Abstract
Introduction
When first line therapy with metformin is insufficient for patients with type 2 diabetes (T2D), the optimal adjunctive therapy is unclear. We assessed the efficacy and safety of adjunctive antidiabetic agents in patients with inadequately controlled T2D on metformin alone.
Materials and Methods
A search of MEDLINE and CENTRAL, clinicaltrials.gov, regulatory websites was performed. We included randomized controlled trials of 3–12 months duration, evaluating Food and Drug Administration or European Union approved agents (noninsulin and long acting, once daily basal insulins) in patients experiencing inadequate glycemic control with metformin monotherapy (≥1500 mg daily or maximally tolerated dose for ≥4 weeks). Random-effects network meta-analyses were used to compare the weighted mean difference for changes from baseline in HbA1c, body weight (BW) and systolic blood pressure (SBP), and the risk of developing hypoglycemia, urinary (UTI) and genital tract infection (GTI).
Results
Sixty-two trials evaluating 25 agents were included. All agents significantly reduced HbA1c vs. placebo; albeit not to the same extent (range, 0.43% for miglitol to 1.29% for glibenclamide). Glargine, sulfonylureas (SUs) and nateglinide were associated with increased hypoglycemia risk vs. placebo (range, 4.00–11.67). Sodium glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 analogs, miglitol and empagliflozin/linagliptin significantly reduced BW (range, 1.15–2.26kg) whereas SUs, thiazolindinediones, glargine and alogliptin/pioglitazone caused weight gain (range, 1.19–2.44kg). SGLT2 inhibitors, empagliflozin/linagliptin, liraglutide and sitagliptin decreased SBP (range, 1.88–5.43mmHg). No therapy increased UTI risk vs. placebo; however, SGLT2 inhibitors were associated with an increased risk of GTI (range, 2.16–8.03).
Conclusions
Adding different AHAs to metformin was associated with varying effects on HbA1c, BW, SBP, hypoglycemia, UTI and GTI which should impact clinician choice when selecting adjunctive therapy.
Introduction
The American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) recommend lifestyle modifications and metformin as first-line therapy in type 2 diabetes mellitus (DM) [1]. However, initial monotherapy with maximally tolerated metformin may be insufficient to achieve hemoglobin A1c (HbA1c) goals of <7%, or given the progressive nature of Type 2 DM, glycemic control can wane over time necessitating combination therapy [1]. When monotherapy alone does not achieve/ maintain an HbA1c target over ~3 months, the next step is often to add a second agent. While there is an extensive list of pharmacologic therapies available for second-line adjunctive treatment of Type 2 DM (alpha-glucosidase inhibitors (AGIs), (dipeptidyl peptidase-4 (DPP-4) inhibitors, bile acid sequestrants, meglitinides, glucagon-like peptide-1 (GLP-1) analogs, long-acting, once-daily basal insulin, sodium glucose co-transporter-2 (SGLT2) inhibitors, sulfonylureas (SUs), thiazolidinediones (TZDs) and combinations of the above agents as either a fixed-dose combination or individual agents), randomized controlled trials (RCTs) directly comparing them are sparse.
Traditional pair-wise meta-analysis can be used to evaluate the efficacy and safety of two drugs based on evidence from RCTs that directly compare them. However, in absence of such direct head-to-head comparisons, network meta-analysis (NMA) provides a statistical framework that incorporates evidence from both direct and indirect comparisons from a network of studies of different therapies and evaluates their relative treatment effects [2–4].
We performed a NMA to assess the comparative efficacy and safety of adjunctive antidiabetic medication therapies in patients with Type 2 DM not adequately controlled on stable and optimized metformin monotherapy.
Materials and Methods
Study Selection
We performed a systematic literature search for all relevant articles from the earliest date through May 2014 in MEDLINE and Cochrane CENTRAL. The search strategy combined the Medical Subject Heading (MeSH) and keywords for “metformin” with terms for Type 2 DM and for glycosylated hemoglobin A1c (HbA1c). Our MEDLINE search strategy is included in S1 Appendix. We also performed a manual search of references from reports of clinical trials and review articles to identify additional relevant studies. Study results of identified studies were supplemented when possible with data identified through searches of www.clinicaltrials.gov, regulatory agency reports and by contacting investigators for clarification or additional data. Two investigators reviewed all potentially relevant citations independently (ESM, CIC).
To be included, studies had to: (1) be published in English; (2) utilize a parallel RCT design (any phase) in adults (≥18 years) with Type 2 DM; (3) compare Food and Drug Administration (FDA) or European Union (EU)-approved antidiabetic drug therapy including non-insulin and long-acting, once-daily basal insulin agents (as a single or combination adjunctive therapy) to another antidiabetic therapy or placebo (in addition to metformin); (4) include only patients who showed inadequate response to stable, optimized metformin monotherapy at randomization; (5) treat patients for 12 to 52 glycemic weeks after randomization; and (6) report change in HbA1c from baseline (our primary endpoint). As in previous NMAs [4], the criterion of stable metformin therapy was considered to be met if a study included patients who received at least 1,500mg/day (or maximum tolerated dose) of metformin or 1,000mg/day (as long as the mean dose in the study was >1,500mg/day) for at least the preceding 4 weeks before randomization.
Validity Assessment
Validity assessment was performed by 2 investigators (ESM, DMS) independently using the Cochrane Risk of Bias Tool [2,5]. This checklist (S2 Appendix) includes 7 validity questions covering the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete data reporting, selective reporting, and other bias (i.e., participants were either not on stable, maximal or near-maximal metformin as monotherapy, received it for less than 12 weeks prior to randomization, and/or the study was not published in a peer-reviewed journal). Each item was scored as a low, unclear or high risk of bias.
Data Extraction
Two investigators (ESM, DMS) used a standardized tool to independently extract all data with disagreements resolved by discussion or a third investigator (CIC). The following information was sought from each trial: (1) author identification; (2) year of publication; (3) study design and methodological quality information needed to complete the Cochrane Collaboration’s tool for assessing risk of bias [2,5]; (4) sample size; (5) inclusion/exclusion criteria; (6) duration of follow-up; (7) metformin and other antidiabetic therapies’, doses and schedules used; and (8) baseline characteristics (age, gender, anthropometrics, baseline HbA1c and duration of Type 2 DM). Endpoint data collected included mean change from baseline in HbA1c, body weight (BW), and systolic blood pressure (SBP) and the number/proportion of patients experiencing finger stick confirmed hypoglycemia, urinary tract infections (UTIs) and genital tract infections (GTIs). In cases where more than 1 published time point or article on an overlapping population was available, the most comprehensive article (e.g., the full analysis population) and primary endpoint time point (between 12 and 52 weeks) were used in the meta-analysis in order to optimize the amount of analyzable data.
Statistical Analysis
We performed traditional meta-analyses analyzing changes in HbA1c, BW and SBP as continuous variables using StatsDirect version 2.7.8 (StatsDirect Ltd, Cheshire, UK) with a P<0.05 considered statistically significant. Separate pair-wise analyses were performed for each antidiabetic therapy, combining data from approved doses of the same therapies using the method recommended by the Cochrane Collaboration [2]. In all cases, weighted (absolute) mean differences (WMDs) and associated 95% confidence intervals (CIs) were calculated using a DerSimonian and Laird random-effects model to account for between study heterogeneity. Net changes in each of the endpoints were calculated as the difference between treatment groups in the changes (baseline to follow-up). In instances where variances for net changes were not reported directly, they were calculated from CIs, P-values or individual variances. If the standard error, standard deviation or 95%CIs) for paired differences were not reported, we calculated it from those at baseline and at the end of follow-up; assuming a correlation coefficient of 0.5 between initial and final values [2]. The proportion of patients experiencing confirmed hypoglycemia, UTI and GTI on each drug therapy (combining data from approved doses of the same therapies) was meta-analyzed using a random-effects model as dichotomous endpoints with weighted averages reported as relative risks (RRs) and associated 95%CIs. We included UTI and GTI as key adverse endpoints in this meta-analysis because they are particularly relevant to the SGLT-2 inhibitors, which are the newest second-line treatment option for patients with Type 2 DM. When at least 3 studies making the same direct comparison were available, the likelihood of statistical heterogeneity (using the I2 statistic with a value >50% representing important statistical heterogeneity) and publication bias (using the Egger’s weighted regression statistic with a P <0.05 suggesting a higher likelihood of publication bias) were assessed.
We then performed NMA, a generalization of traditional pairwise meta-analysis that compares all pairs of treatments within a set of treatments for the same disease state (in this case Type 2 DM) [3]. Along with analyzing direct within-trial comparisons between two treatments, the NMA framework enables incorporation of indirect comparisons constructed from two trials that have one treatment in common [3]. This type of analysis safeguards the within-trial randomized treatment comparison of each trial while combining all available comparisons between treatments; allowing for more precise estimates for compared interventions. We used the package ‘netmeta’ (version 0.5–0) in R (version 3.0.2, The R Foundation for Statistical Computing) to perform NMA [6]. The package uses a novel graph-theory methodology that exploits the analogy between treatment networks and electrical networks to construct a NMA model accounting for the correlated treatment effects in multi-arm trials [6]. We implemented a random-effects model assuming common heterogeneity across all comparisons. Inconsistency was assessed by statistically comparing the results from direct and indirect estimates, whenever indirect estimates could be calculated using a single common comparator. We ascribed clinical superiority if one therapy improved HbA1c, BW or SBP by at least 0.3% [7], 2.3 kg (5 pounds) [8] or 5 mmHg [9], respectively, versus a competitor and with 95% certainty (i.e., for one therapy to be clinically superior to another for change in HbA1c, BW or SBP the lower bound of the 95%CI must depict a reduction greater than 0.3%, 2.3 kg or 5 mmHg, respectively) (S1 Fig). To address the inherent limitations of multiple comparisons in our meta-analysis, we performed sensitivity analyses calculating more conservative 99%CIs.
Results
Study Characteristics
The literature search yielded 1,005 citations, and we identified 17 additional RCTs through other sources (Fig 1). After removal of duplicates and title/abstract screening, 326 articles were eligible for full-text screening. After screening, 62 RCTs (n = 32,185 participants) were included in the NMA [10–71]. Twenty-five treatments, as well as placebo, were analyzed including AGIs (miglitol and acarbose), DPP-4 inhibitors (alogliptin, linagliptin, saxagliptin, sitagliptin and vildagliptin), the bile acid sequestrant (colesevelam), meglitinides (repaglinide and nateglinide), GLP-1 analogs (exenatide, lixisenatide and liraglutide), long-acting, once-daily basal insulin (insulin glargine), SGLT2 inhibitors (canagliflozin, dapagliflozin and empagliflozin), SUs (glibenclamide, gliclazide, glimepiride and glipizide), TZDs (rosiglitazone and pioglitazone), and combinations of the above agents as either a fixed-dose combination or individual agents (alogliptin/pioglitazone and empagliflozin/linagliptin).
Fig 1. Results of the Literature Search.
CCTR = Cochrane controlled trials register; EU = European Union; FDA = Food and Drug Administration; HbA1c = hemoglobin A1c.
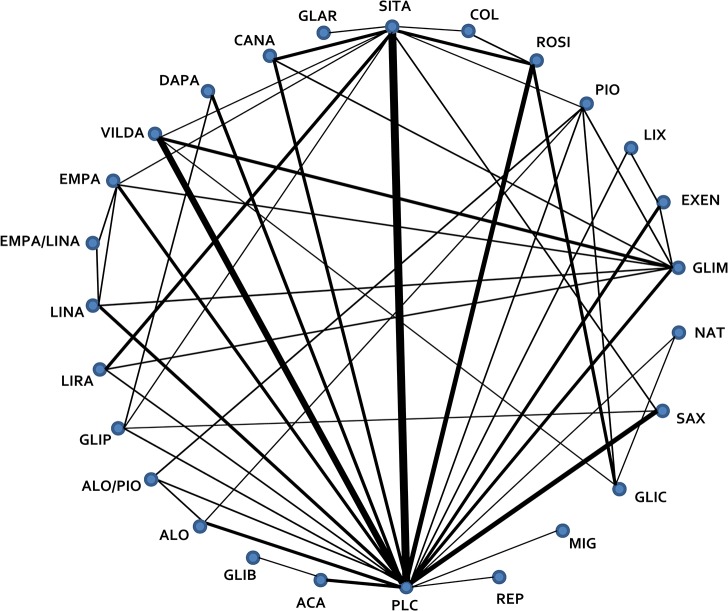
Characteristics of the included trials are described in S1 Table. The mean [range] trial duration was 29 [12–52] weeks; mean age ranged from 50–62 years; mean [range] BMI was 30.8 [25.0–34.6] kg/m2; mean [range] SBP was 131 [126–142] mmHg; and mean [range] baseline HbA1c was 8.0% [6.4–9.3%]. Fig 2 illustrates the network of RCTs with available direct comparisons, and individual RCT endpoint data are presented in Table 1. The overall quality of RCTs was rated as good to unclear with the majority of studies having few domains with a high risk of bias (S2 Fig).
