Abstract
Activation of AMPA receptors in the nucleus accumbens is necessary for the reinstatement of cocaine-seeking behavior, an animal model of drug craving and relapse. AMPA receptors are tetrameric protein complexes that consist of GluA1-GluA4 subunits, of which GluA2 imparts calcium permeability. Adenosine deaminase acting on RNA (ADAR2) is a nuclear enzyme that is essential for editing GluA2 pre-mRNA at Q/R site-607. Unedited GluA2(Q) subunits form calcium permeable AMPA receptors (CP-AMPARs), whereas edited GluA2(R) subunits form calcium impermeable channels (CIAMPARs). Emerging evidence suggests that the reinstatement of cocaine seeking is associated with increased synaptic expression of CP-AMPARs in the nucleus accumbens. However, the role of GluA2 Q/R site editing and ADAR2 in cocaine seeking is unclear. In the present study, we investigated the effects of forced cocaine abstinence on GluA2 Q/R site editing and ADAR2 expression in the nucleus accumbens. Our results demonstrate that 7 days of cocaine abstinence is associated with decreased GluA2 Q/R site editing and reduced ADAR2 expression in the accumbens shell, but not core, of cocaine-experienced rats compared to yoked saline controls. In order to examine the functional significance of ADAR2 and GluA2 Q/R site editing in cocaine seeking, we used viral-mediated gene delivery to overexpress ADAR2b in the accumbens shell. Increased ADAR2b expression in the shell attenuated cocaine priming-induced reinstatement of drug seeking and was associated with increased GluA2 Q/R site editing and surface expression of GluA2-containing AMPARs. Taken together, these findings support the novel hypothesis that an increased contribution of accumbens shell CP-AMPARs containing unedited GluA2(Q) promotes cocaine seeking. Therefore, CP-AMPARs containing unedited GluA2(Q) represent a novel target for cocaine addiction pharmacotherapies.
Keywords: psychostimulant, addiction, relapse, AMPA receptor, RNA editing, ADAR2
Introduction
AMPA receptor calcium permeability depends on whether the GluA2 subunit is incorporated into the tetramer1-3. The ability of the GluA2 subunit to regulate calcium influx depends upon RNA editing. GluA2 transcripts are edited at amino acid 607 such that the genomic glutamine (Q) codon is converted to a codon encoding arginine (R)4. This post-transcriptional modification involves enzymatic deamination of an adenosine residue in the GluA2 pre-mRNA prior to splicing5. The primary enzyme that catalyzes GluA2 Q/R site editing is adenosine deaminase acting on RNA 2 (ADAR2b)6, 7. Thus, AMPA receptors are calcium impermeable (CI-AMPARs) if they contain the edited GluA2(R) subunit and calcium permeable (CP-AMPARs) if they contain the unedited GluA2(Q) subunit or if they are GluA2-lacking8. While there is strong evidence that GluA2-lacking AMPA receptors contribute to normal brain function and disease9, 10, including drug addiction11, the functional significance of unedited GluA2(Q) in the brain is not clear8.
Chronic cocaine exposure produces profound changes in glutamate transmission in limbic nuclei including the nucleus accumbens. Specifically, increased glutamate transmission and AMPA receptor activation in the nucleus accumbens are necessary for the reinstatement of cocaine-seeking behavior, an animal model of drug craving and relapse12. Moreover, cocaine seeking is associated with increased synaptic expression of accumbens CP-AMPARs (i.e., GluA2-lacking AMPA receptors), which enhance synaptic strength13-15. Cocaine-induced AMPA receptor plasticity is a dynamic process that is mediated, in part, by cellular mechanisms regulating trafficking of both GluA1 and GluA2 subunits11. Since 99% of GluA2 subunits expressed in the adult brain are edited GluA2(R)16, 17, cocaine seeking is likely associated with decreased synaptic expression of CI-AMPARs containing GluA2(R). Alternatively, decreased GluA2 Q/R site editing during cocaine abstinence could result in increased synaptic expression of CP-AMPARs containing unedited GluA2(Q) subunits.
The objective of the present study was to investigate the role of GluA2 Q/R site editing in the reinstatement of cocaine-seeking behavior. ADAR2b expression and GluA2 Q/R transcript levels were measured in the nucleus accumbens core and shell subregions of cocaine-experienced rats following 7 days of forced drug abstinence. Our results demonstrate that cocaine abstinence decreased GluA2 Q/R site editing in the nucleus accumbens shell, but not the accumbens core, and was associated with decreased shell ADAR2 expression. Moreover, increased expression of ADAR2b in the accumbens shell attenuated cocaine priming-induced reinstatement of drug seeking and was associated with increased expression of edited GluA2(R) and increased surface expression of GluA2-containing AMPARs in the accumbens shell. These novel findings suggest that nucleus accumbens shell GluA2 Q/R site editing and ADAR2b function play a critical role in cocaine craving and relapse.
