Abstract
Progressive debilitating neurological defects characterize feline GM1 gangliosidosis, a lysosomal storage disease caused by deficiency of lysosomal β-galactosidase. No effective therapy exists for affected children, who often die before age 5. In the current study, an adeno-associated viral vector carrying the therapeutic gene was injected bilaterally into two brain targets (thalamus and deep cerebellar nuclei) of a feline model of GM1 gangliosidosis. Gene therapy normalized β-galactosidase activity and storage throughout the brain and spinal cord. The mean survival of 12 treated GM1 animals was >38 months compared to 8 months for untreated animals. Seven of the 8 treated animals remaining alive demonstrated normalization of disease, with abrogation of many symptoms including gait deficits and postural imbalance. Sustained correction of the GM1 gangliosidosis disease phenotype after limited intracranial targeting by gene therapy in a large animal model suggests that this approach may be useful for treating the human version of this lysosomal storage disorder.
Introduction
GM1 gangliosidosis is an autosomal recessive lysosomal storage disease (LSD) caused by deficiency of β-galactosidase (βgal, EC 3.2.1.23), the enzyme that hydrolyzes terminal galactose residues from numerous molecules. βgal deficiency leads to neuronal storage of GM1 ganglioside and its asialo derivative (GA1), resulting in progressive neurodegeneration and death (1, 2). Three clinical forms exist and are thought to result from differing levels of residual enzyme activity (3). Infantile- and juvenile-onset forms are fatal by ages 5 and 15, respectively, while the adult-onset phenotype varies considerably, with some patients living into the sixth decade (2). No effective therapy exists for GM1 gangliosidosis.
As βgal deficiency affects the central nervous system (CNS) globally, successful treatment strategies must target widespread areas of the brain and spinal cord. Whereas the circulatory system provides global access to the CNS for select molecules, lysosomal enzymes are excluded by the blood-brain barrier (BBB), making the task of enzyme replacement challenging. Therapeutic challenges posed by the BBB may be overcome by several key characteristics of lysosomal enzymes. First, even low concentrations of enzyme may be therapeutic, with activity in adult-onset LSD patients and clinically normal carriers reported at 2–4% and 11% of homozygous normal, respectively (4). Also, diseased cells are able to endocytose and use normal lysosomal enzymes, so a locus of treated cells may “cross-correct” a broad sphere of untreated neighboring tissue (5, 6). Finally, lysosomal enzymes are distributed in the CNS by multiple mechanisms such as diffusion (7), axonal transport (8–10) and cerebrospinal fluid (CSF) flow (8, 11–14).
Recently, adeno-associated viral (AAV) vectors have achieved widespread CNS distribution of lysosomal enzymes after injection into highly interconnected brain structures such as the ventral tegmental area, striatum, thalamus and deep cerebellar nuclei (9, 14–17). Most GM1 mice treated by AAV injection of the thalamus and deep cerebellar nuclei survived for 52 weeks (the experimental endpoint) compared to a median survival of 38 weeks in untreated animals. βgal activity was restored to a presumed therapeutic level (>10% wildtype mice) throughout the CNS, with normalization of substrate storage in the brain and a 50% reduction of substrate storage in the spinal cord (17). Because the mouse brain is 1000 times smaller than that of a human infant, we tested AAV-based gene therapy in a feline model of GM1 gangliosidosis.
First described in 1971, naturally occurring feline GM1 gangliosidosis is an accurate model of the human juvenile-onset disease in terms of enzymatic deficiency (<10% normal βgal activity), storage levels, and CNS and peripheral organ pathology (18–21). Clinical disease progression is remarkably stereotypical, with affected cats reaching a humane endpoint at 8.0 (± 0.6) months. The naturally occurring feline mutation is analogous to a well-characterized human mutation that generates normal amounts of enzymatically defective βgal protein (i.e., positive for cross-reactive material, or CRM+) (22–25). The feline brain is ~20 times smaller than an infant’s brain and provides a good approximation of enzyme and vector distribution challenges that need to be overcome for human gene therapy. In this study, GM1 cats were treated by intracranial injection of AAV vectors encoding feline βgal. Short-term and long-term assessments of therapeutic effect included clinical and MRI-based analyses accompanied by postmortem assays to evaluate normalization of biochemical defects.
Results
Treatment groups and distribution of βgal and AAV
Twenty three GM1 cats were treated before the average age of clinical disease onset by bilateral injection of the thalamus and deep cerebellar nuclei with AAV vectors expressing feline βgal from a hybrid cytomegalovirus enhancer/chicken β-actin (CBA) promoter (serotypes AAV1, n = 8, or AAVrh8, n = 15). Cats were treated with either a traditional serotype already in human use (AAV1) or a new serotype that has shown promise in mouse experiments (AAVrh8). Outcomes were assessed at 16 weeks post-injection (short-term, n = 7) or at the humane endpoint (long-term, n = 16), defined by the inability to stand on two consecutive days. Table 1 summarizes the study design.
Table 1.
Treatment groups and current clinical status of AAV-treated GM1 cats.
| DurationA | Serotype | DoseB | Cat | Gender | Tx age (mos.) | Endpoint or current age (mos.) | Clinical description | ± Seizures* |
|---|---|---|---|---|---|---|---|---|
| Long-term | AAV1 | High | 9-1356 | F | 1.9 | 52.8 | Mild hind limb weakness, carpal hyperextension | + |
| 8-1483 | F | 1.9 | 41.4 | Normal | + | |||
| 8-1485 | F | 2.0 | 41.2 | Normal | - | |||
| Full | 9-1545 | F | 2.3 | 25.3 | Deceased | - | ||
| 8-1551 | F | 2.1 | 34.6 | Normal | + | |||
| AAVrh8 | Full | 8-1364 | M | 1.6 | 50.5 | Mild hind limb weakness | - | |
| 8-1378 | M | 1.3 | 17.5 | Deceased | - | |||
| 8-1397 | F | 1.7 | 47.9 | Deceased# | + | |||
| 9-1424 | F | 2.9 | 44.9 | Normal | - | |||
| 8-1435 | M | 2.8 | 44.8 | Mild hind limb weakness | + | |||
| 9-1515 | M | 1.9 | 29.0 | Deceased# | - | |||
| 8-1626 | M | 3.0 | 29.4 | Weakness of carpi and limbs (hind/fore), pelvic ataxia, tremors | + | |||
| One-tenth | 8-1574 | M | 1.8 | 21.2 | Deceased | + | ||
| 8-1578 | F | 1.8 | 13.9 | Deceased | - | |||
| 8-1576 | F | 1.8 | 14.2 | Deceased | - | |||
| 9-1620 | F | 2.3 | 19.1 | Deceased | - | |||
|
| ||||||||
| Short-term (16 weeks) | AAV1 | Full | 9-1553 | F | 2.3 | N/A | Normal | - |
| 9-1555 | F | 2.3 | N/A | Normal | - | |||
| 8-1617 | M | 2.5 | N/A | Normal | - | |||
| AAVrh8 | Full | 9-1494 | M | 1.9 | N/A | Normal | - | |
| 9-1502 | M | 1.8 | N/A | Normal | - | |||
| 8-1525 | F | 1.9 | N/A | Normal | - | |||
| 8-1526 | F | 1.9 | N/A | Normal | - | |||
Cats in the long-term cohort were euthanized at clinical humane endpoint. Short-term cats were euthanized 16 weeks post-injection. Cats received bilateral injections of the thalamus and DCN.
Doses: High, 1.2 × 1013 vg; Full, 3–4 × 1012 vg; one-tenth dose, 3 × 1011 vg.
Animal developed seizures that were well controlled by medication. The mean age of seizure onset was 20.1 ± 7.4 months. #Cat 8-1397 had normal gait at 47.9 months of age but did not recover from an anesthetic procedure and was euthanized. Cat 9-1515 was clinically normal at 29.0 months of age but did not recover properly from anesthesia for MRI and was euthanized.
Abbreviations: Tx = treatment; mos. = months; N/A = not applicable.
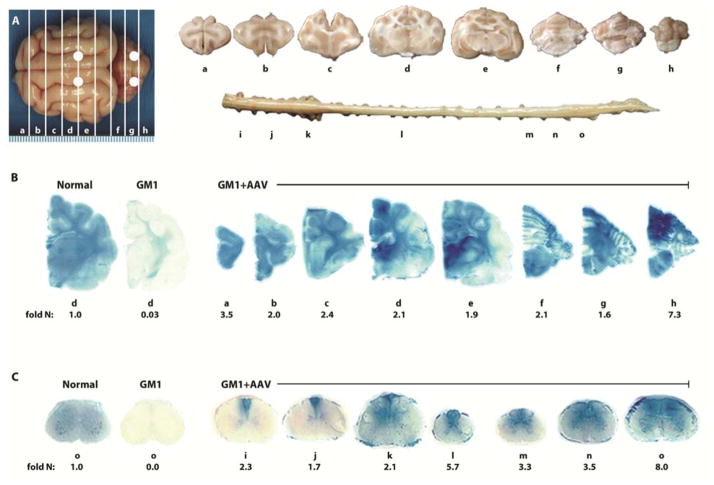
Sixteen weeks after a single surgery lasting ~90 minutes, βgal activity exceeded normal (homozygous for the wildtype allele) concentrations throughout the brain and spinal cord of GM1 cats (Fig. 1A–C). Activity was highest at the injection sites (Fig. 1B) and decreased with distance from the thalamus and deep cerebellar nuclei, yet βgal staining was normal or above normal in most brain regions. Specific activity measured with a synthetic fluorogenic substrate ranged from 1.1 – 4.1 fold that of normal across all coronal brain blocks for cats treated with AAVrh8 (Table 2). In AAV1-treated cats, βgal brain activity ranged from 1.4 – 4.2 fold that of normal, and there was no statistical difference in βgal concentrations when compared to the AAVrh8 cohort (P ≥ 0.22 for each block; Table 2 and fig. S1).
