Abstract
Background
Host-derived lipids including cholesteryl esters (CEs) such as cholesteryl linoleate have emerged as important antibacterial effectors of innate immunity in the airways and cholesteryl linoleate has been found elevated in the context of inflammation. Cystic fibrosis (CF) patients suffer from chronic infection and severe inflammation in the airways. Here, we identified and quantified CEs in bronchoalveolar lavage fluid (BALF) from CF patients and non-CF disease controls, and tested whether CE concentrations are linked to the disease.
Materials and Methods
CEs in BALF from 6 pediatric subjects with CF and 7 pediatric subjects with non-CF chronic lung disease were quantified by mass spectral analysis using liquid chromatography coupled with tandem mass spectrometry and multiple reaction monitoring. BALFs were also examined for total lipid, total protein, albumin, and, as a marker for inflammation, human neutrophil peptide (HNP) 1–3 concentrations. Statistical analysis was conducted after log 10 transformation of the data.
Results
Total lipid/protein ratio was reduced in CF BALF (p = 0.018) but the concentrations of CEs, including cholesteryl linoleate, were elevated in the total lipid fraction in CF BALF compared to non-CF disease controls (p < 0.050). In addition, the concentrations of CEs and HNP1-3 correlated with one another (p < 0.050).
Conclusions
The data suggests that the lipid composition of BALF is altered in CF with less total lipid relative to protein but with increased CE concentrations in the lipid fraction, likely contributed by inflammation. Future longitudinal studies may reveal the suitability of CEs as a novel biomarker for CF disease activity which may provide new information on the lipid mediated pathophysiology of the disease.
Introduction
Cystic fibrosis (CF), one of the most common autosomal recessive genetic disorders, is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene coding for a chloride channel that resides on the apical surface of epithelial cells where it regulates ion transport and hydrates the airways [1]. The hallmark of CF is viscous mucus and defective mucociliary clearance in the respiratory tract [2]. Affected individuals suffer chronic airway infections with characteristic pathogens including Pseudomonas aeruginosa, as well as chronic neutrophil-driven inflammation [3]. Gradual remodeling of the lung architecture ultimately leads to lung failure and premature death [3]. The pathogenesis of opportunistic infections with P. aeruginosa is multifactorial and involves a breach of general and innate immune defenses. Some aspects of impaired defenses in the CF airway affect epithelial cells and include defective bacterial clearance [4], defective apoptotic machinery [5], or dysregulated or inactivated antimicrobial (poly)peptides secretion [6–9].
More recently, lipids have emerged as effector molecules of innate immunity with direct antimicrobial activity when applied alone [10] or in synergism with antimicrobial (poly)peptides [11,12]. For example, the linoleate and arachidonate cholesteryl esters (CEs), which are present in human nasal fluid, exert antimicrobial activity against P. aeruginosa, and the non-polar lipid component of nasal fluid exhibits synergistic activity with the antimicrobial peptide HNP2 [13]. As recently shown, CEs and non-polar lipids are elevated in sinus washes obtained from chronic rhinosinusitis patients [14] whereby potential sources for CEs are epithelial cells [13,14] as well as monocytes and macrophages [15–17].
CF patients require lifelong and complex therapy. Outcomes are variable with some patients remaining relatively stable over many years, while others experience more rapid disease progression in childhood [18]. Lung function is the most commonly used surrogate outcome measure in CF clinical trials and frequently guides clinical practice [19,20]. As treatment of CF improves, new and more sensitive biomarkers of disease activity are needed to detect treatment effects and predict those at risk for more rapid disease progression [21]. Airway neutrophil elastase activity has recently been shown to be associated with subsequent lung function decline [22] and airway structural injury [23]. Other suggested markers include sweat chloride, which appears to be useful in a subset of CF patients treated with CFTR modulators [24], and miRNA’s measured in nasal epithelial tissues, although their clinical significance has yet to be established [25,26]. Considering the role of antimicrobial CEs in innate immunity and inflammation, we sought to test whether CEs were increased in CF BALF compared to non-CF disease control BALF and could also be used as biomarkers of CF.
Materials and Methods
Ethics Statement
All human subjects materials used in this study were collected under full institutional review board approval for studies with human subjects (Colorado Multiple IRB# 99–113) and appropriate written consent.
Human Specimens
Banked BALF from 6 cystic fibrosis (CF) pediatric subjects and 7 non-CF disease control subjects (Non-CF) was used with personal identifiers removed [27]. Non-CF disease control subjects included patients who were undergoing a clinical bronchoscopy for pulmonary indications other than CF. Immediately following collection, BALF samples were centrifuged (250 × g, 10 min, 4°C), and the supernatant was transferred to a sterile polypropylene tube and centrifuged again (4000 × g, 20 min, 4°C). The remaining supernatant was further clarified by filtration through a 0.2 μm filter. The clarified samples were then stored at -70°C. Table 1 summarizes the study population and the BALF characteristics.
