Abstract
Numerous hypotheses of temporal lobe epileptogenesis have been proposed, and several involve hippocampal mossy cells. Building on previous hypotheses we sought to test the possibility that after epileptogenic injuries surviving mossy cells develop into super-connected seizure-generating hub cells. If so, they might require more cellular machinery and consequently have larger somata, elongate their dendrites to receive more synaptic input, and display higher frequencies of miniature excitatory synaptic currents (mEPSCs). To test these possibilities pilocarpine-treated mice were evaluated using GluR2-immunocytochemistry, whole-cell recording, and biocytin-labeling. Epileptic pilocarpine-treated mice displayed substantial loss of GluR2-positive hilar neurons. Somata of surviving neurons were 1.4-times larger than in controls. Biocytin-labeled mossy cells also were larger in epileptic mice, but dendritic length per cell was not significantly different. The average frequency of mEPSCs of mossy cells recorded in the presence of tetrodotoxin and bicuculline was 3.2-times higher in epileptic pilocarpine-treated mice compared to controls. Other parameters of mEPSCs were similar in both groups. Average input resistance of mossy cells in epileptic mice was reduced to 63% of controls, which is consistent with larger somata and would tend to make surviving mossy cells less excitable. Other intrinsic physiological characteristics examined were similar in both groups. Increased excitatory synaptic input is consistent with the hypothesis that surviving mossy cells develop into aberrantly super-connected seizure-generating hub cells, and soma hypertrophy is indirectly consistent with the possibility of axon sprouting. However, no obvious evidence of hyperexcitable intrinsic physiology was found. Furthermore, similar hypertrophy and hyper-connectivity has been reported for other neuron types in the dentate gyrus, suggesting mossy cells are not unique in this regard. Thus, findings of the present study reveal epilepsy-related changes in mossy cell anatomy and synaptic input but do not strongly support the hypothesis that mossy cells develop into seizure-generating hub cells.
Keywords: dentate gyrus, dendrites, hypertrophy, miniature EPSC, GluR2
Introduction
Temporal lobe epilepsy is common and can be difficult to treat effectively (Engel et al., 1997). Numerous hypotheses on underlying mechanisms of temporal lobe epilepsy have been proposed, and several involve hippocampal mossy cells. Normally mossy cells receive convergent synaptic input from nearby granule cells (Scharfman et al., 1990; Sik et al., 2006). In turn, they extend axon projections to the inner molecular layer of the dentate gyrus far along the septotemporal axis of the hippocampus (Buckmaster et al., 1996). Many mossy cells die in patients with temporal lobe epilepsy (Margerison and Corsellis, 1966; Babb et al., 1984; Blümcke et al., 1999; Swartz et al., 2006) and in rodent models (Nadler et al., 1980; Obenaus et al., 1993; Buckmaster and Dudek, 1997; Jiao and Nadler, 2007), and their axon projections in the inner molecular layer degenerate (Sloviter, 1991; Suzuki et al., 1997; Silva and Mello, 2000). Nevertheless, some mossy cells survive in patients with temporal lobe epilepsy (Blümcke et al., 2000; Seress et al., 2009) and in rodent models (Buckmaster and Jongen-Rêlo, 1999; Scharfman et al., 2001; Tang et al., 2005).
The role of mossy cells in temporal lobe epileptogenesis is controversial. The dormant basket cell hypothesis contends that mossy cells normally synapse preferentially with and drive the activity of basket cells, thereby generating inhibition (Sloviter, 1987, 1994; Sloviter et al., 2003). Mossy cells do synapse with and excite interneurons (Scharfman, 1995; Larimer and Strowbridge, 2008). However, in contrast to predictions of the dormant basket cell hypothesis, in control animals ~95% of synapses formed by mossy cell axons are with granule cells (Buckmaster et al., 1996), and mossy cell ablation reduces dentate gyrus excitability (Ratzliff et al., 2004) or transiently increases excitability without causing seizures (Jinde et al., 2012).
The irritable mossy cell hypothesis contends that after epileptogenic injuries, surviving mossy cells amplify hyperexcitable activity patterns of granule cells (Santhakumar et al., 2000; Ratzliff et al., 2002). It also has been suggested that in temporal lobe epilepsy surviving mossy cells might serve as a powerful trigger for the seizure-generating dentate gyrus network by retrogradely bridging from bursting CA3 pyramidal cells to granule cells (Scharfman et al., 2001). In the present study we sought to test the hypothesis that circuit changes of mossy cells themselves could exacerbate their potential amplifying role. In patients with temporal lobe epilepsy surviving mossy cells might develop into aberrant network hubs, establish an excessive positive-feedback circuit with granule cells, and contribute to the generation of spontaneous seizures. Hub cells are highly interconnected with other neurons (Bonifazi et al., 2009), and computer simulations of the dentate gyrus suggest addition of a small number of hub cells with enhanced incoming and outgoing synapses could render an entire network hyperexcitable (Morgan and Soltesz, 2008).
If mossy cells develop into seizure-generating hub cells they would be expected to have abnormally high levels of synaptic input and output. To accommodate increased synaptic input, one might predict surviving mossy cells to elongate dendritic processes. To support axon sprouting and the development and maintenance of additional synapses one might predict hub cells to require more cellular machinery and consequently to have larger somata, because in other parts of the nervous system neuronal cell body size and the number of axonal targets tend to correlate (McPhedran et al., 1965; Easter, 1979). To begin testing the hypothesis that surviving mossy cells develop into aberrant seizure-generating hubs we asked: Does their soma hypertrophy? Do their dendrites elongate? And do they receive more excitatory synaptic input?
Materials and Methods
Animals
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by an institutional animal care and use committee at Stanford University. Male and female GIN mice (FVBTg(GadGFP)4570Swn/J, The Jackson Laboratory) were treated with pilocarpine (300 mg/kg, i.p.) approximately 45 min after atropine methylbromide (5 mg/kg, i.p.) at 60 ± 3 d old. Diazepam (10 mg/kg, i.p.) was administered 2 h after the onset of stage 3 or greater seizures (Racine, 1972) and repeated as needed to suppress convulsions. During recovery, mice received lactated ringers with dextrose subcutaneously. There were no significant sex differences in any of the parameters analyzed in the present study. Control mice included animals that were treated identically but did not develop status epilepticus, as well as naïve mice. There were no significant differences in results between naïve and pilocarpine-treated control mice, so data were combined.
