Abstract
Adverse drug reactions (ADRs) cause considerable mortality and morbidity but no recent reviews are currently available for the European region. Therefore, we performed a review of all epidemiological studies quantifying ADRs in a European setting that were published between 1 January 2000 and 3 September 2014. Included studies assessed the number of patients who were admitted to hospital due to an ADR, studies that assessed the number of patients who developed an ADR during hospitalization, and studies that measured ADRs in the outpatient setting. In total, 47 articles were included in the final review. The median percentage of hospital admissions due to an ADR was 3.5 %, based on 22 studies, and the median percentage of patients who experienced an ADR during hospitalization was 10.1 %, based on 13 studies. Only five studies were found that assessed ADRs occurring in the outpatient setting. These results indicate that the occurrence of ADRs in the European hospital setting—both ADRs that result in hospitalization and ADRs that occur during the hospital stay—is significant. Furthermore, the limited number of studies that were performed in the outpatient setting identify a lack of information regarding the epidemiology of ADRs in this setting.
Key Points
| Based on our review, recent studies demonstrate that the burden of adverse drug reactions (ADRs), in both in- and outpatient settings, is substantial. |
| Data regarding the burden of ADRs in the outpatient setting, especially those ADRs that do not result in healthcare use, are largely lacking as we were only able to identify a handful of studies. |
| Despite the large number of studies we identified, several countries had no recent studies available. Therefore, studies in all European countries, as well as studies on ADR occurrence in the outpatient setting, are needed. |
Introduction
In Europe, adverse drug reactions (ADRs) cause a considerable amount of morbidity and mortality [1]. It has been estimated that approximately 5 % of all hospital admissions are caused by ADRs, that 5 % of hospitalized patients will experience an ADR during their hospital stay, and that ADRs cause 197,000 deaths annually throughout the EU [1]. These estimates formed the foundation of a major reform of the European regulatory system for pharmacovigilance, which was implemented in July 2012. This renewed system for postmarketing surveillance of medicines intends to improve public health in Europe by reducing the substantial burden of disease resulting from ADRs, through better monitoring of medicines in the postmarketing setting, improving the pharmacovigilance systems of companies, by involving stakeholders, and by a set of other adaptations to the regulatory system [1].
These ADR occurrence rates were based on a review published in 2004 that reported nine epidemiological studies [2]. These studies were all published before the year 2000, and some of the studies were performed outside of Europe [2]. Furthermore, the estimated 197,000 annual deaths resulting from ADRs in Europe is an extrapolation of a meta-analysis of studies performed in US hospitals [3]. Since the year 2000, many new medicines have become available and medical practice might also have changed. Furthermore, differences in available medicines, prescribing practices, and medical practice could result in different epidemiology of ADR occurrence in European and US hospitals. More recent estimates on the burden of ADRs in Europe are needed but we were unable to identify any recent reviews of epidemiological studies that focused specifically on the European setting. In addition, we were not able to identify reviews of studies that have assessed the epidemiology of ADRs in the outpatient setting. Therefore, we performed a review of observational studies that have estimated the epidemiology of ADRs in hospital settings, performed in a European country and published since 1 January 2000, and performed an exploratory review of similar studies performed in outpatient settings.
Methods
Adverse Drug Reactions (ADRs)
An ADR is defined as “an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product” [4]. Historically, the main source of information on the occurrence of ADRs has been spontaneous reporting by healthcare professionals. However, the source population (the total number of patients using a certain medicine) is generally not known in such systems and the total number of patients experiencing an ADR is also not known as reporting is usually voluntary and underreporting of ADRs can be as high as 94 % [5]. Therefore, a prospective or retrospective observational study in which the total population at risk of ADRs is included in the study is required to estimate the epidemiology of ADRs. Most studies that assess the occurrence of ADRs focus on one of two different types of at-risk populations: either all users of a certain type of medicine are included in the study, or all patients who are treated within a certain healthcare setting are included. This second study type is able to assess all ADRs that occur, regardless of the actual medicine that caused the ADR. Therefore, when one wishes to assess the total burden of ADRs at the population level, such study types are more informative than studies that assess ADRs for specific medicines only.
Figure 1 depicts the total burden of disease caused by ADRs in Europe and summarizes the types of studies that we included in the review: (1) studies that included all patients who were admitted to a hospital during a specified period of time and assessed how many patients or admissions were the result of an ADR; (2) studies that assessed all hospitalized patients during a specified period and reported how many patients experienced an ADR; and (3) studies that assessed how many people experienced an ADR in outpatient settings that did not result in hospitalization.
Fig. 1.
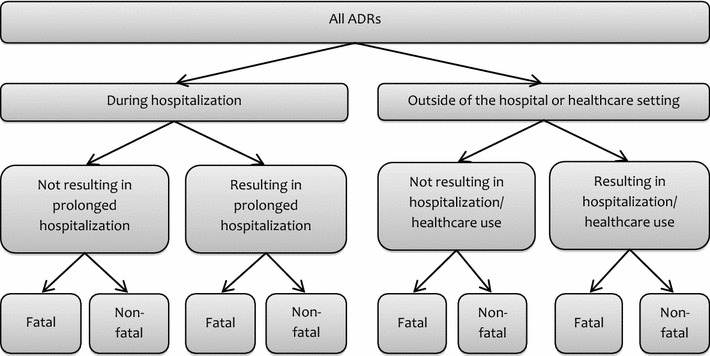
Different settings in which ADRs can occur and, when combined, make up the total morbidity and mortality resulting from ADRs in the hospital and outpatient settings. ADRs adverse drug reactions
Study Eligibility
Eligible study designs were prospective or retrospective observational studies that measured the ADR occurrence rate by assessing (1) the presence of an ADR in a patient that was admitted to a hospital or visited the emergency department (hospitalization caused by ADR); (2) studies that measured the number of patients who developed an ADR during their hospital stay (in-hospital ADR occurrence); or (3) studies that measured ADRs occurring in the outpatient setting. For all three defined settings, we only included studies that were performed within a defined clinical setting (i.e. a hospital, hospital ward, outpatient setting) during a specified period of time, that did not focus on ADRs of one medicinal product specifically but which measured all ADRs regardless of the medicine used, and which were conducted in a European country (European Economic Area countries plus Switzerland). However, it is important to note that the search for outpatient-setting studies was more exploratory as there are different types of study settings that could be considered relevant; we therefore expected there to be much more variation among these studies.
Search Strategy
An electronic search of PubMed/MEDLINE (3 September 2014) was performed using the following search string: (adverse drug reaction OR adverse drug reactions OR side effect OR side effects OR drug induced OR drug related OR tolerability OR toxicity OR adverse effect OR adverse effects OR adverse event OR adverse outcome OR adverse outcomes AND (incidence OR prevalence OR occurrence) AND (hospital* OR admission* OR admitted OR visit) AND (observational OR retrospective OR prospective OR cohort OR population-based) NOT (clinical trial[Publication Type]). All search terms were limited to the title and/or abstract, and only papers published in English were included.
We conducted a search for papers published from 1 January 2000; however, only studies that started data collection after 1 January 1995 (meaning that patients included in the study were treated after 1994) were included. We rigorously assessed study designs in order to minimize variability among the included studies, as well as to ensure the quality of the included studies. Studies that executed non-random sample selection, such as those studies that only included patients who were admitted during working hours or during weekdays, were excluded. Furthermore, studies that reported missing data for more than 20 % of all patients admitted during the study period were also excluded. Those studies that only used a subsample of all patients admitted during the study period were included, but only if inclusion was non-selective (i.e. only if a random sample of the entire patient population was used), so as to minimize the possibility of selection bias in the included studies. Based on the ADR detection method, we limited the inclusion of studies to those that used intensive chart review or voluntary reporting by healthcare professionals combined with measures to stimulate voluntary reports. In other words, we excluded studies that used hospital discharge records, voluntary reporting by patients, national hospital databases, or national causes of death databases where coding for ADRs was used to select cases, so as to minimize variability in estimates caused by ADR detection methods. Less rigorous selection methods were used for the inclusion of studies performed in the outpatient setting as this part of the search was more exploratory.