Table 1. Description of Study Population and BALF Characteristics.
| Disease | Subject Identifier | Sex | Age (y) | BALF Volume (mL) | Infection Status e | Nucleated Cells/μL | Red Blood Cells/μL | Neutrophils/μL | |
|---|---|---|---|---|---|---|---|---|---|
| Non-CF | CLD a /ILD b | 1 | Female | 11.7 | 10 | Negative | 548 | 25 | 33 |
| Stenosis c | 3 | Male | 4.7 | 8 | Positive | 134 | 18 | 1 | |
| NEHI d | 4 | Male | 1.8 | 7 | Positive | 77 | 10 | 0 | |
| ILD | 6 | Male | 10.1 | 8.5 | Negative | 321 | 136 | 125 | |
| ILD | 9 | Female | 4.8 | 8 | Negative | 495 | 40 | 45 | |
| ILD | 10 | Female | 9.5 | 7 | Negative | 220 | 242 | 77 | |
| ILD | 12 | Female | 2.2 | 7 | Negative | 251 | 735 | 38 | |
| CF f | F508/F508 | 2 | Female | 16.6 | 25 | Negative | 193 | 18 | 69 |
| F508/F508 | 5 | Female | 7.6 | 10 | Positive | 1495 | 525 | 1181 | |
| F508/F508 | 7 | Female | 16.4 | 12 | Positive | 783 | 200 | 532 | |
| F508/G542X | 8 | Male | 13 | 12 | Negative | 386 | 59 | 274 | |
| F508/F508 | 11 | Male | 13.9 | 35 | Negative | 2725 | 2450 | 1281 | |
| F508/F508 | 13 | Female | 12.1 | 23 | Positive | 4808 | 4733 | 4183 | |
a Chronic lung disease;
b Interstitial lung disease;
c Subglottic;
d Neuroendocrine hyperplasia of infancy;
e Negative indicates no growth or normal microbiota isolated, positive indicates isolation of probable pathogens;
f CF genotype is given.
Cholesteryl Ester Standards
Cholesteryl arachidonate, linoleate, oleate, palmitate, stearate, and octanoate-1- 13C1 were purchased from Sigma-Aldrich (St. Louis, MO), and stock solutions were prepared in dichloromethane at 1 mg/mL and stored under an atmosphere of N2 at -20°C (Table 2). Cholesteryl octonate-1- 13C1 is a cholesteryl ester not described in humans and was used as an internal standard (IS).
Table 2. Cholesteryl Esters Described in this Study.
| Common name | Fatty acid residue a | Mass | |
|---|---|---|---|
| Cholesteryl - | octonate b | C8:0 | 531.5 |
| palmitate | C16:0 | 642.6 | |
| palmitoleate | C16:1 | 640.5 | |
| stearate | C18:0 | 670.5 | |
| oleate | C18:1 | 668.6 | |
| linoleate | C18:2 | 666.6 | |
| linolenate | C18:3 | 664.5 | |
| arachidate | C20:0 | 698.5 | |
| paullinate | C20:1 | 696.5 | |
| arachidonate | C20:4 | 690.6 |
a Carbon number: number of double bonds;
b in stable 13C1 isotope form.
Lipid Extraction
BALF samples (100 μL) were diluted with dH2O (1.7 mL) in glass screw-capped test tubes, the IS (250 pmol in 25 μL of dichloromethane) was added, and total lipid extracts were prepared as described previously [13,28]. Extracts were dried in a water bath at 35°C under a gentle stream of N2 gas, and stored at -20°C in the dark until further analysis by liquid chromatography/tandem mass spectrometry with multiple reaction monitoring.
Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS)
Liquid chromatography was used with tandem MS to identify and quantify individual cholesteryl esters. Dried lipid extracts from 100 μL BALF were re-dissolved in MeOH/CHCl3 containing 5 mM ammonium acetate (50/50, v/v, 50 μl). Aliquots (typically 5 μl) were injected onto a reverse phase HPLC column (Phenomenex Kinetex XB-C18, 100 x 2.1 mm, 1.7 μm particle size, 100 Å pore diameter) equilibrated in solvent A (MeOH/H2O, 90/10, v/v, containing 5 mM ammonium acetate) and eluted with an increasing concentration of solvent B (CHCl3/H2O, 500/0.2, v/v, containing 5 mM ammonium acetate: min/%B/μl per min; 0/0/200, 3/0/200, 3.01/0/100, 20.5/100/100, 23/100/100, 24/0/200, 30/0/200). The effluent from the column was directed to an electrospray ion source (Agilent Jet Steam, Agilent, Santa Clara, CA) coupled to a triple quadrupole mass spectrometer (Agilent 6460) operating in the positive ion multiple reaction monitoring (MRM) mode in which the intensity of pre-selected (M+NH4)+ parent→fragment ion transitions (cholesteryl-C20:0, 698.5→369.4;-C20:1, 696.5→369.4;-C20:4, 690.6→369.4;-C18:0, 670.5→369.4;-C18;1, 668.6→369.4;-C18:2, 666.6→369.4,-C18:3, 664.5→369.4;-C16:0, 642.6→369.4;-C16:1, 640.5→369.4; and 13C1-cholesteryl-C8:0 internal standard (IS), 531.5→369.4; Table 2) were recorded using instrument manufacturer-supplied software (Agilent MassHunter). With each batch of samples a series of standards were prepared containing the same amount of IS (250 pmol) and varying amounts of authentic cholersteryl-C20:4,-C18:0,-C18:1,-C18:2, and C16:0 (0, 5, 10, 50, and 100 pmol, all in duplicate). The amount of each CE in each sample was calculated by interpolation from the curves constructed using the data for the standard samples (ordinate, ratio peak area cholesteryl ester/peak area of the IS; abscissa, pmol of each authentic ester). Those species for which no authentic standard was available, the curve for the species with the closest mass was used. The detection limit of this method is 20–50 fmol per injection.
Total Lipid Quantification with Nile Red
The fluoroprobe Nile red [29] (Sigma-Aldrich) was used to quantify total lipids in BALF. Ten μL of a Nile red stock solution (100 μg/mL in DMSO) was added to 90 μL BALF in duplicate in a 96-well flat bottom black microtiter plate (Corning Life Sciences—Axygen Inc., Union City, CA). After incubation in the dark for 10 min the relative fluorescence was quantified using a TECAN fluorescence reader (excitation at 485 nm, emission at 535 nm; Genios 2760063, TECAN Systems, San Jose, CA).
