Abstract
Patients developing posttransplant antibodies against HLA and non-HLA antigens expressed by the endothelium of the graft undergo more frequent episodes of rejection and have decreased long-term graft survival. Antibodies against the endothelium can alter/damage the cells of the graft through several mechanisms. Historically, antibodies were thought to elicit endothelial cell injury via complement-dependent mechanisms. New research has shown that antibodies can also contribute to the process of transplant rejection by stimulating proinflammatory and proproliferation signals. Antibody ligation leads to several functional alterations in EC including Weibel Palade body exocytosis, leukocyte recruitment, growth factor expression and cell proliferation. In contrast, under certain circumstances, antibodies may induce prosurvival signals and graft accommodation. The signaling events regulating accommodation vs. rejection appear to be influenced by the specificity and concentration of the anti-HLA antibody and the degree of molecular aggregation. Knowledge of the HLA and non-HLA antibody-mediated signaling pathways has the potential to identify new therapeutic targets to promote accommodation and prevent acute and chronic antibody-mediated rejection.
Keywords: Antibody-mediated rejection, endothelium, endothelial cells, signal transduction, transplantation
Introduction
Antibody-mediated rejection (AMR) remains a major obstacle to solid organ transplantation. The endothelium at the interface between the allograft and recipient blood is directly targeted by antibodies in AMR. Patients exhibiting posttransplant HLA antibodies are at higher risk for acute and chronic AMR (1). In addition, production of antibodies to non-HLA antigens expressed by endothelial cells (EC) have been implicated in AMR (2). Although many of these non-HLA antibodies remain poorly defined, alloantibodies to MICA and ABO and autoantibodies to vimentin, angiotensin II type 1 (AT1) receptor, heat shock proteins and phospholipids have been reported in association with acute and chronic AMR (2).
It is well established that antibodies can mediate EC injury through complement-dependent mechanisms (3). Emerging data indicate that antibodies also contribute to alterations in EC function through complement-independent mechanisms by transducing signals. Anti-EC antibodies transduce signals that are both proinflammatory and proproliferative, suggesting a mechanistic role in both acute and chronic allograft rejection. A definitive causal relationship between antibody-induced signaling and development of chronic rejection is still missing and represents an exciting new area for future research. In contrast, under certain conditions, antibodies to EC antigens have been shown to portend a beneficial effect on graft outcome by inducing graft accommodation through upregulation of cytoprotective proteins or complement regulatory proteins (4). The intracellular signaling events regulating accommodation vs. cell proliferation are dependent on the degree of molecular aggregation, which is determined by the specificity and concentration of the antibody (5). This novel signal transduction paradigm has particular implications for transplant outcomes in recipients producing posttransplant anti-donor antibodies. Patients with high titered anti-donor antibodies may have a greater risk for developing chronic rejection, whereas patients with low titered anti-donor antibodies may develop accommodation. This review focuses on the effects of antibodies on EC activation and accommodation emphasizing the molecular pathways through which these divergent outcomes may be regulated.
Role of Antibodies in Endothelial Cell Activation
Antibody ligation on endothelial cells activates intracellular signaling cascades
In addition to the well-established role in antigen presentation, class I molecules control cellular functions by transducing intracellular signals. We and others have shown that a substantial effect of antibody ligation of class I molecules on the surface of EC is to induce proliferation in a model relevant to transplant vasculopathy (TV). TV is a progressive vasculo-occlusive disease resulting in ischemic injury and deterioration of organ function. The histological appearance of TV shows marked proliferation and hyperplasia of vascular smooth muscle cells and ECs, suggesting that anti-HLA antibodies play a role in cell proliferation and angiogenesis. Consistent with this possibility, Coupel and our group showed engagement of class I by anti-HLA antibodies induced Rho activation, stimulated tyrosine phosphorylation of intracellular proteins, including Src, focal adhesion kinase (FAK) and paxillin (6). Class I mediated stimulation of Rho and its target protein Rho kinase induces the assembly of contractile actin in EC, a necessary step for cell survival and proliferation. Inhibition of Rho kinase suppresses neointima formation in experimental models of transplantation confirming the importance of this pathway in TV (7).