Fig. 1. Therapeutic enzyme distribution in the CNS of GM1 cats after AAV treatment.
GM1 cats were injected bilaterally in the thalamus and deep cerebellar nuclei with AAVrh8-CBA-βgal-WPRE (3–4 × 1012 vg total) and tissues were collected 16 weeks later. (A) Shown are injection sites (white circles) and 0.6 cm coronal blocks of the brain (a–h) and spinal cord (i–o) collected at necropsy. Blocks were halved and analyzed for sialic acid concentration (left) or for enzyme activity (right). Lysosomal βgal activity (blue) detected with Xgal at acidic pH was visualized throughout the brain (B) and spinal cord (C) of a representative, treated GM1 cat (GM1+AAV; 8-1526). Corresponding βgal activity is shown below each block as fold increase over concentrations in untreated normal healthy cats (fold N). Similar distribution occurred with AAV1 (fig. S1). Representative control sections are shown from untreated normal healthy cats along with untreated GM1 cats, which express ≤0.10 fold normal βgal activity in brain blocks ah (B,d) and ≤0.04 fold normal βgal activity in spinal cord blocks i-o (C, o). The range of specific activities for normal control blocks were: brain, 12.5 (a) – 42.1 (e); spinal cord, 3.2 (l) – 8.8 (k) nmol 4MU/mg/hr.
Table 2.
βgal activity in brain, spinal cord, CSF, and liver of AAV-treated and untreated GM1 cats.
| Fold-normal βgal specific activity, mean (s.d)
|
|||||||
|---|---|---|---|---|---|---|---|
| Region | Block | Short term, full doseA AAVrh8* | Short term, full doseB AAV1* | Long term, 1/10th doseC AAVrh8 | Long term, full doseD AAVrh8 | Long term, full doseE AAV1 | GM1 no tx |
| Cerebrum | a | 2.7 (0.85) | 4.1 (4.4) | 0.48 (0.24) | 0.96 (0.37) | 0.48 | 0.00 (0.00) |
| b | 1.8 (0.62) | 2.3 (2.4) | 0.42 (0.11) | 0.71 (0.30) | 0.42 | 0.00 (0.00) | |
| c | 1.7 (0.56) | 2.2 (2.0) | 0.63 (0.57) | 0.56 (0.17) | 0.61 | 0.01 (0.01) | |
| d | 1.7 (0.70) | 2.6 (1.1) | 0.62 (0.40) | 0.78 (0.27) | 1.2 | 0.02 (0.07) | |
| e | 1.1 (0.56) | 1.4 (1.1) | 0.33 (0.16) | 0.78 (0.35) | 0.82 | 0.05 (0.02) | |
|
| |||||||
| Cerebellum | f | 1.5 (1.2) | 3.0 (1.2) | 0.55 (0.90) | 2.9 (1.5) | 0.40 | 0.10 (0.04) |
| g | 2.2 (1.8) | 4.2 (2.1) | 0.33 (0.23) | 2.5 (0.96) | 0.33 | 0.04 (0.02) | |
| h | 4.1 (2.6) | 1.7 (1.1) | 0.27 (0.34) | 1.4 (1.6) | 0.30 | 0.04 (0.03) | |
|
| |||||||
| Spinal cord | i | 2.0 (1.5) | 1.0 (0.38) | 0.00 (0.00) | 0.74 (0.65) | 0.07 | 0.00 (0.00) |
| j | 1.6 (1.4) | 0.85 (0.60) | 0.03 (0.06) | 1.2 (0.93) | 0.04 | 0.00 (0.00) | |
| k | 1.9 (1.0) | 0.89 (0.28) | 0.05 (0.08) | 1.5 (1.1) | 0.10 | 0.03 (0.05) | |
| l | 3.2 (2.5) | 1.0 (0.46) | 0.07 (0.09) | 0.93 (0.62) | 0.00 | 0.03 (0.06) | |
| m | 2.1 (1.4) | 2.2 (1.9) | 0.07 (0.13) | 0.75 (0.68) | 0.05 | 0.00 (0.00) | |
| n | 2.3 (1.1) | 1.8 (1.5) | 0.09 (0.15) | 1.3 (0.98) | 0.05 | 0.04 (0.07) | |
| o | 4.8 (3.1) | 4.2 (3.2) | 0.38 (0.61) | 6.6 (8.5) | 0.17 | 0.01 (0.02) | |
|
| |||||||
| CSF | N/A | 28 (20) | 42 (28) | 2.7 (1.3) | 32 (42) | 8.3 | 0.03 (0.08) |
|
| |||||||
| Liver | N/A | 0.38 (0.25) | 0.24 (0.16) | 0.17 (0.11) | 0.20 (0.19) | 0.08 | 0.05 (0.01) |
βgal activity was not significantly different between AAVrh8 (n = 4) and AAV1 (n = 3) cohorts at the 16 week time point (a–h and i–o, P ≥ 0.22 for each block; CSF, P = 0.19; liver, P = 0.43).
n = 4; βgal specific activity was significantly higher than untreated GM1 cats (n = 4) in a-h and i-o (P ≤ 0.015 for each block), CSF (P = 0.026, n = 3, no sample available for 8-1526), and liver (P = 0.015).
n = 3; βgal specific activity was significantly higher than untreated GM1 cats (n = 4) in a-h and i-o (P ≤ 0.026 for each block), CSF (P = 0.026), and liver (P = 0.026).
n = 4; βgal specific activity was significantly higher than untreated GM1 cats (n = 4) in a-e, g, and h (P ≤ 0.021 for each block), CSF (P = 0.015), and liver (P = 0.015), but not in f (P = 0.15) or i-o (P ≥ 0.20 for each block).
n = 3; βgal specific activity was significantly higher than untreated GM1 cats (n = 4) in a-h, j-l, and n-o (P ≤ 0.025 for each block), as well in CSF (P = 0.025), but not in blocks i and m (P = 0.061) or liver (P = 0.054).
Not enough subjects currently available for statistical analysis.
The Wilcoxon signed-rank test was used for statistical comparisons.
Abbreviations: no tx = no treatment; N/A = not applicable.
βgal activity in the spinal cord at 16 weeks post-injection also was near- or above-normal, with no statistical difference between the two AAV vectors (P ≥ 0.22 for each block; Table 2). When measured in 7 coronal blocks from the cervical, thoracic and lumbar spinal cord, βgal activity ranged from 1.6 – 4.8 fold that of normal in the AAVrh8 cohort and from 0.9 – 4.2 fold that of normal in the AAV1 cohort (Fig. 1C, fig. S1, and Table 2). Residual βgal activity in untreated GM1 cats ranged from 0.0 – 0.1 and 0.0 – 0.04 fold that of normal in the brain and spinal cord, respectively. Both vector cohorts demonstrated statistically significant increases in βgal activity versus untreated animals (P ≤ 0.026 for each block). Vector genomes (vg) were detected in all brain and spinal cord blocks, demonstrating widespread dissemination from the injection sites, although vector levels did not always correlate with βgal activity (table S1).
CSF βgal activity was 28 fold that of normal in the AAVrh8 cohort and 42 fold that of normal in the AAV1 cohort, which was significantly higher than in untreated GM1 cats (P = 0.026 for each cohort; Table 2). Additionally, βgal activity and vector genomes were present in the liver of both AAV-treated cohorts demonstrating dissemination of enzyme and vector to peripheral tissues following intraparenchymal injection (Table 2 and table S1). Liver βgal activity measured 0.38 and 0.24 fold that of normal in the AAVrh8 and AAV1 cohort, respectively, which was significantly higher than in untreated GM1 cats (AAVrh8, P = 0.015; AAV1, P = 0.026).
