Abstract
Recent studies demonstrate a differential trajectory for cannabinoid receptor expression in cortical and sub-cortical brain areas across postnatal development. In the present study, we sought to investigate whether chronic systemic exposure to a synthetic cannabinoid receptor agonist causes morphological changes in the structure of dendrites and dendritic spines in adolescent and adult pyramidal neurons in the medial prefrontal cortex (mPFC) and medium spiny neurons (MSN) in the nucleus accumbens (Acb). Following systemic administration of WIN 55,212-2 in adolescent (PN 37–40) and adult (P55–60) male rats, the neuronal architecture of pyramidal neurons and MSN was assessed using Golgi–Cox staining. While no structural changes were observed in WIN 55,212-2-treated adolescent subjects compared to control, exposure to WIN 55,212-2 significantly increased dendritic length, spine density and the number of dendritic branches in pyramidal neurons in the mPFC of adult subjects when compared to control and adolescent subjects. In the Acb, WIN 55,212-2 exposure significantly decreased dendritic length and number of branches in adult rat subjects while no changes were observed in the adolescent groups. In contrast, spine density was significantly decreased in both the adult and adolescent groups in the Acb. To determine whether regional developmental morphological changes translated into behavioral differences, WIN 55,212-2-induced aversion was evaluated in both groups using a conditioned place preference paradigm. In adult rats, WIN 55,212-2 administration readily induced conditioned place aversion as previously described. In contrast, adolescent rats did not exhibit aversion following WIN 55,212-2 exposure in the behavioral paradigm. The present results show that synthetic cannabinoid administration differentially impacts cortical and sub-cortical neuronal morphology in adult compared to adolescent subjects. Such differences may underlie the disparate development effects of cannabinoids on behavior.
Keywords: Medial prefrontal cortex; Nucleus accumbens; Cannabinoid; WIN 55,212-2; Golgi staining
Introduction
Cannabis is the most widely used drug of abuse and its onset of use often begins during adolescence (Crowley et al. 1998; Schneider 2008). Adolescence is a critical phase of development where dynamic cellular and anatomical modifications occur in the brain (De Bellis et al. 2001; de Fonseca and Schneider 2008). Emerging evidence in humans and animal models has shown particular vulnerability in the adolescent brain to drugs of abuse and long-lasting behavioral disruption (Chambers et al. 2003; Schneider 2008). Based on studies of the general population using diagnostic criteria, adolescents and young adults generally exhibit higher rate of experimental use and substance use disorders compared to adults (Wagner and Anthony 2002; Anthony and Petronis 1991; Warner et al. 1995; Grant and Dawson 1997). Moreover, addictive disorders identified in adults most commonly commenced during adolescence or young adulthood (Kandel et al. 1992; Wagner and Anthony 2002). Using animal models, a chronic treatment of a synthetic cannabinoid receptor agonist WIN 55,212-2, during puberty causes persistent behavioral disturbances in adulthood (Schneider and Koch 2003). Acute and chronic WIN 55,212-2 treatment differentially affect recognition and behavior in adolescent versus adult rats (Schneider et al. 2008). Behavioral deficits following acute WIN 55,212-2 treatment were more pronounced during the pubertal period as compared to adult and chronic WIN 55,212-2 treatment-induced persistent recognition and behavioral deficits during the pubertal period compared to adult rats. While synthetic cannabinoid agonist WIN 55,212-2 was utilized in those studies (Schneider and Koch 2003; Schneider et al. 2008), other synthetic cannabinoids have also been shown to impair cognition and memory in humans and animal models during adolescence period (Castellanos et al. 2011; McGuinness and Newell 2012; Renard et al. 2013). These results may suggest that adolescence is a critical period of high vulnerability for anatomical modifications that may potentially translate to adverse neurobehavioral sequelae of exogenous cannabinoid exposure (Schneider 2008; Schneider et al. 2008). However, it is possible that since synthetic receptor agonists are man-made and are changed rapidly, they may not affect neuronal morphology in a similar manner. In the mammalian central nervous system, cannabinoid receptors are abundant, ubiquitously distributed and highly expressed in the forebrain (Herkenham et al. 1991; Mailleux and Vanderhaeghen 1992a; Matsuda et al. 1993). As the main cannabinoid receptor type in the brain (Piomelli 2003), type 1 cannabinoid receptors (CB1rs) are abundant in pyramidal and non-pyramidal neurons of layers II–III and V–VI in cortical circuits (Bodor et al. 2005; Fortin and Levine 2007; Mailleux and Vanderhaeghen 1992b, and Matsuda et al. 1993) as well as subcortical circuits, including the nucleus accumbens (Acb) (Yuan et al. 2013; Winters et al. 2012). Recent molecular, biochemical and electrophysiological studies (Ellgren et al. 2008 and Heng et al. 2011) have demonstrated an age-dependent downregulation of cortical CB1r expression, an effect that is most particularly pronounced in the medial prefrontal cortex (mPFC; Heng et al. 2011). Specifically, CB1r expression and resultant inhibition of synaptic activity have been reported to be highest in adolescents and lowest in adults (Heng et al. 2011). Whether developmental changes in the endocannabinoid system may lead to subsequent subcellular adaptations in the morphology of neurons remains largely unknown (Spiga et al. 2011; Rubino et al. 2009a, b).
It has been proposed that excessive stimulation of CB1 receptors during sensitive periods of development (e.g., adolescence) can produce enduring dysfunction in limbic/associative cortical circuits and pre-dispose to future psychiatric disorders (D’Souza et al. 2004, 2005; Caspi et al. 2005; Henquet et al. 2005; Realini et al. 2009; Rubino et al. 2009a, b). Exposure to other addictive drugs, including nicotine, cocaine, or morphine, produces persistent changes in the structure of dendrites and dendritic spines of neurons, particularly in the mPFC and Acb, brain regions involved in cognitive and reward functions, respectively (Kolb et al. 2003; Li et al. 2003). In the present study, using Golgi–Cox staining, we investigated changes in structural morphology of dendrites in mPFC and Acb following acute or chronic WIN 55,212-2 in adolescent and adult male rats. We selected the synthetic CB1r agonist WIN 55,212-2 as synthetic cannabinoid receptor agonists are becoming increasingly popular with adolescents as an abused substance (Crowley et al. 1998; Schneider 2008; Schneider et al. 2008). The emergence of new designer cannabinoid-based drugs, such as K2, Spice, and “bath salts”, has created an entirely new class of drugs that are not regulated by current laws and whose purity and toxicity profiles are largely unknown. Emerging evidence shows a wide range of responses to these compounds, including paranoia, aggressive behavior, anxiety, and short-term memory deficits (Seely et al. 2013; Kelly et al. 2013; Rosenbaum et al. 2012). Using the conditioned place paradigm, we have previously shown that WIN 55,212-2 induces aversion in adult rats and involves noradrenergic transmission in the Acb (Carvalho et al. 2010). It has been suggested that adult subjects may exhibit aversive-like responses to WIN 55,212-2 compared to adolescent rats because of decreased dendritic complexity in adult rats that is paralleled by decreased inhibition of synaptic activity by lower expression of CB1rs (Heng et al. 2011). Taken together, we hypothesized that differential effects on structural morphology of dendrites exist between adult and adolescent rat brains following WIN 55,212-2 exposure and we also hypothesized that WIN 55,212-2 induces aversion in adult rats while adolescent rat may not, using the conditioned place paradigm. Therefore, to investigate whether potential differences in neuronal morphology induced by WIN 55,212-2 are correlated with behavioral differences, WIN 55,212-2-induced conditioned place aversion was compared between adolescent and adult groups.
Materials and methods
Subjects
Thirty-six male Sprague–Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) with ages between 27–30 days (adolescent group) and 55–60 days (adult group) were housed two or three per cage in a controlled environment (12-h light schedule, temperature at 20 °C). Given the difficulty of characterizing absolute boundaries during which the first transition to adolescence begins in non-human animals including the rat (Spear 2000), we based the determination of age groups, adolescent versus adult on our previous report (Fox et al. 2009) as well as that of other laboratories (Varlinskaya and Spear 2008; Broadwater et al. 2013). Food and water were provided ad libitum. The care and use of animals were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the care and use of laboratory animals and to the guidelines of the European Communities Council Directive 2010/63/EU. All efforts were made to minimize animal suffering and reduce the number of animals used.
