Abstract
Background
Major depressive disorder (MDD) is a leading cause of disability worldwide and occurs commonly first during adolescence. The insular cortex (IC) plays an important role in integrating emotion processing with interoception and has been implicated recently in the pathophysiology of adult and adolescent MDD. However, no studies have yet specifically examined the IC in adolescent MDD during processing of faces in the sad- happy continuum. Thus, the aim of the present study is to investigate the IC during sad and happy face processing in adolescents with MDD compared to healthy controls (HCL).
Methods
Thirty-one adolescents (22 female) with MDD and 36 (23 female) HCL underwent a well-validated emotional processing fMRI paradigm that included sad and happy face stimuli.
Results
The MDD group showed significantly less differential activation of the anterior/middle insular cortex (AMIC) in response to sad versus happy faces compared to the HCL group. AMIC also showed greater functional connectivity with right fusiform gyrus, left middle frontal gyrus, and right amygdala/parahippocampal gyrus in the MDD compared to HCL group. Moreover, differential activation to sad and happy faces in AMIC correlated negatively with depression severity within the MDD group.
Limitations
Small age-range and cross-sectional nature precluded assessment of development of the AMIC in adolescent depression.
Conclusions
Given the role of the IC in integrating bodily stimuli with conscious cognitive and emotional processes, our findings of aberrant AMIC function in adolescent MDD provide a neuroscientific rationale for targeting the AMIC in the development of new treatment modalities.
Keywords: adolescent major depressive disorder, anterior insular cortex, functional magnetic resonance imaging, functional connectivity
Introduction
Major depressive disorder (MDD) is considered the fourth leading global cause of disease burden (Ustun et al., 2004) and it is the leading cause of disability among Americans aged 15 to 44, with about 11 percent of U.S. adolescents having a depressive disorder by age 18 (Merikangas et al., 2010). Adolescent-onset depression is related to more severe depressive symptoms and an elevated risk of future recurrent episodes (Berndt et al., 2000; Hollon et al., 2006; Zisook et al., 2007), compared to adult onset of MDD. Elucidating the neurobiological mechanisms of emotional processing early in the disease course may help us more precisely target future treatment of adolescent depression and thereby more efficiently reduce prevalence of and decrease the propensity for recurrent depressive episodes.
Prior studies examining the neurobiological mechanisms of MDD have demonstrated that using emotional face tasks in conjunction with fMRI reliably activates the neural circuitry implicated in MDD (Fusar-Poli et al., 2009; Joormann and Gotlib, 2006; Mayberg, 1997; Phillips et al., 2003; Stuhrmann et al., 2011). Differences at multiple levels in the processing of emotional faces have been shown between MDD and healthy controls: 1. sensory occipital areas such as the fusiform gyrus and the superior temporal sulcus, 2. a more extended system supporting the interpretation of facial information, including the amygdala, insula and orbitofrontal areas and 3. prefrontal areas engaged in cognitive control (Drevets et al., 2008; Haxby et al., 2000; Mayberg, 1997; Price and Drevets, 2012; Stuhrmann et al., 2011). The literature also supports the notion that biases in the processing and interpretation of human facial emotional expressions as social cues may be one of the underlying mechanisms of MDD (Gotlib et al., 2004; Joormann and Gotlib, 2006; Joormann et al., 2007; Kujawa et al., 2011). These biases (detailed below) may contribute to the development, maintenance, and relapse of depressive symptoms (Foland-Ross and Gotlib, 2012; Lai, 2014; Mathews and MacLeod, 2005; Stuhrmann et al., 2011). Importantly, depressed individuals do not exhibit impaired performance on emotion identification, but rather show a specific bias to sad (tending to identify neutral faces as sad) and away from happy faces (tending to identify happy faces as neutral) (Arce et al., 2009; Joormann and Gotlib, 2006). Similarly, depressed adolescents do not show deficits in the ability to accurately recognize sad versus happy facial expressions, but a bias towards perceiving low-intensity expressions as sad (Schepman et al., 2012). Whether this specific depression-related bias in interpreting sad and happy facial expressions is due to dysfunctions in sensory regions, regions involved in interpretation of facial expressions and/or cognitive control remains unclear.
As previously mentioned the insular cortex is a brain area involved in the interpretation of facial information and it ahs been highlighted as an integrative hub between the sensory, interpretive and cognitive regions involved in emotional processing (Avery et al., 2013; Manoliu et al., 2013; Sprengelmeyer et al., 2011), Craig and others (Craig, 2009, 2011) and others (Chang et al., 2013; Menon and Uddin, 2010) have suggested that the insular cortex utilizes sensory information from ascending homeostatic afferents together with contextual input from other cortical brain areas to form a conscious “feeling” or an emotional moment in time (Craig, 2011). The posterior part receives information about the physiological state of the body, which is the integrated with information from higher order sensory cortices, as well as from the amygdala and the anterior cingulate cortex (ACC) in the middle and anterior part of the insular cortex where emotional awareness, self-recognition and other functions are represented (Craig, 2010; Nieuwenhuys, 2012).
Thus, it should not be surprising that the insular cortex is implicated in the pathophysiology of depression and may be dysfunctional in individuals with affective disorders. For example, structural differences of the anterior insular cortex have been shown in adult MDD compared to healthy controls (Bora et al., 2012; Peng et al., 2011; Soriano-Mas et al., 2011; Sprengelmeyer et al., 2011; Stratmann et al., 2014; Takahashi et al., 2010) and task-based fMRI studies have shown both increased (Hamilton et al., 2012; Sliz and Hayley, 2012) and decreased activation (Townsend et al., 2010) of the anterior insular cortex in adults with MDD compared to healthy controls.
While neuroimaging in adult depression suggests a road map to understanding depressive symptoms in the developing brain, direct translation of the adult findings of differences in insular cortex activation as characteristics of adolescent depressive symptoms may be obscured by the major re-organization of the brain occurring from puberty to early adulthood (Gogtay et al., 2004). Indeed, developmental differences account for much of the variation in fMRI activation patterns between non-depressed adults and adolescents during emotion processing (Cho et al., 2012). Furthermore, cortical thickness of distinct brain regions develops differently depending on their organization and function (Huttenlocher and Dabholkar, 1997; Shaw et al., 2008). Specifically, changes in the composition of the insular cortex during adolescence correspond to improved emotional regulatory skills and refinement of functioning such as improved impulse-control (Sisk and Zehr, 2005; Steinberg et al., 2009). Paradoxically, the maturation process of the neural circuits involved in emotional processing (Giedd et al., 2006; Kelly et al., 2009; Romeo and McEwen, 2006) may contribute to increased vulnerability to depression during adolescence (Weir et al., 2012). It is also possible that depressive illness during this specific developmental period alters neural pruning, which normally supports the acquisition of emotion regulatory skills (Weir et al., 2012). Both scenarios may result in decreased ability to integrate information about bodily states with higher-order cognitive and emotional processes in depressed adolescents, which may explain the depressive symptomatology, the recurrent course, and long-term functional disability related to this disorder.
