Abstract
Aging is associated with increasing predisposition to multiple chronic diseases. One fundamental aging process that is often operative at sites of the pathology underlying chronic age-related diseases is cellular senescence. Small molecule senolytic agents are being developed. For successful drug development: 1) appropriate animal models of human age-related diseases need to be devised. 2) Models have to be made in which it can be proven that beneficial phenotypic effects are actually caused through clearing senescent cells by putative senolytic agents, as opposed to “off-target” effects of these agents on non-senescent cells. 3) Models are needed to test efficacy of drugs and to uncover potential side effects of senolytic agents. Development of the optimal animal models and clinical trial paradigms for senolytic agents warrants an intensive effort, since senolytic agents, if successful in delaying, preventing, alleviating, or reversing age-related diseases as a group would be transformative.
Keywords: cellular senescence, senolytic, senescence-associated secretory phenotype, healthspan
1. Introduction
Aging is associated with increasing predisposition to multiple chronic diseases: atherosclerosis, cancers, dementias, diabetes, arthritis, and many others (Goldman et al., 2013; Kirkland, 2013a; Kirkland, 2013b; Research, 2012). Chronological age is the biggest risk factor for many of these diseases and in some cases is a better predictor than all other known risk factors combined. One fundamental aging process that is often operative at sites of pathology underlying chronic age-related diseases is cellular senescence (Kirkland, 2013a; Tchkonia et al., 2013).
Cellular senescence refers to the essentially irreversible cell cycle arrest caused by potentially oncogenic and metabolic insults (Tchkonia et al., 2013). Senescent cells can acquire a senescence-associated secretory phenotype (SASP) that involves release of pro-inflammatory cytokines, chemokines, pro-thrombotic factors, and extracellular matrix proteases that cause tissue damage, as well as extracellular matrix proteins that can contribute to dysfunctional tissue architecture or fibrosis. Thus, the adverse pathogenic mechanisms at the tissue level that could be promoted by cellular senescence include chronic inflammation, loss of functional progenitor cells, clotting, and extracellular matrix dysfunction.
Senescent cells accumulate in multiple tissues with advancing age (Tchkonia et al., 2013; Waaijer et al., 2012). Senescent cell burden is, in turn, associated with lifespan. At 18 months of age, long-lived Ames dwarf, Snell dwarf, and growth hormone receptor knockout (GHRKO) mice have fewer senescent cells in their fat tissue than age-matched control wild-type animals, while short lived growth hormone over-expressing mice have more (Stout et al., 2014). Caloric restriction sufficient to increase lifespan in mice is associated with decreased expression of p16Ink4a, a senescence marker, in multiple tissues compared to ad libitum-fed animals (Krishnamurthy et al., 2004). Progeroid mice, including mouse models of Werner's and Hutchinson-Guilford progerias, as well as Klotho-deficient, Ercc−/−, and BubR1H/H mice have increased senescent cells (Baker et al., 2008; Chen et al., 2013; Eren et al., 2014b; Tchkonia et al., 2013). In comparisons across longer- vs. short-lived mouse cohorts, senescent cell accumulation in liver and intestinal crypts predicts mean and maximum lifespan (Jurk et al., 2014). Cellular senescence can occur at any point during life, even in blastocysts (Meuter et al., 2014) and in the placenta (Rajagopalan et al., 2012). Indeed, senescence is important in remodeling during embryogenesis (Munoz-Espin et al., 2013; Storer et al., 2013).
These associations between cellular senescence, aging, and age-related pathologies prompted testing if senescent cell clearance ameliorates dysfunction. Genetically targeting senescent cells in INK-ATTAC;BubR1H/H progeroid mice that express a drug-activatable “suicide” gene only in senescent cells enhanced healthspan (Baker et al., 2011), the portion of the lifespan during which freedom from pain, disability, and dependence is enjoyed (Kirkland et al., 2009). Even clearing only around 30 percent of senescent cells from these mice led to partial reversal of age-related lipodystrophy and decreased progression of frailty, sarcopenia, and cataract formation (Baker et al., 2011; Tchkonia et al., 2013).
These findings have spurred development of small molecule senolytic agents and other approaches to decrease senescent cell burden, including peptides, RNA interference, and vaccines. For this effort to succeed: 1) appropriate animal models of human age-related diseases need to be developed. 2) Models have to be made to prove that beneficial effects are actually caused through clearing senescent cells by putative senolytic agents. Without this proof, it would be possible that the candidate agent leads to senescent cell clearance, but that phenotypic improvement is due to “off-target” effects on non-senescent cells, not directly through senescent cell clearance. 3) Models are needed in which possible side effects of senolytic agents can be tested. It should be noted that even though continual genetic clearance of senescent cells from mice did not lead to any overt side effects during 20 months of observation (Baker et al., 2011), there is evidence that cellular senescence has beneficial effects under some circumstances. For example, cellular senescence protects against cancer development, helps to resolve tissue fibrosis during healing, is involved in immune responses, and can contribute to tissue remodeling (Krizhanovsky et al., 2008; Tchkonia et al., 2013; Xue et al., 2007).
