ABSTRACT
A contentious point in memory research is whether or not the hippocampus plays a time‐limited role in the consolidation of declarative memories. A widely held view is that declarative memories are initially encoded in the hippocampus, then transferred to the neocortex for long‐term storage. Alternate views argue instead that the hippocampus continues to play a role in remote memory recall. These competing theories are largely based on human amnesic and animal lesion/inactivation studies. However, in vivo electrophysiological evidence supporting these views is scarce. Given that other studies examining the role of the hippocampus in remote memory retrieval using lesion and imaging techniques in human and animal models have provided mixed results, it would be particularly useful to gain insight at the in vivo electrophysiological level. Here we report hippocampal single‐neuron and theta activity recorded longitudinally during acquisition and remote retrieval of trace eyeblink conditioning. Results from conditioned rabbits were compared to those obtained from yoked pseudo‐conditioned control rabbits. Results reveal continued learning‐specific hippocampal activity one month after initial acquisition of the task. Our findings yield insight into the normal physiological responses of the hippocampus during memory processes and provide compelling in vivo electrophysiological evidence that the hippocampus is involved in both acquisition and retrieval of consolidated memories. © 2014 The Authors Hippocampus Published by Wiley Periodicals, Inc.
Keywords: hippocampus, in vivo electrophysiology, rabbits, trace eyeblink conditioning, consolidation
INTRODUCTION
Involvement of the medial temporal lobe in mnemonic processes has been extensively studied and it is well known that the hippocampus plays a critical role in the encoding of new declarative memories (Scoville and Milner, 1957; Milner et al., 1968; Corkin, 1984). However, there is considerable debate regarding whether the hippocampus also plays a role in the retrieval of remotely acquired memories. Examination of amnesic patients and experimental animals has led to the view that memories are initially encoded in hippocampal‐cortical networks, which are then gradually redistributed to cortico‐cortical networks for storage as a more permanent form of memory storage through consolidation (Squire, 1992; Squire and Alvarez, 1995; Frankland and Bontempi, 2005). According to this view, known as the standard consolidation theory (SCT), retrieval of consolidated memories is independent of the hippocampus and is instead mediated by regions within the cerebral cortex.
Compelling evidence for SCT is provided by lesion studies utilizing trace eyeblink conditioning (EBC), a cerebellar and forebrain dependent associative memory paradigm that models declarative memories (Clark, 2011). Trace EBC consists of non‐contiguous paired presentations of a conditioned stimulus (CS) and an unconditioned stimulus (US) that result in the learning of the CS‐US association as measured by the expression of conditioned responses (CRs). These studies have demonstrated that hippocampal lesions, consistent with SCT, severely disrupt recent recall of trace EBC but not remote retention of the paradigm tested one month after initial acquisition (Kim et al., 1995; Takehara et al., 2003). Conversely, lesions of some areas within the medial prefrontal cortex had no effect on recent memory recall but impaired remote retention of the paradigm assessed one month after initial acquisition (Powell et al., 2001; Takehara et al., 2003; Oswald et al., 2010).
Despite these findings supporting SCT, other theories such as the multiple trace theory, argue instead that the hippocampus is critical not just for encoding, but also for retrieval of consolidated episodic memories (Nadel and Moscovitch, 1997; Moscovitch and Nadel, 1998; Moscovitch et al., 2005; Winocur et al., 2010; McKenzie and Eichenbaum, 2011). However, these competing views are largely based on lesion studies and examination of human amnesics. Lesion‐based studies do not provide insight into the normal physiological properties mediating memory and may be confounded by several factors including the extent of lesions, collateral damage, and compensatory mechanisms that can obscure hippocampal contributions (Goshen et al., 2011). Therefore, electrophysiological recordings in vivo are better suited to describing neuronal network activity occurring naturally during memory processes. At present, cortical recordings, specifically from the prelimbic medial prefrontal cortex, have been performed longitudinally during trace EBC and have shown, in agreement with SCT, robust activity during remote memory retrieval (Takehara‐Nishiuchi and McNaughton, 2008; Hattori et al., 2014). However, no study to date has examined longitudinally, in vivo hippocampal activity during recent and remote memory recall. Thus, it remains unclear how the neurophysiological response properties of hippocampal neurons change over successive stages of memory. Given that other studies examining the role of the hippocampus in remote memory retrieval using primarily lesion and imaging techniques in human and animal models have provided mixed results, it would be particularly useful to examine the hippocampus at the in vivo electrophysiological level.
Here we examined multiple single‐neuron activity and theta oscillations from dorsal CA1 hippocampus in rabbits as they underwent acquisition of whisker‐signaled trace EBC and retention testing administered one month after initial acquisition of the task. A retention interval of one month was chosen based on previous lesion studies that reported trace EBC memories to become extra‐hippocampal by this time period (Kim et al., 1995; Powell et al., 2001; Takehara et al., 2003; Oswald et al., 2010). Results from conditioned rabbits were compared to those obtained from yoked pseudo‐conditioned control rabbits. We report results that provide in vivo electrophysiological support for learning specific continued hippocampal involvement during remote memory recall.
MATERIALS AND METHODS
Subjects
Subjects were nine female New Zealand white albino rabbits (Oryctolagus cuniculus, five conditioned and four pseudo‐conditioned controls), approximately three to six months of age. All subjects were individually housed with ad lib access to food and water and maintained on a 12 h light/dark cycle.
Surgery
All surgeries were performed in accordance with NIH guidelines and protocol approved by the Northwestern University Institutional Animal Care and Use Committee. Subjects were anesthetized via intramuscular injection of ketamine (60 mg/kg) and xylazine (10 mg/kg). Under aseptic conditions, rabbits were positioned in a stereotaxic frame and an incision made to expose the skull. The skulls were leveled with lambda positioned 1.5 mm below bregma. Burr holes were drilled for insertion of self‐tapping anchoring screws. Craniotomies were performed on the left hemisphere, contralateral to the eye being conditioned. The dura was slit and a custom made 96 channel microdrive was lowered at the recording site for each rabbit. Kwik‐Sil (WPI), a silicone elastomer, was used to seal the craniotomies and dental acrylic was used to secure the microdrives to the skulls. Headbolts for atraumatic head fixation during behavioral training were also implanted. After seven days of recovery, tetrodes were advanced over 7–14 days, relying on turn counts and firing characteristics (sharp waves, theta amplitude, complex‐cell spiking) to reach the dorsal CA1 stratum pyramidale. After the experiments, 25 μA of current were passed among the 4 wires of each tetrode for 25 s to create marking lesions, followed by perfusion and histological confirmation of tetrode locations. Data recorded from tetrodes missing the CA1 stratum pyramidale were excluded from subsequent analyses.
Behavioral Training and Analysis
Trace EBC consisted of 250 ms 62.5 Hz whisker vibration CS, 500 ms stimulus‐free trace interval, and 150 ms 3 psi corneal airpuff US, administered to the right eye, contralateral to the recording site. Rabbits received three days of habituation to the conditioning environment before commencement of training. Rabbits were swaddled in a cotton wrap, placed inside a Plexiglas restrainer, and placed inside a light and sound attenuating chamber (Industrial Acoustics). During conditioning, the head was restrained using a fixed, light‐weight aluminum rod that attached to the implanted headbolt. Four to five whiskers in the B row of the right side of the face were attached to a vibrotactile transducer (Piezo Systems) using a thin strip of the adhesive portion of a Post‐it note to deliver the CS (Das et al., 2001). The right eyelids were held open using dress hooks to prevent closure of the eyelids. An infrared reflective sensor measured the sweep of the nictitating membrane in volts (Thompson et al., 1994). A small plastic tube directed at the cornea delivered the US. All stimulus delivery and behavioral data collection were controlled by a computer running custom Labview software (National Instruments).
All rabbits (except for 1 conditioned rabbit) were trained in yoked pairs where one rabbit within the pair underwent trace EBC with paired CS‐US trials while the other underwent pseudo‐trace EBC. Each rabbit received two phases of conditioning: (1) Acquisition and (2) Retention. For acquisition, conditioned rabbits were trained daily (80 paired trials per session, 45 s average inter‐trial interval, 30–60 s range) until 5 days after reaching behavioral criterion, the day the rabbit exhibited eight adaptive conditioned responses (CR) within a sliding set of 10 trials within a session. An adaptive CR was defined as a blink with a voltage amplitude exceeding the mean baseline amplitude (250 ms pre‐CS) by 4 SD for 15 ms minimum within the 20 ms interval prior to US onset. After acquisition training, conditioned rabbits spent one month in their home cages before returning for five days of retention testing. Pseudo‐conditioned rabbits received the same training schedule as their yoked partner but received 160 trials of randomized presentations of the CS or US (80 trials per stimulus) per session (average intertrial‐interval 22.5 s, 15–30 s range).