Fig 2. Network Diagram of Randomized Controlled Trials Evaluating Antidiabetic Therapies in Addition to Optimized Metformin in Type 2 Diabetes.
All agents are in combination with optimized metformin and include oral and subcutaneous agents and long-acting, once daily basal insulins. Lines represent the presence of direct comparison trial(s). The width of the line is proportional to the number of trials with direct comparisons. ACA, acarbose; ALO, alogliptin; ALO/PIO, alogliptin/pioglitazone; CANA, canagliflozin; COL, colesevelam; DAPA, dapagliflozin; EMPA, empagliflozin; EMPA/LINA, empagliflozin/linagliptin; EXEN, exenatide; GLAR, insulin glargine; GLIB, glibenclamide; GLIC, gliclazide; GLIM, glimeperide; GLIP, glipizide; LINA, linagliptin; LIRA, liraglutide; LIX, lixisenatide; MIG, miglitol; NAT, nateglinide; PIO, pioglitizone; PLC, placebo; REP, repaglinide; ROSI, rosiglitizone; SAX, saxagliptin; SITA, sitagliptin; VILDA, vildagliptin
Table 1. Outcomes Reported In Randomized Controlled Trials Evaluating Antidiabetic Therapies Added to Metformin in Adult Patients With Type 2 Diabetes.
| Author, Year, N | Interventions Evaluated | Change in HbA1c, mean, % (SE) | Change in Body Weight, mean, kg (SE) | Change in Systolic Blood Pressure, mean, mmHg (SE) | Confirmed Hypoglycemia (n/N) | UTI (n/N) | GTI (n/N) |
|---|---|---|---|---|---|---|---|
| Defronzo 2014, N = 686 | Empagliflozin 25 mg /Linagliptin 5 mg | -1.19 (0.06) | -3.0 (0.3) | -5.6 (1.0) | 2/137 | 11/137 | 2/137 |
| Empagliflozin 10 mg /Linagliptin 5 mg | -1.08 (0.06) | -2.6 (0.3) | -4.1 (0.9) | 2/136 | 6/136 | 6/136 | |
| Empagliflozin 25 mg | -0.62 (0.06) | -3.2 (0.3) | -5.1 (0.9) | 4/141 | 12/141 | 12/141 | |
| Empagliflozin 10 mg | -0.66 (0.06) | -2.5 (0.3) | -4.0 (0.9) | 2/140 | 9/140 | 7/140 | |
| Linagliptin 5 mg | -0.70 (0.06) | -0.7 (0.3) | -1.0 (0.9) | 2/132 | 16/132 | 1/132 | |
| Bolli 2014, N = 484 | Lixisenatide 20 μg, 1-step titration | -0.9 (0.10) | -2.63 (0.4) | NR | 3/161 | NR | NR |
| Lixisenatide 20 μg, 2-step titration | -0.8 (0.1) | -2.68 (0.4) | NR | 4/161 | NR | NR | |
| Placebo | -0.4 (0.1) | -1.63 (0.4) | NR | 1/160 | NR | NR | |
| Derosa 2014, N = 167 | Glimepiride 2 mg TID | -1.0 (0.07) | 1.2 (0.74) | NR | NR | NR | NR |
| Vildagliptin 50 mg BID | -1.0 (0.08) | -1.5 (0.70) | NR | NR | NR | NR | |
| Haring 2014, N = 637 | Empagliflozin 10 mg | -0.70 (0.05) | -2.08 (0.17) | -4.5 (0.7) | 4/217 | 11/217 | 8/217 |
| Empagliflozin 25 mg | -0.77 (0.05) | -2.46 (0.17) | -5.2 (0.7) | 3/213 | 12/213 | 10/213 | |
| Placebo | -0.13 (0.05) | -0.45 (0.17) | -0.4 (0.7) | 1/207 | 10/207 | 0/207 | |
| Nauck 2014, N = 1098 | Sitagliptin 100 mg | -0.61 (0.05) | -1.49 (0.19) | -1.9 (0.7) | NR | 11/315 | NR |
| Placebo | 0.03 (0.08) | -1.59 (0.26) | 1.1 (0.9) | NR | 9/177 | NR | |
| Ridderstråle 2014, N = 1549 | Empagliflozin 25 mg | -0.73 (0.03) | -3.2 (0.10) | -3.6 (0.43) | 12/765 | 95/765 | 90/765 |
| Glimepiride 1–4 mg (mean dose = 2.7 mg/d) | -0.66 (0.03) | 1.6 (0.10) | 2.2 (0.46) | 159/780 | 99/780 | 17/780 | |
| White 2014, N = 160 | Saxagliptin 2.5 mg BID | -0.56 (0.09) | -0.32 (0.33) | 1.59 (1.51) | 0/74 | NR | NR |
| Placebo | -0.22 (0.08) | -0.40 (0.22) | 0.83 (1.26) | 1/86 | NR | NR | |
| Charbonnel 2013, N = 650 | Sitagliptin 100 mg | -1.32 (0.05) | -0.4 (0.20) | 0.9 (0.69) | 39/326 | NR | NR |
| Liraglutide 1.2 mg | -1.42 (0.05) | -2.8 (0.23) | -1.9 (0.71) | 13/324 | NR | NR | |
| Chawla 2013, N = 52 | Sitagliptin 100 mg | -0.66 (0.04) | -0.58 (NR) | NR | NR | NR | NR |
| Pioglitazone 30 mg | -0.75 (0.07) | 0.90 (NR) | NR | NR | NR | NR | |
| Cefalu 2013, N = 1450 | Glimepiride 1–8 mg (mean dose = 5.6 mg/d) | -0.81 (0.04) | 0.7 (0.2) | 0.2 (0.6) | 165/482 | 22/482 | 8/482 |
| Canagliflozin 100 mg | -0.82 (0.04) | -3.7 (0.2) | -3.3 (0.6) | 27/483 | 31/483 | 43/483 | |
| Canagliflozin 300 mg | -0.93 (0.04) | -4.0 (0.2) | -4.6 (0.6) | 23/485 | 31/485 | 54/485 | |
| Derosa 2013, N = 167 | Vildagliptin 50 mg BID | -1.2 (0.06) | -5.8 (0.6) | NR | 0/84 | NR | NR |
| Placebo | -0.8 (0.07) | -5.1 (0.6) | NR | 0/83 | NR | NR | |
| Lavalle-González 2013, N = 1284 | Canagliflozin 100 mg | -0.79 (0.04) | -3.3 (0.2) | -3.8 (0.6) | 10/368 | 18/368 | 20/368 |
| Canagliflozin 300 mg | -0.94 (0.04) | -3.6 (0.2) | -5.1 (0.6) | 7/367 | 13/367 | 22/367 | |
| Sitagliptin 100 mg | -0.82 0.04) | -1.1 (0.2) | -1.8 (0.6) | 0/366 | 12/366 | 5/366 | |
| Placebo | -0.17 (0.06) | -1.1 (0.2) | 1.5 (0.8) | 2/183 | 2/183 | 1/183 | |
| Rosenstock 2013, N = 495 | Empagliflozin 10 mg | -0.56 (0.08) | -2.7 (0.33) | -4.39 (1.55) | 0/71 | 3/71 | 7/71 |
| Empagliflozin 25 mg | -0.55 (0.08) | -2.6 (0.31) | -8.51 (1.53) | 0/70 | 4/70 | 0/70 | |
| Sitagliptin 100 mg | -0.45 (0.10) | -0.8 (0.33) | -1.79 (1.38) | 2/71 | 3/71 | 2/71 | |
| Placebo | 0.15 (0.08) | -1.2 (0.33) | -2.23 (1.76) | 0/71 | 2/71 | 0/71 | |
| Rosenstock 2013, N = 634 | Lixisenatide 20 μg | -0.79 (0.05) | -2.96 (0.23) | NR | 8/318 | NR | NR |
| Exenatide 10 μg BID | -0.96 (0.05) | -3.98 (0.23) | NR | 25/316 | NR | NR | |
| Aschner 2012, N = 515 | Insulin glargine w/titration | -1.72 (0.06) | 0.44 (0.22) | NR | 56/237 | NR | NR |
| Sitagliptin 100 mg | -1.13 (0.06) | -1.08 (0.2) | NR | 12/264 | NR | NR | |
| Bergenstal 2012, N = 666 | Sitagliptin 100 mg | -0.89 (0.06) | -0.9 (0.3) | NR | NR | NR | NR |
| Placebo | -0.1 (0.08) | -0.5 (0.4) | NR | NR | NR | NR | |
| DeFronzo 2012, N = 1554 | Placebo | -0.13 (0.08) | -0.66 (0.29) | NR | NR | 6/129 | NR |
| Alogliptin 12.5 mg | -0.64 (0.08) | -0.02 (0.29) | NR | NR | 6/128 | NR | |
| Alogliptin 25 mg | -0.90 (0.08) | -0.67 (0.29) | NR | NR | 5/129 | NR | |
| Pioglitazone 15 mg | -0.75 (0.08) | 0.94 (0.29) | NR | NR | 7/129 | NR | |
| Pioglitazone 30 mg | -0.92 (0.08) | 1.88 (0.29) | NR | NR | 8/129 | NR | |
| Pioglitazone 45 mg | -1.00 (0.08) | 1.65 (0.29) | NR | NR | 8/129 | NR | |
| Alogliptin 12.5 mg + pioglitazone 15 mg | -1.34 (0.08) | 1.25 (0.29) | NR | NR | 5/130 | NR | |
| Alogliptin 12.5 mg + pioglitazone 30 mg | -1.39 (0.08) | 1.89 (0.29) | NR | NR | 4/130 | NR | |
| Alogliptin 12.5 mg + pioglitazone 45 mg | -1.55 (0.08) | 2.30 (0.29) | NR | NR | 5/130 | NR | |
| Alogliptin 25 mg + pioglitazone 15 mg | -1.27 (0.08) | 1.27 (0.29) | NR | NR | 6/130 | NR | |
| Alogliptin 25 mg + pioglitazone 30 mg | -1.39 (0.08) | 2.10 (0.29) | NR | NR | 4/130 | NR | |
| Alogliptin 25 mg + pioglitazone 45 mg | -1.60 (0.08) | 2.25 (0.29) | NR | NR | 5/130 | NR | |
| Derosa 2012, N = 171 | Exenatide 10 μg BID | -1.2 (0.02) | -6.4 (0.6) | NR | 0/81 | NR | NR |
| Placebo | -0.4 (0.03) | -2.3 (1.0) | NR | 0/82 | NR | NR | |
| Derosa 2012, N = 178 | Sitagliptin 100 mg | -1.4 (0.01) | -2.5 (0.5) | NR | NR | NR | NR |
| Placebo | -0.7 (0.02) | -2.3 (0.6) | NR | NR | NR | NR | |
| Gallwitz 2012, N = 1029 | Exenatide 10 μg BID | -0.66 (0.04) | NR | -3.1 (0.97) | 165/511 | NR | NR |
| Glimepiride, 1mg—max tolerable dose (mean dose = 2.0 mg/d) | -0.48 (0.04) | NR | Referent | 332/508 | NR | NR | |
| Gallwitz 2012, N = 1551 | Linagliptin 5 mg | -0.36 (0.03) | -1.12 (0.13) | NR | 41/776 | 50/776 | NR |
| Glimepiride 1–4 mg (mean dose = 2.7 mg/d) | -0.57 (0.03) | 1.38 (0.14) | NR | 249/775 | 52/775 | NR | |
| Ljunggren 2012, N = 182 | Dapagliflozin 10 mg | -0.38 (0.06) | -4.39 (0.5) | NR | 4/89 | 0/89 | 0/89 |
| Placebo | 0.02 (0.06) | -2.03 (0.4) | NR | 2/91 | 0/91 | 0/91 | |
| Pan 2012, N = 438 | Vildagliptin 50 mg | -0.92 (0.08) | NR | NR | 0/148 | 4/148 | NR |
| Vildagliptin 50 mg BID | -1.05 (0.08) | NR | NR | 1/146 | 1/146 | NR | |
| Placebo | -0.54 (0.08) | NR | NR | 0/144 | 0/144 | NR | |
| Rizzo 2012, N = 90 | Sitagliptin 100 mg | -1.2 (0.14) | NR | -1.0 (2.31) | NR | NR | NR |
| Vildagliptin 50 mg BID | -1.0 (0.09) | NR | -3.0 (2.40) | NR | NR | NR | |
| Rosenstock 2012, N = 451 | Placebo | -0.22 (0.09) | -0.78 (0.3) | -1.3 (1.5) | 1/65 | 4/65 | 1/65 |
| Canagliflozin 100 mg | -0.76 (0.13) | -2.25 (0.3) | 1.0 (1.3) | 1/64 | 2/64 | 4/64 | |
| Canagliflozin 300 mg | -0.92 (0.09) | -2.88 (0.3) | -4.9 (1.5) | 0/64 | 2/64 | 2/64 | |
| Sitagliptin 100 mg | -0.74 (0.08) | -0.43 (0.3) | -0.8 (1.4) | 3/65 | 1/65 | 1/65 | |