Drug preparation and administration
The synthetic cannabinoid receptor agonist, WIN 55,212-2 (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 5 % dimethyl sulfoxide (DMSO) (Fisher Scientific, Fair Lawn, NJ, USA) (Carvalho and Van Bockstaele 2011; Carvalho et al. 2010; Reyes et al. 2009) in saline and injected intraperitoneally (i.p.) (3.0 mg/kg, Carvalho and Van Bockstaele 2011; Carvalho et al. 2010) in a volume of 1 ml/kg body weight. In our previous investigation, we examined different doses of WIN 55,212-2, and we showed that 3.0 mg/kg body weight caused a significant elevation of norepinephrine efflux in the FC (Oropeza et al. 2005). Vehicle injections consisted of 5 % DMSO in saline (Carvalho and Van Bockstaele 2011; Carvalho et al. 2010); Reyes et al. 2009).
Histological processing and morphological analysis
To evaluate the effect of chronic WIN 55,212-2 administration on dendritic and spine morphology, six adolescent and six adult rats received a single daily i.p. injection of WIN 55,212-2 or vehicle between the hours of 9–11 a.m. for 14 days. One day after the last injection, rats were anesthetized with isoflurane and transcardially perfused. Brains were dissected and processed for Golgi–Cox staining using FD Rapid GolgiStain Kit (FD Neuro Technologies Consulting and Services, Ellicott City, MD, USA) following the procedures we previously described (Falowski et al. 2011). Briefly, the brains were prepared using the FD Rapid Golgi Stain kit (FD Technologies (FD Neurotechnologies). Brains were placed in 20 ml Golgi–Cox solution (potassium dichromate, mercuric chloride and potassium chromate), where they were stored in the dark for 14 days. Following this incubation, brains were then transferred to a 30 % sucrose solution for at least 3 days and coronal sections (100 µm) containing PFC and Acb were obtained using a freezing microtome (Microm HM550 cryostat, Richard-Allan Scientific, Kalamazoo, MI, USA). Sections were collected onto gelatin-coated slides and dehydrated through a graded series of ethanol, cleared in xylene and coverslipped. Individual neurons and their processes were visualized at 100× using camera lucida.
Dendritic arborization and numbers of spines were analyzed for pyramidal neurons in the mPFC (layer II–III) and medium spiny neurons (MSNs) in the Acb. The criteria used to select neurons for reconstruction were adapted from a previous report (Cerqueira et al. 2007). Briefly, criteria for selection included: (a) the location of the soma to the area of interest (i.e., mPFC and Acb); (b) full impregnation of the neurons along the entire length of the dendritic tree; (c) dendrites without significant truncation of branches and (d) relative isolation from neighboring impregnated neurons, astrocytes or blood vessels. Six to ten neurons were reconstructed per animal (maximum of four neurons per section), resulting in the analysis of twenty to twenty-five neurons per experimental group. Three rats per group were used for the analysis.
For each neuron selected for analysis, all branches of the dendritic tree were reconstructed at 600× magnification using a motorized microscope (Axioplan 2, Carl Zeiss, Germany), with oil objectives, and attached to a camera (DXC-390, Sony Corporation, Tokyo, Japan) and Neurolucida software (Microbrightfield®, Williston, VT, USA). A 3D analysis of the reconstructed neurons was performed using NeuroExplorer software (Microbrightfield®). Several characteristics of dendritic morphology were examined: total dendritic length; number of dendritic branches; and arrangement of dendritic material by a 3D version of Sholl analysis of intersections (see Cerqueira et al. 2007 for details). Dendritic spine density was determined in randomly selected distal dendritic branches for MSNs and in basal (proximal—<50 µm from soma, and distal) and apical dendrites in pyramidal neurons. Distal dendritic branches were included in our analysis along with the basal and proximal dendrites because previous reports (Li et al. 2003, 2004) using amphetamine have focused on distal dendrites demonstrating that long-term psychostimulant-induced spine changes occur in distal but not in proximal dendrites. However, using cocaine, the group of Dumitriu et al. (2012) focused on proximal dendrites. Li’s group used female rats while Dumitriu used male mice, and because we used male adolescent and adult rats we included distal, basal and proximal dendrites to determine the effect of WIN 55,212-2 on cortical and accumbal neuronal morphology
Place conditioning paradigm
Twenty-four rats were used for place conditioning paradigm. An unbiased place conditioning procedure was used so that the side of the apparatus used to conditioned animals was counterbalanced in all the groups. Automated CPP apparatuses (Place Preference System, San Diego Instruments Inc., San Diego, CA, USA) that recorded their movement/location in each chamber using a computer-controlled 4 × 16 array of photobeams were used. The paradigm consisted of three phases: pre-test, conditioning, and test. On pre-test day (day 1), animals were placed in the apparatus and allowed to freely explore both sides of the apparatus for 15 min. The time spent in each side was automatically recorded by the apparatus and animals with preference for one side higher than 200 s were removed from the study. During the conditioning phase (days 2–6), rats were injected twice daily. In the morning, animals were injected with vehicle and confined to one side of the apparatus for 45 min. In the afternoon, animals were injected with WIN 55,212-2 (3.0 mg/kg) and confined to the opposite side for 45 min. Control groups of animals received vehicle in both sessions. On the test day (day 14), animals were placed in the apparatus and allowed to explore both sides for 15 min. The time spent in each side was automatically recorded by the apparatus. No injection was given to the animals on pre-test and test days. On the test day, the last day of experiment, the animals were not exposed to WIN 55,212-2 for 24 h.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 software. Each neuron was treated as an independent measure. Results are presented as mean ± SEM. Data were compared using two-way ANOVA (age × treatment), followed by unpaired t test analysis. Sholl analysis data were compared across groups using repeated measures ANOVA (“age × treatment” as the between-subject factors and “distance from soma” as the within-subject factor) followed by t test analysis. Significance was set at p < 0.05.