2. Associations between diseases in humans and cellular senescence
Cellular senescence is associated with many of the chronic diseases and disabilities that are the leading drivers of morbidity, mortality, and health costs (Kirkland, 2013a; Tchkonia et al., 2013; Zhu et al., 2014). Senescent cells have been identified at sites of pathology in a number of these conditions and may have systemic effects that predispose to others. These include: 1) metabolic conditions (diabetes, obesity, metabolic syndrome, and age-related lipodystrophy (Minamino et al., 2009; Tchkonia et al., 2010)), 2) cardiovascular disorders (atherosclerosis, hypertension, heart failure, and peripheral vascular disease (Holdt et al., 2011; Kirkland, 2013a; Minamino et al., 2002; Wang et al., 2012; Westhoff et al., 2008)), 3) frailty (sarcopenia (Baker et al., 2011; Tchkonia et al., 2013)), 4) blindness (cataracts, glaucoma, macular degeneration (Baker et al., 2011; Kozlowski, 2012; Liton et al., 2005)), 5) loss of resilience (side effects shortly after or many years after chemotherapy or radiation, delayed recovery after elective surgery or acute events such as myocardial infarction (Kirkland, 2013a; Le et al., 2010; Marcoux et al., 2013; Roninson, 2003; Tchkonia et al., 2013)), 6) neurodegenerative diseases (Alzheimer's disease and “tau-opathies”, Parkinson's, “chemo brain” after, for example, cis-platinum, HIV dementia (Chinta et al., 2013; Golde et al., 2009; Kirkland, 2013a; Krull et al., 2013)), 7) bone disorders (osteoporosis, osteoarthritis, fracture non-union (Bajada et al., 2009; Chen et al., 2013; Freund et al., 2010; Price et al., 2002)), 8) lung conditions (idiopathic pulmonary fibrosis, bleomycin lung and other drug- or environmental-toxin related lung diseases, and chronic obstructive lung disease (Aoshiba et al., 2009; Barnes, 2013; Minagawa et al., 2011a; Tsuji et al., 2004; Tsuji et al., 2009)), 9) liver disease (primary biliary cirrhosis(Tabibian et al., 2014), 10) genitourinary dysfunction (age-related glomerulosclerosis, predisposition to acute tubular necrosis, diabetic renal disease, prostatic hypertrophy (Castro et al., 2003; Choi et al., 2000; Clements et al., 2013; Kirkland, 2013a; Kitada et al., 2014)), 11) skin disorders: melanocytic naevi, chronic skin ulcers (bedsores)(Gray-Schopfer et al., 2006; Vande Berg et al., 2005), 12) cancers (Campisi et al., 2007; Kirkland, 2013a; Liu et al., 2007), 13) toxin exposures and drug or radiation treatments (drugs: alkylating and other chemotherapeutic agents (Roninson, 2003), HIV protease inhibitors (Torres et al., 2014), hormones: long term growth hormone treatment (Stout et al., 2014), toxins (Welford et al., 2010), long term effects of therapeutic or accidental radiation (Marcoux et al., 2013)), 14) genetic disorders (such as progerias (Benson et al., 2010)), 15) infections (notably, HIV (Torres et al., 2014)), and 16) chronological aging itself (Tchkonia et al., 2013; Waaijer et al., 2012). Some of these conditions will likely be the subjects of proof-of-concept clinical studies with senolytics to test whether clearing senescent cells provides clinical benefit.
3. Potential scenarios for initial proof-of-concept studies of senolytic agents
Initial clinical studies of senolytic agents will most likely involve indications in which short term administration leads to measurable clinical benefits in already symptomatic subjects, rather than studies of lifespan or healthspan. The first clinical studies may need to be publicly funded in academic settings if they involve repurposed agents that are off-patent. Existing agents still under patent, patentable methods of administration (e.g., aerosol, topically, or ophthalmic drops), novel drug combinations, or new chemical entities will be more attractive to the pharmaceutical industry. The time to get new chemical entities to the point of proof-of-principle studies will be longer than for approved, repurposed agents. However, companies with approved agents under patent may be willing to explore new indications.
Animal models are needed that reflect the designs of initial human studies in order to facilitate preclinical testing of candidate senolytic agents. The first human studies may be small proof-of-principle trials of repurposed agents already approved for other applications that turn out to have senolytic activity. If this assumption is correct, possible scenarios in which such agents might first be tested include: A) amelioration of multiple co-morbidities, frailty, or loss of resilience, B) accelerated aging-like conditions, C) otherwise fatal conditions associated with cellular senescence, and D) localized conditions associated with senescence.
A) Amelioration of multiple co-morbidities, frailty, and loss of resilience
Fundamental aging mechanisms, including cellular senescence, are associated with multiple age-related chronic diseases. These diseases frequently occur within the same older individuals. Therefore, senolytic or SASP-protective agents may simultaneously ameliorate recognized short-term problems related to several different diseases within older subjects with multiple co-morbidities. Testing this will require novel clinical study paradigms. To date, clinical trials have focused on younger subjects with a single target condition, excluding older subjects with co-morbidities. A potential scenario for initial small-scale proof-of-principle trials of candidate senolytic or SASP-protective drugs would be to study their effect on multiple endpoints in elderly subjects with combinations of 2 or more of: atherosclerosis, hypertension, memory impairment, diabetes, COPD, renal dysfunction, or other cellular senescence-related conditions. These endpoints could be surrogate endpoints already recognized by regulatory agencies, such as circulating lipids, left ventricular function or hypertrophy, blood pressure, memory tests, fasting glucose or HOMA, pulmonary function tests, etc. To increase statistical power and reduce the number of subjects required, the endpoints could be combined into a composite score, although this approach carries the risk that an effective drug may seem less than effective if one of the composite endpoint components is affected in a direction opposite to that expected. For example, rapamycin may lead to improvements in a number of age-related measures of function, but also results in decreased glucose tolerance (Lamming et al., 2012).
