Abstract
Hyperacute kidney rejection is unusual in crossmatch positive recipients of simultaneous liver–kidney transplants (SLKT). However, recent data suggest that these patients remain at risk for antibody-mediated kidney rejection. To further investigate the risk associated with donor-specific alloantibodies (DSA) in SLKT, we studied 86 consecutive SLKT patients with an available pre-SLKT serum sample. Serum samples were analyzed in a blinded fashion for HLA DSA using single antigen beads (median florescence intensity ≥ 2,000 = positive). Post-SLKT samples were analyzed when available (76%). Thirty patients had preformed DSA, and nine developed de novo DSA. Preformed class I DSA did not change the risk of rejection, patient or allograft survival. In contrast, preformed class II DSA was associated with a markedly increased risk of renal antibody mediated rejection (AMR) (p = 0.006), liver allograft rejection (p = 0.002), patient death (p = 0.02), liver allograft loss (p = 0.02) and renal allograft loss (p = 0.045). Multivariable modeling showed class II DSA (pre-formed or de novo) to be an independent predictor of patient death (HR = 2.2; p = 0.043) and liver allograft loss (HR = 2.2; p = 0.044). These data warrant reconsideration of the approach to DSA in SLKT.
Keywords: Antibody-mediated rejection, donor-specific antibodies, graft outcomes, liver transplant, renal transplant, simultaneous liver-kidney transplant
Introduction
Clinical experience has shown that the most serious consequence of preformed HLA-donor-specific alloantibodies (DSA), hyperacute rejection, is unusual following simultaneous liver-kidney transplant (SLKT). As such, preexisting DSA is not currently considered a contraindication for SLKT. However, recent data have suggested that DSA still represent a potential risk, particularly to the kidney, in the SLKT setting (1), and a recent registry analysis has demonstrated that highly sensitized SLKT recipients and those with a positive crossmatch experienced impaired patient and renal allograft survival (2). This is consistent with prior data associating preformed DSA with increased rates of graft and patient loss following liver transplantation (3–5). Similarly, although Olausson and colleagues showed that a partial auxiliary liver transplant mitigated the risk of hyperacute rejection in patients with a positive crossmatch, 4/7 patients had persistently positive B-cell crossmatches after reperfusion, one developed definitive and a second possible renal antibody mediated rejection (AMR) (6). Thus, although hyperacute rejection is preventable, the ‘protection’ afforded to the kidney by the liver from DSA, especially from class II, may be incomplete. For example, Dar and colleagues have recently examined the role of DSA in a more granular fashion, examining the DSA class and Median Florescence Intensity (MFI) of alloantibodies in six SLKT patients with a positive crossmatch (1). All five patients with preformed class I DSA experienced rapid DSA clearance, and as expected, hyperacute rejection did not occur. However, 4 patients with preformed class II DSA with high MFI (> 10 000) were diagnosed early posttransplant with renal AMR. Interestingly, the class II DSA persisted among these patients. In the one patient with class II DSA who did not experience AMR the alloantibodies were cleared posttransplant. Fortunately, hyperacute rejection is generally avoided in SLKT recipients despite the presence of DSA or a positive lymphocyte crossmatch; however significant risk may still exist for crossmatch positive SLKT patients, in the form of less immediate forms of alloimmune injury and/or the consequences of therapies deployed to counteract their effects. Additionally, DSA may be associated with adverse outcomes as a biomarker for unchecked alloimmunity without having direct pathological effects. Given the significant competing risks that could be associated with a selective allocation strategy that considered DSA-associated risks, we sought to investigate this issue taking advantage of a large, prospectively assembled biorepository of serum samples derived from liver and SLKT recipients. Using modern solid phase alloantibody determination and complete patient follow-up afforded by a single center registry, we find that class-II, but not class I, DSA segregates with a significant risk of patient death and graft loss following SLKT.
Methods
Patients
All patients who received a SLKT between June 1985 and July 2011 with at least one pre-SLKT sample available for analysis were selected from the Annette C. and Harold C. Simmons Transplant Institute’s biorepository. All patients were consented in an Institutional Review Board-approved tissue acquisition protocol to facilitate their participation in the prospective collection of serum and cells at the time of SLKT, with the understanding that the samples would be used for research. Sera from 86 patients were blinded and sent to Emory University for analysis of class I and II DSA. These patients represent 88% of all SLKT patients transplanted during that time period; the remaining 12% (n = 12) did not have samples available for analysis. 76% of patients tested (n = 65) also had a post-SLKT sample available for analysis; 26% (n = 17) were collected from postoperative day 1 to 6 months, 69% (n = 45) were collected between 6 months and 2 years, and 5% (n = 3) were collected >2 years after transplant.