Materials and Methods
Animals and housing
Male Sprague-Dawley rats (Rattus norvegicus) weighing 250-300 g were obtained from Taconic Laboratories. Animals were individually housed with food and water available ad libitum in their home cage. A 12/12 hr light/dark cycle was used with the lights on at 7:00 a.m. All experimental procedures were performed during the light cycle. The experimental protocols were all consistent with the guidelines issued by the U.S. National Institutes of Health and were approved by the University of Pennsylvania's Institutional Animal Care and Use Committee.
Surgery
Prior to surgery, rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine (i.p.; Sigma-Aldrich, St. Louis, MO). An indwelling silastic catheter was inserted into the right jugular vein and sutured in place. The catheter was then passed subcutaneously over the shoulder blade and routed to a mesh backmount platform (CamCath, Cambridge, UK) that was positioned subcutaneously dorsal to the shoulder blades. Catheters were flushed daily with 0.3 ml of antibiotic (Timentin, 0.93 mg/ml) dissolved in heparinized saline and sealed with plastic obturators when not in use. For viral overexpression experiments, rats were mounted in a stereotaxic apparatus immediately following catheter implantation. Guide cannulas (14 mm, 24 gauge) for microinjections were implanted bilaterally 2mm dorsal to the nucleus accumbens shell. Guide cannulas were cemented in place by affixing dental acrylic to stainless steel screws secured in the skull. The coordinates for the ventral ends of the guide cannulas, relative to bregma according to the atlas of Paxinos and Watson18, were as follows: +1.0 mm A/P, ±1.0 mm M/L, and −7.0 mm D/V.
Cocaine self-administration and yoked saline controls for molecular experiments
Rats were allowed 7 days to recover from surgery before cocaine self-administration commenced. Cocaine was obtained from the National Institute on Drug Abuse (Rockville, MD) and dissolved in bacteriostatic 0.9% saline. Rats were randomly assigned to one of two groups: cocaine self-administering animals and yoked saline controls. Each rat trained to respond for contingent cocaine infusions was paired with a yoked subject that received infusions of saline. Lever pressing for the saline-yoked rats had no scheduled consequences, but these animals received the same number and temporal pattern of infusions as self-administered by the paired cocaine-experimental rat.
Initially, cocaine-experimental rats were placed in the modular operant chambers (Med Associates, St. Albans, VT) and allowed to lever press for intravenous cocaine infusions (0.25 mg cocaine/59 μl saline, infusion over 5 s) on a fixed-ratio 1 (FR1) schedule of reinforcement. Once a cocaine-experimental rat achieved at least 20 infusions of cocaine in a single operant session under the FR1 schedule, the response requirement was switched to a FR5 schedule of reinforcement. For responding on both FR1 and FR5 schedules, the maximum number of cocaine infusions was limited to 30 per daily self-administration session and a 20 s time-out period followed each cocaine infusion. Daily 2 h operant sessions (5-6 days/week) were conducted for a total of 21 days. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during both the FR1 and FR5 training sessions. After the 21st operant session, cocaine-experimental and yoked saline control rats were returned to their home cages where they remained for 7 days. On the 7th day of cocaine abstinence, brains were removed and the nucleus accumbens core and shell were dissected on ice and stored at −80°C until further analysis.
RNA extraction and reverse transcription
RNA was extracted from dissected accumbens tissue from cocaine-experimental or yoked saline control rats using RNeasy kit (Qiagen, Valencia, CA) according to manufacturer's instructions and as described previously19. Superscript First-Strand Synthesis System for RT-PCR (Invitrogen) was used to generate cDNA. Briefly, 1.75 μg of total RNA was used in each 20 μl reaction and reactions were primed using random hexamers. Reverse transcription reactions were performed using specific primers in an iCycler (Bio-Rad) (25°C for 10 min, 42°C for 50 min, 70°C for 15 min) to quantify the amount of gene expression as compared to a standard curve.
