Summary
σ32 controls expression of heat shock genes in Escherichia coli and is widely distributed in proteobacteria. The distinguishing feature of σ32 promoters is a long −10 region (CCCCATNT) whose tetra-C motif is important for promoter activity. Using alanine-scanning mutagenesis of σ32 and in vivo and in vitro assays, we identified promoter recognition determinants of this motif. The most downstream C (−13) is part of the −10 motif; our work confirms and extends recognition determinants of −13C. Most importantly, our work suggests that the two upstream Cs (−16, −15) constitute an ‘extended −10’ recognition motif that is recognized by K130, a residue universally conserved in β- and γ-proteobacteria. This residue is located in the α-helix of σDomain 3 that mediates recognition of the extended −10 promoter motif in other δs. K130 is not conserved in α- and δ-/εproteobacteria and we found that σ32 from the α-proteobacterium Caulobacter crescentus does not need the extended −10 motif for high promoter activity. This result supports the idea that K130 mediates extended −10 recognition. σ32 is the first Group 3σ shown to use the ‘extended −10’ recognition motif.
Introduction
Bacterial transcription initiation is mediated by RNA polymerase holoenzyme, consisting of a 5-subunit core RNA polymerase (α2ββ’ω) and a dissociable subunit, σ. σs contain many of the promoter recognition determinants (Gross et al., 1998). Most transcription in exponentially growing cells is initiated by RNA polymerase holoenzyme carrying the housekeeping σ, called σ70 in Escherichia coli. Alternative ss mediate transcription of regulons activated by specific stress conditions, or by growth or developmental transitions. The σ70 family of proteins has been divided into four groups, based on their phylogenetic relationships and modular structure (Lonetto et al., 1992; Gruber and Gross, 2003). Group 1 σs are the essential housekeeping ss and contain four domains, of which the first is unique to the Group 1 σs. Group 2 ss are most closely related to Group 1 σs, but are not essential. Most Group 3 σs contain Domains 2–4, and the Group 4 σs contain only Domains 2 and 4 (Gruber and Gross, 2003).
Promoters recognized by σ70 and other Group 1 σs have been extensively studied. Promoters are multipartite, and can have the following potential elements to mediate recognition: (i) the ‘UP element’, generally located from −61 to −41 relative to the transcription start point (Ross et al., 1993; Gourse et al., 2000), (ii) the −35 region, a hexamer (TTGACA) centred about 35 bp upstream of the transcription start point (Gross et al., 1998), (iii) the ‘extended −10 motif’ (TG) located immediately upstream of the −10 region (Mitchell et al., 2003), (iv) the −10 region, a hexamer (TATAAT) centred about 10 bp upstream of the transcription start (Helmann and deHaseth, 1999) and (v) the discriminator region (GGGa) located downstream of the −10 region of the promoter (Feklistov et al., 2006; Haugen et al., 2006). With the exception of the UP element, recognized by the C-terminal domain of α, promoter elements are recognized by the σ subunit. The −35 element is recognized by Region 4.2 in sDomain 4; the extended −10 element by an α-helix in σDomain 3; the −10 by Region 2.4 in σDomain 2 and the discriminator by σRegion 1.2 (Campbell et al., 2002; Feklistov et al., 2006; Haugen et al., 2006). The number of elements in promoters is variable.
Promoters recognized by the housekeeping σ in E. coli can be roughly divided into those with an extended −10 motif (‘extended −10 promoters’) and those without this motif. Extended −10 promoters have a lower match to the consensus −35 motif than do those lacking the extended −10 motif (Mitchell et al., 2003). Moreover, at least in vitro, extended −10 promoters can function without σRegion 4.2, the −35 recognition region (Kumar et al., 1993; Ponnambalam et al., 1986; Minakhin and Severinov, 2003; Young et al., 2004). Together these results imply that the extended −10 may functionally substitute for the −35 promoter region. Interestingly, in Bacillus subtilis and other Gram-positive bacteria, this distinction does not hold: > 50% of the promoters have high consensus to all three elements (−35, extended −10 and −10) (Graves and Rabinowitz, 1986; Bashyam and Tyagi, 1998; Helmann, 1995; Sabelnikov et al., 1995).
The ‘discriminator’ region serves as an ancillary element in promoters utilized by the E. coli housekeeping s. It is most important for stable RNA promoters, which characteristically form unstable complexes with RNA polymerase; their instability is largely attributable to a very disfavoured discriminator sequence (Haugen et al., 2006). However, for the thermophilic bacterium Thermus aquaticus housekeeping σ, the discriminator, like the extended −10 motif, can substitute for a −35 element (Feklistov et al., 2006; Barinova et al., 2008). The UP element enhances promoter activity, especially in weaker promoters (Ross et al., 1998; Miroslavova and Busby, 2006).
Promoters of the Group 3 σs and their σ recognition determinants have not been nearly as well studied. However, one contrast between many Group 3 σ promoters and those recognized by housekeeping σs has been noted. Promoters of the Group 3 σs often have a relatively long −10 promoter region, whose upstream end is GC-rich, separated by only 13 or 14 bp from the −35 region of the promoter (Table 1). This configuration suggests the possibility that the long −10 promoter region includes an ‘extended −10’ region adjacent to the −10 region of the promoter. That known ‘extended −10's include either G or C nucleotides (TG: Group 1 σs; T/GC σS, a Group 2 σ) would be consistent with this idea (Helmann, 1995; Becker and Hengge-Aronis, 2001; Mitchell et al., 2003). However, studies to date, on promoters recognized by Group 3 σs provided no evidence for this idea. Neither of the promoters recognized by σE and σH, two B. subtilis Group 3 σs, had been found to have an extended −10 motif (Daniels et al., 1990; Tatti et al., 1991). The major goal of this work was to determine whether an extended −10 recognition element is used by a Group 3 σ.
Table 1.
Consensus sequence of Group 3 s promoters.
| Promoter sequence |
|||
|---|---|---|---|
| σs | –35 | –10 | Reference |
| E. coli σ70 | -TTGACA--------------TG-TATAAT | Gross et al. (1998) | |
| E. coli σ32 | -TTGAAA--------------CCCCAT-T- | Nonaka et al. (2006) | |
| E. coli σ28 | --TAAAGTTT-----------GCCGATAA- | Zhao et al. (2007) | |
| B. subtilis σD | --TAAAG--------------GCCGATAT | Serizawa et al. (2004) | |
| B. subtilis σB | -GTTTAA--------------GGGTAT--- | Petersohn et al. (2001) | |
| B. subtilis σF | -GTATWW--------------GG--A---T | Amaya et al. (2001) | |
| B. subtilis σE | -TCATATT--------------CATA-A-T | Eichenberger et al. (2003) | |
| B. subtilis σH | RNAGGAWWW-----------R- - GAATWW- | Britton et al. (2002) | |
R,Aor G; W,Aor T.
We chose to determine whether E. coli σ32 contains an extended −10 element both because we can build on the extensive studies already performed on σ32 and because it is widely distributed in proteobacteria (Nakahigashi et al., 1995). Several global studies of promoters recognized by σ32 suggested that the consensus sequence of σ32 promoter is TTGAAA – N13-14 – CCCCATNT (Zhao et al., 2005; Nonaka et al., 2006; Wade et al., 2006). A careful mutational analysis of PgroE, a strong σ32 promoter, conducted by deHaseth and collaborators, validated that the conserved −35 region and the entire −10 region, including the tetra C-motif (−16C to −13C), are important for promoter activity (Wang and deHaseth, 2003). More recently, deHaseth has examined promoter recognition determinants in σ32, selecting residues for substitution based on known structures of various ss and by comparison with known promoter recognition determinants in σ70 (Kourennaia et al., 2005). These studies showed that recognition of the −35 region was mediated by Region 4.2 residues in σ32 analogous to those used by s70. In contrast, residues in Region 2.4 analogous to those in σ70 did not mediate recognition of the −10 element. However, W108, at the border between Regions 2.3 and 2.4, located at the end of the α-helix proposed to mediate −10 recognition, did affect recognition of −13C. This work established that the most downstream C is part of the −10 region of the promoter, but leaves unsettled whether the upstream Cs are an extended −10 motif.
σ32 controls the heat shock response and is highly regulated to maintain homeostasis (Guisbert et al., 2008). This presents a challenge when investigating promoter recog- nition of σ32. Both the amount and activity of σ32 can be adjusted by negative feedback loops when cellular amounts of key members of the regulon change. As a consequence, when a mutant σ32 defective in promoter recognition produces insufficient amounts of key regulon members, the regulatory system compensates by overex-pressing the defective σ32, thereby obscuring promoter recognition defects. To address this problem, we developed a new assay system, using an allele of σ32 that is no longer subject to regulation (Yura et al., 2007). Using this assay system, newly constructed libraries of promoter mutants and an alanine scanning mutagenesis library encompassing Region 2.4 in Domain 2 and Region 3.0 in Domain 3 of σ32, we examined recognition of the −10 region. Our studies indicate that the −10 region is a composite element. The two upstream Cs are recognized by Domain 3 and thus likely correspond to an extended −10 element, whereas the downstream C is recognized by two Region 2.4 residues and is therefore part of the −10 element. Thus, in contrast to the σ70 paradigm, E. coli σ32 promoters require both a canonical −35 region and the extended −10 promoter region for high activity. Interestingly, in some groups of proteobacteria, the σ32 residue in Domain 3 that recognizes the upstream Cs is missing. Investigation of one such σ32 reveals that it no longer needs the extended −10 promoter region for high activity.