Blink amplitudes for each animal were normalized to the maximal blink amplitude within the session to account for subtle changes in the position of the infrared reflective eyeblink sensor that could occur during training. Normalized blinks were then adjusted for a common baseline amplitude for all trials.
In Vivo Electrophysiology
Rabbits were implanted with custom‐made 96‐channel chronically implantable microdrives equipped with 24 independently moveable tetrodes and three reference tetrodes aimed at the dorsal CA1 hippocampus (3.0 mm posterior, 3.0 mm lateral to bregma), anterior thalamus, and the prelimbic prefrontal cortex. Only data for the dorsal CA1 hippocampus are reported here. Each tetrode consisted of four, 12.7 µm, heavy‐formvar insulated Stablohm 800 wires (California Fine Wire) with the tips gold‐plated to lower the impedance to 200 kΩ at 1 KHz. Electrical signals recorded from the tetrodes were referenced to a common ground screw or a reference tetrode, amplified 10,000×, digitized, band‐pass filtered at 600–6000 Hz, and saved for offline analysis using Cheetah Data Acquisition Software (Neuralynx).
Single‐Neuron Isolation and Pyramidal Neuron Classification
Clusters of single‐unit activity were isolated offline based on waveform characteristics (peak, valley, first principle component, energy, square root of energy) using Plexon Offline Sorter (Plexon). Waveforms of isolated clusters were subjected to a custom written signal‐to‐noise (SNR) program in MATLAB (Mathworks) to separate well‐isolated neurons from noisy ones. For each cluster, the waveform of each spike was divided into 32 points with the peak forced to the 8th point. Then, the mean waveform and variance waveform was computed for each neuron. Noise, N, was defined as , where n = 32 points and σ 2 = variance waveform. The signal, S, was defined as the difference between the peak and the trough in the mean waveform. The quotient of S and N yielded the SNR for each cluster. Clusters with a SNR ≥ 5 were deemed to be well‐isolated units and all units with SNR < 5 were excluded from further analyses. Cells recorded from each day were conservatively treated as unique neurons.
Single neurons that met the SNR criteria were histologically verified to be recorded from the dorsal CA1 statrum pyramidale. Recorded hippocampal neurons were classified as pyramidal or interneuron based on extracellular features. A common methodology used to differentiate putative pyramidal neurons from interneurons is to examine the spontaneous firing rate and spike width of individual neurons (Fox and Ranck, 1975). However, studies have shown a myriad of interneurons with diverse morphological and physiological characteristics (Maccaferri and Lacaille, 2003; Markram et al., 2004) thus, results based solely on these extracellular features are not immune to misclassifications. Previously, a juxtacellular recording study with morphological identification of hippocampal pyramidal neurons and interneurons has demonstrated that pyramidal neurons preferentially fire to the trough of hippocampal theta oscillations and to the peak of sharp‐wave ripples whereas, hippocampal interneurons do not (Klausberger et al., 2003). Other studies have reported similar phase relationships between excitatory and inhibitory populations (Skaggs et al., 1996; Csicsvari et al., 1999). Thus, after initially classifying pyramidal neurons as those having spontaneous firing rates ≤10 Hz and spikewidth ≥0.2 ms, we further verified their classification by examining the phase relationship between single‐cell and theta activity, and the timing relationship between single‐cell and sharp‐wave ripple activity (Fig. 2C,D). Local field potentials (LFPs) collected at 1–400 Hz were bandpass filtered offline to the theta rhythm (4–8 Hz) using a Butterworth filter. The Hilbert transform was applied to obtain the instantaneous phase and amplitude of the theta rhythm. For each neuron, each spike was assigned to a given phase of the theta cycle. Then, all theta cycles were superimposed to examine the firing probability of each neuron at a given phase of the theta cycle (20° bins). For detection of sharp‐wave ripples, LFPs were filtered at 90–140 Hz. Then, the power (root mean square amplitude) of the filtered signal was calculated in 10 ms windows. Sharp‐wave episodes were defined as epochs where the power exceeded 5 SD above the mean power. The beginning and end of the sharp wave episodes were defined as where the power exceeded 1 SD above the mean power. The maximum amplitude of the sharp‐wave was detected by a peak‐finding algorithm. Since sharp waves are often not symmetrical, the periods between the beginning and the maximum ripple amplitude, and the maximum ripple amplitude and end of sharp‐wave episodes were each divided into 4 bins. Spikes were then sorted into the bins. Neurons that did not exhibit pyramidal neuron‐like characteristics in relation to spontaneous firing rate, spike width, theta, and sharp‐wave activity were removed from subsequent analyses.
Figure 2.
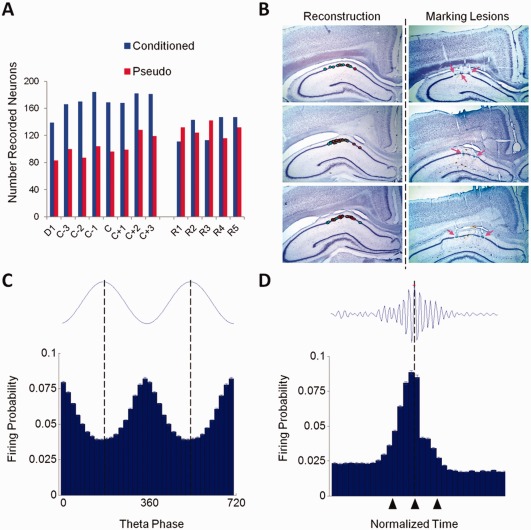
Recorded CA1 pyramidal neurons. (A) Number of recorded pyramidal neurons from conditioned (n = 5) and pseudo‐conditioned (n = 4) animals plotted over acquisition and retention sessions. D1: Day 1, C: Criterion, R: Retention. (B) Histological verification of recording sites. Left, reconstruction of recording sites (cyan = conditioned, red = pseudo‐conditioned). Right, example marking lesions (arrows). After classifying putative pyramidal neurons based on spontaneous firing rate and spike width, pyramidal neurons were further verified by examining their firing probabilities in relation to theta oscillations and sharp‐wave spindles. (C) Firing probabilities (mean ± SEM) of pyramidal neurons (n = 3612) to CA1 theta (4–8 Hz). For clarity, two theta cycles are shown; 0° and 360° mark the trough of theta cycles. (D) Discharge probabilities of pyramidal neurons (n = 3612) to sharp‐wave ripples (90–140 Hz). The start, maximum amplitude, and end of the normalized sharp‐wave episodes are marked by triangles, respectively. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Data Analysis
All analyses were conducted using custom written MATLAB scripts. Data from the first day of training, seven sessions centered on the day of criterion, and 5 retention sessions were analyzed in this study. Examining the data in relation to the day of criterion enabled comparisons among animals that learned at different rates (mean day of criterion, 6.6 ± 1.16 sem., range 4–10).
For each pyramidal neuron, peristimulus time histograms encompassing 1 s pre‐CS onset and 2 s post‐CS onset were generated for each training day using 10 ms bins. The Mann–Whitney U (MWU) nonparametric test comparing 1 s pre‐CS baseline activity to activity during the CS and Trace intervals of the trial was used to separate responsive neurons from nonresponsive neurons and to classify them as rate increasing (RI) or rate decreasing (RD). The MWU test produces a z‐value and neurons with |z| ≥ 1.96 during the CS‐Trace interval were regarded as significantly responsive. Responsive neurons with positive z‐values were classified as RI whereas those with negative z‐values were regarded as RD. The MWU test is useful in the same situations as the Student's paired t‐test; however, the MWU is preferred since as it compares the sums of ranks, it is less likely than the t‐test to spuriously indicate significance based on the presence of outliers. In addition, it allows the comparison of intervals with different durations (e.g., 1 s baseline vs. 750 ms CS‐Trace intervals).
To detect temporal firing rate changes in a population of neurons that have different baseline firing rates, raw firing rates for each responsive neuron were converted to z‐scores: , where FRi = firing rate of the i th bin of the peristimulus period, FRbl = mean baseline firing rate taken from 1 s before CS onset, and SDbl = standard deviation of the baseline firing rate. Z‐scores were averaged for each session as a measure of population response.
