, CTSA Grant UL1TR000457; Teri Flynn, MSN, ANP and Amy Sbrolla BSN, RN - Massachusetts General Hospital CRS (Site 101) Grant 2UM1AI069412-08; CFAR grant P30 AI060354; Sharon Riddler, MD and Lisa Klevens, BSN- University of Pittsburgh CRS (Site 1001) Grant UM1 AI069494.
Additional ACTG 5257 Team Members
George Bishopric, University of Miami, 520 Northeast 10th Avenue, Fort Lauderdale FL 33301
Carmen M. Irizarry M.T., Medical Science Campus, Pathology 606-A, San Juan PR 00935
Michael M. Lederman M.D., The Foley Building, Room 401-A, 2061 Cornell Road, Cleveland OH 44106-5083
Rita Patel M.S., WB292, 1441 West Montgomery Avenue, Rockville MD 20850
Susan Pedersen R.N., B.S.N., University of North Carolina at Chapel Hill, Bioinformatics Building, Suite 2100, 130 Mason Farm Road, Chapel Hill NC 27599-7215
Lynette Purdue Pharm. D., 5601 Fishers Lane, Room 9E28, Rockville MD 20852
Thuy Tran M.S., Harvard School of Public Health, FXB Building, Room 508, Boston MA 02115
Footnotes
The ClinicalTrials.gov registration number for this study is: 00811954
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed 3 June 2014]. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 2.World Health Organization. WHO Conslolidated Guildeines on the use of Antiretroviral Drugs for Treating and Preventing HIV Infection: recommendations for a public health approach. Geneva, Switzerland: 2013. pp. 1–272. [PubMed] [Google Scholar]
- 3.Periard D, Telenti A, Sudre P, Cheseaux JJ, Halfon P, Reymond MJ, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100(7):700–5. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 4.D’Ascenzo F, Cerrato E, Biondi-Zoccai G, Moretti C, Omede P, Sciuto F, et al. Acute coronary syndromes in human immunodeficiency virus patients: a meta-analysis investigating adverse event rates and the role of antiretroviral therapy. Eur Heart J. 2012;33(7):875–80. doi: 10.1093/eurheartj/ehr456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worm SW, Kamara DA, Reiss P, Fontas E, De Wit S, El-Sadr W, et al. Evaluation of HIV protease inhibitor use and the risk of sudden death or nonhemorrhagic stroke. J Infect Dis. 2012;205(4):535–9. doi: 10.1093/infdis/jir788. [DOI] [PubMed] [Google Scholar]
- 6.Boyd SD, Maldarelli F, Sereti I, Ouedraogo GL, Rehm CA, Boltz V, et al. Transmitted raltegravir resistance in an HIV-1 CRF_AG-infected patient. Antivir Ther. 2011;16(2):257–61. doi: 10.3851/IMP1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young B, Fransen S, Greenberg KS, Thomas A, Martens S, St Clair M, et al. Transmission of integrase strand-transfer inhibitor multidrug-resistant HIV-1: case report and response to raltegravir-containing antiretroviral therapy. Antivir Ther. 2011;16(2):253–6. doi: 10.3851/IMP1748. [DOI] [PubMed] [Google Scholar]
- 8.Stein JH, Brown TT, Ribaudo HJ, Chen Y, Yan M, Lauer-Brodell E, et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS. 2013;27(6):929–37. doi: 10.1097/QAD.0b013e32835ce27e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [Accessed March 28, 2014]; http://www.niaid.nih.gov/LabsAndResources/resources/DAIDSClinRsrch/Documents/daidsaegradingtable.pdf.
- 10.Ribaudo H, Lennox J, Currier J, Kuritzkes D, Gulick R, Haubrich R, et al. Virologic failure endpoint definition in clinical trials: Is using HIV-1 RNA threshold <200 copies/mL better than <50 copies/mL: An analysis of ACTG studies; 16th Conference on Retroviruses and Opportunistic Infections; February 8–11, 2009; Montreal Canada. Abstract #580. [Google Scholar]
- 11.Wiens BL, Iglewicz B. Design and analysis of three treatment equivalence trials. Control Clin Trials. 2000;21(2):127–37. doi: 10.1016/s0197-2456(99)00052-5. [DOI] [PubMed] [Google Scholar]
- 12.Prentice RL, Kalbfleisch JD. Hazard rate models with covariates. Biometrics. 1979;35(1):25–39. [PubMed] [Google Scholar]
- 13.Currier J, Averitt Bridge D, Hagins D, Zorrilla CD, Feinberg J, Ryan R, et al. Sex-based outcomes of darunavir-ritonavir therapy: a single-group trial. Ann Intern Med. 2010;153(6):349–57. doi: 10.1059/0003-4819-153-6-201009210-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monteiro P, Perez I, Pich J, Gatell JM, Martinez E. Creatine kinase elevation in HIV-1-infected patients receiving raltegravir-containing antiretroviral therapy: a cohort study. J Antimicrob Chemother. 2013;68(2):404–8. doi: 10.1093/jac/dks416. [DOI] [PubMed] [Google Scholar]
- 15.Aberg JA, Tebas P, Overton ET, Gupta SK, Sax PE, Landay A, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retroviruses. 2012;28(10):1184–95. doi: 10.1089/aid.2011.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeJesus E, Rockstroh JK, Henry K, Molina JM, Gathe J, Ramanathan S, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet. 2012;379(9835):2429–38. doi: 10.1016/S0140-6736(12)60918-0. [DOI] [PubMed] [Google Scholar]
- 17.Elion R, Cohen C, Gathe J, Shalit P, Hawkins T, Liu HC, et al. Phase 2 study of cobicistat versus ritonavir each with once-daily atazanavir and fixed-dose emtricitabine/tenofovir df in the initial treatment of HIV infection. AIDS. 2011;25(15):1881–6. doi: 10.1097/QAD.0b013e32834b4d48. [DOI] [PubMed] [Google Scholar]
- 18.Gallant JE, Koenig E, Andrade-Villanueva J, Chetchotisakd P, DeJesus E, Antunes F, et al. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: week 48 results. J Infect Dis. 2013;208(1):32–9. doi: 10.1093/infdis/jit122. [DOI] [PubMed] [Google Scholar]
- 19.Clotet B, Feinberg J, van Lunzen J, Khuong-Josses MA, Antinori A, Dumitru I, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;S0140–6736(14):60084–2. doi: 10.1016/S0140-6736(14)60084-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.