Abstract
In contrast to kidney transplantation where donorspecific anti-HLA antibodies (DSA) negatively impact graft survival, correlation of DSA with clinical outcomes in patients after orthotopic liver transplantation (OLT) has not been clearly established. We hypothesized that DSA are present in patients who develop chronic rejection after OLT. Prospectively collected serial serum samples on 39 primary OLT patients with biopsy-proven chronic rejection and 39 comparator patients were blinded and analyzed for DSA using LABScreen single antigen beads test, where a 1000 mean fluorescence value was considered positive. In study patients, the median graft survival was 15 months, 74% received ≥ one retransplant, 20% remain alive and 87% had ≥ one episode of acute rejection. This is in contrast to comparator patients where 69% remain alive, and no patient needed retransplant or experienced rejection. Thirty-six chronic rejection patients (92%) and 24 (61%) comparator patients had DSA (p = 0.003). Chronic rejection versus comparator patients had higher mean fluorescence intensity (MFI) DSA. Although a further study with larger numbers of patients is needed to identify clinically significant thresholds, there is an association of high-MFI DSA with chronic rejection after OLT.
Keywords: Chronic allograft rejection, donor-specific antibodies, HLA antibodies, Liver Transplantation
Introduction
A positive crossmatch is usually considered a contraindication to all solid organ transplantation except orthotopic liver transplantation (OLT). This stems from early reports that found a positive crossmatch was unrelated to impaired graft or patient survival (1,2). However, in the 1980s graft loss rates were high and the technology for detecting anti- HLA antibodies was limited to the cytotoxic crossmatch and PRA. Since the early 1990s several groups have reported increased graft loss rates in OLT patients with a positive crossmatch compared to OLT patients with a negative crossmatch (3–7). Takaya and colleagues evaluated 600 OLT patients and reported a 29% 1-month graft loss rate in patients with a positive crossmatch compared to a 16% 1-month graft loss rate in patients with a negative crossmatch (p < 0.05) (5). Similarly, Ogura and colleagues reported a 47% 1-month graft loss rate in patients with a positive crossmatch, and a 12% 1-month graft loss rate in patients with a negative crossmatch (p = 0.001) (3).
Almost two decades later, Castillo-Rama and colleagues extended this finding in 896 consecutive OLTs using solidphase assay and Luminex technology which unambiguously demonstrated an increased graft loss rate in patients with preformed DSA (6). In addition, a separate analysis showed preformed class I donor-specific anti-HLA antibodies (DSA) markedly decreased graft survival after liver retransplantation (7).
The role of HLA antibodies that develop after liver transplantation has been poorly investigated. Kasahara and colleagues evaluated 58 patients for post-OLT DSA (8). Of the 12 patients who tested positive for antibodies by flow cytometry crossmatch, 100% experienced an acute rejection episode during the first month post-OLT, while only 17% of patients who tested negative had rejection (p < 0.001). This suggested that antidonor antibodies are associated with early acute rejection. In a short report published last year, Fontana and colleagues, utilizing the sensitive Luminex technology, found post-OLT anti-HLA antibodies in 24% of liver recipients (9). Of those, 75% had biliary complications when the allograft biopsy was analyzed, and 50% had biopsy-proven chronic rejection.
Despite the reports suggesting a link between donorspecific antibodies and acute rejection and graft survival, clinical practice has not changed. We continue to transplant patients with a positive crossmatch who have DSA. We believe that this occurs because antibody-mediated rejection has never been pathologically identified in OLT recipients. While antibody deposition in liver allografts with chronic rejection was described in 1987, sensitive solid phase PRA assays that utilize purified HLA class I and class II antigens were not available, and the PRA of peritransplant sera was found to be elevated in only 7 of 22 patients analyzed (10). Although intriguing, this did not prove an association between DSA and chronic rejection.
We hypothesized that antibody-mediated rejection in OLT patients is manifested histologically and clinically as chronic rejection.
Methods
Patients
In 1985, a Baylor Transplant biorepository was established to prospectively collect protocol serial serum samples from all donors and recipients of OLT. Patients are consented for their participation in this prospective collection at the time of OLT, with the understanding that the samples will be used for research purposes. Serum and lymphocytes are collected from the donor and recipient at OLT. In addition, samples are collected from the recipient at years 1, 2, 5, 10, 15 and 20. In parallel, we maintain a prospective research database, which contains clinical, demographic and event data on all OLT patients since the program’s inception. The patient’s cause of death is obtained from death certificates and hospital records, and reviewed by a physician before it is entered into the database. Institutional review board approval was granted prior to the initiation of this evaluation of prospectively gathered material and information.
