INTRODUCTION
According to the National Institutes of Health as of April, 2010, 28% of adults aged 18 and older consume alcohol at levels that put them at risk for developing alcoholism, liver disease, and other medical and psychosocial problems (NIAAA, 2010). Alcohol use disorders (AUD) include a spectrum extending from episodic binge drinking to full-blown alcoholism. There is no perfect definition of alcoholism, but most diagnoses require individuals to have been drinking heavily over an extended period of time and to have subsequently suffered multiple major life problems due to their alcohol consumption (Schuckit, 1987; First and Tasman, 2004). At-risk drinking increases one’s chances of injuries; health problems, including liver disease, heart disease, sleep disorders, depression, stroke, bleeding from the stomach, sexually transmitted diseases and several types of cancer; and drinking during pregnancy increases the risk of birth defects for the unborn child (NIAAA, 2010). However, chronic alcohol exposure or severe abuse can cause more direct effects on the brain and is the subject of this chapter.
Alcohol is a central nervous system (CNS) depressant that produces euphoria, decreased anxiety, and behavioral excitation at low blood concentrations, and a spectrum of behavioral responses comprising acute intoxication (drowsiness, ataxia, slurred speech, stupor, and coma) at greater concentrations. Much progress has been made in understanding cellular and molecular mechanisms underlying alcohol actions in the nervous system (Vengeliene et al., 2008; Spanagel, 2009). Ligand-gated ion channels have been identified as specific targets for alcohol, and specific brain regions and pathways involved in addictive behavior have been described. Molecular and genetic studies in both humans and animal models promise new advances in understanding the molecular pathophysiology and genetics of alcoholism. Alcohol is used by a large segment of the population in social situations with minimal risk (e.g. complications of intoxication, fetal alcohol spectrum disorder), but a subset of alcohol consumers develop problems due to AUD. The development of AUD is caused by brain plasticity events that produce craving and habitual alcohol-seeking behavior. In addition, such chronic or high-dose alcohol use causes other toxic or adaptive responses within the CNS, as well as in virtually every organ system. This widespread effect on multiple organ systems makes alcohol a unique drug of abuse. Other chapters of this volume describe the more acute molecular and clinical brain responses to alcohol, alcohol withdrawal, and the neurobiology of addiction in alcoholism. Therefore, in this chapter, we will review current concepts on the neurologic complications of chronic, excessive exposure to alcohol and molecular mechanisms underlying these disorders.
MOLECULAR MECHANISMS OF CHRONIC ALCOHOL ACTIONON THE BRAIN
Alcohol (ethanol) is a simple two-carbon molecule that rapidly diffuses into virtually every biologic compartment in the body upon ingestion. Brain levels of alcohol rise within minutes of consuming an alcoholic beverage and signs of intoxication can be observed within minutes of high-dose alcohol administration. Thus it is largely assumed that acute alcohol actions occur at the level of direct or indirect effects on ion channels or neurotransmission. Such actions of alcohol are discussed in other chapters of this volume. However, the development of alcohol craving and addiction and other neurologic sequelae of chronic alcohol exposure (discussed below) clearly entail additional mechanisms.
Genetic contributions to alcoholism
Elegant studies in both humans and animal models over the last several decades have clearly shown that “genes” influence the development of alcoholism and other behavioral or molecular responses to alcohol. Genetic predisposition contributes to approximately 50% of the vulnerability for developing alcohol dependence (Goodwin et al., 1974; Prescott and Kendler, 1999). However, there has been only limited success in actually identifying candidate genes that contribute to the variable occurrence of alcohol dependence, much less other sequelae such as alcoholic dementia. Success stories include the identification of polymorphisms in isoforms of alcohol dehydrogenase and aldehyde dehydrogenase genes, which influence alcohol metabolism and the risk for alcoholism (Park et al., 2013). Additionally, recent studies on polymorphisms of the mu-opioid receptor gene show promise as a pharmacogenetic risk factor altering responses to naltrexone, a mu-opioid receptor antagonist that is approved by the Food and Drug Administatration for treatment of alcoholism (Ray and Hutchison, 2007; Ramchandani et al., 2011). Recent genomewide association studies suggest additional candidate loci of genes, but the majority of the genetic contribution to alcohol dependence still has not been identified (Kapoor et al., 2013; Kos et al., 2013).
The difficulty with existing human genetic studies is likely due to the occurrence of rare polymorphisms of small effect size in a large number of genes being causal elements in complex traits such as alcoholism. Moreover, even in cases where candidate genes for alcoholism have been implicated, the detection of regulatory network-wide systems is usually beyond the power of these approaches. Additionally, complex interactions between environmental factors (e.g., social stress) and genetic background may further complicate the identification of genetic risk factors. This leaves the question unanswered as to how to identify mechanisms underlying the development of alcohol dependence and alcohol-related neurologic disease.
Modulation of gene expression with chronic alcohol
Although identifying genes associated with genetic risk for alcoholism in humans has been difficult, there has been robust demonstration that acute or chronic alcohol exposure can alter expression of the genome both in cultured neural cells and in vivo with brain tissue from animals or humans (Miles et al., 1991; Miles, 1995; Kerns et al., 2005; Contet, 2012). Alterations in mRNA transcript abundance evoked by alcohol or substance abuse have been proposed as mechanisms underlying enduring neuroadaptations leading to abuse and addiction (Miles, 1995; Nestler and Aghajanian, 1997). Disrupted homeostatic control of gene networks is also a possible mechanism underlying CNS toxicity from compulsive alcohol or drug use. Furthermore, genetic differences in gene expression responses to alcohol are also thought to be an important mechanism underlying a predisposition to alcoholism or other complex traits (Schadt et al., 2003; Chesler et al., 2005). Thus, studies in animal models or humans on gene expression networks associated with alcoholism or alcohol-related behaviors have been an area of intense investigation. Such work has, over the last decade, generated large collections of alcohol-responsive or alcohol consumption-related genes through use of whole-genome expression profiling techniques such as microarrays or, more recently, RNA-seq (Kerns et al., 2005; Mulligan et al., 2006; Farris and Miles, 2012).
Identifying high-throughput lists of genes relevant to alcohol brain responses has not automatically resulted in improved understanding of mechanisms underlying alcoholism or new therapeutics for the disease. Recent work has used sophisticated statistical approaches at deriving “gene networks” from the genomewide expression data (Ponomarev et al., 2012; Wolen and Miles, 2012; Iancu et al., 2013). This results in the identification of so-called “hub genes” that may serve as master regulators for entire networks of alcohol-responsive genes, and thus be optimal targets for future drug development efforts (Mehrabian et al., 2005). Some investigators have also employed either integration of such networks across species and/or combining them with expression and behavioral genetic studies so as to more accurately identify networks relevant to alcohol behaviors or other brain responses (Tabakoff et al., 2009; Zhao et al., 2012; Vanderlinden et al., 2013). Using such approaches, some investigators have identified novel genes that show cross-species modulation of alcohol behaviors and hold promise for the development of future innovative therapeutic approaches (Bhandari et al., 2012). Taken together, genomic studies on alcohol suggest that alteration in the regional and cell-specific expression of specific gene networks in brain is a major mechanism underlying the neuroplasticity constituting alcohol dependence and alcohol-related toxicity (Fig. 10.1).
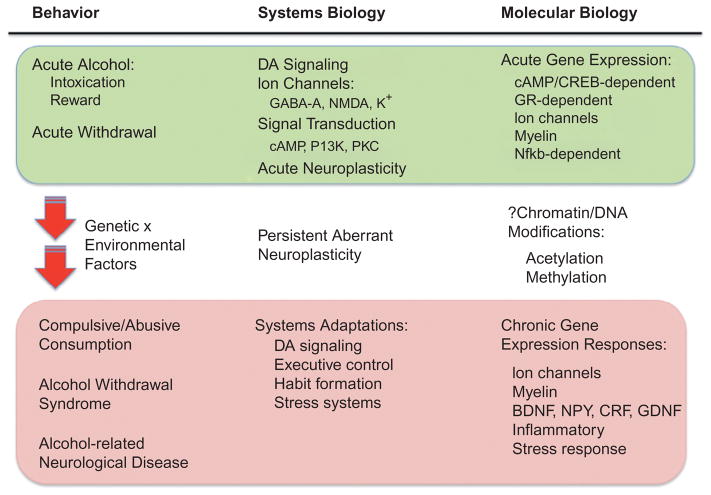
Fig. 10.1.
Overview of molecular adaptive events leading to alcoholism and neurologic complications of alcohol use disorders (AUD). The figure documents major aspects of current knowledge regarding behavioral, systems biology, and molecular mechanisms leading from acute alcohol exposure to chronic consequences such as AUD and neurologic complications. Schema are illustrative only and not meant to be all-inclusive. Acute alcohol has major direct effects on ion channels (γ-aminobutyric acid A (GABA-A), N-methyl-D-aspartate (NMDA), and K+). Alcohol-induced release of dopamine (DA) is a major factor in the rewarding properties of the drug. Alteration in numerous signaling cascades (e.g., cAMP, PI-3-kinase (PI3K), and protein kinase C (PKC)) and transcription factors (GR, glucocorticoid receptor; CREB; and Nfkb, Nf-kappa-B) may function in acute and chronic neuroplasticity by altering gene expression at the transcriptional, translational, or posttranslational level. Gene expression alterations are thought to play a central role in both acute and chronic neuroadaptation to alcohol. Epigenetic changes (DNA or histone modifications) are proposed as a mechanism underlying persistence of gene expression/neuroadaptive events, leading to clinical sequelae such as compulsive drinking, recidivism, and alcohol-related neurologic disease. BDNF, brain-derived neurotropic factor; NPY, neuropeptide Y; CRF, corticotropin-releasing factor; GDNF, glial cell-derived neurotropic factor.