Total Protein Quantification
To determine total protein concentration in BALF, the Bicinchoninic acid (BCA) Protein Assay Kit (Thermo scientific, Rockford, IL) was used according to the manufacturer's instructions following the microtiter plate procedure with bovine serum albumin as the standard.
Quantification of Albumin
A sandwich ELISA colorimetric assay (Bethyl Laboratories, Inc., Montgomery, TX) was employed to quantify albumin in BALF according to the manufacturer's instructions using a 96-well flat bottom microtiter plate (Nunc Nalgene International, Rochester, NY).
Western Immunoblot Quantification of HNP1-3
To quantify the antimicrobial peptide HNP1-3, BALF was subjected to SDS-PAGE followed by Western Immunoblotting as described previously [14,30] using a polyclonal rabbit anti-HNP1-3 antibody (1:400 dilution, kindly provided by Dr. Tomas Ganz, UCLA). The concentration of HNP1-3 was interpolated from a standard curve derived from purified HNP2 peptide (also provided by Dr. Tomas Ganz) using the Versadoc Imaging System and QuantityOne Software (BioRad, Hercules, CA). The polyclonal antibody is cross-reactive with all three major HNP forms.
Data Analysis
Raw data were initially analyzed with Excel 2013 and Sigmaplot version 9.0 was used for graphing. IBM SPSS version 20.0 was used for statistical analysis and unless stated otherwise. Statistical significance was determined by first testing for equality of variances using Levene’s test. If there was no evidence of unequal variances, a one-tailed Student’s t-test for independent samples was used; otherwise, Welch's t-test for unequal variances was used. Probability levels less than 0.05 were flagged as statistically significant. With the exception of calculating statistical significance for differences in age, BALF volume, and infection status, statistical tests were run after log transformation of the data (log10 [1+data]).
Results
Study population and BALF characteristics
Table 1 shows disease status, genotype for CF, age, and BALF characteristics for the 6 cystic fibrosis (CF) and 7 non-CF disease control pediatric subjects (Non-CF). There was a statistically significant difference between Non-CF and CF subjects in age (5.8 ± 1.6 versus 13.3 ± 1.4 years, means ± S.E.M, p = 0.007) and BALF volume (7.93 ± 0.41 versus 19.5 ± 4 mL, means ± S.E.M, p = 0.035), respectively). There was no statistically significant association between disease (Non-CF versus CF) and infection status (p = 0.500 using Fisher’s one-sided exact test for a 2x2 contingency table). Compared to Non-CF, cell counts were elevated in BALF collected from CF subjects, namely nucleated cells 288 ± 78 versus 1732 ± 721, red blood cells 178 ± 117 versus 1331 ± 777, and neutrophils 32 ± 12 versus 1253 ± 618 (cells/ μL for Non-CF versus CF, means ± S.E.M, respectively), and for nucleated cells and neutrophils the differences reached statistical significance after log transformation (p = 0.021 and p = 0.003, respectively).
CE identification and quantification
On the basis of mass concordance and HPLC retention time, the molecular species of CEs detected in BALF were palmitate (16:0), palmitoleate (16:1), oleate (18:1), linoleate (18:2), linolenate (18:3), arachidate (20:0), paullinate (20:1) and arachidonate (20:4) (Table 2). The CE profile for each patient is shown in Fig 1. The most abundant CE was linoleate, which averaged 88.5 ± 54.5 pmol/mL in Non-CF BALF and 166.9 ± 55.3 pmol/mL in CF BALF (means ± S.E.M.). The next most abundant esters were oleate (24.0 ± 17.1 pmol/mL in Non-CF, and 68.8 ± 25.8 pmol/mL in CF) and palmitate (21.5 ± 12.4 pmol/mL in Non-CF disease controls, and 53.2 ±17.9 pmol/mL in CF). Cholesteryl arachidonate concentrations were 16.7 ± 9 pmol/mL in Non-CF and 24.5 ± 8.4 pmol/mL in CF. Cholesteryl linolenate levels averaged 2.8 ± 2 pmol/mL in Non-CF and 5.0 ±1.8 pmol/mL in CF. Of these, the linoleate and arachidonate esters are known to have antimicrobial activity against P. aeruginosa [13]. Signals corresponding to the palmitoleate, arachidate, and paullinate esters were detected, but at very low levels (< 2 pmol/mL). None of the observed differences in raw cholesteryl ester concentrations reached statistical significance and we queried their levels relative to total lipid.
Fig 1. CE concentrations in BALF collected from non-CF disease control subjects (top) and CF patients (bottom).
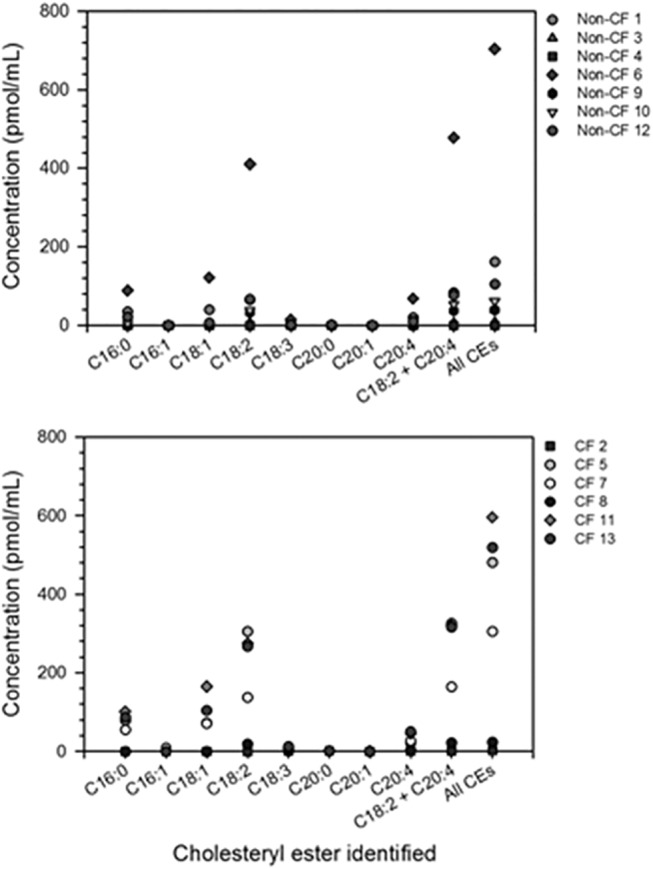
CEs are identified by the fatty acid species (number of carbons: number of unsaturated bonds) attached to the cholesterol molecule (C). Individual CE concentrations are given for each molecular species identified as well as for cholesteryl linoleate (C18:2) and cholesteryl arachidonate (C20:4) combined and all CEs combined.