Studies that reported adverse drug events (ADEs) were also included medication errors [6], and studies that reported drug-related problems (DRPs), which also include failure to treat with a drug and non-compliance [7]. Medication errors are also included in ADEs and DRPs. Whenever these studies also reported on the proportion of ADRs in the article, this information was extracted from the study. As we intended to include as many studies as possible from different European countries, we did not want to exclude studies based on ADR definition used to limit the possibility of underrepresentation of certain regions due to local difference in commonly used definitions. Although we use the term ‘ADR occurrence rate’ throughout, it is possible that some of the rates are in fact ADEs or DRPs.
Data Extraction
Data extraction was performed by two researchers (JCB and MLDB) who independently assessed all selected articles in order to extract the total sample size and the number of patients who experienced at least one ADR from all studies. Disagreement was solved through consensus. The inclusion process was performed by only one researcher (JCB), but when there was any doubt about whether a study should be included or not, a second researcher was consulted (MAK). All reasons for exclusion were recorded in order to increase the transparency of our review process.
To ensure the ADR occurrence rate was calculated in the same way for all included studies, the total number of patients who were admitted to hospital during the study period and the number of patients who were admitted due to an ADR were used to calculate the percentage of hospital admissions or emergency department visits due to an ADR. For calculations of the in-hospital ADR occurrence, the total number of patients who were hospitalized during the study period was used to calculate the percentage of patients who experienced at least one ADR. If only the number of admissions during the study period was reported, this information was used instead as patients could be admitted more than once during a study period but will not necessarily be admitted twice due to an ADR. For all studies, the total sample size that was the basis for the estimate of our calculations was reported.
A number of other study characteristics were collected, including the year(s) covered by the study (i.e. for retrospective studies, the years during which the ADRs occurred), setting, country, duration, population (a number of studies reported on a subpopulation of children or the elderly), population size, ADR detection method used, ADR definition used, what type of causality assessment was used, and what type of seriousness assessment was used. Some articles reported on all patients with an ADR at admission as well as the number of patients for whom the ADR was the cause of the admission, as it is possible that a patient who uses medication reports an ADR but is admitted to the hospital due to other reasons; in those cases, the percentage of ADRs that caused the admission was used.
Reporting and Analysis
All three different study types that were included (ADR at hospital admission, ADR during hospitalization, ADR in outpatient settings) are reported in separate tables. Furthermore, we differentiated between studies performed in unselected patient populations (i.e. adult patients) and those studies that focused on ADRs in pediatric or elderly patients only. For both the studies that assessed ADR occurrence rates among patients admitted to the hospital, and all those studies that assessed the ADR occurrence rate during hospitalization, the median and average ADR occurrence rates were calculated based on all studies that were performed in unselected patient populations. Due to differences in the design of studies performed in outpatient settings, we did not summarize these studies, and only report on the findings per individual study. No additional analyses were performed.
Results
Search Results
The initial search resulted in a total of 1688 articles. All search results were subsequently scanned, based on the title and abstract, to determine whether the article should be included or not, resulting in a total of 59 articles (Fig. 2). Scanning the reference lists of these 59 articles resulted in another 45 articles. The full-text of all 104 articles was read to determine whether the identified studies met all inclusion criteria, and 57 articles were subsequently excluded for various reasons, which are summarized in Fig. 2. The most common reasons for exclusion were the use of a non-random sample and the use of different ADR detection methods.
Fig. 2.

Selection process of all studies included in the review. In total, these articles resulted in 22 ADR occurrence rates at hospital admission, 32 ADR occurrence rates during hospitalization, and six ADR occurrence rates in other settings. ADR adverse drug reaction
The final sample of 47 articles included 20 articles that reported the ADR occurrence rate at hospital admission, 10 articles that reported the ADR occurrence rate during hospitalization, and 11 articles that reported both of these ADR occurrence rates. Furthermore, six studies were identified that estimated the ADR occurrence rate in another setting: four articles measured ADRs occurring in an outpatient setting, one article reported ADR-related hospital deaths in one hospital through intensive chart review of all hospital deaths during a 1-year period, and one article reported the ADR occurrence rate at hospital admission, during hospitalization, and in the outpatient setting. This resulted in 32 articles that reported an ADR occurrence rate at hospital admission, 22 articles that reported an ADR occurrence rate during hospitalization, and five articles that reported ADRs occurring in the outpatient setting.
ADR as the Cause of Hospital Admission
A total of 32 articles encompassing 110,427 patients reported the number of patients for which an ADR was the reason for hospital admission or visit to the emergency department. Twenty-two of the studies reported in these articles were performed in unselected patient populations (i.e. not in pediatric or elderly subpopulations) (Table 1), and nine of these studies were multicenter studies. Furthermore, seven studies were performed in pediatric patient populations, and three studies were performed in the elderly (Table 2). The 32 studies were performed in 12 different countries: France (7), UK (5), Germany (5), Italy (5), Switzerland (2), Greece (1), Spain (1), Romania (1), Slovenia (1), Austria (1), The Netherlands (1), and Norway (1). In addition, one multi-country study (UK and Germany) was included [35]. Patients included in the studies were admitted between 1998 and 2009, and the mean sample size per study was 3346 patients (median 919 patients).
Table 1.
