Abstract
A blood cell type termed crystal cell in Drosophila functions in clotting and wound healing and requires Notch for specification and maintenance. We report that crystal cells express elevated levels of Sima protein orthologous to mammalian hypoxia-inducible factor–α (Hif-α) even under conditions of normal oxygen availability. In these platelet-like crystal cells, Sima activates full-length Notch receptor signaling via a noncanonical, ligand-independent mechanism that promotes hemocyte survival during both normal hematopoietic development and hypoxic stress. This interaction initiates in early endosomes, is independent of Hif-β (Tango in Drosophila), and does not activate hypoxia response targets. Studies in vertebrate myeloid cells have shown a similar up-regulation of Hif-α protein in well-oxygenated environments. This study provides a mechanistic paradigm for Hif-α/Notch interaction that may be conserved in mammals.
The Drosophila lymph gland (hematopoietic organ) gives rise to myeloid blood cells: plasmatocytes, crystal cells, and lamellocytes (1). A majority of these cells are macrophages; a small fraction become crystal cells that function during wound healing (2, 3). Hemolectin (Hml) is the earliest marker expressed as cells initiate differentiation. The Runt-domain protein Lozenge (Lz) (4, 5) is essential for commitment to crystal-cell fate (6), and its expression initiates in these Hml+ precursors, which then discontinue Hml expression (6). Mature crystal cells express prophenol oxidase (ProPO), essential for melanization (5), whereas macrophages remain Hml+.
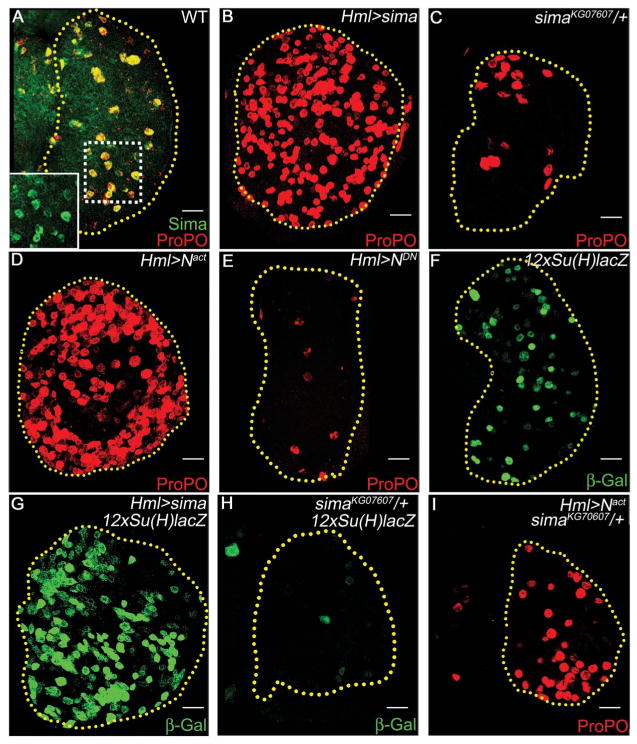
Sima is the Drosophila ortholog of hypoxia-inducible factor–α [Hif-α (7)], the key mediator of responses to hypoxia (8). Sima is stably expressed in mature crystal cells even under normal oxygen tension (Fig. 1A). Overexpression of sima in the lymph gland causes dramatic expansion of crystal cells (Fig. 1B and fig. S1A), whereas single-copy loss of sima reduces their number (Fig. 1C and fig. S1A). These phenotypes are similar to phenotypes resulting from Notch gain and loss of function (5) (Fig. 1, D and E, and fig. S1A). Furthermore, altering Sima can change Notch reporter [12xSu(H)lacZ] expression (Fig. 1, F to H, and fig. S1B), and a single mutant allele of sima suppresses the excess crystal-cell phenotype caused by Notchactivated (N act) (Fig. 1I and fig. S1A). Thus, Sima and Notch signaling appear to function in the same pathway in this tissue.
Fig. 1.