Results
Effects of WIN 55,212-2 on prefrontal cortical neuronal morphology
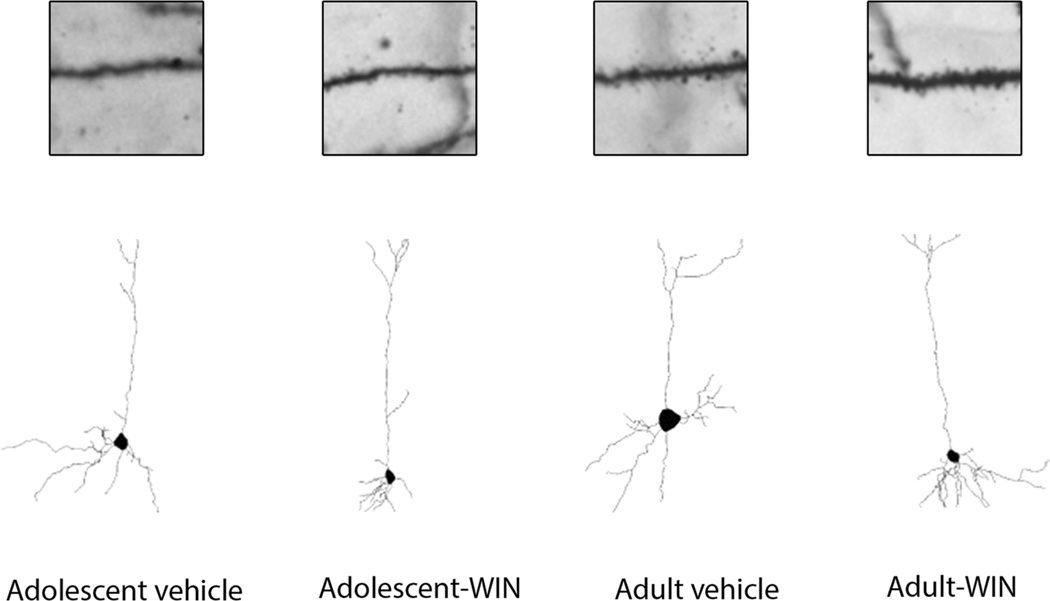
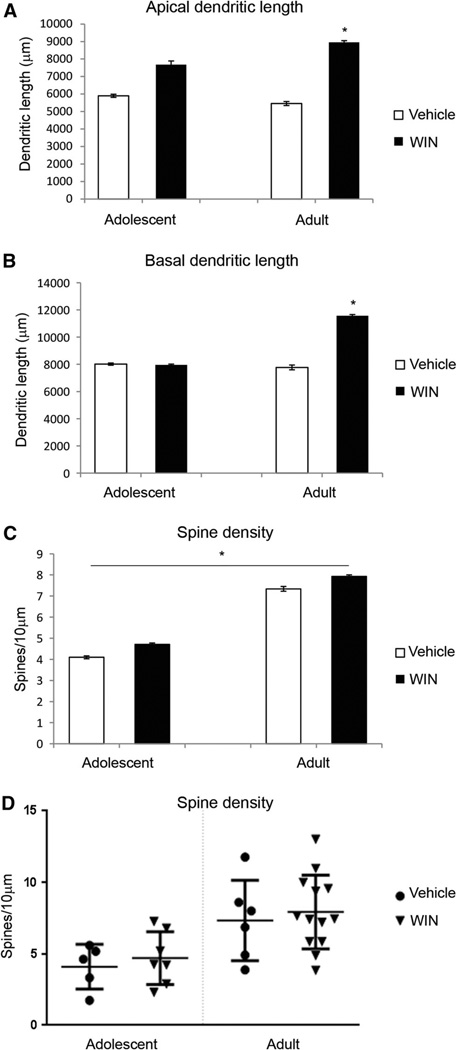
To investigate the effect of WIN 55,212-2 on the neuronal morphology of mPFC, the pyramidal neurons in layers II and III of the cingulate cortex regions 1–3 (Cg1–3) of the mPFC were analyzed. Within the mPFC, layers II and III are readily identifiable in Golgi-stained tissues section based on its characteristic cytoarchitecture (Cerqueira et al. 2007). It is located ventrally from layer I that also contains the distal tufts of layers II and III pyramidal cells; and it serves as the dorsal boundary to layer V. Indeed, this boundary is pronounced because of the greater cell-packing density and smaller somata of pyramidal cells in layers II–III relative to layer V in this region (Cajal 1995; Zilles and Wree 1995). Prefrontal cortical neurons exhibit both basal and apical dendrites. Dendritic length, number of dendritic branches and spine density of both basal and apical dendrites were analyzed. In addition, spine density in basal dendrites was quantified proximally (<50 µm) and distally to the soma. Figure 1 shows representative camera lucida drawings from layers II and III pyramidal cells of mPFC following chronic WIN 55,212-2 administration. These drawings clearly demonstrate that chronic WIN 55,212-2 administration increased basal and apical dendritic length and branching in layers II and III pyramidal cells of mPFC in adult rats. WIN 55,212-2 administration leads to an increase in apical (t(19) = −2.389, p = 0.027) dendritic and basal (t(18) = −2.223, p = 0.039) lengths in adult animals only (Fig. 2a, b) using unpaired t test. Two-way ANOVA revealed an effect of age on spine density in the proximal portion of basal dendrites (F(1, 27) = 6.794, p = 0.015, Fig. 2c), suggesting that adult animals showed higher spine density proximally to the soma. Figure 2d shows cumulative distribution plots for spine density in the mPFC. No significant effects were seen in the number of dendritic branches (data not shown).
Fig. 1.
Representative spine micrographs and camera lucida drawings of cf 2–3 from the medial prefrontal cortex following chronic WIN 55,212-2 administration in adolescent and adult Sprague–Dawley male rats
Fig. 2.
Effect of chronic administration of WIN 55,212-2 on medial prefrontal cortex (mPFC) pyramidal neuronal morphology. Chronic administration of WIN 55,212-2 (3.0 mg/kg, 14 days) increased dendritic length of mPFC neurons in adult rats. Spine density was not affected by WIN 55,212-2 treatment, but was affected by age where adult rats present higher density of spines near the soma, *p < 0.05
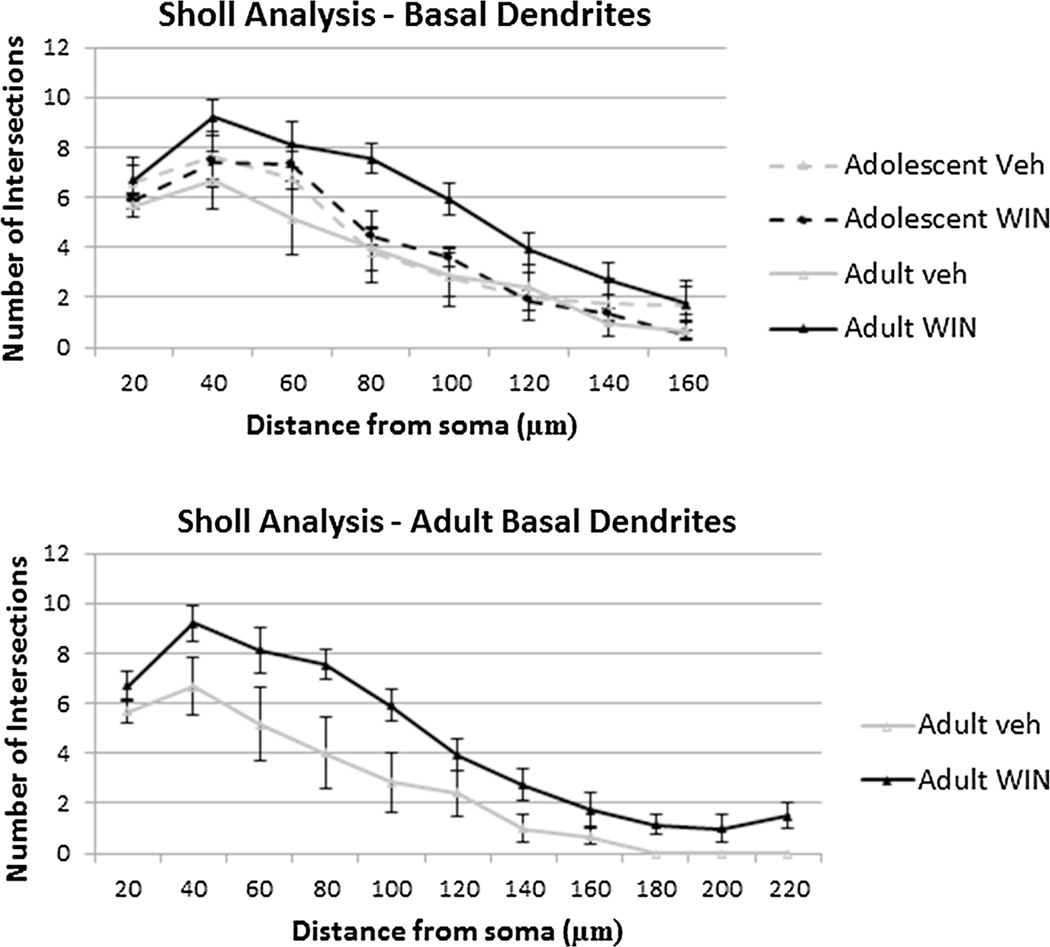
Regarding the Sholl analysis of basal dendrites of the pyramidal neurons (number of intersections as a function of their distance to the soma), two-way repeated measures ANOVA revealed an effect of age in between subjects (F(1, 20) = 5.139, p = 0.035). Further analysis revealed that adult WIN-treated animals showed an increase in the number of intersections from 80 to 140 µm from soma (p < 0.05, Fig. 3), suggesting an increase in dendritic content in a radius distally to the soma, showing no differences at distances to soma smaller than 80 µm or bigger than 140 µm (Fig. 3).
Fig. 3.
Rearrangement of dendrites in medial prefrontal cortex (mPFC) pyramidal neurons after chronic administration of WIN 55,212-2. Sholl analysis-derived distribution of mPFC layer II–III pyramidal cell dendrites between consecutive 20-µm-spaced concentric spheres of cg 1–3. Results revealed that chronic administration of WIN 55,212-2 increases the number of dendritic branches (at 80–140 µm from soma), *p < 0.05. Means and standard error of mean are plotted
Effect of repeated cannabinoid administration on neuronal morphology in Acb
The analysis of the MSNs in Acb included the core and shell subregions of Acb. Core subregion surrounds the anterior commissure, while the shell subregion is located medial and ventral to the lateral ventricle.