Chronic rejection patients were chosen for analysis if they were >18 years of age, had undergone OLT without another organ transplant, had biopsy- proven chronic ductopenic rejection (defined as ≥50% of portal tracts with bile duct loss in the absence of recurrent primary sclerosing cholangitis, with or without foamy arteriopathy) (11) diagnosed by a hepatopathologist, did not have hepatitis C (HCV) or primary biliary cirrhosis, and had never experienced hepatic artery stenosis or thrombosis, portal vein thrombosis or any other known cause of graft ischemia post-OLT.
Comparator patients were chosen who had no history of rejection (acute or chronic) and were matched to chronic rejection patients for gender, year of transplant within 5 years (median 2), age within 5 years (median 3) and calcineurin inhibitor used at 1 month. This was achieved in all patients except six who were not able to be matched with a comparator patient within 5 years of age. The name ‘comparator’ was used instead of control as there were differences found between these patients and the chronic rejection patients. Although, HCV patients were excluded from the chronic rejection group, one HCV patient who achieved a sustained virologic response (virus eradication) pre-OLT was included as a comparator patient.
HLA tissue typing
All patients and donors were typed for HLA-A, -B, -Cw, -DR and -DQ using commercially available serologic typing trays or by molecular methods (Terasaki HLA Tissue Typing Trays and Micro SSP™ or LabType SSO, respectively; One Lambda Inc., Canoga Park, CA, USA). All typing was performed serologically prior to 1998. Since 1998, all donor class I and II HLA typing, as well as patient class II, has been performed by molecular methods; patient class I is still performed by serology.
Detection of anti-HLA antibodies
Samples from chronic rejection and comparator patients were blinded and sent to the Terasaki Foundation Laboratory for evaluation. The detection of HLA antibodies was performed using LABScreen Single Antigen class I (Lot 006) and class II (Lot 008) beads (One Lambda Inc.). The assay was performed according to the manufacturer’s protocol. Briefly, 20 μL of test serum (diluted 1:3 in 1× phosphate buffered saline) was incubated with the beads for 30 min at room temperature in the dark. Next, samples were washed three times and 100 μL of 1:100 phycoerythrin-conjugated goat antihuman IgG (One Lambda Inc.) was added. After a second incubation step in the dark, samples were washed twice and finally the samples were read on a Luminex platform (the LABscan 100 flow analyzer [One Lambda Inc.). Trimmed mean values were obtained from the output file generated by the flow analyzer and normalized using the formula: (sample #N bead– sample negative control (NC) bead)–(negative control serum #N bead— negative control serum NC bead).
Determination of DSA specificities
To identify the DSA specificities, the donor-recipient mismatched HLAs were compared to the antibody profile for each patient’s sample, and a normalized value over 1000 MFI was defined as positive.
Statistical analysis
Patient characteristics were compared using chi-squared and Wilcoxon rank sum tests, and median values are reported in tables when appropriate. Graft survival was evaluated by Kaplan–Meier analysis. Patient survival was markedly impacted by retransplantation in the majority of chronic rejection patients and therefore was not evaluated in this analysis. Statistical significance was defined as a p<0.05. SAS 9.1 was used for all statistical analyses.
Results
Table 1 displays the characteristics of chronic rejection and comparator patients. Most patients in both groups were young white men with a low-calculated model for the end-stage liver disease (MELD) score who received a young donor, and had a short cold ischemia time. Although not statistically significant, the comparator group had a higher percentage of Caucasians than the chronic rejection group. Chronic rejection and comparator patients were matched based on CNI used at 1 month; cyclosporine was more commonly used as the CNI in both groups. The use of chronic steroid therapy and antimetabolite medications was similar in both groups. Cytomegalovirus infection was more commonly seen in chronic rejection than comparator patients (p = 0.02). Only 35% of patients wereable to be matched for primary liver disease; however, the overall distribution of liver diseases was similar between the two groups (p = NS). There are differences between the two groups because comparator patients were chosen for their prolonged graft survival and absence of rejection. Chronic rejection patients had a 15-month median graft survival, and comparator patients had a 159-month median graft survival (p < 0.001). Most chronic rejection patients had at least one episode of biopsy-proven acute cellular rejection, and 44% had at least one episode of biopsy-proven steroid resistant rejection. Only 20% of chronic rejection patients remain alive and 74% have been retransplanted at least once. Sixty-nine percent of comparator patients remain alive. All comparator patients either remain alive or died with a functioning graft and never experienced any type of rejection.