| Ross 2012, N = 491 | Linagliptin 2.5 mg BID | -0.46 (0.05) | -0.4 (0.3) | NR | 5/223 | NR | NR |
| Linagliptin 5 mg | -0.52 (0.05) | -1.0 (0.2) | NR | 2/224 | NR | NR | |
| Placebo | 0.28 (0.11) | -1.1 (0.3) | NR | 1/44 | NR | NR | |
| Arechavaleta 2011, N = 1035 | Sitagliptin 100 mg | -0.47 (0.04) | -0.8 (0.15) | NR | 36/516 | NR | NR |
| Glimepiride 1–6 mg (mean dose = 2.1 mg/d) | -0.54 (0.04) | 1.2 (0.15) | NR | 114/518 | NR | NR | |
| Nauck 2011, N = 801 | Dapagliflozin 2.5–10 mg (mean dose = 9.2 mg/d) | -0.52 (0.04) | -3.22 (0.18) | -5.0 (0.84) | 7/406 | 30/406 | 50/406 |
| Glipizide 5–20 mg (mean dose = 16.4 mg/d) | -0.52 (0.04) | 1.44 (0.18) | Referent | 147/408 | 17/408 | 11/408 | |
| Pfützner 2011, N = 288 | Pioglitazone 15 mg BID | -0.83 (0.07) | 0.7 (0.36) | -2.5 (1.23) | 2/146 | NR | NR |
| Glimepiride 1 mg BID | -0.95 (0.07) | 0.7 (0.36) | 0.5 (1.55) | 5/142 | NR | NR | |
| Taskinen 2011, N = 700 | Placebo | 0.15 (0.06) | -0.5 (NR) | NR | 5/177 | 7/177 | NR |
| Linagliptin 5 mg | -0.49 (0.04) | -0.4 (NR) | NR | 3/523 | 16/523 | NR | |
| Wang 2011, N = 55 | Acarbose 100 mg TID | -0.7 (0.15) | -1.5 (0.4) | NR | 0/28 | NR | NR |
| Glibenclamide 5 mg TID | -1.2 (0.30) | 0.8 (0.5) | NR | NR | NR | NR | |
| Yang 2011, N = 570 | Saxagliptin 5 mg | -0.78 (0.05) | -1.05 (0.1) | -5.22 (NR) | 0/283 | 13/283 | NR |
| Placebo | -0.37 (0.05) | -0.97 (0.1) | -3.10 (NR) | 0/287 | 8/287 | NR | |
| Bailey 2010, N = 546 | Placebo | -0.30 (0.07) | -0.9 (0.3) | -0.2 (1.2) | 4/137 | 11/137 | 7/137 |
| Dapagliflozin 5 mg | -0.70 (0.07) | -3.0 (0.2) | -4.3 (1.3) | 5/137 | 10/137 | 18/137 | |
| Dapagliflozin 10 mg | -0.84 (0.07) | -2.9 (0.2) | -5.1 (1.3) | 5/135 | 11/135 | 12/135 | |
| Filozof 2010, N = 1007 | Vildagliptin 50 mg BID | -0.81 (0.06) | -1.28 (0.39) | NR | NR | NR | NR |
| Gliclazide 80–320 mg (mean dose = 230 mg/d) | -0.85 (0.06) | Referent | NR | NR | NR | NR | |
| Goke 2010, N = 858 | Saxagliptin 5 mg | -0.74 (0.05) | -1.1 (0.19) | -4.1 (NR) | 0/428 | NR | NR |
| Glipizide 5–20 mg (mean dose = 14.7 mg/d) | -0.80 (0.04) | 1.1 (0.16) | -1.2 (NR) | 38/430 | NR | NR | |
| Pratley 2010, N = 665 | Liraglutide 1.2 mg | -1.24 (0.07) | -2.86 (0.3) | -0.55 (0.9) | 12/221 | NR | NR |
| Liraglutide 1.8 mg | -1.50 (0.07) | -3.38 (0.3) | -0.72 (0.9) | 11/218 | NR | NR | |
| Sitagliptin 100 mg | -0.90 (0.07) | -0.96 (0.3) | -0.94 (0.9) | 10/219 | NR | NR | |
| Rigby 2010, N = 169 | Colesevelam 3.75 g | -0.3 (0.13) | -0.64 (0.3) | NR | NR | NR | NR |
| Rosiglitazone 4 mg | -0.6 (0.13) | 0.26 (0.5) | NR | NR | NR | NR | |
| Sitagliptin 100 mg | -0.4 (0.13) | -1.15 (0.3) | NR | NR | NR | NR | |
| Scheen 2010, N = 801 | Saxagliptin 5 mg | -0.52 (0.04) | -0.4 (NR) | NR | 13/406 | 23/403 | NR |
| Sitagliptin 100 mg | -0.62 (0.04) | -0.4 (NR) | NR | 11/393 | 21/398 | NR | |
| DeFronzo 2009, N = 743 | Placebo | 0.13 (0.07) | -0.92 (NR) | -4.5 (1.3) | 1/179 | 8/179 | NR |
| Saxagliptin 2.5 mg | -0.59 (0.07) | -1.43 (NR) | -4.3 (1.2) | 1/192 | 10/192 | NR | |
| Saxagliptin 5 mg | -0.69 (0.07) | -0.87 (NR) | -3.6 (1.2) | 1/191 | 10/191 | NR | |
| Ferrannini 2009, N = 2789 | Vildagliptin 50mg BID | -0.44 (0.02) | -0.23 (0.11) | NR | 23/1389 | NR | NR |
| Glimepiride 2–6 mg(mean dose = 4.5 mg/d) | -0.53 (0.02) | 1.56 (0.12) | NR | 224/1383 | NR | NR | |
| Goodman 2009, N = 370 | Vildagliptin 100mg | -0.60 (0.08) | 0.75 (0.31) | NR | 2/248 | NR | NR |
| Placebo | 0.17 (0.11) | Referent | NR | 0/122 | NR | NR | |
| Nauck 2009, N = 527 | Placebo | -0.1 (0.1) | -0.39 (0.27) | NR | 3/104 | 4/104 | NR |
| Alogliptin 12.5 mg | -0.6 (0.1) | -0.39 (0.19) | NR | 2/213 | 14/213 | NR | |
| Alogliptin 25 mg | -0.6 (0.1) | -0.67 (0.20) | NR | 0/210 | 6/210 | NR | |
| Nauck 2009, N = 1087 | Liraglutide 0.6 mg | -0.7 (0.1) | -1.8 (0.2) | NR | 7/242 | NR | NR |
| Liraglutide 1.2 mg | -1.0 (0.1) | -2.6 (0.2) | -3.2 (1.29) | 7/240 | NR | NR | |
| Liraglutide 1.8 mg | -1.0 (0.1) | -2.8 (0.2) | -2.7 (1.36) | 7/242 | NR | NR | |
| Glimepiride 4 mg | -1.0 (0.1) | 1.0 (0.2) | Referent | 41/242 | NR | NR | |
| Placebo | 0.1 (0.1) | -1.5 (0.3) | NR | 4/121 | NR | NR | |
| Hamann 2008, N = 596 | Rosiglitazone 4 mg | -0.78 (0.06) | 2.7 (0.3) | -2.6 (1.38) a | 1/294 | 7/294 a | NR |
| Gliclazide 80–320mg(mean dose = 238.1 mg/d) | -0.86 (0.06) | 1.6 (0.3) | Referent | 21/301 | 9/301 | NR | |
| Khanolkar 2008, N = 50 | Rosiglitazone 4 mg | -1.19 (0.11) | NR | NR | NR | NR | NR |
| Gliclazide 80 mg | -1.0 (0.13) | NR | NR | NR | NR | NR | |
| Raz 2008, N = 190 | Sitagliptin 100 mg | -0.98 (0.14) | -0.5 (NR) | NR | 1/96 | 4/96 | NR |
| Placebo | 0.04 (0.14) | -0.5 (NR) | NR | 0/94 | 3/94 | NR | |
| Scott 2008, N = 273 | Placebo | -0.22 (0.07) | -0.8 (0.2) | NR | 2/91 | NR | NR |
| Sitagliptin 100 mg | -0.73 (0.07) | -0.4 (0.2) | NR | 1/94 | NR | NR | |
| Rosiglitazone 8 mg | -0.79 (0.07) | 1.5 (0.2) | NR | 1/87 | NR | NR | |
| Bosi 2007, N = 544 | Vildagliptin 50 mg | -0.5 (0.1) | -0.4 (0.3) | NR | 1/143 | NR | NR |
| Vildagliptin 100 mg | -0.9 (0.1) | 0.2 (0.3) | NR | 1/143 | NR | NR | |
| Placebo | 0.2 (0.1) | 1.0 (0.3) | NR | 1/130 | NR | NR | |
| Nauck 2007, N = 1172 | Sitagliptin 100 mg | -0.67 (0.04) | -1.5 (0.28) | NR | 29/588 | 44/588 | NR |
| Glipizide 5–20 mg (mean dose = 10.3 mg/d) | -0.67 (0.04) | 1.1 (0.28) | NR | 187/584 | 25/584 | NR | |
| Ristic 2006, N = 262 | Nateglinide 60–180 mg TID (59.4% of patients on 540 mg/d) | -0.41 (0.08) | NR | NR | 28/130 | NR | NR |
| Gliclazide 80–240 mg (35.7% of patients on 240 mg/d) | -0.57 (0.08) | NR | NR | 28/126 | NR | NR | |
| DeFronzo 2005, N = 336 | Placebo | 0.1 (0.1) | -0.3 (0.3) | NR | 6/113 | NR | NR |
| Exenatide 5 μg BID | -0.4 (0.1) | -1.6 (0.4) | NR | 5/110 | NR | NR | |
| Exenatide 10 μg BID | -0.8 (0.1) | -2.8 (0.5) | NR | 6/113 | NR | NR | |
| Feinglos 2005, N = 122 | Glipizide 2.5 mg | -0.66 (0.1) | 2.1 (0.64) | NR | 9/61 | NR | NR |
| Placebo | -0.19 (0.1) | Referent | NR | 2/61 | NR | NR | |
| Matthews 2005, N = 630 | Pioglitazone 15–45 mg (mean dose = 39 mg/d) | 0.02 (0.09) | 1.5 (NR) | NR | 4/317 | NR | NR |
| Gliclazide 80–320 mg (mean dose = 212 mg/d) | Referent | 1.4 (NR) | NR | 35/313 | NR | NR | |
| Ahren 2004, N = 71 | Vildagliptin 50 mg | -0.6 (0.1) | -0.4 (0.2) | NR | 2/56 | 1/56 | NR |
| Placebo | 0.1 (0.1) | -0.5 (0.2) | NR | 0/51 | 3/51 | NR | |
| Gomez-Perez 2002, N = 116 | Placebo | 0.3 (0.3) | -0.86 (0.5) | NR | 0/34 | NR | NR |
| Rosiglitazone 2 mg BID | -0.7 (0.2) | 0.26 (0.6) | NR | 0/35 | NR | NR | |
| Rosiglitazone 4 mg BID | -1.2 (0.3) | 2.42 (0.6) | NR | 0/36 | NR | NR | |
| Marre 2002, N = 467 | Nateglinide 60 mg TID | -0.36 (0.12) | 0.4 (0.2) | NR | 0/155 | NR | NR |
| Nateglinide 120 mg TID | -0.51 (0.12) | 1.0 (0.2) | NR | 5/160 | NR | NR | |
| Placebo | Referent | 0.1 (0.2) | NR | 1/152 | NR | NR | |
| Charpentier 2001, N = 372 | Placebo | 0.07 (0.14) | -0.74 (0.3) | -0.65 (1.41) | 11/75 | NR | NR |
| Glimepiride 1–6 mg (41% of patients on 6 mg/d) | -0.74 (0.08) | 0.6 (0.2) | -0.14 (1.09) | 22/147 | NR | NR | |
| Van Gaal 2001, N = 152 | Miglitol 100 mg TID | -0.21 (0.1) | -2.5 (0.4) | NR | 0/78 | NR | NR |
| Placebo | 0.22 (0.1) | -0.7 (0.3) | NR | 0/75 | NR | NR | |
| Halimi 2000, N = 129 | Acarbose 50–100 mg TID | -0.7 (0.16) | NR | NR | NR | NR | NR |
| Placebo | 0.2 (0.16) | NR | NR | NR | NR | NR | |
| Fonseca 2000, N = 348 | Placebo | 0.45 (0.12) | Referent | NR | 2/116 | NR | NR |
| Rosiglitazone 4 mg | -0.56 (0.11) | 1.9 (0.49) | NR | 3/119 | NR | NR | |
| Rosiglitazone 8 mg | -0.78 (0.19) | 3.1 (0.80) | NR | 5/113 | NR | NR | |
| Moses 1999, N = 83 | Repaglinide 4 mg | -1.41 (0.23) | 2.41 (0.5) | NR | 9/27 | NR | NR |
| Placebo | -0.33 (0.24) | -0.86 (0.51) | NR | 0/27 | NR | NR | |
| Rosenstock 1998, N = 148 | Acarbose 50–100 mg TID | -0.71 (0.18) | -0.10 (0.42) | NR | 1/73 | NR | NR |
| Placebo | Referent | Referent | NR | 2/74 | NR | NR |
BMI = body mass index; DM = diabetes mellitus; HbA1c = hemoglobin A1c; n = number of patients; NR = not reported; SE = standard error
a Not included in network meta-analysis because neither rosiglitazone nor gliclazide are connected to the network of trials.