Results
In vivo assay of σ32 activity
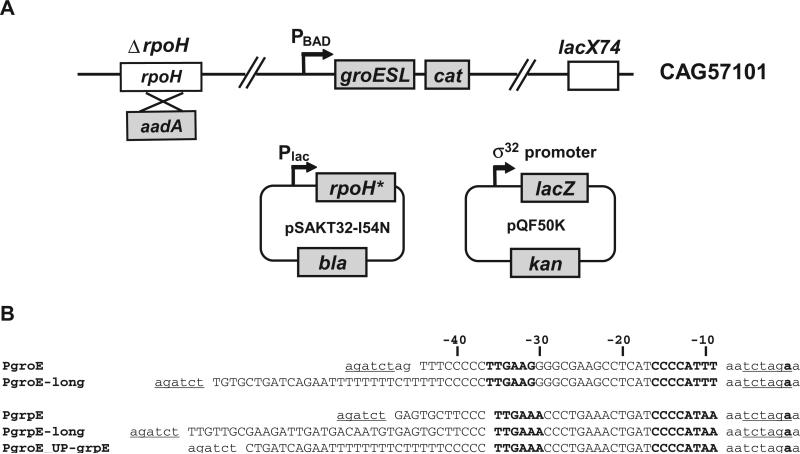
A robust in vivo assay system for the activity of mutant σ32 proteins must be solely dependent on the properties of the mutant protein, and impervious to the regulatory loops that can adjust the amount of σ32 to compensate for its activity deficit. To achieve these goals, the previous assay system used a host background with two attributes: (i) a chromosomal rpoH locus that encoded a σ32 with very reduced function and (ii) a partially disabled regulatory circuit [by substituting dnaK756 for wild-type (wt) dnaK] (Wang and deHaseth, 2003). This system allowed assay of altered function σ32 above a background of expression mediated by the endogenous locus. Our new assay system goes further; it eliminates chromosomal σ32 and completely disables the σ32 regulatory loop. The assay system consists of a host strain lacking σ32 and two plasmids, one supplying mutant σ32 alleles, and the other supplying σ32 promoter mutants of the groE and grpE σ32 promoters fused to the lacZ reporter (Fig. 1A). Overexpression of GroESL is known to permit survival in the absence of σ32 (Kusukawa and Yura, 1988). Thus, the host strain can survive in the absence of σ32 because the σ32 promoter of the groESL operon is replaced with the regulatable σ70 PBAD promoter and GroESL can be over-expressed constitutively by addition of arabinose in medium. The plasmid-encoded mutant σ32 alleles are under the control of an IPTG-inducible promoter and were constructed in a σ32 backbone that contains the I54N substitution, which renders σ32 impervious to regulation of its activity or stability (Yura et al., 2007). In this assay system, the wt groE and grpE promoters (Fig. 1B, PgroE, PgrpE, containing sequences from −44 to −9 of each promoter) had a basal β-galactosidase activity of < 1 and 3 Miller units respectively. Following induction of I54N σ32, the activity of these promoters were ~130 and 90 Miller units respectively. Thus, this assay system has excellent signal to noise ratio. Moreover, as discussed below, the I54N σ32 variant does not alter promoter recognition.
Fig. 1.
Components of in vivo assay systems used in this study.
A. Activity of σ32-dependent promoters in the presence of σ32 variants was determined by β-galactosidase assay from the ΔrpoH strain CAG57101 carrying the plasmids pSAKT32 and pQF50K. σ32 expression was induced from pSAKT32 and derivatives (pBK116–pBK151), which has a p15A replication origin and rpoH under control of the lac promoter. Note that most of the σ32 derivatives contain the additional substitution I54N that improves stability and decreases negative regulation. The reporter plasmid, pQF50K and derivatives (pBK501–pBK549), has a pMB1 replication origin and lacZ under control of σ32 promoter.
B. Sequences of σ32-dependent promoters used in this study. Only wt sequences of each promoter are shown: single- or double-base changed promoter mutants were constructed from these parent promoters. The native sequences of the groE and grpE promoter regions are shown in capital letters; vector sequences are in lower case; BglII and XbaI cloning sites are underlined; −35 and −10 regions, and the
Comparison of base dependence of σ32 promoters with different spacing
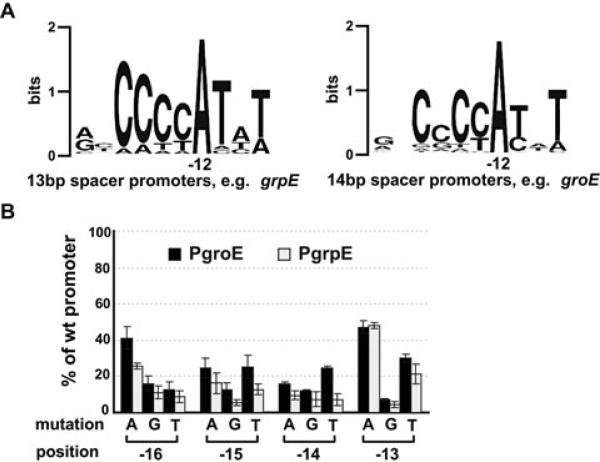
Virtually all σ32 promoters have a spacing of either 13 or 14 bp between the −35 and −10 region, with a 14 bp spacing being optimal (Wang and deHaseth, 2003; Nonaka et al., 2006). Before analysing the effects of mutant σ32s on −10 promoter recognition, it was important to determine whether promoters with each spacing showed similar dependence on positions in the −10 region. We used both a bioinformatics and experimental approach to examine this issue. We classified the 50 σ32 promoters validated in our previous work (Nonaka et al., 2006) by their spacer length and then aligned them to see whether spacer length affected consensus. Our new alignment showed only one difference between promoters with a different spacer length: the upstream CC motif was highly conserved in the 16 promoters with a 13 bp spacer; the second C of this motif is less conserved in 27 promoters with a 14 bp spacer (Fig. 2A). To examine this issue experimentally, we created two identical libraries that are mutant at every conserved position in the −10 region, one in the grpE promoter (13 bp spacing) and a second in the groE promoter (14 bp spacing) (Fig. 1B) and examined the relative transcription of each promoter variant in both backgrounds (Fig. 2B). This permitted two conclusions: (i) the relative effects of single mutant changes in the −10 region are similar regardless of spacing and (ii) the promoter preference of I54N σ32 on the groE library (Fig. 2B) is virtually identical to that reported by deHaseth using wt σ32 (Wang and deHaseth, 2003), eliminating any concern that using the I54N σ32 variant might perturb results.
Fig. 2.
Sequence conservation in the −10 region of E. coli σ32 promoters and effects of single-base mutations at the −10 region tetra-C motif on activities of groE or grpE promoter.
A. Sequence logos (http://weblogo.berkeley.edu; Crooks et al., 2004) denoting sequence conservation within the −10 region of 13 bp spacer and 14 bp spacer promoters. Sequences of 16 σ32 promoters with a 13 bp spacer and 27 promoters with a 14 bp spacer (previously identified by Nonaka et al., 2006) were used to generate sequence logos of the −10 region.
B. Activity of each mutant groE or grpE promoter with wt σ32 is shown as a percentage of measured β-galactosidase activity of its respective wt promoter. Assay strains are as described in Fig. 1A. Promoter mutations and positions are shown on the x-axis. All values are averages of at least three independent experiments; error bars indicate one standard deviation; black bars, groE promoter activities; grey bars, grpE promoter activities.
General strategy for in vivo analysis of mutant σs and overview of results
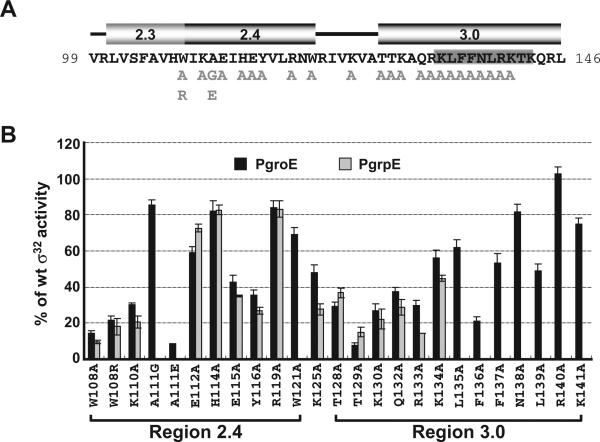
The major goal of this work was to determine whether part of the tetra-C motif in the −10 promoter region is an extended −10 motif. In both Group 1 and Group 2 σs, recognition of this motif is carried out by an α-helix at the N-terminus of Domain 3. By analogy, this same helix should mediate extended −10 recognition in σ32. We therefore performed extensive alanine substitution mutagenesis in this region of Domain 3 of I54N σ32. In addition, we mutagenized the RpoH box region, a σ32-specific insertion in Region 3.0 in Domain 3 that is the most highly conserved region among all σ32 orthologues. Finally, we remade the Region 2.4 substitutions initially assayed by deHaseth and tested them in our new assay system to see if the improved resolution of this system provided new results. In addition, we tested a few additional substitutions in this region (Fig. 3A). Assays of transcriptional activity on both wt PgroE and PgrpE revealed that with the exception of R140A, each mutant s showed some impairment of transcriptional capacity, ranging from mild to severe (Fig. 3B).
Fig. 3.