Another scenario to test senolytics would be to ascertain if they ameliorate frailty. Frailty is an age-related syndrome that entails loss of resilience, with failure to respond well to, or recover from, acute challenges, such as chemotherapy, surgery, pneumonia, stroke, influenza, heart attacks, dehydration, immunization, or fractures (Bandeen-Roche et al., 2009; Bandeen-Roche et al., 2006; Fried et al., 2001; Kanapuru et al., 2009; Kirkland, 2013b; Leng et al., 2007; Qu et al., 2009; Rockwood et al., 2006; Walston et al., 2006; Walston et al., 2002; Walston et al., 2009). Frailty can be diagnosed using scales that are reasonably, but not completely, sensitive and specific. These involve combinations of assessments of muscle weakness and sarcopenia, fatigue, weight loss, low activity, and chronic disease and disability burden (Bandeen-Roche et al., 2006; Fried et al., 2001; Lucicesare et al., 2010; Rockwood et al., 2011). Frailty or multi-system dysfunction potentially related to cellular senescence might occur in younger subjects with major diseases such as cancers, HIV, or post-chemotherapy or post-radiation syndromes. Frailty also may be related to senescent cell burden, since elimination of senescent cells alleviates frailty-like signs in older progeroid mice (Baker et al., 2011).
Subjects with mild or moderate degrees of frailty or sarcopenia (i.e., Fried class 2 or 3 (Ferrucci et al., 2004)) would likely be better candidates for initial trials of effects of senolytics on frailty-related outcomes than would be subjects with advanced, potentially irreversible frailty. Measures such as timed walking distances, strength measurements, senescence-associated secretory phenotype markers such as circulating IL-6, MCP-1, or PAI-1, pulmonary function (e.g., VO2), renal function tests, and other indices are related to frailty, predictive of mortality, associated with cellular senescence, or beginning to be recognized by drug regulators (McLean et al., 2014; Tchkonia et al., 2013; Villareal et al., 2011)). Another option would be to test if senolytic agents enhance resilience of subjects with mild degrees of frailty or other predictors of poor outcomes after homeostatic perturbations such as elective surgery, chemotherapy, therapeutic radiation, bone marrow transplantation, or immunization response, or acute events such as delayed recovery after acute infection or myocardial infarction. In these situations, senolytic or SASP-protective agents could be administered before the perturbation or after the acute event, with measurement of outcomes such as frailty, muscle strength, or time to return to independent living.
B) Accelerated aging-like conditions
Several conditions associated with senescent cell accumulation have features resembling an accelerated aging-like state, including obesity and diabetes, long term effects of chemotherapy or radiation, and progeroid syndromes (Tchkonia et al., 2013). These conditions are a potential starting point for proof-of-principle studies of candidate senolytic or SASP-protective agents. Senescent cells accumulate in fat and other tissues in obesity and diabetes (Minamino et al., 2009; Tchkonia et al., 2010). Obesity and diabetes are associated with accelerated onset of other aging- and senescence-associated conditions, including atherosclerosis, vascular dysfunction, sarcopenia, cognitive impairment and dementia, early menopause, and cancers, including non-hormone-dependent cancers (De Felice et al., 2014; Joost, 2014; Tchkonia et al., 2010). Similarly, survivors of cancers who were treated during childhood with chemotherapy or radiation can develop frailty and sarcopenia, cardiac disease, diabetes, second, unrelated cancers, and cognitive impairment (“chemo-brain”) by mid-adulthood (Hudson et al., 2013). Chemotherapy and radiation can both induce cellular senescence (Marcoux et al., 2013; Roninson, 2003). Progeroid syndromes with phenotypes resembling an accelerated aging-like state have been associated with increased senescent cell burden (Benson et al., 2010). Short term effects of candidate senolytics on muscle strength, metabolic, cardiovascular, cognitive, or other functional measures in these subjects could be tested in initial proof-of-principle trials. Resilience measures, such as recovery after elective surgery or response to immunization could be measured in subjects treated with senolytics vs. controls. Intermediate outcomes, such as rate of progression of frailty, loss of independence, or time to development of additional conditions, could be examined.
C) Fatal conditions for which no highly effective treatments are available
Another scenario for initial proof-of-concept trials of senolytics may be their impact on otherwise fatal conditions that have been associated with cellular senescence and for which either very invasive or no effective treatments are currently available. These include certain cancers, cancer predisposition syndromes, idiopathic pulmonary fibrosis, primary biliary cirrhosis, Alzheimer's, tau-, HIV-related, and other dementias, Parkinson's disease, and progerias (Kirkland, 2013a; Tabibian et al., 2014; Tchkonia et al., 2013). In some cancers, senescent cell clearance might facilitate giving higher chemotherapy or radiation doses or enhance effectiveness of these treatments.
D) Conditions associated with cellular senescence that are amenable to topical or localized drug delivery
Especially for a repurposed senolytic agent that is off-patent or nearing the end of the patent protection period, a way for pharmaceutical firms to refresh protection and support development of the agent is to find indications where it can be administered topically, by aerosol, joint injection, or as ophthalmic drops or injections. Among the senescence-associated conditions that would benefit from non-pill forms of treatment are idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, osteoarthritis, fracture non-union, skin conditions, and eye diseases.