Sima functions with Notch during crystal-cell development. Crystal cells marked with ProPO (red). Scale bars indicate 20 μm. (A) Wild-type lymph gland, crystal cells show elevated levels of Sima (green; yellow because of ProPO co-localization). (Inset) Magnified view of crystal cells expressing Sima (green). (B) Overexpression or (C) single-copy loss of sima causes crystal-cell expansion or reduction, respectively. (D) Overexpression of activated Notch (Nact) that functions as a gain of function Notch or (E) dominant-negative Notch (NDN) that functions as a loss of function Notch causes crystal-cell expansion and reduction, respectively. (F) Wild-type Notch reporter activity [12xSu(H)-lacZ, green] (G) increases with sima gain of function and (H) decreases with single-copy loss of sima. β-Gal, β-galactosidase. (I) Single-copy loss of sima suppresses Nact-driven crystal-cell expansion. Compare with (D).
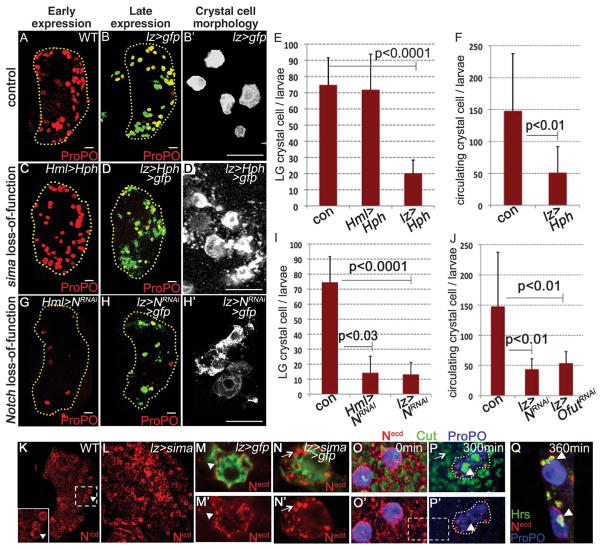
We examined the temporal requirement of Sima during crystal-cell development. Hph (hydroxy prolyl hydroxylase), which marks Sima for degradation (9), or sima RNA interference (simaRNAi) was expressed in Hml+ precursors by using Hml-gal4 as the driver that is switched off once a cell becomes Lz+ (10). Neither has any effect on crystal-cell number (Fig. 2, A, C, and E, and fig. S1C). However, expression of Hph or simaRNAi later in Lz+ crystal-cell precursors causes a significant reduction in crystal-cell number (Fig. 2, B and D to F, and fig. S1C, P < 0.0001). This loss is associated with bursting (compare Fig. 2, D′ with B′) of these cells, visualized by membrane green fluorescent protein (GFP) expression, a phenomenon (3) important for crystal cell–mediated blood clotting because of release of enzymes (3).
Fig. 2.
Sima stabilizes Notch in mature crystal cells, which is necessary for their maintenance and survival. Crystal cells marked with ProPO (red). Scale bars, 20 μm. (A) Wild-type (WT) lymph gland. (B) Control lymph glands expressing membrane GFP in crystal cells. Magnified in (B′). Overexpression of Hph (C) early does not affect crystal cells [compare with (A)]; (D) late expression in crystal cells causes their reduction and (D′) rupturing [compared with (B′)]. Quantification of (E) lymph gland (n = 8) and (F) circulating crystal-cell (n = 8) data. Error bars indicate standard deviation. Expressing NRNAi (G) early and (H) late causes reduction in crystal cells [compared with (A) and (B)]. Loss of Notch late from crystal cells causes their rupturing [(H′) similar to (D′) compared with (B′)]. Quantification of (I) lymph gland (n = 12) data and (J) circulating crystal-cell data expressing NRNAi (n = 8) and OfutRNAi (n = 8). (K) Wild-type lymph glands with elevated Notch protein [Notch intracellular domain (Nicd), red] in crystal cells (arrowheads, magnified in inset). (L) sima overexpression causes further Notch (Nicd, red) accumulation. Antibody against the extracellular domain of Notch (Necd, red) antibody detects (M and M′) Notch in vesicles (arrowheads) in crystal cells from control lymph glands expressing GFP and (N and N′) overexpressing sima further increases Notch (Necd, red) accumulation in vesicles (arrows, compare with M and M′). (O to Q) Live endocytic trafficking assay: Notch antibody-recognizing epitope on Necd (red) in wild-type lymph glands marked with nuclear (Cut, green) and crystal-cell (ProPO, blue) markers. (O and O′) Notch protein is detected on all membranes at 0 min. (P) and (P′) At 300 min, endocytosed Notch is degraded from surrounding cells [compare Necd levels in the dashed areas in (O′) and (P′)] except in crystal cells (arrowheads) (Q) that retain Notch (red) in Hrs-positive (green, arrowheads) early endosomes. Necd (red) and Hrs (green) co-localize (yellow).