Effects of single amino acid substitutions in Regions 2.4 and 3.0 of σ32 on activity of the groE and grpE promoters.
A. Amino acid sequence of Regions 2.3, 2.4 and 3.0 of σ32. Numbers at each end of the sequence indicate amino acid positions. Single-amino acid substitutions of σ32 used in this study are shown in light grey below the main sequence. The location of the RpoH box is denoted as a grey box (Nakahigashi et al., 1995); positions of the αhelices in each region are shown above the sequence. These data are derived both from structural data indicating that these s subregions are virtually identical in σ70, a Group 1 σ, and σ28, a Group 3 σ (Campbell et al., 2002; Sorenson et al., 2004), and secondary structure predictions for σ32 that are completely consistent with these assignments (E. Campbell, data not shown).
B. Activity of the σ32 Regions 2.4 and 3.0 mutants at the wt groE and grpE promoters. β-galactosidase activity driven by each mutant σ32 from the wt groE or grpE promoters is shown as a percentage of that driven by wt σ32. Assay strains are as described in Fig. 1A. The different σ32 amino-acid substitutions and their locations are shown on the x-axis. Note the effects of several σ32 substitutions (W108R, A111G, A111E, W121A and from L135 to K141) were not measured at the grpE promoter. All values are averages of at least three independent experiments; error bars indicate one standard deviation; black bars, groE promoter activities; grey bars, grpE promoter activities.
We next determined whether any of these mutant ss were specifically impaired in their interaction with a particular base in the promoter, using a well-validated allele-specific suppression criterion that has been applied to identify such interactions in both transcription factors and in other σs (Ebright et al., 1984; Ebright, 1986; Hochschild and Ptashne, 1986; Hochschild et al., 1986; Siegele et al., 1989). Basically, if the residue in σ that recognizes the consensus base at position X in the promoter is altered, then expression mediated by this mutant σ should be relatively unaffected by base changes at position X, but as sensitive as wt σ to changes at all other positions in the promoter. In the best-case scenario, promoters having a mutation at position X will be transcribed equivalently to the wt promoter by the s mutant lacking the residue mediating recognition.
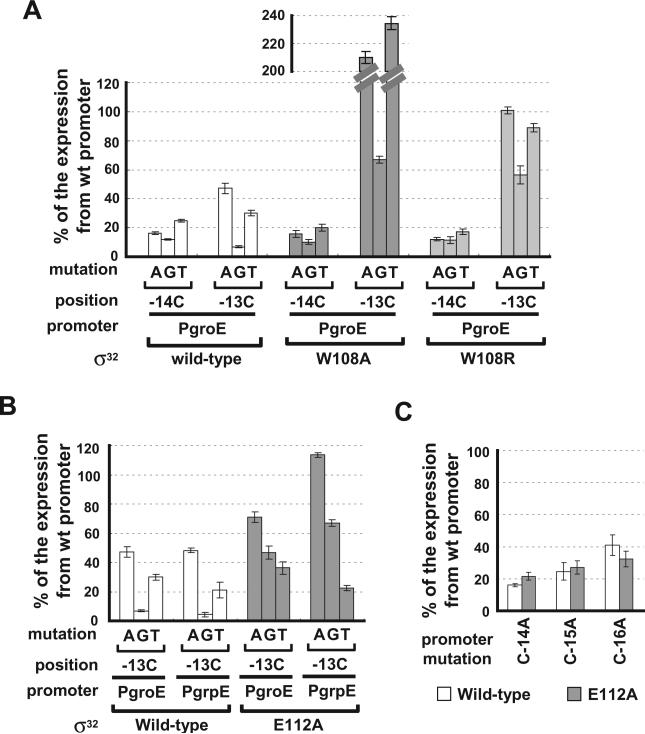
To this end, we assayed the wt σ and all mutant ss on a PgroE library in which all positions except N in the −10 region CCCCATNT had been changed to every other base and on a PgrpE library in which each of the four Cs had been changed to every other base. Wt and mutant ss showed equivalent decreases at almost all promoters assayed, as expected (data not shown). However, several base-specific interactions with the tetra-C motif were detected: two amino acids in Region 2.4 recognized the downstream C (−13; Fig. 4) and an amino acid in the N-terminal helix of Domain 3 recognized the two upstream Cs (−16, −15; Fig. 5). Thus the tetra-C motif is a hybrid element, whose upstream end is an extended −10 motif and whose downstream end is part of the core −10 region.
Fig. 4.
Effects of substitutions in Region 2.4 of σ32 in suppressing promoter mutations at positions −14 and −13. A. Effect of W108A σ32 and W108R σ32 on the groE promoter with single-base changes at the −14 or −13 positions.
B. Effect of E112A σ32 on groE and grpE promoters with single-base changes at the −13 position.
C. Effect of E112A σ32 on groE promoter with a single-base changes at positions −14, −15 and −16. β-galactosidase activity driven by each σ32 variant from mutant groE or grpE promoters is shown as a percentage of β-galactosidase activity driven by each σ32 variant from the wt promoter. Assay strains are as described in Fig. 1A. Below the x-axis, mutation and position indicate the base change at the different promoter positions; promoter indicates the groE or grpE promoter; σ32 indicates the σ32 wt or mutant derivative. All values are averages of at least three independent experiments; error bars indicate one standard deviation.
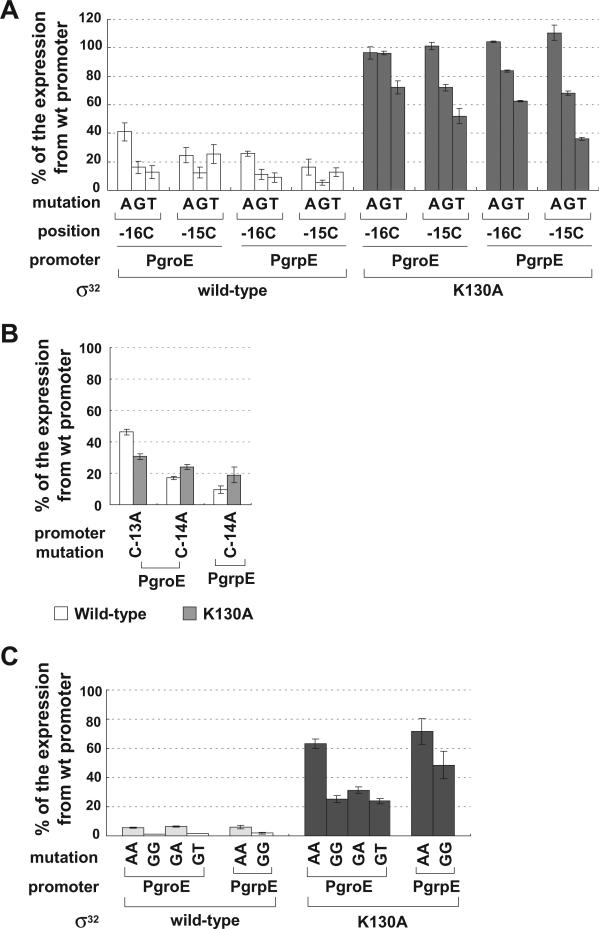
Fig. 5.
Effect of substitution K130A in Region 3.0 of σ32 in suppressing promoter mutations at positions −16 and −15. A. Effect of wt and K130A σ32 on promoters with single-base changes at position −16 or −15.
B. Effect of K130A σ32 on groE and grpE promoters with single-base changes at other positions in the promoter.
C. Effect of wt and K130A of σ32 on promoters with double-base changes at positions −16 and −15. β-galactosidase activity driven by each σ32 variant on mutant groE or grpE promoters is shown as a percentage of β-galactosidase activity of each σ32 variant from the wt promoter. Assay strains are as described in Fig. 1A. Below the x-axis, mutation and position indicate the base change at the different promoter positions; promoter indicates the groE or grpE promoter; σ32 indicates the σ32 wt or mutant derivative. All values are averages of at least three independent experiments; error bars indicate one standard deviation.
Identification of base-specific interactions between Region 2.4 of σ32 and the −10 region of promoter
A previous study implicated W108 in recognition of −13C (Kourennaia et al., 2005). However, somewhat unexpectedly, they found that C-13T was not suppressed by W108A (Kourennaia et al., 2005). Results in our new assay system paint a simpler picture. W108 recognizes −13C: W108A σ32 is almost completely insensitive to changes at −13 (Fig. 4A). Suppression is specific as it is not manifest on promoters with changes at −14C (Fig. 4A) or at any other promoter variant tested (data not shown). Interestingly, the W108A mutant showed almost twofold higher activity on the C-13A and C-13T promoters than on the wt promoter. Possibly, W108A may make adventitious contacts at C-13A/T promoters that are not possible in wt σ32, leading to the increased activity. Consistent with this idea, substituting a bulky residue at position 108 (W108R) led to suppression but not increased activity at C-13A/T (Fig. 4A). Both the W108A and R substitutions also specifically suppressed −13C changes in grpE promoter variants (data not shown).
Our results also implicate E112 in −13C recognition. E112A decreased expression from the wt promoter only about 20–40% (Fig. 3B), but partially suppressed promoter mutations at −13C, especially C-13A and C-13G (Fig. 4B). Suppression is specific as E112A did not suppress promoter mutations at C-14, −15 or −16 (Fig. 4C). E112 may participate with W108 in recognizing −13C. Alternatively, E112 may inhibit other nearby residues from interacting with the −13C position. 112A might allow these adventitious interactions and thereby suppress the deleterious effect of base changes at −13C.