GluR2 immunocytochemistry
Beginning one month after pilocarpine treatment mice used for GluR2-immunocytochemistry were video-monitored to confirm that all animals that experienced status epilepticus developed epilepsy and displayed spontaneous, recurrent motor seizures of grade 3 or greater (Racine, 1972). None of the control mice was observed to have a seizure. Two months after status epilepticus mice were killed by urethane overdose (2 g/kg i.p.), perfused through the ascending aorta at 15 ml/min for 2 min with 0.9% sodium chloride, 5 min with 0.37% sodium sulfide, 1 min with 0.9% sodium chloride, and 30 min with 4% formaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). Brains were post-fixed overnight at 4°C. Then, the right hippocampus was isolated, cryoprotected in cryopreservation solution consisting of 30% sucrose in PB, gently straightened, frozen, and sectioned transversely with a microtome set at 40 μm. Sections were collected in 30% ethylene glycol and 25% glycerol in 50 mM PB and stored at -20°C until they were processed. For processing, sections were rinsed in PB and treated with 1% H2O2 for 2 h. After rinses in PB and 0.1 M tris-buffered saline (TBS, pH 7.4), sections were treated with blocking solution consisting of 3% goat serum (Vector Laboratories), 2% bovine serum albumin (BSA), and 0.3% Triton X-100 in 0.05 M TBS for 2 h. Sections were rinsed in TBS and incubated for 7 d at 4°C in rabbit anti-GluR2 serum (0.5 μg/ml, Millipore, #AB1768) diluted in 1% goat serum, 0.2% BSA, and 0.3% Triton X-100 in 0.05 M TBS. After rinses in TBS, sections incubated for 2 h in biotinylated goat anti-rabbit serum (1:500, Vector Laboratories) in secondary diluent consisting of 2% BSA, and 0.3% Triton X-100 in 0.05 M TBS. After rinses in TBS, sections incubated for 2 h in avidin-biotin-horseradish peroxidase complex (1:500, Vector Laboratories) in secondary diluent. After rinses in TBS and 0.1 M tris buffer (TB, pH 7.6), sections were placed for 5 min in chromogen solution consisting of 0.02% diaminobenzidine, 0.04% NH4Cl, and 0.015% glucose oxidase in TB and then transferred to fresh chromogen solution with 0.1% β-D-glucose until staining reached a desired intensity, which usually took 13 min. The reaction was stopped in rinses of TB. Sections were mounted and dried on gelatin-coated slides, dehydrated, cleared, and coverslipped with DPX. A section two-thirds the distance from the septal to the temporal pole of the hippocampus was selected for analysis. A microscope and Neurolucida system (MBF Biosciences) were used to outline the hilus of the dentate gyrus and count the number of hilar neuron profiles with a soma of diameter >12 μm. A 100X objective was used to outline somata and measure soma area.
Slice preparation
Hippocampal slices were prepared from mice when they were 83 ± 7 d old, an average of 40 d (range 30–49 d) after pilocarpine-induced status epilepticus (n=8). Mice used for slice experiments were not seizure monitored. However, it is likely that they were epileptic, because the identical pilocarpine treatment protocol in the same mouse strain results in epilepsy in virtually 100% of previously evaluated animals (Buckmaster and Lew, 2011; Heng et al., 2013). The control group consisted of 6 naïve and 2 pilocarpine-treated controls. Mice were deeply anesthetized with urethane (1.5 g/kg, i.p.) and decapitated. Tissue blocks including the dentate gyrus were removed rapidly and stored for 3 min in ice-cold modified artificial cerebrospinal fluid (mACSF) containing (in mM): 230 sucrose, 2.5 KCl, 10 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 2.5 CaCl2, and 10 D-glucose, which was aerated continuously with a mixture of 95% O2 and 5% CO2. Horizontal slices (400 μm) were prepared with a microslicer (Leica VT1000S). Slices were incubated at 32°C for 30 min in a submersion-type holding chamber that contained 50% mACSF and 50% normal ACSF, which consisted of (in mM): 126 NaCl, 3 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, and 10 D-glucose. After that, slices were transferred to normal ACSF at 32°C for 1 h. Then, slices were maintained at room temperature until used for recording.
Recording
Cells were visualized with Nomarski optics (40X, Nikon) and an infrared-sensitive video camera (Hamamatsu Photonics). Use of GIN mice permitted selection of hilar neurons that did not express green fluorescent protein and therefore were less likely to be somatostatin-immunoreactive interneurons. Hilar neurons with a large soma and thick proximal dendrites were selected for recording. Whole-cell patch-clamp recordings were obtained at 32 ± 1°C. Cells were recorded in either voltage-clamp mode or current-clamp mode (Axopatch 200B, Molecular Devices). Pipette solution contained (in mM): 100 potassium gluconate, 40 HEPES, 20 biocytin, 10 EGTA, 5 MgCl2, 2 disodium ATP, and 0.3 sodium GTP. The measured liquid junction potential was 7 mV, and all membrane potentials were corrected accordingly. Patch electrodes were pulled from borosilicate glass (1.5 mm outer diameter, 0.75 mm inner diameter, 3–4 MΩ). Seal resistance was >5 GΩ, and only data obtained from electrodes with access resistance <20 MΩ and <20% change during recordings were included. Series resistance was 80% compensated, and compensation was readjusted during experiments, when necessary. Membrane currents and potentials were low-pass filtered at 2 kHz, digitized at 10 kHz, acquired (pCLAMP, Molecular Devices), and stored on computer for off-line analysis.
Intrinsic electrophysiological properties of mossy cells were evaluated in current-clamp recordings. Input resistance was determined by measuring peak voltage responses to current steps ≤ ±10 pA and calculating slopes of regression lines in current-voltage plots. Membrane time constant was calculated from single-exponential fits to voltage responses to hyperpolarizing currents. Action potential amplitude was measured from resting membrane potential to the peak.
Tetrodotoxin (TTX, 1 μM, Tocris Bioscience) and bicuculline (10 μM) were bath applied for voltage-clamp recordings of miniature excitatory postsynaptic currents (mEPSCs) at a holding potential of −60 mV. Miniature EPSCs were analyzed using Mini Analysis (Synaptosoft). Threshold for event detection was 3 times root mean square noise level, which was not significantly different between experimental groups. Epochs for analysis were at least 1 min. Rise time was measured as the interval between points corresponding to 10% and 90% of peak amplitude during the rising phase. Amplitude was measured as the difference between the peak amplitude and baseline. Decay time (τ) was measured as the interval between points corresponding to 100% and 37% of peak amplitude during the falling phase. Charge transfer per event was measured as area under individual detected events. Charge transfer per second was measured as cumulative charge transfer per second.
Biocytin-labeling and mossy cell reconstruction
After recording, slices were placed in 4°C 4% formaldehyde in PB at least overnight and then stored at −20°C in cryopreservation solution. Slices were rinsed in 0.5% Triton X-100 and 0.1 M glycine in PB and then placed in blocking solution containing 0.5% Triton X-100, 2% goat serum, and 2% BSA in PB for 4 h. Slices incubated with mouse antibody to NeuN (1:1000, MAB377; Chemicon) in blocking solution overnight. NeuN-immunoreactivity, which labeled cell layers, was used to confirm mossy cell position in the hilus of the dentate gyrus (not shown). After rinsing, slices incubated with Alexa Fluor 594 streptavidin (5 μg/ml) and Alexa Fluor 488 goat anti-mouse serum (10 μg/ml; Invitrogen) in blocking solution overnight. Slices were rinsed, mounted on slides, and coverslipped with Vectashield (Vector Laboratories). Biocytin-labeled neurons were scanned with a confocal microscope (LSM 5 Pascal; Zeiss) at a magnification large enough to include the entire dendritic arbor. Stack height was adjusted to include all dendritic processes. Optical section interval was 3 μm. Somata and dendrites were reconstructed from confocal image stacks and measured using Neurolucida (MBF Biosciences).