For each session, the mean z‐score during the CS and Trace intervals were computed separately for each neuron, then averaged separately to compare the change in rate modulation for the population over sessions. Separate averages and analyses were done for RI and RD neurons. Statistical comparisons of changes in firing rate were performed using two‐way analysis of variance (ANOVA) with training group and sessions as factors. Significant group × session interactions were followed by one‐way ANOVAs for each group to determine significant within‐group changes across sessions. To determine significant differences in mean CS and Trace interval z‐scores between groups at particular time points, a Bonferroni corrected post hoc unpaired t‐test was performed after testing for homogeneity of variance. Changes in behavioral performance were examined using repeated measures ANOVA of the percent of trials with CRs. A chi‐square goodness of fit test was employed to compare differences in the number of RI and RD neurons that were recorded from each group.
Onset and offset times of rate decrease for RD neurons were determined using a thresholding method based on the baseline SD of z‐score peristimulus time histograms. For each conditioned group RD neuron, peristimulus time histograms encompassing 1s pre CS onset and maximum time before presentation of next trial (i.e., shortest intertrial interval) were generated using 10 ms bins, then converted to z‐scores based on a 1 s baseline. Z‐score histograms were smoothed using a 100 ms moving average filter. By definition, the mean and SD of the z‐scores during the baseline period equals 0 and 1, respectively. The first time point after CS presentation where the z‐score crossed below −0.25 SD for a minimum 100 ms duration was determined as the onset time of rate decrease. The first time point after rate decrease onset where the z‐score returned above −0.25 SD of mean baseline for a minimum 100 ms duration was taken as the offset of the rate decrease. The difference between the onset and offset times expressed in seconds was defined as the duration of rate decrease. The same algorithm was employed for RD neurons of the pseudo group where the search for the rate decrease onset time commenced from CS onset for CS‐only trials and from US onset for US‐only trials.
To examine longitudinal changes in theta activity, LFP activity was filtered for the theta band in rabbit [4–8 Hz, (Green and Arduini, 1954)] for each trial of each session for each animal. Instantaneous amplitudes obtained by Hilbert transform of each trial were normalized to baseline (1 s pre‐CS), then averaged to obtain the mean amplitude of a session. Mean amplitudes of a session were averaged across animals to obtain a group response. For examination of phase‐resetting, circular statistics were used to obtain the mean resultant vector (MRV) of each animal per session. MRV yields the mean angular direction (phase) of the signal. The circular mean of the MRVs of animals within a session was then taken to get a group response for the session. As a measure of the circular spread (i.e., variability) of the animals MRVs within a session, resultant vector lengths (RVL) were computed per session. A RVL of 1 indicates that the data sample is more concentrated around the MRV and a RVL of 0 indicates that the data is less concentrated around the MRV. A circular analog of a two‐way ANOVA [Harrison‐Kanji test, (Harrison and Kanji, 1986)] was used to determine whether the MRVs changed significantly over sessions and between groups. A nonparametric Friedman's test was used to determine any longitudinal changes in the RVLs of the two groups. To quantify the difference in magnitude of phase resetting between conditioned and pseudo‐conditioned animals, Cohen's d index of effect size was calculated as , where is the mean RVL of the conditioned group, is the mean RVL of the pseudo‐conditioned group, and , where and is the standard deviation of the RVL of the conditioned and pseudo‐conditioned groups, respectively. A bootstrap sampling method (10,000 iterations) with replacements was used to compute confidence limits for effect size at 95% confidence. A similar bootstrapping procedure was used to compute 95% bootstrapped confidence limits for theta amplitudes.
RESULTS
Behavior
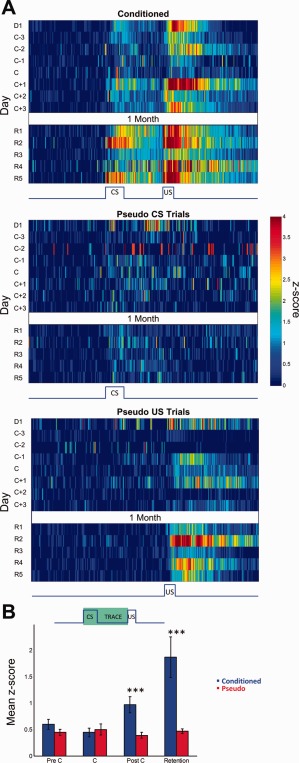
Trace EBC consisted of 250 ms whisker vibrations as the CS and a 150 ms corneal airpuff as the US, where the CS offset and US onset were separated in time by a 500 ms stimulus‐free trace interval (Fig. 1A). Pseudo‐conditioning consisted of random presentations of unpaired, CS‐only and US‐only trials (Fig. 1A). Behavioral and electrophysiological recording data from conditioned animals were examined in relation to the day of achieving a behavioral criterion (8 CRs within a sliding set of 10 trials within a session). This enabled comparison of data across animals that learned at different rates. Conditioned rabbits readily acquired trace EBC and continued to exhibit CRs when brought back for sessions after a one month training‐free hiatus, whereas pseudo‐conditioned rabbits failed to exhibit more than 10% CRs during CS‐only trials of any session (Fig. 1B). During acquisition sessions, a significant increase in the percentage of CRs was observed for conditioned animals but not for pseudo‐conditioned rabbits (Repeated measures ANOVA, interaction: F 7,49 = 36.07, P < 0.0001; ANOVA conditioned group, F 7,28 = 45.04, P < 0.001; ANOVA pseudo group, ns). This difference between groups was maintained during retention sessions (Repeated measures ANOVA, Main effect group F 1,7 = 533.36, P < 0.0001) and both groups continued to exhibit a similar percentage of CRs as on the last day of acquisition training (Conditioned group, 78 ± 5% on C+3 vs. 81 ± 3% on R1:R5; Pseudo group CS‐Trials, 6 ± 2% on C+3 vs. 7 ± 1% on R1:R5).
Figure 1.
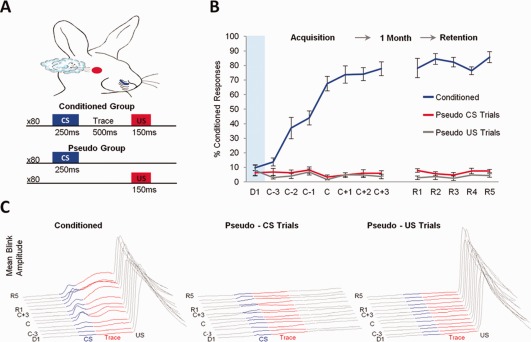
Experimental design and behavioral results. (A) Training protocol for conditioned and pseudo‐conditioned animals. Pseudo‐conditioned animals received random presentations of CS‐alone and US‐alone trials. (B) Mean percent CRs exhibited by conditioned (n = 5) and pseudo (n = 4) rabbits during acquisition and retention of trace EBC. During acquisition, performance was plotted in relation to the day of achieving learning criterion. D1: Day 1, C: Criterion, R: Retention. Error bars represent SEM among subjects. Conditioned animals exhibited a significant increase in the percentage of CRs, whereas pseudo‐conditioned rabbits did not (Repeated measures ANOVA, interaction: F 7,49 = 36.07, P < 0.0001; ANOVA conditioned group, F 7,28 = 45.04, P < 0.001; ANOVA pseudo group, ns). This group difference was maintained during retention sessions (Repeated measures ANOVA, Main effect group F 1,7 = 533.36, P < 0.0001) (C) Average normalized blink amplitudes of conditioned and pseudo‐conditioned animals across training sessions. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Time‐Dependent Analysis of CA1 Hippocampal Activity by Response Type and Group
The activity of 1,426 putative CA1 pyramidal neurons from conditioned rabbits and 808 neurons from pseudo‐conditioned rabbits was analyzed for acquisition sessions. During retention sessions, a total of 732 CA1 pyramidal neurons from conditioned rabbits and 646 neurons from pseudo‐conditioned rabbits were analyzed (Fig. 2A). Recorded pyramidal neurons were histologically verified to be acquired from the dorsal CA1 stratum pyramidale (Fig. 2B). Pyramidal neurons were differentiated from interneurons based on several extracellular features including spontaneous firing rate, spike width (Fox and Ranck, 1975), and firing probability in relation to theta oscillations and sharp‐wave ripples (Figs. 2C,D; see Materials and Methods section). The latter two methods, based on results of juxtacellular recording studies with use of morphological imaging (Klausberger et al., 2003), greatly improve pyramidal neuron discrimination since discrimination based on spontaneous firing rate and spike width alone is not definitive due to the myriad of interneurons that exist with diverse morphological and physiological features (Maccaferri and Lacaille, 2003).