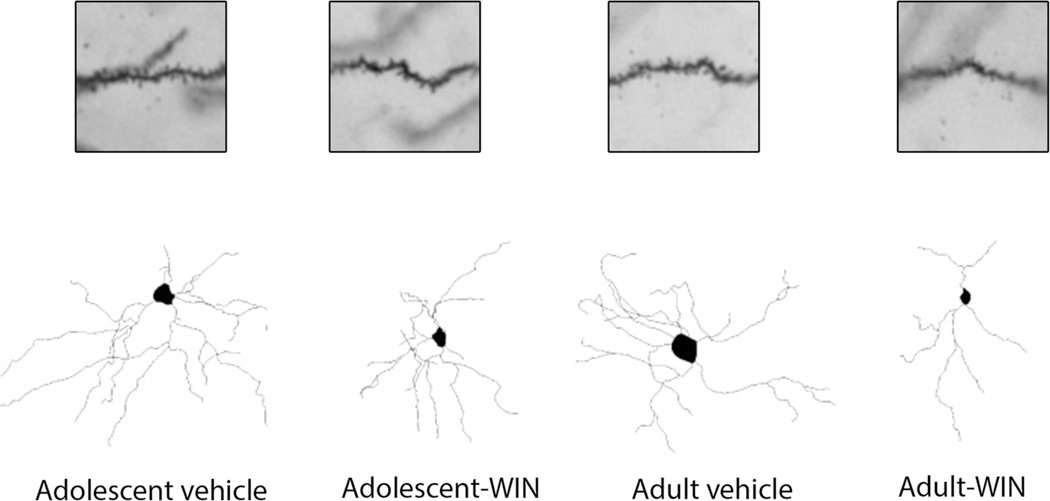
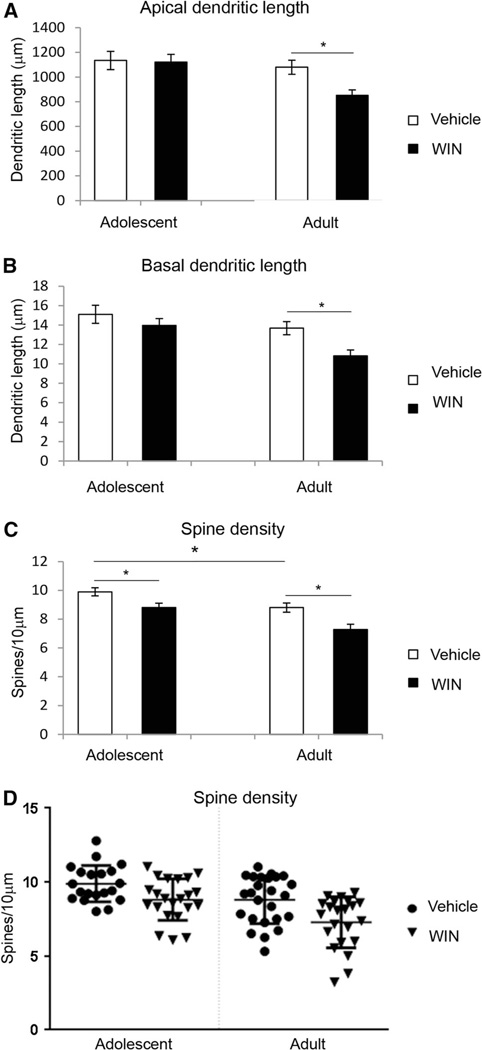
Figure 4 shows representative camera lucida drawings from the Acb neurons following chronic WIN 55,212-2 administration in adolescent and adult male rats. Included in the analysis are the core and shell subregions of Acb. Dendritic length, number of dendritic branches and spine density of MSNs of the Acb were evaluated in adolescent and adult animals chronically treated with WIN 55,212-2 or vehicle. Two-way ANOVA revealed an effect of age in dendritic length (F(1, 85) = 7.885, p = 0.006), number of dendritic branches (F(1, 85) = 10.554, p = 0.002) and spine density (F(1, 85) = 16.616, p < 0.0001). Statistical analysis revealed an effect of drug treatment in number of dendritic branches (F(1, 85) = 6.904, p = 0.01) and spine density (F(1, 85) = 16.453, p < 0.0001) but not in dendritic length (Fig. 5a). No interaction was seen between age and drug treatment for any of the parameters analyzed. Further analysis showed that WIN 55,212-2 administration decreased dendritic length (t(46) = 3.112, p = 0.003) and number of dendritic branches (t(46) = 3.121, p = 0.003) only in adult rats (Fig. 5b). Data analysis showed that control (vehicle-treated) adolescent animals show a higher spine density than control (vehicle-treated) adult animals (t(43) = 2.51, p = 0.016) (Fig. 5c). Moreover, WIN 55,212-2 administration reduced spine density in both adolescent (t(40) = 2.652, p = 0.011) and adult (t(45) = 3.131, p = 0.003) animals (Fig. 5c). Figure 5d shows cumulative distribution plots for spine density in the Acb.
Fig. 4.
Representative spine micrographs and camera lucida drawings of nucleus accumbens (Acb) neuron following chronic WIN 55,212-2 administration in adolescent and adult Sprague–Dawley male rats. Included in the analysis are the core and shell subregions of Acb. Core subregion surrounds the anterior commissure, while the shell subregion is located medial and ventral to the lateral ventricle
Fig. 5.
Effect of chronic administration of WIN 55,212-2 on medium spiny neurons in the nucleus accumbens. Chronic administration of WIN 55,212-2 (3.0 mg/kg, 14 days) resulted in a decrease in dendritic length and dendritic branches in adult rats but not in adolescent rats. Moreover, it is shown that adult animals have a lower spine density than adolescent rats and that WIN 55,212,2 administration decreases spine density in both adult and adolescent rats, *p < 0.01
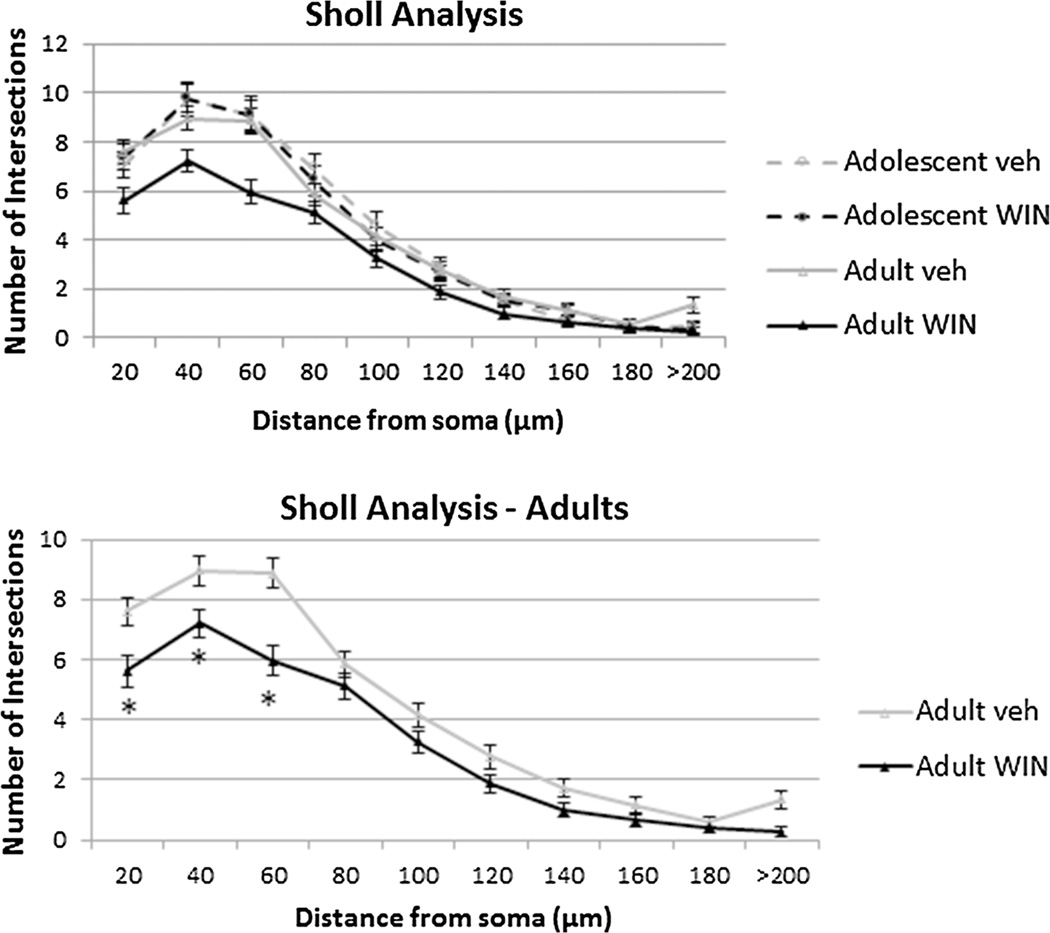
Regarding the Sholl analysis (number of intersections as a function of their distance to the soma), two-way repeated measures ANOVA revealed an effect of age (F(1, 86) = 5.976, p = 0.017) but no interaction between age and drug treatment. In between-subjects analysis showed an effect of age (F(1, 86) = 6.661, p = 0.012) and drug treatment (F(1, 86) = 5.64, p = 0.02), showing that in all groups there was a decrease in the number of intersections as dendritic distance from soma decreases (Fig. 6). As age impacted Sholl analysis, further analysis revealed that drug impacts Sholl analysis in adult animals (F(1, 46) = 5.972, p = 0.018) but not in adolescent animals (F(1, 40) = 0.001, p = 0.979). Moreover, adult WIN-treated animals showed a lower number of intersections until 60 µm from soma (t(46) = 4,119, p < 0.001, Fig. 6), suggesting a decrease in dendritic content in a radius of 60 µm from soma, showing no differences at distances to soma bigger than 80 µm (t(46) = 1,144, p = 0.259, Fig. 6).