All chemicals and drugs were obtained from Sigma unless specified otherwise. Results are reported as mean ± s.e.m. SigmaPlot 12.5 (Systat) was used for statistical analyses with p<0.05 considered significant. Average values from experimental groups were compared with two-tailed t tests. The Shapiro-Wilk test was used to measure normality. Equal variance was tested by checking the variability about the group means. If data failed normality or equal variance tests, a Mann-Whitney rank sum test was used instead of a t test.
Results
GluR2-immunocytochemistry
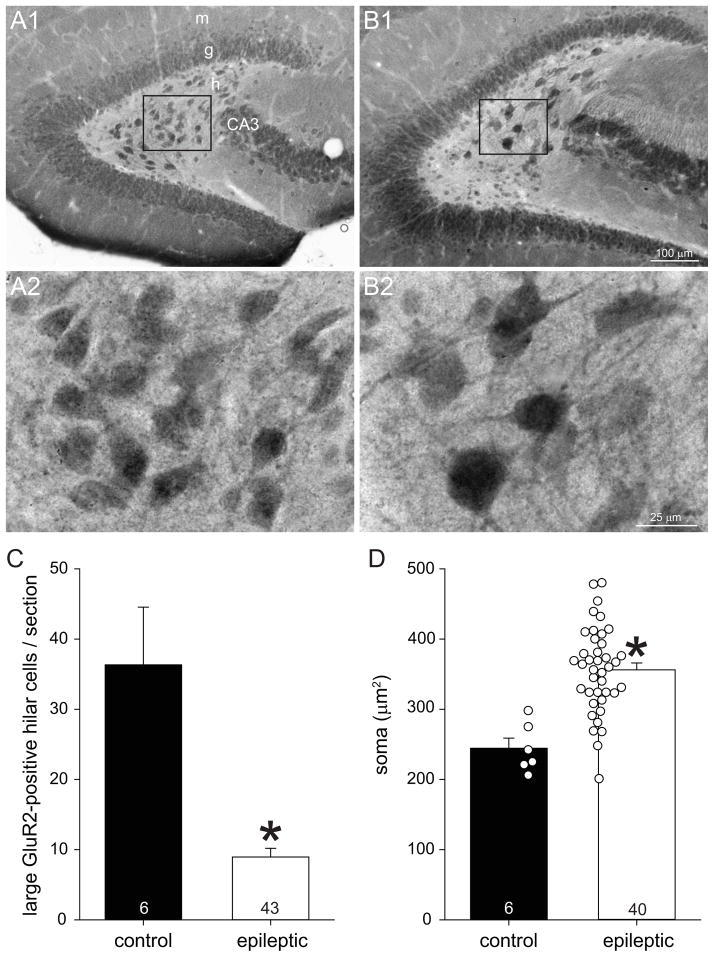
GluR2 is a useful marker for mossy cells, because it is highly expressed by glutamatergic compared to GABAergic neurons in the hippocampus of multiple species (Leranth et al., 1996), including mice (Fujise et al., 1998; Fujise and Kosaka, 1999). Similar to those previous reports, in control mice GluR2-immunolabeling revealed cells in the pyramidal and granule cell layers of the hippocampus and in the hilus of the dentate gyrus (Figure 1A1). Most GluR2-positive hilar neurons in control mice had somata >12 μm in diameter. In epileptic mice the dentate gyrus appeared larger (Figure 1B1), consistent with previous reports (Zhang et al., 2009). In the present study, average hilar area was 0.074 ± 0.007 mm2 in control mice and 1.4-times larger in epileptic animals (0.104 ± 0.005 mm2, p=0.018, Mann-Whitney rank sum test). Compared to controls the hilus in epileptic mice appeared to contain more small GluR2-positive cells, which were similar in size and shape to granule cells in the granule cell layer, consistent with the generation of ectopic granule cells in rats (Parent et al., 1997) and mice (Buckmaster and Lew, 2011) after pilocarpine-induced status epilepticus. On the other hand, the number of large GluR2-positive hilar neurons appeared reduced in animals that had experienced status epilepticus, similar to previous reports (Tang et al., 2005; Jiao and Nadler, 2007; Volz et al., 2011; Hester and Danzer, 2013). Counting only GluR2-positive hilar neuron profiles with soma diameter >12 μm revealed that epileptic animals had, on average, only 25% of controls (9.0 ± 1.2 versus 36.3 ± 8.2 cells/section, p<0.001, Mann-Whitney rank sum test) (Figure 1C), which is similar to the extent of neuron loss found in rats after status epilepticus of comparable duration (Jiao and Nadler, 2007). No large GluR2-positive hilar neurons were found in 3/43 epileptic mice. These findings confirmed mossy cell loss in epileptic pilocarpine-treated mice.
Figure 1.
GluR2-immunoreactivity in the dentate gyrus of a control (A) and epileptic pilocarpine-treated mouse (B). Boxed regions in A1 and B1 are shown at higher magnification in A2 and B2. m=molecular layer, g=granule cell layer, h=hilus, CA3=CA3 pyramidal cell layer. C Large (soma diameter >12 μm) GluR2-positive hilar cell profiles per section. Values represent mean ± s.e.m. Number of mice per group indicated at base of bars. *p<0.001, Mann-Whitney rank sum test). D Soma area of large GluR2-positive hilar cell profiles. Markers indicate average values per mouse.
Surviving GluR2-positive cells with soma diameter >12 μm (to avoid counting ectopic granule cells) in epileptic animals appeared to be larger than in controls (Figure 1A2,B2). All hilar somata >12 μm that could be outlined accurately were evaluated, and a total of 218 cells in controls and 307 cells in epileptic mice were measured. Average soma areas per mouse were calculated and used to generate group averages (Figure 1D). Average soma area of GluR2-positive hilar neurons with soma diameter >12 μm was 245 ± 14 μm2 in controls and over 1.4-times larger in epileptic mice (356 ± 10 μm2, p<0.001, t test). The range of soma areas in the epileptic group extended higher than that of controls, and 83% of epileptic mice had average soma areas larger than the control group maximum, which was 298 μm2.