Pyramidal neurons were separated into those that exhibited either a significant stimulus induced increase (RI) or decrease (RD) in activity during the CS‐Trace interval compared to a 1 s baseline period according to a Mann–Whitney U test (Fig. 3). The percentage of responsive neurons was significantly larger for conditioned animals than pseudo animals during both acquisition (39% Conditioned vs. 19% Pseudo, χ 2(1, N = 711) = 96.94, P < 0.001) and retention sessions (41% Conditioned vs. 18% Pseudo, χ 2(1, N = 420) = 83.44, P < 0.001). To examine how the population response patterns of the neurons changed across acquisition and retention sessions, peristimulus time histograms encompassing 1 s before and 2 s after CS onset for each neuron were generated, then normalized relative to 1 s pre‐CS baseline activity. Then, a mean population response of RI and of RD neurons was calculated for each training session (Figs. 4A and 5A).
Figure 3.
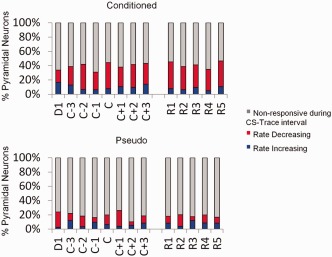
Percentage of responsive CA1 pyramidal neurons. The Mann–Whitney U nonparametric statistical test comparing 1 s pre‐CS baseline activity to activity during the CS and Trace intervals of the trial were used to separate responsive neurons from nonresponsive neurons and to classify the responsive neurons as rate increasing (RI) or rate decreasing (RD). The percentage of RI, RD, and nonresponsive neurons of the total number of recorded pyramidal neurons (y‐axis) are plotted over training sessions (x‐axis).
Figure 4.
Activity of rate increasing neurons. (A) Mean z‐score peristimulus time histograms encompassing 1 s before and 2 s after CS onset (x‐axis) expressed in pseudo‐color for trials with paired CS‐US presentations from conditioned animals (top) and CS‐only (middle) and US‐only (bottom) trials of pseudo‐conditioned animals over training days (y‐axis). The activity of the US‐only trials from pseudo‐conditioned animals are from the same cells that were responsive during the CS‐only trials. D1: Day 1, C: Criterion, R: Retention. (B) Average CS‐Trace interval z‐scores (y axis) for conditioned and pseudo‐conditioned subjects across learning stages (x axis). Error bars represent SEM preserving the variability among neurons. Two‐way ANOVA indicated a significant group × session interaction in mean CS‐Trace z‐score activity (Interaction: F 3,223 = 4.04, P = 0.008). Activity were significantly different among the groups on PostC and Retention sessions (Bonferroni corrected post hoc two‐tailed unpaired t‐tests, *** P < 0.001).
Figure 5.
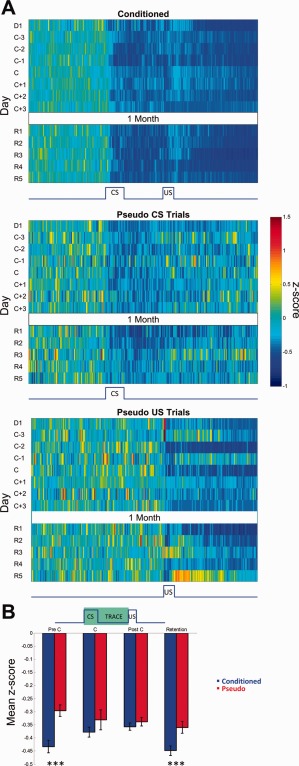
Activity of rate decreasing neurons. (A) Mean z‐score peristimulus time histograms encompassing 1 s before and 2 s after CS onset (x axis) expressed in pseudo‐color for trials with paired CS‐US presentations from conditioned animals (top) and CS‐only (middle) and US‐only (bottom) trials of pseudo‐conditioned animals over training days (y axis). The activity of the US‐only trials from pseudo‐conditioned animals are from the same cells that were responsive during the CS‐only trials. D1: Day 1, C: Criterion, R: Retention. (B) Average CS‐Trace interval z‐scores (y axis) for conditioned and pseudo‐conditioned subjects across learning stages (x axis). Error bars represent SEM preserving the variability among neurons. Two‐way ANOVA indicated a significant group difference in the magnitude of rate decrease (Main effect of group: F 1,729 = 6.88, P < 0.01). Bonferroni corrected two‐tailed unpaired t tests revealed significant group differences on PreC and Retention sessions (*** P < 0.001).
RI Neurons
Analysis of the mean CS and Trace interval z‐scores of RI neurons of both groups for precriterion, criterion, post‐criterion, and retention sessions indicated a significant group x session interaction for both intervals (CS interval two‐way ANOVA, F3,223 = 2.98, P = 0.03; Trace interval two‐way ANOVA, F3,223 = 3.34, P = 0.02). The memory trace is believed to be triggered by the CS and persist into the trace interval (Kalmbach et al., 2009). Given the similar results between the two periods the responses during the CS and Trace periods were combined and analyzed together for subsequent analyses to reflect the entirety of the CS‐triggered memory trace (Fig. 4B). The mean z‐score of RI neurons for the CS‐Trace intervals revealed a significant group × session interaction (Fig. 4B, two‐way ANOVA, F 3,223 = 4.04, P = 0.008) where RI neurons of conditioned animals exhibited a significant increase in mean CS‐Trace interval z‐scores over training sessions (one‐way ANOVA: F 3,121 = 4.61, P < 0.005) and RI neurons of pseudo‐conditioned animals did not. Bonferroni corrected post hoc unpaired t‐tests revealed no significant difference in the mean CS‐Trace interval z‐score of RI neurons between conditioned and pseudo groups during pre‐criterion and criterion sessions, but a significant difference was observed for post‐criterion (P < 0.001) and retention sessions (P < 0.001).
According to SCT and based on the results of hippocampal lesion studies (Moyer et al., 1990; Kim et al., 1995; Takehara et al., 2003), we hypothesized an initial increase in hippocampal activity during early phases of acquisition followed by a gradual decline in activity that would continue well into retention sessions where the CS‐US associations are purportedly extra‐hippocampal. Our results however do not support this view. First, while more neurons were recruited for learning as seen in the larger percentage of responsive neurons in conditioned animals than pseudo animals (Fig. 3), we did not observe a significant increase in mean z‐scores of conditioned CA1 RI neurons during initial acquisition sessions compared to those of pseudo‐conditioned control animals. The similar magnitude of rate modulation between neurons of conditioned and pseudo animals during precriterion sessions suggest that in conditioned animals, dorsal CA1 neurons are possibly encoding the association between the CS and US within the training environment whereas, in the pseudo animals, dorsal CA1 neurons are encoding the nonpaired occurrence of the CS or US within the training environment. Thus, learning during acquisition might be reflected more by neuronal recruitment rather than rate‐modulation.
Second, the robust increase in CS‐Trace interval RI activity observed in postcriterion and retention sessions is unexpected based upon SCT and findings of previous behavioral lesion studies where hippocampal lesions were not found to impair expression of remotely acquired CRs (Kim et al., 1995; Takehara et al., 2003). Consequently, data reported here actually indicate robust involvement of dorsal CA1 in the recall of remotely acquired associations.
RD Neurons
In addition to RI activity, we also examined the response patterns of RD CA1 neurons during recent and remote trace EBC (Fig. 5). Overall, the magnitude of rate decrease for conditioned animals was significantly larger (lower z‐scores) than those of control animals (Fig. 5B, conditioned: −0.413 ± 0.01 SEM; pseudo: −0.342 ± 0.012 SEM, two‐way ANOVA main effect of group: F 1,729 = 6.88, P < 0.01) but unlike RI neurons, RD neurons did not exhibit a significant group × session interaction in mean CS‐Trace interval z‐score activity. Interestingly, the majority of responsive neurons recorded in this study were classified as RD rather than RI for both groups (Conditioned: 30% RD vs. 9% RI, χ 2(1, N = 859) = 293.01, P < 0.001; Pseudo: 11% RD vs. 7% RI, χ 2(1, N = 272) = 12.72, P < 0.001). In addition, the duration of rate decrease of conditioned group RD neurons over acquisition and retention sessions lasted for an average of 3.53 ± 0.14 s as compared to 1.18 ± 0.10 s for pseudo group RD neurons during CS‐only trials (Fig. 6). The duration of rate decrease for RD neurons from pseudo‐conditioned animals during US‐only trials was 3.29 ± 0.12 s. The similar duration of rate decrease between conditioned RD neurons and pseudo RD neurons during US‐only presentations suggest that the long duration of rate‐decrease arises largely out of presentations of salient, behaviorally relevant cues. From a functional point of view, activity of RD neurons in conditioned animals may increase the signal‐to‐noise ratio of the regional network, thereby allowing other responsive neurons in the region (e.g., RI neurons) to exert a larger effect on the overall neuronal circuit that are pertinent to the encoding, consolidation, and retrieval of the CS‐US association. The large magnitude of RD responses we observed here in vivo is convergent with ex vivo data we recently reported where a substantial increase in CA1 interneuron activity was observed (i.e., more inhibition onto CA1 pyramidal neurons) after learning (McKay, 2013).