There is accumulating evidence that links insular cortex dysfunction to adolescent MDD, including aberrant patterns of cerebral perfusion (Ho et al., 2013), and activation during emotional face fMRI paradigms (Ho et al., 2014b; Yang et al., 2010) and altered functional connectivity (FC) during resting state (Connolly et al., 2013; Cullen et al., 2009) as well as during emotional processing (Ho et al., 2014a; Perlman et al., 2012). However, to our knowledge, no fMRI study to date has examined the insular cortex activation and FC during the processing of sad versus happy facial stimuli in adolescent depression. This would contribute to our understanding of the role of the insular cortex specifically in relation to how aberrant integration of sensory and cognitive processing support the emotional biases in adolescent depression.
Thus, the aim of the present study is to investigate the insular cortex and its related circuitry during sad and happy face processing in adolescents with MDD compared to well-matched healthy controls (HCL). We used an emotional face processing task that has been well-validated in healthy, depressed, anxious, and at-risk adolescents (Beesdo et al., 2009; Monk et al., 2008; Nelson et al., 2003; Pine et al., 2004; Roberson-Nay et al., 2006), which we adapted by substituting sad for angry faces. First, based on the reviewed literature, we hypothesized that the insular cortex, especially the anterior part would show aberrant activation in MDD in response to sad versus happy faces compared to HCL corresponding to decreased ability to integrate information about bodily states with cognitive and emotional processes, (i.e., to process and differentiate sad and happy emotions). Second, based on previous studies examining face processing in MDD (Stuhrmann et al., 2011), we theorized that FC between the insular cortex and other brain areas involved in the different stages of facial emotion processing, including the fusiform gyrus, limbic structures, and frontal regions, would be altered. We also hypothesized that insular cortex activation and FC would correlate with depression symptom severity.
Methods
Participants
We recruited a total of 81 participants: 43 HCL and 38 with MDD. One participant from each group was dropped due to missing behavioral data and a further 12 (six from each group) were dropped due to excessive motion during scanning. The final groups consisted of 31 (22 female) MDD subjects and 36 (23 female) HCL.
All MDD subjects met full criteria for a current primary diagnosis of MDD according to the DSM-IV (American Psychiatric Association, 2000) and were excluded from the study if they had a primary diagnosis of any other psychiatric disorder (for full description of exclusion criteria, see the Supplement). All adolescents with MDD were naïve to any psychotropic medications except for five: four had stopped their medication more than a year prior to scanning and one subject four months prior to scanning. HCL adolescents were excluded from the study for any of the exclusionary criteria for the depressed group, as well as any current or lifetime Axis I psychiatric disorder or any family history of mood or psychotic disorders in first- or second-degree relatives. See Table 1 for a full summary of the clinical and demographic characteristics of the sample. The institutional review boards of the University of California (UC) San Diego, UC San Francisco, Rady Children’s Hospital, and The County of San Diego approved this study. Participants provided written informed assent and their parent/legal guardian proved written informed consent. All the participants were financially compensated.
Table 1.
Characteristics of depressed subjects and healthy controls.
| Characteristic | Control a | MDD a | Statisticb,c | p Value | Effect Size (95% CI) d |
|---|---|---|---|---|---|
| Number of participants recruited (n) | 43 | 38 | |||
| Overall recruitment gender (M / F) | 18 / 25 | 10 / 28 | |||
| Rejected due to missing data (n) | 1 | 1 | |||
| Rejected due to excessive motion and outlier count (n)* | 6 | 6 | |||
| Number of participants (n) | 36 | 31 | |||
| Gender (M / F) | 13 / 23 | 9 / 22 | χ2(1.00) = 0.13 | 0.72 | |
| Age at time of scan (years) | 16.1 ± 0.2 (13.1–17.9) | 16 ± 0.3 (13.1–18.1) | t(56.38) = 0.41 | 0.69 | |
| Hollingshead Socioeconomic Score | 29 ± 16.3 (0–77)† | 30 ± 17.8 (11–70)† | W = 458 | 0.21 | |
| Tanner Score | 4 ± 0.7 (3–5)† | 4 ± 0.7 (3–5)† | W = 535 | 0.77 | |
| Wechsler Abbreviated Scale of Intelligence | 107.2 ± 3.4 (84–204) | 100.7 ± 2 (82–120) | t(55.69) = 1.66 | 0.1 | |
| Children's Global Assessment Scale | 90 ± 5.9 (75–100)† | 65 ± 13.3 (27–85)† | W = 1107 | <0.001 | PS(MDD > NCL)=0.004 (0, 0.03) |
| Children's Depression Rating Scale (Standardized) | 33.6 ± 0.9 (30–55) | 73.3 ± 1.4 (61–85) | t(51.64) = −23.96 | <0.001 | g=−5.8 (−6.91, −4.69) |
| Beck Depression Inventory II | 2.9 ± 0.6 (0–12) [1] | 29.1 ± 1.7 (13–45) | t(37.15) = −14.70 | <0.001 | g=−3.56 (−4.34, −2.78) |
| Multidimensional Anxiety Scale for Children (Standardized) | 43 ± 1.6 (26–61) [2] | 59.8 ± 1.7 (40–78) | t(61.92) = −7.35 | <0.001 | g=−1.78 (−2.35, −1.21) |
| Comorbid Diagnoses in the MDD Group | |||||
| No current comorbid diagnoses e | 25 | ||||
| Generalized Anxiety Disorder (Current) | |||||
| Generalized Anxiety Disorder (Past) | 8 | ||||
| Specific Phobia (Current) | 3 | ||||
| Specific Phobia (Past) | 3 | ||||
| Anxiety Disorder Not Otherwise | |||||
| Specified (Current) | 1 | ||||
| Anxiety Disorder Not Otherwise | |||||
| Specified (Past) | 1 | ||||
| Post Traumatic Stress Disorder (Current) | 2 | ||||
| Post Traumatic Stress Disorder (Past) | 4 |
Mean ± SEM (min - max) or median ± MAD (min - max) if indicated by †. The optional number in [] indicates the number of missing data points.
Statistic: W, Wilcox rank sum test; χ2, χ2 test for equality of proportions; t, Welch T test.
Statistics for clinical scales refer only to participants with behavioral data and those surviving motion and outlier correction.
Effect size: g, Hedge’s g; PS, Probability of Superiority. Effect sizes are provided only where p < 0.1.