Allele specific qPCR primers
Edited GluA2(R) and unedited GluA2(Q) mRNA transcript expression was determined using qPCR. GluA2 Q/R site editing occurs at residue 607 (CAG->CGG). Unedited GluA2(Q) transcripts were identified using the following primer pair: “GluA2, F” (5’-GCGAATTCAGAAGTCCAAACCAGGAG-3’)20 with “GluA2, Q” (5’-GCGAAATATCGCATCCTTGCT-3’). Edited GluA2(R) transcripts were identified using the “GluA2, F” forward primer with “GluA2, R” (5’-GCGAAATATCGCATCCTTGCC-3’)20. As a control for our allele-specific GluA2 reactions, we generated primers for the GluA1 transcript at the homologous Q (CAG) site as compared to GluA2 as the GluA1 transcript is not edited. Unedited GluA1 transcripts were detected using “GluA1, F” (5’- GCGAATTCGCTGGAGGGGACAACT CAA G-3’)20 with “GluA1, Q” (5’-GGGAAATGTCACATC CTT GCT-3’). Negative control, “edited” reactions were amplified by “GluA1, F” and “GluA1, R” (5’-GGG AAATGTCACATCCTTGCC-3’). The following primers were used for real-time PCR in order to quantify GAPDH expression: “GAPDH, F” (5’-AACAGCAACTCCCATTCTTC-3’) with “GAPDH, R” (5’-TGGTCCAGGGTTTCTTACTC-3’). Quantitative real time-PCR was performed in an iCycler with the use of SYBR-green PCR Master Mix (Applied Biosystems, Foster City, CA) through 50 PCR cycles (95°C for 30 sec, 57°C for 60 sec, 72°C for 90 sec). The threshold cycle for each sample was chosen from the linear range and converted to a starting quantity by interpolation from a standard curve run on the same plate for each set of primers. mRNA levels were normalized to GAPDH mRNA levels. Threshold amplification cycle numbers (CT) were determined using iCycler software as previously described21.
Bbvl digestion
Edited GluA2(R) and unedited GluA2(Q) mRNA transcript expression was further confirmed by Bbvl digestion. The enzyme Bbvl only recognizes the sequence of the unedited GluA2(Q) receptor subunit around the edited base and thus provides additional support for the RT-qPCR experiments20. RNA was extracted from nucleus accumbens shell and core tissue and cDNA was synthesized as described above. GluA2 transcripts were amplified using the following PCR primers: “GluA2, F” (5’-GCGAATTCAGAAGTCCAAACCAGGAG-3’) with “GluR2, cut” (5’-GCGGTACCGTGTAGGAGGAGATTATGAT-3’)20. Quantitative real time-PCR was performed through 35 cycles at 60°C with 0.2 μg of cDNA in a 50 μl reaction volume. Following PCR cycling, 10 μl of PCR product was digested in a total volume of 20 μl with 1 unit of BbvI per reaction overnight at room temperature. Control reactions omitted the BbvI enzyme.
Western blotting
Whole cell extracts from accumbens tissue were homogenized with a Polytron (Brinkman Instruments) in ice-cold TEVP buffer (10 mm Tris-HCl, pH 7.4, 5 mm NaF, 1 mm Na3VO4, 1 mm EDTA, 1 mm EGTA) and sonicated for 8 s. Protein content was determined with a Bio-Rad protein assay kit. 20 μg of tissue homogenate was boiled in the presence of sample buffer for 5 min before separation on 4-20% SDS- polyacrylamide gel. Proteins were transferred to nitrocellulose membranes, which were then blocked with 5% nonfat dry milk dissolved in Tris-buffered saline containing 0.2% Tween 20 (TBST) for 60 min. Membranes were then incubated overnight at 4°C with anti-ADAR2 antibody 1:500 (Santa Cruz Biotechnology). Primary antibody incubation was followed by 6 washes (10 min, rocking, RT) in TBST before incubation with the secondary antibody (HRP-conjugated goat anti-rabbit IgG, Jackson ImmunoResearch Laboratories). Antibody/protein complexes were visualized using the ECL detection system (NEN) and their intensities were quantified using computer-assisted densitometry (Alpha Innotech). For data analysis, ADAR2 bands were normalized to GAPDH.
Viral-mediate gene transfer
Herpes simplex virus (HSV) vectors were constructed and packaged at the Viral Gene Transfer Core at the McGovern Institute for Brain Research (MIT, MA) as described previously22. Briefly, ADAR2b cDNA was inserted into the HSV amplicon HSV-PrpUC, packaged and re-suspended in 10% sucrose. The average titer of the resulting HSV stocks was 4.0 × 108 infectious units/ml. Transgene expression was regulated by HSV IE 4/5 vectors, which produced a transient increase in transgene expression that was maximal between 3-4 d after infusion and dissipated completely by 6-7 days22. Therefore, reinstatement test sessions were conducted 3 and 6 days after stereotaxic surgery to introduce HSV viral vectors (Figure 4A).
Figure 4. Increased ADAR2b expression in the accumbens shell is associated with increased surface expression of GluA2/3-containing AMPARs in the shell.