Figure 6.
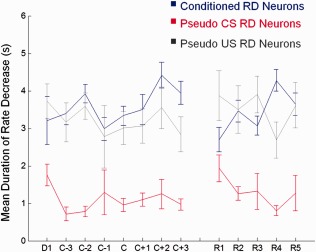
Mean duration of rate decrease. The mean duration of rate decrease (y axis) for RD neurons recorded within a session from conditioned animals (blue) and pseudo‐conditioned animals during CS‐only (red) and US‐only presentations (gray) were plotted across sessions (x axis). The mean duration of rate decrease over all sessions was 3.53 ± 0.14 s for conditioned animals, 1.18 ± 0.10 s for CS‐only trials from pseudo‐conditioned animals, and 3.29 ± 0.12 s for US‐only trials from pseudo‐conditioned animals. The similar duration of rate decrease between conditioned RD neurons and pseudo RD neurons during US‐only presentations suggest that the long decrease observed in conditioned group neurons arise largely out of the salient, US presentations and may contribute to increasing the signal‐to‐noise of the regional network to enhance the encoding, consolidation, and retrieval of behaviorally relevant information.
Theta Oscillations Are Specific to Behaviorally Relevant Stimuli During Successive Stages of Memory
Accumulating evidence has emphasized the importance of the theta rhythm to learning and memory (Hasselmo and Eichenbaum, 2005). To examine how theta activity varies at successive stages of learning and memory, we examined stimulus‐induced theta phase resetting and changes in theta amplitude between conditioned and pseudo‐conditioned rabbits. In conditioned animals, presentation of the CS and US induced theta phase resetting as evidenced by the strong synchronization of the mean resultant vectors (circular mean direction) that occurred upon stimuli presentation (Fig. 7A). This effect was observed throughout acquisition and retention of trace EBC. In pseudo‐conditioned animals, CS‐alone presentations did not induce phase resetting. These observations were confirmed by a Harrison–Kanji test performed on the mean resultant vector over early acquisition (EA, D1∼C‐1), late acquisition (LA, C∼C+3), and retention sessions (R, R1∼R5), which indicated a significant group (conditioned vs. pseudo‐conditioned) × session interaction for the mean resultant vector during the CS interval (χ 2(4) = 27.15, P < 0.001). Phase resetting was observed during US‐alone presentations of pseudo‐conditioned animals, suggesting that behaviorally relevant stimuli, whether it be a salient US or a CS that conveys stimulus contingencies, induce phase resetting.
Figure 7.
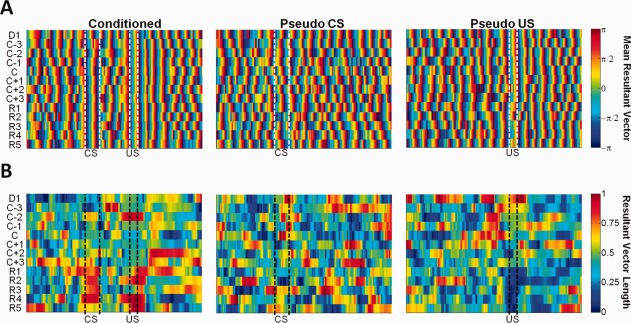
Theta phase resetting. (A) Circular statistics were used to compute the mean resultant vector indicating the preferred theta phase for paired trials of conditioned animals (left) and CS‐alone (middle) and US‐alone trials (right) of pseudo‐conditioned animals across training sessions (y axis). (B) The resultant vector length, indicating the degree at which the data sample is concentrated around the mean direction (1 = concentrated; 0 = nonconcentrated), is shown below each preferred theta phase plot in pseudo‐color.
To quantify the magnitude of phase resetting, we examined the resultant vector length (RVL) among the animals, which describes the degree at which the phase is concentrated around the mean direction (value of 1 indicates uniformity while 0 indicates randomness, Fig. 7B). Results indicated that over EA, LA, and R sessions, the mean RVL during the CS and US interval of conditioned animals increased longitudinally as confirmed by a Friedman's test (CS: 0.28 at EA, 0.49 at LA, 0.77 at R, (χ 2(2) = 6.5, P = 0.03; US: 0.58 at EA, 0.41 at LA, 0.77 at R (χ 2 (2) = 6.5, P = 0.03). In Pseudo‐conditioned animals, no significant change in the mean RVL was observed over sessions (CS: 0.37 at EA, 0.35 at LA, 0.28 at R, χ 2(2) = 0.5, P = 0.78; US: 0.48 at EA, 0.35 at LA, 0.21 at R, χ 2(2) = 3.5, P = 0.17). We further computed the effect size (equivalent to a z‐score of a standard normal distribution), which quantifies the difference between the two groups' mean RVL values, in conjunction with a bootstrap sampling method with replacements to obtain 95% confidence limits. For the CS interval, the effect size gradually increased over sessions [EA: −1.01 (−1.73, 4.29); LA: 1.28 (−0.91, 7.19); Retention: 6.84 (1.40, 13.40)]. A similar trend for the US interval was observed where the effect size was greatest during retention sessions [EA: 0.09 (−1.94, 35.90); LA: 0.49 (−6.36, 50.56); R: 19.56 (1.85, 62.70)]. Collectively, these results indicate that when learning occurs, the strength of phase resetting to behaviorally relevant stimuli becomes greater over successive stages of memory.
We further examined changes in the instantaneous amplitude of theta oscillations across sessions. Theta amplitudes within a trial were normalized to a 1 s baseline period for each animal, averaged across a session, then averaged across animals (Fig. 8). In conditioned animals, theta amplitudes were specific to CS and US stimuli throughout acquisition and remote retention of trace EBC. In comparison, theta amplitudes from US trials but not CS trials of pseudo‐conditioned animals exhibited longitudinal specificity to the stimuli. These observations were confirmed by a 3‐way mixed‐design ANOVA of interval (CS vs. Trace) × group × session, which indicated a significant interval × group interaction (F 1,207 = 7.79, P = 0.01) where the mean theta amplitude z‐score was greater during the CS interval than the trace interval in paired trials of conditioned animals (CS: 0.13 ± 0.04, 95% bootstrapped confidence limits (0.103, 0.214); Trace: 0.03 ± 0.04, 95% bootstrapped confidence limits (0.006, 0.100); F 1,51 = 6.70, P = 0.01) but not in CS‐only trials of pseudo‐conditioned animals (CS: 0.12 ± 0.02, 95% bootstrapped confidence limits (0.109, 0.168); Trace: 0.17 ± 0.04, 95% bootstrapped confidence limits (0.150, 0.250); F 1,51 = 1.585, P = 0.21). US interval theta amplitudes did not differ between paired trials of conditioned animals and US‐trials of pseudo‐conditioned animals [conditioned: 0.23 ± 0.06, 95% bootstrapped confidence limits (0.192, 0.340); pseudo‐conditioned: 0.32 ± 0.05, 95% bootstrapped confidence limits (0.294, 0.410)]. Although theta amplitude during the CS interval appeared highest during acquisition for the conditioned animals, such a trend was not supported statistically. (No significant session × interval interaction was observed, F 12,207 = 1.15, P = 0.37). These results further support the notion that hippocampal theta activity is specific to behaviorally relevant stimuli throughout both acquisition and retention of associative memories.
Figure 8.