We have shown that class I induced phosphorylation of FAK and Src prompts the assembly of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2, resulting in concomitant activation of cell survival and proliferation signaling pathways (8). mTORC1 stimulates cell proliferation by activating p70 ribosomal protein S6 kinase (S6K) that promotes translation and growth by phosphorylating S6 ribosomal protein (S6RP) and 4E-BP1 protein. Inhibition of mTORC1 by siRNA or rapamycin, a widely used immunosuppressive agent, blocks class I antibody induced EC proliferation and therefore may be an effective therapy to prevent TV (9). One potential limitation of rapamycin-based therapy is that it suppresses mTORC2/Akt-inducible prosurvival signals that may promote EC apoptosis (10).
To characterize the role of MHC signaling in the pathogenesis of AMR, we developed an MHC mismatched mouse cardiac allograft model. Long-term treatment with antidonor class I antibodies was highly correlated with increased phosphorylation of S6K and S6RP in capillary EC of the graft. We found an analogous situation occurring in human AMR. Increased phosphorylation of S6RP in endomyocardial biopsies was significantly associated with circulating antibodies and diagnosis of AMR (11).
We also found that antibody ligation of class I on ECs stimulates proliferation by upregulating the expression of fibroblast growth factor receptors (FGFRs) on the cell surface in a dose-dependent fashion (6). Notably, the highest concentration of anti-class I antibodies stimulates maximal FGFR expression (5,6). Thus, the level of FGFR expression correlates with the amount of antibody bound to the EC. Upregulation of FGFR expression on the EC surface can contribute to the process of TV by increasing fibroblast growth factor (FGF) ligand binding that in turn stimulates EC proliferation via the MAPK/ERK pathway (6). Related studies have shown that anti-class I antibodies have the capacity to induce expression of FGFR1 in murine tracheal allografts (12).
Circulating anti-HLA class II antibodies have been identified as a key contributing factor to chronic rejection. Although, little is known about signal transduction in response to class II ligation on EC, recent data suggest that similar to class I, anti-class II antibodies stimulate cell proliferation by activating the S6K and S6RP pathways. Studies from our laboratory showed that production of anti-HLA class II antibody was significantly associated with allograft rejection and a positive S6RP phosphorylation status in cardiac biopsies (11). Furthermore, we confirmed treatment with class II antibodies leads to increased phosphorylation of S6RP in primary cultures of EC. Clearly, the potential of class II antibodies to promote chronic allograft via activation of signaling networks that elicit cell proliferation deserves further investigation.
Non-HLA antigens are receiving increased attention as targets of the humoral response in solid organ transplantation. Although the immune response to HLA antigens plays the central role in allograft rejection, recent reports show that alloimmune-mediated injury of the endothelium leads to the aberrant expression of self-proteins and subsequent generation of autoantibodies (13). Interestingly, the lack of evidence for complement activation by non-HLA antibodies implies that they mediate graft injury via complement-independent mechanisms. Consistent with this possibility, Dragun showed that antibodies against the AT1 receptor cause renal allograft rejection by binding to the second extracellular loop of AT1 receptor and activating intracellular signals as an allosteric receptor agonist (14). Anti-AT1 receptor antibody induces ERK phosphorylation and consequently increases DNA-binding activity of transcription factors activator protein 1 (AP-1) and nuclear factor-κB (NF-κB) that in turn increase tissue factor expression. Dragun also showed that transfer of antibodies against the AT1 receptor induced vasculopathy and hypertension in a rat-kidney transplantation model, which could be prevented using the AT1 antagonist losartan (14).
Antibody ligation on endothelial cells promotes leukocyte recruitment
The core changes of EC activation include expression of leukocyte adhesion molecules and cytokine production. Anti-EC antibodies eluted from acutely rejected allografts upregulate VCAM-1 and ICAM-1 expression in ECs, which leads to increased leukocyte adhesion (15). Yamakuchi demonstrated that treatment of EC with anti-HLA antibodies stimulates the release of von Willebrand Factor (vWF) and P-selectin by triggering calcium-mediated Weibel–Palade body exocytosis (16). The release of P-selectin in turn leads to platelet and leukocyte adherence. Similar to the effect of anti-HLA antibodies on FGFR expression, the maximum release of vWF is induced by high concentrations of anti-HLA antibodies. Crosslinking of HLA appears to be critical to stimulate exocytosis because only the bivalent F(ab’)2 of class I antibody W6/32 is effective in trigging exocytosis. The ability of anti-HLA antibodies to trigger exocytosis in vivo was further confirmed in immunodeficient/beige mice transplanted with human skin grafts. Injection with anti-class I antibodies caused release of vWF and increased the adherence of neutrophils to microvessels in skin grafts (16). Ligation of MHC class I molecules by antibodies also leads to a dose-dependent increase in the production of monocyte chemattractant protein-1 and neutrophil chemattractant growth-related oncogene α that attract macrophages to the graft (17).