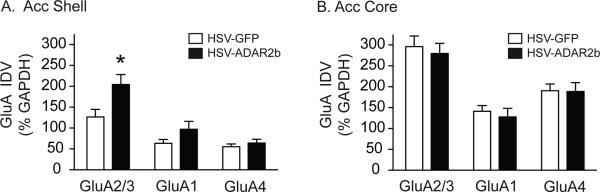
(A) GluA2/3, but not GluA1 or GluA4, surface expression was significantly increased in the shell following ADAR2b overexpression in the shell (*P<0.01). (B) No significant effects of ADAR2b overexpression in the shell on GluA2/3, GluA1 or GluA4 surface expression were noted in the core (P>0.05). (shell: n=8/treatment; core: n=4/treatment).
Four ADAR2 isoforms have been identified in the rat brain23. ADAR2b preferentially edits GluA2 premRNA at the Q/R site7. Therefore, HSV was used to express exogenous ADAR2b in vivo. ADAR2b constructs were identical to those used previously to over-express ADAR2b in the rat brain24. ADAR2b over-expression increases GluA2 Q/R site editing and synaptic expression of functional Ca2+ impermeable AMPA receptors (i.e., edited GluA2(R)-containing AMPA receptors) in the hippocampus24.
Transient ADAR2b overexpression in the accumbens shell and the reinstatement of cocaine seeking
Rats underwent catheterization surgery as described above. After a 7 d recovery period, rats were trained to lever press for intravenous cocaine on a fixed-ratio 5 (FR5) schedule of reinforcement. Daily operant sessions were conducted for a total of 21 d. Cocaine self-administration behavior was then extinguished by replacing the cocaine with 0.9% saline. The extinction phase continued until responding on the active lever was <20% of the response rate maintained by cocaine self-administration. It took approximately 3-5 days for rats to meet their extinction criteria.
After 5 days of extinction, animals were injected with either HSV expressing GFP or ADAR2b directly into the nucleus accumbens shell. HSV-GFP and HSV-ADAR2b (2 μl/hemisphere) were administered bilaterally into the accumbens shell via 16 mm, 33 gauge microinjectors. Each 2 μl microinjection was made over 10 min and the microinjectors were left in place for an additional 2 min. Two consecutive daily extinction sessions were then conducted to ensure that cocaine self-administration behavior was extinguished. Reinstatement of cocaine seeking was tested 72 h following intra-accumbens shell microinjection of viral vectors. An acute priming injection of cocaine (10 mg/kg, i.p.) was administered and rats were placed immediately into the operant chambers. Satisfaction of the response requirement during reinstatement test sessions resulted in a saline rather than a cocaine infusion (which was identical to extinction conditions). Two consecutive days of extinction followed the first reinstatement test. Cocaine priming-induced reinstatement was again tested 72 h after the first reinstatement test (i.e., 6 d following viral infusions).
BS3 cross-linking
Alterations in the surface expression of GluA subunits were measured using Bis(sulfosuccinimidyl)suberate (BS3) cross-linking methods as described previously (Anderson et al., 2008). Briefly, the nucleus accumbens shell and core were dissected and incubated in 2mM BS3 in artificial cerebrospinal fluid (aCSF) for 30 min at 4°C. The reaction was quenched with 100mM glycine, washed with aCSF, resuspended in lysis buffer (10 mm Tris-HCl, pH 7.4, 5 mm NaF, 1 mm Na3VO4, 1 mm EDTA, 1 mm EGTA) and prepared for immunoblots. The samples were used for western blots as described above using the following antibodies GluA1, GluA2/3, and GluA4 (1:700, Millipore). All bands were normalized to GAPDH.
Statistics
Brain region-specific changes in GluA2 Q/R transcripts, Bbvl digest, total GluA2 transcripts and ADAR2 expression were analyzed using un-paired Student's t-tests. Active and inactive lever responses during cocaine reinstatement test sessions were also analyzed with un-paired Student's t-tests. Membrane bound expression of GluA subunits in the core and shell was analyzed with separate 2-way ANOVAs followed by Bonferroni post-hoc tests (p<0.05).