The explosion of genomic information about alcohol and the brain that has been produced since the turn of the century is almost bewildering in magnitude and scope (Contet, 2012; Farris and Miles, 2012). How can such a simple molecule produce such a scale of changes in brain gene expression and what are their functional implications? Despite the complexity, several major conclusions are beginning to become evident about alcohol and the brain transcriptome:
Many ligand-gated ion channels (e.g., γ-aminobutyric acid-A (GABA) receptor subunits) or other neuromodulatory molecules (such as brain-derived neurotrophic factor or neuropeptide Y) that have been implicated in alcohol actions by behavioral pharmacology or physiology studies are also regulated at the level of gene expression by acute or chronic alcohol exposure (Spanagel, 2009; Contet, 2012; Farris and Miles, 2012). This reinforces the crucial role of these molecules in alcohol behaviors and also validates genomic studies as a tool for identifying novel alcohol targets.
Neuroinflammatory signaling genes are prominently regulated by acute alcohol and chronic exposure in both humans and animal models (Blednov et al., 2011, 2012). These suggest that neuroinflammation could function in both chronic behavioral responses to alcohol and alcohol-induced neurologic disorders.
Alcohol regulates multiple myelin-related genes both acutely and with chronic exposure and basal myelin expression levels can affect both sensitivity to acute alcohol and alcohol consumption (Lewohl et al., 2000; Kerns et al., 2005; Farris and Miles, 2012; Wolen and Miles, 2012). These data suggest that “myelin tone” may be both a risk factor and a target in alcoholism (Fig. 10.2).
Although multiple signaling cascades have been implicated by genomic studies, cascades linked to insulin-like growth factor and signaling by AKT kinase appear throughout multiple genomic and other molecular studies (Kerns et al., 2005; Neasta et al., 2011).
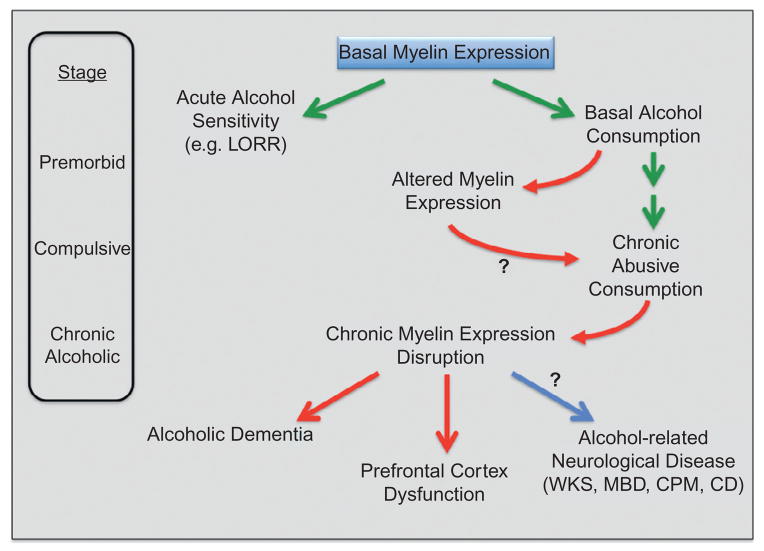
Fig. 10.2.
Hypothetic scheme for the role of myelin in alcoholism and alcohol-related neurologic disorders. Basal myelin expression is pictured as affecting initial sensitivity to alcohol, such as with loss-of-righting reflex (LORR), and perhaps relatedly, alcohol consumption. Acute alcohol, however, also alters myelin gene expression, which may contribute to frontal-lobe dysfunction and the consequent development of abusive alcohol consumption. Chronic alcohol exposure produces prominent decreases in myelin gene expression and myelin structural abnormalities. These are envisioned as contributing to frontal-lobe dysfunction in alcoholism and alcoholic dementia. Additionally, alcohol-induced myelin pathology may contribute as a risk factor to other alcohol-related neurologic diseases, such as Wernicke–Korsakoff’s syndrome (WKS), Machiafava–Bignami disease (MBD), central pontine myelinolysis (CPM), and cerebellar degeneration (CD).
These data have suggested these and related pathways as novel targets for therapeutic development (Barak et al., 2013; Ron and Messing, 2013).
Persistence of gene expression changes – role for epigenetic regulation?
Suggesting that gene networks regulated by acute or chronic alcohol have a functional role in the development of alcohol dependence or other alcohol-related neurologic disorders presents one major mechanistic hurtle – persistence. Explaining how such changes in gene expression could persist during periods of even prolonged abstinence, and thus influence the high rate of recidivism seen in alcoholics, is a challenging question. Recently, there has been a large expansion of studies in the area of “epigenetics” as an effort to explain non-genetic, long-lasting alterations in the transcriptome that could be causal in alcohol dependence or neurologic disorders. Epigenetics refers to a modification of the chromatin structure/function (e.g., by methylation or acetylation of histones) or DNA bases (e.g., by methylation), rather than changes in the actual DNA sequence, as a mechanism for altering gene expression in a potentially lifelong or transgenerational fashion (Borrelli et al., 2008; Kaminen-Ahola et al., 2010; Starkman et al., 2012). Expression of genes regulating epigenetic modifications have been found to correlate with individual variations in alcohol consumption in mice and drugs modifying histone acetylation produced alterations in alcohol consumption in such models (Wolstenholme et al., 2011).
NEUROLOGYOF CHRONIC ALCOHOL ACTION IN THE CENTRAL NERVOUS SYSTEM
Overview of clinical syndromes
Neurologic responses to abusive or chronic alcohol intake are often classified as direct versus secondary, but this assumes one knows the underlying mechanisms of the disorder. Alcoholism is a chronic disease characterized by uncontrolled craving for alcohol and physical dependence on the drug. The latter produces a characteristic severe withdrawal syndrome when alcohol drinking is suddenly decreased. In addition to craving and physical dependence, a set of characteristic neurologic disorders may also accompany long-term abusive drinking (Table 10.1). Additional prominent neurologic sequelae of alcohol abuse include fetal alcohol spectrum disorder and peripheral end-organ damage such as neuropathy and myopathy, but these are discussed elsewhere in this volume. Indirect neurologic complications of alcoholism, such as hepatic encephalopathy and traumatic brain injury, are also beyond the scope of this chapter.
Table 10.1.
Neurologic consequences of chronic exposure to alcohol
| Withdrawal-induced | Chronic/abusive exposure |
|---|---|
| Alcohol-withdrawal seizures | Alcohol blackouts (fragmentary/en bloc) |
| Alcohol-withdrawal syndrome and delirium tremens | Craving |
| Tolerance | |
| Wernicke–Korsakoff syndrome | |
| Cerebellar degeneration | |
| Primary alcoholic dementia | |
| Central pontine myelinolysis | |
| Machiafava–Bignami disease |
Alcohol blackouts
Consumption of large quantities of alcohol, particularly rapidly, can cause episodes of memory impairment, termed “alcohol blackouts.” Although alcohol blackouts can be considered to occur as an acute response to alcohol, they were initially described as a risk factor for alcoholism and thus will be discussed here as relevant to neurologic responses to chronic alcohol. Alcohol blackouts occur in the absence of any loss of consciousness or seizure activity and represent a type of anterograde amnesia. Such memory loss can be partial or fragmentary, such as not recalling names or isolated aspects of an experience, or they can be “en bloc,” where there is complete lack of memory formation for a defined period of time (Goodwin et al., 1969; White, 2003). En bloc episodes appear to represent deficits of memory transfer from short-term to longer-term storage and the individuals can function with apparent intact short-term memory and conduct complicated tasks such as conversations. Fragmentary alcohol blackouts are more common and there is a suggestion that they occur at lower blood alcohol levels. For both types of blackout, however, there is at least substantial anecdotal evidence to suggest that the events are associated with rapidly rising blood alcohol levels, but a linear relationship to absolute blood alcohol levels has also been described (Perry et al., 2006).
Early work suggested that alcoholics exclusively suffered such memory blackouts. Indeed, “alcoholic blackouts” were considered a premonitory symptom of incipient alcoholism (Jellinek, 1946). However such work suffered from the fact that most studies were indeed conducted only on alcoholics. More recent work has clearly shown that apparently normal individuals undergoing binge social drinking episodes can experience fragmentary or en bloc alcohol blackouts, with the former occurring about three times more frequently in studies on college students (Hartzler and Fromme, 2003; Rose and Grant, 2010). Because of the high prevalence of binge drinking among college students, many recent studies on alcohol-related blackouts have been conducted on that population. The occurrence of such blackouts is apparently not a benign event as there are higher rates of violence, accidents, vandalism, and unprotected sex amongst college students experiencing blackouts (White et al., 2002). Furthermore, although Jellinek’s original premise of alcohol blackouts as a predictor of future alcoholism might not have been correct, there have been a variety of studies suggesting that, particularly with individuals having a history of repeated alcohol blackouts, there is a greater incidence of early-onset problem drinking (Rose and Grant, 2010). Twin studies have shown a substantial genetic component to the occurrence of alcohol-related blackouts and shared genetic risk with the frequency of intoxication (Hurley et al., 2011).