Crystal cell–fate specification requires canonical Notch signaling (6, 11). Expressing NotchRNAi in early differentiating Hml+ cells causes loss of crystal cells (Fig. 2, G and I). Additionally, late loss of Notch from already-specified crystal-cell precursors by either expressing NRNAi or OfutRNAi [modification of Notch by Ofut is required for proper Notch function (12)] causes a bursting phenotype (Fig. 2, H to J) as seen with loss of Sima (Fig. 2D′). Thus, Notch function is required continuously: first in specifying the Lz+ precursor and then in expansion and maintenance of crystal cells.
High endogenous Notch expression in crystal cells (Fig. 2K) is further increased upon Sima overexpression (Fig. 2L), without change in Notch RNA (fig. S2, A to C). The majority of Notch protein in crystal cells is seen in intracellular vesicles (Fig. 2, M and N). In live trafficking assays (13), surface-labeled Notch internalized from the plasma membrane is rapidly degraded in all cells (Fig. 2, O and P) except in mature crystal cells, where Notch persists in Hepatocyte growth factor regulated tyrosine kinase substrate (Hrs)–positive early endocytic vesicles (Fig. 2, P and Q) and co-localizes with Sima (fig. S2, D to D‴).
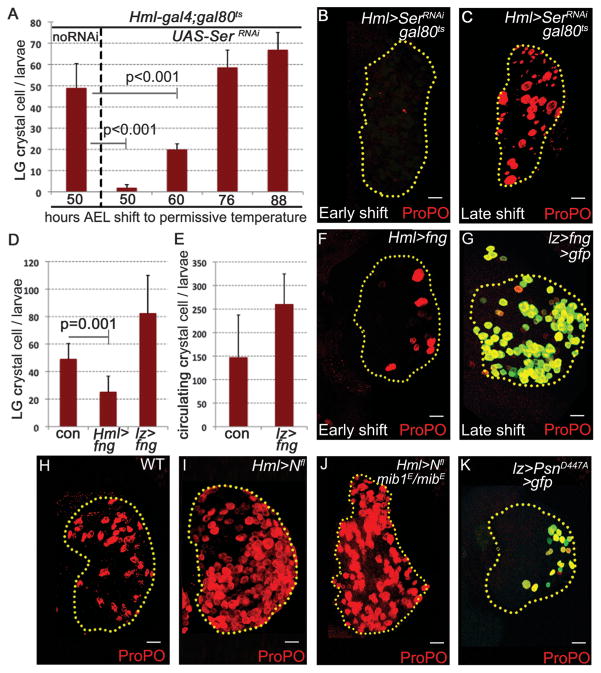
Canonical Notch signaling requires the interaction of Notch with its membrane-bound ligand on an adjacent signaling cell (14, 15). Crystal cell–fate specification requires ligand-Serrate (Ser) (6, 11), but at late stages Ser is expressed in a restricted number of cells (6), not adjacent to a Ser-positive cell. This issue is even more acute for individualized circulating crystal cells, not in direct contact with a neighbor, that continue to require Notch function for their maintenance (Fig. 2J). The circulating crystal cells are of embryonic origin (16) and are not derived from the larval lymph gland (17). The other Notch ligand, Delta, lacks any obvious role in crystal-cell development (6, 11) (fig. S2E). Removal of Ser early prevents crystal cell–fate specification (Fig. 3, A and B), but unlike Notch and Sima, removing Ser after crystal cell–fate specification does not affect crystal-cell number or morphology (Fig. 3, A and C), suggesting that Sima-mediated Notch activation occurs independent of its ligand.
Fig. 3.