Refers to the absence of current and past diagnoses of: PTSD, ADHD, enuresis, conduct disorder, and oppositional defiance disorder.
Abbreviations: CI, Confidence Interval; SEM, standard error of the mean; MAD, median ab solute deviation; PTSD, Post-traumatic stress disorder; ADHD, Attention deficit hyperactivity disorder; M, male; F, female.
Assessment
For potentially depressed participants, an MDD diagnosis was validated with the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version Task (Kaufman et al., 1997). The presence of Axis I disorders in HCL was determined using the Diagnostic Interview Schedule for Children Version 4.0 (Shaffer et al., 2000) and the Diagnostic Predictive Scale (Lucas et al., 2001). In all subjects, depression severity was measured with the Children’s Depression Rating Scale-Revised (CDRS-R) (Poznanski, 1996) and Beck Depression Inventory-II (BDI-II) (Beck et al., 1996); anxiety symptoms with the Multidimensional Anxiety Scale for Children (MASC) (March et al., 1997), and level of global functioning by the Children’s Global Assessment Scale (CGAS) (Dyrborg et al., 2000). The MDD and HCL groups were well-matched on age, gender, general IQ (WASI) (Wechsler, 1999), pubertal status (Tanner, 1963), and socioeconomic status (Hollingshead Two Factor Index of Social Position, HSP)(Hollingshead, 1957). A full description of the test battery is available elsewhere (Connolly et al., 2013; Ho et al., 2014a; Ho et al., 2013; Ho et al., 2014b; Yang et al., 2010)
Experimental Stimuli and Task
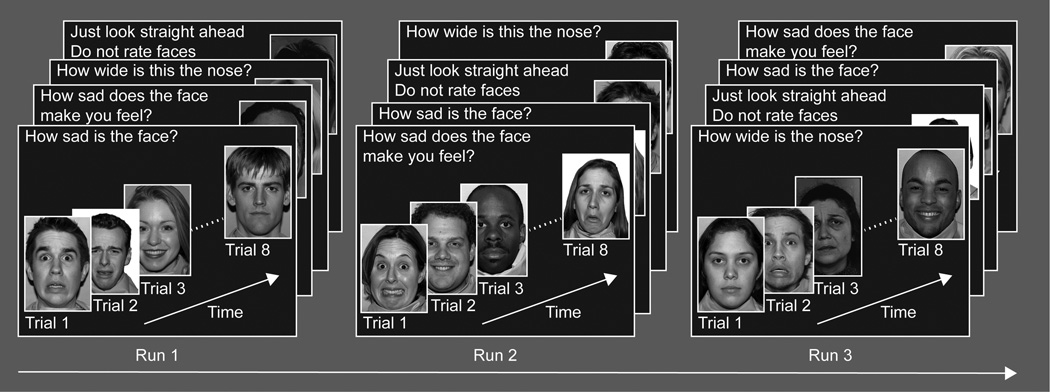
All subjects underwent a well-validated emotional face processing fMRI paradigm for adolescents (Beesdo et al., 2009; Monk et al., 2008; Nelson et al., 2003; Pine et al., 2004; Roberson-Nay et al., 2006). However, we modified the task and replaced angry faces with sad faces. In brief the task consisted of two phases: encoding and recall. The encoding phase required participants to view 32 adult actors depicting four sad, happy, fear, and neutral (8 instances each). The pairings of actor with face-emotion were randomized across participants; thus, participants saw the same set of 32 actors but each actor displayed a different emotion for each participant. All together, the encoding phase constituted a 160 trial run that lasted 14min 20sec. The 160 trials were divided into four 40-trial epochs that were further subdivided into four blocks, one for each of the four instructional sets: 1) the sadness level of the face (“How sad is the face?”); 2) the participant’s emotional reaction to the face (“How sad does the face make you feel?”); 3) the size of a non-emotional facial feature (“How wide is the nose?”); 4) passive viewing of the face. With the exception of the passive viewing condition, subjects made ratings according to each instruction based on a 4-point scale. Each instruction block consisted of 10 pseudo-randomly presented trials: eight of which were faces plus two fixation events. Instructions lasted 3000ms and were presented prior to each block. Each face image was presented for 4000ms, during which participants made their responses. Each event (face or fixation) was followed by a variable inter-trial interval of 750 – 1250ms. The same image of each actor was presented to each participant for each of the four viewings. Figure 1 illustrates the task. The recall phase took the form of an unannounced memory test that occurred outside the scanner at the end of the scanning session. The image set used in the test consisted of 24 previously seen actors and 24 novel actors, each depicting a neutral expression, which participants rated as old or new.
Figure 1.
The experimental design for the emotional face processing task.
MR Data Acquisition and Preprocessing
Scanning was performed on a 3T GE MR750 (Milwaukee, WI). One 14min 20sec T2*-weighted echo planar image (EPI) scan (430 volumes TR/TE=2s/30ms, flip angle=90°, 64×64 matrix, 3×3×3mm voxels, 40 axial slices) was acquired. A T1-weighted scan (TR/TE=8.1ms/3.17ms, flip angle=12°, 256×256 matrix, 1×1×1mm voxels, 168 sagittal slices) was acquired to permit spatial normalization and functional localization.
MR Data Analysis
Analyses were conducted using AFNI (Cox, 1996) and FSL (Smith et al., 2004). T1-weighted images were skull-stripped and transformed to MNI152 space using an affine transform (Jenkinson et al., 2002; Jenkinson and Smith, 2001) followed by nonlinear refinement (Andersson et al., 2007a; Andersson et al., 2007b). EPI images were slice-time and motion corrected, aligned to the T1 images (Saad et al., 2009), smoothed with a 4.2mm full-width at half-maximum (FWHM) Gaussian filter, grand-mean scaled, and transformed to MNI152 space at 3×3×3mm resolution. Censoring of outlier volumes and those with excessive motion was performed (see supplement for details). Preprocessed time-series were subjected to generalized least squares regression that estimates the serial correlation structure of the noise with an ARMA(1, 1) model. Time-series of interest were created such that they started at the onset of a face-emotion image and ended when the next image was displayed. Four regressors-of-interest were defined: happy, fearful, neutral, and sad. Regressors were convolved with a gamma-variate prototypical hemodynamic response function (HRF) (Boynton et al., 1996) and normalized to a peak amplitude of 1. All time-points not accounted for by these regressors constituted the baseline. Six nuisance motion regressors (three translation and three rotational) and a 2nd order Lagrange polynomial (accounting for slow signal drift) were included in the baseline. General linear tests (GLTs) of each pair of face-emotions (e.g., sad versus happy) were computed for each participant. Finally, brain activation was operationally defined as percentage signal change relative to baseline.