Change in HbA1c
SGLT2 Inhibitors
The SGLT2 inhibitors had similar effects on reducing HbA1c when compared to placebo in the NMA, ranging from 0.48% for dapagliflozin to 0.72% for canagliflozin (S3 Fig). When compared to the other active single agents, canagliflozin was associated with statistically significant reductions in HbA1c compared with dapagliflozin, nateglinide and saxagliptin; and empagliflozin was significantly more efficacious compared to dapagliflozin and saxagliptin. Dapagliflozin was inferior in reducing HbA1c when compared to 11 (50%) of the other active single agents. Along with statistical significance, all SGLT-2 inhibitors were found to be clinically superior to placebo (lower bound of the 95%CI depicted an HbA1c reduction greater than 0.3%), however none of the SGLT2 inhibitors were clinically superior in reducing HbA1c to any other active agents analyzed.
Combination Agents
Both combination agents were associated with significant reductions in HbA1c when compared to placebo in the NMA (alogliptin/pioglitazone: 1.24, 95% CI: 1.02–1.45%; empagliflozin/linagliptin: 1.13%, 95% CI: 0.92–1.34%). When comparing the combination medications to the other active single agents, alogliptin/pioglitazone significantly reduced HbA1c when compared to all other therapies except for insulin glargine, glibenclamide and repaglinide; whereas empagliflozin/linagliptin was more efficacious when compared to all other active single agents except for insulin glargine, glibenclamide, repaglinide and acarbose.
In terms of clinical superiority (lower bound of the 95%CI depicted an HbA1c reduction greater than 0.3%) alogliptin/pioglitazone and empagliflozin/linagliptin were clinically superior to 52% and 24% of the other antidiabetic medications analyzed, respectively. Alogliptin/pioglitazone was clinically superior to all DPP-4 inhibitors, colesevelam, dapagliflozin, glipizide, lixisenatide, miglitol, nataglinide, empagliflozin and pioglitazone. Empagliflozin/linagliptin was clinically superior to canagliflozin, dapagliflozin, glipizide, miglitol, nateglinide and saxagliptin.
All Other Agents
All antidiabetic agents were associated with statistically significant reductions in HbA1c relative to placebo, ranging from 0.43% for miglitol to 1.29% for glibenclamide (Table 2). When comparing single active agents, insulin glargine was associated with statistically significant reductions in HbA1c compared with all other therapies, except glibenclamide and repaglinide. Exenatide showed significant reductions in HbA1c when compared to the DPP-4 inhibitors, lixisenatide, miglitol, nateglinide, glipizide and dapagliflozin.
Table 2. Results of Network Meta-Analysis and Traditional Pair-Wise Meta-Analysis Comparing Antidiabetic Therapies’ Effect on Change in HbA1c, Body Weight and Systolic Blood pressure.
| Change in HbA1c, % | Change in Body Weight, kg | Change in Systolic Blood Pressure, mmHg | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparison vs. Placebo | No. of Trials a | Direct Estimate, WMD (95%CI) | NMA Estimate, WMD (95%CI) | No. of Trials a | Direct Estimate, WMD (95%CI) | NMA Estimate, WMD (95%CI) | No. of Trials a | Direct Estimate, WMD (95%CI) | NMA Estimate, WMD (95%CI) |
| Acarbose | 2 | -0.78 (-1.06, -0.50) | -0.79 (-1.09,-0.48) | 1 | -0.10 (-0.92, 0.72) | -0.1 (-1.15, 0.95) | 0 | — | — |
| Alogliptin | 2 | -0.60 (-0.76, -0.43) | -0.57 (-0.76,-0.38) | 2 | 0.05 (-0.41, 0.51) | 0.09 (-0.53, 0.72) | 0 | — | — |
| Alogliptin/pioglitazone | 1 | -1.29 (-1.49, -1.09) | -1.24 (-1.45,-1.02) | 1 | 2.50 (1.74, 3.26) | 2.39 (1.55, 3.23) | 0 | — | — |
| Canagliflozin | 2 | -0.67 (-0.79, -0.55) | -0.72 (-0.85,-0.59) | 2 | -2.10 (-2.65, -1.55) | -2.15 (-2.63, -1.67) | 2 | -3.52 (-8.74, 1.70) | -4.14 (-5.8, -2.48) |
| Colesevelam | 0 | — | -0.5 (-0.85,-0.14) | 0 | — | 0.89 (-0.11, 1.9) | 0 | — | — |
| Dapagliflozin | 2 | -0.43 (-0.55, -0.31) | -0.48 (-0.62,-0.33) | 2 | -2.10 (-2.62, -1.59) | -2.17 (-2.78, -1.57) | 1 | -4.5 (-7.54, -1.46) | -4.5 (-7.97, -1.03) |
| Empagliflozin | 2 | -0.64 (-0.75, -0.52) | -0.69 (-0.81,-0.57) | 2 | -1.74 (-2.10, -1.37) | -2.08 (-2.52, -1.63) | 2 | -4.41 (-5.96, -2.87) | -5.14 (-6.8, -3.48) |
| Emapgliflozin/linagliptin | 0 | — | -1.13 (-1.34,-0.92) | 0 | — | -2.07 (-2.95, -1.19) | 0 | — | -5.43 (-8.39, -2.47) |
| Exenatide | 2 | -0.79 (-0.86, -0.72) | -0.8 (-0.92,-0.67) | 2 | -2.76 (-4.85, -0.67) | -2.26 (-3.15, -1.37) | 0 | — | -2.84 (-5.89, 0.22) |
| Insulin glargine | 0 | — | -1.23 (-1.49,-0.97) | 0 | — | 1.73 (0.8, 2.66) | 0 | — | — |
| Glibenclamide | 0 | — | -1.29 (-2.01,-0.56) | 0 | — | 2.2 (0.45, 3.95) | 0 | — | — |
| Gliclazide | 0 | — | -0.7 (-0.85,-0.56) | 0 | — | 1.19 (0.39, 1.99) | 0 | — | — |
| Glimepiride | 2 | -0.95 (-1.23, -0.67) | -0.73 (-0.82,-0.64) | 2 | 1.92 (0.78, 3.06) | 2.19 (1.84, 2.54) | 1 | 0.51 (-3.08, 4.10) | 0.26 (-1.44, 1.96) |
| Glipizide | 1 | -0.47 (-0.74, -0.20) | -0.55 (-0.68,-0.42) | 1 | 2.10 (0.85, 3.35) | 2.44 (1.88, 3) | 0 | — | 0.5 (-3.69, 4.69) |
| Linagliptin | 2 | -0.68 (-0.81, -0.54) | -0.64 (-0.77,-0.52) | 1 | 0.40 (-0.50, 1.30) | -0.04 (-0.62, 0.54) | 0 | — | -1.58 (-4.81, 1.64) |
| Liraglutide | 1 | -1.00 (-1.26, -0.75) | -0.86 (-1.0,-0.71) | 1 | -0.90 (-1.49, -0.31) | -1.6 (-2.12, -1.08) | 0 | — | -3.04 (-5.05, -1.03) |
| Lixisenatide | 1 | -0.45 (-0.69, -0.22) | -0.56 (-0.76,-0.37) | 1 | -1.02 (-1.96, -0.08) | -1.15 (-2.05, -0.26) | 0 | — | — |
| Miglitol | 1 | -0.43 (-0.70, -0.16) | -0.43 (-0.76,-0.1) | 1 | -1.80 (-2.78, -0.82) | -1.8 (-2.98,-0.62) | 0 | — | — |
| Nateglinide | 1 | -0.44 (-0.60, -0.28) | -0.48 (-0.67,-0.29) | 1 | 0.60 (0.13, 1.07) | 0.6 (-0.2, 1.4) | 0 | — | — |
| Pioglitazone | 1 | -0.76 (-0.98, -0.54) | -0.69 (-0.83,-0.55) | 1 | 2.14 (1.32, 2.96) | 2.06 (1.31, 2.81) | 0 | — | -2.74 (-6.82, 1.35) |
| Repaglinide | 1 | -1.08 (-1.73, -0.43) | -1.08 (-1.75,-0.41) | 1 | 3.27 (1.88, 4.66) | 3.27 (1.73, 4.81) | 0 | — | — |
| Rosiglitazone | 3 | -0.91 (-1.38, -0.44) b | -0.75 (-0.9,-0.6) | 3 | 2.27 (1.84, 2.70) | 2.15 (1.65, 2.66) | 0 | — | — |
| Saxagliptin | 3 | -0.50 (-0.74, -0.26) b | -0.51 (-0.62,-0.39) | 2 | -0.06 (-0.32, 0.20) | 0.06 (-0.45, 0.57) | 2 | 0.64 (-1.86, 3.13) | 0.64 (-2.13, 3.41) |
| Sitagliptin | 8 | -0.67 (-0.73, -0.60) | -0.64 (-0.71,-0.57) | 7 | 0.16 (-0.12, 0.45) | 0.21 (-0.1, 0.53) | 4 | -2.11 (-3.90, -0.32) c | -1.88 (-3.38, -0.38) |
| Vildagliptin | 5 | -0.63 (-0.83, -0.43) b | -0.63 (-0.72,-0.53) | 4 | -0.16 (-1.02, 0.70) b | 0.04 (-0.37, 0.44) | 0 | — | -3.88 (-10.79, 3.02) |
CI = confidence interval; HbA1c = hemoglobin A1c; NMA = network meta-analysis; WMD = weighted mean difference
a Number of trials with direct comparisons
b I2>50%
c Egger’s p-value <0.5.
Along with statistical significance, all therapies were found clinically superior to placebo (lower bound of the 95%CI depicted an HbA1c reduction greater than 0.3%) except for colesevelam, nateglinide and miglitol (lower limit of 95%CI = -0.14, -0.28 and -0.16, respectively). In head-to-head comparisons, and insulin glargine was clinically superior to 40% of the other antidiabetic medications analyzed. Insulin glargine was found to be clinically superior to all DPP-4 inhibitors, colesevelam, dapagliflozin, glipizide, lixisenatide, miglitol, and nataglinide.