HLA typing
All patients and donors were typed for HLA-A, -B, -C, DR and DQ antigens (Terasaki HLA Tissue Typing Trays and Micro SSP™ or LabTypeOR SSO; One Lambda Inc., Conoga Park, CA, USA). Typing was performed serologically prior to 1998. Thereafter, all donor class I and II HLA typing, as well as patient class II typing, was performed by molecular methods; patient class I typing is still preformed serologically.
Detection of donor-specific-HLA antibodies
All samples were tested with a flow cytometric microparticle screening assay (FlowPRA; One Lambda, Canoga Park, CA, USA) that simultaneously identified the presence/absence of HLA class I and/or class II antibodies (7). HLA antibody-positive samples (class I and/or class II) were reflexed to a single antigen bead microparticle assay (SAB; One Lambda, Canoga Park, CA, USA) to define antibody specificities to HLA-A, B, C (class I), DRB1, DQB1, DRB 3,4 5, DQA and/or DPB1 (class II) antigens. Specificity testing utilized microparticles coated with individual HLA proteins (class I or class II) and assessed by Luminex™ technology (8) using a modified and validated version of the manufacturer’s recommended procedure. Briefly, 50 μl of serum was added to 10 μl of class I or 5 μl of class II single antigen beads (SAB; One Lambda, Canoga Park, CA, USA). The mixture was incubated in the dark for 30 min at room temperature and washed four times. Then, 20 μl of biotinylated goat antihuman IgG (Jackson Laboratories, Bar Harbor, ME) was added and beads were incubated an additional 30 min and washed twice. Finally, 2011 of Strepavidin conjugated phycoerythrin (SAPE, One Lambda, Canoga Park, CA, USA) was added and the SAB were incubated for a final 15 min, washed twice and resuspended in 100 μl of buffer. Samples were then run and analyzed on a Luminex LABScan 200™ instrument. A positive result was defined as a baseline normalized MFI >2000. The donor specificity of HLA alloantibodies was determined by comparison of the HLA antibody specificities with the HLA typing of the donor.
Clinical and Laboratory Data and Statistical Analysis
Patient and donor clinical, demographic, laboratory and event data (such as biopsy proven rejection) are prospectively collected and locked in the Liver Transplant Research Database (LTRDS). All causes of death in LTRDS have been reviewed and certified by Dr. Göran Klintmalm. Median patient characteristics were calculated when applicable and compared using the Wilcoxon rank-sum test. Chi-squared analyses were utilized to compare proportions. Patient and graft survival was evaluated by Kaplan–Meier analysis and Cox proportional hazards ratios. All cause-mortality was utilized as the primary endpoint since this analysis was designed to detect an association between DSA and adverse outcome, rather than ascribe a cause and effect between DSA and a specific mechanism of illness or injury. Liver failure was grouped together as an endpoint since DSA’s importance in liver allografts is currently under active investigation, and as such, it remains unclear exactly how DSA influences outcome. Cogent arguments can be built for DSA to influence numerous causes of death or graft loss, and it is unlikely that just one complication would capture the potential influence of unchecked humoral immunity. For example, primary graft nonfunction, biliary complications, rejection, even recurrent HCV can relate to the direct effects of DSA, indirect consequences of alloimmunity-directed therapies and DSA’s role as a biomarker for alloimmunity in general. In the future, a prospective evaluation of a large number of transplant patients will be needed to ascribe individual causes of DSA to specific pathologic outcomes. Univariate analysis was undertaken to discover the variables associated with patient survival, and those with a p <0.2 were entered into stepwise multivariable modeling. Only variables in multivariable modeling with a p <0.05 were kept in the final model. Since no patient underwent treatment with interferon therapy, this was not analyzed as a potential confounder. Statistical significance was defined as p <0.05 for all analyses, and SAS was employed for all statistical analyses.