Results
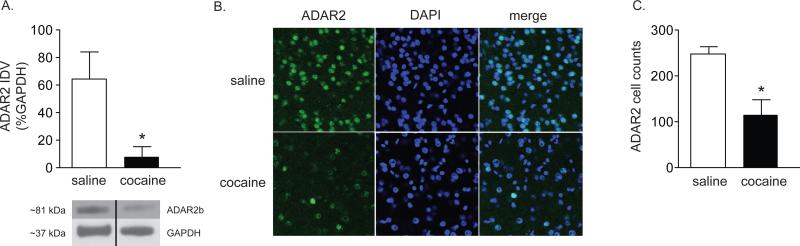
Previous studies have demonstrated that cocaine seeking is associated with increased synaptic expression of CP-AMPARs (i.e., GluA2-lacking AMPA receptors)13-15 in the accumbens. However, it is not clear whether GluA2 Q/R site editing and increased expression of CP-AMPARs containing unedited GluA2(Q) subunits play a role in cocaine seeking. Decreased GluA2 Q/R site editing during cocaine abstinence would result in increased expression of unedited GluA2(Q) subunits that would in turn form CP-AMPARs containing GluA2(Q). In order to investigate this hypothesis, GluA2 Q/R transcripts were measured in rat nucleus accumbens subregions following 1 and 7 days of forced cocaine abstinence. While GluA2 Q/R site editing was not altered following 1 day of cocaine abstinence [t(4)=0.70, P=0.52](Supplemental Figure 1A), there was a significant decrease in GluA2 Q/R editing (as reflected by an increase in the ratio of GluA2(Q):GluA2(R) transcripts) in the accumbens shell as measured by RT-qPCR [t(13)=2.84, P<0.01] and Bbvl digestion [t(8)=3.58, P<0.01] following 7 days of abstinence (Figure 1A & B). In contrast, no effects of cocaine abstinence on GluA2 editing in the accumbens core were noted following 1 day [t(4)=0.05, P=0.97](Supplemental Figure 1B) or 7 days (RT-qPCR [t(14)=0.70, P=0.50] or Bbvl digestion [t(8)=1.09, P=0.31](Figure 1D & E)) of cocaine abstinence. Furthermore, 7 days of cocaine abstinence was not associated with altered expression total GluA2 mRNA in the shell [t(4)=0.24, P=0.82](Figure 1C) or core [t(4)=0.22, P=0.83](Figure 1F). We next assessed ADAR2 expression, which was decreased in the accumbens shell as measured by western blots [t(4)=3.05, P<0.05] (Figure 2A) and immunohistochemistry [t(5)=4.23, P<0.01](Figure 2B &C). Taken together, these findings indicate that cocaine self-administration decreases shell ADAR2 thereby, increasing expression of CP-AMPARs containing unedited GluA2(Q).
Figure 1. Cocaine abstinence is associated with disrupted GluA2 Q/R site editing in the accumbens shell.
The ratio of GluA2 Q:R transcripts in the accumbens shell (A), but not core (D), was significantly increased in cocaine-experienced rats compared to yoked saline control animals following 7 days of forced cocaine abstinence as measured by RT-qPCR (n=8/treatment). Bbvl digestion of GluA2 PCR product demonstrated a significant increase in the ratio of GluA2 Q:R transcripts in the accumbens shell (B), but not core (E), in cocaine-experienced rats compared to yoked saline control animals following 7 days of forced cocaine abstinence (n=4/treatment). No change in total GluA2 mRNA expression was observed in the shell (C) or core (F) following 7 days of abstinence (n=4/treatment). *P<0.05.
Figure 2. Cocaine abstinence is associated with decreased ADAR2 expression in the accumbens shell.
(A) ADAR2 protein expression was significantly decreased in the accumbens shell during cocaine abstinence as measured by western blots. (B) Representative images of ADAR2 immunostaining in the accumbens shell following 7 days of forced abstinence. (C) There was a significant decrease in the number of ADAR2 positive cells in the accumbens shell during cocaine abstinence as measured by immunohistochemistry. (n= 3/treatment), *P<0.05.
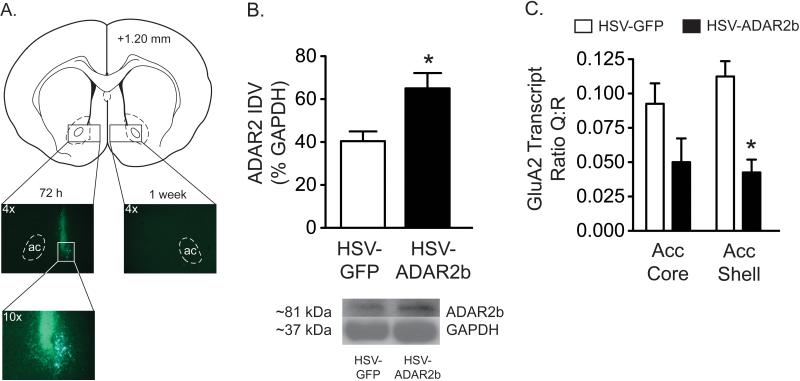
In order to assess the functional significance of cocaine-induced changes in ADAR2, viral-mediated gene transfer was used to express ADAR2b in the accumbens shell prior to cocaine priming-induced reinstatement of drug seeking, an animal model of drug craving and relapse. Transgene expression was maximal 72 h following infusion of HSV into the shell and completely dissipated 7 d post injection (Figure 3A). ADAR2b protein expression [t(19)=2.284, P<0.05](Figure 3B) and GluA2 Q/R site editing [t(6)=4.80, P<0.01](Figure 3C) were increased 72 h following injection of HSV-ADAR2b into the accumbens shell. While there was a trend toward increased GluA2 Q/R site editing in the accumbens core following transgene expression in the shell, these effects were statistically not significant [t(6)=1.86, P=0.11] (Figure 3C).