Body Weight
SGLT2 Inhibitors
All SGLT2 inhibitors were associated with significant weight loss when compared to placebo in the NMA (range: 2.08–2.17 kg) (S4 Fig). When comparing active drugs, SGLT2 inhibitors were associated with statistically greater weight loss compared to all other agents analyzed except GLP-1 analogs, empagliflozin/linagliptin and miglitol. Moreover, SGLT2 inhibitors were associated with both statistically significant and clinically superior weight loss compared to SUs, TZDs and insulin glargine (range: 3.81–4.61 kg).
Combination Agents
Alogliptin/pioglitazone was associated with significant increases in BW when compared with placebo in the NMA (2.39 kg, 95% CI: 1.55–3.23 kg) whereas empagliflozin/linagliptin was associated with significant weight loss (2.07 kg, 95% CI: 1.19–2.95 kg). When compared to other single active agents, empagliflozin/linagliptin was associated with significant weight loss compared to all other agents except SGLT-2 inhibitors, and GLP-1 analogs. Alogliptin/pioglitazone caused significant increases in BW compared to all other active agents except SUs, repaglinide and TZDs. In terms of clinically superior weight gain, (lower bound of the 95%CI depicted a decrease in weight less than 2.3 kg), alogliptin/pioglitazone was associated with clinically superior weight gain compared to SGLT2 inhibitors, empagliflozin/linagliptin, GLP-1 analogs, and miglitol (range: 3.54–4.65 kg).
All Other Agents
The SUs, TZDs, insulin glargine and repaglinide were associated with significant increases in BW when compared with placebo in the NMA (range: 1.19–2.44 kg). GLP-1 analogs and miglitol were associated with significant weight loss (range: 1.15–2.26 kg) but there was no weight change with acarbose, any DPP-4 inhibitor, colesevelam and nateglinide when compared to placebo. When comparing active agents, GLP-1 analogs were associated with statistically greater weight loss when compared to all other agents except SGLT2 inhibitors and miglitol. While several agents exhibited statistically significant weight loss, no agent demonstrated clinically superior weight loss compared to placebo (lower bound of the 95%CI depicted a decrease in weight less than 2.3 kg). When comparing the clinical superiority of single active agents, TZDs were associated with clinically superior weight gain when compared to GLP-1 analogs (range: 3.22–4.41 kg).
Systolic Blood Pressure
SGLT2 Inhibitors
All SGLT2 inhibitors were associated with a decrease in SBP compared with placebo in the NMA (range: 4.14–5.14 mmHg. When comparing active agents, SGLT2 inhibitors significantly reduced SBP when compared to the SUs (glimepiride, glipizide) (range: 4.4–5.64 mmHg), and saxagliptin and sitagliptin (range: 2.26–5.79 mmHg) (S5 Fig). No SGLT2 inhibitor showed clinical superiority (lower bound of the 95%CIs depicted a decrease in SBP less than 5 mmHg) compared to placebo or another active agent.
Combination Agents
Empagliflozin/linagliptin was associated with a decrease in SBP when compared with placebo in the NMA (5.43 mmHg, 95% CI: 2.47–8.39 mmHg). In head-to-head comparisons, empagliflozin/linagliptin significantly reduced SBP when compared to SUs, linagliptin, saxagliptin and sitagliptin; however it did not show clinical superiority compared to any other active agents. There were no data to evaluate alogliptin/pioglitazone for this endpoint.
All Other Agents
Liraglutide (3.04 mmHg, 95% CI: 1.03–5.05 mmHg) and sitagliptin (1.88 mmHg, 95% CI: 0.38–3.38 mmHg) were associated with a decrease in SBP compared with placebo. No medication showed clinical superiority (lower bound of the 95%CIs depicted a decrease in SBP less than 5 mmHg) compared to placebo or another active agent; however, there were no data to evaluate 12 (48%) of the agents for this endpoint.
Confirmed Hypoglycemia
SGLT2 Inhibitors
Upon NMA, the SGLT2 inhibitors were not associated with an increased risk of confirmed hypoglycemia compared with placebo (S6 Fig). In the active drug comparisons, insulin glargine, nateglinide and all SUs were associated with significantly higher rates of confirmed hypoglycemia compared to any SGLT2 inhibitor (RR range, 4.14–22.93).
Combination Agents
Empagliflozin/linagliptin was not associated with increased risk of hypoglycemia compared with placebo in the NMA (0.38, 95% CI: 0.06–2.34). In the active drug comparisons, insulin glargine, nateglinide, both meglitinides and all SUs were associated with significantly higher rates of confirmed hypoglycemia compared to empagliflozin/linagliptin (RR range, 10.54–49.88). There were no data to evaluate alogliptin/pioglitazone for this endpoint.
All Other Agents
Insulin glargine, all SUs, and nateglinide were associated with significantly higher rates of confirmed hypoglycemia compared with placebo upon NMA (RR range, 4.00–11.67) (Table 3). All GLP-1 analogs, DPP-4 inhibitors, TZDs, repaglinide and acarbose were not associated with an increased risk of confirmed hypoglycemia compared with placebo. In the active drug comparisons, insulin glargine and all SUs were associated with significantly higher rates of confirmed hypoglycemia compared to any SGLT2 or DPP-4 inhibitor (RR range, 4.32–71.29). There were no data to evaluate glibenclamide, colesevelam and miglitol for this endpoint.
Table 3. Results of Network Meta-Analysis and Traditional Meta-Analysis Comparing Antidiabetic Therapies’ Effect on Confirmed Hypoglycemia, Urinary and Genital Tract Infection.
| Confirmed Hypoglycemia | Urinary Tract Infection | Genital Tract Infection | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparison vs. Placebo | No. of Trials a | Direct Estimate, RR (95%CI) | NMA Estimate, RR (95%CI) | No. of Trials a | Direct Estimate, RR (95%CI) | NMA Estimate, RR (95%CI) | No. of Trials a | Direct Estimate, RR (95%CI) | NMA Estimate, RR (95%CI) |
| Acarbose | 1 | 0.51 (0.05, 5.47) | 0.51 (0.04, 7.23) | 0 | — | — | 0 | — | — |
| Alogliptin | 1 | 0.16 (0.03, 0.97) | 0.16 (0.02, 1.4) | 2 | 1.05 (0.51, 2.15) | 1.06 (0.51,2.17) | 0 | — | — |
| Alogliptin/pioglitazone | 0 | — | — | 1 | 0.80 (0.34, 1.89) | 0.87 (0.41,1.85) | 0 | — | — |
| Canagliflozin | 2 | 1.55 (0.43, 5.62) | 0.91 (0.33, 2.51) | 2 | 1.38 (0.18, 10.90) | 1.25 (0.78,2) | 2 | 5.85 (1.39, 24.65) | 8.03 (2.44,26.39) |
| Colesevelam | 0 | — | — | 0 | — | — | 0 | — | — |
| Dapagliflozin | 2 | 1.50 (0.54, 4.18) | 0.97 (0.34, 2.76) | 1 | 0.96 (0.48, 1.93) | 1.28 (0.77,2.14) | 1 | 2.16 (0.97, 4.79) | 2.16 (0.97,4.82) |
| Empagliflozin | 1 | 3.45 (0.43, 27.86) | 0.51 (0.17, 1.49) | 2 | 1.20 (0.63, 2.32) | 0.86 (0.57,1.3) | 2 | 11.70 (1.59, 86.34) | 6.84 (1.92,24.37) |
| Emapgliflozin/linagliptin | 0 | — | 0.38 (0.06, 2.34) | 0 | — | 0.6 (0.31,1.19) | 0 | — | 2.95 (0.66,13.28) |
| Exenatide | 1 | 0.93 (0.35, 2.45) | 1.82 (0.7, 4.76) | 0 | — | — | 0 | — | — |
| Insulin glargine | 0 | — | 6.78(1.46, 31.4) | 0 | — | — | 0 | — | — |
| Glibenclamide | 0 | — | — | 0 | — | — | 0 | — | — |
| Gliclazide | 0 | — | 10.02 (2.07, 48.56) | 0 | — | — | 0 | — | — |
| Glimepiride | 2 | 2.19 (0.42,11.50) | 4.0 (2.16, 7.41) | 0 | — | 0.89 (0.59,1.33) | 0 | — | 1.28 (0.36,4.53) |
| Glipizide | 1 | 4.5 (1.01, 19.98) | 11.67 (4.41, 30.87) | 0 | — | 0.55 (0.29,1.04) | 0 | — | 0.47 (0.17,1.33) |
| Linagliptin | 2 | 0.30 (0.09, 0.97) | 0.46 (0.18, 1.2) | 1 | 0.77 (0.32, 1.85) | 0.95 (0.6,1.5) | 0 | — | 0.77 (0.07,8.18) |
| Liraglutide | 1 | 0.88 (0.31, 2.51) | 0.78 (0.31, 1.96) | 0 | — | — | 0 | — | — |
| Lixisenatide | 1 | 3.48 (0.43, 28.03) | 0.93 (0.22, 3.83) | 0 | — | — | 0 | — | — |
| Miglitol | 0 | — | — | 0 | — | — | 0 | — | — |
| Nateglinide | 1 | 2.41 (0.28, 20.47) | 7.19 (1.34, 38.65) | 0 | — | — | 0 | — | — |
| Pioglitazone | 0 | — | 1.28(0.25, 6.48) | 1 | 1.25 (0.5, 3.15) | 1.4 (0.65,3.02) | 0 | — | — |
| Repaglinide | 1 | 19.00 (1.16, 310.66) | 18.92 (0.9, 398.91) | 0 | — | — | 0 | — | — |
| Rosiglitazone | 2 | 1.35 (0.37, 4.90) | 1.01 (0.26, 3.97) | 0 | — | — | 0 | — | — |
| Saxagliptin | 2 | 0.68 (0.10, 4.61) | 0.88 (0.26, 2.96) | 2 | 1.37 (0.76, 2.46) | 1.19 (0.75,1.89) | 0 | — | — |
| Sitagliptin | 5 | 1.19 (0.31, 4.56) | 1.3 (0.62, 2.72) | 5 | 1.02 (0.51, 2.01) | 0.96 (0.62,1.48) | 3 | 2.27 (0.52, 9.90) | 2.33 (0.62,8.8) |
| Vildagliptin | 4 | 1.79 (0.43, 7.45) | 0.75 (0.26, 2.14) | 2 | 1.11 (0.06, 19.52) | 0.9 (0.15,5.24) | 0 | — | — |
CI = confidence interval; NMA = network meta-analysis; RR = relative risk
a Number of trials with direct comparisons.
Urinary and Genital Tract Infection
No treatment was associated with an increased risk of UTI when compared to placebo; however, there were no data to evaluate 13 (52%) of the agents for this endpoint (S7 Fig). NMA suggested canagliflozin and empagliflozin were associated with an increased risk of GTI when compared with placebo; with dapagliflozin (RR 2.16, 95% CI 0.97–4.82) trending towards an increased risk versus placebo. However, only 10 identified RCTs evaluating 8 of 25 agents reported GTI data (S8 Fig).
Direct and Indirect Meta-Analysis Coherence
Results from all pair-wise traditional meta-analyses for each of the six endpoints are reported in S2–S7 Tables. When direct evidence stemming from traditional pair-wise meta-analysis (or a single RCT) was compared to the indirect evidence estimates, we found incoherence in eight of the 151 possible comparisons (2 for the HbA1c endpoint, 2 for body weight, and 4 for confirmed hypoglycemia).
Sensitivity Analysis
Results from the sensitivity analysis are reported in S9–S14 Figs. The HbA1c endpoint found 91 of 124 comparisons that were still statistically significant at the more stringent 99% CI cutoff (resulting in only 33 comparisons that were changed). For the BW endpoint, only 24 comparisons became nonsignificant (from 219 to 195). For the change in SBP, originally 27 comparisons were significant; however using the stricter set of CIs, only about half (15) remained significant. For the confirmed hypoglycemia endpoint, 74 significant comparisons decreased to 39 after a stricter CI cutoff was applied. No comparisons were altered in the UTI endpoint after the application of a more stringent cutoff and only 4 changed (originally 12) for the GTI endpoint.
Discussion
When HbA1c goals are not met or maintained with lifestyle modifications and metformin monotherapy, the ADA/EASD guidelines recommend patients initiate an additional antidiabetic agent from 1 of 5 classes (SUs, TZDs, DPP-4 inhibitors, GLP-1 analogs or basal insulin) but do not endorse specific agents in the overall Type 2 DM population or specific patient subtypes [2]. These guidelines were published before SGLT2 inhibitors were commercially available. Our NMA is the first to assess the comparative efficacy and safety of adjunctive therapy with agents in the 10 antidiabetic classes, including the new SGLT2 inhibitors, combination therapies and colesevelam (which is mentioned in current guidelines), when added to optimized but inadequate metformin therapy.