Fig. 6.
Rearrangement of medium spiny neurons (MSNs) after chronic administration of WIN 55,212-2. Sholl analysis-derived distribution of MSNs in nucleus accumbens between consecutive 20-µm-spaced concentric spheres. Chronic administration of WIN resulted in a significant decrease in the amount of dendritic branches proximal to the soma only in adult rats, *p < 0.05. Means and standard error of mean are plotted
WIN 55,212-2 induces aversion only in adult rats
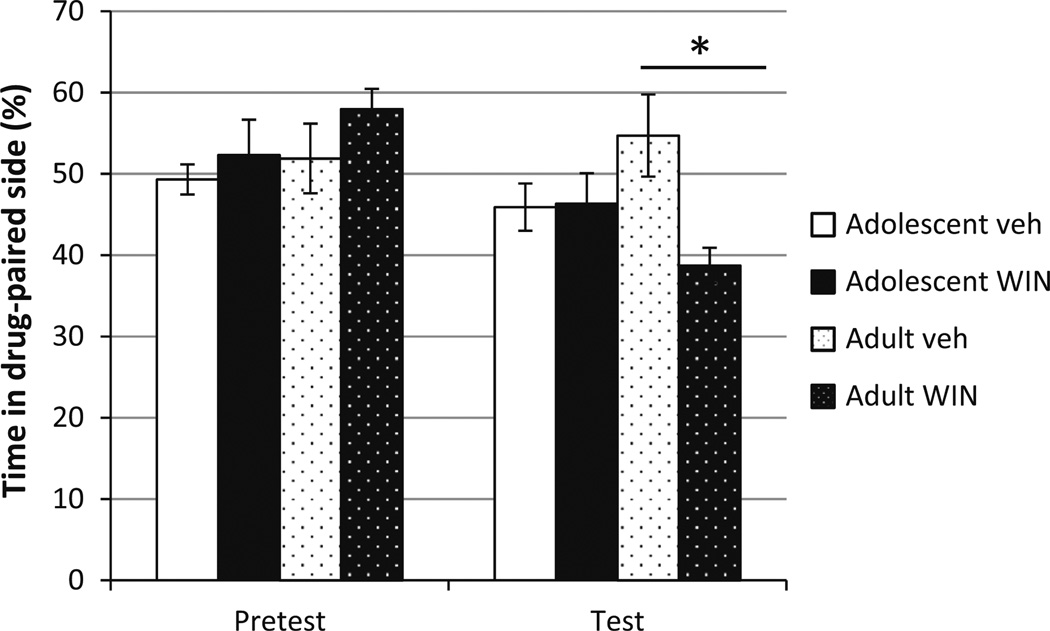
The place conditioning paradigm was used to test whether the aversive properties of WIN 55,212-2 were similar in adolescent versus adult subjects. Repeated measures analysis revealed that the there was an overall effect of time of testing (F(1, 19) = 5.294, p = 0.033), indicating that the conditioning phase affected the performance of the animals on the test day (Fig. 7). The analysis also showed an interaction between the phase of testing and drug (F(1, 19) = 4.831, p = 0.041). Further analysis showed that adult animals that received WIN 55,212-2 spent less time in the drug-paired chamber than the respective vehicle group (t(9) = 3.098, p = 0.027), indicating that WIN 55,212-2 induced aversive-like behaviors (Fig. 7). In contrast, adolescent animals exposed to WIN 55,212-2 did not show aversion when compared to the respective vehicle group (t(10) = −0.093, p = 0.695) (Fig. 7) suggesting that adult and adolescent animals exhibit different sensitivities to WIN 55,212-2.
Fig. 7.
Cannabinoids induce aversion in adult rats. Chronic administration of WIN 55,212-2 (3.0 mg/kg) induced aversion only in adult animals as seen by decreased time spent in the drug-paired chamber, *p < 0.05
Discussion
The present study builds on previous work from our group as well as results from others showing that the effect of cannabinoids on brain function varies by age. Here, results are provided showing that administration of the synthetic cannabinoid receptor agonist WIN 55,212-2 differentially impacts neuronal morphology in the mPFC versus Acb in adolescent compared to adult male rats. Deficits in working memory and decision making are adverse consequences of long-term use of cannabis (Solowij et al. 2002) which implicate functioning of prefrontal cortex (Casey et al. 2000; Luna et al. 2004; Segalowitz and Davies 2004; Bunge and Wright 2007). Dysfunction of prefrontal cortex is a hallmark of psychiatric disorders (Cullen et al. 2006; Nugent et al. 2006). Adaptations in neuronal architecture of MSN in the Acb following WIN 55,212-2 exposure may contribute to differences in behavior in adolescent versus adult rats in the conditioned place paradigm. As previously reported, conditioned place paradigm is a simple method that offers several advantages including observation of both rewarding and aversive effects (Bardo and Bevins 2000; Cunningham and Gremel 2006; Cunningham et al. 2006; Tzschentke 2007). Specifically, conditioned place paradigm offers a choice between the drug-associated cue and a neutral cue (Cunningham et al. 2006). However, the conditioned place paradigm also presents several limitations. One of the major limitations is the potential influence of a novelty seeking effect that could potentially thwart results since rats would prefer novelty over a familiar context (Hughes 1968; Cain et al. 2006; Bardo et al. 2006). To circumvent the issue of novelty in our present experiment, we followed the previous approach that allows the rats to show a preference for the drug-paired context relative to the novel context (Mucha and Iversen 1984; Mucha and Herz 1985; Parker 1992) thus nullifying any consequence related to novelty. Another limitation in conditioned place paradigm is the difficulty in determining the type of dose–effect information that is normally expected in behavioral pharmacology (Bardo and Bevins 2000). As described in the “Materials and methods” section, we selected a dose that caused a significant elevation of nor-epinephrine efflux in the FC that is accompanied by a significant increase in neuronal activity in the locus coeruleus and significant behavioral changes (Oropeza et al. 2005; Page et al. 2007, 2008).