Clearance of storage material
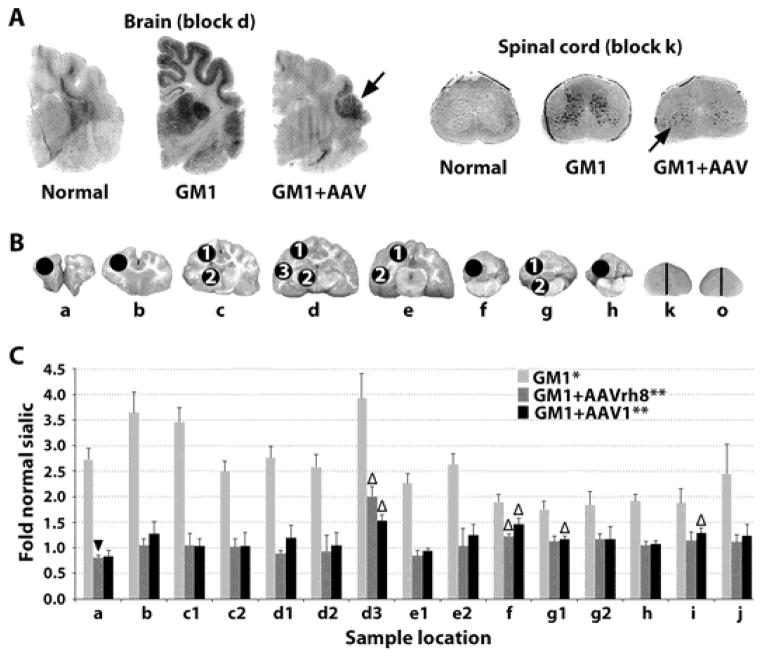
As glycosphingolipids with complex oligosaccharide side chains containing one or more sialic acid residues (26), gangliosides can be detected colorimetrically by a variety of methods. To evaluate the clearance of stored substrate by vector-generated βgal, Periodic Acid-Schiff (PAS) staining in the CNS was performed in GM1 cats 16 weeks post-treatment. Storage levels were substantially normalized throughout most of the brain and spinal cord, but clearance was incomplete in focal areas of the temporal lobe and cervical spinal cord (Fig. 2A) that corresponded with sites of minimal βgal activity in Fig. 1.
Fig. 2. Storage in the CNS of GM1 cats 16 weeks post-treatment.
(A) Storage in untreated GM1 cats was visualized by dark PAS staining in the gray matter and thalamus. In treated brains, residual ganglioside storage was present in focal areas (black arrows) of the temporal lobe (block d in panel B) and cervical spinal cord (block k in panel B). (B) Sample sites for sialic acid quantitation (circles) in brain (a–h) and spinal cord (k and o correspond to Fig. 1A; half of each block was used). (C) Sialic acid levels were measured in untreated GM1 cats (n = 4) and after treatment with AAVrh8 (n = 4) or AAV1 (n = 3) treated GM1 cats for comparison to normal healthy cats (n = 4). *, all samples from untreated GM1 cats were significantly higher than normal (P ≤ 0.015 for each block); **, all samples from treated GM1 cats were significantly lower than untreated GM1 cats (P ≤ 0.026 for each block) except AAV1 block h, as only 2 samples were available (P = 0.053); △, indicates samples from treated GM1 cats that were significantly higher than normal (P ≤ 0.035); ▼, indicates a sample from treated GM1 cats that was significantly lower than normal (P = 0.033). The Wilcoxon signed rank test was used for statistical comparisons.
When quantitative sialic acid assays were performed on 15 samples throughout the CNS (Fig. 2B, C), concentrations in untreated GM1cats were significantly higher than normal in the cerebrum (2.5 – 3.9 fold normal; P ≤ 0.015), cerebellum (1.8 – 1.9 fold normal; P ≤ 0.015) and spinal cord (1.9 – 2.5 fold normal; P ≤ 0.015). Treatment with AAV1 or AAVrh8 significantly reduced storage in all CNS samples (P ≤ 0.026), with the exception of the caudal cerebellum from AAV1-treated cats, for which samples from only 2 animals were available (Fig. 2B, C, block h, P = 0.053). Though reduced by gene therapy, storage remained above normal in 4/15 samples including the temporal lobe (sample d3 in Fig. 2B), rostral and middle cerebellum (samples f and g1 in Fig. 2B) and cervical spinal cord (sample k in Fig. 2B). In one sample from the frontal pole of AAVrh8-treated cats, sialic acid was reduced to 0.83-fold of normal (sample a in Fig. 2B; P = 0.033).
Normalized activity of other lysosomal enzymes
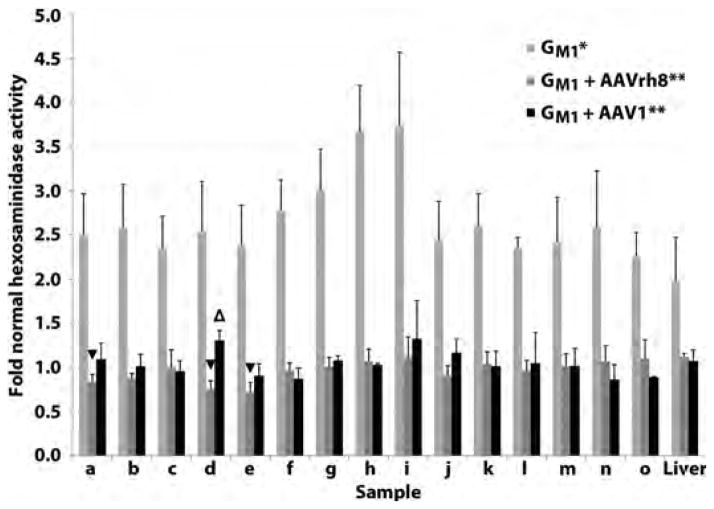
In βgal deficiency, the activity of other lysosomal enzymes is above normal (18, 27). For example, lysosomal β-N-acetlyhexosaminidase (EC 3.2.1.52) activity was elevated up to 3.7 fold that of normal in the CNS and 2.0 fold that of normal in the liver of untreated GM1 cats (P ≤ 0.015 for each CNS block; P = 0.015 for liver; Fig. 3). Treatment for 16 weeks with either AAV serotype reduced hexosaminidase activity significantly in the brain (P ≤ 0.026 for each block), spinal cord (P ≤ 0.026 for each block) and liver (P ≤ 0.026), demonstrating normalization of a secondary lysosomal biomarker after gene therapy (Fig. 3).
Fig. 3. Normalization of lysosomal hexosaminidase activity in the CNS and liver of GM1 cats 16 weeks post-treatment.
GM1 cats were injected bilaterally in the thalamus and deep cerebellar nuclei with 3–4 × 1012 vg of AAVrh8 (n = 4, dark gray bars) or AAV1 (n = 3, black bars). Tissues were collected 16 weeks post-treatment and hexosaminidase activity compared to untreated GM1 cats (n = 4, light gray bars) and normal healthy cats (n = 4) in the brain (a–h), spinal cord (i–o), and liver. Lettering of brain and spinal cord blocks corresponds to Fig. 1A. *, all samples from untreated GM1 cats were significantly higher than normal (P ≤ 0.015 for each sample); **, all samples from treated GM1 cats were significantly lower than untreated GM1 cats (P ≤ 0.026 for each sample); △, indicates a sample from treated cats that was significantly higher than normal (P = 0.026); ▼, indicates samples from treated cats that were significantly lower than normal (P ≤ 0.030). The Wilcoxon signed rank test was used for statistical comparisons.
Long-term clinical benefit of AAV treatment
Long-term clinical benefit in GM1 cats was assessed after treatment with AAV1 (n = 5) or AAVrh8 (n = 7) serotypes. As shown in Table 1, animals were treated with 3–4 × 1012 vg (“full dose,” n = 9) or 1.2 × 1013 vg when AAV1 titers made this “high dose” possible (n = 3). Statistically significant increases in survival have been achieved for both the AAV1 (P = 0.0004) and AAVrh8 (P < 0.0001) cohorts (Fig. 4A). Currently, mean survival of treated GM1 cats is >4.7 times that of untreated cats: 39.1 (± 10.1) months for the AAV1 cohort and 37.7 (± 12.4) months for the AAVrh8 cohort. Eight of 12 treated cats remain alive, most with subtle or no disease signs. The oldest cats from each vector cohort remain alive and well at 52.8 (AAV1) and 50.5 (AAVrh8) months, or >6.3 times the life span of untreated cats. Of the 4 cats no longer living, 2 had normal gait at 29.0 months (9–1515) and 47.9 months (8–1397) but were euthanized due to abnormal recovery from anesthesia.
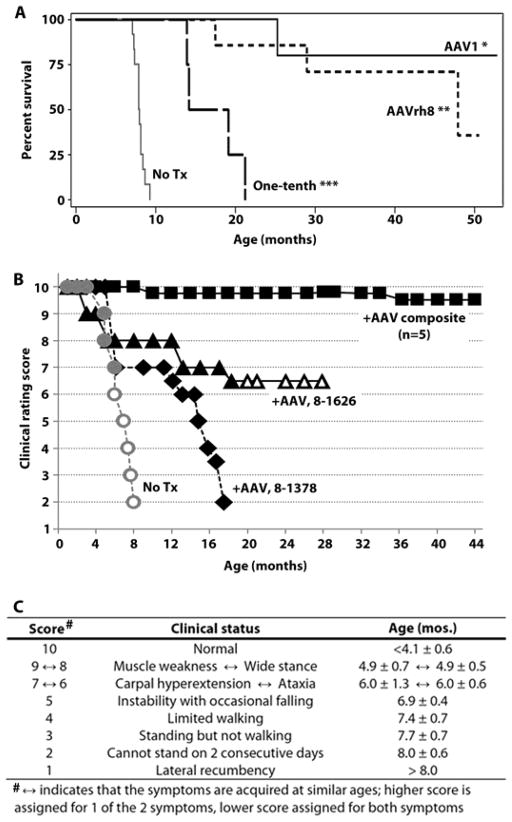
Fig. 4. Survival and clinical progression of AAV-treated GM1 cats.