Figure 3. Increased ADAR2b expression in the accumbens shell is associated with increased GluA2 Q/R editing.
(A) Photomicrographs depicting transient HSV-GFP expression in the accumbens shell. Maximal expression was detected 72 h post infusion and dissipated completely 7 days following microinjection into the shell. (B) ADAR2 protein expression was increased in the accumbens shell 72 h following infusion of HSV-ADAR2b (n=14) compared to rats that received HSV-GFP (n=7) (*P<0.05). (C) The ratio of GluA2 Q:R transcripts in the accumbens shell was significantly decreased 72 h following intra-shell infusion of HSV-ADAR2b compared to HSV-GFP (*P<0.01). No significant effects of transgene expression on GluA2 editing were noted in the accumbens core (P=0.11).
Next, we measured alterations in the surface expression of accumbens GluA1-4 subunits using BS3 cross-linking following ADAR2b overexpression in the shell. These data were analyzed with separate two-way ANOVAs with a between-subjects factor of treatment (HSV-GFP versus HSV-ADAR2b) and a within-subjects factor of GluA subunits. The results of the analyses revealed significant main effects of virus [F(1, 28)=4.659, P<0.05] and GluA subunits [F(2, 28)=57.44, P<0.0001] for membrane bound proteins in the accumbens shell (Figure 4A), but not core [F(2, 12)=0.164, P=0.70] (Figure 4B). Subsequent post-hoc analyses revealed a significant increase in surface GluA2/3, but not GluA1 or GluA4, expression in the shell of rats treated with HSV-ADAR2b versus rats treated with HSV-GFP (Figure 4A).
Expression of exogenous ADAR2b in the accumbens shell significantly attenuated cocaine priming-induced reinstatement (i.e. reinstatement test #1) [t(18)=2.51, P<0.05](Figure 5B). This effect paralleled ADAR2b expression as no differences in cocaine seeking were observed [t(18)=0.58, P=0.57](Figure 5C) in the same animals 72 h following the first reinstatement test (i.e. reinstatement test #2), a time point consistent with full loss of transgene expression in the accumbens shell (Figure 3A). No effect of viral treatment on inactive lever responding was noted during the 1st [t(18)=1.38, P=0.18](Figure 5B) or the 2nd [t(18)=0.83, P=0.42](Figure 5C) reinstatement tests suggesting that attenuation of drug seeking is not due to general motor suppressant effects of ADAR2b overexpression. Viral infusions were targeted at the medial accumbens shell and are shown in Figure 5D.
Figure 5. Increased exogenous ADAR2b expression in the accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior.
(A) Timeline of the experimental design used to test the effects of ADAR2b over-expression on cocaine seeking. (B) Cocaine seeking was attenuated in rats that received HSV-ADAR2b (n= 9) into the shell compared to HSV-GFP (n=11) 72 h following infusion of viral vectors (*P<0.05). (C) Cocaine seeking was not significantly different between rats treated with HSV-ADAR2b (n=9) or HSV-GFP (n=11) 1 week following virus infusions into the shell (P>0.05). (D) Coronal sections taken at the level of the striatum depicting virus infusion sites within the medial nucleus accumbens.
Discussion
This is the first study to demonstrate that GluA2 Q/R site editing can modulate behavior, specifically in a model of cocaine craving. Here, we show that 7 days of forced cocaine abstinence decreased expression of ADAR2b, the primary enzyme responsible for catalyzing GluA2 Q/R site editing5-7, and GluA2 editing in the nucleus accumbens shell, but not core. These results suggest that increased synaptic expression of CP-AMPARs containing unedited GluA2(Q) subunits in the shell may facilitate cocaine seeking. In order to investigate the functional significance of ADAR2b and GluA2 Q/R site editing in drug addiction, we utilized a viral-mediated gene transfer to over-express ADAR2b in the accumbens shell of cocaine abstinent rats prior to tests of cocaine seeking. Increased ADAR2b expression in the accumbens shell attenuated cocaine priming-induced reinstatement of drug seeking and was associated with an increased GluA2 Q/R site editing and surface expression of GluA2-containing AMPARs. Previous studies have demonstrated that decreased surface expression of GluA2 is associated with cocaine seeking13-15. The present results suggest that increased expression of CP-AMPARs in the accumbens shell during cocaine seeking is due, in part, to disrupted GluA2 Q/R site editing and possibly increased surface expression of CP-AMPARs containing unedited GluA(Q) subunits. Collectively, these findings indicate that GluA2 editing in combination with GluA2 endocytosis14 may contribute to cocaine craving and relapse.