Our HbA1c results showed both statistical differences and clinical superiority between antidiabetic therapies. All therapies significantly reduced HbA1c, but to differing degrees when compared to placebo. Combination therapies (empagliflozin/linagliptin and alogliptin/pioglitazone) and insulin glargine were statistically and clinically superior in reducing HbA1c compared to a majority of other antidiabetic agents. As a class, the SGLT2 inhibitors were similar in efficacy to other non-insulin monotherapies recommended by the ADA as add-ons to metformin, which warrants an update to clinical practice guidelines to include them as a treatment option. Since the FDA uses a 0.3–0.4% noninferiority margin to assess clinical comparative efficacy, colesevelam, nateglinide and miglitol were not clinically superior to placebo (although they did statistically significantly decrease HbA1c) making them less attractive as adjunctive therapies [7].
A therapy’s efficacy, or ability to lower HbA1c, must be weighed against a therapy’s propensity to cause hypoglycemia. In fact, guidelines emphasize the importance of preventing even mild hypoglycemia [2]. Insulin glargine and the SUs caused significantly higher rates of confirmed hypoglycemia when compared to SGLT2 inhibitors, DPP-4 inhibitors and placebo making them less attractive options for patients with Type 2 DM. Of note, we required studies to report the more rigorous definition of “confirmed” hypoglycemia to be included in our meta-analysis. While this endpoint likely carries greater validity than other hypoglycemia definitions, it is also associated with lower event rates making it more difficult to detect differences between therapies.
The majority of patients with Type 2 DM are overweight which may have notable impacts on insulin resistance, glycemic control and cardiovascular risk [2, 73]. SUs, insulin glargine, TZDs, repaglinide and alogliptin/pioglitazone were associated with weight gain compared to placebo. SGLT2 inhibitors, GLP-1 analogs, empagliflozin/linagliptin and miglitol resulted in weight loss, while all other medications were weight neutral compared to placebo. There is no universal threshold for clinically significant weight loss, however reductions greater than 2.3 kg (5 pounds) have been operationally defined [8,72] and associated with improved comorbid outcomes (e.g. improved serum lipids and SBP) [73]. In our analysis, no agent was found to be clinically superior to placebo. However, SGLT2 inhibitors were superior, both statistically and clinically, to SUs, TZDs and insulin glargine for weight loss.
SGLT2 inhibitors, empagliflozin/linagliptin, liraglutide and sitagliptin were associated with a significant decrease in SBP compared with placebo. No antidiabetic therapy met our a priori definition of clinical superiority (lower bound of the 95%CI depicting a decrease in SBP of at least 5 mmHg [9]) over other active agents or placebo. The SBP lowering effect of SGLT2 inhibitors observed in our meta-analysis are consistent with a recently published meta-analysis by Baker and colleagues [74] which reported significant reductions in SBP with SGLT2 inhibitors when compared to placebo (WMD -3.8 mmHg, 95%CI -4.4 to -3.2) and active comparators (WMD -4.2 mmHg, 95%CI -4.9 to -3.5).
No agents in any therapy class were found to increase the risk of UTI when compared to placebo. SGLT2 inhibitors were associated with an increased risk of GTI but there was limited reporting of this outcome in RCTs, and future studies are warranted to confirm these findings.
Our NMA has several strengths worth noting. We used stringent inclusion criteria for defining metformin use to assure we were truly assessing therapy in addition to stable metformin therapy. Our NMA is the most comprehensive to date. We evaluated individual agents as compared to classes to assess whether there were within class differences in effect and included almost double the number of trials and therapy comparisons than in previously published NMAs [5, 75]. For comparisons previously assessed in other meta-analyses, our results were generally consistent [5, 75–77]. However, even here we included a substantial amount of additional data on DPP-4 inhibitors and GLP-1 analogs, which had limited data to assess their efficacy/safety in previous meta-analyses. We included SGLT2 inhibitors in our NMA. NMAs focusing on SGLT2 inhibitors have been published, but did not include all potential antidiabetic therapies in their network, thus suppressing potential indirect data [78]. Methods papers suggest not including all possible and relevant linked therapies can negatively impact conclusions of a NMA. We assessed outcomes like UTI and GTI not included in previous meta-analyses which augments the safety data available for interclass comparisons.
Our NMA has several limitations. We saw little evidence of incoherence in our network (as evidenced by finding only 8 out of 151 possible direct versus indirect evidence comparisons statistically significant); however, incoherence cannot be completely ruled out. The eight statistically significant findings resulting from 151 statistical tests are consistent with 7–8 findings that would be expected due to chance alone. We only included English-language RCTs, but due to the vast number of trials included in our meta-analysis, a limited number of trials missed for this reason would likely have minimal impact on our results. We found sparse reporting of certain endpoints (UTI and GTI) which makes it difficult to draw concrete conclusions (potential for type 2 error). At the same time, our NMA cross- compared 25 therapies plus placebo on 6 different endpoints; thus multiple hypothesis testing can lead to erred conclusions of statistical differences between therapies when they do not truly exist (type 1 error). However, we conducted a sensitivity analysis using 99% CIs as a more stringent cutoff for statistical significance and found the majority of comparisons to still be statistically significant. Other agent-specific endpoints (e.g. cardiovascular events, pancreatitis, renal dysfunction, heart failure) could not be assessed due to the limited data and short follow-up periods of the included trials. Lastly, publication bias is always a concern in a meta-analysis, but we minimized this concern with our systematic search strategy, backwards citation tracking and multi-pronged grey literature searches.
Conclusions
In persons with Type 2 DM inadequately-controlled on stable metformin monotherapy, all agents analyzed in our NMA proved effective (lowered HbA1c vs. placebo) but with differing reductions in HbA1c. Evaluated agents differed in their effects on BW, SBP, hypoglycemia and GTI; however no medication increased the risk of UTI when compared to placebo. The newest class of antidiabetic agents, the SGLT2 inhibitors, was found to provide similar HbA1c efficacy to other non-insulin monotherapies (albeit not oral combination therapies) with the added benefits of weight loss, reduced SBP and a low risk of hypoglycemia; but at a cost of an increased risk of GTI. Combination therapies resulted in some of the largest reductions in HbA1c and may be appropriate for patients requiring profound (>1%) HbA1c reductions after failing optimized metformin.
Supporting Information
(DOC)
(PDF)
GTI = genital tract infection; HbA1c = glycated hemoglobin; IVRS = interactive voice response system; SGLT2 = sodium glucose co-transporter-2; SU = sulfonylurea; TZD = thiazolidinedione; UTI = urinary tract infection.
(PDF)
In this figure (x) is defined as the minimally important clinical difference between two treatments (or a treatment and placebo). Using the example of HbA1c reduction, line A displays an HbA1c difference between therapies that is not statistically significant because the 95% confidence interval (CI) cross the line of no effect; lines B and C both show drug A to be statistically significantly better at lowering HbA1c than drug B, but that clinical superiority cannot be claimed in either case because the lower bound of the 95% CI crosses the (dashed) line marking the minimally important clinical difference, (MICD) x (or in the case of HbA1c reduction, 0.3%); line D depicts a situation where drug A is both statistically significant and clinically superior in reducing HbA1c compared to drug B as evidenced by the lower bound of the 95%CI not crossing the line marking the MICD. In our network meta-analysis, the comparison of empagliflozin/linagliptin vs. dapagliflozin (change in HbA1c = -0.65, 95%CI -0.91, -0.39) would met these criteria for statistical significance and clinical superiority. MICD = minimally important clinical difference; RR = relative risk.
(PDF)
+ = low risk of bias;? = unclear risk of bias;– = high risk of bias.
(PDF)
Therapies are reported in alphabetical order. HbA1c results are reported in WMD, % (95% CI). Results for changes in HbA1c on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in HbA1c, negative values favor the first agent in alphabetical order. Statistically significant results are bolded. Clinically superior results are underlined. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; HbA1c = hemoglobin A1c; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results are reported in WMD, kg (95% CI). Results for changes in weight on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in weight, negative values favor the first agent in alphabetical order. Statistically significant results are bolded. Clinically superior results are underlined. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results are reported in WMD, mmHg (95% CI). Results for changes in systolic blood pressure (SBP) on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in SBP, negative values favor the first agent in alphabetical order. Statistically significant results are bolded. Clinically superior results are underlined. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; PIO = pioglitazone; PLC = placebo; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of confirmed hypoglycemia on the top portion of the matrix represent relative risks (RRs) of hypoglycemia in the row-defining treatment vs. those the column-defining treatment (referent). For confirmed hypoglycemia, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results are bolded. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. ACA = acarbose; ALO = alogliptin; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of urinary tract infection (UTI) on the top portion of the matrix represent relative risks (RRs) of UTI in the row-defining treatment vs. those the column-defining treatment (referent). For UTI, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results are bolded. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. ALO/PIO = alogliptin/pioglitazone; ALO = alogliptin; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PIO = pioglitazone; PLC = placebo; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of genital tract infection (GTI) on the top portion of the matrix represent relative risks (RRs) of GTI in the row-defining treatment vs. those the column-defining treatment (referent). For GTI, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results are bolded. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PLC = placebo; SITA = sitagliptin.
(PDF)
Therapies are reported in alphabetical order. HbA1c results are reported in WMD, % (99% CI). Results for changes in HbA1c on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in HbA1c, negative values favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; HbA1c = hemoglobin A1c; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results are reported in WMD, kg (95% CI). Results for changes in weight on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in weight, negative values favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results are reported in WMD, mmHg (95% CI). Results for changes in systolic blood pressure (SBP) on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in SBP, negative values favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; PIO = pioglitazone; PLC = placebo; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of confirmed hypoglycemia on the top portion of the matrix represent relative risks (RRs) of hypoglycemia in the row-defining treatment vs. those the column-defining treatment (referent). For confirmed hypoglycemia, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. ACA = acarbose; ALO = alogliptin; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of urinary tract infection (UTI) on the top portion of the matrix represent relative risks (RRs) of UTI in the row-defining treatment vs. those the column-defining treatment (referent). For UTI, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. ALO/PIO = alogliptin/pioglitazone; ALO = alogliptin; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PIO = pioglitazone; PLC = placebo; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of genital tract infection (GTI) on the top portion of the matrix represent relative risks (RRs) of GTI in the row-defining treatment vs. those the column-defining treatment (referent). For GTI, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PLC = placebo; SITA = sitagliptin.
(PDF)
BID = twice daily; BMI = body mass index; DM = diabetes mellitus; FPG = fasting plasma glucose; HbA1c = hemoglobin A1c; n = number of patients; NR = not reported; OAD = oral antidiabetic drug; SBP = systolic blood pressure; SU = sulfonylurea; SE = standard error; TID = three times a day; qAM = daily AM dosing; qPM = daily PM dosing; QW = weekly. aNumber of study participants evaluated for change in A1c from baseline (sample size may vary for other endpoints). bDoses were given daily, unless otherwise specified. cData given in median (range).
(PDF)
ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = insulin glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitizone; PLC = placebo; REP = repaglinide; ROSI = rosiglitizone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin. aI2>50%.
(PDF)
ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = insulin glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitizone; PLC = placebo; REP = repaglinide; ROSI = rosiglitizone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin. aI2>50%.
(PDF)
CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = insulin glargine; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; PIO = pioglitizone; PLC = placebo; SAX = saxagliptin; SBP = systolic blood pressure; SITA = sitagliptin; VILDA = vildagliptin. aEgger’s p-value <0.05
(PDF)
ACA = acarbose; ALO = alogliptin; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = insulin glargine; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; NAT = nateglinide; PIO = pioglitizone; PLC = placebo; REP = repaglinide; ROSI = rosiglitizone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PIO = pioglitizone; PLC = placebo; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PLC = placebo; SITA = sitagliptin.
(PDF)
Data Availability
All relevant data are contained within the paper and Supporting Information files.
Funding Statement
This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICJME) and were fully responsible for all content and editorial decisions, and were involved in all stages of manuscript development. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–79. 10.2337/dc12-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org. (Last accessed on June 27, 2014).