Given our hypotheses on sad versus happy face processing, we focused our analyses on GLTs involving sad and happy face stimuli (i.e., happy versus sad, sad versus neutral, happy versus neutral). Between-group voxel-wise analyses of the GLTs were accomplished using linear mixed effects models (LMEs) implemented in R (R Development Core Team, 2014) where participant was treated as a random effect. Follow-up post-hoc t-tests were conducted to ascertain the source of differences in regions-of-interest identified from this analysis.
Functional Connectivity Analysis
We assessed the functional connectivity (FC) between the anterior middle insular cortex (AMIC) and the whole brain using the psychophysiological interaction (PPI) method (Friston et al., 1997) using the same method as described in our previously published work (Fonzo et al., 2010; Ho et al., 2014a; Ho et al., 2014b; Perlman et al., 2012; Simmons et al., 2008). See the supplement for details. Briefly, the interaction time-series for each GLT were convolved with a gamma-variate function HRF (Boynton et al., 1996). The 10 regressors detailed above, the seed (AMIC) time-series, and the interaction regressor (happy versus sad) were then subjected to multiple linear regression analysis. Correlation coefficients representing the correlation between each interaction regressor and every other voxel were calculated and converted to z-scores using Fisher’s r-to-z transform. These resulting Z-scores were then subject to LMEs, as outlined above, to assess differences in connectivity related to between-group differential face-emotion processing.
Voxel-wise Thresholds and Correction for Multiple Comparisons
For both task-related and functional connectivity analyses, all significant voxels were required to pass a voxel-wise statistical threshold (F(1, 65)=3.99, p<0.05, uncorrected) and were further required to be part of a cluster of at least 1669 μL to control for multiple comparisons. The cluster threshold was determined using a Monte Carlo simulation that, in combination with the voxel-wise threshold, resulted in a 5% probability of that cluster surviving due to chance (i.e., cluster-wise p<0.05).
Behavioral Analysis
We used the d’ measure from signal detection theory to assess participants’ ability to correctly distinguish previously viewed actors (“signal”) from those never seen before (“noise”). Between-group differences in false alarm rate were examined using a Welch t-test. A one-sample t-test determined whether overall recall differed significantly from chance. An ANOVA was conducted to determine whether group, face-emotion, and their interaction influenced recall. For a more detailed description see the supplement.
Demographic and Clinical Scales Analysis
Between-group differences in age, WASI, BDI-II, CDRS-R and MASC were assessed using Welch t-tests. Effect sizes for these measures were determined using Hedge’s g (Hedges & Olkin 1985). Wilcox rank sum test was used to assess group differences on Tanner stage, CGAS, and HSP, with effect sizes computed using the probability of superiority (PS; range 0 – 1) (Mahwah, 2005). PS represents the probability that a randomly selected MDD participant reported a greater value on the corresponding measure than a randomly selected control. Confidence intervals for the PS were computed using a bootstrap method (Ruscio J, 2012). Differences in the number of participants per group and gender ratios were assessed using χ2 test of equal proportions.
Correlational Analysis
Within the MDD group, the relationship between BDI-II and the contrast of activation in the AMIC to happy and sad faces was examined using Spearman’s rank correlation test. A similar investigation was conducted to assess the relationship between BDI-II and age of MDD onset with the average Z-score from each 3 regions (right fusiform gyrus (FG), left middle frontal gyrus (MFG), and right amygdala/parahippocampal gyrus (PHG); see results below) showing connectivity differences with the AMIC seed as identified by the PPI analysis. Bonferroni correction for multiple comparisons was applied: p=0.05/2=0.025 for the correlation tests involving the happy-sad contrast differences in the AMIC and p=0.05/6=0.008 for the PPI results.
Results
Demographics and Clinical Scales
MDD and HCL groups did not differ significantly on age, gender, HSP, Tanner stage, or WASI (all p>0.1). Higher depression symptom scores on the BDI-II and CDRS-R (all p<0.05) and higher anxiety scores on MASC (p<0.001) and lower levels of global functioning with CGAS (p<0.001) were observed in the MDD compared to the HCL. See Table 1.
Behavioral Task Results
No group differences in false alarm rate were present (t(60.93)=0.06, p>0.1). Collapsing across groups, the overall d’ significantly differed from 0 (t(66)=17.09, p<0.001), indicating that participants were better at recalling previously seen actors than chance levels. Upon examining the effect of group, face-emotion and their interaction on d’, there was no effect of group (F(1, 260)=0.23, p>0.1) or group × face-emotion interaction (F(1, 260)=0.73, p>0.1). However, face-emotion was significant (F(1, 260)=9.85, p<0.001) with follow-up pairwise t-tests indicating that recall for neutral faces was greater than all non-neutral faces (all p<0.05).
fMRI Task Results
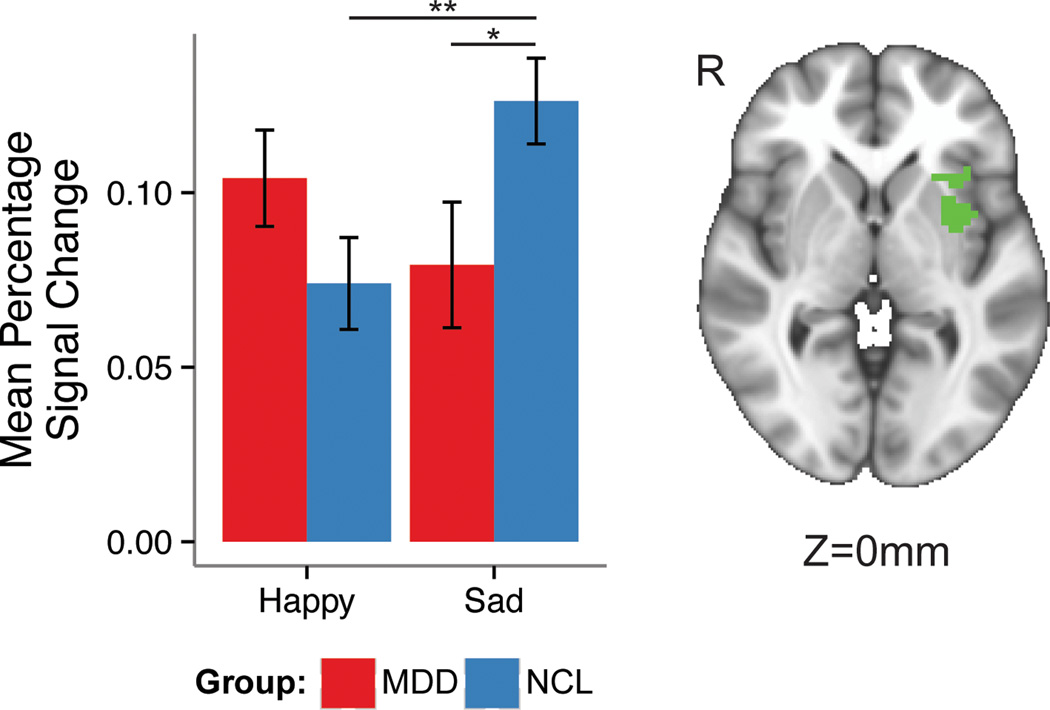
We found several regions showing between-group differences in the contrast happy versus sad faces (Table S1). First, the happy-sad comparison revealed significant differences in a cluster composed of the left claustrum extending laterally to include anterior and middle insular cortex (AMIC) (2,322μL, x=31, y=−7, z=0, average F(1, 65)=6.00; see Figure 2). The happy-sad contrast also showed significant differences in the right lingual gyrus (LG), bilateral FG, right thalamus, and right declive of the cerebellum (Table S1). Post-hoc tests examining the directionality of these effects (Table S2), showed the following: the left AMIC from the happy-sad contrast showed lower activation levels in the MDD compared to the HCL group. Compared to HCL, the MDD group exhibited reduced activation in the right LG, bilateral FG and right declive of the cerebellum, while exhibiting greater activation in the right thalamus during happy versus sad face processing. Within the MDD group, sad faces elicited stronger activation than happy faces in the left and right FG and the right declive of the cerebellum.