It is also apparent that not all individuals, despite high blood alcohol levels, will experience alcohol blackouts. Twin studies, mentioned above, showing a greater than 50% heritability rate for alcohol blackouts, support the concept that some individuals are predisposed to these events. Recent longitudinal neuroimaging studies have suggested that individuals susceptible to future alcohol blackouts have underlying neurobiologic differences in memory-related tasks (Wetherill et al., 2013). Other neu-roimaging studies have shown differences in neural activity during contextual learning tasks only in the presence of alcohol for individuals with a history of fragmentary alcohol blackouts (Wetherill et al., 2012).
Neuroimaging and clinical laboratory studies in humans and behavioral pharmacology studies in animals models have contributed greatly of late to our understanding of possible mechanisms underlying different neurobiologic aspects of memory and alcohol-related blackouts (Rose and Grant, 2010). Some investigators initially hypothesized that alcohol-related blackouts represented an example of “state-dependent learning,” such that the memories laid down during an alcohol-drinking binge could only be recalled in the presence of alcohol intoxication (Goodwin, 1974). More recent findings, however, have not fully supported such theories (Weissenborn and Duka, 2000). Studies over the last two decades have shown that alcohol impairs explicit memory and spatial memory tasks, which are highly dependent on intact hippocampal function, perhaps by interfering with septohippocampal regulation of hippocampal neuronal circuitry (Givens et al., 2000).
The exact mechanisms for alcohol impairment of hippocampal circuits are not clear, but alcohol does produce dose-dependent inhibition of long-term potentiation, likely by actions at N-methyl-D-aspartate (NMDA)-type glutamatergic synaptic currents (Lovinger et al., 1989; Morrisett and Swartzwelder, 1993). Other work has suggested that alcohol effects on hippocampal NMDA receptor function occur at higher alcohol concentrations and that, at lower doses, increases in septohippocampal GABA receptor activity might also function in alcohol-related blackouts. As suggested by the time course, epidemiology, and neuroanatomic localization of alcohol-related blackouts, this disorder appears distinct from the mechanisms underlying Korsakoff’s psychosis and primary alcoholic dementia (see below).
Craving
The current definition of craving is “the desire to experience the effect(s) of a previously experienced psycho-active substance” (Vengeliene et al., 2008). This topic is discussed elsewhere in this volume but is also briefly discussed here since craving is a major neuroadaptive event leading to AUD. Despite experiencing adverse neurologic and environmental consequences of alcohol, alcoholics crave alcohol and exhibit habitual, uncontrolled drinking. Thus, these three components typify alcoholism – uncontrolled craving, habitual consumption, and loss of normal judgment regarding adverse consequences. Craving can occur following long-term abstinence from alcohol and can be elicited by exposure to alcohol and alcohol-associated stimuli in abstinent alcoholics (Heinz et al., 2003; Vengeliene et al., 2008). Functional magnetic resonance imaging (MRI) and positron emission tomography studies have identified critical brain regions and neurocircuitry underlying alcohol craving (George et al., 2001; de Greck et al., 2009; Fryer et al., 2013; Jasinska et al., 2013).
Such neuroimaging studies, combined with decades of neurophysiology and behavioral pharmacology work on alcohol in animal models, have led to the development of an elegant model describing the transition from social alcohol use to AUD (Koob and Volkow. 2010), although the model also applies to drugs of abuse. This model describes three stages of drug use: intoxicating/binge, withdrawal/negative affect, and a final stage of preoccupation/anticipation or “craving.” Data supporting such a model evoke interplay of different neural pathways and neurotransmitter systems at each stage. Craving is thought to have major influences from hippocampus, basal lateral amygdala, orbital frontal cortex, and medial prefrontal cortex (cingulate). Glutamate projections from medial prefrontal cortex and basal lateral amygdala to the ventral striatum are thought to have a major role in the craving stage, producing changes in dopaminergic reward pathways.
Clinical observations indicate that craving for alcohol persists for very extended periods (if not permanently) in abstinent alcoholics and that recidivism is a major obstacle to treatment of alcoholism. The mechanism underlying such prolonged risk for recidivism is unknown and obviously is an area of intense study. As discussed above, one major hypothesis for the mechanism of AUD and abuse of other drugs concerns alterations in brain gene expression as a direct or indirect consequence of drug exposure (Miles, 1995; Nestler and Aghajanian, 1997). Changes in gene expression evoked by chronic alcohol exposure, perhaps codified by epigenetic alterations of DNA and chromatin, could thus lead to persistent alterations in synaptic plasticity, and ultimately long-lived behavioral consequences (Fig. 10.1).
Tolerance and dependence
Once chronic abusive alcohol intake is established, one prevalent brain adaptive response concerns the development of tolerance to the intoxicating effects of alcohol. Such individuals can appear sober at blood alcohol concentrations of 89–108 mmol/L (Urso et al., 1981), well above the legal limits of intoxication (<22 mmol/L). The highest recorded blood alcohol level to be survived is 328 mmol/L, which was measured in an ambulatory alcoholic who stopped drinking 3 days previously (Johnson et al., 1982). These data highlight early observations by Goldberg (1941), showing that naïve individuals became ataxic at lower blood alcohol levels than heavy drinkers. Tolerance thus represents a CNS pharmacodynamic adaptive response which improves neurologic function at a given blood alcohol level.
Prolonged alcohol consumption leads to the development of tolerance and physical dependence. Physical dependence refers to the existence of spontaneous behavioral disturbances, which are produced by alcohol removal and suppressed by alcohol replacement. Physical dependence underlies the alcohol withdrawal syndrome. Animal models have suggested that there is an inverse genetic relationship between the severity of alcohol withdrawal symptoms and the drive for alcohol consumption (Metten et al., 1998). The mechanisms of physical dependence are less well understood than those responsible for acute intoxication. However, it is known that tolerance and physical dependence occur due to compensatory changes in the function of alcohol’s acute targets, including many of the ion channels and second-messenger systems discussed earlier (Spanagel, 2009) and in other chapters of this volume.
Changes in the function or localization of GABA-A and NMDA receptors occur with acute or chronic alcohol exposure in a variety of different in vivo and in vitro models (Allan and Harris, 1987; Charlton et al., 1997; Anderson et al., 2007; Diaz et al., 2011). Numerous studies have identified changes in GABA-A receptor or NMDA receptor subunit mRNA/protein following chronic alcohol, suggesting that adaptive changes in the expression of these two major targets of acute alcohol action might have a major contribution in the development of tolerance/dependence. Alcohol-induced decreases in GABA-A receptor subunits could explain the cross-tolerance between alcohol and barbiturates or benzodiazepines, which also act on GABA-A receptors. A role for GABA-A receptors in alcohol tolerance is also implied by the usefulness of benzodiazepines or barbiturates in treating alcohol withdrawal.
Whereas acute alcohol exposure inhibits the function of NMDA receptors, chronic alcohol treatment results in increased NMDA receptor expression (Iorio et al., 1992; Allgaier, 2002). This increase in the number of NMDA receptors offsets the acute inhibitory actions of alcohol on the NMDA receptor and may contribute to hyperactivity duringwithdrawal. Additionally, hyperactivity during withdrawal could result from changes in the subunit composition of the NMDA receptor, as it has been shown that chronic alcohol consumption increases NMDA NR1 and NR2A (Snell et al., 1996) and NR2B (Kalluri et al., 1998) subunit levels in certain brain regions.
Alcohol withdrawal syndrome overview
Approximately 2 million Americans experience the symptoms of alcohol withdrawal yearly (Kosten and O’Connor, 2003; Bayard et al., 2004). As discussed in detail above, prolonged alcohol consumption leads to compensatory functional changes in alcohol’s target molecules and alcohol withdrawal syndrome occurs when alcohol use is abruptly discontinued and these changes are unmasked. Alcohol withdrawal syndrome may occur intentionally if the person voluntarily ceases alcohol consumption or unintentionally if abstinence is due to illness or injury (Hall and Zador, 1997). In any case, alcohol withdrawal syndrome indicates the development of a physical dependence on alcohol (Uzbay, 2012). Depending on the amount of alcohol consumed and the duration of an individual’s drinking habit, alcohol withdrawal syndrome can range from minor symptoms such as irritability, agitation, anxiety, headache, anorexia, diaphoresis, palpitations, insomnia, nausea and vomiting, and tremors; to moderate symptoms, including confusion and autonomic hyperactivity; to more severe symptoms, including muscle rigidity, seizures, and delirium tremens (Kosten and O’Connor, 2003; Bayard et al., 2004). Minor withdrawal symptoms occur within the first 6–12 hours following the cessation of alcohol use (Hall and Zador, 1997; Bayard et al., 2004). Mild hallucinations can occur 12–14 hours following the cessation of alcohol and usually resolve within 48 hours, but they may last up to 6 days (Bayard et al., 2004; Corfee, 2011). Alcohol withdrawal seizures typically begin 24–48 hours following cessation of drinking (Hall and Zador, 1997; Bayard et al., 2004; Rogawski, 2005; Corfee, 2011). Finally, 48 hours to 5 days following the cessation of alcohol use, delirium tremens can begin and includes marked hallucinations, disorientation, tachycardia, hypertension, fever, agitation, and diaphoresis – symptoms that usually peak 5 days following drinking cessation (Hall and Zador, 1997; Bayard et al., 2004; Corfee, 2011). In most cases, the symptoms of moderate alcohol withdrawal end 2–7 days following consumption of the last drink and do not require medical intervention. However, in more severe cases, individuals may need to be withdrawn from alcohol under medical supervision (Hall and Zador, 1997).