In a series of papers, Rose showed that vimentin is another EC protein recognized by the sera of patients with TV (18). Vimentin, an intermediate filament protein, is mainly expressed in the intima and media of coronary arteries. Production of anti-vimentin antibody correlates with accelerated rejection in a murine cardiac transplant model. Antivimentin antibodies activate ECs by inducing expression of P-selectin on the microvessels of hearts. Anti-vimentin antibodies may also indirectly trigger EC activation by stimulating leukocytes to release platelet-activating factor and subsequent platelet activation and adherence to the endothelium.
These data imply that antibodies can contribute to the pathogenesis of AMR by activating human EC exocytosis and leukocyte trafficking. How crosslinking of HLA class I molecules is coupled to the exocytic machinery of the EC appears to be dependent upon calcium (16), which is likely activated following the generation of inositol phosphate (19). Drugs specifically targeting the exocytosis machinery may be effective in treating AMR, as well as other antibody-induced inflammatory disorders.
Role of Antibodies in Graft Accommodation
Under certain circumstances, anti-EC antibodies may be beneficial to graft outcome by promoting accommodation. Accommodation is an acquired resistance of an organ graft to antibody-mediated injury. Accommodation was first observed in ABO-incompatible renal transplants. In these patients, anti-blood group antibodies were depleted and graft accommodation was achieved despite the return of blood group antibody and high level of ABO antigen expression on the endothelium of graft (20). How antibodies promote accommodation has been extensively studied in vitro and in vivo in experimental xenotransplants where the recipients had natural antibodies specific for carbohydrate antigens on the graft. The mechanisms underlying this process are thought to involve antibody-induced expression of prosurvival and cytoprotective proteins and/or regulation of the terminal components of complement (4). Notably, similar to ABO incompatible transplants, accommodation in the setting of xenotransplantation can be induced by lowering the titer of anti-donor antibodies (21). ECs of an accommodated cardiac xenograft, but not a rejected heart, expressed the prosurvival genes A20, Bcl-2, Bcl-xL and the cytoprotective proteins hemoxygenase (HO) and nitric oxide (NO) (22). Furthermore, rejected hearts had severe TV where accommodated hearts did not. Sustained Bcl-2 expression is accompanied by inducible NO synthase generation and increased production of NO by EC (21). Recent data question whether sustained expression of protective genes is required for maintenance of accommodation. Park found no increase in HO-1, Bcl-2 and Bcl-xL expression in long-term surviving renal allografts and accommodated ABO-incompatible long-term renal allografts (23). Instead tumor necrosis factor β, regulatory protein SMAD5 and transforming growth factor β are downregulated whereas expression of the immunoregulatory protein mucin-1 and protein tyrosine kinase signaling molecule GFRA1 is increased. Thus, increased expression of cytoprotective genes may not represent the late process of accommodation.
Accommodation can also be induced by inhibiting complement activation. Wang found that blockade of complement activation by anti-C5 complement antibodies prevented AMR through inhibition of complement activation and upregulation of Bcl-2 and Bcl-xL expression in a murine model mimicking presensitized transplant recipients (24). Williams used a swine-to-baboon cardiac xenograft model and found that some grafts experienced accommodation despite the fact these grafts expressed the donor-specific epitope Galα1-3Gal at a comparable level to grafts with rejection and contained donor-specific antibodies against Galα1-3Gal (25). Additionally, the accommodated grafts had both IgM and IgG bound to large and small vessels and the amount of antibody was similar to that in the rejected grafts. The difference was that the grafts with accommodation lacked the membrane attack complex that can disrupt the vascular EC surface by lysis or cause activation of ECs. These results indicate that complement activation is inhibited in grafts with accommodation. Increased expression of heparin sulfate proteoglycan in accommodated grafts is suggested to account for the interruption of complement activation and protection from graft injury (25).