Figure 2.
Between-group differences in mean percent signal change during the happy-sad condition in a cluster composed of the left claustrum extending laterally to include primarily anterior and middle insula.
Functional Connectivity Results
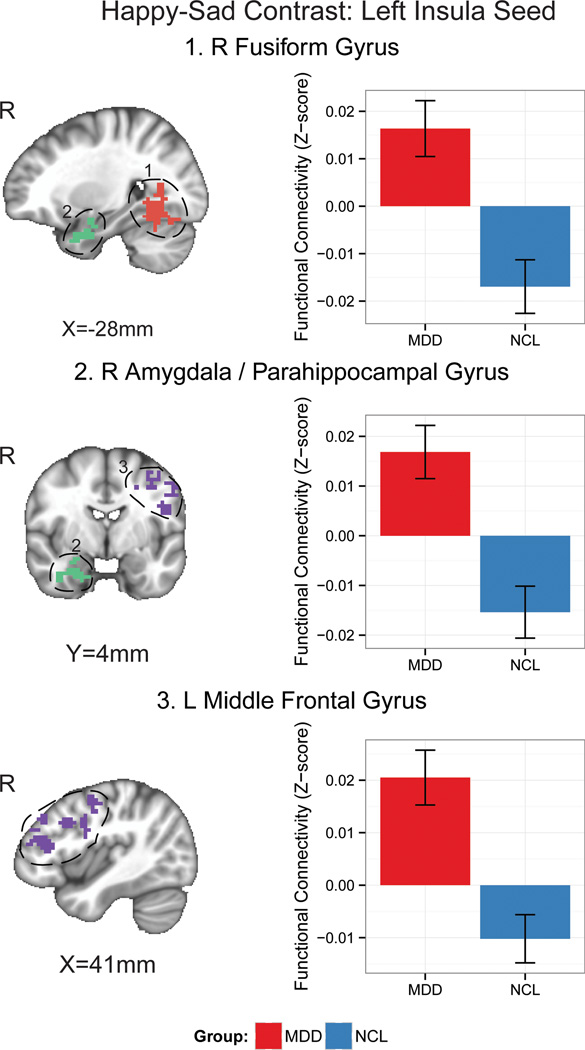
Among the regions identified by the task-related analysis (Table 2), we specifically focused on the AMIC region. The left AMIC seed identified in the happy-sad contrast was positively connected to the right FG, left MFG, and right amygdala/PHG in the MDD group but was negatively connected to these same regions in the HCL group (Figure 3).
Table 2.
Mean connectivity z-scores between the left insular seed and functionally connected brain structures in the happy-sad contrast in the depressed group (MDD) and healthy controls (HCL).
| Structure | Hemisphere | Volume (μL) |
Center of mass |
Average F valuea |
Mean connectivity z-score |
|||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | MDD | HCL | ||||
| Happy-sad Contrast: L Insula Seed | ||||||||
| Declive | L | 14,040 | 20 | 60 | −19 | 5.93 | 0.02 | −0.01 |
| Fusiform Gyrus | R | 13,797 | −28 | 56 | −13 | 6.18 | 0.02 | −0.02 |
| Middle Frontal Gyrus | L | 13,068 | 42 | −18 | 30 | 6.19 | 0.02 | −0.01 |
| Precuneus | L | 5,994 | 12 | 76 | 37 | 5.85 | 0.02 | −0.01 |
| Culmen | R | 5,373 | −3 | 41 | −28 | 6.57 | 0.02 | −0.01 |
| Inferior Parietal Lobule | L | 3,672 | 58 | 41 | 26 | 5.98 | 0.02 | −0.02 |
| Precuneus | R | 2,997 | −18 | 67 | 25 | 5.43 | 0.02 | −0.02 |
| Amygdala/Parahippocampal Gyrus | R | 2,538 | −29 | 1 | −29 | 6.15 | 0.02 | −0.02 |
| Cingulate Gyrus | L | 2,322 | 12 | 16 | 35 | 7.00 | 0.02 | −0.02 |
| Precuneus | R | 2,160 | −21 | 81 | 41 | 6.00 | 0.02 | −0.01 |
F(1, 65)
Figure 3.
PPI results of the left anterior/middle insular cortex/claustrum seed identified in the happy-sad contrast in the depressed adolescents (MDD) and in the healthy controls (HCL).
Correlational Analysis Results
Those MDD individuals who showed relatively less activation left AMIC cluster identified from the happy-sad contrast reported higher BDI-II scores (ρ=-0.58, p<0.001). No significant correlations between BDI-II or age of MDD onset and the strength of functional connectivity of the AMIC seed and connected regions (i.e., right FG, left MFG, or right amygdala/PHG) were observed within the MDD group (all p>0.1).
Discussion
The present study aimed to elucidate the activation patterns of the insular cortex in response to sad versus happy facial exposure using fMRI in a sample of adolescents diagnosed with MDD compared to well-matched HCL. Our investigation yielded several important findings. First, the differential activation in the left anterior/middle insular cortex (AMIC) during sad versus happy face processing was less pronounced in the MDD group compared to HCL (Figure 2). Second, in the MDD group, there was greater functional connectivity between AMIC and right fusiform gyrus, left middle frontal gyrus, and right amygdala/parahippocampal gyrus (PHG) during sad versus happy face processing. Third, reduced differential activation to sad and happy faces in this same AMIC region correlated negatively with depression symptom severity scores in the depressed adolescents. Taken together, these findings are consistent with a dysfunction of the AMIC when processing emotional information in adolescents with MDD. Moreover, given the role of the AMIC integrating bodily states and emotions, this finding raises the possibility that adolescents with MDD show poorer integration of emotion processing with bodily sensations.