Table 1.
Patient demographics
| Chronic rejection (N = 39) | Comparator (N = 39) | p-Value | |
|---|---|---|---|
| Age1 | 43 | 45 | NS |
| Male sex | 70% | 69% | NS |
| Race | |||
| White | 79% | 92% | NS |
| Hispanic | 5% | 3% | |
| AA | 15% | 3% | |
| Other | 1% | 3% | |
| Donor age1 | 29 | 20 | NS |
| MELD at OLT1 | 17 | 17 | NS |
| Cold ischemia time1 | 7.8 | 9.3 | NS |
| Year OLT1 | 1991 | 1994 | NS |
| Month 1 immunosuppression | |||
| Tacrolimus | 28% | 28% | NS |
| Cyclosporine | 72% | 72% | |
| Steroids | 94% | 92% | NS |
| MMF | 17% | 5% | NS |
| Azathioprine | 25% | 26% | |
| Cytomegalovirus | 49% | 21% | p = 0.02 |
| Primary liver disease | |||
| PSC | 14 | 10 | NS |
| Cryptogenic | 7 | 6 | |
| Alcoholic liver disease | 5 | 9 | |
| Acute liver failure | 5 | 1 | |
| Autoimmune hepatitis | 1 | 0 | |
| Metabolic/congenital | 4 | 4 | |
| HBV/SVR HCV | 1 | 5 | |
| Other | 2 | 4 | |
| Graft survival (months)1 | 15 | 159 | p < 0.001 |
| Alive | 20% | 69% | p < 0.001 |
| Re-OLT | 74% | 0 | p < 0.001 |
| Acute cellular rejection | |||
| 0 | 13% | 100% | p < 0.001 |
| 1 | 18% | ||
| 2 | 15% | ||
| ≥3 | 54% | ||
| Steroid resistant rejection | |||
| 0 | 56% | 100% | p < 0.001 |
| ≥1 | 44% | 0% | |
Median values.
AA, African American; MELD, model for end-stage liver disease; OLT, orthotopic liver transplant; PSC, primary sclerosing cholangitis; MMF, mycophenolate mofetil; HBV, hepatitis B; SVR HCV, sustained virologic response to hepatitis C (patients were only included if this occurred pre-OLT).
Nearly all chronic rejection patients had DSA (92% vs. 61% for comparator patients p = 0.003) (Table 2). Preformed DSA were present in 60% of chronic rejection patients and only 41% of comparator patients (p = NS). De novo DSA occurred post-OLT in 62% of chronic rejection patients versus 38% of comparator patients (p = 0.047); however, this occurred in 44% of chronic rejection patients within the first year post-OLT versus 13% of comparator patients (p = 0.004). When pre- and post-OLT DSA in chronic rejection patients are evaluated together, 10% had class I DSA only, 38% had class II DSA only, and 44% had both class I and II DSAs present. In comparator patients 5% had class I DSA only, 33% had class II DSA only, and 23% had both class I and II DSAs present (p = 0.008).
Table 2.
Anti-HLA donor-specific antibodies
| Chronic rejection (N=39) | Comparator (N=39) | p-Value | |
|---|---|---|---|
| Any DSA ever | 92% | 61% | p = 0.003 |
| Preformed | 60%* | 41% | NS |
| De novo | 62%** | 38% | p =0.047 |
| De novo <1 year | 44%** | 13% | p =0.004 |
| DSA post-OLT | 79%** | 56% | p =0.047 |
| Any DSA ever | |||
| Class I | 10% | 5% | p =0.008 |
| Class II | 38% | 33% | |
| Class I and II | 44% | 23% | |
| None | 8% | 39% | |
Number of patients analyzed was 39 except
N = 35,
N = 34.
OLT, orthotopic liver transplant.