Biopsies
Liver and renal allograft rejection are always biopsy proven; empiric therapy for rejection is never given. A follow-up liver biopsy to document resolution is also always performed. Renal allograft biopsies were blinded and rereviewed by one renal-transplant pathologist (Xin J. Zhou). C4d stains of all renal allograft biopsies were re-reviewed when available and performed on those who previously did not have one on paraffin embedded tissue), except when there was not enough tissue to perform the analysis (n = 3).
Results
Table 1 depicts the characteristics of 86 SLKT patients and their donors. Sixty-seven percent of recipients were male, 56% were white, with a median age of 51, and they were transplanted with donors who had a median age of 22 with 8.7 h of cold ischemia time. The primary liver disease was HCV in 32% of patients and 10% received a re-transplant. The indications for renal transplant were highly variable but diabetic and hypertensive nephropathy were the most common at 26%, followed by glomerulonephritis (14%), polycystic kidney disease (12%) and prolonged dialysis for hepatorenal syndrome or acute tubular necrosis (11%). Tacrolimus was the calcineurin inhibitor of choice at 3 months in 59% of patients and 96% were also on oral steroids as part of their immunosuppression regiment at that time.
Table 1.
Patient and donor characteristics
| Age | Recipient | 51 |
| Donor | 22 | |
| Cold ischemia time | 8.7 h | |
| MELD | 23 | |
| Male | 67% | |
| Race | Black | 13% |
| White | 56% | |
| Other | 31% | |
| Liver diagnosis | Hepatitis C | 36% |
| Alcoholic liver disease | 15% | |
| NASH/CC/metabolic | 15% | |
| Retransplant | 10% | |
| Hepatitis B | 5% | |
| PSC/AIH/PBC | 4% | |
| Other | 15% | |
| Hepatocellular carcinoma | 12% | |
| Immunosupression 3 months | Tacrolimus | 59% |
| Cyclosporine | 37% | |
| Steroids | 96% | |
| Renal indications for Transplant | Diabetic or hypertensive nephropathy | 26% |
| Polycystic kidney disease | 12% | |
| Glomerulonephritis | 14% | |
| HRS/ATN | 11% | |
| Retransplant | 7% | |
| CNI toxicity | 6% | |
| Oxalosis | 5% | |
| Amyloid/IN/reflux | 5% | |
| IgA nephropathy | 2% | |
| Other | 12% |
MELD = Model for End-Stage Liver Disease; NASH = nonalcoholic steatohepatitis; CC = cryptogenic cirrhosis; PSC, primary sclerosing cholangitis; AIH, autoimmune hepatitis; PBC = primary biliary cirrhosis; HRS = hepatorenal syndrome; ATN = acute tubular necrosis; IN = interstitial
Of the 86 patients, 30 (35%) had preformed DSA (Table 2A). Of these, 10 only had preformed class I alloantibodies, 10 only had preformed class II alloantibodies, and 10 had both preformed class I and II alloantibodies. In patients with preformed class I alloantibodies, the median MFI was 18 550 with an interquartile range of 5000–23 000. In patients with preformed class II alloantibodies, the median MFI was 19 400 with an interquartile range of 9100–25 100.
Table 2.
(A) Donor-specific antibody characteristics. (B) Rejection episodes in patients with preformed class II DSA versus no class II DSA (none).
| (A) | Preformed MFI>2000 |
De novo MFI >2000 |
|---|---|---|
| Class I | 10 (11.6%) | 0 |
| Class II | 10 (11.6%) | 8 (10%) |
| Classes I and II | 10 (11.6%) | 1 (1%) |
| None | 56 (65%) | 56 (65%) |
| No sample | 0 | 21 (24%) |
| II DSA (none). |
| (B) | Preformed class II DSA | None | p-value | |
|---|---|---|---|---|
| Rejection < 3 months | 0 | 50% | 83% | 0.01 |
| 1 | 50% | 10% | ||
| 2 | 0 | 7% | ||
| Rejection > 3 months | 0 | 79% | 90% | NS |
| 1 | 21% | 10% | ||
| SRR < 3 months | 0 | 79% | 98% | 0.01 |
| 1 | 21% | 0 | ||
| 2 | 0% | 2% |
MFI = median fluorescence intensity; SRR = Steroid resistant
Seventy six percent of patients had a post-SLKT sample available for analysis. In this group, we identified eight patients with de novo class II DSA, and one patient with de novo class I and II DSA.