Identification of a base-specific interaction between Region 3.0 of σ32 and the −10 region of promoter
Of the 14 residues mutated in Domain 3, only K130 was identified as mediating a base-specific interaction. K130A had a significant defect in transcribing both PgroE and PgrpE (about 25% activity of wt σ32; Fig. 3B) and suppressed all mutational changes at −16C and −15C to a variable extent (Fig. 5A). C to A changes at both positions were completely suppressed in both promoters, exhibiting the same activity as the wt promoter. C to G changes were either completely or almost completely suppressed and C to T changes were moderately suppressed. Suppression is specific as K130A did not suppress promoter mutations at −13C or −14C (Fig. 5B). Taken together, these results suggest that the K130 residue makes base-specific contacts with both −16C and −15C. To test this idea, we determined whether K130A was able to suppress promoters with base changes at both −16C and −15C (Fig. 5C). K130A suppressed these mutations effectively in both groE and grpE promoters. Importantly, the CC to AA change was almost completely suppressed, consistent with the fact that each individual change was also completely suppressed (Fig. 5C). As expected, the double mutant promoters were expressed very poorly in cells with wt σ32 (Fig. 5C). The fact that the activity of the double mutants was less than that of the K130A mutant could result from negative interactions of the changed bases with K130; changing K130 to A could alleviate these clashes. Alternatively, other residues might be able to partially substitute for K130. K130 is located in the N-terminal α-helix of Domain 3. Residues in this helix mediate extended −10 recognition in all Group 1 and Group 2 ss examined (Barne et al., 1997; Becker and Hengge-Aronis, 2001; Sanderson et al., 2003). This leads us to conclude that −16, −15 CC constitute an extended −10 recognition motif.
Do all proteobacteria have σ32 promoters with an extended −10 motif?
σ32 orthologues are widely distributed in proteobacteria and one would imagine that residues mediating recognition would be highly conserved. Indeed, W108 is universally conserved in all proteobacteria, consistent with its proposed role in −13C recognition. In contrast, K130 is universally conserved in β- and γ-proteobacteria, but is not conserved in the α- and δ-/ε-groups (Fig. S1). It is noteworthy that the amino acids positioned homologously to K130 in the α- and δ-/ε-groups of σ32 are alanine, serine or glutamine, which are unlikely to substitute for K130 in mediating recognition. Intriguingly, it has been reported that CC motif of the upstream −10 region is less well conserved in the two α-proteobacteria examined, Caulobacter crescentus and Rhodobacter sphaeroides (Green and Donohue, 2006; McGrath et al., 2007), than in the γ-proteobacteria. Together, these results raised the possibility that α- and δ-/ε-proteobacteria do not utilize an extended −10 motif. We tested this idea by examining the −10 promoter recognition properties of C. crescentus σ32, which has an alanine at the position comparable to K130 (Fig. 6A). The assay system used was the one described in Fig. 1A except that C. crescentus σ32 was the sole source of σ32 and it was not modified with the I54N substitution. Instead, we disabled the regulatory loops that control the amount and activity of σ32 by using an assay strain in which wt dnaK was replaced by dnaK756. This alteration in DnaK prevents it from participating in feedback inhibition and degradation of σ32, thereby disabling the regulatory loops (Georgopoulos et al., 1979; Straus et al., 1990; da Silva et al., 2003). C. crescentus σ32 was produced at a level comparable to that of E. coli σ32 (data not shown) and is generally less sensitive than E. coli σ32 to mutations at the −10 region (compare Figs 6B and 2B). Most striking is that C. crescentus σ32 has little or no sequence selectivity at the −16 and −15 positions, with even a promoter mutated at both −16C and −15C showing only slightly less activity than the wt promoter (Fig. 6B). Thus, loss of the conserved K130 residue correlates with loss of extended −10 recognition.
Fig. 6.
Recognition of the −10 region of groE promoter by C. crescentus σ32.
A. Amino acid sequence alignment of E. coli σ32 Regions 2.4–3.0 with the corresponding region of C. crescentus σ32. Amino acids shown to interact with specific promoter bases in E. coli σ32 are shown in bold.
B. Activities of C. crescentus σ32 on mutant E. coli groE promoters are shown as a percentage of measured β-galactosidase activity for wt groE promoter driven by C. crescentus σ32. CAG57102 strain was used for this assay with plasmid pBK151 expressing C. crescentus σ32 and derivatives of pQF50K_groE carrying the groE promoter lacZ reporter. Promoter mutations and positions are shown on the x-axis. All values are averages of at least three independent experiments; error bars indicate one standard deviation.
It is also interesting to note that E112, implicated by our results in Region 2.4 recognition of −13C, is universally conserved in β- and γ-proteobacteria, but not in the α- and δ-/ε-groups (Fig. S1). The amino acid corresponding to E112 is serine in C. crescentus σ32 and C. crescentus σ32 showed the same activity on the C-13T promoter variant as on the wt promoter (Fig. 6B). Possibly a serine at this position permits recognition of both T and C at the −13 position.
In vitro effect of the K130A substitution in E. coli has little or no sequence 32 and C. crescentus s32 on transcription
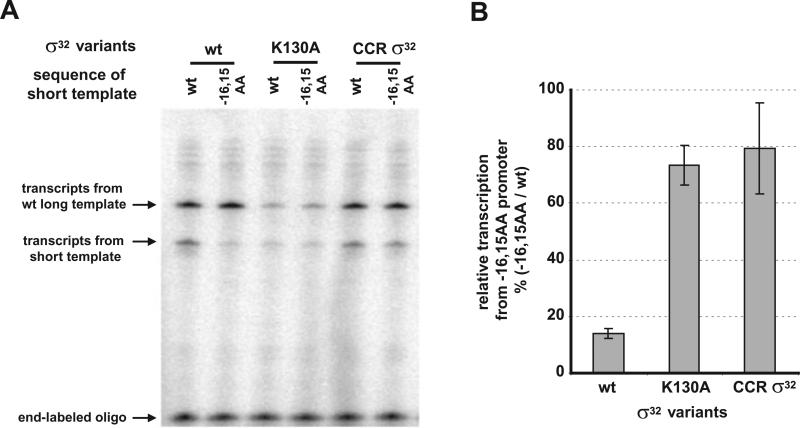
We performed single-round in vitro transcription assays to examine the promoter preferences of E. coli and C. crescentus σ32 as well as the K130A variant of E. coli has little or no sequence 32. The linear templates were based on PgroE and were identical to those used for the in vivo assay. In addition to the test template, each assay also contained a competitor template to serve as an internal control. The competitor template encodes wt PgroE and gave a longer transcript than the test template (Fig. 7A). Whereas wt E. coli σ32 initiates transcription from the wt promoter about sixfold better than a promoter mutated with A at positions −16 and −15, the K130A mutant initiates transcription from each promoter equivalently (Fig. 7B). Additionally, C. crescentus shows only a slight defect on the doubly mutant promoter as compared with the wt promoter (Fig. 7B). That the in vivo expression phenotype is reproduced in vitro argues that it is a direct effect of the particular σ32 present during transcription.
Fig. 7.
Effect of substitution at K130 of σ32 on promoter recognition in vitro. Single-round in vitro transcriptions were performed on linear DNA templates containing wt or −16,−15 AA mutant groE promoters by RNA polymerase containing E. coli wt σ32, K130A σ32 or C. crescentus s 32(CCRσ32).
A. In vitro transcription from groE promoter variants with σ32 variants. Upper bands in each lane are from long template of wt groE promoter (served as internal control); lower bands are from short template of either wt or −16,−15 AA mutant groE promoter (see Experimental procedures); bottom bands (end-labelled 35 nt oligomer) are shown as loading control.
B. Quantification of the in vitro transcripts to show the effect of substitution at K130 position on promoter recognition. The bars indicate the relative transcription from −16, −15 AA mutant promoter as a percentage transcript from wt promoter for each σ32 variant. Each value was calculated as follows: (i) each short transcript was expressed as a fraction of total transcript in the lane (long + short transcript) and called normalized short transcript and (ii) normalized short transcript from −16, −15 AA promoter was divided by normalized short transcript from the wt promoter. All values are averages of three independent experiments; error bars indicate one standard deviation.