Pulmonary disease
Idiopathic pulmonary fibrosis is especially attractive, as it is associated with accumulation of senescent cells in the lung, can be fatal, and lacks effective treatment options (Minagawa et al., 2011b; Takasaka et al., 2014). Subjects with this condition might benefit from aerosol delivery of senolytic or SASP-protective agents. A potential problem is that the fibrosis in idiopathic pulmonary fibrosis could be exacerbated by removing senescent cells, since increased fibrosis occurs during liver regeneration following partial hepatectomy if senescence is inhibited (Krizhanovsky et al., 2008; Vincent et al., 2012). Senolytic aerosols may need to be combined with anti-fibrotic agents, such as the MMP's that are currently being studied as treatments for fibrotic pulmonary diseases (Ganesan et al., 2010). This needs to be tested first in animal models, such as in mice with pulmonary fibrosis induced by aerosolized bleomycin (Moore et al., 2008) crossed with animals from which senescent cells can be cleared genetically or treated with senolytic drugs.
Arthritis
Cellular senescence is associated with osteoarthritis, an inflammatory arthritis that can affect multiple joints and that becomes increasingly common in old age (Ryu et al., 2014). Currently, osteoarthritis is treated with oral analgesics, anti-inflammatories, and sometimes intra-articular injections with steroids. The oral agents need to be administered frequently, usually daily. Steroid joint injections have effects that last for days or weeks, but often have to be administered repeatedly. Unfortunately, repeated steroid injections eventually add to joint damage. Given the accumulation of senescent cells and the inflammatory features of osteoarthritis, perhaps senolytic agents administered in a single systemic course or by an injection into affected joints will have a more sustained effect and fewer side effects than current treatments. Work in animal models to test if local injection of senolytic agents into sites of fracture non-union, topical application for skin diseases associated with cellular senescence (e.g., psoriasis, hair loss, canities) or ophthalmic administration for macular degeneration and other conditions might ameliorate these conditions.
4. Animal models of cellular senescence-associated diseases
Animal models reflective of the potential indications for senolytics in humans considered above are needed for the preclinical studies required before proceeding to proof-of-principle human trials. In some cases, genetically modified mice or relevant disease-inducing genetic, pharmacological, or dietary manipulations are available. However, in many cases there are either no models or only ones with imperfections. In the case of human progerias or other syndromes that result from single gene mutations, mouse models are available that are reasonably good approximations. Progeroid mice that have increased senescent cells include lamin A-mutated, Klotho-deficient, Ercc−−, and BubR1H/H mice (Baker et al., 2008; Chen et al., 2013; Eren et al., 2014b; Tchkonia et al., 2013). Candidate senolytic agents could be tested in such mice to determine if the drugs resolve multiple aging-like and healthspan phenotypes, such as impaired grip strength, exercise endurance, activity, glucose homeostasis and other metabolic dysfunction, and cognitive dysfunction.
In polygenic, multifactorial human diseases that first become clinically manifest in late life, animals with single gene mutations that develop superficially similar syndromes in early life can be problematic for drug development. Examples include single gene mutations that lead to findings resembling Alzheimer's disease in very young mice. Perhaps animals with dysfunctionprovoking mutations that can be induced in later life will be better for testing senolytic agents. At least the context of an aging tissue microenvironment could be modeled in such mice. This may be particularly important in the case of senescence-associated diseases, since senescent cells can profoundly influence the tissues around them because of the SASP, matrix proteins, and metabolites that they secrete and the adaptive immune cells that they chemo-attract.
Manipulations that can be used in experimental animals to induce cellular senescence and model human senescence-associated disorders include; high fat feeding, localized or systemic radiation, systemic pharmacological interventions (e.g., alkylating agents or other chemotherapy, HIV protease inhibitors, streptozotocin, Parkinson's-inducing agents), localized pharmacological interventions (e.g., inhaled bleomycin or cigarette smoke, psoralogens), topical carcinogens, cancer xenografts, skin wounding, and surgical joint damage. Effects of candidate senolytic agents on a panel of such models could be helpful in testing candidate agents and selecting potential clinical applications for each new senolytic drug. In some cases, such as Alzheimer's disease, tests of senolytic agents may be more informative in human cell culture systems that recapitulate disease pathology better than currently available animal models (Choi et al., 2014).
There are advantages and drawbacks to each of these models. For example, there is no precise mouse counterpart of human osteoarthritis, although guinea pigs can develop a condition similar to human osteoarthritis in some respects (Vincent et al., 2012). Mouse models of arthritis generally involve some form of surgical or chemical trauma. This approach could be valuable in modeling human post-traumatic arthritis, but is perhaps less faithful in modeling the common form of age-related human osteoarthritis.
Effectiveness of candidate senolytic agents in alleviating effects of clinically-relevant homeostatic perturbations could be modeled in animals to test their effectiveness on resilience. For example, older, obese, or progeroid mice could be treated with candidate agents. The homeostatic perturbations might include chemotherapy, radiation, surgical wounding, food or water deprivation, kainic acid, cardiostimulants such as epinephrine, isoproterenol, or dobutamine, cardiac or renal injury/ re-perfusion, cancer xenografts, carcinogens, stem cell or organ transplantation, or immobilization by hind-limb suspension. The impact of these perturbations on a range of physical, metabolic, cardiovascular, and cognitive senescence-associated healthspan phenotypes could be determined.
Complications and side effects of candidate senolytic agents need to be examined before proof-of-principle human studies can begin. Partial hepatectomy, skin wound, lung fibrosis, vascular plaque, and other models will be useful in a search for potential fibrotic, plaque rupture, or other adverse effects of candidate senolytic agents. Preclinical studies of potential complications, toxicity, and effectiveness will need to be done using more than one species. In each of the above models, effects of candidate senolytic agents on multiple age-related endpoints within the same animals vs. placebo could be evaluated. They could then be combined into a single overall “healthspan” score to enhance statistical power.