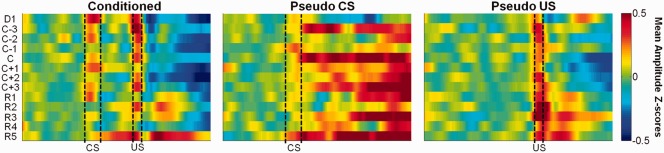
Theta amplitude. Mean instantaneous theta amplitude in z‐scores are expressed in pseudo‐color for paired trials of conditioned animals (left) and CS‐alone (middle) and US‐alone trials (right) of pseudo‐conditioned animals across training sessions (y axis).
DISCUSSION
The present study, aimed to characterize longitudinally how hippocampal activity changes over successive stages of memory as memories are encoded, consolidated, and retrieved. Our results provide insight into the normal physiological responses at the single neuron and field potential levels that occur during acquisition and remote retention of trace EBC and provide compelling support for a continued role of the hippocampus during remote time points. The exact role of hippocampal engagement during remote time points is however, subject to interpretation and further investigation.
One possible interpretation is that the present results are counter to SCT and support theories that posit continued hippocampal involvement at remote time points. In particular, increased activity in dorsal CA1 neurons during post learning and remote retention are consistent with views such as multiple trace theory (MTT) and reconsolidation theory. Specifically, MTT posits that episodic memories are qualitatively transformed into semantic memories via the formation of distinct traces every time memories are retrieved. According to MTT, memories initially stored in hippocampal‐cortical circuits are episodic (context‐rich). Reactivations of the memory create multiple distinct traces from which the common information, i.e., the semantics which lack contextual detail, is extracted and integrated within pre‐existing semantic networks in the cortex (Nadel and Moscovitch, 1997; Moscovitch and Nadel, 1998; Moscovitch et al., 2005; Winocur et al., 2010; McKenzie and Eichenbaum, 2011). Thus, episodic memories rich in contextual detail require hippocampal activation whereas, semantic memory is mediated independently of the hippocampus. The response of neurons observed in the present study during remote retrieval could therefore reflect reactivation of episodic components of the CS‐US association within each memory trace.
The findings here may also be consistent with reconsolidation theory. According to SCT, consolidated memories are believed to be stable. However, Pavlovian fear conditioning studies have suggested that consolidated fear memories, upon recall, become labile since they are affected by immediate infusions of protein synthesis inhibitors but not by infusions made hours later (Nader et al., 2000). These studies suggest that reactivated memories require subsequent consolidation (i.e., reconsolidation) to prevent their erasure (Nader et al., 2000; Sara, 2000; McKenzie and Eichenbaum, 2011). The increased hippocampal RI activity observed during retention sessions could reflect a process of stabilizing a reactivated and labile memory trace.
Additionally, the present results may be consistent with a model of the functional organization of the medial temporal lobe proposed by Eichenbaum and colleagues. They propose that initial encoding of memories involves convergence within the hippocampus of information about events and their context. The outputs of hippocampal processing are sent back to widespread neocortical areas via the parahippocampal cortex. Then, during memory retrieval, a previously experienced cue drives the circuit to reactivate the convergent representation in the hippocampus, which allows for the recall of contextually‐rich memories (Eichenbaum et al., 2012). The increase in RI activity observed during retention sessions of conditioned rabbits may therefore reflect a reactivation of the convergent representation of the CS‐US association within the testing environment. Thus, the hippocampus is consistently engaged during memory acquisition and whenever detailed episodic information is recalled. This is in agreement with reports of amnesic patients, including patient H.M., which showed spared semantic memory but severe autobiographical memory impairments with no temporal gradient (Steinvorth et al., 2005).
However, the present results do not refute SCT entirely. A common misconception is that if the hippocampus is not required for the behavioral expression of consolidated memories as reported by lesion studies, then it is expected that hippocampal neurons are not engaged at remote time points. This is not necessarily true. Engagement of hippocampal neurons during remote memory retrieval, as we report, may be related to accessing certain aspects of the memory representation (e.g., rich episodic detail), which are not necessary for the actual behavioral expression of the CR itself. The internal representation for the significance of the CS that leads to expression of a CR may be mediated by other neural structures, such as the neocortex as SCT posits. A prime example exists in the place cell literature where hippocampal place cells exhibit spatial firing patterns even during random foraging for food (O'Keefe and Dostrovsky, 1971; O'Keefe and Conway, 1978), an act that is not hippocampal‐dependent. But place cells also carry context‐specific information as demonstrated by conjunctive item‐place coding during hippocampal‐dependent conditional discrimination tasks (Komorowski et al., 2013, 2009). Thus, the present electrophysiological results do not address whether the representation of the CS‐US association is purged from the hippocampus after consolidation or if the expression of remote memories becomes independent of the hippocampus after consolidation.
Another interesting possibility, consistent with SCT, is that the hippocampal activity we observed after memory consolidation could reflect activation as a result of input from cortical regions where the memory is permanently stored, such as the prefrontal cortex (Squire and Alvarez, 1995; Powell et al., 2001; Takehara et al., 2003; Frankland and Bontempi, 2005; Takehara‐Nishiuchi and McNaughton, 2008; Oswald et al., 2010; Hattori et al., 2014). Given the complex patterns of afferent drive from higher order cortical regions, such input would seem quite feasible. Future work, using a combination of electrophysiological recordings and neuronal inactivation techniques, can seek to examine longitudinal interactions between multiple cognitive systems in order to dissociate hippocampal involvement from requirement for remote memory retrieval.
The activity of rate‐decreasing neurons is of additional interest given that the responses were observed throughout successive stages of memory, demonstrating further evidence for continued hippocampal engagement during remote time points. Specifically, RD neurons of conditioned animals exhibited much larger magnitudes of rate‐decrements than neurons of pseudo‐conditioned animals. RD neurons also exhibited longer durations of rate decrements in response to behaviorally relevant stimuli. We speculate that these responses may be involved in augmenting the signal‐to‐noise of the regional network to help propagate the signals of other neurons involved in the encoding and retrieval of trace EBC more efficiently. Though further work is needed to test the functional role(s) of RD neurons, from a neural coding perspective, neurons that exhibit rate decrements are just as likely as neurons that exhibit rate increases to convey meaningful information in sculpting cognition and behavior (Maunsell et al., 1991; Miller and Desimone, 1994; Romo et al., 1999; Pagan and Rust, 2014), as evidenced by several recent studies that highlight this important class of responses (Wirth et al., 2003; Yanike et al., 2004; Hattori et al., 2014). Thus the present results represent an incentive that will further motivate the investigation of the roles of these response types during cognitive processes.
In addition to the single neuron recording results, the observation that CA1 theta oscillations (phase and amplitude) are specific to behaviorally relevant stimuli throughout successive stages of memory provide further support for continued hippocampal involvement during remote memory retrieval. Neural oscillations are generally thought to reflect the synchronous activity of large populations of neurons. The theta rhythm in particular is believed to be critical for managing information processing of activated neuronal ensembles via modification of synaptic weights and thereby regulating alternating phases of encoding and retrieval (Brankack et al., 1993; Buzsaki, 2002; Hasselmo et al., 2002; Hyman et al., 2003; Hasselmo and Eichenbaum, 2005). Specifically, various studies have demonstrated that induction of long‐term potentiation (LTP) and depression are closely associated with the theta rhythm (Larson et al., 1986; Pavlides et al., 1988; Orr et al., 2001; Hyman et al., 2003), and that stimuli‐induced theta resetting (Buzsaki et al., 1979; Givens, 1996; Tesche and Karhu, 2000) produce optimal conditions for LTP induction (McCartney et al., 2004). Consistent with these observations, pharmacological manipulations of theta activity was shown to affect memory processes where blockage led to impairments (Winson, 1972; Givens and Olton, 1990; Mizumori et al., 1990; Pan and McNaughton, 1997) and facilitation led to improvements (Kinney et al., 1999; Markowska et al., 1995) in performance in various memory paradigms. As such, the stimuli‐specific theta activity observed in the present study during acquisition and remote retention may reflect the encoding and subsequent strengthening of existing representations of behaviorally relevant cues through modification of synaptic plasticity. Given the observations that theta amplitude was significantly different between groups but not across sessions, the importance of theta in regulating information processing appears to be consistent throughout all stages of memory for the conditioned rabbits.