Withdrawal seizures
Alcohol abuse accounts for 20–40% of all new-onset seizures seen in emergency departments, particularly in male patients between 30 and 60 years of age (Tardy et al., 1995; McMicken and Liss, 2011). Additionally, another large clinical study showed that alcohol use was one of the four leading etiologies for status epilepticus (Lowenstein and Alldredge, 1993). Alcohol withdrawal seizures are usually tonicclonic, although partial seizures can also occur. Because alcohol withdrawal seizures are pharmacologically induced, the pathophysiologic mechanisms of the seizures are different from those of the seizures occurring in genetic and acquired epilepsy (Freedland and McMicken, 1993; Rogawski, 2005). Withdrawal seizures are more common in patients who have a history of recurrent detoxifications (Ballenger and Post, 1978; Bayard et al., 2004; Duka et al., 2004) and the severity and duration of withdrawal seizures increase with repeated withdrawal experiences (Becker et al., 1998). Alternate causes for seizures other than alcohol withdrawal syndrome should be considered if there is no recent abstinence from drinking, if the patient has suffered from a traumatic event or has a fever, if the seizures are focal, and if the seizures occur more than 48 hours following an individual’s last drink (Bayard et al., 2004). Treatment and prevention of alcohol withdrawal seizures are remarkably resistant to traditional anticonvulsants such as carbamazepine or phenytoin but benzodiazepines are effective (Rogawski, 2005; McMicken and Liss, 2011).
Rodent models of the human alcohol withdrawal-related tonicclonic seizures have been used to identify their underlying mechanisms. Rodents chronically exposed to alcohol via intragastric alcohol administration, inhalation, or feeding of a liquid diet for periods of 2–21 days exhibit sound-evoked audiogenic seizures 1–3 days following cessation of alcohol intake (McCown and Breese, 1990; Devaud et al., 1997; Becker et al., 1998; Becker and Veatch, 2002). The audiogenic seizures are the best-studied type of alcohol withdrawal seizure and are mediated largely in the brainstem, which makes the mechanisms mediating these seizures unique from those believed to mediate other clinical seizure types (Rogawski, 2005). The mechanisms of alcohol withdrawal seizures are not fully understood, but it appears that the inferior colliculus plays a major role in alcohol withdrawal-induced audiogenic seizures in rodents. For example, multiple alcohol withdrawal episodes in rats facilitate the development of inferior colliculus kindling (McCown and Breese, 1990). Alterations in GABA-A receptor localization or receptor gene expression, or NMDA receptor expression, likely play a critical mechanistic role in the development of alcohol withdrawal-related seizures (Rogawski, 2005). Rodent models suggest that kindling, a form of neuroplasticity causing increased neuronal excitability, can occur with repeated alcohol withdrawal episodes (McCown and Breese, 1990; Becker et al., 1998). Such neuroplasticity could further contribute to withdrawal seizures, and possibly the genesis of long-standing seizure disorders in the absence of alcohol withdrawal.
Delirium tremens
Delirium tremens is the most severe manifestation of the alcohol withdrawal syndrome. It is characterized by severe disorientation, exaggerated sympathetic activity, psychomotor agitation, and marked hallucinations. Risk factors include concurrent acute medical illness, daily heavy alcohol consumption, history of delirium tremens or withdrawal seizures, old age, abnormal liver function, and severe withdrawal symptoms (Bayard et al., 2004; Corfee, 2011). Although many symptoms of alcohol withdrawal syndrome begin early following cessation of the drug, the manifestations of delirium tremens can develop abruptly several days following alcohol cessation. Delirium tremens can mimic alternate critical illnesses such as sepsis or head injury, may precipitate respiratory and cardiovascular collapse, and, if left untreated, death may occur from respiratory or cardiovascular collapse (Hall and Zador, 1997; Corfee, 2011). In one study, 24% of 200 consecutive alcohol-dependent patients admitted to an inner-city US hospital developed delirium tremens and 8% of those individuals died from complications of delirium tremens (Ferguson et al., 1996). Among other factors, those who developed delirium tremens were more likely to be unemployed, homeless, and to show signs of more severe alcohol-related end-organ disease (Ferguson et al., 1996; Eyer et al., 2011). While high mortality rates (30–50%) from delirium tremens were reported in earlier clinical studies, mortality today has generally been reduced to less than 3–5% (Lukan et al., 2002) and is usually associated with other alcohol-related diseases such as hepatitis, aspiration, and fatal cardiac arrhythmias (Eastes, 2010; Carlson et al., 2012).
Wernicke–Korsakoff syndrome
Wernicke–Korsakoff syndrome (WKS) describes a constellation of neurologic and cognitive problems that comprises both Wernicke’s encephalopathy (WE) and Korsakoff syndrome (KS). WE is an acute, potentially reversible, neuropsychiatric disorder caused by thiamine deficiency (Donnino et al., 2007; Isenberg-Grzeda et al., 2012). The altered cognition of WE can progress to KS, a chronic and usually permanent impairment in formation of new memories with relative preservation of other mental functions (Toth et al., 2002). Additionally, KS has additional causes other than WE, including primary CNS lymphoma (Toth et al., 2002) and mammillothalamic tract infarction (Yoneoka et al., 2004). Approximately 80% of patients with acute WE will develop KS (Donnino et al., 2007). With early recognition and treatment, morbidity associated with WKS can be prevented. WE and KS were first described separately by Wernicke and Korsakoff as distinct pathologies (Donnino et al., 2007; Isenberg-Grzeda et al., 2012). It was not until 1956 that it was noted that patients diagnosed with KS revealed neuropathologic lesions in the identical brain regions as patients with WE (Malamud and Skillicorn, 1956).
The classic clinical triad of WE consists of mental status impairment, ophthalmoplegia, and gait ataxia, but the complete triad may only appear in as few as 10% of cases (Harper et al., 1986). Therefore, reliance on the complete triad as the sole criterion for the disease is inadequate and may lead to misdiagnoses. The most common component of the disease determined from retrospective autopsy studies is mental status change (Harper et al., 1986). The degree and nature of cognitive impairment can range from apathy or confusion to, although rare, coma (Donnino et al., 2007). Unfortunately, WE is often recognized only on autopsy (Torvik et al., 1982; Harper et al., 1986). One study at Ulleva °l Hospital in Oslo showed that, among 8735 autopsies performed over 5 years, there were 70 cases of WE. Examination of the clinical records showed that stupor and coma were the dominating symptoms in active cases of WE (Torvik et al., 1982).
Treatment of WE involves the timely administration of thiamine. Thiamine is a cofactor for several essential enzymes in the Krebs cycle and the pentose phosphate pathway. In the presence of adenosine triphosphate, thiamine is converted to thiamine pyrophosphate, which functions in carbohydrate metabolism as a coenzyme in the decarboxylation of pyruvic and α-ketoglutaric acid. A serious deficiency of thiamine leads to alterations in metabolism, including the accumulation of pyruvate and α-ketoglutarate in the blood, and a decrease in the activity of transketolase, an enzyme in the pentose phosphate pathway (Hoyumpa, 1980). Traditionally in the treatment of WE, 100 mg thiamine is administered once per day intramuscularly or orally, but there is some controversy as to whether this dose is appropriately high to support passage of thiamine across the blood–brain barrier (Chataway and Hardman, 1995; Cook et al., 1998). In one study, Ambrose et al. (2001) compared five groups of subjects, all of whom were detoxifying from alcohol, in a randomized, double-blind, multidose study of thiamine treatment. Subjects were given different doses (range 5–200 mg) of intramuscular thiamine for 2 consecutive days. The posttreatment performance of these groups then was examined on a test of working memory, the delayed alternation task. The subjects taking the higher doses of thiamine performed best on the delayed alternation task, but the results did not show a stepwise dose response (Ambrose et al., 2001). Thus, a common treatment for possible thiamine deficiency consists of intravenous thiamine (10 mg) for several days, followed by daily oral thiamine (100 mg) for at least a month, if not indefinitely (Donnino et al., 2007).
Common misconceptions about WKS persist, including the idea that it is a rare disease and that it is only a disease seen in alcoholics (Donnino et al., 2007; Weathers and Lewis, 2009). Alcoholism appears to be the most common factor predisposing individuals in the United States to WE; however, WE can occur in any patient with nutritional deficiency (Donnino et al., 2007). Older autopsy series reports have revealed WE in 10% of patients with acquired immunodeficiency syndrome (AIDS) (Boldorini et al., 1992), 6% of patients with bone marrow transplants (Bleggi-Torres et al., 2000), and 12.5% of alcoholics (Torvik et al., 1982).
Cerebellar degeneration
The most common degenerative condition affecting the cerebellum is chronic alcoholism, which typically occurs after 10 or more years of alcohol abuse (Baker et al., 1999; Andersen, 2004). Alcoholic cerebellar degeneration is characterized by ataxia of stance and gait. Individuals with cerebellar degeneration may adopt a wide-based gait with shortened steps to compensate for their loss of balance (Andersen, 2004). Additionally, eye movement disorders such as nystagmus, poor handwriting, incoordination of the upper extremities, and mild dysarthria may be present (Diener et al., 1984; Johnson-Greene et al., 1997).