One approach to show if clearance of senescent cells is associated with phenotypic improvement would be to compare effects of candidate senolytic drugs to clearance in genetic models of p16Ink4a-related senescence, such as INK-ATTAC mice, or in models with senescence-related promoters in the p53 pathway, such as PAI-1. Mice based on a PAI-1 promoter-suicide gene construct may be informative, since PAI-1 has been implicated in inducing cellular senescence and spread of senescence from cell to cell (Eren et al., 2014a). Recently, PAI-1 inhibitors have been shown to extend lifespan in progeroid Klotho-deficient mice in tandem with decreased senescent cell burden (Eren et al., 2014b), implicating PAI-1 in the genesis, spread, or viability of senescent cells. To do these studies, work is needed to develop senescence-associated disease animal models fully, breed them to animal models in which senescent cells can be tracked in vivo or in tissues, and from which senescent cells can be cleared using inducible suicide genes as positive controls.
5. Conclusions
Indications we feel could be particularly promising for initial proof-of-principle clinical studies as senolytic or SASP-protective agents become available will be in mitigating short- or long-term effects of chemotherapy or radiation, aerosol delivery for idiopathic pulmonary fibrosis, treatment of primary biliary cirrhosis, and injection for osteoarthritis. Disease-emulating animal models reflecting each of these conditions, proof-of-concept testing in animals bred by crosses between these models and INK-ATTAC mice or other mice from which senescent cells can be cleared genetically, and demonstration that candidate senolytic drugs can both clear senescent cells and resolve disease are needed before initial clinical studies begin. Studies of effectiveness of senolytics for preventing vs. treating senescence-associated conditions need to be tested in these preclinical animal models. This will provide important information for determining the point during disease progression when senolytics should start to be administered during clinical trials.
A critical challenge to claims that a candidate senolytic agent actually works by clearing senescent cells will be to prove that observed decreases in senescent cell burden are the cause of phenotypic effects of the drug (Fig. 1). A cause and effect mechanism must be distinguished from a non-causal association between decreases in senescent cell burden and phenotypic improvement. Many agents, including highly selective small molecules or even antibodies, can act through “off-target” mechanisms, potentially leading to phenotypic effects by working through pathways in cell types other than those targeted. Showing that changes in phenotypes associated with administering a candidate drug parallel changes in phenotypes due to genetic clearance of senescent cells from models, such as INK-ATTAC mice, would suggest an association between clearing senescent cells and phenotype improvement. However, this type of comparison is not sufficient to prove cause and effect. For example, a drug such as ibuprofen reduces spread of senescence and therefore eventually will also reduce senescent cell abundance (Jurk et al., 2014), but may do so without actually being senolytic. Ibuprofen alleviates phenotypes due to inflammation, mimicking the effects anticipated by removing senescent cells. To rule out off-target mechanisms definitively, new types of models need to be developed.
Figure 1. Causation vs. association.
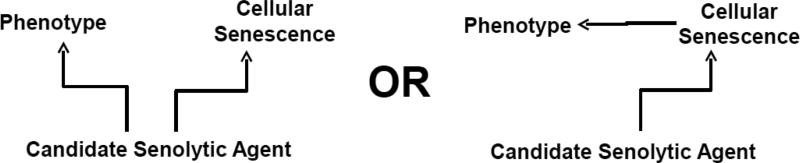
Do candidate senolytic drugs actually cause phenotypic improvement by targeting senescent cells? Alternatively, do they affect non-senescent cells to ameliorate phenotypes and, as an epiphenomenon, simultaneously induce loss of senescent cells? Causal effects of a candidate drug due to senescent cell clearance may combine with “off-target” effects on non-senescent cells to result in phenotypic effects.
One way to establish causality would be to make animal models in which a constitutively active drug target is expressed only in senescent cells. These animals could then be used to test if senescence-related phenotypes are affected by the potentially senolytic agent being investigated. If the drug were actually senolytic, these phenotypes should not be resolved in the animals that express the drug target constitutively. However, they should be corrected by senolytic agents in animals that do not express the constitutively active drug target. Such an approach could be used to estimate relative contributions of senescent cell clearance vs. effects of “off-target” mechanisms. Also, such an approach would be required to prove cause and effect. Cause and effect would not be proven by merely finding a correlation between phenotype resolution following candidate senolytic drug treatment vs. resolution following induction of a senescent cell suicide gene in genetically-modified mice.
There will very likely be unforeseen drawbacks to senolytic treatment, in addition to those already known, perhaps including increased fibrosis. A search for side effects will be needed for each candidate agent using animal models that recapitulate the context in which the agent is to be used clinically. For example, if the goal is to develop a particular senolytic agent as an aerosol for treating idiopathic pulmonary fibrosis, it would be necessary to show that the agent does not make the fibrosis worse in, for example, older (as opposed to young) mice in which pulmonary fibrosis has been induced by bleomycin or smoke inhalation.
There are a number of potentially important advantages of clearing senescent cells over other treatment approaches. Unlike agents that ameliorate the SASP, it may be feasible to administer senolytic treatments intermittently, perhaps for a day or two every few months or once a year during periods of good health. Since, unlike cancer cells or microbes, senescent cells do not divide, resistance to senolytics of the type encountered in treating cancer or infectious diseases is unlikely to be a problem. Targeting only a fraction of senescent cells may have beneficial effects. Consistent with this possibility, only 30 percent of p16Ink4a-expressing senescent cells were removed from INK-ATTAC;BubR1H/H mice by AP20187. Removal of this fraction of senescent cells enhanced healthspan markedly (Baker et al., 2011). Possibly, those senescent cells that are susceptible to removal differ from those that remain after genetic or pharmacological targeting. The susceptible fraction could be most responsible for causing functional impairment and disease.