The importance of theta rhythms is not restricted to just the hippocampus, however. Numerous studies have demonstrated that synchronized theta activity between brain regions is critical for memory (von Stein and Sarnthein, 2000; Fell et al., 2003; Hoffmann and Berry, 2009; Anderson et al., 2010; Wikgren et al., 2010; Takehara‐Nishiuchi et al., 2011). Functionally, synchronization of neural oscillatory signals among brain regions may enhance neural communication and promote synaptic plasticity between two or more brain areas, thus enabling large scale integration of sensory stimuli and top‐down processing to produce coherent and behaviorally adequate responses (von Stein and Sarnthein, 2000; Varela et al., 2001; Fell and Axmacher, 2011). Given our results, theta synchrony and neural communication among the hippocampus and other brain regions is likely not just during acquisition of memories, but during remote memory retrieval as well. This process could form the basis for not only the encoding and consolidation of memories in hippocampal‐cortical networks, but for re‐linking of individual episodic components of a memory trace within the hippocampus for the retrieval of remotely acquired associations.
While the present study provides electrophysiological support for continued hippocampal involvement during remote memory recall, how can the present results be reconciled with the findings of lesion studies (Moyer et al., 1990; Kim et al., 1995; Takehara et al., 2003) demonstrating a time‐limited role of the hippocampus during trace EBC acquisition and retention? A plausible explanation is that the hippocampus is normally involved in remote memory recall but hippocampal lesions result in compensatory mechanisms that enable successful memory retrieval after consolidation, or the retrieved memory following hippocampal lesions is not qualitatively similar to that from an intact subject. A study by Goshen et al. (2011) examined real‐time contribution of dorsal CA1 neurons to remote fear conditioning memory. They showed that recall of remotely acquired fear memory can be reversibly abolished by temporally precise optogenetic inhibition of CA1, but not by extended optical stimulation that matches the typical time course of pharmacological inhibition (i.e., hippocampal‐dependence of remote memory converted to hippocampal‐independence), thereby suggesting that long‐term memory retrieval normally depends on the hippocampus but can adaptively shift to alternate structures (Goshen et al., 2011). In fact, altered hippocampal activity has been shown to be more detrimental than ablation of the entire structure for delay EBC conditioning (Solomon et al., 1983). Since both trace EBC and contextual fear conditioning require the hippocampus (Kim and Fanselow, 1992), a similar process may be engaged during remote trace EBC recall.
As discussed above, the findings of the current study have important implications for theories of memory consolidation. While we view the heightened activity during remote trace EBC to be reflective of mnemonic processing, alternate explanations are possible. For example, the activity during remote trace EBC can be argued to reflect heightened fear or attention developed 1 month after conditioning. However, the hippocampus is not traditionally regarded as an area mediating fear or attentions, which are instead believed to be mediated more by the amygdala (Davis, 1992; Rogan et al., 1997; LeDoux, 2003) and prefrontal cortex (Knight et al., 1995; Bush et al., 1998; Carter et al., 1999; Daffner et al., 2000), respectively. Nevertheless, emotional arousal and attention are closely associated with learning and memory, thus the activity observed during remote time points is likely to include interactions with additional cognitive systems.
The present study provides for the first time, longitudinal insight into the electrophysiological response patterns of CA1 neurons during acquisition and retention of a hippocampal‐dependent memory. These results offer compelling evidence for continued hippocampal involvement during remote memory recall and cast doubt on the view that the hippocampus plays a time‐limited role in memory processes. Electrophysiological results obtained during successive stages of memory, such as the ones we report, can be used to model the normal functional organization of the intact hippocampal system. Such studies will be invaluable to translational efforts being undertaken to restore memory function in individuals with amnesia, including efforts using nootropics and pharmacological approaches (Burgdorf et al., 2011; Shema et al., 2011), and those using neuroprosthetic approaches to develop biomimetic, artificial hippocampi (Hampson et al., 2011; Robinson et al., 2012). These findings may compel further longitudinal in vivo recording studies in multiple hippocampal subregions and associated areas during a variety of behavioral paradigms to dissociate the functional role(s) and organization of the medial temporal lobe during the encoding, consolidation, and retrieval of declarative memories.
Acknowledgments
The authors thank Dr. Matthew Oh for helpful comments about the manuscript and Dr. Jody Dyan Ciolino of the Northwestern University Biostatistics Collaboration Center for assistance with statistical analyses.
REFERENCES
- Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M. 2010. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cereb Cortex 20:1604–1612. [DOI] [PubMed] [Google Scholar]
- Brankack J, Stewart M, Fox SE. 1993. Current source density analysis of the hippocampal theta rhythm: Associated sustained potentials and candidate synaptic generators. Brain Res 615:310–327. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Weiss C, Matthews E, Disterhoft JF, Stanton PK, Moskal JR. 2011. The N‐methyl‐d‐aspartate receptor modulator GLYX‐13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol Aging 32:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. 1998. The counting Stroop: An interference task specialized for functional neuroimaging—Validation study with functional MRI. Hum Brain Mapp 6:270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. 2002. Theta oscillations in the hippocampus. Neuron 33:325–340. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Grastyan E, Tveritskaya IN, Czopf J. 1979. Hippocampal evoked potentials and EEG changes during classical conditioning in the rat. Electroencephalogr Clin Neurophysiol 47:64–74. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. 1999. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci 10:49–57. [DOI] [PubMed] [Google Scholar]
- Clark RE. 2011. Eyeblink conditioning and systems consolidation: An ironic yet powerful pairing. Neurobiol Learn Mem 95:118–124. [DOI] [PubMed] [Google Scholar]
- Corkin S. 1984. Lasting consequences of bilateral medial temporal lobectomy—clinical course and experimental findings in Hm. Semin Neurol 4:249–259. [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. 1999. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci 19:274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P. 2000. The central role of the prefrontal cortex in directing attention to novel events. Brain 123 (Pt 5):927–939. [DOI] [PubMed] [Google Scholar]
- Das S, Weiss C, Disterhoft JF. 2001. Eyeblink conditioning in the rabbit (Oryctolagus cuniculus) with stimulation of the mystacial vibrissae as a conditioned stimulus. Behav Neurosci 115:731–736. [DOI] [PubMed] [Google Scholar]
- Davis M. 1992. The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15:353–375. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. 2012. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev 36:1597–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Axmacher N. 2011. The role of phase synchronization in memory processes. Nat Rev Neurosci 12:105–118. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernandez G. 2003. Rhinal‐hippocampal theta coherence during declarative memory formation: Interaction with gamma synchronization? Eur J Neurosci 17:1082–1088. [DOI] [PubMed] [Google Scholar]
- Fox SE, Ranck JB Jr. 1975. Localization and anatomical identification of theta and complex spike cells in dorsal hippocampal formation of rats. Exp Neurol 49 (1 Pt 1):299–313. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. 2005. The organization of recent and remote memories. Nat Rev Neurosci 6:119–130. [DOI] [PubMed] [Google Scholar]
- Givens B. 1996. Stimulus‐evoked resetting of the dentate theta rhythm: Relation to working memory. Neuroreport 8:159–163. [DOI] [PubMed] [Google Scholar]
- Givens BS, Olton DS. 1990. Cholinergic and GABAergic modulation of medial septal area: Effect on working memory. Behav Neurosci 104:849–855. [DOI] [PubMed] [Google Scholar]
- Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K. 2011. Dynamics of retrieval strategies for remote memories. Cell 147:678–689. [DOI] [PubMed] [Google Scholar]
- Green JD, Arduini AA. 1954. Hippocampal electrical activity in arousal. J Neurophysiol 17:533–557. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Marmaralis V, Shin DC, Gerhardt GA, Song D, Chan RH, Sweatt AJ, Granacki J, Berger TW, Deadwyler SA. 2011. Restorative encoding memory integrative neural device: “REMIND”. Conf Proc IEEE Eng Med Biol Soc 2011:3338–3341. [DOI] [PubMed] [Google Scholar]
- Harrison D, Kanji GK. 1986. Analysis of variance for circular data. J Appl Stat 13:123–138. [Google Scholar]
- Hasselmo ME, Eichenbaum H. 2005. Hippocampal mechanisms for the context‐dependent retrieval of episodes. Neural Net 18:1172–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelon C, Wyble BP. 2002. A proposed function for hippocampal theta rhythm: Separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput 14:793–817. [DOI] [PubMed] [Google Scholar]