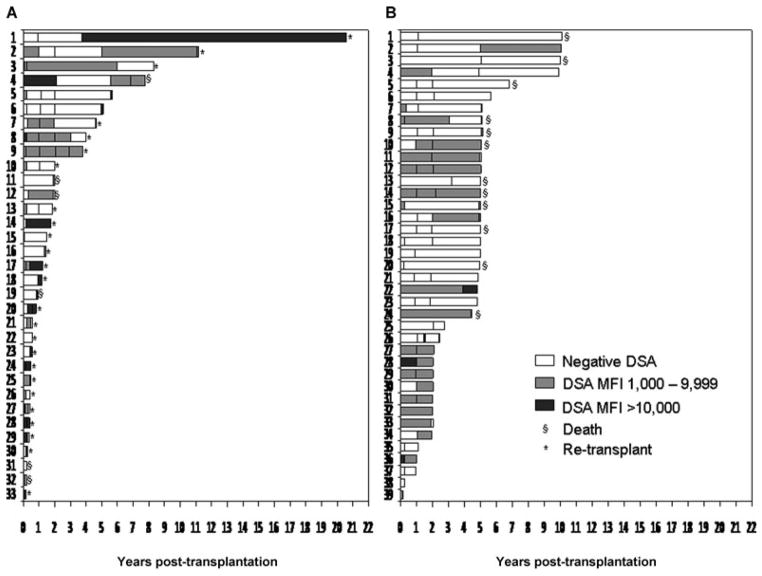
Figure 1A shows the antibody profile and the follow-up period for the chronic rejection patients. Six patients from this group were not included because they only had one serum sample analyzed. Every patient is represented by a bar and the DSA status is represented by different color filling. Figure 1B shows the antibody profile in the comparator group. More patients in the chronic rejection group had high MFI DSA than in the comparator group, evidenced by the dark gray bars. Fifty-two percent of chronic rejection patients had DSA MFI >10 000 in at least one sample either pre- or post-OLT in contrast to only 13% of comparator patients (p < 0.001).
Figure 1.
Antibody profile of the (A) chronic rejection group and (B) comparator group. Each patient is represented by a bar and the DSA status is represented by different colors. (Six patients in the chronic rejection group are not depicted because only one sample was available for analysis.) All patients in the comparator group who died, dies >5 years after transplant with a normally functioning graft.
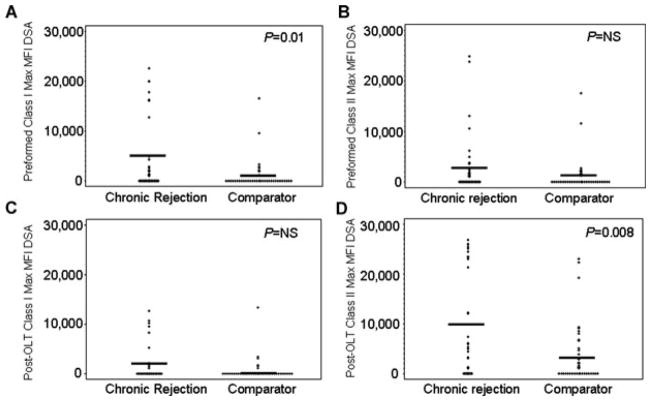
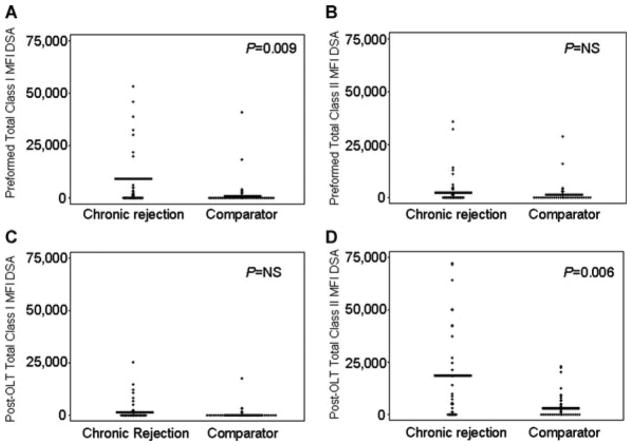
Figure 2 graphically depicts the maximum MFI present for each patient (represented as a +) and the mean value for the group (represented as a line) for chronic rejection versus comparator patients: (a) preformed class I (mean MFI 4075 vs. 1027; p = 0.01), (b) preformed class II (mean MFI 2849 vs. 1209; p = NS), (c) post-OLT class I (mean MFI 1858 vs. 600; p = NS), and (d) post-OLT class II (mean MFI 9930 vs. 3637; p = 0.008). Post-OLT DSA in Figure 2C and D represent both preformed persistent and de novo DSA. Since many chronic rejection patients had more than one anti-HLA DSA, we assessed the ‘total MFI’ which was calculated by adding the maximum MFI of each distinct DSA together for each patient. The total exposure to DSA could not be calculated accurately as the samples were not collected close enough together to know precisely when patients developed or lost DSA. Figure 3 graphically depicts the ‘total MFI’ present for each patient (represented as a +) and the mean value for the group (represented as a line) for chronic rejection versus comparator patients: (a) preformed class I (mean MFI 7381 vs. 1941; p = 0.009), (b) preformed class II (mean MFI 3782 vs. 1813; p = NS), (c) post-OLT class I (mean MFI 2610 vs. 728; p = NS) and (d) post-OLT class II (mean MFI 17 363 vs. 3840; p = 0.006).