(A) Kaplan-Meier survival curves for untreated GM1 cats (No treatment (Tx), n = 12) and GM1 cats treated long-term with gene therapy, which have significantly increased survival compared to untreated animals using the logrank test: *AAV1, P = 0.0004 (n = 5); **AAVrh8, P < 0.0001 (n = 7); ***One-tenth dose AAVrh8 group, P = 0.0012 (n = 4). Survival of AAVrh8 treated cats was significantly higher for full dose versus one-tenth dose (P = 0.0046). (B) Full dose AAVrh8-treated cats were scored on a scale based on disease progression in untreated GM1 cats (No Tx). A composite of 5 cats that responded robustly to treatment is shown (+AAV composite), whereas 2 cats that responded less favorably are depicted separately (8-1626, 8-1378). Solid lines signify living cats; dashed lines represent deceased cats. Open symbols denote whole-body tremors, which occurred in all untreated cats but only 1 treated animal. (C) Age of symptom onset is shown for untreated GM1 cats (mean ± SD, n = 12). The scale is based on gait defects, which ultimately defined the humane endpoint, with initial deficits appearing at 4.9 months. However, disease onset begins at 4.1 months with fine head and tail tremors that progress to whole-body tremors at 6.2 months. For clarity, only whole-body tremors are depicted in panel B.
Disease onset in treated cats was delayed versus untreated GM1 controls, whose stereotypical disease progression began at 4.1 (± 0.6) months of age with fine tremors of the head and tail. Clinical symptoms in untreated animals progressed to generalized muscle weakness, wide-based stance, ataxia, carpal hyperextension (in 80% of cats), instability with occasional falling, loss of ambulation, and finally the inability to stand, which defined the humane endpoint at 8.0 (± 0.6) months. Of 12 treated GM1 cats, disease onset is yet to be determined in 4 animals ranging from 34.6 to 44.9 months of age. Also, 3 cats whose disease onset occurred at 10.2, 36.6 and 37.0 months currently have only mild disease at 44.8, 50.5 and 52.8 months, respectively (8–1435, 9–1356 and 8–1364).
Quality of life for treated GM1 cats has improved dramatically, as measured by a clinical rating scale that reflects an animal’s departure from normal function (Fig. 4B, C). Clinical rating scores decrease from 10 (normal function) to 1 (lateral recumbency) as animals become more debilitated by gait defects and balance disturbances/instability. Currently, minimal disease progression has been documented for 5/7 animals in the AAVrh8 cohort (Fig. 4B, C) and 4/5 animals in the AAV1 cohort (fig. S2), whose composite clinical rating scores at 40 months of age were 9.5 (± 0.6) and 9.7 (± 0.6), respectively. While untreated GM1 cats have severe dysequilibrium and gait defects by 7 months of age (video S1), most treated GM1 cats remain overtly normal (video S2; 8–1485 at 34.7 months old) or have only subtle gait abnormalities (video S3; 8–1435 at 39.0 months old). No difference in therapeutic benefit is discernible between AAV1 and AAVrh8 cohorts.
In 3/12 AAV-treated cats, therapeutic response was clearly positive but less dramatic. One cat that was symptomatic at the time of surgery (8–1626) has moderately progressive disease at 29.4 months of age, though current symptom acquisition has been delayed ~3-fold. Two animals had disease onset at 6.1 (8–1378) and 4.6 (9–1545) months of age, ultimately reaching humane endpoint at 17.5 and 25.3 months, respectively. Conclusions regarding why 3/12 treated animals responded less robustly than others await tissue analysis from more cats in the long-term cohort. However, diminished enzyme activity is a reasonable hypothesis since both cats that reached the humane endpoint (8–1378, 9–1545) had low βgal concentrations in the spinal cord (≤0.4-fold normal and ≤0.14-fold that of samples from the 16 week time point). In contrast, two cats that had normal gait at 29.0 and 47.9 months of age (9–1515 and 8–1397, respectively) had above-normal βgal activity in the spinal cord that was comparable to the 16 week cohort (fig. S3). βgal activity in CSF was above normal (2.5–63.1 fold) in all cats from the long-term cohort, perhaps explaining why there was no correlation between CSF activity and clinical outcome/lifespan (fig. S3).
Half of all treated cats in the long-term cohort (6/12) experienced seizure activity, well-controlled by medication, with a mean onset of 20.1 (± 7.4) months (Table 1). Common symptoms included blank stares, salivation, and facial twitches, but progression to undirected running and tonic-clonic seizures did occur in some animals. In at least 3/6 animals, seizures were demonstrably inducible by sounds such as running water. No seizures occurred in GM1 cats treated for only 16 weeks (n = 7). Seizures are a known feature of late-stage feline GM1 gangliosidosis (19, 21), though no untreated GM1 cats in the current study had seizures because they occur well beyond the humane endpoint used.
Normalization of MRI brain architecture
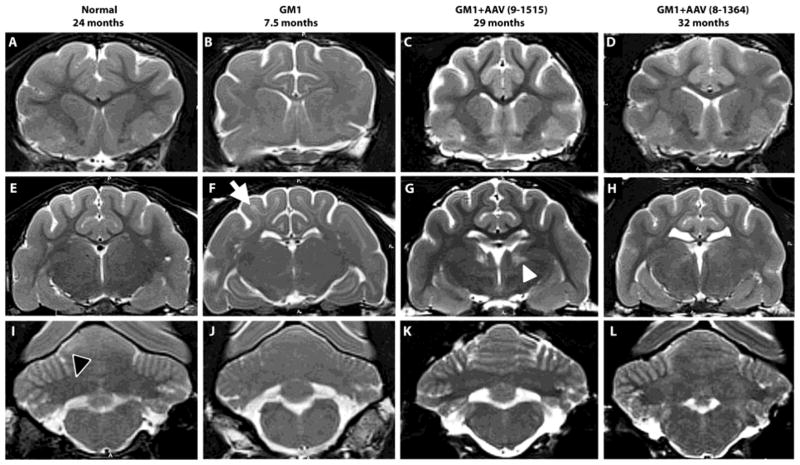
On T2-weighted MRI, cortical white matter is hypointense to (i.e., darker than) gray matter in normal cats but is hyperintense to (i.e., lighter than) gray matter in untreated GM1 cats with moderate to severe disease. Also, the deep cerebellar nuclei area is hypointense to cerebellar gray matter in normal cats but becomes isointense with disease progression in untreated GM1 cats. Therefore, MRI provides an easily discernible, non-invasive biomarker of disease progression (Fig. 5). In AAV-treated GM1 cats, hypointensity of white matter to gray matter is substantially normalized in the cortex to at least 32 months of age (cat 8–1364), or >5 times longer than in untreated cats. Additionally, hypointensity of the deep cerebellar nuclei area to surrounding gray matter is largely preserved after AAV treatment. Overall brain architecture of treated GM1 cats is normal, though slight widening of sulci is appreciated in some animals.
Fig. 5. MRI evaluation of GM1 cats.
T2-weighted MRI images (3 Tesla) were taken at the level of the caudate nucleus (A–D), thalamus (E–H) and deep cerebellar nuclei (I–L). Cortical white matter is hypointense to (i.e., darker than) gray matter in normal healthy cats, but hyperintense to (i.e., lighter than) gray matter in untreated GM1 cats (white arrow in panel F). Also, the deep cerebellar nuclei area is hypointense to surrounding gray matter in normal healthy cats (outlined black arrowhead in panel I), but becomes isointense with disease progression in untreated GM1 cats. In AAV-treated GM1 cats (GM1+AAV), hypointensity of cortical white to gray matter and deep cerebellar nuclei area to cerebellar gray matter was largely preserved, indicating reduced myelin loss after treatment. Both AAV-treated cats were clinically normal at the time of imaging. In 5 of 11 treated cats tested, a locus of hyperintensity was noted in the thalamus (white arrowhead in panel G), though no clinical or histopathological correlates have been identified to date. Ages in months are shown for each cat.