The first comprehensive study of alcoholic cerebellar degeneration was performed by Victor et al. (1959), who established that degeneration of the Purkinje cells in the central lobule and the anterior folia of the vermis resulted in lower-limb incoordination and ataxia. Since this study, additional reports have documented neuropathologic alterations in the structure of the cerebellum following long-term alcohol consumption ( Johnson-Greene et al., 1997; Baker et al., 1999; Harper et al., 2003). In one such study, Baker et al. reported a significant decrease in Purkinje cell density and molecular layer volume in the cerebellar vermis in thiamine-deficient chronic alcoholics compared to chronic alcoholics and non-alcoholics. These thiamine-deficient alcoholics also had a significant decrease in the estimated Purkinje cell number in the flocculi (Baker et al., 1999). In this study, chronic alcoholics did not differ significantly from the non-alcoholic controls in all cerebellar measures, suggesting that chronic alcohol consumption per se does not necessarily damage human cerebellar tissue. Along these lines, it has been suggested that alcoholic cerebellar degeneration might be caused by thiamine deficiency and liver disease, and represents the same disease as WE (Butterworth, 1995; Baker et al., 1999). In support of this theory, cerebellar dysfunction frequently improves or stabilizes with abstinence and improved nutrition (Diener et al., 1984). However, other studies have documented cerebellar atrophy in well-nourished alcoholics and noted a relationship with duration and severity of alcohol intake (Nicolas et al., 2000), showing graded volume deficits from controls to uncomplicated alcoholics, to alcoholics with KS (Sullivan et al., 2000; Sullivan and Pfefferbaum, 2009).
Primary alcoholic dementia
In recent decades it has become evident that chronic alcohol use can cause lasting cognitive impairment, or alcoholic dementia, even in the absence of nutritional deficiency or cerebral trauma. Alcohol-related dementia has been estimated to represent approximately 10% of all cases of dementia (Gupta and Warner, 2008). In a review of epidemiologic, neurologic, cognitive, and imaging data by Smith and Atkinson (1995), “heavy alcohol use” (roughly defined) was seen as a contributing factor in 21–24% cases of dementia. Many alcoholics demonstrate gradually progressive multidomain cognitive impairment rather than a more restrictive amnestic disorder which might be more typical of WKS (Brust. 2010). There is some suggestion that alcoholic dementia is at least partially reversible on cessation of alcohol consumption.
Numerous pathologic and neuroimaging studies have documented brain shrinkage with alcoholism, even in the apparent absence of thiamine deficiency (Melgaard et al., 1990; Pfefferbaum et al., 1992; Kril and Halliday, 1999). Such brain tissue loss appears to be primarily due to shrinkage of myelin, although dendritic abnormalities and neuronal loss have also been observed (reviewed in Harper et al., 2003). Myelin and neuronal loss appears to particularly involve the prefrontal and frontal lobe areas, with older alcoholics showing even more severe tissue loss (Pfefferbaum et al., 1992, 1997). Frontocerebellar tracts and interhemispheric white-matter tracts appear to convey aspects of the cognitive disruption seen with alcoholic dementia (Fadda and Rossetti, 1998; Chanraud et al., 2010, 2011; Ridley et al., 2013). Diffusion tensor imaging studies of white-matter abnormalities in alcoholics highlight a disruption of myelin microstructure in prefrontal areas and the corpus callosum (Pfefferbaum and Sullivan, 2005) that appears distinct from callosal atrophy seen with Alzheimer’s disease (Pitel et al., 2010). Recent neuroimaging studies on a primate model of chronic oral alcohol consumption clearly document that alcohol itself, rather than just nutritional factors, can cause cerebral cortical gray-matter loss (Kroenke et al., 2013).
There are numerous neuroimaging reports documenting that myelin and brain tissue loss with alcoholism is at least partially reversible on cessation of drinking. In one study by Gazdzinski et al. (2005), the authors showed marked brain tissue volume recovery in alcoholics that were abstinent for 1 month and the tissue volume continued to increase over the next 6–9 months of abstinence, but at a slower rate than the first month following initial abstinence.
The mechanisms underlying alcoholic dementia are currently unknown. There is considerable animal model evidence suggesting that chronic alcohol exposure can cause neurotoxicity, perhaps related to withdrawal-induced glutamate excitotoxicity (Hoffman, 1995) or oxidative stress (Brust, 2010). Additionally, the alcohol metabolite acetaldehyde binds to select proteins, resulting in the formation of adducts. There have been reports suggesting a relationship between acetaldehyde adducts in the brain and brain damage (Nakamura et al., 2000).
Molecular changes evoked by chronic alcohol consumption also alter expression of numerous brain gene expression networks (Figs 10.1 and 10.2), which could contribute to neurotoxicity or functional disruption in alcoholic dementia. As mentioned earlier, alcohol has both acute and chronic effects on the expression of myelin-related genes (Fig. 10.2). Given the prominent disruption of myelin seen with neuropathologic and neuroimaging studies on alcoholic dementia, it is possible that alcohol modulation of myelin gene expression is functionally related to at least aspects of alcoholic dementia. Intriguingly, a recent study demonstrated that variation in myelin gene expression levels correlate with a measure of acute sensitivity to alcohol (loss-of-righting reflex, LORR), in mouse models (Farris and Miles, 2013). Since acute sensitivity to alcohol is a well-established risk factor for development of AUD, myelin expression and structure could have a central mechanistic role in both the genesis of AUD and the development of alcohol-related neurologic disorders such as alcoholic dementia (Fig. 10.2).
Central pontine myelinolysis
Central pontine myelinolysis (CPM) is characterized by a loss of oligodendrocytes and myelin in the central pons (Adams et al., 1959). Extrapontine myelinolysis (EPM) has also been described most often involving the white matter of the cerebellum, lateral geniculate body, putamen, thalamus, hippocampus, and cerebral transition of white and gray matter (Martin, 2004). The most common cause of CPM is an overly rapid correction of hyponatremia in patients with conditions such as alcoholism that lead to nutritional or electrolyte stress. Hyponatremia developing over a 48-hour period is considered acute and causes a more critical patient presentation. Chronic hyponatremia takes greater than 48 hours to develop and can indicate a higher risk for CPM (Lien and Shapiro, 2007).
The central pons may be especially vulnerable to osmotic injury because, unlike most areas of the brain, gray matter and white matter are intermixed. Typically, oligodendrocytes are embedded within white matter and physically isolated from gray matter. It has been proposed that, in CPM, oligodendrocytes in close proximity to gray matter are exposed to a myelin toxic substance following osmotic stress. The symptoms of CPM are varied and include confusion, impaired cognition, dysarthria, dysphasia, gait instability, weakness or paralysis, and generalized seizures (Hurley et al., 2011).
Despite considerable evidence supporting hyponatremia as the proximal cause of CPM, there are both clinical and model organism data suggesting that alcohol itself may perturb myelin structure or that alcoholics may have an underlying “myelin tone” which predisposes them to both alcoholism and other environmental insults that might cause myelinolysis (Fig. 10.2). As discussed elsewhere in this chapter, alcoholics show widespread (but particularly frontal, callosal, and hippocampal) myelin abnormalities, some of which are reversible with abstinence (Alhassoon et al., 2012). Alcoholics show a down-regulation of multiple myelin-related genes, particularly in hippocampus and prefrontal areas (Lewohl et al., 2000; McClintick et al., 2013). Animal models show that acute alcohol regulates myelin gene expression in pre-frontal cortex (Kerns et al., 2005) and that a gene network consisting of multiple myelin genes shows strong correlations with both acute alcohol sensitivity and alcohol consumption in mice (Farris and Miles, 2012, 2013). Furthermore, recent MRI studies suggest that adolescents prone to AUD have premorbid abnormalities in myelin structure, as has been predicted by animal studies (Kerns et al., 2005; De Bellis et al., 2008; Farris and Miles, 2012, 2013). Thus, it remains a possibility that alcoholics are predisposed to myelin disorders by environmental insults such as hyponatremia with CPM.
There is a considerable range in prognosis in patients with clinically symptomatic CPM or EPM (Menger and Jorg, 1999; Mochizuki et al., 2003; Odier et al., 2010). Menger and Jorg examined 44 cases, most (42/44) with chronic alcoholism. Of the 32 patients with follow-up data who survived (2 died in the acute phase), 34% (11/32) experienced no significant functional deficits; 34% (11/32) experienced minor neurologic deficits; and 31% (10/32) remained dependent on personal help (Menger and Jorg, 1999). Mochizuki et al. also studied patients with alcoholism and reported that 44% (4/9) of the patients in their study were free of neurologic deficits 6 months following CPM. Additionally, the authors noted that most of their cohort (8/9) did not have an episode of acute correction of hyponatremia (Mochizuki et al., 2003). Treatment algorithms include considerations of current symptoms, whether hyponatremia is acute or chronic, presence of chronic malnourishment, underlying medical illnesses or complications, and other accompanying electrolyte abnormalities (Hurley et al., 2011). Hyponatremia occurs when the serum sodium drops below 135 mmol/L and can be treated by restoring sodium levels to normal. However, this must be done at a rate not exceeding 0.5 mmol/hour (Lien and Shapiro, 2007; Hurley et al., 2011).
Marchiafava–Bignami disease
Another neurologic disorder affecting primarily myelin and occurring with chronic alcohol abuse is Marchiafava–Bignami disease (MBD). Originally described as the “red wine drinker’s encephalopathy,” MBD is a rare and somewhat poorly understood disease that causes mania, depression, paranoia, dementia, seizures, paresis, and ataxia (Brust, 2010). Corpus callosum demyelination is the hallmark of MBD disease (Tozakidou et al., 2011), but demyelination may extend to the optic chiasm and tracts, cerebellar peduncle, subcortical region, neighboring white matter, and, rarely, cortical gray matter (Kim et al., 2007). The disease may present in two major clinical forms: acute and chronic. Acutely, severe impairment of consciousness, seizures, and muscle rigidity often result in death after several days. In the chronic form, an interhemispheric disconnection syndrome, such as limb apraxia, tactile agraphia, unilateral agraphia, hemialexia, and dementia, can be seen and can last for months to years (Kim et al., 2007). Clinicians can confuse MBD with WE, and some reports have shown that MBD and WE often occur concurrently ( Johkura et al., 2005; Kim et al., 2007). Furthermore, there are scattered reports of response to thiamine replacement in cases diagnosed as MBD (Aggarwal et al., 2011). Thus, thiamine replacement should be given in cases of suspected MBD but it is not clear as to whether thiamine disorders are mechanistically involved with most cases of MBD. Furthermore, MBD-like lesions on MRI scans have been infrequently reported with a number of other clinical conditions such as ischemia, multiple sclerosis, lupus, and posterior reversible encephalopathy syndrome. Some of these may be responsible for cases of MBD having milder or reversible courses.