Biocytin-labeled mossy cells
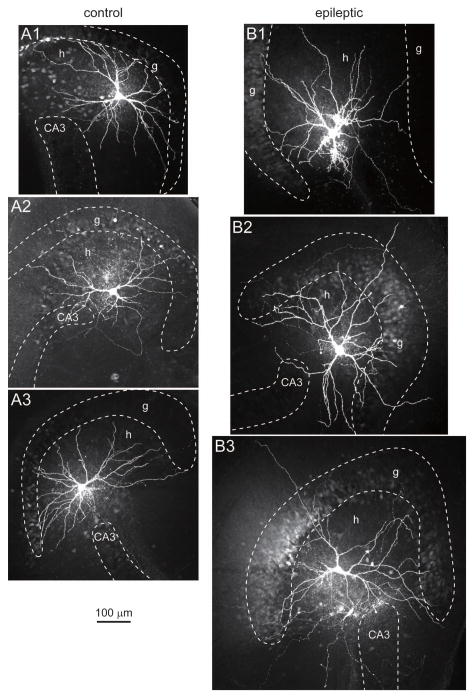
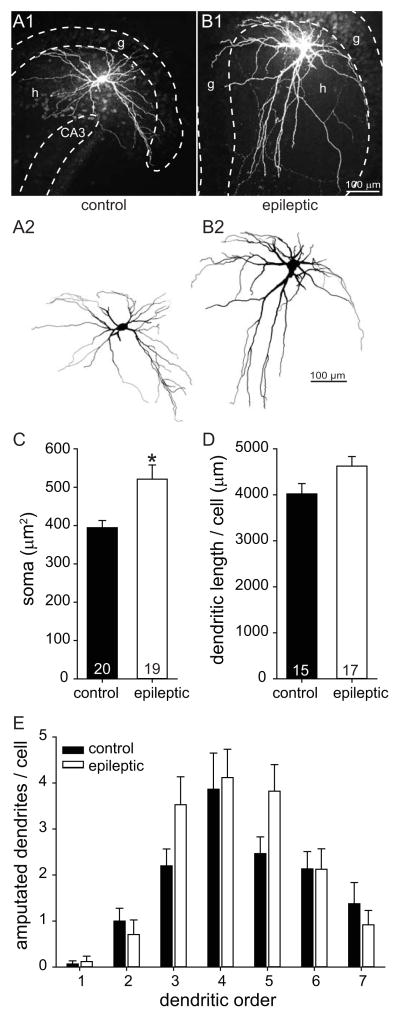
GluR2-immunocytochemistry results suggested that surviving mossy cells hypertrophy in epileptic pilocarpine-treated mice. To test that possibility more directly, hippocampal slices were prepared, and putative mossy cells were visualized and selected for recording, labeling, and anatomical verification (Figure 2). Of 52 biocytin-labeled hilar neurons, 48 displayed thorny excrescences, which is a defining feature of mossy cells (Amaral, 1978), and only those cells were included for further analysis. Biocytin-labeled mossy cell somata and dendrites were reconstructed using a Neurolucida system (Figure 3AB). Average soma area of biocytin-labeled mossy cells was 1.3-times larger in epileptic mice compared to controls (521 ± 37 μm2 versus 394 ± 19 μm2, n=19 and 20, p=0.007, Mann-Whitney rank sum test) (Figure 3C). Average soma area of mossy cells in control mice was slightly larger than that reported for control rats (323 μm2) (Buckmaster et al., 1993), probably because of less shrinkage with the whole-mount protocol used in the present study compared to staining and coverslipping protocols that use alcohols, including the GluR2-immunocytochemical protocol described above.
Figure 2.
Examples of mossy cells recorded and biocytin-labeled in control (A) and epileptic (B) mice. Cell layers were identified by NeuN-immunoreactivity (not shown) and are indicated by dashed lines. g=granule cell layer, h=hilus, CA3=CA3 pyramidal cell layer.
Figure 3.
Increased soma size of biocytin-labeled surviving mossy cells in epileptic mice. A Confocal images (A1,B1) and reconstructions (A2,B2) of representative mossy cells from a control (A) and epileptic (B) mouse. g=granule cell layer, h=hilus, CA3=CA3 pyramidal cell layer. C Soma size of mossy cells was 1.3-times larger in epileptic mice compared to controls (*p=0.007, Mann-Whitney rank sum test). Values represent mean ± s.e.m. Number of cells per group indicated at base of bars. D Cumulative dendritic length per cell was not statistically different. E Number of amputated dendrites with respect to dendritic branching order was similar in control and epileptic mice.
Average cumulative dendritic length per cell in epileptic mice (4625 ± 207 μm, n=17) was not significantly different than that of controls (4020 ± 224 μm, n=15, p=0.057, t test) (Figure 3D). Slice preparation can amputate dendrites. To estimate how this might have affected results, the number of cut dendrites per cell was counted with respect to dendritic branching order. In both control and epileptic mice average numbers of amputated dendrites peaked at the relatively distal fourth branching order, and there were no obvious differences between groups (Figure 3E), suggesting effects of dendrite amputation were similar. Control mice had 34.8 ± 1.2 dendritic endings (normal plus amputated) per cell, and epileptic animals had 40.6 ± 2.6, which was not a significant difference (p=0.179, Mann-Whitney rank sum test). These findings from biocytin-labeling confirmed surviving mossy cells in epileptic mice had larger somata but their dendrite length was not significantly different than controls.
In rodents mossy cell dendrites are largely confined to the hilus but occasionally extend through the granule cell layer and into the molecular layer of the dentate gyrus (Amaral, 1978). The number of mossy cells with at least one dendritic branch that extended into the molecular layer was 2/15 (13%) in controls and 8/17 (47%) in epileptic mice, but the difference was not statistically significant (p=0.068, Fisher exact test). Most mossy cells with dendritic extensions into the molecular layer had only one, but one mossy cell in the control group and two in the epileptic group had two dendrites per cell that extended into the molecular layer. The average number of dendritic branches extending into the molecular layer per mossy cell was 0.2 ± 0.2 and 0.6 ± 0.2 in control and epileptic animals, respectively, which was not significantly different (p=0.078, Mann-Whitney rank sum test).
Intrinsic physiology of mossy cells
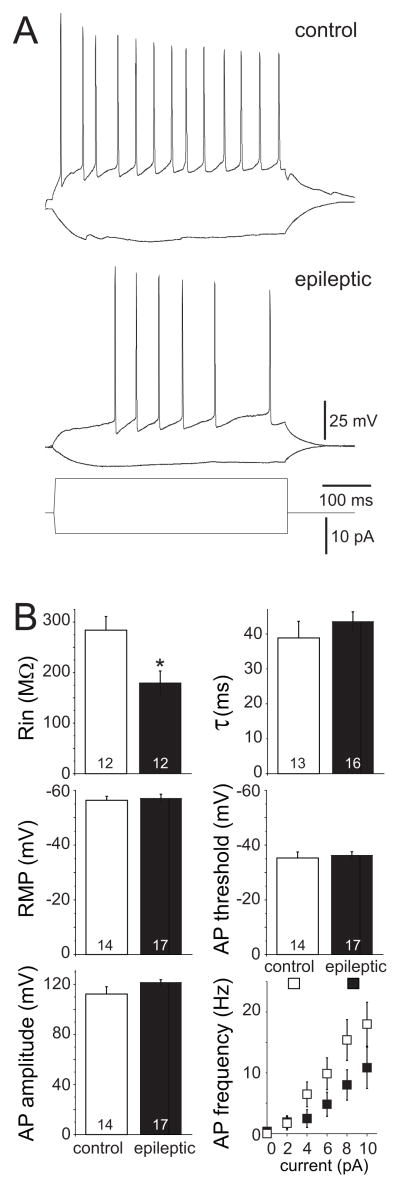
Intrinsic physiology values of control mossy cells in the present study were similar to mossy cells of mice and rats evaluated previously with whole-cell recording (Lübke et al., 1998; Jinno et al., 2003; Kerr and Copagna, 2007). Increased soma size might affect intrinsic physiological properties of surviving mossy cells in epileptic mice. Input resistance of mossy cells in epileptic mice was reduced to 63% of controls (180 ± 24 MΩ versus 284 ± 27 MΩ, n=12/group, p=0.008, t test) (Figure 4). However, all other evaluated parameters -- membrane time constant, resting membrane potential, action potential threshold and amplitude -- were similar in control and epileptic mice. A 500 ms intracellular current was injected in mossy cells to test action potential firing properties. There was a trend of lower firing frequency in epileptic mice, which likely is attributable to the decreased input resistance, but the difference was not statistically significant. Together, these results reveal little change or reduced intrinsic excitability of surviving mossy cells in epileptic mice.