Effect of UP element on promoter recognition by σ32
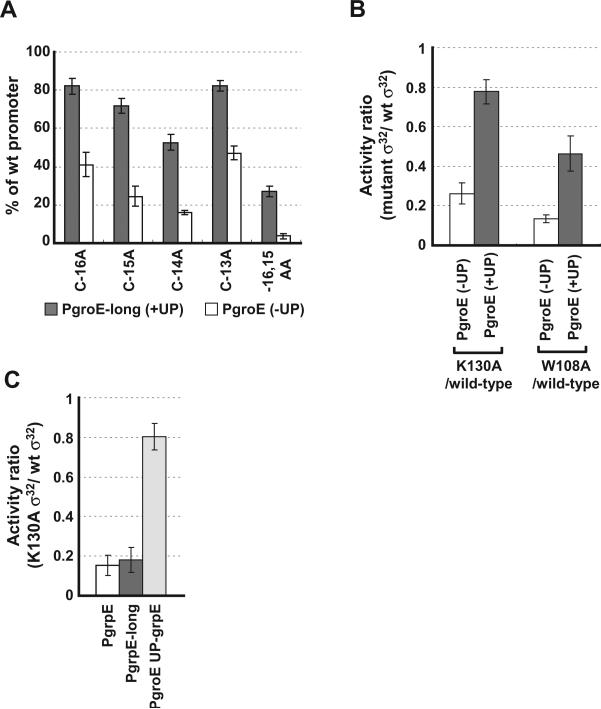
The studies described thus far have been performed on PgroE and PgrpE core promoters (−44 to −7). The UP element can often compensate for defects in the core promoter (Miroslavova and Busby, 2006). Both bioinformatic analysis (Nonaka et al., 2006) and experimental studies (V.A. Rhodius, unpublished) suggest that many σ32 promoters contain UP elements. We therefore repeated key experiments in a PgroE variant that contained the UP element (−66 to −7). Expression from complete PgroE was about fourfold higher than from core PgroE, validating that the groE promoter contains a functional UP element (data not shown). Importantly, addition of the UP element partially suppressed single-base changes at every position in the tetra-C motif of the −10 region of the promoter (Fig. 8A). Moreover, expression from double-base changed −16,−15 AA promoter was increased to 20% of wt promoter. This finding led us to test whether the UP element also suppressed the deleterious effects of mutant σ32s implicated in recognizing this motif. Indeed, the deleterious effects of both the K130A and W108A σ32 mutants were significantly suppressed (Fig. 8B). Whereas the K130A mutant had a fourfold defect when initiating transcription from the core groE promoter, it was 80% as effective as wt when initiating transcription from the complete groE promoter. Likewise, W108A was about threefold more effective in initiating transcription from the complete promoter than the core promoter. We also tested the two variants of the grpE promoter. We find that grpE does not have a functional UP element, as the activities of both the complete and core promoter variants were the same (data not shown). Consistent with this, the complete grpE promoter does not suppress the deleterious effects of K130A σ32 (Fig. 8C). However, addition of the functional UP element from the groE promoter to the grpE promoter did suppress K130A (Fig. 8C), demonstrating that UP suppression of defective recognition of the tetra-C motif is likely to be a general phenomenon.
Fig. 8.
Effect of A/T-rich UP element on promoter recognition.
A. Effect of UP element on mutations at the −10 region of σ32 promoter. Activity of each mutant groE promoter with/without UP element are shown as a percentage of measured β-galactosidase activity of each wt promoter with/without UP element driven by wt σ32. Grey bars, PgroE-long (with UP element); clear bars, PgroE (without UP element).
B. Effect of UP element on promoter recognition mutant σ32. The bars show the activity ratios of mutant σ32 versus wt σ32. Grey bars, PgroE-long (with UP element); clear bars, PgroE (without UP element).
C. Suppression of promoter recognition mutant σ32 at grpE promoter by UP element from groE promoter (PgroE_UP-grpE). Assay strains are as described in Fig. 1A; promoter sequences are shown in Fig. 1B. Promoter mutations and variants, and σ32 derivatives are indicated below the x-axis. All values are averages of at least three independent experiments; error bars indicate one standard deviation.
Discussion
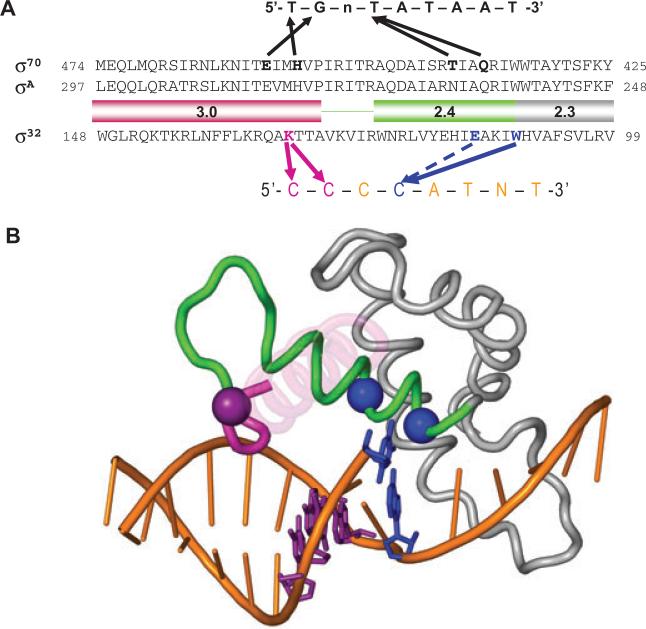
Sigma factors are composed of a variable number of domains, each with a particular function in promoter recognition. Thus, Domain 4 recognizes the −35 region, Domain 3 the extended −10 region and Domain 2 the −10 region of the promoter. Most Group 3 σs possess sDomain 3, and the promoters recognized by these ss have longer than normal −10 regions, often a hallmark of a composite region composed of both the extended −10 and −10 recognition units. However, no Group 3 σ had been shown to carry out recognition of the extended −10 region. The principal accomplishment of this work was to provide strong evidence that E. coli σ32 promoters have an extended −10 motif that is recognized by sDomain 3. Additionally, we provide information concerning residues in Region 2.4 recognizing the −10 region. Our findings are represented in schematic from in Fig. 9A. A structural model of the interactions we propose, based on the T. aquaticus holoenzyme/fork-junction promoter DNA complex, is presented in Fig. 9B.
Fig. 9.
Structural model of the recognition of the −10 region of promoter by σ32.
A. Comparison of the recognition of the −10 region of promoters by σ70 and σ32. Amino acid sequences of Regions 2.3, 2.4 and 3.0 of σ70, σA and σ32 are shown. The −10 consensus promoter sequences for each σ is indicated above or below the amino acid sequences. Thick arrows indicate contacts suggested by the previous studies for σ70 and in this study for σ32. Arrow with dotted line indicates contact to increase selectivity.
B. Shown is the structure of σA Regions 2.1–3.0 and the −10 element region of the fork-junction promoter DNA from the T. aquaticus holoenzyme/fork-junction promoter DNA complex (Murakami et al., 2002). The rest of the protein and DNA are not shown. The σA Region 2.4 is coloured green and Region 3.0 is magenta. Most of the 3.0 helix is transparent for clarity. The positions corresponding to residues of σ32 that make base-specific contacts with the promoter are shown as α-carbon spheres, and colour-coded along with the corresponding DNA base pair: W108 (W257 in T. aquaticus σA)/−11CG, blue; E112 (A261)/−11CG, blue; and K130 (M279)/−13CG/−14CG, purple. This figure was prepared using PyMol (http://pymol.sourceforge.net/).
Two independent lines of evidence support the proposition that the upstream edge of the tetra-C motif (positions −16C and −15C) constitutes an extended −10 motif in σ32 promoters. First, allele-specific suppression analysis identifies a single residue that mediates recognition of the −16, −15 CC position. That residue, K130, is located in sDomain 3, in a position similar to extended −10 recognition determinants identified for Group 1 and Group 2 ss (Becker and Hengge-Aronis, 2001; Sanderson et al., 2003). Briefly, when K130 is present, the consensus C is preferred at positions −16 and −15; all substitutions decrease promoter activity. When K130 is altered to A130, expression from the consensus promoter decreases, but base changes at −16C and −15C no longer significantly decrease promoter activity. This behaviour constitutes genetic evidence that K130 mediates base-specific recognition of −16,−15 CC. Lysine has two hydrogen bond donors. Therefore, K130 could interact with the major groove hydrogen bond acceptors, −16G and −15G, located in the template strand. This explains how a single amino acid could mediate dual recognition. This kind of interaction has been shown in other DNA binding proteins (Luscombe et al., 2001). Second, phylogenetic analysis supports the assignment of K130 as the recognition determinant for −16, −15 CC. As reported in the supplementary figure, K130 is universally conserved in β- and γ-proteobacteria, but is substituted by alanine, serine or gluatmine in the α- and δ-/ε-groups. For example, σ32 from the α-proteobacteria, C. crescentus and R. sphaeroides are not conserved for K130. Both promoter analysis of C. crescentus σ32 (McGrath et al., 2007) and R. sphaeroides σ32 (Green and Donohue, 2006) and, more importantly, the direct analysis of the promoter recognition properties of C. crescentus σ32 (Fig. 6B) indicate that these σ32 do not recognize the extended −10 motif. Thus, both allele-specific suppression analysis and the concordance between distribution of K130 and requirement of −16,−15 CC for promoter function support the idea that −16, −15 CC is an extended −10 motif whose recognition is mediated by sDomain 3. We note Domain 3 of σE and σH, the two B. subtilis Group 3 ss studied previously, is rather discrepant from other Group 3 σs (Gruber and Gross, 2003), providing a rationale for why these σs do not have extended −10 recognition.
Parenthetically, our results rule out a role for the RpoH box in −10 recognition. This nine-amino-acid insertion in sDomain 3 is the most conserved portion of σ32 (Nakahigashi et al., 1995). Although alanine substitutions of these residues result in transcription defects, we could identify no promoter-specific defects associated with altering the RpoH box. Previous work indicated that at least one residue in the RpoH Box is involved in interaction with RNA polymerase, and that may be the function of this motif (Joo et al., 1998).