In light of recent studies demonstrating that increased surface expression of GluA1 subunits is associated with cocaine seeking13, 25, it is likely that GluA1 insertion and GluA2 endocytosis occur in parallel in the accumbens to promote drug-seeking behavior11. The present findings, however, indicate that this model should be expanded to include the effects of cocaine abstinence on GluA2 editing and subsequent insertion of unedited GluA2(Q)-containing CP-AMPARs into the plasma membrane. In the nucleus accumbens, expression of GluA1 and GluA2 subunits is high, GluA3 expression is low and GluA4 expression is very low13. Our data indicate that ADAR2b-induced GluA2 site editing promotes increased surface expression of GluA2/3, but not GluA1 and GluA4, AMPAR subunits in the accumbens shell. Therefore, it is likely that increased ADAR2b-mediated GluA2 editing affects trafficking of GluA2 subunits only. Taken together, these results indicate that cocaine seeking may be driven, in part, by the complex and dynamic processes of GluA1 insertion, GluA(Q)-containing AMPAR insertion and GluA2(R)-containing CI-AMPAR endocytosis in the accumbens shell.
In addition to channel gating, GluA2 Q/R site editing also influences GluA2 trafficking, AMPAR assembly and subunit composition27. Retention of GluA2 in the endoplasmic reticulum (ER) is controlled by Q/R site editing. The majority of edited GluA2(R) resides stably in the ER before being trafficked to the cell surface where it forms heterodimers with GluA128. In contrast, unedited GluA2(Q) is rapidly released from the ER and expressed on the cell surface28. Moreover, the insertion rate of edited GluA2(R) into the plasma membrane is slower than that of unedited GluA2(Q)29. Thus, GluA2 Q/R site editing regulates trafficking, subunit composition and ion conduction through AMPA receptors. Here, we show that cocaine abstinence decreases GluA2 Q/R site editing in the shell, suggesting that there may be increased expression of CP-AMPARs containing unedited GluA2(Q) on the cell surface.
It is not possible to differentiate edited GluA2(R) protein from unedited GluA2(Q) protein at the synapse in vivo8. While functional CP-AMPARs can be identified electrophysiologically, there are no methods currently available to differentiate GluA2-lacking AMPA receptors from AMPA receptors containing unedited GluA2(Q) subunits. Cocaine seeking is associated with increased synaptic expression of CP-AMPARs in the accumbens shell13. Moreover, AMPA receptors on nucleus accumbens medium spiny neurons display inward rectification, a hallmark of CP-AMPARs, in rats during cocaine abstinence30. These results are consistent with the present finding that cocaine self-administration decreased GluA2 Q/R site editing in the shell. However, it is not possible to separate the exact contributions of GluA2-lacking AMPA receptors versus AMPA receptors containing unedited GluA2(Q) to changes in rectification during cocaine abstinence. Since only a small proportion of GluA2 subunits are unedited, cocaine abstinence-induced disruptions in GluA2 Q/R expression could have a disproportionate effect on AMPA receptor calcium permeability and synaptic plasticity in the accumbens shell. New methods are needed to distinguish GluA2-lacking CP-AMPARs from CPAMPARs containing unedited GluA2(Q) subunits in order to determine the precise contributions of these CP-AMPAR populations to cocaine-induced changes in neuronal excitability and the reinstatement of cocaine-seeking behavior.
This is the first in vivo evidence that CP-AMPARs containing unedited GluA2(Q) influence behavior. Specifically, the current results define a critical role of accumbens shell ADAR2b-dependent GluA2 Q/R site editing in the reinstatement of cocaine-seeking behavior. These findings indicate that increased expression of CP-AMPARs in the accumbens shell during cocaine seeking may be due, in part, to disrupted GluA2 Q/R site editing, suggesting that GluA2 editing contributes to cocaine craving and relapse. Thus, the present results indicate that regulation of ADAR2b expression and GluA2 Q/R site editing represent novel targets for the development of treatments for cocaine craving and addiction.
Supplementary Material
Supplemental Figure 1: GluA2 Q/R site editing in the accumbens is not altered following 1 day of cocaine abstinence. The ratio of GluA2 Q:R transcripts in the accumbens shell (A) and core (B) was not different in cocaine-experienced rats when compared to yoked saline control animals following 1 day of forced cocaine abstinence (n=3/treatment).