Figure 4.
Intrinsic physiology of mossy cells in control and epileptic mice. A Representative voltage responses to hyperpolarizing (−5 pA) and depolarizing (10 pA) current pulses in mossy cells of control (top) and epileptic (bottom) mice. B Input resistance (Rin), membrane time constant (τ), resting membrane potential (RMP), action potential (AP) threshold and amplitude of mossy cells. Values represent mean ± s.e.m. Number of cells per group indicated at base of bars. Input resistance in epileptic mice was significantly reduced (*p=0.008, t test). There were no significant differences in other parameters.
Excitatory synaptic input to mossy cells
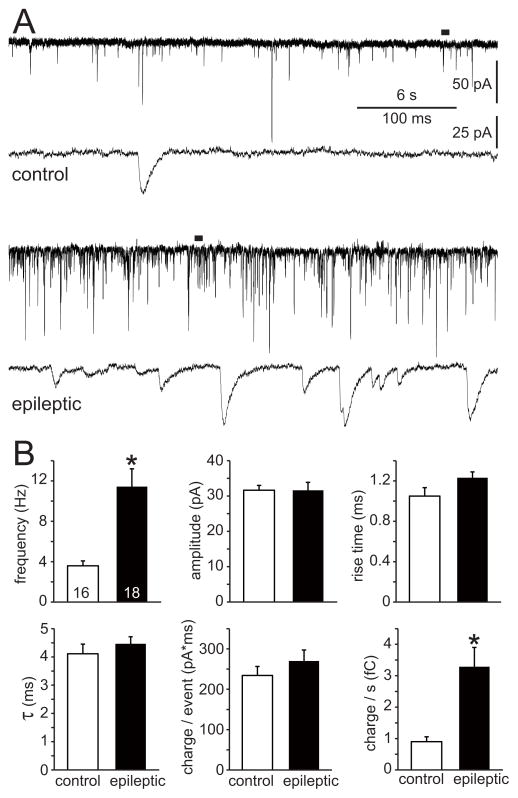
To test whether surviving mossy cells in epileptic mice receive more excitatory synaptic input, mEPSCs were recorded at a holding potential of -60 mV in the presence of tetrodotoxin and bicuculline (Figure 5A). Average frequency of mEPSCs in epileptic mice was 3.2 times higher than in controls (11.4 ± 1.8 Hz versus 3.6 ± 0.5 Hz, n=18 and 16, p<0.001, Mann-Whitney rank sum test) (Figure 5B). There were no significant differences in average amplitude, rise time, decay time, or charge transfer per event between epileptic and control mice. Consistent with increased mEPSC frequency, charge transfer per second in epileptic mice was 3.6 times that of controls (3.3 ± 0.6 fC versus 0.9 ± 0.2 fC, p<0.001, Mann-Whitney rank sum test). These findings suggest surviving mossy cells in epileptic mice receive more excitatory synaptic input.
Figure 5.
Excitatory synaptic input to mossy cells was increased in epileptic mice. A Representative miniature EPSC recordings of mossy cells from a control (top) and epileptic (bottom) mouse. Expanded views indicated by bars. B Frequency, amplitude, rise time (10–90%), decay time (τ), charge transfer per event, and charge transfer per second of mEPSCs. Values represent mean ± s.e.m. Number of cells per group indicated at base of bars. Frequency and charge transfer per second were increased in epileptic mice (*p<0.001, Mann-Whitney rank sum test). There were no significant differences in other parameters.
Discussion
The principal findings of the present study are that the soma area of large, hilar GluR2-positive cells increased, the soma area of biocytin-labeled mossy cells increased, input resistance decreased, and the frequency of mEPSCs increased in epileptic pilocarpine-treated mice compared to controls. These findings suggest surviving mossy cells enlarge and receive increased excitatory synaptic input in a mouse model of temporal lobe epilepsy.
Surviving mossy cells hypertrophy
Results of GluR2-immunocytochemistry and biocytin-labeling suggested average soma area of surviving mossy cells in epileptic mice was 1.3–1.4 times larger than in controls. The difference is unlikely to be attributable to increased antigen (GluR2) expression, because it also was evident in cells labeled with biocytin. The difference is unlikely to be attributable to selective loss of small mossy cells, because the range of soma sizes in epileptic animals extended far above that of the largest mossy cells in the control group. Surviving mossy cells in epileptic rats were reported to be qualitatively larger than in controls (Pierce et al., 2007). These findings suggest mossy cell hypertrophy occurs in rodent models of temporal lobe epilepsy, which is indirect evidence, albeit tenuous, consistent with the hypothesis that they sprout axon collaterals and form additional synapses.
However, epilepsy-related hypertrophy is not unique to mossy cells. In epileptic pilocarpine-treated mice, somata of surviving hilar somatostatin-immunoreactive interneurons display greater hypertrophy than mossy cells, 1.6-times control values (Zhang et al., 2009). Surviving somatostatin interneurons also display dendritic elongation, whereas there was no significant elongation of mossy cell dendrites in epileptic mice. Similarly, dendrites of surviving mossy cells in rats were reported to be qualitatively similar to those in controls (Scharfman et al., 2001). Mouse models of temporal lobe epilepsy also display hypertrophy of granule cells (Guilhem et al., 1996), which because of their large numbers, probably account for hypertrophy of the entire dentate gyrus (Bouilleret et al., 1999; Zhang et al., 2009). Increased signaling of the mammalian or mechanistic target of rapamycin (mTOR) pathway causes granule cell hypertrophy (Backman et al., 2001; Kwon et al., 2001, 2003, 2006). And epilepsy-related granule cell hypertrophy requires BNDF (Guilhem et al., 1996) and mTOR signaling (Buckmaster and Lew, 2011; Lew and Buckmaster, 2011; Heng et al., 2013). It is unclear whether BDNF and mTOR signaling also is necessary for mossy cell hypertrophy.
Are surviving mossy cells hyper-connected?