Studies reporting the percentage of patients or hospital admission due to an ADR—unselected populations
| References | Year | Country | Type | Length | Setting | Sample size | ADR occurrence rate (%) | Detection method | ADR definition | Causality assessment | Fatal ADRs (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fattinger et al. [8] | 1996–1998 | Switzerland | Multicenter (2) | 2 years | Internal medicine department | 4431 | 3.3 | ICR | ADR | NR | 0.07 |
| Pouyanne et al. [9] | 1998 | France | Multicenter (33) | 2 weeks | 62 Departments; 33 hospitals | 3137 | 3.2 | ICR | ADR (WHO) | NR | 0.13 |
| Hardmeier et al. [10] | Up to 2000 | Switzerland | Multicenter (2) | Cross-sectional | 2 Hospitals, department of internal medicine | 6383 | 2.9 | ICR | ADE | NR | NR |
| Pirmohamed et al. [11] | 2001–2002 | UK | Multicenter (2) | 6 months | Teaching + general hospital | 18,820 | 6.4 | ICR | ADR (WHO) | 0.7 % Definite, 69.5 % probable, 29.8 % possible | 0.15 |
| Capuano et al. [12] | 2000 | Italy | Multicenter (2) | 20 days | Emergency departments | 1049 | 1.2 | ICR | ADE | NR | NR |
| Trifirò et al. [13] | 2000 | Italy | Multicenter (22) | 20 days | Emergency department | 18,854 | 3.3 | ICR | ADE | NR | 0.01 |
| Capuano et al. [14] | 2005 | Italy | Multicenter | 20 days | Emergency department | 7861 | 1.2 | ICR | ADE | NR | 0 |
| Sánchez Muñoz-Torrero et al. [15] | 2009 | Spain | Multicenter (2) | 10 weeks | Internal medicine; teaching hospitals | 405 | 5.9 | ICR | ADR | NR | 0.49 |
| Benard-Laribiere et al. [16] | 2006–2007 | France | Multicenter (61) | 2 weeks | Medical wards of teaching (25) and general (36) hospitals | 2692 | 3.6 | ICR | ADR | NR | 0.04 |
| Lagnaoui et al. [17] | 1996–1997 | France | Single-center | 4 months | Internal medicine unit | 444 | 7.2 | ICR | ADR | NR | 0 |
| Green et al. [18] | 1996 | UK | Cross-sectional random sample | Cross-sectional | University hospital | 200 | 7.5 | ICR | ADR (WHO) | 40 % Probable/likely, 53.3 % possible | 1 |
| Bordet et al. [19] | NS | France | Single-center | 18 months | Cardiology hospital | 16,916 | 0.5 | VR | ADR (WHO) | NR | 0.11 |
| Olivier et al. [20] | 1998 | France | Single-center | 4 weeks | Emergency department | 671 | 6.1 | ICR | ADR | NR | 0 |
| Thuermann et al. [21] | 1999 | Germany | Single-center | 2 months | Department of neurology; teaching hospital | 600 | 0.8 | ICR | ADR (WHO) | 56.1 % Possible, 35.4 % probable, 4.8 % definite | 0.33 |
| Dormann et al. [22] | 1998–1999 | Germany | Single-center | 13 months | Department of Medicine; university hospital | 915 | 8.5 | ICR | ADR (WHO) | 46.1 % Possible, 43.1 % probable, 10.8 % definite | 0.14 |
| Bednall et al. [23] | 1999 | UK | Single-center | 2 weeks | Emergency department | 2636 | 1.3 | ICR | DRP | NR | NR |
| Alexopoulou et al. [24] | 2005 | Greece | Single-center | 6 months | Department of medicine, university hospital | 548 | 12.8 | ICR | ADR (WHO) | 74.3 % Probable or definite, 25.7 % possible | NR |
| Hopf et al. [25] | NS | UK | Single-center | 15 days | Teaching hospital | 2371 | 1.3 | ICR | ADR (WHO) | 43.3 % Possible, 53.7 % probable | 0.08 |
| Schwake et al. [26] | 2003 | Germany | Single-center | 1 year | Intensive care unit; university hospital | 1554 | 6.4 | ICR | ADR (WHO) | NR | 0.13 |
| Brvar et al. [27] | 2006 | Slovenia | Single-center, cross-sectional | 1 year | University hospital | 520 | 5.8 | ICR | ADR (WHO) | NR | 0 |
| Farcas et al. [28] | 2009 | Romania | Single-center | 1 year | Internal medicine ward, university hospital | 1854 | 3.6 | VR | ADR (WHO) | NR | NR |
| Hofer-Dueckelmann et al. [29] | 2007–2008 | Austria | Single-center | 6 months | Departments of gastroenterology, nephrology, cardiology | 3190 | 7.7 | ICR | ADR (WHO) | NR | 0.13 |
ADR occurrence was calculated by dividing the number of patients who experienced at least one ADR, or the number of admissions with at least one ADR, by the total number of patients or admissions included in the study. The ADR definition that was reported is stated, including whether the WHO definition was used in the study
ADR adverse drug reaction, WHO World Health Organization, DRP drug-related problem, ADE adverse drug event, ICR intensive chart review, VR voluntary reporting, NS not stated, NR not reported
Table 2.
Studies reporting the percentage of patients or hospital admissions due to an ADR—studies in children or the elderly
| References | Year | Country | Type | Length | Setting | Sample size | ADR occurrence rate (%) | Detection method | ADR definition | Causality assessment | Fatal ADRs (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jonville-Béra et al. [30] | 1998 | France | Single-center | 1 week | Emergency department; pediatric hospital | 260 | 1.5 | ICR | ADR | NR | 0 |
| Buajordet et al. [31] | 1996 | Norway | Single-center | 5 months | University hospital; pediatric department | 919 | 5.8 | VR + ICR | ADR (WHO) | NR | NR |
| Haffner et al. [32] | 2001 | Germany | Single-center | 13 weeks | Pediatric teaching hospital | 703 | 1.8 | ICR | ADR (WHO) | 71.3 % Probable or definite | NR |
| Gallagher et al. [33] | 2008 | UK | Single-center | 2 weeks | Emergency department; pediatric hospital | 847 | 3.2 | ICR | ADR (WHO) | 63 % Probable, 37 % possible | 0 |
| Posthumus et al. [34] | 2008 | The Netherlands | Single-center | 18 weeks | Emergency department; pediatric hospital | 683 | 6.9 | ICR | ADR (WHO) | NR | 0 |
| Rashed et al. [35] | 2008–2009 | UK + Germany | Single-center | 3 months | Pediatric ward | 313 (UK) | 1.7 (both countries combined) | ICR | ADR (WHO) | NR | NR |
| 376 (Germany) | |||||||||||
| Gallagher et al. [36] | 2009–2009 | UK | Single-center | 1 year | Tertiary pediatric hospital | 6821 | 2.6 | ICR | ADR (WHO) | NR | 0 |
| Franceschi et al. [37] | 2004–2005 | Italy | Single-center | 2 months | Geriatic department | 1756 | 5.8 | ICR | ADR (WHO) | 6.8 % Definite, 91.2 % probable, 2 % possible | 0 |
| Olivier et al. [38] | 2002–2003 | France | Single-center | 4 weeks | Emergency department; university hospital | 789 | 8.4 | ICR | ADR (WHO) | NR | 0.38 |
| Conforti et al. [39] | 2009 | Italy | Single-center | 6 months | Geriatric ward | 909 | 28.2 | ICR | ADR (WHO) | NR | NR |
ADR occurrence was calculated by dividing the number of patients who experienced at least one ADR, or the number of admissions with at least one ADR, by the total number of patients or admissions included in the study. The ADR definition that was reported is stated, including whether the WHO definition was used in the study
ADR adverse drug reaction, WHO World Health Organization, ICR intensive chart review, VR voluntary reporting, NR not reported
The median ADR occurrence rate in the 22 studies concerning the general population was 3.6 % of all hospital admissions (mean 4.6 %; Fig. 3). In these studies, the percentage of patients who were admitted to the hospital due to an ADR varied from 0.5 % [21] to 12.8 % of all patients [24].
Fig. 3.
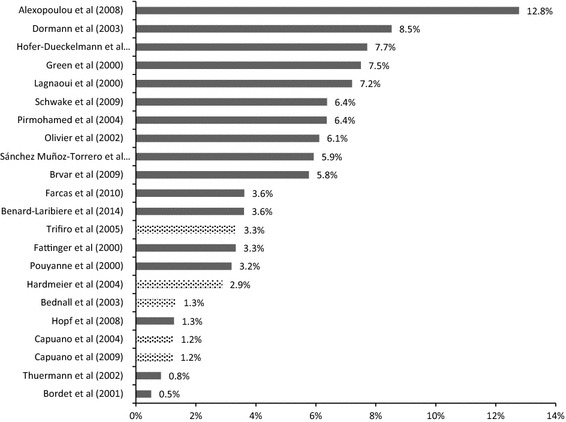
Variation in the reported percentage of hospital admissions caused by ADRs (all studies in unselected patient populations). Graph shows all studies that reported the percentage of hospital admissions caused by ADRs in various settings, excluding those studies that focused on children or the elderly. The median of these studies was 3.6 % and the mean was 4.6 % of all admissions. Light bars indicate that the study used ADEs/DRPs instead of ADRs. ADRs adverse drug reactions, ADE adverse drug event, DRP drug-related problem
Four of the studies collected ADEs and one study collected DRPs, which also includes intentional overdose. Twenty-one studies explicitly stated that they used the WHO definition of an ADR. Furthermore, 28 studies used intensive chart review as the data collection method, which means that the charts or medical records of all admitted patients during the study period were screened for possible ADRs. Two studies used voluntary reporting by nurses and/or doctors combined with measures to stimulate voluntary reporting of ADRs, one study used both methods, and one study did not report the method used.