To date, the only abnormality associated with AAV treatment is an irregularly-shaped locus of T2 hyperintensity in the thalamus of 5/11 cats evaluated (Fig. 5). In previous studies of gene therapy in feline GM2 gangliosidosis (Sandhoff disease), similar hyperintense loci were thought to be associated with eosinophilic, botryoid neurons in H&E sections from the dorsal thalamic nuclei (28). In treated GM1 cats in the current study, fine eosinophilic granules were found in scattered cortical and hippocampal neurons dorsal to the injection site (fig. S4), but not in thalamic regions corresponding to T2 hyperintensities. The underlying cause of hyperintense thalamic loci remains to be defined. Detected in AAV-treated cats by 6 months of age, T2 hyperintensities were not present in untreated cats at any age, including the humane endpoint of ~8 months. No histological or MRI anomalies were found in 3 normal cats injected with saline and followed for >18 months.
Dose response
When GM1 cats were treated with one-tenth of the full AAVrh8 dose, mean survival was 17.1 (± 3.6) months, significantly lower than the full dose cohort (P = 0.0046, Fig. 4A). Of 4 cats treated with the one-tenth dose, 2 reached the humane endpoint at 14.2 and 19.1 months old, 1 was euthanized at 13.9 months old due to chronic weight loss and lethargy, and 1 was euthanized at 21.2 months due to dysphagia, also reported in juvenile-onset GM1 patients (29). Nevertheless, mean survival of the tenth dose cohort was 2.1 times greater than untreated GM1 cats (P = 0.0012). βgal activity and vector copies for the one-tenth dose cohort are detailed in Table 2 and table S1. Liver βgal activity of only 0.17-fold that of normal was sufficient to fully normalize hexosaminidase activity (P = 0.015 versus untreated GM1 cats; fig. S5).
Restoration of breeding function
In >40 years of study, no female GM1 cat has become pregnant and no GM1 male has been fertile. After AAV injection, 2 treated males bred 2 treated females and 4 untreated carrier females, producing a total of 9 litters (27 kittens). Treated GM1 cats bred successfully from 9.8 to at least 28.6 months of age. Fertility onset for carrier or normal colony cats is ~7 months. GM1 × GM1 matings produced litters composed entirely of GM1 kittens, which had stereotypical disease progression and life span. In agreement with previous reports (30, 31), vector was detected only transiently in gonads and no evidence of germ line gene transmission was found in treated cats or their offspring (table S2).
Discussion
As a group, LSD prevalence is ~1 in 7,700 live births (32), similar to that of cystic fibrosis or hemophilia. Like most lysosomal diseases, GM1 gangliosidosis is neurodegenerative and fatal, with only palliative measures currently available to patients. Promising results have been achieved with intracranial AAV injections in rodent models of gangliosidosis. Treated GM2 mice demonstrate near normalization of life span, representing a 5-fold survival increase versus untreated GM2 mice (14). Also encouraging are data from large animal models of neurodegenerative lysosomal diseases. In dogs with mucopolysaccharidosis I or IIIb, intracranial gene therapy with AAV vectors improved storage and histological lesions throughout most of the brain, though no clinical or survival benefit was demonstrated (33, 34). Cats with α-mannosidosis treated by intracranial injections of an AAV1 vector through 14 burr holes had only mild neurological disease at the untreated humane endpoint of 18 weeks, with restoration of α-mannosidase activity to ~4% normal in the brain. Because of the study design, improved survival benefit was not achieved, although 1 of 2 treated cats followed long-term had only mild disease when euthanized at 56 weeks of age (35).
The current report documents above-normal βgal activity throughout the GM1 cat brain and spinal cord after intracranial injections of AAV vectors carrying the therapeutic gene through only 4 burr holes in a surgery lasting ~90 minutes. The successful translation of a treatment approach from GM1 mice to GM1 cats, with a ~70-fold scale-up of brain size suggests that this approach may be beneficial in human patients. Although the human brain is ~20 times larger than the cat brain, the thalamus and deep cerebellar nuclei also are proportionally larger, allowing an equivalent AAV dose per kg of brain weight. In 12 treated animals followed long-term, mean life span was extended >4.7-fold and continues to increase, as the majority of treated cats are disease-free or have only subtle symptoms at present. Quality of life improved profoundly, with only 2 of 12 treated animals having progressed to the most debilitating symptoms such as dysequilibrium and severe ataxia. Clinical outcomes were similar for the 2 vector serotypes tested: AAV1, currently in human clinical trials (36–39) or approved for human use (40, 41), and AAVrh8, a new serotype (42) that showed promise in a related feline model (28).
Little toxicity was apparent from the vector. The eosinophilic granules in brain neurons of treated GM1 animals may represent a less extreme version of the large, botryoid hypereosinophilic inclusions previously reported in Sandhoff and normal cats treated with AAVrh8 vectors that overexpressed feline hexosaminidase, often at >50 times normal (28). In our treated GM1 cats, typical βgal expression is ≤4-fold normal, so it is possible that fine eosinophilic granules represent inclusions with mildly over-expressed βgal. Additionally, there was limited toxicity from the injection procedure. A thalamic hemorrhage documented by MRI in 1 animal (8–1435) caused ataxia, head tilt and cortical blindness that resolved after 1 week of treatment with mannitol and steroids. Now >44 months of age, 8–1435 (video S3) has only slight hind limb gait deficits and is the first fertile male in the 40-year history of the research colony.
Although ~half of AAV-treated GM1 cats had hyperintense thalamic loci on T2-weighted images, no histopathological or clinical correlates were identified. In related ongoing studies, similar loci were found in the thalamus of 4/6 normal cats treated with AAVrh8 vectors expressing feline hexosaminidase, none of which had seizures in >18 months of follow-up. Since no seizure activity was observed in 3 of the 5 treated GM1 cats with T2 anomalies, a causal relationship between hyperintense thalamic loci and seizures is unlikely. Known to occur in late-stage GM1 disease in both humans (29, 43, 44) and cats (19, 21), seizures in treated animals may result from progressive disease in the temporal lobe, one of the few brain regions with incomplete restoration of βgal activity (Fig. 1B) and only partial normalization of storage (Fig. 2A, C). In fact, seizures in treated GM1 cats often resemble temporal lobe epilepsy, with short duration, initial motionless stare, impaired awareness, involuntary motor behaviors and salivation (45–47). Improved treatment and abrogation of seizure activity may be achieved by adding injection sites that target areas of suboptimal therapeutic response.
Above-normal βgal activity in the spinal cord after injection of the brain parenchyma was among the surprising findings of this study, even with injection targets (thalamus and deep cerebellar nuclei) (16, 17) chosen for a high degree of interconnectivity with other CNS structures. βgal activity and vector genomes were detected throughout the spinal cord, up to 14 cm from the deep cerebellar nuclei injection site at the time of surgery. We hypothesize that a portion of vector transport to the spinal cord occurred through CSF, known to be an effective medium for AAV transfection of the CNS (12, 13). For our thalamic injections, the needle transects the lateral ventricle, which may provide a path of least resistance for vector backflow through the needle tract. Similarly for cerebellar injections, vector may travel into the subarachnoid space dorsal to the cerebellum. Varying degrees of vector leakage into the CSF due to slight differences in needle placement or anatomy may explain the reduced βgal activity in the spinal cord of cats that responded less robustly to treatment.
Vector likely gained access to peripheral organs via blood vessels disrupted during the injection procedure and via CSF, which ultimately is reabsorbed into the circulatory system. To date, there have been no apparent peripheral disease symptoms in AAV-treated GM1 animals. It is likely that residual βgal activity (~5% normal) in GM1 cats delays development of clinical peripheral disease, but the increased βgal activity in peripheral tissues of AAV-treated GM1 cats also may contribute to therapeutic success. Normalized liver activity of lysosomal hexosaminidase in all AAV-treated groups indicates that even partial restoration of liver βgal activity is beneficial (fig. S5).
Although it was not possible to blind investigators to the treatment status of cats in the present study, clinical rating scores were assigned independently by two investigators to attempt to limit bias. Survival and MRI data provided objective clinical measures, and biochemical analyses from the deceased long-term cats supported their clinical scores. The promising therapeutic results in this study after intraparenchymal injections through only 4 needle tracts, with little evidence of toxicity, support the initiation of AAV-based clinical trials for GM1 gangliosidosis in human patients. Although deep cerebellar nuclei injection was effective in restoring enzyme activity to the cerebellum, a similar strategy in human patients must weigh the benefit of direct cerebellar treatment versus the risk of hemorrhage in the posterior fossa. Safety studies in non-human primates will assess potential vector and procedural toxicity in human patients. Should the risk of deep cerebellar nuclei injection be considered too great, CSF-mediated approaches to treating the cerebellum may be incorporated, such as those deemed successful in mice and dogs (11, 13). When predicting outcomes in human patients it must be considered that GM1 cats in this study were treated pre-symptomatically while most human patients are diagnosed after disease onset, so gene therapy may be less efficacious when administered later in the disease course. Nevertheless, as evidence for the benefits of AAV gene therapy continues to build in animal models, addition of future clinical trials to those already underway for human lysosomal storage diseases (48, 49) will determine whether this therapeutic potential is realized for GM1 gangliosidosis and for other lysosomal storage diseases.