Mossy cells appear to receive more excitatory synaptic input in epileptic animals than in controls. That would account for their significantly higher frequency of mEPSCs, although higher release probability also could contribute. In pilocarpine-treated rats, mossy cells have longer duration excitatory postsynaptic potentials in response to stimulation of the outer molecular layer (Scharfman et al., 2001). In rat models of temporal lobe epilepsy, granule cell axons branch (Wenzel et al., 2000), sprout within the hilus (Buckmaster and Dudek, 1999), and form synapses with newly generated granule cell basal dendrites (Ribak et al., 2000) and ectopic granule cells (Pierce et al., 2005), which raises the possibility that granule cells form new synapses with surviving mossy cells, too. In epileptic animals axons from CA3 pyramidal cells also appear to sprout in the dentate gyrus where they might synapse with mossy cells. Tracer injections reveal enhanced retrograde CA3 pyramidal cell axon projections to the dentate gyrus after kainate-induced status epilepticus in rats (Siddiqui and Joseph, 2005). Similarly, transplanted CA3 pyramidal cells develop retrograde axon projections to the dentate gyrus in kainate-treated rats (Shetty et al., 2005). Thus, substantial excitatory axon sprouting and synaptogenesis occurs in areas where mossy cell dendrites and somata are located.
In turn, it is possible that surviving mossy cells sprout axons and form new synapses. Although not tested directly in the present study, soma hypertrophy is indirectly, albeit tenuously, consistent with this possibility (McPhedran et al., 1965; Easter, 1979). Mossy cells have been proposed to sprout axons and form new synapses with granule cells as a mechanism of learning and memory (Namgung et al., 1997). After lesions of the entorhinal cortex, mossy cell axons sprout and form new synapses with granule cell dendrites (Del Turco et al., 2003). In hippocampal slices from pilocarpine-treated rats, laser-scanning photostimulation of hilar neurons, which probably includes surviving mossy cells, evokes more excitatory monosynaptic input to granule cells compared to controls, suggesting surviving mossy cells sprout axons and form new synapses with granule cells after epileptogenic injuries (Zhang et al., 2012).
Thus, evidence suggests surviving mossy cells in epileptic animals might become hyper-connected, but they are not unique in this respect. In epileptic pilocarpine-treated mice surviving somatostatin interneurons receive more excitatory synaptic input (Halabisky et al., 2010), sprout axons, and form more synapses with granule cells (Zhang et al., 2009). Similarly, in rat models of temporal lobe epilepsy granule cells receive more excitatory synaptic input (Wuarin and Dudek, 2001) and form new synapses with other granule cells (Buckmaster et al., 2002; Scharfman et al., 2003). However, epilepsy-related hyper-connectivity does not appear to be universal, because dentate basket cells receive less excitatory synaptic input in epileptic pilocarpine-treated rats compared to controls (Zhang and Buckmaster, 2009).
Do surviving mossy cells become super-connected seizure-generating hub cells?
Theoretically super-connected seizure-generating hub cells do not require hyperexcitable intrinsic physiology (Morgan and Soltesz, 2008). However, hyperexcitability could exacerbate an aberrant amplifying role. Sharp electrode recordings revealed no significant differences in intrinsic physiological properties of surviving mossy cells in epileptic pilocarpine-treated rats (Scharfman et al., 2001). The present study discovered no obviously hyperexcitable intrinsic physiological characteristics in surviving mossy cells. The only significant change found was reduced input resistance, which is consistent with increased soma size and would tend to make mossy cells less excitable not more. However, in response to depolarizing current injection, action potential firing was not significantly reduced in mossy cells of mice that had experienced status epilepticus. The present study did not evaluate mossy cell responses to incoming excitatory synaptic input or spontaneous action potential discharges. It remains possible that mossy cells in epileptic animals are more active and spread that activity to many granule cells.
Surviving mossy cells in mice 30–49 d after pilocarpine-induced status epilepticus displayed no change in resting membrane potential and reduced input resistance. Mossy cells in rats one week after traumatic brain injury display depolarized resting membrane potential and no change in input resistance (Howard et al., 2007). It is unclear whether differences are attributable to the timing or the type of initial insult or some other factor. In both cases, however, spike frequency in response to depolarizing current injection was not changed significantly.
Despite findings that surviving mossy cells receive more synaptic input and perhaps develop more synaptic output, other results raise doubts that they drive seizures as a consequence of aberrantly enhanced connectivity. The mTOR inhibitor rapamycin blocks granule cell axon sprouting after epileptogenic treatments (Buckmaster et al., 2009; Zeng et al., 2009; Huang et al., 2010; Sunnen et al., 2011; van Vliet et al., 2012; Guo et al., 2013). Other neuronal types also depend on mTOR activation for axon sprouting and synaptogenesis (Campbell and Holt, 2001; Verma et al., 2005; Park et al., 2008; Li et al., 2010; Buckmaster and Wen, 2011). Stereological electron microscopy that detects all types of synaptic boutons, including those of mossy cells, reveals that rapamycin prevents excitatory axons from synapsing with dendrites of granule cells in the inner molecular layer, which is precisely where mossy cells axons would be expected to sprout and synapse (Yamawaki et al., 2014). Yet, rapamycin treatment has no effect on the development or frequency of spontaneous seizures in pilocarpine-treated mice (Buckmaster and Lew, 2011; Heng et al., 2013). Mossy cell axons might sprout in other areas, including the hilus, where they might synapse with and excite other neurons. If that synaptic reorganization were not inhibited by rapamycin, it could affect network excitability, perhaps powerfully, but that has not been evaluated. Although there are caveats, results of rapamycin treatment experiments challenge the hypothesis that mossy cell synaptic reorganization is necessary for the development of temporal lobe epilepsy. However, rigorous testing of whether surviving mossy cells develop a hub-like nature requires more direct quantification of their connectivity and electrophysiology in vivo.
Acknowledgments
Grant sponsor: NINDS and OD of NIH. Grant numbers: NS40276 and OD10989
The authors are grateful to Yui Yin Chu and Dr. Xiling Wen for assistance with immunoprocessing and Dr. Khushdev Thind for assistance with confocal microscopy.