CEs adjusted to lipid content
To quantify total lipid content, a protocol that employs the fluoroprobe Nile red was adapted [29]. Nile red emits red fluorescence in lipophilic environments, thus as more lipid is present red fluorescence increases. Total lipid content did not significantly differ between CF and Non-CF (Table 3), but the ratio of total lipid to total protein was significantly decreased in CF BALF (25.9 ± 10.1 versus 6.7 ± 1.2, means ± S.E.M. of Non-CF versus CF, p = 0.018) and when CE concentrations were adjusted to total lipid, the CEs of palmitate (p = 0.03), oleate (p = 0.028), linoleate (p = 0.021), linolenate (p = 0.016), and arachidonate (p = 0.038), as well as cholesteryl linoleate and cholesteryl arachidonate combined, and all CEs combined, were significantly elevated in CF compared to Non-CF (Table 4 and Fig 2). We then questioned whether this increase correlates with inflammation.
Table 3. Total Lipid and Protein Analysis of BALF.
| Disease | Subject Identifier | Total Lipid (Nile Red RFU a ) | Total Protein (μg/mL) | Albumin (μg/mL) | HNP1-3 (μg/mL) |
|---|---|---|---|---|---|
| Non-CF | 1 | 18839.5 | 222.70 | 16.81 | 0.37 |
| 3 | 1968.5 | 85.60 | 12.25 | ND b | |
| 4 | 1245.5 | 71.97 | 9.72 | ND | |
| 6 | 6638.5 | 282.03 | 69.88 | 1.45 | |
| 9 | 2811.0 | 457.61 | 200.71 | ND | |
| 10 | 1585.0 | 208.27 | 19.62 | 1.78 | |
| 12 | 6185.5 | 330.14 | 35.75 | ND | |
| CF | 2 | 1502.5 | 152.95 | 9.44 | 2.41 |
| 5 | 2086.5 | 548.21 | 80.16 | 43.48 | |
| 7 | 1301.5 | 334.14 | 35.52 | 5.19 | |
| 8 | 935.0 | 205.06 | 35.68 | 1.12 | |
| 11 | 3724.5 | 367.02 | 16.24 | 37.29 | |
| 13 | 4472.5 | 577.88 | 32.92 | 34.88 |
a RFU: relative fluorescence units;
b ND: not detectable.
Table 4. Significance Levels for Total Lipid Adjusted Cholesteryl Ester Concentrations in BALF from Non-CF vs. CF Pediatric Patients.
| CE a | Levene's Test for Equality of Variances (Significance) | t-test for Equality of Means (Significance one-tailed) | |
|---|---|---|---|
| C16:0 | Equal variances assumed | .008 | .014 |
| Equal variances not assumed | .030 | ||
| C16:1 | Equal variances assumed | .020 | .150 |
| Equal variances not assumed | .182 | ||
| C18:1 | Equal variances assumed | .009 | .013 |
| Equal variances not assumed | .028 | ||
| C18:2 | Equal variances assumed | .070 | .021 |
| Equal variances not assumed | .035 | ||
| C18:3 | Equal variances assumed | .193 | .016 |
| Equal variances not assumed | .024 | ||
| C20:0 | Equal variances assumed | .136 | .092 |
| Equal variances not assumed | .103 | ||
| C20:1 | Equal variances assumed | .345 | .292 |
| Equal variances not assumed | .284 | ||
| C20:4 | Equal variances assumed | .435 | .038 |
| Equal variances not assumed | .048 | ||
| CA + CL | Equal variances assumed | .082 | .021 |
| Equal variances not assumed | .033 | ||
| Total CE | Equal variances assumed | .027 | .015 |
| Equal variances not assumed | .028 |
a For each cholesteryl ester the fatty acid residue esterified to cholesterol is given. One-tailed significance was calculated with log10 (1+ [CE concentration as pmol per 1 RFU Nile red]) testing whether the individual CEs are elevated in CF compared to Non-CF. CL: cholesteryl linoleate; CA: cholesteryl arachidonate. P-values indicating statistical significance are shown in bold.
Fig 2. CE fraction of total lipid.
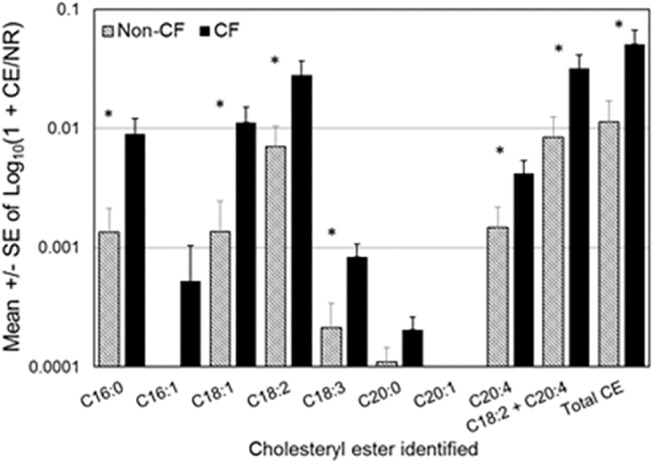
CEs are identified by the fatty acid species (number of carbons: number of unsaturated bonds) attached to the cholesterol molecule (C). Individual CE concentrations are given for each molecular species identified as well as for cholesteryl linoleate (C18:2) and cholesteryl arachidonate (C20:4) combined and all CEs combined. Shown are the means ± S.E.M of log transformed data with n = 7 for Non-CF and n = 6 for CF. * indicates a p value of < 0.050 in one-tailed t-test.