Materials and Methods
Study Design
Objective and subjects
The study objective was to evaluate intracranial AAV-mediated gene therapy in a feline model of GM1 gangliosidosis. The feline GM1 breeding colony is maintained at Auburn University, whose institutional animal care and use committee (IACUC) approved the research described herein.
Experimental design
Treatment groups for this controlled laboratory experiment are detailed in Table 1. GM1 cats were treated at 1.3–3.0 months of age. The precise age of treatment was dependent on logistical considerations, but all cats were treated at least 1 month before the average age of clinical disease onset at 4.1 ± 0.6 months. Animals were treated in the thalamus and deep cerebellar nuclei with 1 of 3 vector doses: “high,” 1.2 × 1013 vg; “full,” 3–4 × 1012 vg; or “one-tenth,” 3 × 1011 vg. One of 2 primary endpoints was used: (1) 16 weeks post-treatment (short-term), for biochemical and tissue-based measures of therapeutic effect or (2) humane endpoint, for evaluation of long-term clinical benefit. The humane endpoint was defined by an animal’s inability to stand on 2 consecutive days. Age-matched normal untreated controls (homozygous for the wildtype allele) were included for the short-term (n = 4) and long-term (n = 3) time points. GM1 untreated controls also were included for the short-term (6.0–7.3 months old, n = 4) and long-term cohorts (humane endpoint, n = 12).
Group sample sizes were based on IACUC guidelines to use the minimum number of animals necessary to derive statistical significance, which was estimated to be ≥3 animals for short-term studies and ≥5 animals for long-term studies, based on previous results from GM1 mice used to inform the experimental design. Larger sample sizes were necessary for long-term cohorts due to anticipated variability of subjective readouts such as clinical rating scores. GM1 cats were randomly assigned to each treatment group, and actual sample size (short-term, n = 3–4; long-term, n = 5–7) was determined by the number of viable kittens born during the treatment phase of the study.
General anesthesia was induced with ketamine (10 mg/kg) and dexmedetomidine (0.04 mg/kg) through an intravenous catheter and maintained using isofluorane (0.5–1.5%) in oxygen delivered through an endotracheal tube. Cats were positioned sternally for intracranial injection using a Horsley-Clark stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) and injected bilaterally in the thalamus and deep cerebellar nuclei with AAV1- or AAVrh8-CBA-βgal-WPRE (see AAV Vector Design and Preparation below). Craniotomy sites were made with a 20 gauge needle at the following distances (in cm) from bony landmarks on the skull: thalamus (relative to bregma), anterior-posterior (AP) −0.7, mediolateral (ML) ±0.4, dorsoventral (DV) −1.6 (from meninges); deep cerebellar nuclei (relative to lambda), AP 0.0, ML ±0.4, DV −1.25 (from meninges). Vector was delivered using a Hamilton syringe (Harvard Apparatus, Holliston, MA) with a non-coring needle (22–25 G). A total of 70 μl was injected into each thalamus in 10–20 μl boluses, and a total of 24 μl was injected into each deep cerebellar nuclei in a 10 μl and 14 μl bolus. Injection rate was 2 μl/min and the needle was raised 0.15 cm between boluses.
Cats were assigned a clinical rating score from the scale in Fig. 4C using 2 separate, independent readouts: (1) neurological exams performed by a veterinarian at 2–4 week intervals and (2) video footage, which was retrospectively analyzed by a second, independent investigator. With the personnel available, it was not possible for evaluators to be completely blinded to treatment status, though their observations were performed independently. The supplementary material contains video footage of 3 cats.
For biochemical analysis, brains were divided into coronal blocks of 0.6 cm from the frontal pole through the cerebellum (Fig. 1A). Coronal blocks from the right hemisphere were frozen in optimum cutting temperature (OCT) medium and used for the following analyses: βgal distribution by histochemical staining, βgal specific activity by 4-methylumbelliferone (4MU) enzyme assays (performed in duplicate for each block), AAV vector distribution by SYBR Green qPCR (performed in triplicate for each block), and ganglioside storage by PAS staining. Coronal blocks from the left hemisphere were halved to 0.3 cm and fixed in 10% formalin or stored at −80 °C for analysis of sialic acid levels using a resorcinol based assay (performed in duplicate). Mean values were reported for all quantitative assays.
AAV Vector Design and Preparation
The feline βgal cDNA sequence was assigned GenBank accession number AF006749. AAV vectors were produced as previously described by triple transfection of 293T cells with vector plasmid (derived from the plasmid pAAV-CBA-EGFP-W by replacing EGFP with the cDNA for feline βgal) (22), a mini adenovirus helper plasmid pFΔ6 and AAVrh8 helper plasmid pAR8 or AAV1 helper plasmid pXR1 (50). The inverted terminal repeats in the vectors are derived from AAV2. Transgene expression is controlled by a hybrid CMV enhancer/chicken β-actin promoter (CBA). All vectors carry the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE).
Assays of lysosomal enzyme activity
For CNS tissue extraction several frozen sections (50 μm) were cut from each coronal block (Fig. 1A), and for liver extraction a sample was taken from tissue stored at −80 °C. Tissue was homogenized manually in 50 mM citrate phosphate buffer, pH 4.4 (50 mM citric acid, 50 mM Na2HPO4, 10 mM NaCl) containing 0.1% TritonX and 0.05% BSA, followed by 2 freeze-thaw cycles and centrifugation at 15,700 g for 5 minutes at 4 °C. CSF samples were analyzed directly from −80 °C. The activity of βgal and total hexosaminidase were measured using synthetic fluorogenic substrates as previously described (51), but for βgal we measured 10 μl of sample for brain and 30 μl for spinal cord. Specific activity was expressed as nmol 4MU/mg/hr after normalization to protein concentration by the Lowry method.
Histochemical staining of βgal activity or storage product
For analysis of βgal activity, frozen sections at 40 μM were thawed and fixed in 0.5% glutaraldehyde in citrate phosphate buffer (50 mM Na2HPO4.7H2O, 50 mM citric acid monohydrate, 10 mM NaCl), pH 4.2 (brain) or pH 5.2 (spinal cord) for 10 minutes followed by washes in citrate phosphate buffer. Tissue sections were then incubated at 37 °C overnight in citrate phosphate buffer, pH 4.2 (brain) or pH 5.2 (spinal cord) containing 20 mM K4Fe(CN)6, 20 mM K3Fe(CN)6, 2 mM MgCl2, 0.02% IGEPAL, 0.01% deoxycholic acid and 2 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (Xgal). Next day, sections were washed, dehydrated and mounted.
Ganglioside storage material was assessed qualitatively with PAS staining, which detects the oligosaccharide side chain. Frozen sections (20 μM) were washed in PBS, fixed for 7 minutes with 3.7% paraformaldehyde in 95% ethanol/5% ddH2O (pH 7.4), washed again with PBS, incubated for 3 minutes in 0.5% Periodic Acid, washed in ddH2O and incubated in Schiff Reagent for 45 seconds (brain) or 60 seconds (spinal cord).
Lipid extraction and sialic acid quantification
Punch biopsies of 8 mm diameter were taken from representative areas of the brain and spinal cord (Fig. 2B) and lyophilized overnight. Next day, 10–25 mg of dry weight sample was rehydrated in 0.5 ml water and total lipids extracted in 5 ml of chloroform: methanol (1:1 by volume). Samples were stirred at room temperature for at least 4.5 hours and centrifuged at 850 g for 20 minutes. Supernatant was collected and the pellet extracted again in 2 ml of chloroform: methanol for 15 minutes before another centrifugation. Supernatants for each sample were combined to measure sialic acid by the method of Svennerholm (52) with modification from Miettinen (53). A 0.25 ml aliquot was evaporated, re-dissolved in 0.5 ml H2O, dissolved in 0.5 ml resorcinol reagent (5 ml distilled H2O, 40 ml HCl, 0.125 ml 0.1M copper sulfate, 5 ml 2% resorcinol stock in distilled H2O), and boiled for 15 minutes. After cooling for 10 minutes, 1 ml butyl acetate:butanol solution (85:15 v/v) was added and each sample was mixed vigorously. The upper phase was removed and read at 580 nm using a Shimadzu UV 160U spectrophotometer (Columbia, MD). Sialic acid concentration was expressed as nmol/mg.
Quantitative PCR for vector genomes
Vector was measured by quantitative PCR using SYBR®Green-based reactions (Applied Biosystems, Warrington, UK) with primers specific for WPRE in the vector (forward 5′-AGTTGTGGCCCGTTGTCA-3′; reverse 5′-GAGGGGGAAAGCGAAAGT-3′). Samples were incubated for 2 minutes at 50 °C, 10 minutes at 95 °C, and then amplified for 40 cycles of 95 °C for 30 seconds, 62 °C for 30 seconds, and 72 °C for 45 seconds. Genomic DNA samples (10–20 ng for spinal cord, 50 ng for brain, 25ng for liver, and 100–250 ng for gonad) were measured in triplicate on a BioRad CFX96 Real-Time System and compared to a standard curve generated from a plasmid containing WPRE (1 × 108–1 × 100 copies). The assay limit of detection (LOD) above background was 20 AAV copies/reaction. Background amplification was determined on DNA from untreated normal and untreated GM1 cats.