Figure 2.
Highest mean fluorescent intensity (MFI) donor-specific antibody (DSA) for (A) preformed class I (mean MFI 4075 vs. 1027), (B) preformed class II (mean MFI 2849 vs. 1209), (C) post-OLT class I (mean MFI 1858 vs. 600) and (D) post-OLT class II (mean MFI 9930 vs. 3637). + represents each patient’s maximum value, line represents the group’s mean value.
Figure 3.
Total mean fluorescent intensity (MFI) of donor-specific antibodies (DSA) = the addition of each individual DSA per patient. (A) Preformed total class I (mean MFI 7381 vs. 1942), (B) preformed total class II (mean MFI 3782 vs. 1813), (C) post-OLT total class I (mean MFI 2610 vs. 728) and (D) post-OLT total class II (mean MFI 17 353 vs. 3839). + represents each patient’s maximum value, line represents the group’s mean value.
Since the MFI of DSA appears to be relevant, we separately assessed graft survival in patients with DSA MFI > 1000 and MFI >5000. In addition, graft survival in all the figures was always calculated after the first positive DSA to eliminate lead-time bias. Figure 4 demonstrates the poor graft survival of patients with DSA whose MFI is >1000 regardless of whether the DSA is preformed or de novo. However, when looking at patients with high MFI (>5000) DSA there was a nonsignificant trend toward a worse outcome in those with preformed antibody. In addition, only 8% of comparator patients had preformed DSA with MFI > 5000, compared to 30% of chronic rejection patients (p = 0.04).
Figure 4.
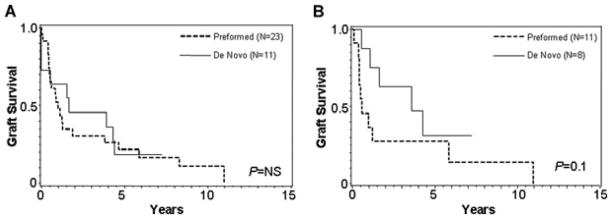
Graft survival after the first positive DSA in patients with preformed versus de novo DSA (A) when positive is MFI > 1000 and (B) when positive is MFI > 5000.
Figure 5 depicts graft survival based on the class of anti-body present, regardless of whether they are preformed or de novo. When an MFI cutoff of 1000 is used, there is no difference in graft survival based on the class of DSA; however when an MFI cutoff of 5000 is used, high MFI class I with or without class II appears to have inferior graft outcomes compared to isolated class II DSA.
Figure 5.
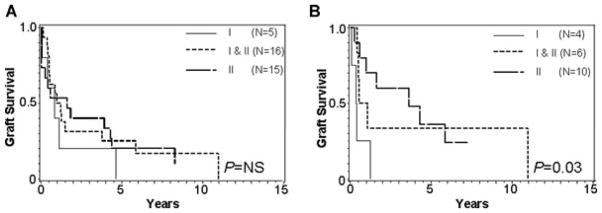
Graft survival after the first positive DSA in patients with class I, class II, and class I and II DSA (A) when positive is MFI > 1000 and (B) when positive is MFI > 5000.
Of greatest interest would be a means to eliminate DSA. Since induction with antilymphocyte antibodies is sometimes employed during the initiation of immunosuppressive therapy and might reduce DSA, we compared those recipients who did or did not receive such induction. Figure 6 shows that graft survival is markedly improved when induction therapy with either daclizumab or OKT3 was used. In fact, 5/5 class I preformed antibodies were eliminated in patients who received antibody induction therapy and had a follow-up sample for analysis. Of note, only 1/5 preformed class II antibodies were eliminated with induction therapy.
Figure 6.
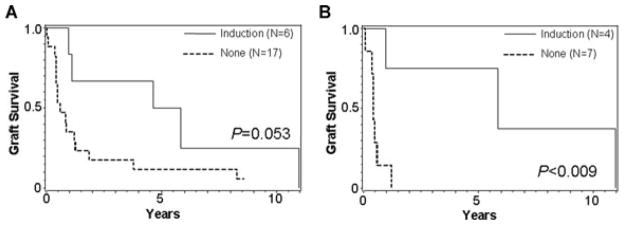
Graft survival after the first positive DSA in the presence and absence of induction with daclizumab or OKT3 (A) when positive is MFI > 1000 and (B) when positive is MFI > 5000.