Markers of inflammation
Inflammation is typically accompanied by an increase in total protein and increased plasma transudation to deliver additional host defense proteins, and an influx of immune cells, particularly neutrophils in CF. Albumin can be used to assess plasma transudation, and the antimicrobial peptides HNP1-3, major constituents of neutrophil granules, are reliable markers for neutrophil infiltration. In addition to neutrophil counts, these markers were used to assess the lung inflammation status of the subjects (Table 3), and then their relationship to CEs was determined.
Total protein and albumin concentrations did not differ significantly between Non-CF and CF subjects; total protein concentrations were 236.9 ± 51.3 versus 364.2 ± 70.8 μg/mL (p = 0.177) and albumin concentrations were 52.1 ± 26.0 versus 35.0 ± 10.1 μg/mL (p = 0.669), Non-CF versus CF, respectively. When CE concentrations were adjusted to total protein and albumin concentrations, there was no statistically significant difference between CF and Non-CF subjects.
Next, HNP1-3 was quantified (Fig 3 and S1 Fig. Original blots presented in cropped and gray tone version in Fig 3 and Table 3). HNP1-3 was present at low levels in Non-CF and significantly elevated in CF patients (0.52 ± 0.3 μg/mL versus 20.7 ± 8.1 μg/mL in Non-CF versus CF patients, means ± S.E.M., p = 0.01 before and p = 0.04 after adjustment to total protein). Furthermore, HNP1-3 concentrations as well as neutrophil counts correlated with the same CEs that represented an increased fraction of total lipid in CF patients (Table 5). In contrast, lung infection status did not correlate with any of the CEs (p values between 0.123 and 0.506).
Fig 3. Quantification of HNP1-3 by Western immunoblot.
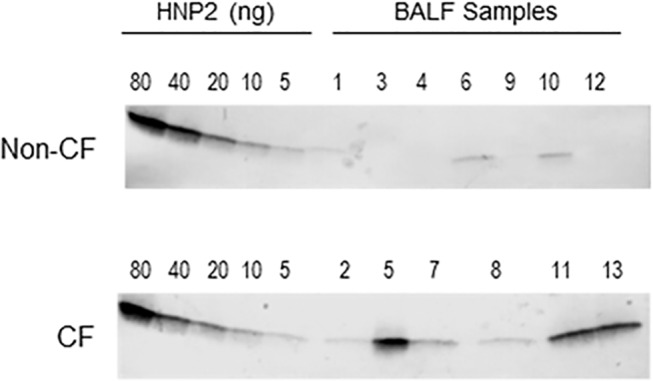
HNP2 peptide standard and bronchoalveolar lavage fluid (BALF) samples were separated by 4–20% SDS PAGE, blotted onto PVDF-PSQ membranes, probed with polyclonal rabbit antibodies against HNP1-3, and antibody binding was visualized with goat-anti rabbit antibodies conjugated to alkaline phosphatase and NBT/BCIP substrate. HNP1-3 differ by one amino acid only and co-migrate in this gel system. Per lane, the equivalent of the following BALF volumes were loaded: for Non-CF samples, 1: 20 μL, 3: 40 μL, 4: 40 μL, 6: 20 μL, 9: 40 μL, 10: 20 μL, 12: 20 μL; for CF samples, 2: 6 μL, 5: 6 μL, 7: 6 μL, 8: 20 μL, 11: 6 μL, 13: 6 μL.
Table 5. Correlation between Cholesteryl Esters and Markers of Inflammation.
| HNP | Neutrophil Count | |||
|---|---|---|---|---|
| CE a | Pearson Correlation | Significance (2-tailed) | Pearson Correlation | Significance (2-tailed) |
| C16:0 | 0.661 * | 0.014 | 0.676 * | 0.011 |
| C16:1 | 0.105 | 0.733 | 0.212 | 0.487 |
| C18:1 | 0.699 ** | 0.008 | 0.688 ** | 0.009 |
| C18:2 (CL) | 0.575 * | 0.040 | 0.731 ** | 0.005 |
| C18:3 | 0.650 * | 0.016 | 0.758 ** | 0.003 |
| C20:0 | 0.043 | 0.889 | 0.134 | 0.663 |
| C20:1 | 0.005 | 0.988 | 0.009 | 0.976 |
| C20:4 (CA) | 0.608 * | 0.028 | 0.775 ** | 0.002 |
| CL + CA | 0.591 * | 0.033 | 0.763 ** | 0.002 |
| Total CE | 0.614 * | 0.025 | 0.768 ** | 0.002 |
a For each cholesteryl ester the fatty acid residue esterified to cholesterol is given. Correlation was calculated with log transformed data (log10 [1+data]).
*Correlation is significant at the 0.05 level (2-tailed).
**Correlation is significant at the 0.01 level (2-tailed). P-values indicating statistical significance are shown in bold. CL: cholesteryl linoleate; CA: cholesteryl arachidonate.