Twenty-three of the 32 studies reported the number of fatal ADRs. Nine of those studies reported no fatal ADRs and, among the remaining studies, the highest percentage of fatal ADRs was 0.49 % of all admissions. Furthermore, none of the studies that focused specifically on ADRs in children reported any fatal ADRs.
ADRs During Hospitalization
A total of 22 articles encompassing 42,279 patients reported the percentage of patients who experienced an ADR during hospitalization. Thirteen studies were performed in unselected patient populations (Table 3), including seven multicenter studies. Six studies were performed in pediatric populations and three studies were performed in elderly populations (Table 4). The 22 studies were performed in nine different countries: Germany (8), France (3), The Netherlands (2), Switzerland (2), UK (2), Norway (2), Italy (2), Romania (1), and Spain (1). In addition, one multi-country study (UK and Germany) was included [35]. Patients included in the studies were hospitalized during 1997–2008, and the mean sample size was 1838 patients (median 595 patients).
Table 3.
All studies reporting the percentage of in-hospital ADR occurrence among patients—unselected populations
| References | Year | Country | Study design | Study length | Study setting | Sample size | ADR occurrence rate (%) | Detection method | ADR definition | Causality | Fatal ADRs (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fattinger et al. [8] | 1996–1998 | Switzerland | Multicenter (2) | 2 years | Internal medicine department | 4187 | 7.6 | ICR | ADR | NR | 0.12 |
| van den Bemt et al. [40] | 1996–1997 | The Netherlands | Multicenter (2) | 2 months | Internal medicine ward; general hospital | 538 | 27.7 | ICR + VR | ADE | NR | NR |
| Hardmeier et al. [10] | Up to 2000 | Switzerland | Multicenter (2) | Cross-sectional | 2 Hospitals, department of internal medicine | 6383 | 7.2 | ICR | ADE | NR | NR |
| Blix et al. [41] | 2002 | Norway | Multicenter (5) | 6 months | Departments of internal medicine/rheumatology; 5 hospitals | 827 | 81.3 | ICR | DRP | NR | NR |
| Zopf et al. [42] | NS | Germany | Multicenter (2) | 6 months | Internal medicine | 907 | 38.0 | ICR | ADR (WHO) | 33.4 % Possible, 61.5 % probable, 4.7 % highly probable | 0.22 |
| Sánchez Muñoz-Torrero et al. [15] | 2009 | Spain | Multicenter (2) | 10 weeks | Internal medicine; teaching hospitals | 381 | 26.8 | ICR | ADR | NR | 0.52 |
| Dequito et al. [43] | 2006–2008 | The Netherlands | Multicenter (2) | 5 months | Geriatric, internal medicine, rheumatology wards | 603 | 50.9 | ICR | ADR | NR | NR |
| Dormann et al. [44] | 1997 | Germany | Single-center | 6 months | Medical ward, university hospital | 379 | 11.9 | ICR | ADR (WHO) | NR | 0.26 |
| Lagnaoui et al. [17] | 1996–1997 | France | Single-center | 4 months | Internal medicine unit | 354 | 7.3 | ICR | ADR | NR | 0 |
| Bordet et al. [19] | NS | France | Single-center | 18 months | Cardiology hospital | 16,830 | 1.7 | VR | ADR (WHO) | NR | 0.11 |
| Thuermann et al. [21] | 1999 | Germany | Single-center | 2 months | Department of neurology; teaching hospital | 595 | 8.4 | ICR | ADR (WHO) | 56.1 % Possible, 35.4 % probable, 4.8 % definite | 0.34 |
| Davies et al. [45] | 2005 | UK | Single-center | 2 weeks | Five wards; teaching hospital | 3695 | 14.7 | ICR | ADR (WHO) | 63 % Possible, 33 % probable, 4 % definite | 0.03 |
| Farcas et al. [28] | 2009 | Romania | Single-center | 1 year | Internal medicine ward, university hospital | 1787 | 2.0 | VR | ADR (WHO) | NR | NR |
The total number of patients admitted during the study period is reported (under sample size). ADR occurrence was calculated by dividing the number of patients who experienced at least one ADR, or the number of admissions during which at least one ADR occurred, by the total number of patients or hospital admissions. The ADR definition that was reported is stated, including whether the WHO definition was used in the study
ADR adverse drug reaction, WHO World Health Organization, DRP drug-related problem, ADE adverse drug event, ICR intensive chart review, VR voluntary reporting, NS not stated, NR not reported
Table 4.
All studies reporting the percentage of in-hospital ADR occurrence among patients–studies in children or the elderly
| References | Year | Country | Study design | Study length | Study setting | Sample size | ADR occurrence rate (%) | Detection method | ADR definition | Causality | Fatal ADRs (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weiss et al. [46] | 1999–2000 | Germany | Single-center | 8 months | Pediatric ward; university hospital | 214 | 21.5 | ICR | ADR (WHO) | NR | 0 |
| Jonville-Béra et al. [30] | 1998 | France | Single-center | 1 week | Pediatric hospital | 256 | 2.3 | ICR | ADR | NR | 0 |
| Buajordet et al. [31] | 1996 | Norway | Single-center | 5 months | University hospital; pediatric department | 880 | 11.9 | VR + ICR | ADR (WHO) | NR | NR |
| Haffner et al. [32] | 2001 | Germany | Single-center | 13 weeks | Pediatric teaching hospital | 690 | 10.3 | ICR | ADR (WHO) | 71.3 % Probable or definite | NR |
| Rashed et al. [35] | 2008–2009 | UK + Germany | Single-center | 3 months | Pediatric ward | 305 (UK) | 18.6 (both countries combined) | ICR | ADR (WHO) | NR | NR |
| 373 (Germany) | |||||||||||
| Oehme et al. [47] | 2008 | Germany | Single-center | 3 months | Department of pediatric medicine; university hospital | 517 | 10.6 | ICR | ADR (WHO) | NR | 0 % |
| Corsonello et al. [48] | 2007 | Italy | Multicenter (11) | 3 months | 11 Acute care wards | 506 | 11.5 | ICR | ADR (WHO) | NR | NR |
| Egger et al. [49] | 2001–2002 | Germany | Single-center | 4 months | Geriatic department | 163 | 60.7 | ICR | ADR (WHO) | 53.6 % Possible, 45.8 % probable, 0.7 % definite | NR |
| Conforti et al. [39] | 2009 | Italy | Single-center | 6 months | Geriatric ward | 909 | 28.2 | ICR | ADR (WHO) | NR | NR |
The total number of patients admitted during the study period is reported (under sample size). ADR occurrence was calculated by dividing the number of patients who experienced at least one ADR, or the number of admissions during which at least one ADR occurred, by the total number of patients or hospital admissions. The ADR definition that was reported is stated, including whether the WHO definition was used in the study
ADR adverse drug reaction, WHO World Health Organization, ICR intensive chart review, VR voluntary reporting, NR not reported
Among the 13 studies that did not focus on a subpopulation of children or the elderly, the ADR occurrence rate during hospitalization was 11.9 % (mean 22.0 %; Fig. 4). The percentage of patients who developed at least one ADR during hospital stay ranged from 1.7 % [19] to 50.9 % of all patients [43]. Furthermore, a multicenter study performed in Norway found that 81.3 % of all patients experienced DRPs [41]. When we excluded this study, the median ADR occurrence rate was 10.1 % of all patients in 12 studies (mean 17.0 %).
Fig. 4.