Our first finding that significantly less differential activation in the anterior/middle insular cortex to sad versus happy face stimuli were seen in the depressed group compared to HCL may correspond to a decreased ability in adolescents with MDD to differentiate between these differently valenced emotional stimuli and thereby contribute to difficulties in interpreting social cues. We also found that the left AMIC showed less activation to sad faces in the MDD group compared to the HCL, which is contradicting previous findings in adult MDD, where anterior insular cortex is shown relatively hyperactive during emotion processing (Hamilton et al., 2012; Sliz and Hayley, 2012). This discrepancy suggests that the decreased activation of AMIC in adolescent depression in response to sad faces may be developmentally specific and corresponds to a limited tendency to successfully process and interpret sad emotions in MDD adolescents. Indeed, recent work suggests that ongoing development of the anterior insular cortex during adolescence may differentially impact cognitive and affective functioning of this structure in adolescents compared to adults (Dupont et al., 2003; Smith et al., 2014). This finding may also be explained by the theory that adolescents with MDD show less activation to mood congruent stimuli, analogous with previous findings showing increase attentional deficit in MDD subjects when exposed to mood incongruent stimuli (Erickson et al., 2005).
Our second finding was a positive functional connectivity (FC) in the MDD group between the left AMIC cluster to other key emotion processing areas: namely, the right FG, right amygdala/parahippocampus gyrus (PHG) and left MFG. In contrast, the functional connections between the AIMC and these regions were negative in the HCL. Importantly, these findings imply that the integrative function of the AMIC in relation to these areas, representing different aspects of facial emotion processing, is altered in adolescent MDD. Further supporting this idea is our finding that the FG, a part of the visual system specialized for facial recognition (Kanwisher et al., 1997), showed a greater activation for sad than happy faces in the depressed group. This implies that aberrant activation in AMIC and FG, and the strength of their functional connection may contribute to biased processing of sad stimuli, as previously described in adolescent depression (Schepman et al., 2012). The positive FC between left AMIC and the amygdala in our depressed sample may indicate dysfunctional processing of bodily sensations to emotional experiences, and more specifically imply increased interaction between perception of emotional cues in the amygdala and increased arousal in the insula, which may be corresponding to the symptomatology of general emotion dys-regulation in adolescent MDD. Finally, the positive FC between AMIC and the MFG, in the adolescent MDD group may reflect a compensatory effort of top-down cognitive control when the insular cortex is activated in response to emotionally valenced stimuli. Interestingly, studies in adult depression showed that increasing levels of sadness were associated with increased activation of anterior insula and decreased activation of prefrontal regions, but that this pattern reverses with recovery from depression (Kennedy et al., 2001; Mayberg et al., 1999).
Lastly, we report that more severe depression symptomatology within the MDD group was significantly associated with less of a difference in activation between sad and happy faces in the AMIC. This result may have implications for the previously described biased processing towards sad stimuli in depressed individuals (Joormann and Gotlib, 2006; Schepman et al., 2012). Studies in healthy adolescents show that insular cortex thickness (Churchwell and Yurgelun-Todd, 2013; Shaw et al., 2008) and connectivity patterns continue to develop into young adulthood (McRae et al., 2012). We suggest that early onset of depression increases the impact of MDD on AMIC development and its function (Gaffrey et al., 2013) with consequences for the development of emotion regulation skills during adolescence.
The major limitations of this study are the sample’s relatively small age-range and the cross-sectional design of this study, which limit the possibility of assessing the development of the insular cortex in adolescent depression. Future studies of longitudinal design following the structural and functional development of the insular cortex in depressed adolescents compared to healthy controls are needed to address these limitations and to confirm the reproducibility of our findings.
Our findings provide a neuroscientific rationale for testing new treatment modalities on the level of integrating bodily stimuli to conscious cognitive and emotional processes. Adaptively switching from ruminative and self-referential processing to an interoceptive focus has been suggested to rely on the insular cortex (Menon and Uddin, 2010) and difficulty in switching between these brain states has been suggested as a potential contributing mechanism of depression (Chang et al., 2013; Manoliu et al., 2013). Furthermore, the practice of switching from rumination to interoceptive awareness has been related to changes in insular activation patterns in fMRI studies of healthy (Farb et al., 2013) and depressed (Farb et al., 2010; Farb et al., 2012; Paul et al., 2013) adults. Thus, an important future direction of research may be to investigate the effects of practicing how to direct attention toward momentary bodily and sensory experience for emotion regulation on depression symptom severity in adolescent MDD (Henje Blom E, 2014). This practice could provide an alternative strategy to therapies requiring top-down cognitive control efforts. The effect of such training on insular cortex activation and FC during the processing of sad and happy faces would more clearly link a possible practice effect to insular cortex function and the previously described biased emotion processing in adolescent depression.
Conclusion
This study showed less functional differentiation in response to sad versus happy faces in depressed adolescents compared to well-matched healthy controls. These finding differed from previous reports in depressed adults and suggests that MDD may alter the developmental trajectory of the AMIC in adolescents. Furthermore, we report that functional connectivity between the AMIC and key brain regions (e.g., the MFG and FG) involved in facial emotion processing and the regulation thereof was increased in the depressed adolescents as compared to healthy controls.. We also found greater depression severity correlated significantly with reduced differential responses to sad and happy faces in the AMIC. We theorize that the developmental trajectory of the AMIC and its related circuitry is impacted by early onset depression and that this may impair the acquisition of emotional regulatory skills during adolescence. Together, our results suggest that this region may serve as a target in the development of more effective future interventions for adolescent depression.
Supplementary Material
Highlights.
Adolescents with MDD show decreased differentiation in activation of the anterior/middle insular cortex between happy and sad faces.
The depressed adolescents show greater functional connectivity between anterior/middle insular cortex to key emotion processing regions.
Reduced functional differentiation between happy and sad faces correlated to increased depression severity in depressed adolescents
Aberrant function of the anterior/middle insular cortex in depressed adolescents provides a neuroscientific rationale for this region as a target for the development of new treatment modalities.
Acknowledgements
We want to thank the teenagers who have participated in the study. We are grateful to Daniel Pine for providing us with the fMRI task and to both Daniel Pine and Ellen Leibenluft for valuable input. We also wish to thank Kevin Han, Mary Luna, Nisreen Mobayed, Audrey Fortezzo for their help in the acquisition and management of the data for this study.