Sima promotes ligand-independent stabilization of full-length Notch protein for crystal-cell maintenance. (A to C) SerRNAi in signaling cells affects crystal cell [(A) and (B)] early [n = 5, 50, and 60 hours after egg laying (AEL)] but not [(A) and (C)] late (n = 5, 76 and 88 hours AEL). Crystal cells marked with ProPO (red). Scale bars, 20 μm. (D to F) fng overexpression [(D and (F)] early in signaling cells (n = 7) causes crystal-cell reduction but not [(D), (E), and (G)] late (n = 4). (H) Wild-type lymph gland. (I) Overexpressing Nfl increases crystal cells [compare with (H)]. (J) Expressing Nfl in mib1 background shows no reduction in crystal cells [compare with (I) and (H)]. (K) Expressing dominant-negative presenilin (PsnD447A) in crystal cells causes reduction.
We addressed the question of ligand independence by using additional genetic criteria. Over-expression of Fng, a glycosyl transferase that inhibits Ser-Notch signaling (18) by using Hml-gal4, dramatically reduces crystal-cell number (Fig. 3, D and F), whereas late expression using lz-gal4 has no effect (Fig. 3, D, E, and G). Mib1 (19) and neuralized (20), which encode E3 ubiquitin ligases necessary for ligand endocytosis and promotion of ligand-dependent Notch signaling, have no role in crystal-cell development (fig. S2, F to I). We conclude that in Lz+ cells, Notch activation is Ser-independent but Sima-dependent for its intracellular stabilization.
Full-length Notch (Nfl) can accumulate in endocytic vesicles in a ligand-independent manner (13, 21). This is also seen in hemocytes expressing Sima (fig. S3A), and Nfl is sufficient to increase crystal-cell number (Fig. 3I). This increase is not suppressed by mib1 (Fig. 3J), but Rab5 co-expression, expected to enhance turnover of endocytic Nfl (22), causes strong suppression of Sima excess crystal-cell phenotype (fig. S3, B to D), confirming the involvement of endocytic vesicles in Nfl/Sima interaction. Although ligand-independent, the signaling entity is still cleaved Notch because expressing a dominant-negative form of presenilin (PsnD447A, Fig. 3K) necessary for S3 cleavage of Notch (23) or feeding larvae with a γ-secretase inhibitor, DAPT {N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester} (fig. S3E), causes bursting of crystal cells. Also, expressing a dominant-negative form of Su(H) in Lz+ cells causes bursting of crystal cells (fig. S2J).
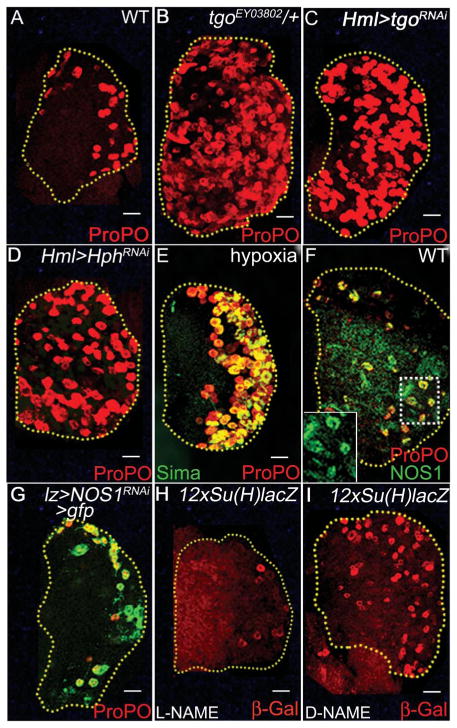
In the context of hypoxia, Hif-α (Sima) functions as a heterodimer with Hif-β [Tango in Drosophila (24)]. However, tgo mutants or tgoRNAi do not cause reduction but increase in crystal cells (Fig. 4, A to C). Crystal cells do not express the Sima/Tgo heterodimer-dependent hypoxia reporter (24) in wild-type (fig. S3F) and in Sima overexpression (fig. S3G) backgrounds. We hypothesize that in this context Tgo functions as a cytosolic sink to sequester Sima and prevents it from interacting with Notch.
Fig. 4.