Variation in the reported percentage of in-hospital ADRs (all studies in unselected patient populations). Graph shows all studies that reported the percentage of patients who experienced an ADR during their hospital stay, and which were performed in various settings, excluding those studies that focused on children or the elderly. The median of all studies was 11.9 % and the mean was 22.0 % of all hospitalizations. When the study of Blix et al. [41] was excluded, the median was 10.1 % and the mean was 17.0 % of all hospitalizations. Light bars indicate that ADEs/DRPs were reported instead of ADRs. ADR adverse drug reaction, ADE adverse drug event, DRP drug-related problem
Two of the 22 studies collected ADEs, one study collected DRPs, and the remaining 19 studies all reported ADRs. Sixteen studies explicitly reported using the WHO definition of ADRs. Furthermore, two studies used voluntary reporting by healthcare professionals to detect ADRs, 18 studies used intensive chart review, and two studies used both voluntary reporting and intensive chart review to detect ADRs.
Eleven studies reported the number of fatal ADRs that occurred during the study period. In four studies, no fatal ADRs occurred at all, and the highest percentage of fatal ADRs was 0.52 % of all admitted patients. None of the studies that focused specifically on children reported fatal ADRs.
Other Study Settings
Five studies that reported the ADR occurrence rate in the outpatient setting, and one study that measured ADRs as a cause of death in the hospital setting, were identified (Table 5). Two of the studies in the outpatient setting were performed in children. Letrilliart et al. [50] reported that an ADR was present in 0.38 % of all patients who contacted a general practitioner within 30 days of hospital discharge (total sample size 7540 patients), while Jonville-Béra et al. [30] reported that an ADR was present in 0.67 % of all patients (sample size 1192 children). Hakkarainen et al. [52] performed a cross-sectional study among the adult Swedish general public and reported a 1-month ADR prevalence of 7.8 % [52], while a second study by the same authors reported a 3-month ADR prevalence of 6.9 % among the Swedish adult general population [53]. The first study consisted of self-reported ADRs, regardless of whether healthcare treatment was sought for the ADR, whereas the second Swedish study used chart review in inpatient and outpatient settings to identify individuals with ADRs. Knopf and Du reported an ADR occurrence rate of 0.9 % in a sample of 17,450 German children (outpatient setting, self-reported ADRs) [51]. The study that reported ADRs as a cause of death used intensive chart review to identify ADRs that contributed to the death of all patients who died during a 1-year period in a Finnish hospital (1511 deaths) [54]. They reported that 5 % of all deaths were caused by an ADR and that 0.05 % of all hospitalizations resulted in a fatal ADR [54]. This is in line with all studies (Tables 1, 2, 3, 4) that reported the rate of fatal ADRs.
Table 5.
Outpatient setting and in-hospital cause-of-death studies
| References | Year | Country | Type | Length | Setting | Sample size | ADR occurrence rate (%) | Detection method | ADR definition | Causality | Fatal ADRs (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Letrilliart et al. [50] | 1997–1999 | France | Observational | 2 years | 305 GPs reported all patients referred to hospital; follow-up of all patients who, within 30 days of discharge, contacted the GP | 7540 | 0.4 | VR | ADR (WHO) | NR | NR |
| Jonville-Béra et al. [30] | 1998 | France | Observational | 1 week | Pediatricians in region of hospital | 1192 outpatient visits | 0.7 | ICR | ADR | NR | 0% |
| Knopf and Du [51] | 2003–2006 | Germany | Cross-sectional | Cross-sectional | Stratified random sample of all German children not hospitalized/institutionalized | 17,450 | 0.9 | SR | ADR | NR | NR |
| Hakkarainen et al. [52] | 2010 | Sweden | Cross-sectional | 1 month | Representative sample of adult general population; SR ADRs during previous month measured through survey | 7099 | 7.8 | SR | ADR (WHO) | NR | NR |
| Hakkarainen et al. [53] | 2008 | Sweden | Observational | 3 months | Random sample of Swedish adult general population; extraction of all medical records; records reviewed for possible ADRs | 4970 | 6.9 | ICR | ADR (WHO) | NR | NR |
| Juntti-Patinen and Neuvonen [54] | 2000 | Finland | Observational (in-hospital cause of death) | 1 year | University hospital | 1511 deaths | 5.0 | ICR | ADR (WHO) | NR | NR |
The ADR definition that was reported is stated, including whether the WHO definition was used in the study
ADR adverse drug reaction, WHO World Health Organization, ICR intensive chart review, VR voluntary reporting, SR self-reported, NR not reported, GPs general practitioner
Causality Assessment
Most studies reported that they used an assessment of causality (32 of 47 articles; data not shown), i.e. for all suspected ADRs it was assessed whether symptoms where possibly caused by an ADR, probably caused by an ADR, or definitely caused by an ADR. Methods that were used for causality assessment varied and included the Naranjo algorithm [55] (18 studies), WHO definition [4] (6 studies), Karch and Lasagna method [56] (2 studies), or other methods (6 studies). However, only 14 articles reported the actual distribution of the causality assessment among all ADRs. Therefore, for the majority of the studies we did not know what the distribution of ‘possible ADR’, ‘probable ADR’, or ‘definite ADR’ was.
Other Characteristics
Most studies reported the medicines or medicine classes that were the suspected cause of the ADRs, but they were reported in various ways, which made summarizing them for all the studies problematic. For example, several studies provided a table that listed brief descriptions of all individual ADRs (e.g. ‘gastrointestinal bleeding after the use of a non-steroid inflammatory drug’) that occurred during the study period, whereas in other studies only categories of ADRs were listed (e.g. ‘bleeding disorders’); different classification systems for aggregate reporting were also used. Furthermore, the majority of studies reported the types of ADRs that patients experienced, but again these were reported in many different ways; therefore, it was impossible to extract these data from all studies in a standardized manner as different grading and classification systems were used. In only one study [37], all ADRs were classified as serious, and 3 % were classified as ‘life-threatening’. Nineteen of the 47 articles reported ADR seriousness and, in most studies, the proportion of serious ADRs was below 30 % of all ADRs (13 of 19 studies).
Discussion
Main Findings
We identified 47 articles, published since 1 January 2000, of prospective or retrospective observational studies that reported the ADR occurrence rate among the European population. In total, 32 studies, performed in 12 different countries, reported the percentage of patients who were admitted to the hospital due to an ADR. Twenty-two studies, performed in eight different countries, reported the percentage of patients who experienced an ADR during hospitalization, five studies reported ADRs occurring in various outpatient settings in three different countries, and one study reported ADRs as a cause of in-hospital deaths in a Finnish hospital. On average, the ADR occurrence rate at hospital admission was 3.6 % of all hospitalizations (median; mean 4.6 %) in 22 studies that reported the ADR occurrence rate in unselected patient populations. Furthermore, the ADR occurrence rate during hospitalization was 10.1 % of all patients (median; mean 17.0 %) in 12 studies that reported in-hospital ADR occurrence in unselected patient populations.
Only five studies were identified that estimated the occurrence of ADRs in the outpatient setting, and the estimated ADR occurrence rate reported by these studies varied considerably. Hakkarainen et al. [52] found that 7.8 % of Swedish adults had experienced an ADR during the month previous to the survey; however, three other outpatient setting studies all found ADR prevalence to be lower than 1 %. Given the variability of study methods used, and settings in which the studies were performed, the prevalence of ADRs among the general population that do not result in healthcare use is largely unknown. As it was estimated that this subtype of ADRs are responsible for 80 % of the total economic burden of ADRs in Europe [1], new observational studies in the outpatient setting of ADRs are warranted.