Magnetic resonance imaging (MRI)
Data were acquired on a 3 Tesla MAGNETOM Verio scanner (Siemens Healthcare, Malvern, PA, USA) using an 8 channel phased array wrist coil (InVivo Corp, Gainesville, FL, USA). Whole-brain anatomical images were acquired using 3D MP RAGE (magnetization-prepared rapid gradient echo) with 0.4 mm isotropic resolution and TR/TE of 1900/3.3 ms, followed by whole-brain multi-slice 2D axial T2 TSE (turbo-spin echo) images with TR/TE of 4630/107 ms, turbo factor of 9 and a resolution of (0.3×0.3×1) mm3. MRI data were analyzed using eFilm 3.2 software (Merge Healthcare, Chicago, IL).
Statistical Analysis
Data is expressed as mean (± standard deviation) throughout the text and figures. Statistics were performed using SAS 9.2 software and a P value ≤ 0.05 was considered significant for all comparisons. The Wilcoxon signed rank test was used for pairwise comparisons of lysosomal enzyme activity, vector distribution, and sialic acid levels. One-sided testing is reported to determine directional significance (i.e., significantly higher or lower), which would not be realized using two-sided testing due to low animal numbers when using a feline model. The logrank test was used for survival comparisons between groups. No data was excluded from analysis and, as outliers are hard to determine with small sample sizes, no data points in the study were defined as outliers.
Supplementary Material
Acknowledgments
Funding: Supported by the US National Institutes of Health (R01HD060576 to M.S.E and D.R.M.; F32NS080488 to H.G.E. and D.R.M.) and the Scott-Ritchey Research Center.
Footnotes
Author Contributions: The study was designed by M.S.E. and D.R.M. in consultation with H.J.B. and N.R.C. D.R.M. managed overall execution of the project. Production and husbandry of affected kittens were managed by A.K.J. with assistance from A.N.R and H.J.B. The anesthesia protocol for 2–3 month old cats was designed and implemented by J.A.J. with assistance from A.K.J. Vector was designed and produced by M.S.E., S.G.L. and S.M. using a feline βgal cDNA cloned by D.R.M. Surgeries were performed by A.K.J. and D.R.M. with assistance from A.N.R. Long-term clinical follow-up of treated cats was performed by A.K.J., A.N.R. and H.G.E. Video recordings were made by A.N.R. and V.J.M., who collated and analyzed video and clinical data for generation of clinical rating scores. N.E.M., M.H., N.R.C. and B.L.B. performed necropsy and histological analyses. Assays for enzymatic activity, storage material and vector quantitation were performed by V.J.M. with participation from N.E.M. and M.H. MRI analyses were performed by H.G.E., N.S., R.J.B. and T.S.D. Data was analyzed and the manuscript was written by V.J.M. and D.R.M. in consultation with M.S.E.
Competing Interests: The authors declare that they have no competing interests.
References and Notes
- 1.Okada S, O’Brien JS. Generalized gangliosidosis: beta-galactosidase deficiency. Science. 1968;160:1002–1004. doi: 10.1126/science.160.3831.1002. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki Y, Oshima A, Nanba E. Beta-galactosidase deficiency (beta-galactosidosis): GM1 gangliosidosis and Morquio B disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8 Vol. 3. McGraw-Hill; New York: 2001. chap. 151. [Google Scholar]
- 3.Leinekugel P, Michel S, Conzelmann E, Sandhoff K. Quantitative correlation between the residual activity of b-hexosaminidase A and arylsulfatase A and the severity of the resulting lysosomal storage disease. Hum Genet. 1992;88:513–523. doi: 10.1007/BF00219337. [DOI] [PubMed] [Google Scholar]
- 4.Conzelmann E, Kytzia H, Navon R, Sandhoff K. Ganglioside GM2 N-acetyl-beta-D-galactosaminidase activity in cultured fibroblasts of late-infantile and adult GM2 gangliosidosis patients and of healthy probands with low hexosaminidase level. Am J Hum Genet. 1983;35:900. [PMC free article] [PubMed] [Google Scholar]
- 5.Hickman S, Neufeld EF. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Comm. 1972;49:992. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- 6.Neufeld EF, Fratantoni JC. Inborn errors of mucopolysaccharide metabolism. Science. 1970;169:141. doi: 10.1126/science.169.3941.141. [DOI] [PubMed] [Google Scholar]
- 7.Taylor RM, Wolfe JH. Decreased lysosomal storage in the adult MPS VII mouse brain in the vicinity of grafts of retroviral vector-corrected fibroblasts secreting high levels of beta-glucuronidase. Nat Med. 1997;3:771–774. doi: 10.1038/nm0797-771. [DOI] [PubMed] [Google Scholar]
- 8.Broekman MLD, Tierney LA, Benn C, Chawla P, Cha JH, Sena-Esteves M. Mechanisms of distribution of mouse beta-galactosidase in the adult GM1-gangliosidosis brain. Gene Ther. 2009;16:303–308. doi: 10.1038/gt.2008.149. [DOI] [PubMed] [Google Scholar]
- 9.Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passini MA, Lee EB, Heuer GG, Wolfe JH. Distribution of a lysosomal enzyme in the adult brain by axonal transport and by cells of the rostral migratory stream. J Neurosci. 2002;22:6437–6446. doi: 10.1523/JNEUROSCI.22-15-06437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broekman MLD, Baek RC, Comer LA, Fernandez JL, Seyfried TN, Sena-Esteves M. Complete correction of enzymatic deficiency and neurochemistry in the GM1-gangliosidosis mouse brain by neonatal adeno-associated virus-mediated gene delivery. Mol Ther. 2007;15:30–37. doi: 10.1038/sj.mt.6300004. [DOI] [PubMed] [Google Scholar]
- 12.Gray SJ, Nagabhushan SK, McCown TJ, Samulski RJ. Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 2013;20:450–459. doi: 10.1038/gt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haurigot V, Marcó S, Ribera A, Garcia M, Ruzo A, Villacampa P, Ayuso E, Añor S, Andaluz A, Pineda M, García-Fructuoso G, Molas M, Maggioni L, Muñoz S, Motas S, Ruberte J, Mingozzi F, Pumarola M, Bosch F. Whole body correction of mucopolysaccharidosis IIIA by intracerebrospinal fluid gene therapy. J Clin Invest. 2013;123:3254–3271. doi: 10.1172/JCI66778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cachon-Gonzalez MB, Wang SZ, McNair R, Bradley J, Lunn D, Ziegler R, Cheng SH, Cox TM. Gene transfer corrects acute GM2 gangliosidosis--potential therapeutic contribution of perivascular enzyme flow. Mol Ther. 2012;20:1489–1500. doi: 10.1038/mt.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cachon-Gonzalez MB, Wang SZ, Lynch A, Ziegler R, Cheng SH, Cox TM. Effective gene therapy in an authentic model of Tay-Sachs-related diseases. Proc Natl Acad Sci U S A. 2006;103:10373–10378. doi: 10.1073/pnas.0603765103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodge JC, Clarke J, Song A, Bu J, Yang W, Taksir TV, Griffiths D, Zhao MA, Schuchman EH, Cheng SH, O’Riordan CR, Shihabuddin LS, Passini MA, Stewart GR. Gene transfer of human acid sphingomyelinase corrects neuropathology and motor deficits in a mouse model of Niemann-Pick type A disease. Proc Natl Acad Sci U S A. 2005;102:17822–17827. doi: 10.1073/pnas.0509062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baek RC, Broekman MLD, Leroy SG, Tierney LA, Sandberg MA, d’Azzo5 A, Seyfried TN, Sena-Esteves M. AAV-mediated gene delivery in adult GM1-gangliosidosis mice corrects lysosomal storage in CNS and improves survival. PLoS One. 2010;5:e13468. doi: 10.1371/journal.pone.0013468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker HJ, Lindsey JR, Jr, McKhann GM, Farrell DF. Neuronal GM1 gangliosidosis in a Siamese cat with beta-galactosidase deficiency. Science. 1971;174:838–839. doi: 10.1126/science.174.4011.838. [DOI] [PubMed] [Google Scholar]
- 19.Baker HJ, Lindsey JR. Animal model: feline GM1 gangliosidosis. Am J Pathol. 1974;74:649–652. [PMC free article] [PubMed] [Google Scholar]
- 20.Farrell DF, Baker HJ, Herndon RM, Lindsey JR, McKhann GM. Feline GM1 gangliosidosis: biochemical and ultrastructural comparisons with the disease in man. J Neuropathol Exp Neurol. 1973;32:1–18. [PubMed] [Google Scholar]
- 21.Baker HJ, Reynolds GD, Walkley SU, Cox NR, Baker GH. The Gangliosidoses: Comparative Features and Research Applications. Vet Pathol. 1979;16:635–649. doi: 10.1177/030098587901600602. [DOI] [PubMed] [Google Scholar]