An additional mechanism of accommodation has been shown by Ding in a rat-to-mouse xenograft model in which accommodation is induced by repeated injection of low-dose anti-donor antibodies (26). Treatment with low-dose antibodies upregulates the expression of the complement regulatory protein decay accelerating actor (DAF), complement receptor-related protein (Crry) and CD59 in the graft endothelium (26). Expression of DAF, Crry and CD 59 negatively regulates complement activation and has been shown to inhibit hyperacute rejection.
Understanding how anti-ABO and anti-Gal antibodies promote accommodation is of importance in clinical transplantation. We reason that the signal cascades activated by antibodies against carbohydrate epitopes, such as ABO and Gal, are likely determined by the nature of the proteins and lipids that carry the carbohydrate epitopes. Many glycoproteins, including protein Band 3 anion transporter, platelet EC adhesion molecule 1 (PECAM1) and integrin α6, carry ABO antigens in renal endothelium (27). Thus, it is plausible that anti-ABO antibodies induce accommodation by crosslinking ABO bearing proteins that elicit survival signaling cascades. Consistent with this possibility, ligation of integrin α6 has been shown to promote cell survival (28). Furthermore, treatment with PECAM1 antibodies protects EC from serum deprivation-induced cell death (29). Moreover, protein Band 3 is coupled to the spectrin–actin cytoskeleton network through ankyrin, which functions in a wide variety of processes including cell survival (30).
Although approximately 25% of patients produce posttransplant donor-specific anti-HLA antibodies, their appearance is not always accompanied by rejection, especially if the antibody titers are low. These data link HLA antibody titer to graft outcome and suggest that like anti-ABO antibodies, low levels of anti-HLA antibodies may promote accommodation. In vitro and in vivo models exploring the role of anti-HLA antibodies in accommodation support this concept. We found that crosslinking of class I molecules with low concentrations of antibody induced cell survival/accommodation by stimulating the mTORC2 pathways (8). mTORC2 can phosphorylate Akt and Bad and promote cell survival through upregulation of the antiapoptotic protein Bcl-2. In accordance with these in vitro studies, we demonstrated increased expression of Bcl-2 on the graft endothelium in biopsy samples from heart transplant recipients with AMR. Interestingly, maximum expression of Bcl-2 and phosphorylation of Akt and Bad was seen when ECs were exposed to low titers of anti-class I antibodies. These results were corroborated by Narayanan and Salama who showed that, preexposure of EC to subsaturating concentrations of class I antibodies conferred resistance of ECs to complement mediated cell death via upregulation of Bcl-2 and Bcl-xL. Similarly, expression of Bcl-xL was upregulated in the endothelium in patients with accommodated renal allografts (31). Ex vivo treatment with subsaturating concentrations of anti-MHC antibodies upregulates antiapoptotic genes in intra islet EC and prevents hyperacute rejection of the islets when transplanted into sensitized recipients (32). These results are consistent with the idea that accommodation is an active process induced by low concentrations of anti-donor antibodies that activate the mTORC2/Akt pathway and upregulate survival proteins in the graft.
A question remains as to whether long-term accommodation can be maintained through persistent exposure of EC to anti-HLA antibodies, even if they are at a low titer. Data from mice and cynomolgus monkeys suggest that long-term exposure to anti-MHC antibodies leads to chronic allograft rejection, rather than accommodation (33,34). We have shown that cardiac allografts in a mouse model treated with anti-donor class I antibody for 15 days are defined by high levels of Akt phosphorylation and Bcl-2 expression, consistent with an accommodation phenotype. In contrast, grafts recovered on day 30 were defined by phoshorylation of proteins involved in cell proliferation. This suggests that the duration of antibody exposure contributes to differences in transplant outcome. Short-term exposure may promote activation of proteins involved in accommodation, whereas long-term exposure might activate proteins associated with cell proliferation and chronic rejection. Because ligation of HLA by antibodies triggers simultaneous activation of mTORC1 (proliferation) and mTORC2 (survival) signaling pathways, we conjecture that class I mediated enhancement of cell survival/accommodation may augment chronic rejection by allowing grafts to survive immunologic injury sufficiently long to develop chronic rejection. Phosphoproteomic analysis of signaling pathways in EC of the allograft represents a future strategy to investigate the mechanisms underlying accommodation and TV.