Fatal ADRs
The rate of fatal ADRs among all studies was quite consistent. Interestingly, no fatal ADRs were reported in any of the pediatric studies, which might suggest that fatal ADRs are either very rare in children, or are underreported in studies specifically focusing on children. Seventy-two percent (23/32) of all studies that reported the ADR occurrence rate at hospital admission also reported the rate of fatal ADRs, and in nine of these studies, no fatal ADRs occurred; the highest reported fatal ADR rate was 0.49 % of all patients admitted because of an ADR. Fifty percent (11/22) of all in-hospital ADR occurrence studies reported the rate of fatal ADRs; in four studies, no fatal ADRs were reported, and the highest fatal ADR rate was 0.52 % of all hospitalized patients. One other article reported that 0.05 % of all patients admitted during a 1-year period in a university hospital died of an ADR (5 % of all ADRs) [54].
When we combined these estimates with the most recent data from the WHO European Hospital Morbidity Database, which provides the number of hospital admissions in all European countries, it can be estimated that 83.8 million patients are hospitalized each year in 31 European countries with a combined population of 504 million people [57]. A rate of 0.5 % of fatal in-hospital ADRs would mean that almost 419,000 people die from fatal ADRs each year in Europe. Given that most studies have reported a fatal ADR rate below 0.5 %, the actual number of fatal ADRs might be lower. Using the reported 0.05 % rate from Juntti-Pattinen and Neuvonen [54] results in an estimated 42,000 deaths due to ADRs. The Impact Assessment for the new pharmacovigilance legislation [1] used an estimate of 197,000 deaths annually, which was based on the extrapolation of a US study [3]. This estimate seems to be in a plausible range based on the studies that were included in the current study, and would suggest that approximately 0.25 % (or 1 in 400 hospitalized patients) of all patients who are not hospitalized due to an ADR will die as a result of an ADR during their stay in a European hospital.
Previous Reviews
Taché et al. [58] reviewed the prevalence of ADEs in ambulatory care and reported that 5.1 % of hospital admissions were due to ADRs, based on 37 studies, the majority of which were performed in the US [58]. Kongkaew et al. [59] found that in 27 studies a median of 5.3 % of admissions were caused by ADRs, of which 17 were performed in Europe. Krähenbühl-Melcher et al. [60] found a median of 6.1 % of all hospitalized patients experienced an ADR during their stay, based on 46 studies (23 studies of non-European origin). A recent systematic review of 21 studies that reported the percentage of hospitalizations resulting from ADRs identified a median of 7 % of all hospitalizations [61], but the review was not limited to European studies and included methods other than intensive chart review and voluntary reporting by healthcare professionals. Our review indicated that the percentage of hospitalizations caused by ADRs is somewhat lower than those reported in earlier reviews, which could be explained by our focus on European studies only and restrictions regarding data collection methods of studies. In addition, we found that the number of patients who experienced an ADR during hospitalization was higher in the European studies we reviewed (10.1 % of all patients) than the estimates reported by Krähenbühl-Melcher et al. [60], but the variability in reported studies was considerable.
Strengths and Limitations
The most important limitation of this study is that we have not performed a systematic review but rather an exploratory review of a large number of studies performed in different settings, in different countries, and in different subpopulations. Therefore, we cannot exclude the possibility of having missed some publications due to the search strategy that was used. We limited our search to Pubmed/MEDLINE only, have not used Medical Subject Heading (MeSH) terms in our search string, and the scanning of titles and abstracts of the search results was only performed by one researcher. The fact that we included approximately half of the studies through searching reference lists indicates that our search strategy might not have been optimal. Nonetheless, we did manage to identify a large number of studies in different settings, which makes the results of our review informative, even though it might not have been exhaustive, especially since no recent similar systematic reviews are available.
There was considerable variability in study length, sample size, and study settings among the studies included. There was also great variability in the reporting of the types of ADRs identified, as well as in the medicines responsible for ADRs, making a summarized report of the most common ADRs in this review problematic. Furthermore, not all patients who are admitted to the hospital will use medicines, even though it is reasonable to assume that the majority of hospitalized patients will receive some form of pharmaceutical treatment. However, the studies that assessed the occurrence of ADRs during hospitalization included all hospitalized patients, regardless of whether or not they were treated with a medicine.
Notwithstanding, this review reports a large number of recent observational ADR studies performed in Europe. Even though we used a cut-off date for publication of 1 January 2000, we identified a total of 47 European studies. Our search strategy of scanning the reference lists of all full-text scanned articles (104 in total) was rigorous and limited the possibility that we may have missed a large number of published studies. Furthermore, our inclusion criteria with regard to study designs were stricter than most other published reviews as we excluded studies that used secondary data sources (such as hospital discharge records) to identify ADRs. We aimed to also include studies that were performed in outpatient settings, making our review more comprehensive than others with regard to the scope of studies included.
Future Research
Most of the studies that we included in our review measured the percentage of hospital admissions that were caused by ADRs (32 studies in total), and we found fewer studies that assessed the occurrence of ADRs during hospitalization (22 studies in total). Reported percentages in these studies varied considerably, making interpretation of these percentages problematic. Furthermore, only one multi-country study was identified, which included hospital settings in the UK and Germany, as well as several non-European countries [35]. Therefore, more observational studies of ADRs occurring during hospitalization, as well as multi-country studies and studies performed in those European countries that were not included in this review, are required. Only five studies were identified that were performed in the outpatient setting, and these studies varied considerably in study methods and design. Therefore, our knowledge regarding the occurrence of ADRs in the outpatient setting, especially those that do not result in healthcare use, is currently very limited and requires further study.
There was great variability among the studies with regard to whether the most common types of ADRs were reported, as well as what medicines most commonly caused ADRs in various settings. However, when designing policies that intend to reduce the burden of ADRs, it is essential to know which ADRs, as well as which medicines, are in fact causing the majority of ADRs in Europe, such that policies that effectively reduce the occurrence of those ADRs can be designed. The development of standardized ways in which types of ADRs and medicines contributing to these ADRs are reported in observational studies would enable better comparison of studies performed in different countries and settings.
Given the large number of recent studies that we identified through this exploratory review, systematic reviews and meta-analyses of ADR occurrence studies would be helpful in the provision of more reliable estimates of ADRs occurring in different settings and populations.
Conclusions
This review indicates that, in Europe, approximately 3.6 % of all hospital admissions are caused by ADRs, and up to 10 % of patients in European hospitals experience an ADR during their stay. Furthermore, the percentage of hospitalizations that end in a fatal ADR is likely to be lower than 0.5 %. Our knowledge concerning the occurrence of ADRs in the outpatient setting that do not result in healthcare use is very limited as only a few studies were identified that have used different methods and settings. Therefore, more epidemiological studies of ADR occurrence in European settings are needed.
Acknowledgments
This study was performed in the context of the Escher project (T6-202), a project of the Dutch Top Institute Pharma (TI Pharma). The sponsor did not play a role in the design, conduct, or analysis of the manuscript, nor the interpretation of data or the writing or review of the manuscript. Jacoline C. Bouvy, Marie L. De Bruin and Marc A. Koopmanschap declare no conflicts of interest.
References
- 1.European Commission. Proposal for a regulation amending, as regards pharmacovigilance of medicinal products for human use. Regulation (EC) No 726/2004. Impact assessment. 2008. Available at: http://ec.europa.eu/health/files/pharmacos/pharmpack_12_2008/pharmacovigilance-ia-vol1_en.pdf. Accessed 3 Sept 2014.
