Abstract
Fetal rats can alter patterns of interlimb coordination after experience with a yoke that links two legs together. Yoke training results in a pronounced increase in conjugate limb movements (CLM). To determine whether yoke motor learning is mediated by spinal cord circuitry, fetal subjects at embryonic day 20 (E20) received yoke training after mid-thoracic spinal cord transection or sham surgery. Both spinal and sham-treated fetuses exhibited an increase in CLM during training. In a second experiment, fetuses received initial yoke training, then were transected or sham treated before a 2nd training. Spinal and sham fetuses that were yoked during both training sessions exhibited a more rapid rise in CLM than those yoked only in the later session. These findings indicate that motor learning in fetal rats can be supported by spinal cord circuitry alone, and that savings implies a form of motor memory localized in the spinal cord.
Keywords: Rattus norvegicus, Norway rat, prenatal behavior, spinal cord, motor learning, motor development, interlimb coordination, kinesthesia, proprioception
“Innate” is one of the most unfortunate terms associated with developmental science. Although it literally means just “present at birth”, it conveys a host of other intended and unintended meanings that assume a sharp divide in the mechanisms that govern development before and after birth (Bateson & Mameli, 2007). “Innate” is typically associated with concepts such as instinct, maturation, genetic programs, biological determinism and “hard-wiring”, implying that there can be little or no role for experience during development before birth or hatching. Although researchers in child development, perinatology, and animal behavior readily acknowledge the rich interplay between the infant and the postnatal environment, the possibility that prenatal sensory experience or motor practice may help shape the abilities and proclivities of the newborn has been more reluctantly considered, and often, summarily dismissed.
Research over the past forty years has produced considerable empirical evidence that experience does contribute to the development of behavior before birth. The pioneering research of Gilbert Gottlieb on avian embryos, which followed the theoretical perspective of Zing-Yang Kuo (1967), provided experimental confirmation that prenatal sensory experience is crucial for establishing perceptual biases that classical ethologists, such as Lorenz (1935/1937) and Hess (1973), presumed were instinctive (see Gottlieb, 1997, for an excellent summary of this research). In the wake of Gottlieb’s avian research, a new cadre of developmental scientists, including Anthony DeCasper, William Fifer, William Smotherman, Jeffrey Alberts, and Barbara Kisilevsky, extended the prenatal research program to investigate the potential for learning in mammalian fetuses, including humans (Smotherman & Robinson, 1988a; Lecanuet, Fifer, Krasnegor, & Smotherman, 1995; Kisilevsky & Low, 1998; James, 2010). However, most of the work in both avian embryos and mammalian fetuses focused on the prenatal development of sensory systems, especially audition and chemosensation, and on associative learning involving these sensory modalities.
A parallel research tradition with origins in the 1960s also focused on behavior in avian embryos. This was the legacy of Viktor Hamburger, who firmly established that embryonic movements were generated spontaneously by the developing nervous system, and that segments of the cervical and lumbosacral spinal cord could function autonomously during embryonic development to produce organized patterns of movement (Hamburger, 1973; Bekoff, 1992). Discovery of pattern generating circuitry within the embryonic spinal cord accorded well with research on central pattern generation of rhythmic motor patterns in reduced preparations of adult animals, such as spinal turtles and cats (Grillner & Zangger, 1979; Robertson, Mortin, Keifer & Stein, 1985). Indeed, EMG recordings from ventral roots of avian embryos or fictive neural activity from isolated spinal cord preparations suggested that the basic locomotor patterns of left-right alternation and flexor-extensor alternation were driven by dedicated circuitry laid down in the embryonic spinal cord (Bekoff, 1992; Cazalets, Grillner, Menard, Crémieux & Clarac, 1990; Kudo & Yamada, 1987). The conclusion: the basic coordination of locomotor activity is innate.