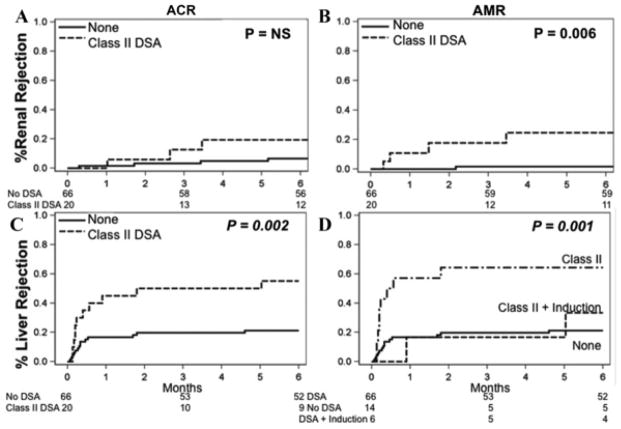
Patients with preformed class I DSA had no change in the risk of acute cellular rejection or antibody mediated rejection of the renal allograft. In addition, there was no change in liver allograft rejection, patient, liver allograft, or renal allograft survival, or renal function when compared to patients without preformed class I DSA (Figure 1A and B). Patients with preformed class II DSA had no change in the incidence of acute cellular rejection of the renal allograft, but had an increased risk of early antibody mediated rejection of the renal allograft and liver allograft rejection (Table 2B and Figure 2A and B). In patients who experienced renal AMR, all but one had C4d positive staining. In those with C4d present, 75% had diffuse peritublar capillary staining and 25% had focal staining.
Figure 1. Risk of (A) all types of renal and (B) liver allograft rejection in patients with preformed class I DSA with MFI > 2000.
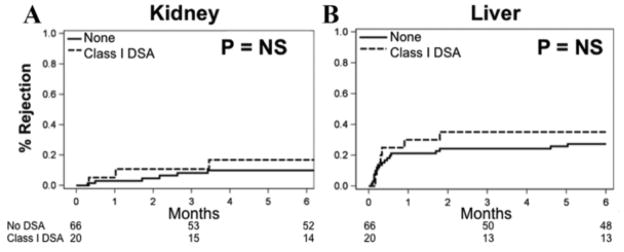
All rejections are biopsy proven. There was no difference in ACR or AMR of the kidney (data not shown).
Figure 2. Risk of (A) renal ACR, (B) renal AMR and (C) liver allograft rejection in patients with preformed class II DSA with MFI > 2000.
(D) Induction decreased the risk of rejection but did not change the overall survival impairment. Induction used was Daclizumab in 16 patients, Daclizumab plus Thymoglobulin in three patients, and OKT3 in two patients. All rejections are biopsy proven
Of note, patients with preformed class II DSA who received induction therapy had a similar (low) risk of liver allograft rejection as patients without preformed class II DSA, unlike those with preformed class II DSA who did not receive induction therapy (Figure 2C and D).
Preformed class II DSA was not only associated with an increased risk of early renal antibody-mediated rejection and liver allograft rejection, but also had a marked negative impact on patient (p = 0.02), liver allograft (p = 0.02) and renal allograft (p = 0.045) survival (Figure 3A–C). Univariate Cox proportional hazards modeling showed a hazards ratio (HR) for death of 2.1 (p = 0.023) in patients with preformed class II DSA. The causes of liver allograft loss or death in patients with class II DSA (either preformed or de novo) were different from those patients without class II DSA (Table 3; p = 0.1). Liver allograft failure occurred in 31% of patients with class II DSA and only 4% of those without class II DSA and the risk of renal failure as the primary cause of death was 11% in those with class II DSA and 6% in those without class II DSA.
Figure 3. Preformed class II DSA (MFI > 2000) decreases (A) patient, (B) liver allograft and (C) renal allograft survival.
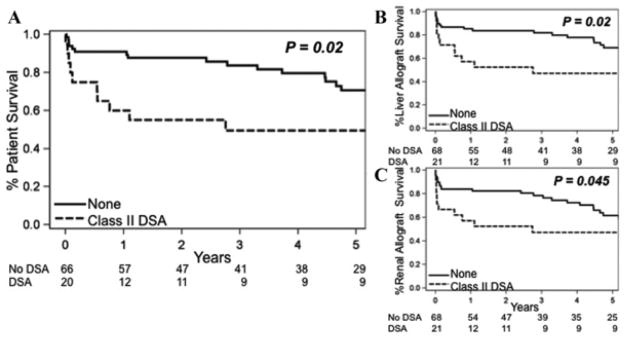
Table 3.