- 2.Lundkvist J, Jonsson B. Pharmacoeconomics of adverse drug reactions. Fundam Clin Pharmacol. 2004;18(3):275–280. doi: 10.1111/j.1472-8206.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 3.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 4.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 5.Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 6.Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician’s guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140:795–801. doi: 10.7326/0003-4819-140-10-200405180-00017. [DOI] [PubMed] [Google Scholar]
- 7.Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: their structure and function. DICP. 1990;24:1093–1097. doi: 10.1177/106002809002401114. [DOI] [PubMed] [Google Scholar]
- 8.Fattinger K, Roos M, Vergères P, Holenstein C, Kind B, Masche U, et al. Epidemiology of drug exposure and adverse drug reactions in two swiss departments of internal medicine. Br J Clin Pharmacol. 2000;49(2):158–167. doi: 10.1046/j.1365-2125.2000.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pouyanne P, Haramburu F, Imbs JL, Bégaud B. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. French Pharmacovigilance Centres. BMJ. 2000;320(7241):1036. doi: 10.1136/bmj.320.7241.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardmeier B, Braunschweig S, Cavallaro M, Roos M, Pauli-Magnus C, Giger M, et al. Adverse drug events caused by medication errors in medical inpatients. Swiss Med Wkly. 2004;134(45–46):664–670. doi: 10.4414/smw.2004.10801. [DOI] [PubMed] [Google Scholar]
- 11.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capuano A, Motola G, Russo F, Avolio A, Filippelli A, Rossi F, et al. Adverse drug events in two emergency departments in Naples, Italy: an observational study. Pharmacol Res. 2004;50(6):631–636. doi: 10.1016/j.phrs.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Trifirò G, Calogero G, Ippolito FM, Cosentino M, Giuliani R, Conforti A, et al. Adverse drug events in emergency department population: a prospective Italian study. Pharmacoepidemiol Drug Saf. 2005;14(5):333–340. doi: 10.1002/pds.1074. [DOI] [PubMed] [Google Scholar]
- 14.Capuano A, Irpino A, Gallo M, Ferrante L, Illiano ML, Rinaldi B, et al. Regional surveillance of emergency-department visits for outpatient adverse drug events. Eur J Clin Pharmacol. 2009;65(7):721–728. doi: 10.1007/s00228-009-0641-8. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez Muñoz-Torrero JF, Barquilla P, Velasco R, Fernández Capitan Mdel C, Pacheco N, Vicente L, et al. Adverse drug reactions in internal medicine units and associated risk factors. Eur J Clin Pharmacol. 2010;66(12):1257–1264. doi: 10.1007/s00228-010-0866-6. [DOI] [PubMed] [Google Scholar]
- 16.Benard-Laribiere A, Miremont-Salame G, Perault-Pochat MC, Noize P, Haramburu F. Incidence of hospital admissions due to adverse drug reactions in France: the EMIR study. Fundam Clin Pharmacol. 2015;29(1):106–111. doi: 10.1111/fcp.12088. [DOI] [PubMed] [Google Scholar]
- 17.Lagnaoui R, Moore N, Fach J, Longy-Boursier M, Bégaud B. Adverse drug reactions in a department of systemic diseases-oriented internal medicine: prevalence, incidence, direct costs and avoidability. Eur J Clin Pharmacol. 2000;56(2):181–186. doi: 10.1007/s002280050738. [DOI] [PubMed] [Google Scholar]
- 18.Green CF, Mottram DR, Rowe PH, Pirmohamed M. Adverse drug reactions as a cause of admission to an acute medical assessment unit: a pilot study. J Clin Pharm Ther. 2000;25(5):355–361. doi: 10.1046/j.1365-2710.2000.00298.x. [DOI] [PubMed] [Google Scholar]
- 19.Bordet R, Gautier S, Le Louet H, Dupuis B, Caron J. Analysis of the direct cost of adverse drug reactions in hospitalised patients. Eur J Clin Pharmacol. 2001;56(12):935–941. doi: 10.1007/s002280000260. [DOI] [PubMed] [Google Scholar]
- 20.Olivier P, Boulbés O, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M. Assessing the feasibility of using an adverse drug reaction preventability scale in clinical practice: a study in a French emergency department. Drug Saf. 2002;25(14):1035–1044. doi: 10.2165/00002018-200225140-00005. [DOI] [PubMed] [Google Scholar]
- 21.Thuermann PA, Windecker R, Steffen J, Schaefer M, Tenter U, Reese E, et al. Detection of adverse drug reactions in a neurological department: comparison between intensified surveillance and a computer-assisted approach. Drug Saf. 2002;25(10):713–724. doi: 10.2165/00002018-200225100-00004. [DOI] [PubMed] [Google Scholar]
- 22.Dormann H, Criegee-Rieck M, Neubert A, Egger T, Geise A, Krebs S, et al. Lack of awareness of community-acquired adverse drug reactions upon hospital admission: dimensions and consequences of a dilemma. Drug Saf. 2003;26(5):353–362. doi: 10.2165/00002018-200326050-00004. [DOI] [PubMed] [Google Scholar]
- 23.Bednall R, McRobbie D, Hicks A. Identification of medication-related attendances at an A and E department. J Clin Pharm Ther. 2003;28(1):41–45. doi: 10.1046/j.0269-4727.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 24.Alexopoulou A, Dourakis SP, Mantzoukis D, Pitsariotis T, Kandyli A, Deutsch M, et al. Adverse drug reactions as a cause of hospital admissions: a 6-month experience in a single center in Greece. Eur J Intern Med. 2008;19(7):505–510. doi: 10.1016/j.ejim.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Hopf Y, Watson M, Williams D. Adverse-drug-reaction related admissions to a hospital in Scotland. Pharm World Sci. 2008;30(6):854–862. doi: 10.1007/s11096-008-9240-5. [DOI] [PubMed] [Google Scholar]
- 26.Schwake L, Wollenschläger I, Stremmel W, Encke J. Adverse drug reactions and deliberate self-poisoning as cause of admission to the intensive care unit: a 1-year prospective observational cohort study. Intensive Care Med. 2009;35(2):266–274. doi: 10.1007/s00134-008-1250-1. [DOI] [PubMed] [Google Scholar]
- 27.Brvar M, Fokter N, Bunc M, Mozina M. The frequency of adverse drug reaction related admissions according to method of detection, admission urgency and medical department specialty. BMC Clin Pharmacol. 2009;9:8. doi: 10.1186/1472-6904-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farcas A, Sinpetrean A, Mogosan C, Palage M, Vostinaru O, Bojita M, et al. Adverse drug reactions detected by stimulated spontaneous reporting in an internal medicine department in Romania. Eur J Intern Med. 2010;21(5):453–457. doi: 10.1016/j.ejim.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Hofer-Dueckelmann C, Prinz E, Beindl W, Szymanski J, Fellhofer G, Pichler M, et al. Adverse drug reactions (ADRs) associated with hospital admissions: elderly female patients are at highest risk. Int J Clin Pharmacol Ther. 2011;49(10):577–586. doi: 10.5414/CP201514. [DOI] [PubMed] [Google Scholar]
- 30.Jonville-Béra AP, Giraudeau B, Blanc P, Beau-Salinas F, Autret-Leca E. Frequency of adverse drug reactions in children: a prospective study. Br J Clin Pharmacol. 2002;53(2):207–210. doi: 10.1046/j.0306-5251.2001.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buajordet I, Wesenberg F, Brørs O, Langslet A. Adverse drug events in children during hospitalization and after discharge in a Norwegian university hospital. Acta Paediatr. 2002;91(1):88–94. doi: 10.1111/j.1651-2227.2002.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 32.Haffner S, von Laue N, Wirth S, Thürmann PA. Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf. 2005;28(5):453–464. doi: 10.2165/00002018-200528050-00008. [DOI] [PubMed] [Google Scholar]