Therapy Implications
Inhibition of EC activation by blocking antibody-induced signal transduction may prevent AMR. This is exemplified by the blockade of the mTORC1 signaling pathway using rapamycin (9). However, prolonged exposure to rapamycin prevented assembly of mTORC2 and increased EC apoptosis (10), making the graft more susceptible to injury. Thus, it will be important to identify drugs that will selectively inhibit cell proliferation and TV while promoting accommodation. Recent studies show that blockade of complement activation by anti-C5 complement antibodies prevents AMR in an animal model (24). Ecluzimab is a humanized monoclonal antibody that prevents activation of C5 and the formation of the membrane attack complex, which may promote accommodation (35). The PKC inhibitor AEB071 (Sotrastaurin) may reduce antibody-induced leukocyte and platelet recruitment by blocking calcium-dependent Weibel–Palade body exocytosis and vWF and P-selectin expression on EC (36,37).
Conclusions
The findings presented herein demonstrate that antibodies against the endothelium play an important role in transplant outcome. High concentrations of anti-HLA antibodies may have a detrimental effect on graft survival by inducing surface expression of FGFR and stimulating release of vWF and externalization of P-selectin. In addition, ligation of class I molecules stimulates cell proliferation by increasing mTORC1 complex formation and activating the S6K signaling pathway. Antibodies against AT1 receptor or vimentin trigger intracellular cascades that also can result in TV (Figure 1). On the other hand, lower concentrations of anti-EC antibodies may play an important role in accommodation by promoting cell survival through upregulation of cytoprotective and antiapoptotic proteins (Figure 2). Accommodation may also be achieved by blockade of complement activation and/or upregulation of the complement regulatory proteins DAF, Crry and CD59 in the graft endothelium (Figure 2). Elucidation of the signaling cascades elicited by anti-EC antibodies holds promise to identify novel therapeutic strategies to prevent acute and chronic AMR.
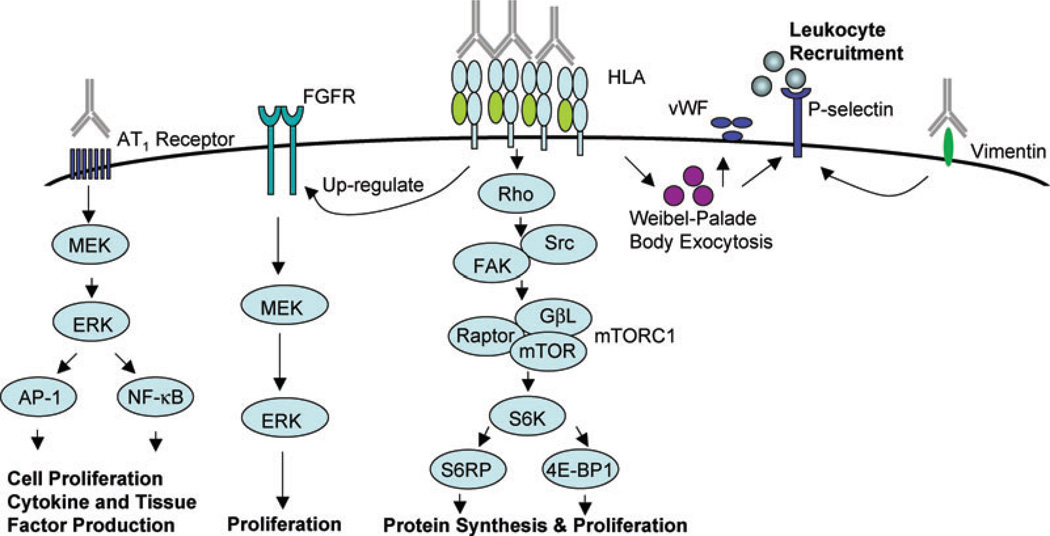
Figure 1. Signaling pathways regulating antibody-mediated activation of endothelial cells.