More needs to be known about senescent cell turnover. Animal models in which all cells can be permanently tagged by pulse-labelling, for example animals expressing the lineage-tracking construct in AdipoChaser mice (Wang et al., 2014), could be crossed with models in which senescent cells can be tracked, for example INK-ATTAC (Baker et al., 2011) or p16Ink4aluciferase mice (Burd et al., 2013). These animals could be used to assess senescent cell turnover with aging as well as in the context of each senescence-related disease state or condition (e.g., high fat feeding, after radiation or chemotherapy, or in senescence-related chronic disease animal models). Information about turnover of senescent cells in different conditions and diseases will be very important in devising appropriate indication-specific dosing intervals. However, while a first approximation of turnover rates can be estimated in animal studies by combining lineage-tracing constructs with a senescence-related promoter, turnover in humans is likely to be very different from mice, which have dissimilar adaptive immune systems from humans. Immune function appears to be a major mechanism for senescent cell clearance (Xue et al., 2007). A great deal of work, especially fine-tuning of senolytic drug dosing intervals, will need to be done in clinical analyses.
In summary, a range of animal models, including species other than mice, are needed for the following reasons. 1) A particularly challenging issue is to prove if decreases in senescent cell number associated with senolytic drug treatment actually cause phenotype resolution, rather than through an off-target effect of the drug on other cell types. 2) Better animal models of human senescence-associated diseases, frailty, and resilience are needed. These models should be designed in tandem with clinical trials strategies, so the most relevant pre-clinical testing that best reflects the particular clinical trials strategy can be done. 3) Senescent cell generation or reappearance rates need to be determined for each senescence-related disease state as a guide to determining intervals between pulses of senolytic treatment. This can be estimated approximately through animal models, but will need to be determined more precisely in clinical studies. 4) A thorough search for side effects of senolytic agents in the context of each senescence-related chronic condition will need to be conducted in age-appropriate pre-clinical animal models, as will comparisons of side effect profiles of different senolytic agents. The development of the right animal models and clinical trials paradigms for senolytic agents warrants intensive exploration, since senolytic agents, if successful in delaying, preventing, alleviating, or reversing the major age-related diseases as a group would be transformative.
Highlights.
Scenarios for proof-of-concept clinical studies of senolytic agents are proposed
Animal models for testing candidate senolytics are considered
Models are needed that reflect potential clinical applications of senolytic agents
Animal models for determining dosing intervals and side effects will be important
Whether senescent cell removal related to drugs is the cause of phenotype resolution must be proven
Acknowledgements
The authors wish to acknowledge the assistance of J. Armstrong in preparing this manuscript. Support was provided by NIH grants AG013975 and AG044396 (Geroscience Network). The authors are grateful for advice and conversations about strategies for translating agents that affect fundamental aging mechanisms into clinical interventions with colleagues during the retreat series supported by the Geroscience Network.
Abbreviations
- SA-βgal
senescence-associated β-galactosidase
- SASP
senescence-associated secretory phenotype
- FDA
Food and Drug Administration
- GHRKO
growth hormone receptor knockout
- ATTAC
apoptosis through targeted activation of caspase 8
- BubR1
Budding Uninhibited By Benzimidazoles-1
- PAI-1
plasminogen activator inhibitor-1
- MMP
matrix metalloproteinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors and Mayo Clinic have a financial interest related to this research. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and is being conducted in compliance with Mayo Clinic Conflict of Interest policies.
References
- Aoshiba K, Nagai A. Senescence hypothesis for the pathogenetic mechanism of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:596–601. doi: 10.1513/pats.200904-017RM. [DOI] [PubMed] [Google Scholar]
- Bajada S, Marshall MJ, Wright KT, Richardson JB, Johnson WE. Decreased osteogenesis, increased cell senescence and elevated Dickkopf-1 secretion in human fracture non union stromal cells. Bone. 2009;45:726–735. doi: 10.1016/j.bone.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Perez-Terzic C, Jin F, Pitel KS, Niederlander NJ, Jeganathan K, Yamada S, Reyes S, Rowe L, Hiddinga HJ, Eberhardt NL, Terzic A, van Deursen JM. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat Cell Biol. 2008;10:825–836. doi: 10.1038/ncb1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res. 2009;12:403–410. doi: 10.1089/rej.2009.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nature reviews Drug discovery. 2013;12:543–559. doi: 10.1038/nrd4025. [DOI] [PubMed] [Google Scholar]
- Benson EK, Lee SW, Aaronson SA. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J Cell Sci. 2010;123:2605–2612. doi: 10.1242/jcs.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, Bardeesy N, Castrillon DH, Beach DH, Sharpless NE. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell. 2013;152:340–351. doi: 10.1016/j.cell.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Castro P, Giri D, Lamb D, Ittmann M. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate. 2003;55:30–38. doi: 10.1002/pros.10204. [DOI] [PubMed] [Google Scholar]