Discussion
Cholesteryl linoleate and arachidonate have been recently identified as antimicrobial effectors in airway fluid, and cholesteryl linoleate concentrations have been found to be elevated in the context of inflammation [13,14]. This study suggests that in BALF collected from pediatric CF subjects, total lipid relative to total protein is decreased, and that when corrected for total lipid content palmitate, oleate, linoleate, linolenate, and arachidonate CEs are significantly elevated, compared to non-CF disease control subjects. Furthermore, CE concentrations correlated with HNP1-3 and neutrophil counts, both measures of inflammation. These data provide additional evidence that in CF airways the lipid composition is altered and that CEs take part in the inflammatory lung response inviting future studies to explore the potential of CEs as a novel biomarker for CF disease activity.
The CE species identified in BALF represented species previously identified in nasal fluid [13], with the exception of C20:1 which has yet not been described in human body fluids. However, the detected concentrations in BALF are low and considering the high sensitivity of the methodology used in this study, this CE species might have been present but undetected in earlier studies.
The total lipid to protein ratio was decreased in CF BALF. This is in line with earlier studies by Meyer et al., who described reduced phospholipid to protein ratios in lipid extracts of BALF collected from young adults with CF [31]. However, CEs of palmitate, oleate, linoleate, linolenate, and arachidonate were elevated in the lipid fraction of BALF collected from CF subjects. While it cannot be ruled out that the age differences in the study populations may have contributed to the observed differences, increased levels of CEs have also been found earlier in expectorated tracheobronchial secretions of CF patients [32], and it has been demonstrated that chronically infected CF patients have significantly higher CE levels in their plasma compared to non-chronically infected CF patients [33]. Decreased cholesteryl ester content of plasma lipoproteins in CF have been reported elsewhere [34,35] but in these studies CE content was indirectly calculated as the difference between total and unesterified cholesterol, and individual cholesteryl esters have not been quantified precluding detection of relative changes among the various CEs. Furthermore, the CE deficiency was attributed to defective hepatic lipase and a dysfunctional cholesteryl ester transfer protein representing metabolic changes of the liver while BALF used in this study reflects CE alterations in the airways.
We have shown previously that epithelial cells secrete CEs in the airways [13]. Here, CEs correlated with HNP1-3 and neutrophil counts, which both are strong indicators of inflammation [36,37]. Within the limitations of the small sample size of this study, CEs did not correlate with the infection status. Thus, their elevated levels do not appear to be simply a surrogate of infection but could reflect an up-regulation of their production and secretion by epithelia in the context of inflammation in CF, independent from infection. A neutrophil elastase-initiated modulation of epithelial cell transcription has been recently reported by Fischer et al. who observed an increased expression of senescence markers in CF airway tissue sections, and an in vitro up-regulation of these markers in neutrophil elastase-treated epithelial cells [38]. Alternatively, neutrophils could deliver CEs to the site of inflammation, but information on CEs in neutrophils is limited. May et al reported that total esterified cholesterol in neutrophils decreased after LPS stimulation [39], which could be consistent with CE secretion. For monocytes and macrophages, significant amounts of stored CEs and their increase after stimulation have been reported [15–17]. Thus, the elevated CE concentrations in BALF collected from CF subjects may be contributed by both epithelial and inflammatory cells. While increased hemorrhagic lesions and plasma transudation have been reported in CF disease [40], lack of statistically significant differences in red blood cells and albumin in BALF make this source less likely, but future studies that measure in parallel plasma and BALF concentrations of albumin and CEs are needed to ascertain this notion. This would also resolve the apparent discrepancy between plasma [34,35] and BALF CE levels as reported here.
While this study suggests that in pediatric CF patients the CEs of palmitate, oleate, linoleate, linolenate, and arachidonate are elevated in the lipid fraction of BALF compared to Non-CF, future studies are needed to determine whether antimicrobial CEs are active in the microenvironment of the CF lung. For example, it has been shown that while the concentrations of the antimicrobial peptide LL-37 are elevated in CF lungs, this antimicrobial peptide is inactive in the CF lung microenvironment [41,42]. Similarly, it has been recently reported that the pH of CF airway secretions is reduced and that the antimicrobial activity of lysozyme and other antimicrobial polypeptides is reduced at the pH observed in the CF fluids [9].
The findings of this study suggest that the observed increase in CEs in the lipid fraction of CF BALF reflects inflammation. Considering that increased CE levels and increased expression of a key enzyme for CE biosynthesis have been also found in chronic rhinosinusitis [14,43], the observed inflammation-associated increase in CEs is not CF specific. Thus, CEs may be explored as a novel biomarker classifying mild versus severe cases and monitoring disease activity. Using CEs as biomarkers of disease activity may add a measure of the epithelial cell response. There have been only few biomarkers described that are known products of epithelial cells, namely the proinflammatory cytokines IL-1β, IL-6, and IL-8 in sputum samples and the chemokine IP-10 in nasal lavage fluid [22,44–46], and chloride secretion [24,47]. A larger, age-controlled longitudinal study that measures in BALF known biomarkers of CF such as neutrophil elastase [22,23] in parallel with CEs is needed to confirm the suitability of CE levels as novel biomarker of CF disease activity that may provide new information on the lipid mediated pathophysiology of the disease.
Conclusions
The lipid composition of BALF is altered in CF with less total lipid relative to protein but increased CE concentrations in the lipid fraction, likely contributed by inflammation. Future longitudinal studies may reveal the suitability of CEs as a novel biomarker for CF disease activity which may provide new information on the lipid mediated pathophysiology of the disease.
Supporting Information
The primary antibody is a polyclonal antibody that shows unspecific reactivity with higher molecular weight components in BALF.
(TIF)
Acknowledgments
We thank Drs. Paul B. McCray Jr. and Jennifer Bartlett for helpful discussions.