The most downstream C in the tetra-C motif, −13C, has been shown by deHaseth to be part of the −10 element, recognized by W108 located in Region 2.4. Our data extend the observations of deHaseth in two respects. First, we provide allele-specific suppression evidence that clarifies the role of W108. When W108 is present, the consensus −13C is preferred and substitutions at that position significantly decrease promoter activity. When W108 is altered to A108, expression from the consensus promoter decreases, but base changes at −13C no longer significantly decrease promoter activity, providing genetic evidence that W108 mediates recognition. The corresponding position in s70, W434, mediates promoter melting process as well as base recognition (Fenton et al., 2000; Tomsic et al., 2001); it is possible that W108 also has a role in both processes. Second, we suggest that E112, likely to protrude from the same surface as W108 (Fig. 9B and Kourennaia et al., 2005), has an auxiliary role in recognizing −13C. Although A112 did not significantly decrease expression from the wt groE or grpE promoters, it did suppress promoter mutations at the −13C position. This kind of interaction between a Group 3 σ and its promoter is reminiscent of that reported by Moran and colleagues for the M124 residue of B. subtilis σE M124A does not significantly decrease expression from wt promoters, but does significantly suppress the deleterious effects of base mutations at the 5′ end of the −10 element, leading Moran to propose interaction of M124 with bases at this position (Tatti et al., 1991; Diederich et al., 1992). Possibly, the function of M124 of sE and of E112 of σ32 is to increase the specificity of promoter recognition. These residues may inhibit non-specific interaction of the ss with the promoter region rather than directly interacting with the base pair themselves.
We report here the first studies of the interplay between the UP element and the composite −10 tetra-C motif. The presence of the UP element diminishes but does not eliminate the effect of effects of base substitutions at positions −16, −15, −14 and −13C, suggesting that initial recognition by a can partially substitute for recognition by s. We repeated these particular assays in wt cells to make sure that compensation still occurs under physiological conditions where the levels of σ32 are very low. UP element compensation for defects in the tetra-C motif is slightly diminished in wt cells, but still occurs (data not shown). We note that most σ32 promoters retain a good consensus tetra-C motif even when they have UP elements, suggesting that incomplete compensation is deleterious to cell growth.
One of the most intriguing findings in these studies is that E. coli σ32, in common with σ32s from other γ-and β-proteobacteria, is constructed so that high promoter activity requires a consensus extended −10 motif in addition to consensus at the −35 and −10 elements of the promoter. This stands in contrast to the paradigm derived from extensive study of σ70, the E. coli housekeeping s, and is reminiscent of promoter requirements in Gram-positive bacteria, which utilize all three elements. In the case of σ32, we know that the alteration in promoter requirements is completely contained with the σ32 itself, as σ32 from the α-proteobacteria, C. crescentus, in combination with E. coli core RNA polymerase efficiently utilizes σ32 promoters lacking the extended −10 region. Our current studies are aimed at identifying the determinants in E. coli σ32 responsible for this phenotype. Understanding this distinction is likely to provide important insights into how alternative ss are constructed.
Experimental procedures
Strains, plasmids and growth conditions
Strains and plasmids used in this study are listed in Table S1. Escherichia coli strains were grown at 30°C or 37°C in Luria–Bertani media (Sambrook and Russell, 2001) supplemented with appropriate antibiotics such as ampicillin (100 mg ml−1), chloramphenicol (30 mg ml−1), kanamycin (20 mg ml−1), spectinomycin (50 mg ml−1) and tetracycline (10 mg ml−1). 0.1% of L-(+)-arabinose was supplemented for growth of rpoH strains to induce expression of GroESL. C. crescentus strain, CB15N, was grown at 30°C in PYE medium consisting of 0.2% peptone, 0.1% yeast extract, 0.8 mM MgSO4 and 0.5 mM CaCl2 (Johnson and Ely, 1977).
Construction of assay strains
To construct the rpoH mutant strains CAG57099, CAG57101 and CAG57102, as a first step CAG57097 (DY330 PBAD-groESL) was constructed by P1 transduction of PBAD-groESL from CAG48176 (McLennan and Masters, 1998; Guisbert et al., 2004) and selecting for the downstream kanamycin marker. This marker was then replaced by cat (CAG57098). Finally, CAG57099 (DY330 PBAD-groESL DrpoH::aadA) was constructed by replacing the rpoH open reading frame by the spectinomycin-resistant gene, aadA, using electroporated linear DNA amplified by PCR and E. coli DY330 as described previously (Yu et al., 2000). aadA was amplified from pDH50 with the following primers: forward primer, 5′-CAGTTGTTGCTACCACTGAAGCGCCAGAAGATATCGATTGAGAGGATTTGAACCGTGGAAACGGATGAAGGC-3′ and reverse primer, 5′-AAACAAAAACCCCGGACTCTCATCCAGGGTTC TCTGCTTAATAGCGGAAAAGGGCTTATTATGCACGCTTAA-3′ (underlined sequences are complementary to the upstream and downstream non-coding sequences immediately adjacent to the rpoH open reading frame). The assay strain used in this study, CAG57101, was constructed by P1 transduction of ΔrpoH::aadA from CAG57100. CAG57102 was constructed by P1 transduction of dnaK756 (Georgopoulos et al., 1979) into CAG57101. The presence of each gene disruption was confirmed by resistance to appropriate antibiotics and by PCR.
Plasmid construction
Genomic DNA from E. coli and C. crescentus were prepared by Blood and Cell Culture DNA Midi kit (Qiagen) and used for templates of PCR for cloning of wt rpoH gene and promoter fragments.
The plasmid pSAKT32 encoding wt E. coli σ32, and pSAKT32 derivatives pBK116–pBK150 encoding E. coli σ32 variants were used for σ32 expression to determine their activities in vivo. The pSAKT32 plasmid derivatives carry the rpoH gene under control of the isopropyl-β-D-thiogalactopyranoside-inducible Plac promoter as well as the lac repressor gene under the control of a strong mutant promoter(iq) (Wang and deHaseth, 2003). All E. coli σ32 variants were constructed using site-directed mutagenesis. To express C. crescentus σ32 in CAG57102, pBK151 was constructed by replacing E. coli rpoH gene of pSAKT32 with the C. crescentus rpoH gene.
Derivatives of pQF50K (pQF50K derivatives and pBK501–pBK549 plasmid series) carrying promoter fragments cloned in the BglII-XbaI sites upstream of lacZ were used for measuring promoter strength and activities of ss (Wang and deHaseth, 2003). Promoter mutant derivatives were constructed using PCR by amplifying the promoter regions using mutagenic primers that encompassed either the BglII or XbaI site.
Derivatives of the pET21a vector (Novagen) encoding σ32 and variants were used to overproduce σ32 protein for purification. The vectors were constructed using PCR by amplifying from the pSAKT32 series using primers to create flanking NdeI and HindIII sites and inserted as NdeI-HindIII fragments into pET21a NdeI-HindIII vector. The resultant ss contain an N-terminal His6-tag and PreScission cleavage site (Patikoglou et al., 2007).
β-Galactosidase assay
Assay systems for in vivo transcription were constructed by transforming derivatives of pSAKT32 and pQF50K into appropriate strains sequentially using electroporation. Overnight cultures in Luria–Bertani media supplemented with 100 mg ml−1 ampicillin and 30 mg ml−1 kanamycin (strains CAG57101 and CAG57102 were also supplemented with 0.1% L-(+)-arabinose) at 30°C were diluted 1:100 into fresh media and grown for 2 h with aeration. Cultures were then induced with 1 mM IPTG and grown for a further 3 h before harvesting (final ODs at 600 nm was approximately 0.6–0.8). β-galactosidase assays were performed as described previously (Miller, 1972).
Overproduction and purification of σ32
Escherichia coli BL21(DE3)/pLysS cells harbouring pET21a encoding wt σ32 or variants were used to overproduce σ32 with an N-terminal His6-tag and PreScission cleavage site for purification. Overnight cultures were diluted 1:100 into fresh media containing 100 mg ml−1 ampicillin and 30 mg ml−1 chloramphenicol, and grown aerobically at 30°C. When the cultures reached OD600 of 0.4, expression of σ32 was induced by addition of IPTG to a final concentration of 1 mM and growth continued at 30°C with aeration for 2 h. Five hundred millilitres of each culture was then harvested by centrifugation and the cell pellets resuspended in buffer A1 (20 mM HEPES, pH 8.0, 300 mM KCl, 1 mM β-mercaptoethanol, 5 mM imidazole, 0.66% isoleucine, 0.83% phenylalnine, 1 mM AEBSF). The cells were disrupted by sonication and the lysates cleared of cell debris by centrifugation at 100 000 g for 90 min. N-terminal His6-σ32 was purified from the soluble fraction by metal affinity chromatography using Talon metal affinity resin (Clontech). The N-terminal His6-tag was then removed using PreScission protease (GE Healthcare) during dialysis into buffer Q (10 mM Tris, pH 7.5, 50 mM NaCl, 1 mM DTT) at 4°C for 16 h. The sample was further purified by a second, subtractive metal affinity chromatography step to remove uncleaved His6-ss and the His6-tag, followed by ion exchange chromatography (HiTrap Q Sepharose; GE Healthcare). As a final step, DnaK-bound σ32 was removed by gel filtration chromatography (Superdex 75; GE Healthcare) and free σ32 fractions were taken and concentrated in buffer B (20 mM Tris, pH 7.5, 200 mM KCl, 1 mM DTT, 0.01% Triton X-100, 50% glycerol).
Purity of all His6-tag free σ32s were > 95% judged by SDS-PAGE and native-PAGE. Protein concentration was determined by the bicinchoninic acid protein assay (Pierce).