- Hattori S, Yoon T, Disterhoft JF, Weiss C. 2014. Functional reorganization of a prefrontal cortical network mediating consolidation of trace eyeblink conditioning. J Neurosci 34:1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann LC, Berry SD. 2009. Cerebellar theta oscillations are synchronized during hippocampal theta‐contingent trace conditioning. Proc Natl Acad Sci U S A 106:21371–21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. 2003. Stimulation in hippocampal region CA1 in behaving rats yields long‐term potentiation when delivered to the peak of theta and long‐term depression when delivered to the trough. J Neurosci 23:11725–11731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD. 2009. Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learn Mem 16:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. 1995. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci 109:195–203. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. 1992. Modality‐specific retrograde amnesia of fear. Science 256:675–677. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Patino P, Mermet‐Bouvier Y, Starrett JE Jr, Gribkoff VK. 1999. Cognition‐enhancing drugs increase stimulated hippocampal theta rhythm amplitude in the urethane‐anesthetized rat. J Pharmacol Exp Ther 291:99–106. [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. 2003. Brain‐state‐ and cell‐type‐specific firing of hippocampal interneurons in vivo. Nature 421:844–848. [DOI] [PubMed] [Google Scholar]
- Knight RT, Grabowecky MF, Scabini D. 1995. Role of human prefrontal cortex in attention control. Adv Neurol 66:21–34; discussion 34–36. [PubMed] [Google Scholar]
- Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, Eichenbaum H. 2013. Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J Neurosci 33:8079–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. 2009. Robust conjunctive item‐place coding by hippocampal neurons parallels learning what happens where. J Neurosci 29:9918–9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. 1986. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long‐term potentiation. Brain Res 368:347–350. [DOI] [PubMed] [Google Scholar]
- LeDoux J. 2003. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23:727–738. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Lacaille JC. 2003. Interneuron Diversity series: hippocampal interneuron classifications—Making things as simple as possible, not simpler. Trends Neurosci 26:564–571. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Olton DS, Givens B. 1995. Cholinergic manipulations in the medial septal area: Age‐related effects on working memory and hippocampal electrophysiology. J Neurosci 15(3 Pt 1):2063–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo‐Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. 2004. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5:793–807. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Sclar G, Nealey TA, DePriest DD. 1991. Extraretinal representations in area V4 in the macaque monkey. Vis Neurosci 7:561–573. [DOI] [PubMed] [Google Scholar]
- McCartney H, Johnson AD, Weil ZM, Givens B. 2004. Theta reset produces optimal conditions for long‐term potentiation. Hippocampus 14:684–687. [DOI] [PubMed] [Google Scholar]
- McKay BM, Oh MM, Disterhoft JF. 2013. Learning increases intrinsic excitability of hippocampal interneurons. J Neurosci 33:5499–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S, Eichenbaum H. 2011. Consolidation and reconsolidation: Two lives of memories? Neuron 71:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Desimone R. 1994. Parallel neuronal mechanisms for short‐term memory. Science 263:520–522. [DOI] [PubMed] [Google Scholar]
- Milner B, Corkin S, Teuber HL. 1968. Further analysis of hippocampal amnesic syndrome—14‐year follow‐up study of Hm. Neuropsychologia 6:215–234. [Google Scholar]
- Mizumori SJ, Perez GM, Alvarado MC, Barnes CA, McNaughton BL. 1990. Reversible inactivation of the medial septum differentially affects two forms of learning in rats. Brain Res 528:12–20. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L. 1998. Consolidation and the hippocampal complex revisited: In defense of the multiple‐trace model. Curr Opin Neurobiol 8:297–300. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. 2005. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat 207:35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR Jr, Deyo RA, Disterhoft JF. 1990. Hippocampectomy disrupts trace eye‐blink conditioning in rabbits. Behav Neurosci 104:243–252. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. 1997. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7:217–227. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. 2000. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722–726. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Conway DH. 1978. Hippocampal place units in the freely moving rat: Why they fire where they fire. Exp Brain Res 31:573–590. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. 1971. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely‐moving rat. Brain Res 34:171–175. [DOI] [PubMed] [Google Scholar]
- Orr G, Rao G, Houston FP, McNaughton BL, Barnes CA. 2001. Hippocampal synaptic plasticity is modulated by theta rhythm in the fascia dentata of adult and aged freely behaving rats. Hippocampus 11:647–654. [DOI] [PubMed] [Google Scholar]
- Oswald BB, Maddox SA, Tisdale N, Powell DA. 2010. Encoding and retrieval are differentially processed by the anterior cingulate and prelimbic cortices: A study based on trace eyeblink conditioning in the rabbit. Neurobiol Learn Mem 93:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan M, Rust NC. 2014. Quantifying the signals contained in heterogeneous neural responses and determining their relationships with task performance. J Neurophysiol 112:1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, McNaughton N. 1997. The medial supramammillary nucleus, spatial learning and the frequency of hippocampal theta activity. Brain Res 764:101–108. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Greenstein YJ, Grudman M, Winson J. 1988. Long‐term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta‐rhythm. Brain Res 439:383–387. [DOI] [PubMed] [Google Scholar]
- Powell DA, Skaggs H, Churchwell J, McLaughlin J. 2001. Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus). Behav Neurosci 115:1029–1038. [DOI] [PubMed] [Google Scholar]
- Robinson BS, Yu GJ, Hendrickson PJ, Song D, Berger TW. 2012. Implementation of activity‐dependent synaptic plasticity rules for a large‐scale biologically realistic model of the hippocampus. Conf Proc IEEE Eng Med Biol Soc 2012:1366–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, LeDoux JE. 1997. Fear conditioning induces associative long‐term potentiation in the amygdala. Nature 390:604–607. [DOI] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernandez A, Lemus L. 1999. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature 399:470–473. [DOI] [PubMed] [Google Scholar]
- Sara SJ. 2000. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem 7:73–84. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Haramati S, Ron S, Hazvi S, Chen A, Sacktor TC, Dudai Y. 2011. Enhancement of consolidated long‐term memory by overexpression of protein kinase Mzeta in the neocortex. Science 331:1207–1210. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. 1996. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6:149–172. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Solomon SD, Schaaf EV, Perry HE. 1983. Altered activity in the hippocampus is more detrimental to classical conditioning than removing the structure. Science 220:329–331. [DOI] [PubMed] [Google Scholar]
- Squire LR. 1992. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev 99:195–231. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. 1995. Retrograde amnesia and memory consolidation: A neurobiological perspective. Curr Opin Neurobiol 5:169–177. [DOI] [PubMed] [Google Scholar]
- Steinvorth S, Levine B, Corkin S. 2005. Medial temporal lobe structures are needed to re‐experience remote autobiographical memories: evidence from H.M. and W.R. Neuropsychologia 43:479–496. [DOI] [PubMed] [Google Scholar]
- Takehara‐Nishiuchi K, Maal‐Bared G, Morrissey MD. 2011. Increased entorhinal‐prefrontal theta synchronization parallels decreased entorhinal‐hippocampal theta synchronization during learning and consolidation of associative memory. Front Behav Neurosci 5:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara‐Nishiuchi K, McNaughton BL. 2008. Spontaneous changes of neocortical code for associative memory during consolidation. Science 322:960–963. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Kirino Y. 2003. Time‐dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci 23:9897–9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesche CD, Karhu J. 2000. Theta oscillations index human hippocampal activation during a working memory task. Proc Natl Acad Sci U S A 97:919–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR Jr, Akase E, Disterhoft JF. 1994. A system for quantitative analysis of associative learning. Part 1. Hardware interfaces with cross‐species applications. J Neurosci Methods 54:109–117. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. 2001. The brainweb: Phase synchronization and large‐scale integration. Nat Rev Neurosci 2:229–239. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. 2000. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int J Psychophysiol 38:301–313. [DOI] [PubMed] [Google Scholar]
- Wikgren J, Nokia MS, Penttonen M. 2010. Hippocampo‐cerebellar theta band phase synchrony in rabbits. Neuroscience 165:1538–1545. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Bontempi B. 2010. Memory formation and long‐term retention in humans and animals: convergence towards a transformation account of hippocampal‐neocortical interactions. Neuropsychologia 48:2339–2356. [DOI] [PubMed] [Google Scholar]
- Winson J. 1972. Interspecies differences in the occurrence of theta. Behav Biol 7:479–487. [DOI] [PubMed] [Google Scholar]
- Wirth S, Yanike M, Frank LM, Smith AC, Brown EN, Suzuki WA. 2003. Single neurons in the monkey hippocampus and learning of new associations. Science 300:1578–1581. [DOI] [PubMed] [Google Scholar]
- Yanike M, Wirth S, Suzuki WA. 2004. Representation of well‐learned information in the monkey hippocampus. Neuron 42:477–487. [DOI] [PubMed] [Google Scholar]