Financial Support
This work was supported by the Swedish Research Council (E.H.B., grant number 350-2012-303); the Swedish Society of Medicine (E.H.B., grant number SLS244671) and the Sweden American Association to EHB; the NARSAD foundation and the National Institute of Mental Health (T.T.Y., grant numbers 7R01MH085734, 3R01MH085734-02S1 and R01MH085734-05S1), (K.Z.L., grant number K01MH097978) and T.C.H from American Foundation of Suicide Prevention PDF-1-064-13. The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Contributors
Conceived and designed the experiments: MPP ANS TTY.
Performed the experiments: JW, NM.
Analyzed the data: CGC.
Contributed reagents/materials/analysis tools: CGC.
Wrote the paper: EHB CGC TCH KZLW LH MPP ANS TTY.
All authors have approved the final version of this article.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TRM. Fourth Edition. American Psychiatric Association; 2000. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-Linear Registration, aka Spatial Normalisation. Oxford, UK: University of Oxford,, FMRIB; 2007a. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-Linear Optimisation. UK: FMRIB, University of Oxford; 2007b. p. http://www.fmrib.ox.ac.uk/analysis/techrep/tr07ja01/tr07ja01.pdf. [Google Scholar]
- Arce E, Simmons AN, Stein MB, Winkielman P, Hitchcock C, Paulus MP. Association between individual differences in self-reported emotional resilience and the affective perception of neutral faces. J Affect Disord. 2009;114:286–293. doi: 10.1016/j.jad.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major Depressive Disorder Is Associated with Abnormal Interoceptive Activity and Functional Connectivity in the Insula. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebko G, Bertocci M, Chase H, Dwojak A, Bonar L, Almeida J, Perlman SB, Versace A, Schirda C, Travis M, Gill MK, Demeter C, Diwadkar V, Sunshine J, Holland S, Kowatch R, Birmaher B, Axelson D, Horwitz S, Frazier T, Arnold LE, Fristad M, Youngstrom E, Findling R, Phillips ML. Decreased amygdala-insula resting state connectivity in behaviorally and emotionally dysregulated youth. Psychiatry Res. 2015;231:77–86. doi: 10.1016/j.pscychresns.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of personality assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt ER, Koran LM, Finkelstein SN, Gelenberg AJ, Kornstein SG, Miller IM, Thase ME, Trapp GA, Keller MB. Lost human capital from early-onset chronic depression. The American journal of psychiatry. 2000;157:940–947. doi: 10.1176/appi.ajp.157.6.940. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cerebral cortex (New York, N.Y.: 1991) 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, Ernst M. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage. 2012;66C:508–521. doi: 10.1016/j.neuroimage.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Yurgelun-Todd DA. Age-related changes in insula cortical thickness and impulsivity: significance for emotional development and decision-making. Developmental cognitive neuroscience. 2013;6:80–86. doi: 10.1016/j.dcn.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, Tapert SF, Banerjee D, Simmons AN, Yang TT. Resting-State Functional Connectivity of Subgenual Anterior Cingulate Cortex in Depressed Adolescents. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature reviews. Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Camchong J, Bell CJ, Houri A, Kumra S, Lim KO, Castellanos FX, Milham MP. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional anatomy of the insula: new insights from imaging. Surgical and radiologic anatomy : SRA. 2003;25:113–119. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- Dyrborg J, Larsen FW, Nielsen S, Byman J, Nielsen BB, Gautre-Delay F. The Children's Global Assessment Scale (CGAS) and Global Assessment of Psychosocial Disability (GAPD) in clinical practice--substance and reliability as judged by intraclass correlations. Eur Child Adolesc Psychiatry. 2000;9:195–201. doi: 10.1007/s007870070043. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CA, Jr, Charney DS, Sahakian BJ. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one's emotions: mindfulness training alters the neural expression of sadness. Emotion (Washington, D.C.) 2010;10:25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Segal ZV. The mindful brain and emotion regulation in mood disorders. Can J Psychiatry. 2012;57:70–77. doi: 10.1177/070674371205700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Mindfulness meditation training alters cortical representations of interoceptive attention. Social cognitive and affective neuroscience. 2013;8:15–26. doi: 10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Gotlib IH. Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Frontiers in psychology. 2012;3:489. doi: 10.3389/fpsyg.2012.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Luby JL, Barch DM. Towards the Study of Functional Brain Development in Depression: An Interactive Specialization Approach. Neurobiol Dis. 2013;52:38–48. doi: 10.1016/j.nbd.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP. Puberty-related influences on brain development. Molecular and cellular endocrinology. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. 2012;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- Hamm LL, Jacobs RH, Johnson MW, Fitzgerald DA, Fitzgerald KD, Langenecker SA, Monk CS, Phan KL. Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biology of mood & anxiety disorders. 2014;4 doi: 10.1186/s13587-014-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Henje Blom E, DL G, Ho TC, Connolly CG, LeWinn KZ, Chesney MA, Hecht FM, Yang TT. The development of a RDoC-based treatment program for adolescent depression: training for awareness, resilience and action (TARA) Front Hum Neurosci. 2014 doi: 10.3389/fnhum.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP, Frank G, Max JE, Wu J, Chan M, Tapert SF, Simmons AN, Yang TT. Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biological Psychiatry In press. 2014a doi: 10.1016/j.biopsych.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Wu J, Shin DD, Liu TT, Tapert SF, Yang G, Connolly CG, Frank GK, Max JE, Wolkowitz O, Eisendrath S, Hoeft F, Banerjee D, Hood K, Hendren RL, Paulus MP, Simmons AN, Yang TT. Altered cerebral perfusion in executive, affective, and motor networks during adolescent depression. J Am Acad Child Adolesc Psychiatry. 2013;52:1076–1091. e1072. doi: 10.1016/j.jaac.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Yang G, Wu J, Cassey P, Brown SD, Hoang N, Chan M, Connolly CG, Henje-Blom E, Duncan LG, Chesney MA, Paulus MP, Max JE, Patel R, Simmons AN, Yang TT. Functional connectivity of negative emotional processing in adolescent depression. J Affect Disord. 2014b;155:65–74. doi: 10.1016/j.jad.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Two Factor Index of Social Position. New Haven, Connecticut: Mimeo, Yale University; 1957. [Google Scholar]