We have previously shown that WIN 55,212-2 induces aversion in adult rats and that noradrenergic transmission in the Acb is responsible for this behavior (Carvalho et al. 2010). Adult subjects may exhibit aversive-like responses to WIN 55,212-2 compared to adolescent rats because of decreased dendritic complexity in adult rats that is paralleled by decreased inhibition of synaptic activity by lower expression of CB1rs (Heng et al. 2011). These data suggest that differences in the structural adaptations to WIN 55,212-2 and a lack of cannabinoid-induced aversion may render adolescents more vulnerable to the negative sequelae of synthetic cannabinoids. As dendrites are the main site of information input into neurons (Urbanska et al. 2008) and dendritic spines are seen as the main site for excitatory synaptic transmission (Fernández-Espejo 2013), we hypothesize that altered input to Acb neurons may contribute to the behavioral expression of aversion.
The mPFC plays a role in the integration of emotional stimuli (Kohn et al. 2014; Davidson 2002) as well as in complex cognitive tasks, including temporal organization of behavior, decision making, rule learning, and behavioral flexibility (Baird et al. 2013; Clark et al. 2004), while the Acb is a key region involved in eliciting cannabinoid-induced aversion (Carvalho and Van Bockstaele 2011; Carvalho et al. 2010). The present study reveals important differences in the effects of cannabinoids on the dendritic structure of neurons in the mPFC and Acb across development. As the main site of information processing in neurons (Urbanska et al. 2008), dendrites and dendritic spines are seen as the main site for excitatory synaptic transmission (Fernández-Espejo 2013). Maturation of dendrites and spines requires intact intrinsic mechanisms as well as sustained afferent input (Libersat and Duch 2004; Libersat 2005). Dendrite development involves dendrite and spine growth followed by higher order branching and spine maturation (Koss et al. 2014; Lu et al. 2013; Maynard et al. 2012; Verslegers et al. 2014). Slight decreases in spine density have been reported with age in areas such as the mPFC, Acb and hippocampus (Sánchez et al. 2011), and are supported by the present study. In situ hybridization histochemical studies demonstrated that CB1r expression in prefrontal cortex follows distinct trajectories (Heng et al. 2011). For instance, CB1r expression in rats is highest in juvenile (postnatal 25) and declines with age, the lowest level is shown at (postnatal day 70). The developmental down-regulation in CB1r expression follows similar trajectory as observed in CB1-dependent inhibition of synaptic transmission (Heng et al. 2011). Morphological analysis of pyramidal neurons in the mPFC showed changes in adult but not in adolescent rats. Considering that cannabinoids are abused substances, increases in dendritic length, number of branches and spine density might not be unexpected since adult rats given or self-administering cocaine, nicotine and amphetamine show similar morphological changes (Robinson and Kolb 2004). The reported effects of WIN 55,212-2 on dendritic architecture in adults but not in adolescents may relate to differences in CB1r expression. Using fluorescent immunosorbent assay, CB1r expression in PFC decreases with age following delta-9-tetrahydrocannabinol (Δ9-THC) exposure (Ellgren et al. 2008). Interestingly, while CB1r expression in mPFC declines with age, expression of endocannabinoids, anandamide and 2-AG, increases following Δ9-THC exposure (Ellgren et al. 2008). As the mPFC is also important for the corticolimbic pathway (Ellgren et al. 2008), we suspect that the changes observed herein may reflect interactions between the PFC and the Acb. It has been shown that analogous developmental effects in mPFC following Δ9-THC exposure parallel CB1r downregulation and endocannabinoid markers up-regulation in Acb following Δ9-THC exposure (Ellgren et al. 2008). Indeed, similar pattern of norepinephrine increases was also evident in mPFC and Acb when WIN 55,212-2 or Δ9-THC was administered systemically (Jentsch et al. 1997; Oropeza et al. 2005; Page et al. 2007).
A recent review (Hurd et al. 2014) points out the vulnerability of adolescent brain to cannabis addiction given the differential developmental expression of CB1r from juvenile to adulthood. However, it also highlighted that some inconsistencies regarding the ontogenic pattern of CB1r expression may be attributed to regional versus global, development differences in the receptor development, differences in mRNA, receptor protein or receptor binding being investigated (Hurd et al. 2014). There is no discrepancy with our present study because we exposed adolescent and adult rats to WIN 55,212-2 and their brains were harvested following a 14-day exposure, while the studies (Biscaia et al. 2008; Singh et al. 2006) cited in the recent review (Hurd et al. 2014) investigated the effect of periadolescent or perinatal exposure to cannabinoid agonist (WIN 55,212-2 or Δ9-THC) in adult. In other words, while we studied the direct effect of WIN 55,212-2 exposure during adolescence and adulthood, the other studies determined the subsequent effect of periadolescent or perinatal cannabinoid exposure in adult (Biscaia et al. 2008; Singh et al. 2006).
It has been reported that chronic treatment with the cannabinoid receptor agonist Δ9-THC leads to an increase in dendritic length and dendritic branches in the Acb (Kolb et al. 2006), a finding that is not consistent with the data presented here. In Kolb’s study, Δ9-THC was injected at a dose (0.5 mg/kg) not known to produce aversion or anxiogenic- like responses. Therefore, at the dose utilized Δ9-THC may be rewarding and thus the increase in dendritic arborization might be expected. It would be interesting to see whether a Δ9-THC dose known to be aversive and anxiogenic (e.g., 5 mg/kg (Quinn et al. 2008) would decrease dendritic length, branches and spine density in adults, similar to the findings reported here. In the Acb, the expression of CB1r in rats progressively increases postnatally until around 30 or 40 days and then decreases into adulthood (Rodríguez de Fonseca et al. 1993). Furthermore, CB1r in adolescent rats (30–35 days old) is less functionally coupled to G proteins and desensitize more slowly in response to Δ9-THC treatment than in adults (Moore et al. 2010). Interestingly, another set of studies has shown that chronic treatment with the cannabinoid agonist HU210, for 14 days, induced a decrease in CB1r binding (measured by [3H] CP55,940 autoradiography), that was more evident in adult animals (Dalton et al. 2009, 2010), suggesting that CB1r in adult animals may be more responsive to cannabinoids than CB1r in adolescents. Thus, changes in Acb neuronal morphology may be due to higher sensitivity of CB1r in adulthood, which ultimately may translate into aversive-like responses.
It is widely accepted that glutamatergic excitatory input to neurons is usually associated with spines, while gamma-aminobutyric acid (GABA)ergic inhibitory input usually occurs onto dendritic shafts (Robinson and Kolb 2004). The MSNs of the Acb possess proximal dendrites with very few spines, while distal dendrites have higher density of spines. GABA terminals in the Acb synapse onto the soma or proximal dendrites (Pickel et al. 1988) and glutamatergic afferents form synapse mainly at distal dendrites (French and Totterdell 2002). The effect of WIN 55,212-2 on spine density in the Acb in both adolescent and adults animals suggests that WIN 55,21-2 may affect glutamatergic transmission in the Acb (possibly decreasing it) in both adolescent and adult rats. The fact that WIN 55,212-2 also induces a decrease in dendritic branching in adults, especially at proximal levels suggests a decrease in GABAergic transmission only in adult rats, which could lead to an overall activation of the Acb triggering aversion (Carlezon and Thomas 2009).
The present study reveals important differences in the behavioral effects of cannabinoids in adolescent versus adult subjects. The aversive and anxiogenic effects of cannabinoid receptor agonists are well described in adult rats (Cagni and Barros 2013; Quinn et al. 2008; Schramm-Sapyta et al. 2007). However, there is greater heterogeneity reported for behavioral responses in adolescent subjects following cannabinoid administration. For instance, cognitive and behavioral studies have shown that WIN 55,212- 2 causes long-lasting deficits in sensorimotor gating, object recognition memory and performance on an instrumental task (Schneider and Koch 2003; Schneider et al. 2005; Quinn et al. 2008), as well as deficits on residual working memory and social interaction (O’Shea et al. 2004, 2006) Several factors can account for these differences: (1) maturation of brain circuitry; (2) differential sensitivities of specific circuits to cannabinoids; (3) differential patterns of CB1r expression. The fact that cannabinoids exhibit a greater effect on affective-like behaviors (e.g., aversion, anxiety) in adult animals suggests that the circuits involved in aversion and anxiety may not be fully established in adolescence (Quinn et al. 2008; Schramm-Sapyta et al. 2007). In fact, MSNs of the striatum do not possess adult-like activity until the end of the first postnatal month (Tepper et al. 1998; Belleau and Warren 2000). The present results showing that adolescent rats findWIN55,212-2 less aversive are similar to the developmental aversive effects observed for other drugs of abuse including alcohol (Philpot et al. 2003) and nicotine (Wilmouth and Spear 2004). This suggests that neural circuits in adolescent brain may be more vulnerable to drugs of abuse because their structural neuronal adaptations are such that they do not elicit behavioral effects (e.g., aversion) that may be protective. This may increase vulnerability to continued use and addiction.