- Chen Q, Liu K, Robinson AR, Clauson CL, Blair HC, Robbins PD, Niedernhofer LJ, Ouyang H. DNA damage drives accelerated bone aging via an NF-kappaB-dependent mechanism. J Bone Miner Res. 2013;28:1214–1228. doi: 10.1002/jbmr.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Lieu CA, Demaria M, Laberge RM, Campisi J, Andersen JK. Environmental stress, ageing and glial cell senescence: a novel mechanistic link to Parkinson's disease? J Intern Med. 2013;273:429–436. doi: 10.1111/joim.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Shendrik I, Peacocke M, Peehl D, Buttyan R, Ikeguchi EF, Katz AE, Benson MC. Expression of senescence-associated beta-galactosidase in enlarged prostates from men with benign prostatic hyperplasia. Urology. 2000;56:160–166. doi: 10.1016/s0090-4295(00)00538-0. [DOI] [PubMed] [Google Scholar]
- Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D'Avanzo C, Chen H, Hooli B, Asselin C, Muffat J, Klee JB, Zhang C, Wainger BJ, Peitz M, Kovacs DM, Woolf CJ, Wagner SL, Tanzi RE, Kim DY. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 2014 doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements ME, Chaber CJ, Ledbetter SR, Zuk A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One. 2013;8:e70464. doi: 10.1371/journal.pone.0070464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63:2262–2272. doi: 10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- Eren M, Boe AE, Klyachko EA, Vaughan DE. Role of plasminogen activator inhibitor-1 in senescence and aging. Semin Thromb Hemost. 2014a;40:645–651. doi: 10.1055/s-0034-1387883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eren M, Boe AE, Murphy SB, Place AT, Nagpal V, Morales-Nebreda L, Urich D, Quaggin SE, Budinger GR, Mutlu GM, Miyata T, Vaughan DE. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proceedings of the National Academy of Sciences of the United States of America. 2014b;111:7090–7095. doi: 10.1073/pnas.1321942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr., Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Amer Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Ganesan S, Faris AN, Comstock AT, Chattoraj SS, Chattoraj A, Burgess JR, Curtis JL, Martinez FJ, Zick S, Hershenson MB, Sajjan US. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir Res. 2010;11:131. doi: 10.1186/1465-9921-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, Miller VM. Proteinopathy-induced neuronal senescence: a hypothesis for brain failure in Alzheimer's and other neurodegenerative diseases. Alzheimers Res Ther. 2009;1:5. doi: 10.1186/alzrt5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DP, Cutler D, Rowe JW, Michaud PC, Sullivan J, Peneva D, Olshansky SJ. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff (Millwood) 2013;32:1698–1705. doi: 10.1377/hlthaff.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Schopfer VC, Cheong SC, Chong H, Chow J, Moss T, Abdel-Malek ZA, Marais R, Wynford-Thomas D, Bennett DC. Cellular senescence in naevi and immortalisation in melanoma: a role for p16? British journal of cancer. 2006;95:496–505. doi: 10.1038/sj.bjc.6603283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdt LM, Sass K, Gabel G, Bergert H, Thiery J, Teupser D. Expression of Chr9p21 genes CDKN2B (p15(INK4b)), CDKN2A (p16(INK4a), p14(ARF)) and MTAP in human atherosclerotic plaque. Atherosclerosis. 2011;214:264–270. doi: 10.1016/j.atherosclerosis.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, Sklar CA, Srivastava DK, Robison LL. Clinical ascertainment of health outcomes among adults treated for childhood cancer. J Am Med Assoc. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost HG. Diabetes and cancer: Epidemiology and potential mechanisms. Diab Vasc Dis Res. 2014 doi: 10.1177/1479164114550813. [DOI] [PubMed] [Google Scholar]
- Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SL, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nature communications. 2014;2:4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanapuru B, Ershler WB. Inflammation, coagulation, and the pathway to frailty. Am J Med. 2009;122:605–613. doi: 10.1016/j.amjmed.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL. Inflammation and cellular senescence: potential contribution to chronic diseases and disabilities with aging. Public Policy and Aging Report. 2013a;23:12–15. [Google Scholar]
- Kirkland JL. Translating advances from the basic biology of aging into clinical application. Exp Gerontol. 2013b;48:1–5. doi: 10.1016/j.exger.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Peterson C. Healthspan, translation, and new outcomes for animal studies of aging. J Gerontol A Biol Sci Med Sci. 2009;64:209–212. doi: 10.1093/gerona/gln063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada K, Nakano D, Ohsaki H, Hitomi H, Minamino T, Yatabe J, Felder RA, Mori H, Masaki T, Kobori H, Nishiyama A. Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complications. 2014;28:604–611. doi: 10.1016/j.jdiacomp.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski MR. RPE cell senescence: a key contributor to age-related macular degeneration. Med Hypotheses. 2012;78:505–510. doi: 10.1016/j.mehy.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Srivastava DK, Gurney JG, Kimberg C, Krasin MJ, Pui CH, Robison LL, Hudson MM. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4407–4415. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM, Baur JA. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le ON, Rodier F, Fontaine F, Coppe JP, Campisi J, DeGregori J, Laverdiere C, Kokta V, Haddad E, Beausejour CM. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell. 2010;9:398–409. doi: 10.1111/j.1474-9726.2010.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005;40:745–748. doi: 10.1016/j.exger.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67:3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- Lucicesare A, Hubbard RE, Searle SD, Rockwood K. An index of self-rated health deficits in relation to frailty and adverse outcomes in older adults. Aging Clin Exp Res. 2010;22:255–260. doi: 10.1007/BF03324805. [DOI] [PubMed] [Google Scholar]