References
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182:851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Babb TL, Brown WJ, Pretorious J, Davenport C, Lieb JP, Crandall PH. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia. 1984;25:729–740. doi: 10.1111/j.1528-1157.1984.tb03484.x. [DOI] [PubMed] [Google Scholar]
- Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao M-S, Shannon P, Bolon B, Ivy GO, Mak TW. Deletion of Pten in mouse brain causes seizures ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. doi: 10.1038/ng782. [DOI] [PubMed] [Google Scholar]
- Blümcke I, Suter B, Behle K, Kuhn R, Schramm J, Elger CE, Wiestler OD. Loss of hilar mossy cells in Ammon’s horn sclerosis. Epilepsia. 2000;41(s6):S174–S180. doi: 10.1111/j.1528-1157.2000.tb01577.x. [DOI] [PubMed] [Google Scholar]
- Blümcke I, Zuschratter W, Schewe J-C, Suter B, Lie AA, Riederer BM, Meyer B, Schramm J, Elger CE, Wiestler OD. Cellular pathology of hilar neurons in Ammon’s horn sclerosis. J Comp Neurol. 1999;414:437–453. doi: 10.1002/(sici)1096-9861(19991129)414:4<437::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, Represa A, Ben-Ari Y, Cossart R. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326:1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Ridoux V, Depaulis A, Marescaux C, Nehlig A, Le Gal La Salle G. Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience. 1999;89:717–729. doi: 10.1016/s0306-4522(98)00401-1. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. In vivo intracellular analysis of granule cell axon reorganization in epileptic rats. J Neurophysiol. 1999;81:712–721. doi: 10.1152/jn.1999.81.2.712. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Jongen-Rêlo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci. 1999;19:9519–9529. doi: 10.1523/JNEUROSCI.19-21-09519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;81:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Strowbridge BW, Schwartzkroin PA. A comparison of rat hippocampal mossy cells and CA3c pyramidal cells. J Neurophysiol. 1993;70:1280–1299. doi: 10.1152/jn.1993.70.4.1281. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Wen X. Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy. Epilepsia. 2011;52:2057–2064. doi: 10.1111/j.1528-1167.2011.03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Wenzel HJ, Kunkel DD, Schwartzkroin PA. Axon arbors and synaptic connections of hippocampal mossy cells in the rat in vivo. J Comp Neurol. 1996;366:270–292. doi: 10.1002/(sici)1096-9861(19960304)366:2<270::aid-cne7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Zhang GF, Yamawaki R. Axon sprouting in a model of temporal lobe epilepsy creates a predominantly excitatory feedback circuit. J Neurosci. 2002;22:6650–6658. doi: 10.1523/JNEUROSCI.22-15-06650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Del Turco D, Woods AG, Gebhardt C, Phinney AL, Jucker M, Frotscher M, Deller T. Comparison of commissural sprouting in the mouse rat fascia dentata after entorhinal cortex lesion. Hippocampus. 2003;13:685–699. doi: 10.1002/hipo.10118. [DOI] [PubMed] [Google Scholar]
- Easter SS., Jr The growth and development of the superior oblique muscle and trochlear nerve in juvenile and adult goldfish. Anat Rec. 1979;195:683–698. doi: 10.1002/ar.1091950408. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy. In: Engel J Jr, Pedley TA, editors. Epilepsy: a comprehensive textbook. Philadelphia: Lippincott-Raven; 1997. pp. 2417–2426. [Google Scholar]
- Fujise N, Kosaka T. Mossy cells in the mouse dentate gyrus: identification in the dorsal hilus and their distribution along the dorsoventral axis. Brain Res. 1999;816:500–511. doi: 10.1016/s0006-8993(98)01202-5. [DOI] [PubMed] [Google Scholar]
- Fujise N, Liu Y, Hori N, Kosaka T. Distribution of calretinin immunoreactivity in the mouse dentate gyrus: II. Mossy cells, with special reference to their dorsoventral difference in calretinin immunoreactivity. Neuroscience. 1998;82:181–200. doi: 10.1016/s0306-4522(97)00261-3. [DOI] [PubMed] [Google Scholar]
- Guilhem D, Dreyfus PA, Makiura Y, Suzuki F, Onteniente B. Short increase of BDNF messenger RNA triggers kainic acid-induced neuronal hypertrophy in adult mice. Neuroscience. 1996;72:923–931. doi: 10.1016/0306-4522(96)00005-x. [DOI] [PubMed] [Google Scholar]
- Guo D, Zeng L, Brody DL, Wong M. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS ONE. 2013;8:e64078. doi: 10.1371/journal.pone.0064078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabisky B, Prada I, Buckmaster PS, Prince DA. Excitatory input onto hilar somatostatin interneurons is increased in a chronic model of epilepsy. J Neurophysiol. 2010;104:2214–2223. doi: 10.1152/jn.00147.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng K, Haney MM, Buckmaster PS. High-dose rapamycin blocks mossy fiber sprouting but not seizures in a mouse model of temporal lobe epilepsy. Epilepsia. 2013;54:1535–1541. doi: 10.1111/epi.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester MS, Danzer SC. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci. 2013;33:8926–8936. doi: 10.1523/JNEUROSCI.5161-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AL, Neu A, Morgan RJ, Echegoyen JC, Soltesz I. Opposing modifications in intrinsic currents and synaptic inputs in post-traumatic mossy cells: evidence for single-cell homeostasis in a hyperexcitable network. J Neurophysiol. 2007;97:2394–2409. doi: 10.1152/jn.00509.2006. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Nadler JV. Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp Neurol. 2007;205:569–582. doi: 10.1016/j.expneurol.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinde S, Zsiros V, Jiang Z, Nakao K, Pickel J, Kohno K, Belforte JE, Nakazawa K. Hilar mossy cell degeneration causes transient dentate granule cell hyperexcitability and impaired pattern separation. Neuron. 2012;76:1189–1200. doi: 10.1016/j.neuron.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S, Ishizuka S, Kosaka T. Ionic currents underlying rhythmic bursting of ventral mossy cells in the developing mouse dentate gyrus. Eur J Neurosci. 2003;17:1338–1354. doi: 10.1046/j.1460-9568.2003.02569.x. [DOI] [PubMed] [Google Scholar]
- Kerr AM, Capogna M. Unitary IPSPs enhance hilar mossy cell gain in the rat hippocampus. J Physiol. 2007;578:451–470. doi: 10.1113/jphysiol.2006.121608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C-H, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C-H, Zhu X, Zhang J, Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci USA. 2003;100:12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C-H, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Gen. 2001;29:404–411. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- Larimer P, Strowbridge BW. Nonrandom local circuits in the dentate gyrus. J Neurosci. 2008;28:12212–12223. doi: 10.1523/JNEUROSCI.3612-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Szeidemann Z, Hsu M, Buzsáki G. AMPA receptors in the rat and primate hippocampus: a possible absence of GluR 2/3 subunits in most interneurons. Neuroscience. 1996;70:731–652. doi: 10.1016/s0306-4522(96)83003-x. [DOI] [PubMed] [Google Scholar]
- Lew FH, Buckmaster PS. Is there a critical period for mossy fiber sprouting in a mouse model of temporal lobe epilepsy? Epilepsia. 2011;52:2326–2332. doi: 10.1111/j.1528-1167.2011.03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R, Banasr M, Dwyer JM, Iwata M, Li X, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübke J, Frotscher M, Spruston N. Specialized electrophysiological properties of anatomically identified neurons in the hilar region of the rat fascia dentata. J Neurophysiol. 1998;79:1518–1534. doi: 10.1152/jn.1998.79.3.1518. [DOI] [PubMed] [Google Scholar]