Prior presentation: Parts of the results described here had been presented at the 2013 North American Cystic Fibrosis Conference, Salt Lake City, Utah, 10.17–10.19. 2013.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study has been supported by the Cystic Fibrosis Foundation (http://www.cff.org; PORTER12I0, ZEMANI11A0), NIH (http://www.nih.gov; 1SC1GM096916 to EP, K23HL114883 to EZ), and NSF/DMS (http://www.nsf.gov;1225529 to RD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mickle JE, Cutting GR (2000) Genotype-phenotype relationships in cystic fibrosis. Med Clin North Am 84: 597–607. [DOI] [PubMed] [Google Scholar]
- 2. Doring G, Gulbins E (2009) Cystic fibrosis and innate immunity: how chloride channel mutations provoke lung disease. Cell Microbiol 11: 208–216. 10.1111/j.1462-5822.2008.01271.x [DOI] [PubMed] [Google Scholar]
- 3. Gifford AM, Chalmers JD (2014) The role of neutrophils in cystic fibrosis. Curr Opin Hematol 21: 16–22. 10.1097/MOH.0000000000000009 [DOI] [PubMed] [Google Scholar]
- 4. Pier GB (2000) Role of the cystic fibrosis transmembrane conductance regulator in innate immunity to Pseudomonas aeruginosa infections. Proc Natl Acad Sci U S A 97: 8822–8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cannon CL, Kowalski MP, Stopak KS, Pier GB (2003) Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am J Respir Cell Mol Biol 29: 188–197. [DOI] [PubMed] [Google Scholar]
- 6. Taggart CC, Greene CM, Smith SG, Levine RL, McCray PB Jr, et al. (2003) Inactivation of human beta-defensins 2 and 3 by elastolytic cathepsins. J Immunol 171: 931–937. [DOI] [PubMed] [Google Scholar]
- 7. Sagel SD, Sontag MK, Accurso FJ (2009) Relationship between antimicrobial proteins and airway inflammation and infection in cystic fibrosis. Pediatr Pulmonol 44: 402–409. 10.1002/ppul.21028 [DOI] [PubMed] [Google Scholar]
- 8. Schmidtchen A, Frick IM, Andersson E, Tapper H, Bjorck L (2002) Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol Microbiol 46: 157–168. [DOI] [PubMed] [Google Scholar]
- 9. Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, et al. (2012) Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487: 109–113. 10.1038/nature11130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen CH, Wang Y, Nakatsuji T, Liu YT, Zouboulis C, et al. (2011) An innate bactericidal oleic acid effective against skin infection of methicillin-resistant Staphylococcus aureus: a therapy concordant with evolutionary medicine. J Microbiol Biotechnol 21: 391–399. [PubMed] [Google Scholar]
- 11. Martinez JG, Waldon M, Huang Q, Alvarez S, Oren A, et al. (2009) Membrane-targeted synergistic activity of docosahexaenoic acid and lysozyme against Pseudomonas aeruginosa. Biochem J 419: 193–200. 10.1042/BJ20081505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tollin M, Bergsson G, Kai-Larsen Y, Lengqvist J, Sjovall J, et al. (2005) Vernix caseosa as a multi-component defence system based on polypeptides, lipids and their interactions. Cell Mol Life Sci 62: 2390–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Do TQ, Moshkani S, Castillo P, Anunta S, Pogosyan A, et al. (2008) Lipids including cholesteryl linoleate and cholesteryl arachidonate contribute to the inherent antibacterial activity of human nasal fluid. J Immunol 181: 4177–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee JT, Jansen M, Yilma AN, Nguyen A, Desharnais R, et al. (2010) Antimicrobial lipids: novel innate defense molecules are elevated in sinus secretions of patients with chronic rhinosinusitis. Am J Rhinol Allergy 24: 99–104. 10.2500/ajra.2010.24.3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asmis R, Buhler E, Jelk J, Gey KF (1997) Concurrent quantification of cellular cholesterol, cholesteryl esters and triglycerides in small biological samples. Reevaluation of thin layer chromatography using laser densitometry. J Chromatogr B Biomed Sci Appl 691: 59–66. [DOI] [PubMed] [Google Scholar]
- 16. Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, et al. (2010) Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med 2: 258–274. 10.1002/emmm.201000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Uda S, Spolitu S, Angius F, Collu M, Accossu S, et al. (2013) Role of HDL in cholesteryl ester metabolism of lipopolysaccharide-activated P388D1 macrophages. J Lipid Res 54: 3158–3169. 10.1194/jlr.M042663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Konstan MW, Wagener JS, VanDevanter DR (2009) Characterizing aggressiveness and predicting future progression of CF lung disease. J Cyst Fibros 8 Suppl 1: S15–19. 10.1016/S1569-1993(09)60006-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schluchter MD, Konstan MW, Davis PB (2002) Jointly modelling the relationship between survival and pulmonary function in cystic fibrosis patients. Stat Med 21: 1271–1287. [DOI] [PubMed] [Google Scholar]
- 20. Rosenfeld M (2007) An overview of endpoints for cystic fibrosis clinical trials: one size does not fit all. Proc Am Thorac Soc 4: 299–301. [DOI] [PubMed] [Google Scholar]
- 21. Mayer-Hamblett N, Ramsey BW, Kronmal RA (2007) Advancing outcome measures for the new era of drug development in cystic fibrosis. Proc Am Thorac Soc 4: 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET (2012) Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med 186: 857–865. 10.1164/rccm.201203-0507OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, et al. (2013) Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med 368: 1963–1970. [DOI] [PubMed] [Google Scholar]