In summary, the present results show that synthetic cannabinoid exposure differentially impacts the neuronal morphology of adult compared to adolescent subjects. Such differences may underlie the disparate developmental effects of cannabinoids on behavior.
Acknowledgments
This work was supported by NIDA DA20129 (EVB).
Abbreviations
- Acb
Nucleus accumbens
- CB1r
Cannabinoid receptor type 1
- CB2r
Cannabinoid receptor type 2
- CNS
Central nervous system
- MSNs
Medium spiny neurons
- PFC
Prefrontal cortex
- VTA
Ventral tegmental area
Contributor Information
A. F. Carvalho, Life and Health Sciences Research Institute (ICVS), School of Health Sciences, University of Minho, Campus Gualtar, 4710-057 Braga, Portugal ICVS/3B’s, PT Government Associate Laboratory, Braga/Guimarães, Portugal; Department of Neuroscience, Farber Institute for Neurosciences, Thomas Jefferson University, Philadelphia, PA 19107, USA.
B. A. S. Reyes, Email: Beverly.Reyes@drexelmed.edu, Department of Pharmacology and Physiology, Drexel University College of Medicine, Philadelphia, PA 19102, USA.
F. Ramalhosa, Life and Health Sciences Research Institute (ICVS), School of Health Sciences, University of Minho, Campus Gualtar, 4710-057 Braga, Portugal ICVS/3B’s, PT Government Associate Laboratory, Braga/Guimarães, Portugal.
N. Sousa, Life and Health Sciences Research Institute (ICVS), School of Health Sciences, University of Minho, Campus Gualtar, 4710-057 Braga, Portugal ICVS/3B’s, PT Government Associate Laboratory, Braga/Guimarães, Portugal.
E. J. Van Bockstaele, Email: Elisabeth.VanBockstaele@DrexelMed.edu, Department of Pharmacology and Physiology, Drexel University College of Medicine, Philadelphia, PA 19102, USA.
References
- Anthony JC, Petronis KR. Epidemiologic evidence on suspected associations between cocaine use and psychiatric disturbances. NIDA Res Monogr. 1991;110:189–210. [PubMed] [Google Scholar]
- Baird B, Smallwood J, Gorgolewski KJ, Margulies DS. Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. J Neurosci. 2013;33(42):16657–16665. doi: 10.1523/JNEUROSCI.0786-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Cain ME, Bylica KE. Effect of amphetamine on response inhibition in rats showing high or low response to novelty. Pharmacol Biochem Behav. 2006;85(1):98–104. doi: 10.1016/j.pbb.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Belleau ML, Warren RA. Postnatal development of electrophysiological properties of nucleus accumbens neurons. J Neurophysiol. 2000;84(5):2204–2216. doi: 10.1152/jn.2000.84.5.2204. [DOI] [PubMed] [Google Scholar]
- Biscaia M, Fernandez B, Higuera-Matas A, Miguens M, Viveros MP, Garcia-Lecumberri C, Ambrosio E. Sex-dependent effects of periadolescent exposure to the cannabinoid agonist CP-55,940 on morphine self-administration behaviour and the endogenous opioid system. Neuropharmacology. 2008;54(5):863–873. doi: 10.1016/j.neuropharm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25(29):6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Effects of voluntary access to sweetened ethanol during adolescence on intake in adulthood. Alcohol Clin Exp Res. 2013;37(6):1048–1055. doi: 10.1111/acer.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Cur Opinion Neurobiol. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cagni P, Barros M. Cannabinoid type 1 receptor ligands WIN 55,212-2 and AM 251 alter anxiety-like behaviors of marmoset monkeys in an open-field test. Behav Brain Res. 2013;240:91–94. doi: 10.1016/j.bbr.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Cain ME, Dotson WF, Bardo MT. Individual differences in the effect of novel environmental stimuli prior to amphetamine selfadministration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2006;14(3):389–401. doi: 10.1037/1064-1297.14.3.389. [DOI] [PubMed] [Google Scholar]
- Cajal S. Histology of the nervous system of man and vertebrates. New York: Oxford University Press; 1995. [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Van Bockstaele EJ. Direct intra-accumbal infusion of a beta-adrenergic receptor antagonist abolishes WIN 55,212-2-induced aversion. Neurosci Lett. 2011;500(1):82–85. doi: 10.1016/j.neulet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Reyes AR, Sterling RC, Unterwald E, Van Bockstaele EJ. Contribution of limbic norepinephrine to cannabinoid-induced aversion. Psychopharmacology. 2010;211(4):479–491. doi: 10.1007/s00213-010-1923-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene × environment interaction. Biol Psychiatry. 2005;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Castellanos D, Singh S, Thornton G, Avila M, Moreno A. Synthetic cannabinoid use: a case series of adolescents. J Adolesc Health. 2011;49(4):347–349. doi: 10.1016/j.jadohealth.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Taipa R, Uylings HB, Almeida FO, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17(9):1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160(6):1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LR, Cools R, Robbins TW. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55(1):41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Macdonald MJ, Whitmore EA, Mikulich SK. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct symptoms and substance use disorders. Drug Alcohol Depend. 1998;50(1):27–37. doi: 10.1016/s0376-8716(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Cullen TJ, Walker MA, Eastwood SL, Esiri MM, Harrison PJ, Crow TJ. Anomalies of asymmetry of pyramidal cell density and structure in dorsolateral prefrontal cortex in schizophrenia. Br J Psychiatry. 2006;188:26–31. doi: 10.1192/bjp.bp.104.008169. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM. Proximal ethanol pretreatment interferes with acquisition of ethanol-induced conditioned place preference. Pharmacol Biochem Behav. 2006;85(3):612–619. doi: 10.1016/j.pbb.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1(4):1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Dalton VS, Wang H, Zavitsanou K. HU210-induced down-regulation in cannabinoid CB1 receptor binding strongly correlates with body weight loss in the adult rat. Neurochem Res. 2009;34(7):1343–1353. doi: 10.1007/s11064-009-9914-y. [DOI] [PubMed] [Google Scholar]
- Dalton VS, Wang H, Zavitsanou K. Cannabinoid effects on CB1 receptor density in the adolescent brain: an autoradiographic study using the synthetic cannabinoid HU210. Synapse. 2010;64(11):845–854. doi: 10.1002/syn.20801. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51(1):68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- de Fonseca FR, Schneider M. The endogenous cannabinoid system and drug addiction: 20 years after the discovery of the CB1 receptor. Addict Biol. 2008;13:143–146. doi: 10.1111/j.1369-1600.2008.00116.x. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57(6):594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. J Neurosci. 2012;32(20):6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Devi LA, Hurd YL. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. 2008;18(11):826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falowski SM, Sharan A, Reyes BA, Sikkema C, Szot P, Van Bockstaele EJ. An evaluation of neuroplasticity and behavior after deep brain stimulation of the nucleus accumbens in an animal model of depression. Neurosurgery. 2011;69:1281–1290. doi: 10.1227/NEU.0b013e3182237346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Espejo E. How does the nucleus accumbens function? Revista de Neurologia. 2013;30(9):845–849. [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex. 2007;17(1):163–174. doi: 10.1093/cercor/bhj133. [DOI] [PubMed] [Google Scholar]
- Fox KM, Sterling RC, Van Bockstaele EJ. Cannabinoids and novelty investigation: influence of age and duration of exposure. Behav Brain Res. 2009;196(2):248–253. doi: 10.1016/j.bbr.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell D. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus. J Comp Neurol. 2002;446(2):151–165. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65(4):278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31(3):608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11(2):563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. Behaviour of male and female rats with free choice of two environments differing in novelty. Anim Behav. 1968;16(1):92–96. doi: 10.1016/0003-3472(68)90116-4. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Michaelides M, Miller ML, Jutras-Aswad D. Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology. 2014;76(Pt B):416–424. doi: 10.1016/j.neuropharm.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Andrusiak E, Tran A, Bowers MB, Jr, Roth RH. Delta 9-tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: blockade of dopaminergic effects with HA966. Neuropsychopharmacology. 1997;16:426–432. doi: 10.1016/S0893-133X(97)00018-3. [DOI] [PubMed] [Google Scholar]
- Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Wells BE, Pawson M, Leclair A, Parsons JT, Golub SA. Novel psychoactive drug use among younger adults involved in US nightlife scenes. Drug Alcohol Rev. 2013;32(6):588–593. doi: 10.1111/dar.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation—an ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci USA. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Limebeer CL, Parker LA. Chronic treatment with delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60(6):429–436. doi: 10.1002/syn.20313. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20(6):1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Libersat F. Maturation of dendritic architecture: lessons from insect identified neurons. J Neurobiol. 2005;64(1):11–23. doi: 10.1002/neu.20142. [DOI] [PubMed] [Google Scholar]
- Libersat F, Duch C. Mechanisms of dendritic maturation. Mol Neurobiol. 2004;29(3):303–320. doi: 10.1385/MN:29:3:303. [DOI] [PubMed] [Google Scholar]
- Lu D, He L, Xiang W, Ai WM, Cao Y, Wang XS, Pan A, Luo XG, Li Z, Yan XX. Somal and dendritic development of human CA3 pyramidal neurons from midgestation to middle childhood: a quantitative Golgi study. Anat Rec (Hoboken) 2013;296:123–132. doi: 10.1002/ar.22616. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75(5):1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992a;48(3):655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Localization of cannabinoid receptor in the human developing and adult basal ganglia. Higher levels in the striatonigral neurons. Neurosci Lett. 1992b;148(1–2):173–176. doi: 10.1016/0304-3940(92)90832-r. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327(4):535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- McGuinness TM, Newell D. Risky recreation: synthetic cannabinoids have dangerous effects. J Psychosoc Nurs Ment Health Serv. 2012;50(8):16–18. doi: 10.3928/02793695-20120703-04. [DOI] [PubMed] [Google Scholar]
- Moore NL, Greenleaf AL, Acheson SK, Wilson WA, Swartzwelder SH, Kuhn CM. Role of cannabinoid receptor type 1 desensitization in greater tetrahydrocannabinol impairment of memory in adolescent rats. J Pharmacol Exp Ther. 2010;335(2):294–301. doi: 10.1124/jpet.110.169359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology. 1985;86(3):274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Reinforcing properties of morphine and naloxone revealed by conditioned place preferences: a procedural examination. Psychopharmacology. 1984;82(3):241–247. doi: 10.1007/BF00427782. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Zarate CA, Pine DS, Price JL, Drevets WC. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. NeuroImage. 2006;30:485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046(1–2):45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- O’Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol. 2006;20:611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86(1):162–168. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Van Bockstaele EJ. Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neurosci Lett. 2008;431(1):1–5. doi: 10.1016/j.neulet.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA. Place conditioning in a three- or four-choice apparatus: role of stimulus novelty in drug-induced place conditioning. Behav Neurosci. 1992;106(2):294–306. doi: 10.1037//0735-7044.106.2.294. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Towle AC, Joh TH, Chan J. Gamma-aminobutyric acid in the medial rat nucleus accumbens: ultrastructural localization in neurons receiving monosynaptic input from catecholaminergic afferents. J Comp Neurol. 1988;272(1):1–14. doi: 10.1002/cne.902720102. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mallet PE, Kashem MA, Matsuda-Matsumoto H, Iwazaki T, McGregot IS. Adolescent rats find repeated delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33(5):1113–1126. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol Res. 2009;60(2):132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Renard J, Krebs MO, Jay TM, Le Pen G. Long-term cognitive impairments induced by chronic cannabinoid exposure during adolescence in rats: a strain comparison. Psychopharmacology. 2013;225(4):781–790. doi: 10.1007/s00213-012-2865-z. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Rosario JC, Piana PM, Van Bockstaele EJ. Cannabinoid modulation of cortical adrenergic receptors and transporters. J Neurosci Res. 2009;87(16):3671–3678. doi: 10.1002/jnr.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Ramos JA, Bonnin A, Fernández-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. NeuroReport. 1993;4(2):135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow…and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8(1):15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Alberio T, Capurro V, Vigano D, Guidali C, Sala M, Fasano M, Parolaro D. The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox Res. 2009a;15(4):291–302. doi: 10.1007/s12640-009-9031-3. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, Guidali C, Pinter M, Sala M, Bartesaghi R, Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009b;19(8):763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Sánchez F, Gómez-Villalobos Mde J, Juarez I, Quevedo L, Flores G. Dendritic morphology of neurons in medial prefrontal cortex, hippocampus, and nucleus accumbens in adult SH rats. Synapse. 2011;65(3):198–206. doi: 10.1002/syn.20837. [DOI] [PubMed] [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schneider M, Drews E, Koch M. Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212-2. Behav Pharmacol. 2005;16:447–454. doi: 10.1097/00008877-200509000-00018. [DOI] [PubMed] [Google Scholar]
- Schneider M, Schomig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 2008;13:345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Scott Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology. 2007;191(4):867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- Seely KA, Patton AL, Moran CL, Womack ML, Prather PL, Fantegrossi WE, Radominska-Pandya A, Endres GW, Channell KB, Smith NH, McCain KR, James LP, Moran JH. Forensic investigation of K2, spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. For Sci Int. 2013;233(1–3):416–422. doi: 10.1016/j.forsciint.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Davies PL. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain Cogn. 2004;55(1):116–133. doi: 10.1016/S0278-2626(03)00283-5. [DOI] [PubMed] [Google Scholar]
- Singh ME, McGregor IS, Mallet PE. Perinatal exposure to delta(9)-tetrahydrocannabinol alters heroin-induced place conditioning and fos-immunoreactivity. Neuropsychopharmacology. 2006;31(1):58–69. doi: 10.1038/sj.npp.1300770. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spiga S, Lintas A, Diana M. Altered mesolimbic dopamine system in THC dependence. Curr Neuropharmacol. 2011;9(1):200–204. doi: 10.2174/157015911795017083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Sharpe NA, Koós TZ, Trent F. Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev Neurosci. 1998;20(2–3):125–145. doi: 10.1159/000017308. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Urbanska M, Blazejczyk M, Jaworski J. Molecular basis of dendritic arborization. Acta Neurobiol Exp (Wars) 2008;68(2):264–288. doi: 10.55782/ane-2008-1695. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague–Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188(2):398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslegers M, Van Hove I, Dekeyster E, Gantois I, Hu TT, D’Hooge R, Arckens L, Moons L. MMP-2 mediates Purkinje cell morphogenesis and spine development in the mouse cerebellum. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0747-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Warner LA, Kessler RC, Hughes M, Anthony JC, Nelson CB. Prevalence and correlates of drug use and dependence in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:219–229. doi: 10.1001/archpsyc.1995.03950150051010. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Ann N Y Acad Sci. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Winters BD, Kruger JM, Huang X, Gallaher ZR, Ishikawa M, Czaja K, Krueger JM, Huang YH, Schluter OM, Dong Y. Cannabinoid receptor 1-expressing neurons in the nucleus accumbens. Proc Natl Acad Sci USA. 2012;109(40):E2717–E2725. doi: 10.1073/pnas.1206303109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan WX, Heng LJ, Ma J, Wang XQ, Qu LJ, Duan L, Kang JJ, Chen LW, Gao GD. Increased expression of cannabinoid receptor 1 in the nucleus accumbens core in a rat model with morphine withdrawal. Brain Res. 2013;1531:102–112. doi: 10.1016/j.brainres.2013.07.047. [DOI] [PubMed] [Google Scholar]
- Zilles K, Wree A. Cortex: areal and laminar structure. In: Paxinos G, editor. The rat nervous system. 2nd edn. San Diego, CA: Academic Press; 1995. pp. 649–688. [Google Scholar]