Acknowledgements
The authors would like to thank Dr. Mary O'Connell for her advice and expertise on ADAR2b antibodies. This work was supported by DA022339 (H.D.S., R.C.P. and G.S-V.), DA033641 (H.D.S., R.C.P. and G.S-V.), K02 DA18678 (R.C.P.) and K01 DA030445 (H.D.S.).
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References
- 1.Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science. 1991;252(5007):851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 2.Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991;253(5023):1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- 3.Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8(1):189–198. doi: 10.1016/0896-6273(92)90120-3. [DOI] [PubMed] [Google Scholar]
- 4.Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, et al. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15(1):193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 5.Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996;379(6564):460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- 6.Bass BL. RNA editing by adenosine deaminases that act on RNA. Annu Rev Biochem. 2002;71:817–846. doi: 10.1146/annurev.biochem.71.110601.135501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rueter SM, Burns CM, Coode SA, Mookherjee P, Emeson RB. Glutamate receptor RNA editing in vitro by enzymatic conversion of adenosine to inosine. Science. 1995;267(5203):1491–1494. doi: 10.1126/science.7878468. [DOI] [PubMed] [Google Scholar]
- 8.Wright A, Vissel B. The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front Mol Neurosci. 2012;5:34. doi: 10.3389/fnmol.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54(6):859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Wiltgen BJ, Royle GA, Gray EE, Abdipranoto A, Thangthaeng N, Jacobs N, et al. A role for calcium-permeable AMPA receptors in synaptic plasticity and learning. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierce RC, Wolf ME. Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. In: Pierce RC, Kenny PJ, editors. Addiction. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2013. pp. 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann NY Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, et al. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11(3):344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- 14.Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, et al. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci. 2008;28(43):11061–11070. doi: 10.1523/JNEUROSCI.1221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454(7200):118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawahara Y, Ito K, Sun H, Kanazawa I, Kwak S. Low editing efficiency of GluR2 mRNA is associated with a low relative abundance of ADAR2 mRNA in white matter of normal human brain. Eur J Neurosci. 2003;18(1):23–33. doi: 10.1046/j.1460-9568.2003.02718.x. [DOI] [PubMed] [Google Scholar]
- 17.Paschen W, Djuricic B. Regional differences in the extent of RNA editing of the glutamate receptor subunits GluR2 and GluR6 in rat brain. J Neurosci Methods. 1995;56(1):21–29. doi: 10.1016/0165-0270(94)00085-u. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1997. [DOI] [PubMed] [Google Scholar]
- 19.Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30(35):11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sommer B, Kohler M, Sprengel R, Seeburg PH. RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell. 1991;67(1):11–19. doi: 10.1016/0092-8674(91)90568-j. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt HD, Sangrey GR, Darnell SB, Schassburger RL, Cha JH, Pierce RC, et al. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J Neurochem. 2012;120(2):202–209. doi: 10.1111/j.1471-4159.2011.07571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neve RL, Neve KA, Nestler EJ, Carlezon WA., Jr Use of herpes virus amplicon vectors to study brain disorders. Biotechniques. 2005;39(3):381–391. doi: 10.2144/05393PS01. [DOI] [PubMed] [Google Scholar]
- 23.Slavov D, Gardiner K. Phylogenetic comparison of the pre-mRNA adenosine deaminase ADAR2 genes and transcripts: conservation and diversity in editing site sequence and alternative splicing patterns. Gene. 2002;299(1-2):83–94. doi: 10.1016/s0378-1119(02)01016-8. [DOI] [PubMed] [Google Scholar]
- 24.Peng PL, Zhong X, Tu W, Soundarapandian MM, Molner P, Zhu D, et al. ADAR2-dependent RNA editing of AMPA receptor subunit GluR2 determines vulnerability of neurons in forebrain ischemia. Neuron. 2006;49(5):719–733. doi: 10.1016/j.neuron.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ, et al. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011;61(7):1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiggins A, Smith RJ, Shen HW, Kalivas PW. Integrins modulate relapse to cocaine-seeking. J Neurosci. 2011;31(45):16177–16184. doi: 10.1523/JNEUROSCI.3816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40(4):763–774. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- 28.Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34(5):759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- 29.Araki Y, Lin DT, Huganir RL. Plasma membrane insertion of the AMPA receptor GluA2 subunit is regulated by NSF binding and Q/R editing of the ion pore. Proc Natl Acad Sci U S A. 2010;107(24):11080–11085. doi: 10.1073/pnas.1006584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31(15):5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: GluA2 Q/R site editing in the accumbens is not altered following 1 day of cocaine abstinence. The ratio of GluA2 Q:R transcripts in the accumbens shell (A) and core (B) was not different in cocaine-experienced rats when compared to yoked saline control animals following 1 day of forced cocaine abstinence (n=3/treatment).