- 3. Hoaglin DC, Hawkins N, Jansen JP, Scott DA, Itzler R, Cappelleri JC et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 2. Value Health. 2011;14(4):429–37. 10.1016/j.jval.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 4. Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303(14):1410–18. 10.1001/jama.2010.405 [DOI] [PubMed] [Google Scholar]
- 5. Higgins JP, Altman DG, Gøtzsche PC, Juni P, Moher D, Oxman AD, et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Package ‘netmeta’. June 24, 2014. Available at: http://cran.r-project.org/web/packages/netmeta/netmeta.pdf (Last accessed on June 27, 2014).
- 7.US Food and Drug Administration. Guidelines for industry: diabetes mellitus: developing drugs and therapeutic biologics for treatment and prevention. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071624.pdf (Last accessed on October 3, 2014).
- 8. St Jeor ST, Brunner RL, Harrington ME, Scott BJ, Daugherty SA, Cutter GR, et al. A classification system to evaluate weight maintainers, gainers, and losers. J Am Diet Assoc. 1997;97(5):481–8. [DOI] [PubMed] [Google Scholar]
- 9. Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29(7):1253–69. 10.1097/HJH.0b013e3283469976 [DOI] [PubMed] [Google Scholar]
- 10.Defronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, et al. Fixed dose combinations of empagliflozin/linagliptin for 24 weeks as add-on to metformin in subjects with type 2 diabetes (T2D). Poster presentation, American Diabetes Association (ADA) 74th Scientific Sessions. 13–17 June 2014, San Francisco, CA.
- 11. Bolli GB, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Wu Y, et al. Efficacy and safety of lixisenatide once daily vs. placebo in people with Type 2 diabetes insufficiently controlled on metformin (GetGoal-F1). Diabet Med. 2014;31(2):176–84. 10.1111/dme.12328 [DOI] [PubMed] [Google Scholar]
- 12. Derosa G, Bonaventura A, Bianchi L, Romano D, Fogari E, D'Angelo A, et al. Vildagliptin compared to glimepiride on post-prandial lipemia and on insulin resistance in type 2 diabetic patients. Metabolism. 2014;63(7):957–67. 10.1016/j.metabol.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 13. Häring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650–9. 10.2337/dc13-2105 [DOI] [PubMed] [Google Scholar]
- 14. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and Safety of Dulaglutide Versus Sitagliptin After 52 Weeks in Type 2 Diabetes in a Randomized Controlled Trial (AWARD-5). Diabetes Care. 2014;37:2149–58. 10.2337/dc13-2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691–700. 10.1016/S2213-8587(14)70120-2 [DOI] [PubMed] [Google Scholar]
- 16. White JL, Buchanan P, Li J, Frederich R. A randomized controlled trial of the efficacy and safety of twice-daily saxagliptin plus metformin combination therapy in patients with type 2 diabetes and inadequate glycemic control on metformin monotherapy. BMC Endocr Disord. 2014;14(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charbonnel B, Steinberg H, Eymard E, Xu L, Thakkar P, Prabhu V, et al. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia. 2013;56(7):1503–11. 10.1007/s00125-013-2905-1 [DOI] [PubMed] [Google Scholar]
- 18. Chawla S, Kaushik N, Singh NP, Ghosh RK, Saxena A. Effect of addition of either sitagliptin or pioglitazone in patients with uncontrolled type 2 diabetes mellitus on metformin: A randomized controlled trial. J Pharmacol Pharmacother. 2013;4(1):27–32. 10.4103/0976-500X.107656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–50. 10.1016/S0140-6736(13)60683-2 [DOI] [PubMed] [Google Scholar]
- 20. Derosa G, Ragonesi PD, Carbone A, Fogari E, D'Angelo A, Cicero AF, et al. Evaluation of the positive effects on insulin-resistance and β-cell measurements of vildagliptin in addition to metformin in type 2 diabetic patients. Pharmacol Res. 2013;73:20–6. 10.1016/j.phrs.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 21. Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56(12):2582–92. 10.1007/s00125-013-3039-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15(12):1154–60. 10.1111/dom.12185 [DOI] [PubMed] [Google Scholar]
- 23. Rosenstock J, Raccah D, Korányi L, Maffei L, Boka G, Miossec P, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care. 2013;36(10):2945–51. 10.2337/dc12-2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aschner P, Chan J, Owens DR, Picard S, Wang E, Dain MP, et al. Insulin glargine versus sitagliptin in insulin-naive patients with type 2 diabetes mellitus uncontrolled on metformin (EASIE): a multicentre, randomised open-label trial. Lancet. 2012;379(9833):2262–9. 10.1016/S0140-6736(12)60439-5 [DOI] [PubMed] [Google Scholar]
- 25. Bergenstal RM, Forti A, Chiasson JL, Woloschak M, Boldrin M, Balena R. Efficacy and safety of taspoglutide versus sitagliptin for type 2 diabetes mellitus (T-emerge 4 trial). Diabetes Ther. 2012;3(1):13 10.1007/s13300-012-0013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeFronzo RA, Burant CF, Fleck P, Wilson C, Mekki Q, Pratley RE. Efficacy and tolerability of the DPP-4 inhibitor alogliptin combined with pioglitazone, in metformin-treated patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97(5):1615–22. 10.1210/jc.2011-2243 [DOI] [PubMed] [Google Scholar]
- 27. Derosa G, Franzetti IG, Querci F, Carbone A, Ciccarelli L, Piccinni MN, et al. Exenatide plus metformin compared with metformin alone on β-cell function in patients with Type 2 diabetes. Diabet Med. 2012;29(12):1515–23. 10.1111/j.1464-5491.2012.03699.x [DOI] [PubMed] [Google Scholar]
- 28. Derosa G, Carbone A, Franzetti I, Querci F, Fogari E, Bianchi L, et al. Effects of a combination of sitagliptin plus metformin vs metformin monotherapy on glycemic control, β-cell function and insulin resistance in type 2 diabetic patients. Diabetes Res Clin Pract. 2012;98(1):51–60. 10.1016/j.diabres.2012.05.022 [DOI] [PubMed] [Google Scholar]
- 29. Gallwitz B, Guzman J, Dotta F, Guerci B, Simó R, Basson BR, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet. 2012;379(9833):2270–8. 10.1016/S0140-6736(12)60479-6 [DOI] [PubMed] [Google Scholar]
- 30. Gallwitz B, Rosenstock J, Rauch T, Bhattacharya S, Patel S, von Eynatten M, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380(9840):475–83. 10.1016/S0140-6736(12)60691-6 [DOI] [PubMed] [Google Scholar]
- 31. Ljunggren Ö, Bolinder J, Johansson L, Wilding J, Langkilde AM, Sjöström CD, et al. Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab. 2012;14(11):990–9. 10.1111/j.1463-1326.2012.01630.x [DOI] [PubMed] [Google Scholar]
- 32. Pan C, Xing X, Han P, Zheng S, Ma J, Liu J, et al. Efficacy and tolerability of vildagliptin as add-on therapy to metformin in Chinese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14(8):737–44. 10.1111/j.1463-1326.2012.01593.x [DOI] [PubMed] [Google Scholar]
- 33. Rizzo MR, Barbieri M, Marfella R, Paolisso G. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care. 2012;35(10):2076–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35(6):1232–8. 10.2337/dc11-1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ross SA, Rafeiro E, Meinicke T, Toorawa R, Weber-Born S, Woerle HJ. Efficacy and safety of linagliptin 2.5 mg twice daily versus 5 mg once daily in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, placebo-controlled trial. Curr Med Res Opin. 2012;28(9):1465–74. [DOI] [PubMed] [Google Scholar]
- 36. Arechavaleta R, Seck T, Chen Y, Krobot KJ, O'Neill EA, Duran L, et al. Efficacy and safety of treatment with sitagliptin or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2011;13(2):160–8. 10.1111/j.1463-1326.2010.01334.x [DOI] [PubMed] [Google Scholar]
- 37. Nauck MA, Del Prato S, Meier JJ, Durán-García S, Rohwedder K, Elze M, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34(9):2015–22. 10.2337/dc11-0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfützner A, Schöndorf T, Tschöpe D, Lobmann R, Merke J, Müller J, et al. PIOfix-study: effects of pioglitazone/metformin fixed combination in comparison with a combination of metformin with glimepiride on diabetic dyslipidemia. Diabetes Technol Ther. 2011;13(6):637–43. 10.1089/dia.2010.0233 [DOI] [PubMed] [Google Scholar]
- 39. Taskinen MR, Rosenstock J, Tamminen I, Kubiak R, Patel S, Dugi KA, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13(1):65–74. 10.1111/j.1463-1326.2010.01326.x [DOI] [PubMed] [Google Scholar]
- 40. Wang JS, Lin SD, Lee WJ, Su SL, Lee IT, Tu ST, et al. Effects of acarbose versus glibenclamide on glycemic excursion and oxidative stress in type 2 diabetic patients inadequately controlled by metformin: a 24-week, randomized, open-label, parallel-group comparison. Clin Ther. 2011;33(12):1932–42. 10.1016/j.clinthera.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 41. Yang W, Pan CY, Tou C, Zhao J, Gause-Nilsson I. Efficacy and safety of saxagliptin added to metformin in Asian people with type 2 diabetes mellitus: a randomized controlled trial. Diabetes Res Clin Pract. 2011;94(2):217–24. 10.1016/j.diabres.2011.07.035 [DOI] [PubMed] [Google Scholar]
- 42. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9733):2223–33. 10.1016/S0140-6736(10)60407-2 [DOI] [PubMed] [Google Scholar]
- 43. Filozof C, Gautier JF. A comparison of efficacy and safety of vildagliptin and gliclazide in combination with metformin in patients with Type 2 diabetes inadequately controlled with metformin alone: a 52-week, randomized study. Diabet Med. 2010;27(3):318–26. 10.1111/j.1464-5491.2010.02938.x [DOI] [PubMed] [Google Scholar]
- 44. Göke B, Gallwitz B, Eriksson J, Hellqvist A, Gause-Nilsson I, D1680C00001 Investigators. Saxagliptin is non-inferior to glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: a 52-week randomised controlled trial. Int J Clin Pract. 2010;64(12):1619–31. 10.1111/j.1742-1241.2010.02510.x [DOI] [PubMed] [Google Scholar]
- 45. Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375(9724):1447–56. 10.1016/S0140-6736(10)60307-8 [DOI] [PubMed] [Google Scholar]
- 46. Rigby SP, Handelsman Y, Lai YL, Abby SL, Tao B, Jones MR. Effects of colesevelam, rosiglitazone, or sitagliptin on glycemic control and lipid profile in patients with type 2 diabetes mellitus inadequately controlled by metformin monotherapy. Endocr Pract. 2010;16(1):53–63. 10.4158/EP09146.OR [DOI] [PubMed] [Google Scholar]
- 47. Scheen AJ, Charpentier G, Ostgren CJ, Hellqvist A, Gause-Nilsson I. Efficacy and safety of saxagliptin in combination with metformin compared with sitagliptin in combination with metformin in adult patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2010;26(7):540–9. 10.1002/dmrr.1114 [DOI] [PubMed] [Google Scholar]
- 48. DeFronzo RA, Hissa MN, Garber AJ, Luiz Gross J, Yuyan Duan R, Ravichandran S, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes Care. 2009;32(9):1649–55. 10.2337/dc08-1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferrannini E, Fonseca V, Zinman B, Matthews D, Ahrén B, Byiers S, et al. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2009;11(2):157–66. 10.1111/j.1463-1326.2008.00994.x [DOI] [PubMed] [Google Scholar]
- 50. Goodman M, Thurston H, Penman J. Efficacy and tolerability of vildagliptin in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Horm Metab Res. 2009;41(5):368–73. 10.1055/s-0028-1104604 [DOI] [PubMed] [Google Scholar]
- 51. Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q; Alogliptin Study 008 Group. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63(1):46–55. 10.1111/j.1742-1241.2008.01933.x [DOI] [PubMed] [Google Scholar]
- 52. Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. 10.2337/dc08-1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hamann A, Garcia-Puig J, Paul G, Donaldson J, Stewart M. Comparison of fixed-dose rosiglitazone/metformin combination therapy with sulphonylurea plus metformin in overweight individuals with Type 2 diabetes inadequately controlled on metformin alone. Exp Clin Endocrinol Diabetes. 2008;116(1):6–13. [DOI] [PubMed] [Google Scholar]
- 54. Khanolkar MP, Morris RH, Thomas AW, Bolusani H, Roberts AW, Geen J, et al. Rosiglitazone produces a greater reduction in circulating platelet activity compared with gliclazide in patients with type 2 diabetes mellitus—an effect probably mediated by direct platelet PPARgamma activation. Atherosclerosis. 2008;197(2):718–24. [DOI] [PubMed] [Google Scholar]
- 55. Raz I, Chen Y, Wu M, Hussain S, Kaufman KD, Amatruda JM, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin. 2008;24(2):537–50. 10.1185/030079908X260925 [DOI] [PubMed] [Google Scholar]
- 56. Scott R, Loeys T, Davies MJ, Engel SS; Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2008;10(10):959–69. 10.1111/j.1463-1326.2007.00839.x [DOI] [PubMed] [Google Scholar]
- 57. Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30(4):890–5. [DOI] [PubMed] [Google Scholar]
- 58. Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9(2):194–205. [DOI] [PubMed] [Google Scholar]
- 59. Ristic S, Collober-Maugeais C, Pecher E, Cressier F. Comparison of nateglinide and gliclazide in combination with metformin, for treatment of patients with Type 2 diabetes mellitus inadequately controlled on maximum doses of metformin alone. Diabet Med. 2006;23(7):757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–100. [DOI] [PubMed] [Google Scholar]