Discussion
The present study demonstrates, for the first time, the association of DSA and chronic rejection after liver transplantation. We found DSA to be present in nearly all (92%) patients with chronic rejection, and in a lower percentage of comparator patients (61%). We found this level of DSA in comparator patients surprising. The higher than expected preformed DSA likely reflects our practice of using blood products pretransplant for volume expansion. The higher than expected de novo DSA post-OLT may either result from this same practice in the perioperative period or from the antigenic stimulus provided by the graft. Fortunately, it allowed us to better analyze the differences between patients with DSA with and without graft dysfunction.
Preformed DSA impair graft survival, and therefore are usually considered a contraindication to all solid organ transplants other than the liver. However, even in renal transplant, where no debate exists about the importance of DSA, the clinically significant threshold for proceeding with an individual transplant remains debatable (12–14). In the last two decades, reports demonstrated an association between preformed antibodies and early graft loss after liver transplantation (3–7). On the other hand, detection of anti-HLA antibodies in the post-transplant period has been associated with poorer outcomes in other solid organ transplants, but their role after liver transplantation remains unclear.
As a first step toward understanding DSA’s role in OLT graft failure, we developed a highly selected cohort of patients to eliminate possible confounders such as HCV recurrence, primary biliary cirrhosis recurrence, or vascular problems that can cause ductopenia and be mistakenly diagnosed as chronic rejection.
Many significant differences existed between our cohort of chronic rejection and comparator patients. There were a larger percent of chronic rejection patients with DSA ever, de novo DSA (both early [<1 year] and late), and post-OLT DSA than comparator patients. However the largest differences were seen when we compared the mean MFI between the groups. These differences were amplified when ‘total MFI’ (defined as the sum of distinct DSA) were analyzed. Both analyses supported that preformed class I antibodies in high MFI seem to be more detrimental to graft outcome than preformed class II antibodies. It has been repeatedly documented that OLT is often able to be performed successfully across a positive crossmatch indicating that not all DSA induce graft failure; however it has also been well documented that a positive crossmatch decreases patient and graft survival (3–7). Our data support these previously well-established findings. Ultimately, liver allografts are likely able to accommodate preformed DSA in low MFI, and only when they reach higher MFI either alone or more likely in aggregate does graft dysfunction in the form of chronic rejection occur.
Although preformed class I DSA seem more detrimental to early graft function, preformed persistent and de novo class II DSA were more prevalent and found in higher MFI in patients with chronic rejection post-OLT. This may indicate that class II DSA are only clinically significant in very high MFI, cause slower graft dysfunction, or may only become relevant when up regulation of class II HLA occurs in the allograft after release of proinflammatory cytokines. Moreover, whereas the level of detectable class I DSA in the serum of combined liver kidney transplant recipients is a function of how much antibody is absorbed by the liver parenchyma, allograft absorption is less likely a factor for class II DSA because of the more limited amount of class II antigen expression (15). This postulate may also help explain why it appears as if class I DSA is less of a factor post-transplant than pretransplant in our study. Further study will be needed to make these determinations.
The time that DSA are present may also play a role in pathogenesis. We found de novo DSA within the first year more commonly occurred in patients with chronic rejection than in comparator patients (44% vs. 13%). This phenomenon has previously been shown in renal allografts and may also be of importance in liver allograft antibody-mediated dys-function (16). DSA may be better accommodated once the endothelium, bile ducts, and even hepatocytes of the graft have achieved either chimerism or replacement with recipient cells (17).
In addition, we cannot rule out the possibility that both the timing and duration of exposure to DSA play a significant role in the pathologic progression of chronic rejection; however, frequently collected serial samples would be needed to determine the precise time of DSA exposure and these were not available. Therefore, further large scale studies are needed to identify clinically significant threshold values that lead to post-transplant graft dysfunction, and likely these values will be higher in liver allografts than other solid organ transplants.
In addition to threshold MFI values, there may be specific antibody characteristics that play a role in their ability to induce graft dysfunction, such as: affinity of binding; ability to activate complement; and ability to mediate antibody- dependent cellular cytotoxicity, opsonization, and other Fc- gamma receptor dependent activities. We did not analyze such antibody characteristics in this study.