Sima function in mature crystal cells is independent of Tgo. ProPO (red) marks crystal cells (red). Scale bars, 20 μm. (A) Wild-type lymph gland. (B) Single-copy loss of tgoEY03802 or (C) expressing tgoRNAi causes an increase in crystal cells. (D) Expressing HphRNAi or (E) exposing second instar wild-type larvae to 5% hypoxic stress increases Sima (green) stabilization and crystal-cell expansion. (F) Crystal cells from third instar WT lymph glands show elevated NOS1 (green, yellow because of overlap with ProPO; see inset). (G) NOS1RNAi in crystal cells causes bursting [compare with (A)]. (H and I) Feeding larvae with (H) L-NAME (NO inhibitor) shows reduction in Notch reporter activity (red), whereas (I) D-NAME (inactive isomer) has no effect.
Although normoxic Sima/Notch function does not require Tgo, the system responds to hypoxia by coopting the same developmental strategy. Conditions that stabilize Sima from degradation, such as blocking Hph (Fig. 4D) or exposing larvae to hypoxic stress (Fig. 4E), are sufficient to mediate an expansion of crystal cells. Under these conditions, the hypoxia-stabilized Sima further enhances Notch signaling independent of Tgo, causing an expansion of crystal cells.
Stimuli reported to stabilize Hif-α under normoxia include nitric oxide (NO) (25), reactive oxygen species (ROS) (26), and cations (27) that inhibit Hph (27) or stabilize Sima (28). Mature crystal cells express high levels of nitric oxide synthase1 (NOS1) (Fig. 4F), and expressing NOS1RNAi causes bursting of crystal cells (Fig. 4G). Additionally, feeding larvae NO inhibitor [L-NAME (NG-nitro-L-arginine methyl ester)] diminishes Notch reporter expression (Fig. 4, H compared to I). Lastly, clones expressing NOS1RNAi have low Sima protein and do not form crystal cells (fig. S3, H and H′). Thus, maturing crystal cells express NOS1, which raises NO level, stabilizing Sima, which then functions with Notch to promote crystal cell maintenance and survival (fig. S4).
Earlier biochemical studies have established direct binding of Notch to Hif-α (29). Here, we describe a hypoxia and Notch ligand-independent developmental role for such an interaction. Normally a cell requiring continuous Notch signal needs to be in constant contact with a ligand-bearing cell, and a circulating cell will be unable to maintain active Notch signal. Crystal cells circumvent this need through noncanonical Notch activation via stabilization of Nfl receptor in the endocytic pathway mediated by Hif-α, even in the absence of ligand binding. Once stabilized, cleavage by presinilin creates an active signaling moiety, likely to be a Nact/Hif-α/Su(H) complex essential for crystal-cell maintenance. The Sima/Notch-dependent phase is a two-step process: the first is an expansion of Hml− Lz+ crystal-cell precursors, followed by maintenance of Lz+ ProPO+ mature crystal cells (see fig. S4 for a summary of all the data presented here). Circulating crystal cells in the larvae originate from the embryonic mesoderm (16) and not the lymph gland (17), and they show a similar Sima-mediated Notch requirement for their maintenance. Circulating Tcells also require Notch function to respond to pathogens (30) and express elevated levels of Hif-α (31). The source of ligand is the antigen-presenting cell, but additional alternate mechanisms are worth investigating.
Sima stabilization is important both for normal development and during response to hypoxia. A similar dual use of a signaling scenario is seen with ROS (32). At low levels, ROS functions as a signaling molecule in the stemlike progenitors, and scavenging ROS retards their differentiation. Under oxidative stress, the sensitized progenitors differentiate rapidly (32). Thus, the Drosophila myeloid system makes dual use of the same ROS/c-Jun N-terminal kinase and Sima/Notch signaling pathways for development and stress responses. Interestingly, vertebrate myeloid cells also maintain high Hif-α in normoxic environments to maintain their cellular energy pools and ability to mount an inflammatory response (33). Because Drosophila hemocytes are functionally most similar to mammalian myeloid cells, the concepts presented here are worthy of further investigation in mammalian systems.
Supplementary Material
Acknowledgments
We thank H.Bellen, C. Frei, S. Artavanis-Tsakonas, C. Delidakis, M. Guo, M. Regulski, B. Shilo, G. Struhl, P. Wappner, National Institute of Genetics, Vienna Drosophila RNAi Center, Flybase, and Bloomington for reagents and D. Walker, G. Weinmaster, and C. Evans for helpful discussion. Supported by NIH grant R01HL067395.