Reasons for death or graft failure in SLKT recipients with either preformed or de novo class II DSA (MFI > 2000) versus those without DSA (p = 0.01).
| Class II DSA | No DSA | |
|---|---|---|
| Alive | 31% | 60% |
| Liver allograft failure1 | 31% | 4% |
| Renal failure | 11% | 6% |
| Cancer/infection/other | 27% | 28% |
| Unknown | 0% | 2% |
Liver allograft failure was found in 8 patients with class II DSA: 2 with primary graft nonfunction, 2 with acute or chronic rejection, 2 with biliary causes, and 1 with recurrent hepatitis C. Two patients died from liver allograft failure without class II DSA: one from recurrent hepatitis C and one from recurrent hepatitis B with vascular thrombosis.
Univariate modeling was performed and factors with a p <0.2 were incorporated into stepwise multivariable modeling. Class II DSA (either preformed or de novo) in the multivariable model had an increased risk of death (HR = 2.2, p = 0.043) and liver allograft loss (HR = 2.2, p = 0.044; Table 4). The risk of renal allograft loss was numerically increased (HR = 2.0, p = 0.066), although did not meet the p < 0.05 cutoff for significance.
Table 4.
Univariate analysis was undertaken and all factors with p < 0.2 (all the factors shown) were entered into a stepwise multivariable model. The final multivariable model shows that only three factors remained significantly associated (p < 0.05) with patient and liver allograft survival. Class II DSA for this analysis was defined as either preformed or de novo.
| Patient
|
Liver graft
|
Renal graft
|
||||
|---|---|---|---|---|---|---|
| HR | p-Value | HR | p-Value | HR | p-Value | |
| Class II DSA | 2.2 | 0.043 | 2.2 | 0.044 | 2.0 | 0.066 |
| Steroids 1 month | 0.03 | 0.004 | 0.03 | 0.004 | 0.07 | 0.022 |
| Recipient age >50 | 6.4 | <0.001 | 6.3 | <0.001 | 2.8 | 0.024 |
| Cytomegalovirus | NS | NS | NS | |||
| Acute cellular rejection | NS | NS | NS | |||
| Hepatocellular carcinoma | NS | NS | NS | |||
| MELD >15 | NS | NS | NS | |||
| Induction | NS | NS | ||||
MELD = Model for End-Stage Liver Disease.
The fate of preformed DSA depended on the class of alloantibody (Supporting Figure S1). Of 20 patients with preformed class I DSA, 15 had a follow-up sample available for analysis. Four patients had persistent class I; however, one patient’s sample was drawn on postoperative day 1 and the second postoperative day 17. Only two patients had persistent class I DSA that was present at the time of transplant and at 4 months and 1 year respectively; in both patients, the MFI values of the DSA displayed a significant decrease in MFI compared to the pretransplant samples. Of the 20 patients with preformed class II DSA, 17 had a follow-up sample available for analysis. Five patients had clearance of their preformed DSA (all with MFI < 12 000), and in 12 patients, the class II DSA persisted.
Discussion
Empiric clinical observation has shown that although kidney transplantation is contraindicated in the presence of a positive crossmatch due to the risk of hyperacute rejection, it can proceed when accompanied by a liver allograft from the same donor. This has led to a general ambivalence regarding the importance of DSA in SLKT. However, as data accumulate from SLKT patients, there is sufficient cause for concern that the role of DSA is worthy of greater attention. This study builds on the alarm sounded by prior case reports (1), registry analyses (2) and other studies (3–6) to examine the issue. Our study has the advantage of reasonably large numbers facilitated by a longstanding prospective database and tissue acquisition policy, combined with the detail afforded by a single center experience with access to granular clinical and histocompatibility data. We find that when studied with this vantage, class II DSA has a significant and concerning impact on the subsequent outcome of SLKT patients, one that may warrant a shift away from the current clinical dismissal of DSA.