- Hollon SD, Shelton RC, Wisniewski S, Warden D, Biggs MM, Friedman ES, Husain M, Kupfer DJ, Nierenberg AA, Petersen TJ, Shores-Wilson K, Rush AJ. Presenting characteristics of depressed outpatients as a function of recurrence: preliminary findings from the STAR*D clinical trial. Journal of psychiatric research. 2006;40:59–69. doi: 10.1016/j.jpsychires.2005.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of comparative neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Is this happiness I see? Biases in the identification of emotional facial expressions in depression and social phobia. J Abnorm Psychol. 2006;115:705–714. doi: 10.1037/0021-843X.115.4.705. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. J Abnorm Psychol. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral cortex (New York, N.Y.: 1991) 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kujawa AJ, Torpey D, Kim J, Hajcak G, Rose S, Gotlib IH, Klein DN. Attentional biases for emotional faces in young children of mothers with chronic or recurrent depression. J Abnorm Child Psychol. 2011;39:125–135. doi: 10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH. Patterns of cortico-limbic activations during visual processing of sad faces in depression patients: a coordinate-based meta-analysis. The Journal of neuropsychiatry and clinical neurosciences. 2014;26:34–43. doi: 10.1176/appi.neuropsych.12060143. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Mahwah N. 2005 Effect Sizes For Research: A broad practical approach. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2005. p. 253. US:. xv. [Google Scholar]
- Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Schwerthoffer D, Zimmer C, Forstl H, Bauml J, Riedl V, Wohlschlager AM, Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual review of clinical psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. The Journal of neuropsychiatry and clinical neurosciences. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social cognitive and affective neuroscience. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, Guardino M, Masten CL, McClure-Tone EB, Fromm S, Blair RJ, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, Ernst M. Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. J Child Psychol Psychiatry. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. The insular cortex: a review. Prog Brain Res. 2012;195:123–163. doi: 10.1016/B978-0-444-53860-4.00007-6. [DOI] [PubMed] [Google Scholar]
- Paul NA, Stanton SJ, Greeson JM, Smoski MJ, Wang L. Psychological and neural mechanisms of trait mindfulness in reducing depression vulnerability. Social cognitive and affective neuroscience. 2013;8:56–64. doi: 10.1093/scan/nss070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Liu J, Nie B, Li Y, Shan B, Wang G, Li K. Cerebral and cerebellar gray matter reduction in first-episode patients with major depressive disorder: a voxel-based morphometry study. European journal of radiology. 2011;80:395–399. doi: 10.1016/j.ejrad.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, Paulus MP, Brown GG, Frank GK, Campbell-Sills L, Yang TT. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. J Affect Disord. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pine DS, Lissek S, Klein RG, Mannuzza S, Moulton JL, 3rd, Guardino M, Woldehawariat G. Face-memory and emotion: associations with major depression in children and adolescents. J Child Psychol Psychiatry. 2004;45:1199–1208. doi: 10.1111/j.1469-7610.2004.00311.x. [DOI] [PubMed] [Google Scholar]
- Poznanski EO. Children's Depression Rating Scale-Revised (CDRS-R) Manual. Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2013;30:234–241. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing Vol.1) Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, Charney DS, Leibenluft E, Blair J, Ernst M, Pine DS. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An FMRI study. Biol Psychiatry. 2006;60:966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Annals of the New York Academy of Sciences. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C, Benson B, Castellanos FX, Milham MP, Pine DS, Ernst M. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–299. e292. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio JMT. Confidence Intervals for the Probability of Superiority Effect Size Measure and the Area Under a Receiver Operating Characteristic Curve. Multivariate Behavioral Research. 2012;47:201–223. doi: 10.1080/00273171.2012.658329. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepman K, Taylor E, Collishaw S, Fombonne E. Face emotion processing in depressed children and adolescents with and without comorbid conduct disorder. J Abnorm Child Psychol. 2012;40:583–593. doi: 10.1007/s10802-011-9587-2. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Paulus MP, Thorp SR, Matthews SC, Norman SB, Stein MB. Functional activation and neural networks in women with posttraumatic stress disorder related to intimate partner violence. Biol Psychiatry. 2008;64:681–690. doi: 10.1016/j.biopsych.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in neuroendocrinology. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front Hum Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AR, Steinberg L, Chein J. The Role of the Anterior Insula in Adolescent Decision Making. Developmental neuroscience. 2014 doi: 10.1159/000358918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Soriano-Mas C, Hernandez-Ribas R, Pujol J, Urretavizcaya M, Deus J, Harrison BJ, Ortiz H, Lopez-Sola M, Menchon JM, Cardoner N. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol Psychiatry. 2011;69:318–325. doi: 10.1016/j.biopsych.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Steele JD, Mwangi B, Kumar P, Christmas D, Milders M, Matthews K. The insular cortex and the neuroanatomy of major depression. J Affect Disord. 2011;133:120–127. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O'Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child development. 2009;80:28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Stratmann M, Konrad C, Kugel H, Krug A, Schoning S, Ohrmann P, Uhlmann C, Postert C, Suslow T, Heindel W, Arolt V, Kircher T, Dannlowski U. Insular and hippocampal gray matter volume reductions in patients with major depressive disorder. PLoS One. 2014;9:e102692. doi: 10.1371/journal.pone.0102692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biology of mood & anxiety disorders. 2011;1:10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Yucel M, Lorenzetti V, Tanino R, Whittle S, Suzuki M, Walterfang M, Pantelis C, Allen NB. Volumetric MRI study of the insular cortex in individuals with current and past major depression. J Affect Disord. 2010;121:231–238. doi: 10.1016/j.jad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Tanner JM. The Regulation of Human Growth. Child development. 1963;34:817–847. doi: 10.1111/j.1467-8624.1963.tb05970.x. [DOI] [PubMed] [Google Scholar]
- Townsend JD, Eberhart NK, Bookheimer SY, Eisenberger NI, Foland-Ross LC, Cook IA, Sugar CA, Altshuler LL. fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Res. 2010;183:209–217. doi: 10.1016/j.pscychresns.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustun TB, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–392. doi: 10.1192/bjp.184.5.386. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York: Harcourt Brace & Company; 1999. [Google Scholar]
- Weir JM, Zakama A, Rao U. Developmental risk I: depression and the developing brain. Child Adolesc Psychiatr Clin N Am. 2012;21:237–259. vii. doi: 10.1016/j.chc.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, Bischoff-Grethe A, Lansing AE, Brown G, Strigo IA, Wu J, Paulus MP. Adolescents with major depression demonstrate increased amygdala activation. J Am Acad Child Adolesc Psychiatry. 2010;49:42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisook S, Lesser I, Stewart JW, Wisniewski SR, Balasubramani GK, Fava M, Gilmer WS, Dresselhaus TR, Thase ME, Nierenberg AA, Trivedi MH, Rush AJ. Effect of age at onset on the course of major depressive disorder. Am J Psychiatry. 2007;164:1539–1546. doi: 10.1176/appi.ajp.2007.06101757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.