Recent data suggest that preformed DSA can cause a pathologic injury to the graft that is often read as ‘ischemic’ or ‘reperfusion’ injury (18,19). Many patients are able to recover from this acute injury, just as they recover from other immunologic insults such as acute cellular rejection. Although our work has started the process, ultimately we need to identify patients with antibody levels and characteristics that lead to poor outcome. Once this is accomplished we can either alter allocation of donors or devise immunosuppressive regimens to treat patients with pathologic DSA. One immunosuppression factor that may play a role in mitigating the effect of preformed DSA is induction antibody therapy. While based on a small number of cases, our findings are intriguing enough to warrant speculation. In nonpathological conditions, class I HLA molecules are expressed by sinusoidal endothelial cells, hepatocytes and biliary epithelial cells. Conversely, class II HLA molecules are only expressed on endothelial cells. If preformed antibodies are to be absorbed by the allograft after transplantation, it is more likely that class I would show a more dramatic change compared to class II antibodies. In addition, the elimination of the T cells (and potentially other cell lineages) by induction therapy agents in the presence of antigenic B-cell stimulation by the liver may constitute a ‘signal one in the absence of signal two’ situation, resulting in high-dose B-cell tolerance (20). This occurring in 5/5 class I preformed DSA but only 1/5 preformed class II DSA is consistent with the reports that class II antibodies are less susceptible to desensitization (21,22).
Indeed, Moonka and colleagues in surveying the UNOS database found improved early graft survival in patients who received induction antibody therapy regardless of their HCV status (23). Given our results, it is interesting to hypothesize that this may be the mechanism of improved graft survival seen by Moonka et al. This warrants a larger scale investigation as the small number of patients with preformed DSA, who received induction therapy in our study limits our ability to make conclusions.
In summary, our study shows that 92% of patients who developed chronic rejection had detectable DSA before chronic rejection induced graft failure compared to 61% of patients who did not have chronic rejection. We also found that patients who developed chronic rejection had significantly higher isolated and additive MFI values of DSA compared to the comparator group. At this time further study is needed to confirm our findings that DSA are associated with chronic rejection, to identify threshold levels and essential characteristics of DSA immunoglobulins that cause graft dysfunction, and to investigate the role of induction antibody therapy as a possible mitigating force. This will need to be done prospectively to identify the predictive value of class I and II preformed and de novo DSA.
Abbreviations
- DSA
donor-specific anti-HLA antibodies
- HCV
hepatitis C
- MFI
mean fluoresce intensity
- MELD
model for end-stage liver disease
- OLT
orthotopic liver transplantation
Footnotes
Personal Financial Interests:
P.I.T.—One Lambda Incorporated Chairman and major share holder. H.K. was a consultant for One Lambda. All the other authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Disclosure
Research Funds: Antibody analysis funded by the Terasaki Foundation Laboratory.
References
- 1.Gordon RD, Fung JJ, Markus B, et al. The antibody crossmatch in liver transplantation. Surgery. 1986;100:705–715. [PMC free article] [PubMed] [Google Scholar]
- 2.Moore SB, Wiesner RH, Perkins JD, Nagorney DM, Sterioff S, Krom RA. A positive lymphocyte cross-match and major histo-compatibility complex mismatching do not predict early rejection of liver transplants in patients treated with cyclosporine. Transplant Proc. 1987;19(Pt 3):2390–2391. [PubMed] [Google Scholar]
- 3.Ogura K, Koyama H, Takemoto S, Terasaki PI, Busuttil RW. Significance of a positive crossmatch on outcome in human liver transplantation. Transplant Proc. 1992;24:1465. [PubMed] [Google Scholar]
- 4.Ogura K, Terasaki PI, Koyama H, Chia J, Imagawa DK, Busuttil RW. High one-month liver graft failure rates in flow cytometry crossmatch-positive recipients. Clin Transplant. 1994;8(Pt1):111–115. [PubMed] [Google Scholar]
- 5.Takaya S, Duquesnoy R, Iwaki Y, et al. Positive crossmatch in primary human liver allografts under cyclosporine or FK 506 therapy. Transplant Proc. 1991;23(Pt 1):396–399. [PMC free article] [PubMed] [Google Scholar]