In vitro transcription
Run-off single-round transcription was performed at 30°C. Linear DNA templates were generated by amplification of promoter region of pQF50K_groE (wt groE promoter) or pBK501 (−16,−15 AA groE promoter) plasmids with following primers: forward primer, IT-F, 5′-ACTCTCAGTACAATCTG CTCTGATG-3′ and reverse primers, IT-R-S, 5′-GCTATTA CGCCAGCTGGCGAAAGG-3′ and IT-R-L, 5′-AAAGCGCC ATTCGCCATTCAGGCTG-3′. Primers IT-F and IT-R-L were used to generate control template (420 bp: 220 nt upstream and 200 nt downstream of the transcription start site) from pQF50K_groE. Short templates (355 bp: 220 nt upstream and 135 nt downstream of the transcription start site) were amplified from pQF50K_groE and pBK501 with IT-F and IT-R-S and used for comparing transcription from wt or mutant promoters.
RNA polymerase core enzyme was purified as described in previous study (Sharp et al., 1999). Holoenzyme was reconstituted by incubation of core RNA polymerase and a threefold excess of σ32 on ice for 30 min in binding buffer (20 mM Tris pH 8.0, 100 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, 1 mM DTT, 100 mg ml−1 BSA, 5% glycerol and 0.05% Tween-20). Holoenzyme was diluted in binding buffer and incubated 30°C for 5 min. After incubation of holoenzyme and DNA template at 30°C for 5 min, transcription was initiated by adding transcription mix and incubated at 30°C for 6 min. Total reaction mixture was given as a 9 ml of 50 nM holoenzyme, 5 nM template DNA (2.5 nM of long template and 2.5 nM of short template), 200 mM ATP, 200 mM GTP, 200 mM UTP, 10 mM CTP, 100 nM [α-32P]-CTP (3000 Ci mmol−1), 100 mg ml−1 heparin in binding buffer. Reactions were stopped by the addition of 7 ml of gel loading solution containing 20 mM EDTA, 80% deionized formamide, 0.1% of bromophenol blue and xylene cyanol FF, and 35 nt 32P end-labelled oligomer. After incubation at 90°C for 2 min, 9 ml of each sample was subjected to electrophoresis in a 6% acrylamide/7M urea/TBE gel. The transcripts were visualized and analysed using Molecular Dynamics Storm 560 PhosphoImager scanning system and ImageQuant 5.2 densitometry software. Sizes of transcripts from long and short templates are 200 and 135 nt respectively.
Supplementary Material
Acknowledgements
We thank Lucy Shapiro for providing C. crescentus CB15N strain, Pieter L. deHaseth for providing pQF50K_groE and pSAKT32 and members of the Gross lab for critically reading of the manuscript. We thank Athanasios Typas and Monica Guo for useful suggestions and Seth A. Darst for critically reading this manuscript. This work was supported by National Institutes of Health Grants GM36278 (to C.A.G.) and GM53759 (to S.A.D.).
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Amaya E, Khvorova A, Piggot PJ. Analysis of promoter recognition in vivo directed by sigma(F) of Bacillus subtilis by using random-sequence oligonucleotides. J Bacteriol. 2001;183:3623–3630. doi: 10.1128/JB.183.12.3623-3630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinova N, Kuznedelov K, Severinov K, Kulbachinskiy A. Structural modules of RNA polymerase required for transcription from promoters containing downstream basal promoter element GGGA. J Biol Chem. 2008;283:22482–22489. doi: 10.1074/jbc.M802445200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barne KA, Bown JA, Busby SJ, Minchin SD. Region 2.5 of the Escherichia coli RNA polymerase sigma70 subunit is responsible for the recognition of the ‘extended-10’ motif at promoters. EMBO J. 1997;16:4034–4040. doi: 10.1093/emboj/16.13.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashyam MD, Tyagi AK. Identification and analysis of ‘extended −10’ promoters from mycobacteria. J Bacteriol. 1998;180:2568–2573. doi: 10.1128/jb.180.9.2568-2573.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G, Hengge-Aronis R. What makes an Escherichia coli promoter sigma(S) dependent? Role of the −13/−14 nucleotide promoter positions and region 2.5 of sigma(S). Mol Microbiol. 2001;39:1153–1165. doi: 10.1111/j.1365-2958.2001.02313.x. [DOI] [PubMed] [Google Scholar]
- Britton RA, Eichenberger P, Gonzalez-Pastor JE, Fawcett P, Monson R, Losick R, Grossman AD. Genome-wide analysis of the stationary-phase sigma factor (sigma-H) regulon of Bacillus subtilis. J Bacteriol. 2002;184:4881–4890. doi: 10.1128/JB.184.17.4881-4890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EA, Muzzin O, Chlenov M, Sun JL, Olson CA, Weinman O, et al. Structure of the bacterial RNA polymerase promoter specificity sigma subunit. Mol Cell. 2002;9:527–539. doi: 10.1016/s1097-2765(02)00470-7. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels D, Zuber P, Losick R. Two amino acids in an RNA polymerase sigma factor involved in the recognition of adjacent base pairs in the −10 region of a cognate promoter. Proc Natl Acad Sci U S A. 1990;87:8075–8079. doi: 10.1073/pnas.87.20.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich B, Tatti KM, Jones CH, Beall B, Moran CP., Jr Genetic suppression analysis of sigma E interaction with three promoters in sporulating Bacillus subtilis. Gene. 1992;121:63–69. doi: 10.1016/0378-1119(92)90162-i. [DOI] [PubMed] [Google Scholar]
- Ebright RH. Evidence for a contact between glutamine-18 of lac repressor and base pair 7 of lac operator. Proc Natl Acad Sci U S A. 1986;83:303–307. doi: 10.1073/pnas.83.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebright RH, Cossart P, Gicquel-Sanzey B, Beckwith J. Mutations that alter the DNA sequence specificity of the catabolite gene activator protein of E. coli. Nature. 1984;311:232–235. doi: 10.1038/311232a0. [DOI] [PubMed] [Google Scholar]
- Eichenberger P, Jensen ST, Conlon EM, van Ooij C, Silvaggi J, Gonzalez-Pastor JE, et al. The sigmaE regulon and the identification of additional sporulation genes in Bacillus subtilis. J Mol Biol. 2003;327:945–972. doi: 10.1016/s0022-2836(03)00205-5. [DOI] [PubMed] [Google Scholar]
- Feklistov A, Barinova N, Sevostyanova A, Heyduk E, Bass I, Vvedenskaya I, et al. A basal promoter element recognized by free RNA polymerase sigma subunit determines promoter recognition by RNA polymerase holoenzyme. Mol Cell. 2006;23:97–107. doi: 10.1016/j.molcel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Fenton MS, Lee SJ, Gralla JD. Escherichia coli promoter opening and −10 recognition: mutational analysis of sigma70. EMBO J. 2000;19:1130–1137. doi: 10.1093/emboj/19.5.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos CP, Lam B, Lundquist-Heil A, Rudolph CF, Yochem J, Feiss M. Identification of the C. coli dnaK (groPC756) gene product. Mol General Genet. 1979;172:143–149. doi: 10.1007/BF00268275. [DOI] [PubMed] [Google Scholar]
- Gourse RL, Ross W, Gaal T. UPs and downs in bacterial transcription initiation: the role of the alpha subunit of RNA polymerase in promoter recognition. Mol Microbiol. 2000;37:687–695. doi: 10.1046/j.1365-2958.2000.01972.x. [DOI] [PubMed] [Google Scholar]
- Graves MC, Rabinowitz JC. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. Evidence for ‘extended’ promoter elements in gram-positive organisms. J Biol Chem. 1986;261:11409–11415. [PubMed] [Google Scholar]
- Green HA, Donohue TJ. Activity of Rhodobacter sphaeroides RpoHII, a second member of the heat shock sigma factor family. J Bacteriol. 2006;188:5712–5721. doi: 10.1128/JB.00405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CA, Chan C, Dombroski A, Gruber T, Sharp M, Tupy J, Young B. The functional and regulatory roles of sigma factors in transcription. Cold Spring Harb Symp Quant Biol. 1998;63:141–155. doi: 10.1101/sqb.1998.63.141. [DOI] [PubMed] [Google Scholar]
- Gruber TM, Gross CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- Guisbert E, Herman C, Lu CZ, Gross CA. A chaperone network controls the heat shock response in E. coli. Genes Dev. 2004;18:2812–2821. doi: 10.1101/gad.1219204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisbert E, Yura T, Rhodius VA, Gross CA. Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiol Mol Biol Rev. 2008;72:545–554. doi: 10.1128/MMBR.00007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen SP, Berkmen MB, Ross W, Gaal T, Ward C, Gourse RL. rRNA promoter regulation by nonoptimal binding of sigma region 1.2: an additional recognition element for RNA polymerase. Cell. 2006;125:1069–1082. doi: 10.1016/j.cell.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Helmann JD. Compilation and analysis of Bacillus subtilis sigma A-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucl Acids Res. 1995;23:2351–2360. doi: 10.1093/nar/23.13.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann JD, deHaseth PL. Protein–nucleic acid interactions during open complex formation investigated by systematic alteration of the protein and DNA binding partners. Biochemistry. 1999;38:5959–5967. doi: 10.1021/bi990206g. [DOI] [PubMed] [Google Scholar]