Ligation of HLA molecules by high titers of anti-HLA antibodies stimulates EC proliferation by upregulating fibroblast growth factor receptor (FGFR) expression, increasing FGF ligand binding and activating the ERK signaling pathway. Antibody ligation of class I molecules also triggers activation of mammalian target of rapamycin complex 1 (mTORC1) and phosphorylation of downstream targets S6 kinase (S6K) and S6 ribosomal protein (S6RP), resulting in protein synthesis and cell proliferation. Antibodies to HLA antigens and vimentin promote platelet and leukocyte recruitment by triggering exocytosis of von Willebrand factor (vWF) and externalization of P-selectin. Anti-angiotensin II type 1 (AT1) receptor antibodies transduce signals activating ERK and the transcription factors activator protein 1 (AP-1) and nuclear factor-κB (NF-κB), resulting in increased expression of proinflammatory cytokines, procoagulatory genes and cell proliferation.
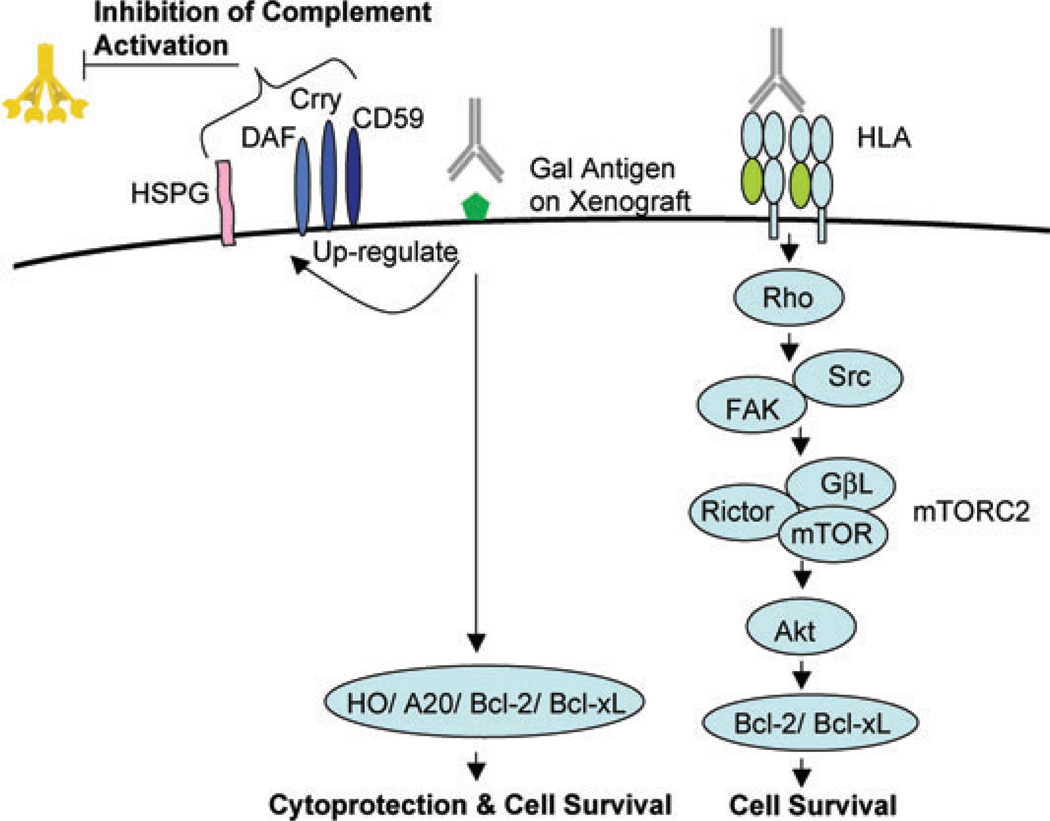
Figure 2. Signaling pathways regulating antibody-mediated survival and accommodation in endothelial cells.
Ligation of HLA molecules by low titers of anti-HLA antibodies activates Rho and Rho kinase and stimulates phosphorylation of Src and focal adhesion kinase (FAK) followed by downstream activation of the mammalian target of rapamycin complex 2 (mTORC2) pathway culminating in increased expression of cell survival proteins. The binding of anti-Gal antibodies to the endothelium of the graft induces expression of the cytoprotective proteins A20, HO and nitric oxide and the survival proteins Bcl-2 and Bcl-xL. Anti-Gal antibodies also promote graft survival by upregulating expression of heparin sulfate proteoglycan (HSPG) and the complement regulatory proteins decay accelerating actor (DAF), complement receptor-related protein (Crry) and CD59 in the graft endothelium.