Several aspects of this work deserve attention. Currently, it appears that the presence of a liver allograft mitigates any substantial risk to a kidney from class I DSA. Indeed, as shown here, preformed class I DSAs, even with MFI values >10 000, are adequately cleared in the vast majority of cases. Although not designed to assess mechanism, this study suggests an absorption effect driven by the ubiquitous nature of class I on liver vascular and parenchymal tissue, and a general resistance of the liver to bound class-I antibody, although downregulation of DSA production after transplantation (especially in those receiving induction therapy) cannot be excluded. In stark contrast, class II DSA, especially with high MFI values (> 10 000), typically persists post-SLKT. These findings are consistent with previous case series observations (1) in suggesting that the liver’s absorptive capacity for class II-directed antibodies is limited. Indeed, not only did class-II DSA portend a worse outcome for the kidney, patients with such preformed class II DSA have a marked increased risk of liver allograft rejection, suggesting that the effects of bound class-II antibody were less well tolerated. This may relate to the distribution of class II on hepatic antigen present cells. Although allograft rejection can be abated by antibody induction, such treatment did not protect patients from graft loss or death (Figure 2D).
In addition to an increased risk of liver rejection, preformed class II DSA also carries a significant risk of renal AMR (1,6). This has been shown by others and confirms the incomplete protective effect of a concomitant liver allograft.
Unfortunately, risk to both the kidney and liver may exist from preformed DSA. Some prior investigators have evaluated the presence of pathologic DSA in liver tissue through immunohistochemistry with C4d staining. This technique, while being an important part of the standard protocol for renal transplant biopsies, plays an undefined role in the assessment of liver transplant biopsies, particularly in paraffin embedded liver tissue. C4d staining when positive can tell you that local complement activation has occurred, but does not confirm or refute the presence of DSA. In the future, it will be important to prospectively evaluate this with serial DSA measurements, and in those who develop rejection, a DSA evaluation at that time combined with a comparison of the cellular infiltrate recruited and complement activation seen in patients with and without DSA present. However, because the DSA MFI was not available at the time of rejection in our current cohort, outcomes such as patient survival, graft loss and renal AMR were the focus of our study.
Most often, the cause of death among patients with class II DSA (preformed or de novo) is liver allograft failure. We grouped liver allograft failure together, regardless of the etiology, since we showed an association between preformed and de novo DSA and accelerated HCV fibrosis progression (9). Therefore, liver allograft failure may be directly caused by DSA in cases of chronic rejection or unexplained biliary complications, or indirectly caused by DSA in cases of accelerated fibrosis from HCV-infection, likely through igniting the immune system against HCV (10–12).
Clearly, not all patients in this study with class II DSA died from liver or kidney failure. As such, there is an incomplete penetrance of the DSA-associated risk. Regardless, the effect size, particularly when considering survival as the ultimate endpoint, warrants attention. Patients may also die from other indirect causes of DSA, such as infection from intense immunosuppression that resulted from treating rejection. For example, the one patient who developed de novo class I and II DSA had repeated rejection episodes and died from pneumonia 6 months after transplant with functioning organs. This study is not powered or designed to assess etiology, but rather raises a clear flag of concern and hopefully will spur prospective analysis. This is needed since our cohort crosses a large time span in transplantation, and we were not powered to assess subgroups from different eras.
Of note, the risk for death was greatest within the first 1–2 years after transplant in patients with preformed class II DSA. This suggests that those with preformed class II DSA experience the detrimental effects earlier on, and that in those who survive, either antibody clearance or accommodation is achieved or the HLA-antibody may not be a true DSA (i.e. it may be directed against a denatured antigen only found on the single antigen beads that is not relevant in vivo). Also of interest, was the finding that steroids at 1 month was protective against death. This warrants further investigation in SLKT recipients in larger trials. However, it was not surprising that older recipient age had a major negative impact on survival in multivariable analysis. Our data suggest that improvement in SLKT outcomes will require a deeper consideration of preformed class II DSA that recipients possess. In those who are positive, follow-up testing for clearance seems prudent. Induction antibody therapy can be considered, and while we anticipate it will decrease the risk of rejection, it may not improve survival. A more aggressive approach would be to consider the antigens complimentary to the class II DSAs as unacceptable, meaning that neither the liver nor the kidney would be allocated to the recipient. These considerations must be made in concert with a measured assessment of the immediate risk of death in patients with decompensated liver disease.