- 61. Feinglos M, Dailey G, Cefalu W, Osei K, Tayek J, Canovatchel W, et al. Effect on glycemic control of the addition of 2.5 mg glipizide GITS to metformin in patients with T2DM. Diabetes Res Clin Pract. 2005;68(2):167–75. [DOI] [PubMed] [Google Scholar]
- 62. Matthews DR, Charbonnel BH, Hanefeld M, Brunetti P, Schernthaner G. Long-term therapy with addition of pioglitazone to metformin compared with the addition of gliclazide to metformin in patients with type 2 diabetes: a randomized, comparative study. Diabetes Metab Res Rev. 2005;21(2):167–74. [DOI] [PubMed] [Google Scholar]
- 63. Ahrén B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27(12):2874–80. [DOI] [PubMed] [Google Scholar]
- 64. Gómez-Perez FJ, Fanghänel-Salmón G, Antonio Barbosa J, Montes-Villarreal J, Berry RA, Warsi G, et al. Efficacy and safety of rosiglitazone plus metformin in Mexicans with type 2 diabetes. Diabetes Metab Res Rev. 2002;18(2):127–34. [DOI] [PubMed] [Google Scholar]
- 65. Marre M, Van Gaal L, Usadel KH, Ball M, Whatmough I, Guitard C. Nateglinide improves glycaemic control when added to metformin monotherapy: results of a randomized trial with type 2 diabetes patients. Diabetes Obes Metab. 2002;4(3):177–86. [DOI] [PubMed] [Google Scholar]
- 66. Charpentier G, Fleury F, Kabir M, Vaur L, Halimi S. Improved glycaemic control by addition of glimepiride to metformin monotherapy in type 2 diabetic patients. Diabet Med. 2001;18(10):828–34. [DOI] [PubMed] [Google Scholar]
- 67. Van Gaal L, Maislos M, Schernthaner G, Rybka J, Segal P. Miglitol combined with metformin improves glycaemic control in type 2 diabetes. Diabetes Obes Metab. 2001;3(5):326–31. [DOI] [PubMed] [Google Scholar]
- 68. Halimi S, Le Berre MA, Grangé V. Efficacy and safety of acarbose add-on therapy in the treatment of overweight patients with Type 2 diabetes inadequately controlled with metformin: a double-blind, placebo-controlled study. Diabetes Res Clin Pract. 2000;50(1):49–56. [DOI] [PubMed] [Google Scholar]
- 69. Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA. 2000;283(13):1695–702. [DOI] [PubMed] [Google Scholar]
- 70. Moses R, Slobodniuk R, Boyages S, Colagiuri S, Kidson W, Carter J, et al. Effect of repaglinide addition to metformin monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 1999;22(1):119–24. [DOI] [PubMed] [Google Scholar]
- 71. Rosenstock J, Brown A, Fischer J, Jain A, Littlejohn T, Nadeau D, Sussman A, Taylor T, Krol A, Magner J. Efficacy and safety of acarbose in metformin-treated patients with type 2 diabetes. Diabetes Care. 1998;21(12):2050–5. [DOI] [PubMed] [Google Scholar]
- 72. Sherwood NE, Jeffery RW, French SA, Hannan PJ, Murray DM. Predictors of weight gain in the Pound of Prevention study. Int J Obes Relat Metab Disord. 2000;24(4):395–403. [DOI] [PubMed] [Google Scholar]
- 73. Unick JL, Beavers D, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TA, et al. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care. 2011;34(10):2152–7. 10.2337/dc11-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8(4):262–75. 10.1016/j.jash.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 75. McIntosh B, Cameron C, Singh SR, Yu C, Ahuja T, Welton NJ, et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5(1):e35–48. [PMC free article] [PubMed] [Google Scholar]
- 76. Bolen S, Wilson L, Vassy J, Feldman L, Yeh J, Marinopoulos S, et al. Comparative Effectiveness and Safety of Oral Diabetes Medications for Adults With Type 2 Diabetes [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US); 2007. Jul. Available from http://www.ncbi.nlm.nih.gov/books/NBK43056/PubMed . [PubMed] [Google Scholar]
- 77. Monami M, Lamanna C, Marchionni N, Mannucci E. Comparison of different drugs as add-on treatments to metformin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract. 2008; 79(2):196–203. [DOI] [PubMed] [Google Scholar]
- 78. Mills EJ, Kanters S, Thorlund K, Chaimani A, Veroniki AA, Ioannidis JP. The effects of excluding treatments from network meta-analyses: survey. BMJ. 2013;347:f5195 10.1136/bmj.f5195 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
GTI = genital tract infection; HbA1c = glycated hemoglobin; IVRS = interactive voice response system; SGLT2 = sodium glucose co-transporter-2; SU = sulfonylurea; TZD = thiazolidinedione; UTI = urinary tract infection.
(PDF)
In this figure (x) is defined as the minimally important clinical difference between two treatments (or a treatment and placebo). Using the example of HbA1c reduction, line A displays an HbA1c difference between therapies that is not statistically significant because the 95% confidence interval (CI) cross the line of no effect; lines B and C both show drug A to be statistically significantly better at lowering HbA1c than drug B, but that clinical superiority cannot be claimed in either case because the lower bound of the 95% CI crosses the (dashed) line marking the minimally important clinical difference, (MICD) x (or in the case of HbA1c reduction, 0.3%); line D depicts a situation where drug A is both statistically significant and clinically superior in reducing HbA1c compared to drug B as evidenced by the lower bound of the 95%CI not crossing the line marking the MICD. In our network meta-analysis, the comparison of empagliflozin/linagliptin vs. dapagliflozin (change in HbA1c = -0.65, 95%CI -0.91, -0.39) would met these criteria for statistical significance and clinical superiority. MICD = minimally important clinical difference; RR = relative risk.
(PDF)
+ = low risk of bias;? = unclear risk of bias;– = high risk of bias.
(PDF)
Therapies are reported in alphabetical order. HbA1c results are reported in WMD, % (95% CI). Results for changes in HbA1c on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in HbA1c, negative values favor the first agent in alphabetical order. Statistically significant results are bolded. Clinically superior results are underlined. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; HbA1c = hemoglobin A1c; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results are reported in WMD, kg (95% CI). Results for changes in weight on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in weight, negative values favor the first agent in alphabetical order. Statistically significant results are bolded. Clinically superior results are underlined. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results are reported in WMD, mmHg (95% CI). Results for changes in systolic blood pressure (SBP) on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in SBP, negative values favor the first agent in alphabetical order. Statistically significant results are bolded. Clinically superior results are underlined. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; PIO = pioglitazone; PLC = placebo; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of confirmed hypoglycemia on the top portion of the matrix represent relative risks (RRs) of hypoglycemia in the row-defining treatment vs. those the column-defining treatment (referent). For confirmed hypoglycemia, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results are bolded. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. ACA = acarbose; ALO = alogliptin; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of urinary tract infection (UTI) on the top portion of the matrix represent relative risks (RRs) of UTI in the row-defining treatment vs. those the column-defining treatment (referent). For UTI, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results are bolded. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. ALO/PIO = alogliptin/pioglitazone; ALO = alogliptin; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PIO = pioglitazone; PLC = placebo; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of genital tract infection (GTI) on the top portion of the matrix represent relative risks (RRs) of GTI in the row-defining treatment vs. those the column-defining treatment (referent). For GTI, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results are bolded. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PLC = placebo; SITA = sitagliptin.
(PDF)
Therapies are reported in alphabetical order. HbA1c results are reported in WMD, % (99% CI). Results for changes in HbA1c on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in HbA1c, negative values favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; HbA1c = hemoglobin A1c; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results are reported in WMD, kg (95% CI). Results for changes in weight on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in weight, negative values favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results are reported in WMD, mmHg (95% CI). Results for changes in systolic blood pressure (SBP) on the top portion of the matrix represent changes in the row-defining treatment vs. those in the column-defining treatment (referent). For changes in SBP, negative values favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. The results on the bottom portion of the matrix represent the reciprocal of the top portion. CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; PIO = pioglitazone; PLC = placebo; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of confirmed hypoglycemia on the top portion of the matrix represent relative risks (RRs) of hypoglycemia in the row-defining treatment vs. those the column-defining treatment (referent). For confirmed hypoglycemia, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. ACA = acarbose; ALO = alogliptin; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = glargine; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; NAT = nateglinide; PIO = pioglitazone; PLC = placebo; REP = repaglinide; ROSI = rosiglitazone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of urinary tract infection (UTI) on the top portion of the matrix represent relative risks (RRs) of UTI in the row-defining treatment vs. those the column-defining treatment (referent). For UTI, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. ALO/PIO = alogliptin/pioglitazone; ALO = alogliptin; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PIO = pioglitazone; PLC = placebo; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
Therapies are reported in alphabetical order. Results for risk of genital tract infection (GTI) on the top portion of the matrix represent relative risks (RRs) of GTI in the row-defining treatment vs. those the column-defining treatment (referent). For GTI, RRs lower than 1 favor the first agent in alphabetical order. Statistically significant results of the sensitivity analysis are colored grey. Sodium glucose co-transporter-2 (SGLT-2) inhibitors are highlighted. To obtain RRs for comparisons in the opposite direction, reciprocals should be taken or the lower portion of the matrix can be used. CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PLC = placebo; SITA = sitagliptin.
(PDF)
BID = twice daily; BMI = body mass index; DM = diabetes mellitus; FPG = fasting plasma glucose; HbA1c = hemoglobin A1c; n = number of patients; NR = not reported; OAD = oral antidiabetic drug; SBP = systolic blood pressure; SU = sulfonylurea; SE = standard error; TID = three times a day; qAM = daily AM dosing; qPM = daily PM dosing; QW = weekly. aNumber of study participants evaluated for change in A1c from baseline (sample size may vary for other endpoints). bDoses were given daily, unless otherwise specified. cData given in median (range).
(PDF)
ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = insulin glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitizone; PLC = placebo; REP = repaglinide; ROSI = rosiglitizone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin. aI2>50%.
(PDF)
ACA = acarbose; ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; COL = colesevelam; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = insulin glargine; GLIB = glibenclamide; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; MIG = miglitol; NAT = nateglinide; PIO = pioglitizone; PLC = placebo; REP = repaglinide; ROSI = rosiglitizone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin. aI2>50%.
(PDF)
CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = insulin glargine; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; PIO = pioglitizone; PLC = placebo; SAX = saxagliptin; SBP = systolic blood pressure; SITA = sitagliptin; VILDA = vildagliptin. aEgger’s p-value <0.05
(PDF)
ACA = acarbose; ALO = alogliptin; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; EXEN = exenatide; GLAR = insulin glargine; GLIC = gliclazide; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; LIRA = liraglutide; LIX = lixisenatide; NAT = nateglinide; PIO = pioglitizone; PLC = placebo; REP = repaglinide; ROSI = rosiglitizone; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
ALO = alogliptin; ALO/PIO = alogliptin/pioglitazone; CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PIO = pioglitizone; PLC = placebo; SAX = saxagliptin; SITA = sitagliptin; VILDA = vildagliptin.
(PDF)
CANA = canagliflozin; DAPA = dapagliflozin; EMPA = empagliflozin; EMPA/LINA = empagliflozin/linagliptin; GLIM = glimepiride; GLIP = glipizide; LINA = linagliptin; PLC = placebo; SITA = sitagliptin.
(PDF)
Data Availability Statement
All relevant data are contained within the paper and Supporting Information files.