- 22.Martin DR, Rigat BA, Foureman P, Varadarajan GS, Hwang M, Krum BK, Smith BF, Callahan JW, Mahuran DJ, Baker HJ. Molecular consequences of the pathogenic mutation in feline GM1 gangliosidosis. Mol Genet Metab. 2008;94:212–221. doi: 10.1016/j.ymgme.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caciotti A, Donati MA, Boneh A, d’Azzo A, Federico A, Parini R, Antuzzi D, Bardelli T, Nosi D, Kimonis V, Zammarchi E, Morrone A. Role of beta-galactosidase and elastin binding protein in lysosomal and nonlysosomal complexes of patients with GM1-gangliosidosis. Hum Mutat. 2005;25:285–292. doi: 10.1002/humu.20147. [DOI] [PubMed] [Google Scholar]
- 24.Georgiou T, Stylianidou G, Anastasiadou V, Caciotti A, Campos Y, Zammarchi E, Morrone A, D’Azzo A, Drousiotou A. The Arg482His mutation in the beta-galactosidase gene is responsible for a high frequency of GM1 gangliosidosis carriers in a Cypriot village. Genet Test. 2005;9:126–132. doi: 10.1089/gte.2005.9.126. [DOI] [PubMed] [Google Scholar]
- 25.Mosna G, Fattore S, Tubiello G, Brocca S, Trubia M, Gianazza E, Gatti R, Danesino C, Minelli A, Piantanida M. A homozygous missense arginine to histidine substitution at position 482 of the beta-galactosidase in an Italian infantile GM1-gangliosidosis patient. Hum Genet. 1992;90:247–250. doi: 10.1007/BF00220071. [DOI] [PubMed] [Google Scholar]
- 26.Kolter T, Proia RL, Sandhoff K. Combinatorial ganglioside biosynthesis. J Biol Chem. 2002;277:25859–25862. doi: 10.1074/jbc.R200001200. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki Y, Crocker AC, Suzuki K. GM1-gangliosidosis: Correlation of clinical and biochemical data. Arch Neurol. 1971;24:58. doi: 10.1001/archneur.1971.00480310086008. [DOI] [PubMed] [Google Scholar]
- 28.Bradbury AM, Cochran JN, McCurdy VJ, Johnson AK, Brunson BL, Gray-Edwards H, Leroy SG, Hwang M, Randle AN, Jackson LS, Morrison NE, Baek RC, Seyfried TN, Cheng SH, Cox NR, Baker HJ, Cachon-Gonzalez MB, Cox TM, Sena-Esteves M, Martin DR. Therapeutic response in feline Sandhoff disease despite immunity to intracranial gene therapy. Mol Ther. 2013;21:1306–1315. doi: 10.1038/mt.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Grandis E, Di Rocco M, Pessagno A, Veneselli E, Rossi A. MR imaging findings in 2 cases of late infantile GM1 gangliosidosis. AJNR Am J Neuroradiol. 2009;30:1325–1327. doi: 10.3174/ajnr.A1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arruda VR, Fields PA, Milner R, Wainwright L, De Miguel MP, Donovan PJ, Herzog RW, Nichols TC, Biegel JA, Razavi M, Dake M, Huff D, Flake AW, Couto L, Kay MA, High KA. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- 31.Favaro P, Downey HD, Zhou JS, Wright JF, Hauck B, Mingozzi F, High KA, Arruda VR. Host and vector-dependent effects on the risk of germline transmission of AAV vectors. Mol Ther. 2009;17:1022–1030. doi: 10.1038/mt.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 33.Ciron C, Desmaris N, PhD, Colle M, Raoul S, Joussemet B, Vérot L, Ausseil J, Froissart R, Roux F, Chérel Y, Ferry N, Lajat Y, Schwartz B, Vanier M, Maire I, Tardieu M, Moullier P, Heard J. Gene therapy of the brain in the dog model of Hurler’s syndrome. Ann Neurol. 2006;60:204–213. doi: 10.1002/ana.20870. [DOI] [PubMed] [Google Scholar]
- 34.Ellinwood NM, Ausseil J, Desmaris N, Bigou S, Liu S, Jens JK, Snella EM, Mohammed EEA, Thomson CB, Raoul S, Joussemet B, Roux F, Chérel Y, Lajat Y, Piraud M, Benchaouir R, Hermening S, Petry H, Froissart R, Tardieu M, Ciron C, Moullier P, Parkes J, Kline KL, Maire I, Vanier M, Heard J, Colle M. Safe, efficient, and reproducible gene therapy of the brain in the dog models of Sanfilippo and Hurler syndromes. Mol Ther. 2011;19:251–259. doi: 10.1038/mt.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vite CH, McGowan JC, Niogi SN, Passini MA, Drobatz KJ, Haskins ME, Wolfe JH. Effective gene therapy for an inherited CNS disease in a large animal model. Ann Neurol. 2005;57:355–364. doi: 10.1002/ana.20392. [DOI] [PubMed] [Google Scholar]
- 36.Smith BK, Collins SW, Conlon TJ, Mah CS, Lawson LA, Martin AD, Fuller DD, Cleaver BD, Clément N, Phillips D, Islam S, Dobjia N, Byrne BJ. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for dhronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gen Ther. 2013;24:630–640. doi: 10.1089/hum.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F, Campbell-Thompson M, Yachnis AT, Sandhaus RA, McElvaney NG, Mueller C, Messina LM, Wilson JM, Brantly M, Knop DR, Ye G, Chulay JD. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum Gen Ther. 2011;22:1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, Craenen JM, Lewis S, Malik V, Shilling C, Byrne BJ, Conlon T, Campbell KJ, Bremer WG, Viollet L, Walker CM, Sahenk Z, Clark KR. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaudet D, Méthot J, Déry S, Brisson D, Essiembre C, Tremblay G, Tremblay K, de Wal J, Twisk J, van den Bulk N, Sier-Ferreira V, van Deventer S. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20:361–369. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wierzbicki AS, Viljoen A. Alipogene tiparvovec: gene therapy for lipoprotein lipase deficiency. Expert Opin Biol Ther. 2013;13:7–10. doi: 10.1517/14712598.2013.738663. [DOI] [PubMed] [Google Scholar]
- 42.Pike LS, Tannous BA, Deliolanis NC, Hsich G, Morse D, Tung CH, Sena-Esteves M, Breakefield XO. Imaging gene delivery in a mouse model of congenital neuronal ceroid lipofuscinosis. Gene Ther. 2011;18:1173–1178. doi: 10.1038/gt.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen CY, Zimmerman RA, Fu-Hwa CL, Yuh Y, Hsiao H. Neuroimaging findings in late infantile GM1 gangliosidosis. AJNR Am J Neuroradiol. 1998;19:1628–1630. [PMC free article] [PubMed] [Google Scholar]
- 44.O’Brien JS, Ho MW, Veath ML, Wilson JF, Myers G, Opitz JM, Zurhein GM, Spranger JW, Hartmann HA, Haneberg B, Grosses FR. Juvenile GM1 gangliosidosis: clinical, pathological, chemical and enzymatic studies. Clin Genet. 1972;3:411–434. doi: 10.1111/j.1399-0004.1972.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 45.Jackson GD, Briellmann RS, Kuzniecky RI. Temporal lobe epilepsy. In: Kuzniecky RI, Jackson GD, editors. Magnetic resonance in epilepsy: neuroimaging techniques. Elsevier Academic Press; Burlington, MA: 2005. pp. 99–176. [Google Scholar]
- 46.Blair RDG. Temporal lobe epilepsy semiology. Epilepsy Res Treat. 2012;2012:1–10. doi: 10.1155/2012/751510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michelucci R, Pasini E, Nobile C. Lateral temporal lobe epilepsies: clinical and genetic features. Epilepsia. 2009;50:52–54. doi: 10.1111/j.1528-1167.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 48.Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, Dyke JP, Ballon D, Heier L, Greenwald BM, Christos P, Mazumdar M, Souweidane MM, Kaplitt MG, Crystal RG. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19:463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- 49.Leone P, Shera D, McPhee SW, Francis JS, Kolodny EH, Bilaniuk LT, Wang DJ, Assadi M, Goldfarb O, Goldman HW, Freese A, Young D, during MJ, Samilski RJ, Janson CG. Long-term follow-up after gene therapy for canavan disease. Sci Transl Med. 2012;4:163. doi: 10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broekman M, Comer L, Hyman B, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or-2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138:501–510. doi: 10.1016/j.neuroscience.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 51.Martin DR, Cox NR, Morrison NE, Kennamer DM, Peck SL, Dodson AN, Gentry AS, Griffin B, Rolsma MD, Baker HJ. Mutation of the GM2 activator protein in a feline model of GM2 gangliosidosis. Acta Neuropathol. 2005;110:443–450. doi: 10.1007/s00401-005-1040-6. [DOI] [PubMed] [Google Scholar]
- 52.Svennerholm L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957;24:604. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- 53.Miettinen T, Takki-Luukkainen I. Use of butyl acetate in determination of sialic acid. Acta Chem Scand. 1959;13:856–858. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.