- 6.Castillo-Rama M, Castro MJ, Bernardo I, et al. Preformed antibodies detected by cytotoxic assay or multibead array decrease liver allograft survival: Role of human leukocyte antigen compatibility. Liver Transpl. 2008;14:554–562. doi: 10.1002/lt.21408. [DOI] [PubMed] [Google Scholar]
- 7.Goh A, Scalamogna M, De Feo T, Poli F, Terasaki PI. Human leukocyte antigen crossmatch testing is important for liver retransplantation. Liver Transpl. 2010;16:308–313. doi: 10.1002/lt.21981. [DOI] [PubMed] [Google Scholar]
- 8.Kasahara M, Kiuchi T, Takakura K, et al. Postoperative flow cytometry crossmatch in living donor liver transplantation: Clinical significance of humoral immunity in acute rejection. Transplantation. 1999;67:568–575. doi: 10.1097/00007890-199902270-00014. [DOI] [PubMed] [Google Scholar]
- 9.Fontana M, Moradpour D, Aubert V, Pantaleo G, Pascual M. Prevalence of anti-HLA antibodies after liver transplantation. Transpl Int. 2010;23:858–859. doi: 10.1111/j.1432-2277.2009.01022.x. [DOI] [PubMed] [Google Scholar]
- 10.Demetris AJ, Markus BH, Burnham J, et al. Antibody deposition in liver allografts with chronic rejection. Transplant Proc. 1987;19 (Suppl 5):121–125. [PubMed] [Google Scholar]
- 11.Demetris A, Adams D, Bellamy C, et al. Update of the international banff schema for liver allograft rejection: Working recommendations for the histopathologic staging and reporting of chronic rejection. An Int Panel Hepatol. 2000;31:792–799. doi: 10.1002/hep.510310337. [DOI] [PubMed] [Google Scholar]
- 12.Mizutani K, Terasaki P, Hamdani E, et al. The importance of anti- HLA-specific antibody strength in monitoring kidney transplant patients. Am J Transplant. 2007;7:1027–1031. doi: 10.1111/j.1600-6143.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 13.Batal I, Zeevi A, Lunz JG, 3rd, et al. Antihuman leukocyte antigen-specific antibody strength determined by complement- dependent or solid-phase assays can predict positive donor-specific crossmatches. Arch Pathol Lab Med. 2010;134:1534–1540. doi: 10.5858/2009-0581-OA.1. [DOI] [PubMed] [Google Scholar]
- 14.Singh N, Djamali A, Lorentzen D, et al. Pretransplant donor-specific antibodies detected by single-antigen bead flow cytometry are associated with inferior kidney transplant. Outcomes Transplant. 2010;90:1079–1084. doi: 10.1097/TP.0b013e3181f6a07b. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal A, Dar W, Watkins C, et al. Donor-directed MHC class I antibody is preferentially cleared in sensitized recipients of combined liver/kidney transplants. Am J Transplant. 2011;11:841–847. doi: 10.1111/j.1600-6143.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee PC, Zhu L, Terasaki PI, Everly MJ. HLA-specific antibodies developed in the first year posttransplant are predictive o chronic rejection and renal graft loss. Transplantation. 2009;88:568–574. doi: 10.1097/TP.0b013e3181b11b72. [DOI] [PubMed] [Google Scholar]
- 17.Hove WR, van Hoek B, Bajema IM, Ringers J, van Krieken JH, Lagaaij EL. Extensive chimerism in liver transplants: Vascular endothelium, bile duct epithelium, and hepatocytes. Liver Transpl. 2003;9:552–556. doi: 10.1053/jlts.2003.50116. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz R, Tomiyama K, Chinnakotla S, et al. The positive crossmatch in liver transplantation: A risk actor for preservation injury?. XXIII Int Cong Transplant Soc; 2010. p. Abstract 060.07. [Google Scholar]
- 19.Kozlowski T, Rubinas T, Nickeleit V, et al. Liver allograft antibody- mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011:17. doi: 10.1002/lt.22233. [DOI] [PubMed] [Google Scholar]
- 20.Cruse J, Lewis R, Wang H. Immunology guidebook. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 21.Zachary AA, Montgomery RA, Leffell MS. Factors associated with and predictive of persistence of donor-specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum Immunol. 2005;66:364–370. doi: 10.1016/j.humimm.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Zachary AA, Leffell MS. Detecting and monitoring human leukocyte antigen-specific antibodies. Hum Immunol. 2008;69:591–604. doi: 10.1016/j.humimm.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Moonka DK, Kim D, Kapke A, Brown KA, Yoshida A. The influence of induction therapy on graft and patient survival in patients with and without hepatitis C after liver transplantation. Am J Transplant. 2010;10:590–601. doi: 10.1111/j.1600-6143.2009.02880.x. [DOI] [PubMed] [Google Scholar]