- 33.Gallagher RM, Bird KA, Mason JR, Peak M, Williamson PR, Nunn AJ, et al. Adverse drug reactions causing admission to a paediatric hospital: a pilot study. J Clin Pharm Ther. 2011;36(2):194–199. doi: 10.1111/j.1365-2710.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 34.Posthumus AA, Alingh CC, Zwaan CC, van Grootheest KK, Hanff LL, Witjes BB, et al. Adverse drug reaction-related admissions in paediatrics, a prospective single-centre study. BMJ Open. 2012;2(4). pii: e000934. [DOI] [PMC free article] [PubMed]
- 35.Rashed AN, Wong IC, Cranswick N, Hefele B, Tomlin S, Jackman J, et al. Adverse drug reactions in children-international surveillance and evaluation (ADVISE): a multicentre cohort study. Drug Saf. 2012;35(6):481–494. doi: 10.2165/11597920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher RM, Mason JR, Bird KA, Kirkham JJ, Peak M, Williamson PR, et al. Adverse drug reactions causing admission to a paediatric hospital. PLoS One. 2012;7(12):e50127. doi: 10.1371/journal.pone.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franceschi M, Scarcelli C, Niro V, Seripa D, Pazienza AM, Pepe G, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1756 patients. Drug Saf. 2008;31(6):545–556. doi: 10.2165/00002018-200831060-00009. [DOI] [PubMed] [Google Scholar]
- 38.Olivier P, Bertrand L, Tubery M, Lauque D, Montastruc JL, Lapeyre-Mestre M. Hospitalizations because of adverse drug reactions in elderly patients admitted through the emergency department: a prospective survey. Drugs Aging. 2009;26(6):475–482. doi: 10.2165/00002512-200926060-00004. [DOI] [PubMed] [Google Scholar]
- 39.Conforti A, Costantini D, Zanetti F, Moretti U, Grezzana M, Leone R. Adverse drug reactions in older patients: an Italian observational prospective hospital study. Drug Healthc Patient Saf. 2012;4:75–80. doi: 10.2147/DHPS.S29287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Bemt PM, Egberts AC, Lenderink AW, Verzijl JM, Simons KA, van der Pol WS, et al. Risk factors for the development of adverse drug events in hospitalized patients. Pharm World Sci. 2000;22(2):62–66. doi: 10.1023/A:1008721321016. [DOI] [PubMed] [Google Scholar]
- 41.Blix HS, Viktil KK, Reikvam A, Moger TA, Hjemaas BJ, Pretsch P, et al. The majority of hospitalised patients have drug-related problems: results from a prospective study in general hospitals. Eur J Clin Pharmacol. 2004;60(9):651–658. doi: 10.1007/s00228-004-0830-4. [DOI] [PubMed] [Google Scholar]
- 42.Zopf Y, Rabe C, Neubert A, Hahn EG, Dormann H. Risk factors associated with adverse drug reactions following hospital admission: a prospective analysis of 907 patients in two German university hospitals. Drug Saf. 2008;31(9):789–798. doi: 10.2165/00002018-200831090-00007. [DOI] [PubMed] [Google Scholar]
- 43.Dequito AB, Mol PG, van Doormaal JE, Zaal RJ, van den Bemt PM, Haaijer-Ruskamp FM, et al. Preventable and non-preventable adverse drug events in hospitalized patients: a prospective chart review in The Netherlands. Drug Saf. 2011;34(11):1089–1100. doi: 10.2165/11592030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Dormann H, Muth-Selbach U, Krebs S, Criegee-Rieck M, Tegeder I, Schneider HT, et al. Incidence and costs of adverse drug reactions during hospitalisation: computerised monitoring versus stimulated spontaneous reporting. Drug Saf. 2000;22(2):161–168. doi: 10.2165/00002018-200022020-00007. [DOI] [PubMed] [Google Scholar]
- 45.Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS One. 2009;4(2):e4439. doi: 10.1371/journal.pone.0004439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss J, Krebs S, Hoffmann C, Werner U, Neubert A, Brune K, et al. Survey of adverse drug reactions on a pediatric ward: a strategy for early and detailed detection. Pediatrics. 2002;110(2 Pt 1):254–257. doi: 10.1542/peds.110.2.254. [DOI] [PubMed] [Google Scholar]
- 47.Oehme AK, Rashed AN, Hefele B, Wong IC, Rascher W, Neubert A. Adverse drug reactions in hospitalised children in Germany are decreasing: results of a nine year cohort-based comparison. PLoS One. 2012;7(9):e44349. doi: 10.1371/journal.pone.0044349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corsonello A, Pedone C, Lattanzio F, Lucchetti M, Garasto S, Di Muzio M, Pharmacosur Veillance in the Elderly Care Study Group et al. Potentially inappropriate medications and functional decline in elderly hospitalized patients. J Am Geriatr Soc. 2009;57(6):1007–1014. doi: 10.1111/j.1532-5415.2009.02266.x. [DOI] [PubMed] [Google Scholar]
- 49.Egger T, Dormann H, Ahne G, Runge U, Neubert A, Criegee-Rieck M, et al. Identification of adverse drug reactions in geriatric inpatients using a computerised drug database. Drugs Aging. 2003;20(10):769–776. doi: 10.2165/00002512-200320100-00005. [DOI] [PubMed] [Google Scholar]
- 50.Letrilliart L, Hanslik T, Biour M, Fagot JP, Guiguet M, Flahault A. Postdischarge adverse drug reactions in primary care originating from hospital care in France: a nationwide prospective study. Drug Saf. 2001;24(10):781–792. doi: 10.2165/00002018-200124100-00006. [DOI] [PubMed] [Google Scholar]
- 51.Knopf H, Du Y. Perceived adverse drug reactions among non-institutionalized children and adolescents in Germany. Br J Clin Pharmacol. 2010;70(3):409–417. doi: 10.1111/j.1365-2125.2010.03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hakkarainen KM, Andersson Sundel K, Petzold M, Hagg S. Prevalence and perceived preventability of self-reported adverse drug events: a population-based survey of 7099 adults. PLoS One. 2013;8(9):e73166. doi: 10.1371/journal.pone.0073166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hakkarainen KM, Gyllensten H, Jonsson AK, AnderssonSundell K, Petzold M, Hagg S. Prevalence, nature and potential preventability of adverse drug events: a population-based medical record study of 4970 adults. Br J Clin Pharmacol. 2013;78(1):170–183. doi: 10.1111/bcp.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juntti-Patinen L, Neuvonen PJ. Drug-related deaths in a university central hospital. Eur J Clin Pharmacol. 2002;58(7):479–482. doi: 10.1007/s00228-002-0501-2. [DOI] [PubMed] [Google Scholar]
- 55.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 56.Karch FE, Lasagna L. Toward the operational identification of adverse drug reactions. Clin Pharmacol Ther. 1977;21:247–254. doi: 10.1002/cpt1977213247. [DOI] [PubMed] [Google Scholar]
- 57.WHO 2013. European Hospital Morbidity Database. Available at: http://data.euro.who.int/hmdb/index.php. Accessed 3 Sept 2014.
- 58.Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45(7–8):977–989. doi: 10.1345/aph.1P627. [DOI] [PubMed] [Google Scholar]
- 59.Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother. 2008;42(7):1017–1025. doi: 10.1345/aph.1L037. [DOI] [PubMed] [Google Scholar]
- 60.Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30(5):379–407. doi: 10.2165/00002018-200730050-00003. [DOI] [PubMed] [Google Scholar]
- 61.Al Hamid A, Ghaleb M, Aljadhey H, Aslanpour Z. A systematic review of hospitalization resulting from medicine-related problems in adult patients. Br J Clin Pharmacol. 2013;78(2):202–217. doi: 10.1111/bcp.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]