- Margerison JH, Corsellis JAN. Epilepsy and the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- McPhedran AM, Wuerker RB, Henneman E. Properties of motor units in a homogeneous red muscle (soleus) of the cat. J Neurophysiol. 1965;28:71–84. doi: 10.1152/jn.1965.28.1.71. [DOI] [PubMed] [Google Scholar]
- Morgan RJ, Soltesz I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc Natl Acad Sci USA. 2008;105:6179–6184. doi: 10.1073/pnas.0801372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JV, Perry BW, Cotman CW. Selective reinnervation of hippocampal area CA1 and the fascia dentata after destruction of CA3-CA4 afferents with kainic acid. Brain Res. 1980;182:1–9. doi: 10.1016/0006-8993(80)90825-2. [DOI] [PubMed] [Google Scholar]
- Namgung U, Matsuyama S, Routtenberg A. Long-term potentiation activates the GAP-43 promotor: selective participation of hippocampal mossy cells. Proc Natl Acad Sci USA. 1997;94:11675–11680. doi: 10.1073/pnas.94.21.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13:4470–4485. doi: 10.1523/JNEUROSCI.13-10-04470.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Melton J, Punsoni M, McCloskey DP, Scharfman HE. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol. 2005;196:316–331. doi: 10.1016/j.expneurol.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Punsoni M, McCloskey DP, Scharfman HE. Mossy cell axon synaptic contacts on ectopic granule cells that are born following pilocarpine-induced seizures. Neurosci Lett. 2007;422:136–140. doi: 10.1016/j.neulet.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Ratzliff AdH, Howard A, Santhakumar V, Osapay I, Soltesz I. Rapid deletion of mossy cells does not result in a hyperexcitable dentate gyrus: implications for epileptogenesis. J Neurosci. 2004;24:2259–2269. doi: 10.1523/JNEUROSCI.5191-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzliff AdH, Santhakumar V, Howard A, Soltesz I. Mossy cells in epilepsy: rigor mortis or vigor mortis? TINS. 2002;25:140–144. doi: 10.1016/s0166-2236(00)02122-6. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428:240–253. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Bender R, Frotscher M, Ross ST, Hollrigel GS, Toth Z, Soltesz I. Granule cell hyperexcitability in the early post-traumatic rat dentate gyrus: the ‘irritable mossy cell’ hypothesis. J Physiol. 2000;524:117–134. doi: 10.1111/j.1469-7793.2000.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Electrophysiological evidence that dentate hilar mossy cells are excitatory and innervate both granule cells and interneurons. J Neurophysiol. 1995;74:179–194. doi: 10.1152/jn.1995.74.1.179. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Kunkel DD, Schwartzkroin PA. Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience. 1990;37:693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Smith KL, Goodman JH, Sollas AL. Survival of dentate hilar mossy cells after pilocarpine-induced seizures and their synchronized burst discharges with area CA3 pyramidal cells. Neuroscience. 2001;104:741–759. doi: 10.1016/s0306-4522(01)00132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Berger RE, Goodman JH. Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting. J Neurophysiol. 2003;90:2536–2547. doi: 10.1152/jn.00251.2003. [DOI] [PubMed] [Google Scholar]
- Seress L, Ábrahám H, Horváth Z, Dóczi T, Janszky J, Klemm J, Byrne R, Bakay RAE. Survival of mossy cells of the hippocampal dentate gyrus in humans with mesial temporal lobe epilepsy. J Neurosurg. 2009;111:1237–1247. doi: 10.3171/2008.11.JNS08779. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Zaman V, Hattiangady B. Repair of the injured adult hippocampus through graft-mediated modulation of the plasticity of the dentate gyrus in a rat model of temporal lobe epilepsy. J Neurosci. 2005;25:8391–8401. doi: 10.1523/JNEUROSCI.1538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui AH, Joseph SA. CA3 axonal sprouting in kainate-induced chronic epilepsy. Brain Res. 2005;1066:129–146. doi: 10.1016/j.brainres.2005.10.066. [DOI] [PubMed] [Google Scholar]
- Sík A, Coté A, Boldogkõi Z. Selective spread of neurotropic herpesviruses in the rat hippocampus. J Comp Neurol. 2006;496:229–243. doi: 10.1002/cne.20921. [DOI] [PubMed] [Google Scholar]
- Silva JG, Mello LEAM. The role of mossy cell death and activation of protein synthesis in the sprouting of dentate mossy fibers: evidence from calretinin and neo-Timm staining in pilocarpine-epileptic mice. Epilepsia. 2000;41(s6):S18–S23. doi: 10.1111/j.1528-1157.2000.tb01551.x. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994;35:640–654. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459:44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- Sunnen CN, Brewster AL, Lugo JN, Vanegas F, Turcios E, Mukhi S, Parghi D, D’Arcangelo G, Anderson AE. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52:2065–2075. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F, Makiura Y, Guilhem D, Sorensen J-C, Onteniente B. Correlated axonal sprouting and dendritic spine formation during kainate-induced neuronal morphogenesis in the dentate gyrus of adult mice. Exp Neurol. 1997;145:203–213. doi: 10.1006/exnr.1997.6469. [DOI] [PubMed] [Google Scholar]
- Swartz B, Houser CR, Tomiyasu U, Walsh GO, DeSalles A, Rich JR, Delgado-Escueta A. Hippocampal cell loss in posttraumatic human epilepsy. Epilepsia. 2006;47:1373–1382. doi: 10.1111/j.1528-1167.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Tang FR, Chia SC, Zhang S, Chen PM, Gao H, Liu CP, Khanna S, Lee WL. Glutamate receptor 1-immunopositive neurons in the gliotic CA1 area of the mouse hippocampus after pilocarpine-induced status epilepticus. Eur J Neurosci. 2005;21:2361–2374. doi: 10.1111/j.1460-9568.2005.04071.x. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Forte G, Holtman L, den Burger JCG, Sinjewel A, de Vries HD, Aronica E, Gorter JA. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia. 2012;53:1254–1263. doi: 10.1111/j.1528-1167.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz F, Bock HH, Gierthmuehlen M, Zentner J, Haas CA, Freiman TM. Stereologic estimation of hippocampal GluR2/3- and calretinin-immunoreactive hilar neurons (presumptive mossy cells) in two mouse models of temporal lobe epilepsy. Epilepsia. 2011;42:1579–1589. doi: 10.1111/j.1528-1167.2011.03086.x. [DOI] [PubMed] [Google Scholar]
- Wenzel JH, Woolley CS, Robbins CA, Schwartzkroin PA. Kainic acid-induced mossy fiber sprouting and synapse formation in the dentate gyrus of rats. Hippocampus. 2000;10:244–260. doi: 10.1002/1098-1063(2000)10:3<244::AID-HIPO5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wuarin JP, Dudek FE. Excitatory synaptic input to granule cells increases with time after kainate treatment. J Neurophysiol. 2001;85:1067–1077. doi: 10.1152/jn.2001.85.3.1067. [DOI] [PubMed] [Google Scholar]
- Yamawaki R, Thind K, Buckmaster PS. Blockade of excitatory synaptogenesis with proximal dendrites of dentate granule cells following rapamycin treatment in a mouse model of temporal lobe epilepsy. J Comp Neurol. 2014 doi: 10.1002/cne.23681. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L-H, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Buckmaster PS. Dysfunction of the dentate basket cell circuit in a rat model of temporal lobe epilepsy. J Neurosci. 2009;29:7846–7856. doi: 10.1523/JNEUROSCI.6199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Huguenard JR, Buckmaster PS. Increased positive-feedback from hilar and CA3 neurons to granule cells in a rat model of temporal lobe epilepsy. J Neurosci. 2012;32:1183–1196. doi: 10.1523/JNEUROSCI.5342-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci. 2009;29:14247–14256. doi: 10.1523/JNEUROSCI.3842-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]