Before a DSA-centric approach could be implemented on a national scale, there must be more definitive data characterizing the class II alloantibodies that are associated with poor patient and graft outcome including characteristics such as IgG subclass and complement fixing ability. (13) Clearly, additional prospective work in larger cohorts is needed, but for now, close follow-up of SLKT patients with preformed high MFI (> 10 000) class II DSA seems prudent. We must learn to distinguish pathologic DSA from nonpathologic DSA, and while we are doing that–through hypervigilancehope to diagnose AMR of the liver and/or kidney earlier leading to improved outcomes (14).
Supplementary Material
Acknowledgments
We would like to acknowledge Linda Jennings PhD for her work on the statistical analysis for this manuscript.
Abbreviations
- AMR
antibody-mediated rejection
- DSA
donor-specific alloantibodies
- LTRDS
Liver Transplant Research Database
- MELD
Model for End-Stage Liver Disease
- MFI
median florescence intensity
- SLKT
simultaneous liver-kidney transplants
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Dar W, Agarwal A, Watkins C, et al. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am J Transplant. 2011;11:841–847. doi: 10.1111/j.1600-6143.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 2.Askar M, Schold JD, Eghtesad B, et al. Combined liver-kidney transplants: Allosensitization and recipient outcomes. Transplantation. 2011;91:1286–1292. doi: 10.1097/TP.0b013e3182184181. [DOI] [PubMed] [Google Scholar]
- 3.Takaya S, Duquesnoy R, Iwaki Y, et al. Positive crossmatch in primary human liver allografts under cyclosporine or FK 506 therapy. Transplant Proc. 1991;23(Pt 1):396–399. [PMC free article] [PubMed] [Google Scholar]
- 4.Ogura K, Koyama H, Takemoto S, Terasaki PI, Busuttil RW. Significance of a positive crossmatch on outcome in human liver transplantation. Transplant Proc. 1992;24:1465. [PubMed] [Google Scholar]
- 5.Castillo-Rama M, Castro MJ, Bernardo I, et al. Preformed antibodies detected by cytotoxic assay or multibead array decrease liver allograft survival: Role of human leukocyte antigen compatibility. Liver Transpl. 2008;14:554–562. doi: 10.1002/lt.21408. [DOI] [PubMed] [Google Scholar]
- 6.Olausson M, Mjornstedt L, Norden G, et al. Successful combined partial auxiliary liver and kidney transplantation in highly sensitized cross-match positive recipients. Am J Transplant. 2007;7:130–136. doi: 10.1111/j.1600-6143.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 7.Pei R, Wang G, Tarsitani C, et al. Simultaneous HLA Class I and Class II antibodies screening with flow cytometry. Hum Immunol. 1998;59:313–322. doi: 10.1016/s0198-8859(98)00020-2. [DOI] [PubMed] [Google Scholar]
- 8.Bradley JA, Baldwin WM, Bingaman A, et al. Antibody-mediated rejection—an ounce of prevention is worth a pound of cure. Am J Transplant. 2011;11:1131–1139. doi: 10.1111/j.1600-6143.2011.03581.x. [DOI] [PubMed] [Google Scholar]
- 9.O’Leary JG, Kaneku H, Suskind BM, Jennings LW, Terasaki PI, GBK high MFI preformed class I & de novo class II DSA accelerate fibrosis progression after liver transplant in HCV-infected patients. Am J Transplant. 2011:Abstract782. [Google Scholar]
- 10.O’Leary JG, Kaneku H, Susskind BM, et al. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection postliver transplant. Am J Transplant. 2011;11:1868–1876. doi: 10.1111/j.1600-6143.2011.03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musat AI, Agni RM, Wai PY, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. 2011;11:500–510. doi: 10.1111/j.1600-6143.2010.03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirohashi T, Chase CM, Della Pelle P, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12:313–321. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaneku H, O’Leary JG, Taniguchi M, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with chronic rejection and graft loss after liver transplantation. Liver Transpl. 2012;18:984–992. doi: 10.1002/lt.23451. [DOI] [PubMed] [Google Scholar]
- 14.Paterno F, Shiller M, Tillery G, et al. Bortezomib for acute antibody-mediated rejection in liver transplantation. Am J Transplant. 2012;12:2526–2531. doi: 10.1111/j.1600-6143.2012.04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.