As discussed above for CPM, it remains an open question as to whether myelin abnormalities either underlying or accompanying AUD could contribute to the occurrence of MBD. In particular, fetal alcohol spectrum disorder is known to sometimes produce striking deficits in myelination, particularly involving the corpus callosum. MBD could thus result from the occurrence of a myelin-toxic environmental event, superimposed on a myelin state that is already compromised. Future longitudinal studies on fetal alcohol spectrum disorder patients or alcoholics would be required to confirm such a hypothesis.
CONCLUSIONS
This chapter highlights current knowledge on the molecular and clinical aspects of chronic alcohol effects on the CNS. This drug is almost ubiquitous, widely enjoyed socially, but produces a diverse spectrum of neurologic disease when abused. We have not detailed all current molecular information since the field is moving extremely rapidly and various molecular underpinnings of alcohol action are elaborated in other chapters of this volume. Alcohol interacts acutely predominantly with GABA-A and NMDA receptors, but triggers diverse signaling events within well-defined neural pathways. These result in adaptive changes in gene expression that ultimately produce two major states: addiction and toxicity. Epigenetic modifications of chromatin could lead to long-lived or even transgenerational changes in gene expression, thus producing aspects of the heritability of AUD and long-term behaviors such as recidivism. The diverse clinical syndromes produced by chronic alcohol actions in the CNS reflect the molecular pathology and predominantly involve aspects of tolerance/withdrawal and selective vulnerability (WKS, CPM, MBD). Additionally, deleterious aspects of chronic alcohol on signaling, synaptic transmission, and cell toxicity lead to primary alcoholic dementia. Genetically determined aspects of myelin structure and alcohol actions on myelin gene expression may be a prominent molecular component underlying predisposition to, or causation of, AUD and multiple other neurologic complications of chronic alcohol. The dramatic progress made in understanding molecular actions of alcohol hold great promise for our eventual treatment or prevention of AUD and neurologic complications from chronic abuse of alcohol.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants: U01AA016667, P20AA017828 (MFM), and F31AA020141 (BNC).
References
- Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry. 1959;81:154–172. [PubMed] [Google Scholar]
- Aggarwal A, Khandelwal A, Jiloha RC. A case of Marchiafava Bignami disease: complete recovery with thiamine. J Neuropsychiatry Clin Neurosci. 2011;23:E28. doi: 10.1176/jnp.23.2.jnpe28. [DOI] [PubMed] [Google Scholar]
- Alhassoon OM, Sorg SF, Taylor MJ, et al. Callosal white matter microstructural recovery in abstinent alcoholics: a longitudinal diffusion tensor imaging study. Alcohol Clin Exp Res. 2012;36:1922–1931. doi: 10.1111/j.1530-0277.2012.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AM, Harris RA. Acute and chronic ethanol treatments alter GABA receptor-operated chloride channels. Pharmacol Biochem Behav. 1987;27:665–670. doi: 10.1016/0091-3057(87)90192-4. [DOI] [PubMed] [Google Scholar]
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Ambrose ML, Bowden SC, Whelan G. Thiamin treatment and working memory function of alcohol-dependent people: preliminary findings. Alcohol Clin Exp Res. 2001;25:112–116. [PubMed] [Google Scholar]
- Andersen BB. Reduction of Purkinje cell volume in cerebellum of alcoholics. Brain Res. 2004;1007:10–18. doi: 10.1016/j.brainres.2004.01.058. [DOI] [PubMed] [Google Scholar]
- Anderson NJ, Daunais JB, Friedman DP, et al. Long-term ethanol self-administration by the nonhuman primate, macaca fascicularis, decreases the benzodiazepine sensitivity of amygdala GABA(A) receptors. Alcohol Clin Exp Res. 2007;31:1061–1070. doi: 10.1111/j.1530-0277.2007.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KG, Harding AJ, Halliday GM, et al. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience. 1999;91:429–438. doi: 10.1016/s0306-4522(98)90664-9. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barak S, Liu F, Ben Hamida S, et al. Disruption of alcohol-related memories by mtorc1 inhibition prevents relapse. Nat Neurosci. 2013;16:1111–1117. doi: 10.1038/nn.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayard M, McIntyre J, Hill KR, et al. Alcohol withdrawal syndrome. Am Fam Physician. 2004;69:1443–1450. [PubMed] [Google Scholar]
- Becker HC, Veatch LM. Effects of lorazepam treatment for multiple ethanol withdrawals in mice. Alcohol Clin Exp Res. 2002;26:371–380. [PubMed] [Google Scholar]
- Becker HC, Veatch LM, Diaz-Granados JL. Repeated ethanol withdrawal experience selectively alters sensitivity to different chemoconvulsant drugs in mice. Psychopharmacology (Berl) 1998;139:145–153. doi: 10.1007/s002130050699. [DOI] [PubMed] [Google Scholar]
- Bhandari P, Hill JS, Farris SP, et al. Chloride intracellular channels modulate acute ethanol behaviors in Drosophila, Caenorhabditis elegans and mice. Genes Brain Behav. 2012;11:387–397. doi: 10.1111/j.1601-183X.2012.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, et al. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011;25 (Suppl 1):S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, et al. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleggi-Torres LF, de Medeiros BC, Werner B, et al. Neuropathological findings after bone marrow transplantation: an autopsy study of 180 cases. Bone Marrow Transplant. 2000;25:301–307. doi: 10.1038/sj.bmt.1702140. [DOI] [PubMed] [Google Scholar]
- Boldorini R, Vago L, Lechi A, et al. Wernicke’s encephalopathy: occurrence and pathological aspects in a series of 400 AIDS patients. Acta Biomed Ateneo Parmense. 1992;63:43–49. [PubMed] [Google Scholar]
- Borrelli E, Nestler EJ, Allis CD, et al. Decoding the epigenetic language of neuronal plasticity. Neuron. 2008;60:961–974. doi: 10.1016/j.neuron.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust JC. Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection: a review. Int J Environ Res Public Health. 2010;7:1540–1557. doi: 10.3390/ijerph7041540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth RF. Pathophysiology of alcoholic brain damage: synergistic effects of ethanol, thiamine deficiency and alcoholic liver disease. Metab Brain Dis. 1995;10:1–8. doi: 10.1007/BF01991777. [DOI] [PubMed] [Google Scholar]
- Carlson RW, Kumar NN, Wong-Mckinstry E, et al. Alcohol withdrawal syndrome. Crit Care Clin. 2012;28:549–585. doi: 10.1016/j.ccc.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Rohlfing T, et al. Dual tasking and working memory in alcoholism: relation to frontocerebellar circuitry. Neuropsychopharmacology. 2010;35:1868–1878. doi: 10.1038/npp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, et al. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011;21:2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton ME, Sweetnam PM, Fitzgerald LW, et al. Chronic ethanol administration regulates the expression of GABAA receptor alpha 1 and alpha 5 subunits in the ventral tegmental area and hippocampus. J Neurochem. 1997;68:121–127. doi: 10.1046/j.1471-4159.1997.68010121.x. [DOI] [PubMed] [Google Scholar]
- Chataway J, Hardman E. Thiamine in Wernicke’s syndrome– howmuchand howlong? PostgradMedJ. 1995;71:249. doi: 10.1136/pgmj.71.834.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler EJ, Lu L, Shou S, et al. Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet. 2005;37:233–242. doi: 10.1038/ng1518. [DOI] [PubMed] [Google Scholar]
- Contet C. Gene expression under the influence: Transcriptional profiling of ethanol in the brain. Curr Psychopharmacol. 2012;1:301–314. doi: 10.2174/2211556011201040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CC, Hallwood PM, Thomson AD. B vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol. 1998;33:317–336. doi: 10.1093/oxfordjournals.alcalc.a008400. [DOI] [PubMed] [Google Scholar]
- Corfee FA. Alcohol withdrawal in the critical care unit. Aust Crit Care. 2011;24:110–116. doi: 10.1016/j.aucc.2010.08.005. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, et al. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Greck M, Supady A, Thiemann R, et al. Decreased neural activity in reward circuitry during personal reference in abstinent alcoholics – a fMRI study. Hum Brain Mapp. 2009;30:1691–1704. doi: 10.1002/hbm.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, et al. Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, et al. Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local gabaergic synapses. J Pharmacol Exp Ther. 2011;337:162–170. doi: 10.1124/jpet.110.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Dichgans J, Bacher M, et al. Improvement of ataxia in alcoholic cerebellar atrophy through alcohol abstinence. J Neurol. 1984;231:258–262. doi: 10.1007/BF00313662. [DOI] [PubMed] [Google Scholar]
- Donnino MW, Vega J, Miller J, et al. Myths and misconceptions of Wernicke’s encephalopathy: what every emergency physician should know. Ann Emerg Med. 2007;50:715–721. doi: 10.1016/j.annemergmed.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Duka T, Gentry J, Malcolm R, et al. Consequences of multiple withdrawals from alcohol. Alcohol Clin Exp Res. 2004;28:233–246. doi: 10.1097/01.alc.0000113780.41701.81. [DOI] [PubMed] [Google Scholar]