- 24. Accurso FJ, Van Goor F, Zha J, Stone AJ, Dong Q, et al. (2014) Sweat chloride as a biomarker of CFTR activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros 13: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Booton R, Lindsay MA (2014) Emerging role of MicroRNAs and long noncoding RNAs in respiratory disease. Chest 146: 193–204. 10.1378/chest.13-2736 [DOI] [PubMed] [Google Scholar]
- 26. Megiorni F, Cialfi S, Cimino G, De Biase RV, Dominici C, et al. (2013) Elevated levels of miR-145 correlate with SMAD3 down-regulation in cystic fibrosis patients. J Cyst Fibros 12: 797–802. 10.1016/j.jcf.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 27. Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, et al. (1995) Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 151: 1075–1082. [DOI] [PubMed] [Google Scholar]
- 28. Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 29. Kimura K, Yamaoka M, Kamisaka Y (2004) Rapid estimation of lipids in oleaginous fungi and yeasts using Nile red fluorescence. J Microbiol Methods 56: 331–338. [DOI] [PubMed] [Google Scholar]
- 30. Porter E, Yang H, Yavagal S, Preza GC, Murillo O, et al. (2005) Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect Immun 73: 4823–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer KC, Sharma A, Brown R, Weatherly M, Moya FR, et al. (2000) Function and composition of pulmonary surfactant and surfactant-derived fatty acid profiles are altered in young adults with cystic fibrosis. Chest 118: 164–174. [DOI] [PubMed] [Google Scholar]
- 32. Slomiany A, Murty VL, Aono M, Snyder CE, Herp A, et al. (1982) Lipid composition of tracheobronchial secretions from normal individuals and patients with cystic fibrosis. Biochim Biophys Acta 710: 106–111. [DOI] [PubMed] [Google Scholar]
- 33. Ollero M, Astarita G, Guerrera IC, Sermet-Gaudelus I, Trudel S, et al. (2011) Plasma lipidomics reveals potential prognostic signatures within a cohort of cystic fibrosis patients. J Lipid Res 52: 1011–1022. 10.1194/jlr.P013722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Levy E, Lepage G, Bendayan M, Ronco N, Thibault L, et al. (1989) Relationship of decreased hepatic lipase activity and lipoprotein abnormalities to essential fatty acid deficiency in cystic fibrosis patients. J Lipid Res 30: 1197–1209. [PubMed] [Google Scholar]
- 35. Levy E, Roy C, Lacaille F, Lambert M, Messier M, et al. (1993) Lipoprotein abnormalities associated with cholesteryl ester transfer activity in cystic fibrosis patients: the role of essential fatty acid deficiency. Am J Clin Nutr 57: 573–579. [DOI] [PubMed] [Google Scholar]
- 36. Beisswenger C, Bals R (2005) Antimicrobial peptides in lung inflammation. Chem Immunol Allergy 86: 55–71. [DOI] [PubMed] [Google Scholar]
- 37. Suzuki T, Chow CW, Downey GP (2008) Role of innate immune cells and their products in lung immunopathology. Int J Biochem Cell Biol 40: 1348–1361. 10.1016/j.biocel.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 38. Fischer BM, Wong JK, Degan S, Kummarapurugu AB, Zheng S, et al. (2013) Increased expression of senescence markers in cystic fibrosis airways. Am J Physiol Lung Cell Mol Physiol 304: L394–400. 10.1152/ajplung.00091.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. May GL, Wright LC, Obbink KG, Byleveld PM, Garg ML, et al. (1997) Increased saturated triacylglycerol levels in plasma membranes of human neutrophils stimulated by lipopolysaccharide. J Lipid Res 38: 1562–1570. [PubMed] [Google Scholar]
- 40. Wattiez R, Falmagne P (2005) Proteomics of bronchoalveolar lavage fluid. J Chromatogr B Analyt Technol Biomed Life Sci 815: 169–178. [DOI] [PubMed] [Google Scholar]
- 41. Chen CI, Schaller-Bals S, Paul KP, Wahn U, Bals R (2004) Beta-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J Cyst Fibros 3: 45–50. [DOI] [PubMed] [Google Scholar]
- 42. Bucki R, Byfield FJ, Janmey PA (2007) Release of the antimicrobial peptide LL-37 from DNA/F-actin bundles in cystic fibrosis sputum. Eur Respir J 29: 624–632. [DOI] [PubMed] [Google Scholar]
- 43. Lee JT, Escobar OH, Anouseyan R, Janisiewicz A, Eivers E, et al. (2014) Assessment of epithelial innate antimicrobial factors in sinus tissue from patients with and without chronic rhinosinusitis. Int Forum Allergy Rhinol 4: 893–900. 10.1002/alr.21404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Colombo C, Faelli N, Tirelli AS, Fortunato F, Biffi A, et al. (2011) Analysis of inflammatory and immune response biomarkers in sputum and exhaled breath condensate by a multi-parametric biochip array in cystic fibrosis. Int J Immunopathol Pharmacol 24: 423–432. [DOI] [PubMed] [Google Scholar]
- 45. Eickmeier O, Huebner M, Herrmann E, Zissler U, Rosewich M, et al. (2010) Sputum biomarker profiles in cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) and association between pulmonary function. Cytokine 50: 152–157. 10.1016/j.cyto.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 46. Solomon GM, Frederick C, Zhang S, Gaggar A, Harris T, et al. (2013) IP-10 is a potential biomarker of cystic fibrosis acute pulmonary exacerbations. PLoS One 8: e72398 10.1371/journal.pone.0072398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sousa M, Servidoni MF, Vinagre AM, Ramalho AS, Bonadia LC, et al. (2012) Measurements of CFTR-mediated Cl- secretion in human rectal biopsies constitute a robust biomarker for Cystic Fibrosis diagnosis and prognosis. PLoS One 7: e47708 10.1371/journal.pone.0047708 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The primary antibody is a polyclonal antibody that shows unspecific reactivity with higher molecular weight components in BALF.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