- Hochschild A, Ptashne M. Homologous interactions of lambda repressor and lambda Cro with the lambda operator. Cell. 1986;44:925–933. doi: 10.1016/0092-8674(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Hochschild A, Douhan J, 3rd, Ptashne M. How lambda repressor and lambda Cro distinguish between OR1 and OR3. Cell. 1986;47:807–816. doi: 10.1016/0092-8674(86)90523-4. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977;86:25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo DM, Nolte A, Calendar R, Zhou YN, Jin DJ. Multiple regions on the Escherichia coli heat shock transcription factor sigma32 determine core RNA polymerase binding specificity. J Bacteriol. 1998;180:1095–1102. doi: 10.1128/jb.180.5.1095-1102.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourennaia OV, Tsujikawa L, Dehaseth PL. Mutational analysis of Escherichia coli heat shock transcription factor sigma 32 reveals similarities with sigma 70 in recognition of the −35 promoter element and differences in promoter DNA melting and −10 recognition. J Bacteriol. 2005;187:6762–6769. doi: 10.1128/JB.187.19.6762-6769.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Malloch RA, Fujita N, Smillie DA, Ishihama A, Hayward RS. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an ‘extended minus 10’ promoter. J Mol Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- Kusukawa N, Yura T. Heat shock protein GroE of Escherichia coli: key protective roles against thermal stress. Genes Dev. 1988;2:874–882. doi: 10.1101/gad.2.7.874. [DOI] [PubMed] [Google Scholar]
- Lonetto M, Gribskov M, Gross CA. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscombe NM, Laskowski RA, Thornton JM. Amino acid-base interactions: a three-dimensional analysis of protein–DNA interactions at an atomic level. Nucl Acids Res. 2001;29:2860–2874. doi: 10.1093/nar/29.13.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath PT, Lee H, Zhang L, Iniesta AA, Hottes AK, Tan MH, et al. High-throughput identification of transcription start sites, conserved promoter motifs and predicted regulons. Nat Biotechnol. 2007;25:584–592. doi: 10.1038/nbt1294. [DOI] [PubMed] [Google Scholar]
- McLennan N, Masters M. GroE is vital for cell-wall synthesis. Nature. 1998;392:139. doi: 10.1038/32317. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY; Cold Spring Harbor Laboratory Press: 1972. [Google Scholar]
- Minakhin L, Severinov K. On the role of the Escherichia coli RNA polymerase sigma 70 region 4.2 and alpha-subunit C-terminal domains in promoter complex formation on the extended −10 galP1 promoter. J Biol Chem. 2003;278:29710–29718. doi: 10.1074/jbc.M304906200. [DOI] [PubMed] [Google Scholar]
- Miroslavova NS, Busby SJ. Investigations of the modular structure of bacterial promoters. Biochem Soc Symp. 2006;73:1–10. doi: 10.1042/bss0730001. [DOI] [PubMed] [Google Scholar]
- Mitchell JE, Zheng D, Busby SJ, Minchin SD. Identification and analysis of ‘extended −10’ promoters in Escherichia coli. Nucl Acids Res. 2003;31:4689–4695. doi: 10.1093/nar/gkg694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami KS, Masuda S, Darst SA. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution. Science. 2002;296:1280–1284. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- Nakahigashi K, Yanagi H, Yura T. Isolation and sequence analysis of rpoH genes encoding sigma 32 homologs from gram negative bacteria: conserved mRNA and protein segments for heat shock regulation. Nucl Acids Res. 1995;23:4383–4390. [PMC free article] [PubMed] [Google Scholar]
- Nonaka G, Blankschien M, Herman C, Gross CA, Rhodius VA. Regulon and promoter analysis of the E. coli heat-shock factor, sigma32, reveals a multifaceted cellular response to heat stress. Genes Dev. 2006;20:1776–1789. doi: 10.1101/gad.1428206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patikoglou GA, Westblade LF, Campbell EA, Lamour V, Lane WJ, Darst SA. Crystal structure of the Escherichia coli regulator of sigma70, Rsd, in complex with sigma70 domain 4. J Mol Biol. 2007;372:649–659. doi: 10.1016/j.jmb.2007.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersohn A, Brigulla M, Haas S, Hoheisel JD, Volker U, Hecker M. Global analysis of the general stress response of Bacillus subtilis. J Bacteriol. 2001;183:5617–5631. doi: 10.1128/JB.183.19.5617-5631.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnambalam S, Webster C, Bingham A, Busby S. Transcription initiation at the Escherichia coli galactose operon promoters in the absence of the normal −35 region sequences. J Biol Chem. 1986;261:16043–16048. [PubMed] [Google Scholar]
- Ross W, Gosink KK, Salomon J, Igarashi K, Zou C, Ishihama A, et al. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- Ross W, Aiyar SE, Salomon J, Gourse RL. Escherichia coli promoters with UP elements of different strengths: modular structure of bacterial promoters. J Bacteriol. 1998;180:5375–5383. doi: 10.1128/jb.180.20.5375-5383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelnikov AG, Greenberg B, Lacks SA. An extended −10 promoter alone directs transcription of the DpnII operon of Streptococcus pneumoniae. J Mol Biol. 1995;250:144–155. doi: 10.1006/jmbi.1995.0366. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- Sanderson A, Mitchell JE, Minchin SD, Busby SJ. Substitutions in the Escherichia coli RNA polymerase sigma70 factor that affect recognition of extended −10 elements at promoters. FEBS Lett. 2003;544:199–205. doi: 10.1016/s0014-5793(03)00500-3. [DOI] [PubMed] [Google Scholar]
- Serizawa M, Yamamoto H, Yamaguchi H, Fujita Y, Kobayashi K, Ogasawara N, Sekiguchi J. Systematic analysis of SigD-regulated genes in Bacillus subtilis by DNA microarray and Northern blotting analyses. Gene. 2004;329:125–136. doi: 10.1016/j.gene.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Sharp MM, Chan CL, Lu CZ, Marr MT, Nechaev S, Merritt EW, et al. The interface of sigma with core RNA polymerase is extensive, conserved, and functionally specialized. Genes Dev. 1999;13:3015–3026. doi: 10.1101/gad.13.22.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegele DA, Hu JC, Walter WA, Gross CA. Altered promoter recognition by mutant forms of the sigma 70 subunit of Escherichia coli RNA polymerase. J Mol Biol. 1989;206:591–603. doi: 10.1016/0022-2836(89)90568-8. [DOI] [PubMed] [Google Scholar]
- da Silva AC, Simao RC, Susin MF, Baldini RL, Avedissian M, Gomes SL. Downregulation of the heat shock response is independent of DnaK and sigma32 levels in Caulobacter crescentus. Mol Microbiol. 2003;49:541–553. doi: 10.1046/j.1365-2958.2003.03581.x. [DOI] [PubMed] [Google Scholar]
- Sorenson MK, Ray SS, Darst SA. Crystal structure of the flagellar sigma/anti-sigma complex sigma(28)/FlgM reveals an intact sigma factor in an inactive conformation. Mol Cell. 2004;14:127–138. doi: 10.1016/s1097-2765(04)00150-9. [DOI] [PubMed] [Google Scholar]
- Straus D, Walter W, Gross CA. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of sigma 32. Genes Dev. 1990;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- Tatti KM, Jones CH, Moran CP., Jr Genetic evidence for interaction of sigma E with the spoIIID promoter in Bacillus subtilis. J Bacteriol. 1991;173:7828–7833. doi: 10.1128/jb.173.24.7828-7833.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsic M, Tsujikawa L, Panaghie G, Wang Y, Azok J, deHaseth PL. Different roles for basic and aromatic amino acids in conserved region 2 of Escherichia coli sigma(70) in the nucleation and maintenance of the single-stranded DNA bubble in open RNA polymerase-promoter complexes. J Biol Chem. 2001;276:31891–31896. doi: 10.1074/jbc.M105027200. [DOI] [PubMed] [Google Scholar]
- Wade JT, Roa DC, Grainger DC, Hurd D, Busby SJ, Struhl K, Nudler E. Extensive functional overlap between sigma factors in Escherichia coli. Nat Struct Mol Biol. 2006;13:806–814. doi: 10.1038/nsmb1130. [DOI] [PubMed] [Google Scholar]
- Wang Y, deHaseth PL. Sigma 32-dependent promoter activity in vivo: sequence determinants of the groE promoter. J Bacteriol. 2003;185:5800–5806. doi: 10.1128/JB.185.19.5800-5806.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BA, Gruber TM, Gross CA. Minimal machinery of RNA polymerase holoenzyme sufficient for promoter melting. Science. 2004;303:1382–1384. doi: 10.1126/science.1092462. [DOI] [PubMed] [Google Scholar]
- Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci U S A. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T, Guisbert E, Poritz M, Lu CZ, Campbell E, Gross CA. Analysis of sigma32 mutants defective in chaperone-mediated feedback control reveals unexpected complexity of the heat shock response. Proc Natl Acad Sci U S A. 2007;104:17638–17643. doi: 10.1073/pnas.0708819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Liu M, Burgess RR. The global transcriptional response of Escherichia coli to induced sigma 32 protein involves sigma 32 regulon activation followed by inactivation and degradation of sigma 32 in vivo. J Biol Chem. 2005;280:17758–17768. doi: 10.1074/jbc.M500393200. [DOI] [PubMed] [Google Scholar]
- Zhao K, Liu M, Burgess RR. Adaptation in bacterial flagellar and motility systems: from regulon members to ‘foraging’-like behavior in E. coli. Nucl Acids Res. 2007;35:4441–4452. doi: 10.1093/nar/gkm456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.