Acknowledgments
Funding Sources: This work was supported by the National Institute of Allergy and Infectious Diseases Grant RO1 AI 42819 and NIH U01AI077821 and the National Heart Lung and Blood Institute Grant RO1 HL 090995 to E.F.R.
References
- 1.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: A prospective trial. Am J Transplant. 2004;4:438–443. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 2.Sumitran-Holgersson S. Relevance of MICA and other non-HLA antibodies in clinical transplantation. Curr Opin Immunol. 2008;20:607–613. doi: 10.1016/j.coi.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Wehner J, Morrell CN, Reynolds T, Rodriguez ER, Baldwin WM., III Antibody and complement in transplant vasculopathy. Circ Res. 2007;100:191–203. doi: 10.1161/01.RES.0000255032.33661.88. [DOI] [PubMed] [Google Scholar]
- 4.Tang AH, Platt JL. Accommodation of grafts: Implications for health and disease. Hum Immunol. 2007;68:645–651. doi: 10.1016/j.humimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jindra PT, Zhang X, Mulder A, et al. Anti-HLA antibodies can induce endothelial cell survival or proliferation depending on their concentration. Transplantation. 2006;82:S33–S35. doi: 10.1097/01.tp.0000231447.34240.3c. [DOI] [PubMed] [Google Scholar]
- 6.Jin YP, Korin Y, Zhang X, Jindra PT, Rozengurt E, Reed EF. RNA interference elucidates the role of focal adhesion kinase in HLA class I-mediated focal adhesion complex formation and proliferation in human endothelial cells. J Immunol. 2007;178:7911–7922. doi: 10.4049/jimmunol.178.12.7911. [DOI] [PubMed] [Google Scholar]
- 7.Hattori T, Shimokawa H, Higashi M, et al. Long-term treatment with a specific Rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94:46–52. doi: 10.1161/01.RES.0000107196.21335.2B. [DOI] [PubMed] [Google Scholar]
- 8.Jindra PT, Jin Y-P, Rozengurt E, Reed EF. HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J Immunol. 2008;180:2357–2366. doi: 10.4049/jimmunol.180.4.2357. [DOI] [PubMed] [Google Scholar]
- 9.Delgado JF, Manito N, Segovia J, et al. The use of proliferation signal inhibitors in the prevention and treatment of allograft vasculopathy in heart transplantation. Transplant Rev (Orlando) 2009;23:69–79. doi: 10.1016/j.trre.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Dormond O, Madsen JC, Briscoe DM. The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem. 2007;282:23679–23686. doi: 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepin EJ, Zhang Q, Zhang X, et al. Phosphorylated S6 ribosomal protein: A novel biomarker of antibody-mediated rejection in heart allografts. Am J Transplant. 2006;6:1560–1571. doi: 10.1111/j.1600-6143.2006.01355.x. [DOI] [PubMed] [Google Scholar]
- 12.Maruyama T, Jaramillo A, Narayanan K, Higuchi T, Mohanakumar T. Induction of obliterative airway disease by anti-HLA class I antibodies. Am J Transplant. 2005;5:2126–2134. doi: 10.1111/j.1600-6143.2005.00999.x. [DOI] [PubMed] [Google Scholar]
- 13.Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I induce autoimmunity: Role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragun D, Muller DN, Brasen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 15.Lucchiari N, Panajotopoulos N, Xu C, et al. Antibodies eluted from acutely rejected renal allografts bind to and activate human endothelial cells. Hum Immunol. 2000;61:518–527. doi: 10.1016/s0198-8859(00)00109-9. [DOI] [PubMed] [Google Scholar]
- 16.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, et al. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci USA. 2007;104:1301–1306. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CY, Lotfi-Emran S, Erdinc M, et al. The involvement of FcR mechanisms in antibody-mediated rejection. Transplantation. 2007;84:1324–1334. doi: 10.1097/01.tp.0000287457.54761.53. [DOI] [PubMed] [Google Scholar]
- 18.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–892. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 19.Bian H, Harris PE, Mulder A, Reed EF. Anti-HLA antibody ligation to HLA class Imolecules expressed by endothelial cells stimulates tyrosine phosphorylation, inositol phosphate generation, and proliferation. Hum Immunol. 1997;53:90–97. doi: 10.1016/S0198-8859(96)00272-8. [DOI] [PubMed] [Google Scholar]