- Eastes LE. Alcohol withdrawal syndrome in trauma patients: a review. J Emerg Nurs. 2010;36:507–509. doi: 10.1016/j.jen.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Eyer F, Schuster T, Felgenhauer N, et al. Risk assessment of moderate to severe alcohol withdrawal – predictors for seizures and delirium tremens in the course of withdrawal. Alcohol Alcohol. 2011;46:427–433. doi: 10.1093/alcalc/agr053. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Farris SP, Miles MF. Ethanol modulation of gene networks: implications for alcoholism. Neurobiol Dis. 2012;45:115–121. doi: 10.1016/j.nbd.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SP, Miles MF. Fyn-dependent gene networks in acute ethanol sensitivity. PLoS One. 2013;8:e82435. doi: 10.1371/journal.pone.0082435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JA, Suelzer CJ, Eckert GJ, et al. Risk factors for delirium tremens development. J Gen Intern Med. 1996;11:410–414. doi: 10.1007/BF02600188. [DOI] [PubMed] [Google Scholar]
- First MB, Tasman A. DSM-IV-TR mental disorders: Diagnosis, etiology, and treatment. John Wiley; Chichester, West Sussex: 2004. [Google Scholar]
- Freedland ES, McMicken DB. Alcohol-related seizures, part I: Pathophysiology, differential diagnosis, and evaluation. J Emerg Med. 1993;11:463–473. doi: 10.1016/0736-4679(93)90251-2. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Jorgensen KW, Yetter EJ, et al. Differential brain response to alcohol cue distractors across stages of alcohol dependence. Biol Psychol. 2013;92:282–291. doi: 10.1016/j.biopsycho.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Meyerhoff DJ. Temporal dynamics and determinants of whole brain tissue volume changes during recovery from alcohol dependence. Drug Alcohol Depend. 2005;78:263–273. doi: 10.1016/j.drugalcdep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Givens B, Williams JM, Gill TM. Septohippocampal pathway as a site for the memory-impairing effects of ethanol. Hippocampus. 2000;10:111–121. doi: 10.1002/(SICI)1098-1063(2000)10:1<111::AID-HIPO12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Goldberg L. Criteria of alcohol intoxication in animals in relation to blood alcohol. Acta Physiol Scand. 1941;3:71–81. [Google Scholar]
- Goodwin DW. Alcoholic blackout and state-dependent learning. Fed Proc. 1974;33:1833–1835. [PubMed] [Google Scholar]
- Goodwin DW, Crane JB, Guze SB. Alcoholic “blackouts”: A review and clinical study of 100 alcoholics. Am J Psychiatry. 1969;126:191–198. doi: 10.1176/ajp.126.2.191. [DOI] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Moller N, et al. Drinking problems in adopted and nonadopted sons of alcoholics. Arch Gen Psychiatry. 1974;31:164–169. doi: 10.1001/archpsyc.1974.01760140022003. [DOI] [PubMed] [Google Scholar]
- Gupta S, Warner J. Alcohol-related dementia: A 21st-century silent epidemic? Br J Psychiatry. 2008;193:351–353. doi: 10.1192/bjp.bp.108.051425. [DOI] [PubMed] [Google Scholar]
- Hall W, Zador D. The alcohol withdrawal syndrome. Lancet. 1997;349:1897–1900. doi: 10.1016/S0140-6736(97)04572-8. [DOI] [PubMed] [Google Scholar]
- Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341–345. doi: 10.1136/jnnp.49.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Dixon G, Sheedy D, et al. Neuropathological alterations in alcoholic brains. Studies arising from the new south wales tissue resource centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Hartzler B, Fromme K. Fragmentary and en bloc blackouts: similarity and distinction among episodes of alcohol-induced memory loss. J Stud Alcohol. 2003;64:547–550. doi: 10.15288/jsa.2003.64.547. [DOI] [PubMed] [Google Scholar]
- Heinz A, Lober S, Georgi A, et al. Reward craving and withdrawal relief craving: assessment of different motivational pathways to alcohol intake. Alcohol Alcohol. 2003;38:35–39. doi: 10.1093/alcalc/agg005. [DOI] [PubMed] [Google Scholar]
- Hoffman PL. Glutamate receptorsinalcohol withdrawal-induced neurotoxicity. Metab Brain Dis. 1995;10:73–79. doi: 10.1007/BF01991784. [DOI] [PubMed] [Google Scholar]
- Hoyumpa AM., Jr Mechanisms of thiamin deficiency in chronic alcoholism. Am J Clin Nutr. 1980;33:2750–2761. doi: 10.1093/ajcn/33.12.2750. [DOI] [PubMed] [Google Scholar]
- Hurley RA, Filley CM, Taber KH. Central pontine myelinolysis: a metabolic disorder of myelin. J Neuropsychiatry Clin Neurosci. 2011;23:369–374. doi: 10.1176/jnp.23.4.jnp369. [DOI] [PubMed] [Google Scholar]
- Iancu OD, Oberbeck D, Darakjian P, et al. Selection for drinking in the dark alters brain gene coexpression networks. Alcohol Clin Exp Res. 2013;37:1295–1303. doi: 10.1111/acer.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio KR, Reinlib L, Tabakoff B, et al. Chronic exposure of cerebellar granule cells to ethanol results in increased N-methyl-D-aspartate receptor function. Mol Pharmacol. 1992;41:1142–1148. [PubMed] [Google Scholar]
- Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff syndrome: under-recognized and under-treated. Psychosomatics. 2012;53:507–516. doi: 10.1016/j.psym.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, et al. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2013;38C:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinek EM. Phases in the drinking history of alcoholics. Q J Stud Alcohol. 1946;7:1–88. [PubMed] [Google Scholar]
- Johkura K, Naito M, Naka T. Cortical involvement in Marchiafava-Bignami disease. AJNR Am J Neuroradiol. 2005;26:670–673. [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Noll EC, Rodney WM. Survival after a serum ethanol concentration of 1 1/2% Lancet. 1982;2:1394. doi: 10.1016/s0140-6736(82)91285-5. [DOI] [PubMed] [Google Scholar]
- Johnson-Greene D, Adams KM, Gilman S, et al. Impaired upper limb coordination in alcoholic cerebellar degeneration. Arch Neurol. 1997;54:436–439. doi: 10.1001/archneur.1997.00550160070018. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Mehta AK, Ticku MK. Up-regulation of nmda receptor subunits in rat brain following chronic ethanol treatment. Brain Res Mol Brain Res. 1998;58:221–224. doi: 10.1016/s0169-328x(98)00112-0. [DOI] [PubMed] [Google Scholar]
- Kaminen-Ahola N, Ahola A, Maga M, et al. Maternal ethanol consumption alters the epigenotype and the phenotype of offspring in a mouse model. PLoS Genet. 2010;6:e1000811. doi: 10.1371/journal.pgen.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Wang JC, Wetherill L, et al. A meta-analysis of two genome-wide association studies to identify novel loci for maximum number of alcoholic drinks. Hum Genet. 2013;132:1141–1151. doi: 10.1007/s00439-013-1318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns RT, Ravindranathan A, Hassan S, et al. Ethanol-responsive brain region expression networks: Implications for behavioral responses to acute ethanol in dba/2j versus c57bl/6j mice. J Neurosci. 2005;25:2255–2266. doi: 10.1523/JNEUROSCI.4372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Kim JK, Yoo BG, et al. Acute Marchiafava-Bignami disease with widespread callosal and cortical lesions. J Korean Med Sci. 2007;22:908–911. doi: 10.3346/jkms.2007.22.5.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos MZ, Yan J, Dick DM, et al. Common biological networks underlie genetic risk for alcoholism in African-and European-American populations. Genes Brain Behav. 2013;12:532–542. doi: 10.1111/gbb.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, O’Connor PG. Management of drug and alcohol withdrawal. N Engl J Med. 2003;348:1786–1795. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM. Brain shrinkage in alcoholics: a decade on and what have we learned? Prog Neurobiol. 1999;58:381–387. doi: 10.1016/s0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- Kroenke CD, Rohlfing T, Park B, et al. Monkeys that voluntarily and chronically drink alcohol damage their brains: a longitudinal MRI study. Neuropsychopharmacology. 2013;39:823–830. doi: 10.1038/npp.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, et al. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Lien YH, Shapiro JI. Hyponatremia: clinical diagnosis and management. Am J Med. 2007;120:653–658. doi: 10.1016/j.amjmed.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology. 1993;43:483–488. doi: 10.1212/wnl.43.3_part_1.483. [DOI] [PubMed] [Google Scholar]
- Lukan JK, Reed DN, Jr, Looney SW, et al. Risk factors for delirium tremens in trauma patients. J Trauma. 2002;53:901–906. doi: 10.1097/00005373-200211000-00015. [DOI] [PubMed] [Google Scholar]
- Malamud N, Skillicorn SA. Relationship between the Wernicke and the Korsakoff syndrome; a clinicopathologic study of seventy cases. AMA Arch Neurol Psychiatry. 1956;76:585–596. doi: 10.1001/archneurpsyc.1956.02330300015003. [DOI] [PubMed] [Google Scholar]
- Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 3):iii22–iii28. doi: 10.1136/jnnp.2004.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, Xuei X, Tischfield JA, et al. Stress-response pathways are altered in the hippocampus of chronic alcoholics. Alcohol. 2013;47:505–515. doi: 10.1016/j.alcohol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Multiple withdrawals from chronic ethanol “kindles” inferior collicular seizure activity: Evidence for kindling of seizures associated with alcoholism. Alcohol Clin Exp Res. 1990;14:394–399. doi: 10.1111/j.1530-0277.1990.tb00492.x. [DOI] [PubMed] [Google Scholar]
- McMicken D, Liss JL. Alcohol-related seizures. Emerg Med Clin North Am. 2011;29:117–124. doi: 10.1016/j.emc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Mehrabian M, Allayee H, Stockton J, et al. Integrating genotypic and expression data in a segregating mouse population to identify 5-lipoxygenase as a susceptibility gene for obesity and bone traits. Nat Genet. 2005;37:1224–1233. doi: 10.1038/ng1619. [DOI] [PubMed] [Google Scholar]