Footnotes
References and Notes
- 1.Jung SH, Evans CJ, Uemura C, Banerjee U. Development. 2005;132:2521. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- 2.Rizki RR, Grell TM. Roux’s Arch Dev Biol. 1980;188:91. doi: 10.1007/BF00848799. [DOI] [PubMed] [Google Scholar]
- 3.Bidla G, Dushay MS, Theopold U. J Cell Sci. 2007;120:1209. doi: 10.1242/jcs.03420. [DOI] [PubMed] [Google Scholar]
- 4.Rizki RR. Genetics. 1981;97:s90. [Google Scholar]
- 5.Lebestky T, Chang T, Hartenstein V, Banerjee U. Science. 2000;288:146. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- 6.Lebestky T, Jung SH, Banerjee U. Genes Dev. 2003;17:348. doi: 10.1101/gad.1052803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacon NC, et al. Biochem Biophys Res Commun. 1998;249:811. doi: 10.1006/bbrc.1998.9234. [DOI] [PubMed] [Google Scholar]
- 8.Kasivisvanathan V, et al. Curr Vasc Pharmacol. 2011;3:333. doi: 10.2174/157016111795495602. [DOI] [PubMed] [Google Scholar]
- 9.Centanin L, Ratcliffe PJ, Wappner P. EMBO Rep. 2005;6:1070. doi: 10.1038/sj.embor.7400528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Materials and methods are available as supporting material on Science Online.
- 11.Duvic B, Hoffmann JA, Meister M, Royet J. Curr Biol. 2002;12:1923. doi: 10.1016/s0960-9822(02)01297-6. [DOI] [PubMed] [Google Scholar]
- 12.Okajima T, Xu A, Irvine KD. J Biol Chem. 2003;278:42340. doi: 10.1074/jbc.M308687200. [DOI] [PubMed] [Google Scholar]
- 13.Vaccari T, Bilder D. Dev Cell. 2005;9:687. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Artavanis-Tsakonas S, Rand MD, Lake RJ. Science. 1999;284:770. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 15.Hansson EM, Lendahl U, Chapman G. Semin Cancer Biol. 2004;14:320. doi: 10.1016/j.semcancer.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 16.vans CJE, Hartenstein V, Banerjee U. Dev Cell. 2003;5:673. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 17.Márkus R, et al. Proc Natl Acad Sci USA. 2009;106:4805. doi: 10.1073/pnas.0801766106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panin VM, Papayannopoulos V, Wilson R, Irvine KD. Nature. 1997;387:908. doi: 10.1038/43191. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Struhl G. Development. 2005;132:2883. doi: 10.1242/dev.01860. [DOI] [PubMed] [Google Scholar]
- 20.Pitsouli C, Delidakis C. Development. 2005;132:4041. doi: 10.1242/dev.01979. [DOI] [PubMed] [Google Scholar]
- 21.Moberg KH, Schelble S, Burdick SK, Hariharan IK. Dev Cell. 2005;9:699. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Jaekel R, Klein T. Dev Cell. 2006;11:655. doi: 10.1016/j.devcel.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Struhl G, Greenwald I. Proc Natl Acad Sci USA. 2001;98:229. doi: 10.1073/pnas.011530298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavista-Llanos S, et al. Mol Cell Biol. 2002;22:6842. doi: 10.1128/MCB.22.19.6842-6853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandau KB, Fandrey J, Brüne B. Blood. 2001;97:1009. doi: 10.1182/blood.v97.4.1009. [DOI] [PubMed] [Google Scholar]
- 26.Gerald D, et al. Cell. 2004;118:781. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Schofield CJ, Ratcliffe PJ. Biochem Biophys Res Commun. 2005;338:617. doi: 10.1016/j.bbrc.2005.08.111. [DOI] [PubMed] [Google Scholar]
- 28.Li F, et al. Mol Cell. 2007;26:63. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafsson MV, et al. Dev Cell. 2005;9:617. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald HR, Wilson A, Radtke F. Trends Immunol. 2001;22:155. doi: 10.1016/s1471-4906(00)01828-7. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura H, et al. J Immunol. 2005;174:7592. doi: 10.4049/jimmunol.174.12.7592. [DOI] [PubMed] [Google Scholar]
- 32.Owusu-Ansah E, Banerjee U. Nature. 2009;461:537. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cramer T, et al. Cell. 2003;112:645. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.