- Marcoux S, Le ON, Langlois-Pelletier C, Laverdiere C, Hatami A, Robaey P, Beausejour CM. Expression of the senescence marker p16INK4a in skin biopsies of acute lymphoblastic leukemia survivors: a pilot study. Radiat Oncol. 2013;8:252. doi: 10.1186/1748-717X-8-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, Kenny AM, Peters KW, Ferrucci L, Guralnik JM, Kritchevsky SB, Kiel DP, Vassileva MT, Xue QL, Perera S, Studenski SA, Dam TT. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69:576–583. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuter A, Rogmann LM, Winterhoff BJ, Tchkonia T, Kirkland JL, Morbeck DE. Markers of cellular senescence are elevated in murine blastocysts cultured in vitro: molecular consequences of culture in atmospheric oxygen. J Assist Reprod Genet. 2014 doi: 10.1007/s10815-014-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011a;300:L391–401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. American journal of physiology Lung cellular and molecular physiology. 2011b;300:L391–401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15:1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. American journal of physiology Lung cellular and molecular physiology. 2008;294:L152–160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M. Programmed cell senescence during mammalian embryonic development. Cell. 2013;155:1104–1118. doi: 10.1016/j.cell.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, Clark IM. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- Qu T, Walston JD, Yang H, Fedarko NS, Xue QL, Beamer BA, Ferrucci L, Rose NR, Leng SX. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009;130:161–166. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Long EO. Cellular senescence induced by CD158d reprograms natural killer cells to promote vascular remodeling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20596–20601. doi: 10.1073/pnas.1208248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research, A.f.A . The Silver Book: Chronic Disease and Medical Innovation in an Aging Nation. Washington, DC: 2012. [Google Scholar]
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54:975–979. doi: 10.1111/j.1532-5415.2006.00738.x. [DOI] [PubMed] [Google Scholar]
- Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- Ryu JH, Moua T, Daniels CE, Hartman TE, Yi ES, Utz JP, Limper AH. Idiopathic pulmonary fibrosis: evolving concepts. Mayo Clin Proc. 2014;89:1130–1142. doi: 10.1016/j.mayocp.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, Keyes WM. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013;155:1119–1130. doi: 10.1016/j.cell.2013.10.041. [DOI] [PubMed] [Google Scholar]
- Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging. 2014 doi: 10.18632/aging.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibian JH, O'Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–2275. doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaka N, Araya J, Hara H, Ito S, Kobayashi K, Kurita Y, Wakui H, Yoshii Y, Yumino Y, Fujii S, Minagawa S, Tsurushige C, Kojima J, Numata T, Shimizu K, Kawaishi M, Kaneko Y, Kamiya N, Hirano J, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Autophagy induction by SIRT6 through attenuation of insulin-like growth factor signaling is involved in the regulation of human bronchial epithelial cell senescence. Journal of immunology. 2014;192:958–968. doi: 10.4049/jimmunol.1302341. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Morbeck DE, von Zglinicki T, van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres RA, Lewis W. Aging and HIV/AIDS: pathogenetic role of therapeutic side effects. Laboratory investigation; a journal of technical methods and pathology. 2014;94:120–128. doi: 10.1038/labinvest.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Aoshiba K, Nagai A. Cigarette smoke induces senescence in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2004;31:643–649. doi: 10.1165/rcmb.2003-0290OC. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence exacerbates pulmonary inflammation in patients with chronic obstructive pulmonary disease. Respiration. 2009;80:59–70. doi: 10.1159/000268287. [DOI] [PubMed] [Google Scholar]
- Vande Berg JS, Rose MA, Haywood-Reid PL, Rudolph R, Payne WG, Robson MC. Cultured pressure ulcer fibroblasts show replicative senescence with elevated production of plasmin, plasminogen activator inhibitor-1, and transforming growth factor-beta1. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2005;13:76–83. doi: 10.1111/j.1067-1927.2005.130110.x. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. The New England journal of medicine. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent TL, Williams RO, Maciewicz R, Silman A, Garside P. Mapping pathogenesis of arthritis through small animal models. Rheumatology. 2012;51:1931–1941. doi: 10.1093/rheumatology/kes035. [DOI] [PubMed] [Google Scholar]
- Waaijer ME, Parish WE, Strongitharm BH, van Heemst D, Slagboom PE, de Craen AJ, Sedivy JM, Westendorp RG, Gunn DA, Maier AB. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell. 2012;11:722–725. doi: 10.1111/j.1474-9726.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, Hadley E, Ferrucci L, Guralnick JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/ National Institute on Aging research conference on frailty in older adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- Walston JD, Matteini AM, Nievergelt C, Lange LA, Fallin DM, Barzilai N, Ziv E, Pawlikowska L, Kwok P, Cummings SR, Kooperberg C, LaCroix A, Tracy RP, Atzmon G, Lange EM, Reiner AP. Inflammation and stress-related candidate genes, plasma interleukin-6 levels, and longevity in older adults. Exp Gerontol. 2009;44:350–355. doi: 10.1016/j.exger.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111:245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- Wang QA, Scherer PE. The AdipoChaser mouse: A model tracking adipogenesis in vivo. Adipocyte. 2014;3:146–150. doi: 10.4161/adip.27656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford SM, Dorie MJ, Li X, Haase VH, Giaccia AJ. Renal oxygenation suppresses VHL loss-induced senescence that is caused by increased sensitivity to oxidative stress. Mol Cell Biol. 2010;30:4595–4603. doi: 10.1128/MCB.01618-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff JH, Hilgers KF, Steinbach MP, Hartner A, Klanke B, Amann K, Melk A. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension. 2008;52:123–129. doi: 10.1161/HYPERTENSIONAHA.107.099432. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Current opinion in clinical nutrition and metabolic care. 2014;17:324–328. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]