- Melgaard B, Henriksen L, Ahlgren P, et al. Regional cerebral blood flow in chronic alcoholics measured by single photon emission computerized tomography. Acta Neurol Scand. 1990;82:87–93. doi: 10.1111/j.1600-0404.1990.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Menger H, Jorg J. Outcome of central pontine and extrapontine myelinolysis (n=44) J Neurol. 1999;246:700–705. doi: 10.1007/s004150050435. [DOI] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, et al. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Miles MF. Alcohol’s effects on gene expression. Alcohol Health Res World. 1995;19:237–243. [PMC free article] [PubMed] [Google Scholar]
- Miles MF, Diaz JE, DeGuzman VS. Mechanisms of neuronal adaptation to ethanol. Ethanol induces hsc70 gene transcription in ng108-15 neuroblastoma x glioma cells. J Biol Chem. 1991;266:2409–2414. [PubMed] [Google Scholar]
- Mochizuki H, Masaki T, Miyakawa T, et al. Benign type of central pontine myelinolysis in alcoholism –clinical, neuroradiological and electrophysiological findings. J Neurol. 2003;250:1077–1083. doi: 10.1007/s00415-003-0157-6. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Swartzwelder HS. Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and gabaergic mechanisms. J Neurosci. 1993;13:2264–2272. doi: 10.1523/JNEUROSCI.13-05-02264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Iwahashi K, Itoh M, et al. Immunohistochemical study on acetaldehyde adducts in alcohol-fed mice. Alcohol Clin Exp Res. 2000;24 (Suppl):93S–96S. [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell QV, et al. Akt signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry. 2011;70:575–582. doi: 10.1016/j.biopsych.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- NIAAA; U.S. Department of Health and Human Services National Institutes of Health , editor. Rethinking drinking. 2010 http://pubs.niaaa.nih.gov/
- Nicolas JM, Fernandez-Sola J, Robert J, et al. High ethanol intake and malnutrition in alcoholic cerebellar shrinkage. QJM. 2000;93:449–456. doi: 10.1093/qjmed/93.7.449. [DOI] [PubMed] [Google Scholar]
- Odier C, Nguyen DK, Panisset M. Central pontine and extrapontine myelinolysis: from epileptic and other manifestations to cognitive prognosis. J Neurol. 2010;257:1176–1180. doi: 10.1007/s00415-010-5486-7. [DOI] [PubMed] [Google Scholar]
- Park BL, Kim JW, Cheong HS, et al. Extended genetic effects of ADH cluster genes on the risk of alcohol dependence: from GWAS to replication. Hum Genet. 2013;132:657–668. doi: 10.1007/s00439-013-1281-8. [DOI] [PubMed] [Google Scholar]
- Perry PJ, Argo TR, Barnett MJ, et al. The association of alcohol-induced blackouts and grayouts to blood alcohol concentrations. J Forensic Sci. 2006;51:896–899. doi: 10.1111/j.1556-4029.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extra-cellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, et al. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, et al. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Chanraud S, Sullivan EV, et al. Callosal microstructural abnormalities in alzheimer’s disease and alcoholism: same phenotype, different mechanisms. Psychiatry Res. 2010;184:49–56. doi: 10.1016/j.pscychresns.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Wang S, Zhang L, et al. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci. 2012;32:1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Umhau J, Pavon FJ, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol Psychiatry. 2011;16:809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Ridley NJ, Draper B, Withall A. Alcohol-related dementia: an update of the evidence. Alzheimers Res Ther. 2013;5:3. doi: 10.1186/alzrt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA. Update on the neurobiology of alcohol withdrawal seizures. Epilepsy Current. 2005;5:225–230. doi: 10.1111/j.1535-7511.2005.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Messing RO. Signaling pathways mediating alcohol effects. Curr Topics Behav Neurosci. 2013;13:87–126. doi: 10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose ME, Grant JE. Alcohol-induced blackout. Phenomenology, biological basis, and gender differences. J Addiction Med. 2010;4:61–73. doi: 10.1097/ADM.0b013e3181e1299d. [DOI] [PubMed] [Google Scholar]
- Schadt EE, Monks SA, Drake TA, et al. Genetics of gene expression surveyed in maize, mouse and man. Nature. 2003;422:297–302. doi: 10.1038/nature01434. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Biological vulnerability to alcoholism. J Consult Clin Psychol. 1987;55:301–309. doi: 10.1037//0022-006x.55.3.301. [DOI] [PubMed] [Google Scholar]
- Smith DM, Atkinson RM. Alcoholism and dementia. Int J Addict. 1995;30:1843–1869. doi: 10.3109/10826089509071058. [DOI] [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL, et al. Regional and subunit specific changes in nmda receptor MRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Brain Res Mol Brain Res. 1996;40:71–78. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Starkman BG, Sakharkar AJ, Pandey SC. Epigenetics –beyond the genome in alcoholism. Alcohol Res. 2012;34:293–305. [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 2009;44:155–165. doi: 10.1093/alcalc/agn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, et al. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology. 2000;14:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Printz M, et al. Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardy B, Lafond P, Convers P, et al. Adult first generalized seizure: etiology, biological tests, EEG, CT scan, in an ED. Am J Emerg Med. 1995;13:1–5. doi: 10.1016/0735-6757(95)90229-5. [DOI] [PubMed] [Google Scholar]
- Torvik A, Lindboe CF, Rogde S. Brain lesions in alcoholics. A neuropathological study with clinical correlations. J Neurol Sci. 1982;56:233–248. doi: 10.1016/0022-510x(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Toth C, Voll C, Macaulay R. Primary CNS lymphoma as a cause of Korsakoff syndrome. Surg Neurol. 2002;57:41–45. doi: 10.1016/s0090-3019(01)00650-4. [DOI] [PubMed] [Google Scholar]
- Tozakidou M, Stippich C, Fischmann A. Teaching neu-roimages: radiologic findings in Marchiafava-Bignami disease. Neurology. 2011;77:e67. doi: 10.1212/WNL.0b013e31822e144b. [DOI] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life Sci. 1981;28:1053–1056. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- Uzbay TI. Atypical antipsychotic drugs and ethanol withdrawal syndrome: a review. Alcohol Alcohol. 2012;47:33–41. doi: 10.1093/alcalc/agr142. [DOI] [PubMed] [Google Scholar]
- Vanderlinden LA, Saba LM, Kechris K, et al. Whole brain and brain regional coexpression network interactions associated with predisposition to alcohol consumption. PLoS One. 2013;8:e68878. doi: 10.1371/journal.pone.0068878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, et al. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M, Adams RD, Mancall EL. A restricted form of cerebellar cortical degeneration occurring in alcoholic patients. Arch Neurol. 1959;1:579–688. [Google Scholar]
- Weathers AL, Lewis SL. Rare and unusual … Or are they? Less commonly diagnosed encephalopathies associated with systemic disease. Semin Neurol. 2009;29:136–153. doi: 10.1055/s-0029-1213734. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Duka T. State-dependent effects of alcohol on explicit memory: the role of semantic associations. Psychopharmacology (Berl) 2000;149:98–106. doi: 10.1007/s002139900349. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Schnyer DM, Fromme K. Acute alcohol effects on contextual memory bold response: differences based on fragmentary blackout history. Alcohol Clin Exp Res. 2012;36:1108–1115. doi: 10.1111/j.1530-0277.2011.01702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Castro N, Squeglia LM, et al. Atypical neural activity during inhibitory processing in substance-naive youth who later experience alcohol-induced blackouts. Drug Alcohol Depend. 2013;128:243–249. doi: 10.1016/j.drugalcdep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM. What happened? Alcohol, memory blackouts, and the brain. Alcohol Res Health. 2003;27:186–196. [PMC free article] [PubMed] [Google Scholar]
- White AM, Jamieson-Drake DW, Swartzwelder HS. Prevalence and correlates of alcohol-induced blackouts among college students: results of an e-mail survey. J Am Coll Health. 2002;51 (117–119):122–131. doi: 10.1080/07448480209596339. [DOI] [PubMed] [Google Scholar]
- Wolen AR, Miles MF. Identifying gene networks underlying the neurobiology of ethanol and alcoholism. Alcohol Res. 2012;34:306–317. [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Warner JA, Capparuccini MI, et al. Genomic analysis of individual differences in ethanol drinking: evidence for non-genetic factors in c57bl/6 mice. PLoS One. 2011;6:e21100. doi: 10.1371/journal.pone.0021100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneoka Y, Takeda N, Inoue A, et al. Acute Korsakoff syndrome following mammillothalamic tract infarction. AJNR Am J Neuroradiol. 2004;25:964–968. [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Guo AY, van den Oord EJ, et al. Multi-species data integration and gene ranking enrich significant results in an alcoholism genome-wide association study. BMC Genomics. 2012;13 (Suppl 8):S16. doi: 10.1186/1471-2164-13-S8-S16. [DOI] [PMC free article] [PubMed] [Google Scholar]