- 20.Chopek MW, Simmons RL, Platt JL. ABO-incompatible kidney transplantation: Initial immunopathologic evaluation. Transplant Proc. 1987;19:4553–4557. [PubMed] [Google Scholar]
- 21.Delikouras A, Hayes M, Malde P, Lechler RI, Dorling A. Nitric oxide-mediated expression of Bcl-2 and Bcl-xl and protection from tumor necrosis factor-alpha-mediated apoptosis in porcine endothelial cells after exposure to low concentrations of xenoreactive natural antibody. Transplantation. 2001;71:599–605. doi: 10.1097/00007890-200103150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Bach FH, Ferran C, Hechenleitner P, et al. Accommodation of vascularized xenografts: Expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997;3:196–204. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- 23.Park WD, Grande JP, Ninova D, et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003;3:952–960. doi: 10.1034/j.1600-6143.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Arp J, Liu W, et al. Inhibition of terminal complement components in presensitized transplant recipients prevents antibody-mediated rejection leading to long-term graft survival and accommodation. J Immunol. 2007;179:4451–4463. doi: 10.4049/jimmunol.179.7.4451. [DOI] [PubMed] [Google Scholar]
- 25.Williams JM, Holzknecht ZE, Plummer TB, Lin SS, Brunn GJ, Platt JL. Acute vascular rejection and accommodation: Divergent outcomes of the humoral response to organ transplantation. Transplantation. 2004;78:1471–1478. doi: 10.1097/01.tp.0000140770.81537.64. [DOI] [PubMed] [Google Scholar]
- 26.Ding JW, Zhou T, Ma L, et al. Expression of complement regulatory proteins in accommodated xenografts induced by anti-alpha-Gal IgG1 in a rat-to-mouse model. Am J Transplant. 2008;8:32–40. doi: 10.1111/j.1600-6143.2007.02016.x. [DOI] [PubMed] [Google Scholar]
- 27.Tasaki M, Yoshida Y, Miyamoto M, et al. Identification and characterization of major proteins carrying ABO blood group antigens in the human kidney. Transplantation. 2009;87:1125–1133. doi: 10.1097/TP.0b013e31819e0054. [DOI] [PubMed] [Google Scholar]
- 28.Farrelly N, Lee YJ, Oliver J, Dive C, Streuli CH. Extracellular matrix regulates apoptosis in mammary epithelium through a control on insulin signaling. J Cell Biol. 1999;144:1337–1348. doi: 10.1083/jcb.144.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: New roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 30.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 31.Salama AD, Delikouras A, Pusey CD, et al. Transplant accommodation in highly sensitized patients: A potential role for Bcl-xL and alloantibody. Am J Transplant. 2001;1:260–269. doi: 10.1034/j.1600-6143.2001.001003260.x. [DOI] [PubMed] [Google Scholar]
- 32.Bharat A, Saini D, Benshoff N, et al. Role of intra-islet endothelial cells in islet allo-immunity. Transplantation. 2007;84:1316–1323. doi: 10.1097/01.tp.0000288192.11396.70. [DOI] [PubMed] [Google Scholar]
- 33.Uehara S, Chase CM, Cornell LD, Madsen JC, Russell PS, Colvin RB. Chronic cardiac transplant arteriopathy inmice: Relationship of alloantibody, C4d deposition and neointimal fibrosis. Am J Transplant. 2007;7:57–65. doi: 10.1111/j.1600-6143.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith RN, Kawai T, Boskovic S, et al. Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in Cynomolgus monkeys. Am J Transplant. 2008;8:1662–1672. doi: 10.1111/j.1600-6143.2008.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locke JE, Magro CM, Singer AL, et al. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9:231–235. doi: 10.1111/j.1600-6143.2008.02451.x. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzi O, Frieden M, Villemin P, Fournier M, Foti M, Vischer UM. Protein kinase C-delta mediates von Willebrand factor secretion from endothelial cells in response to vascular endothelial growth factor (VEGF) but not histamine. J Thromb Haemost. 2008;6:1962–1969. doi: 10.1111/j.1538-7836.2008.03138.x. [DOI] [PubMed] [Google Scholar]
- 37.Vincenti F, Kirk AD. What’s next in the pipeline. Am J Transplant. 2008;8:1972–1981. doi: 10.1111/j.1600-6143.2008.02403.x. [DOI] [PubMed] [Google Scholar]