The relative poverty of research on prenatal motor experience coupled with the clear demonstration of autonomously active regions of the spinal cord that are functional before birth has led to a prevalent attitude, if not belief, that the motor system develops without significant need of “practice” before birth. Spontaneous movement is known to contribute in important ways to physical development, including the growth of mechanical elements of the motor system, such as muscles, bones, and connective tissue (Drachman & Sokoloff, 1966; Moessinger, 1983; Muller, 2003). There also is growing appreciation for the role of spontaneous neural activity in pruning and refining various parts of the nervous system (Katz & Shatz, 1996; Navarrete & Vrbova, 1993; Thompson, 1983; see also Blumberg, Freeman & Robinson, 2010, for recent reviews), and in a cascade of effects on subsequent neural and behavioral development (see Kleven & Bellinger, this issue). But Hamburger’s classic experiments—which demonstrated that the embryonic spinal cord, isolated from the brain and deafferented bilaterally, nevertheless could produce patterned motor activity (Hamburger, Wenger & Oppenheim, 1966; Narayanan & Malloy, 1974) —implied that the embryo or fetus did not require feedback from motor performance to build or refine neural systems involved in motor control (cf., Sharp, this issue).
From a theoretical perspective, however, it is difficult to see how experience cannot be involved in the prenatal development of coordinated movement. Producing temporally organized discharges in the isolated spinal cord is one thing, but coordinating the movement of dozens of muscles in a physical body is quite another. We have previously argued that physical growth poses a significant challenge for the developing motor system, because the dimensions of limb segments, relative proportions of body parts, strength of muscles and body mass changes abruptly during perinatal development. In the rat, for instance, the period of the last five days of gestation (E17–21) entails a nearly exponential rate of growth, including a 215% increase in body length (crown-rump length grows from 17.4 to 37.4 mm) and 550% increase in body mass (1.0 g to 5.5 g) (Angulo y Gonzalez, 1932; Robinson, 1989). During this same period, fetal motor behavior undergoes a profound change from seemingly random jerks and kicks to a repertoire of coordinated action that foreshadows postnatal behavior (Brumley & Robinson, 2010; Robinson & Kleven, 2005; Robinson & Smotherman, 1992). Because body dimensions change so extensively during prenatal development, the timing and strength of contraction of various muscles must change in order to produce movements of the same kinematic form. For coordinated movement to develop, or just be maintained, in the face of the changing geometry of the body, the motor system must continually recalibrate. There seems to be no logical alternative but for the nervous system to attend to sensory feedback from motor performance to solve this “Calibration Problem” (Robinson & Kleven, 2005).
Much of the behavior of the rat fetus appears to be supported by spinal cord circuitry. The temporal organization of spontaneous motor activity persists unabated after cervical spinal cord transection (Robinson, Blumberg, Lane & Kreber, 2000). Hindlimb movements continue to be expressed, albeit at a reduced frequency, after mid-thoracic spinal cord transection (Robertson & Smotherman, 1990). Coordinated movement, including species-typical patterns such as the leg-extension response (LER) evoked by anogenital stimulation and locomotor-like stepping induced by dopaminergic or serotonergic drugs, are not abolished by spinal transection (Brumley & Robinson, 2005; Smotherman & Robinson, 1988b; Strain, Kauer, Kao & Brumley, 2014; see also Brumley, Kauer & Swann in this issue). These facts imply that spinal motor systems, which are developing concurrent with massive changes in the fetal body, must be flexible, plastic and responsive to sensory feedback.
Although spinal neural systems are often taken to be synonymous with reflexive, rigid or pre-programmed responses, there is a growing literature on the ability of the spinal cord to support learning, including habituation and sensitization, Pavlovian and instrumental conditioning (Grau, 2014; Grau, et al., 2006; Thompson & Spencer, 1966; Wolpaw, 2006, 2007). Various forms of locomotor training, such as stepping on a treadmill or with a robotic assistive device, are helpful in promoting recovery of locomotor function after spinal cord trauma (Edgerton & Roy, 2009; Raineteau & Schwab, 2001; see also Teulier, Lee & Ulrich, this issue). A particularly germane example is the developmental plasticity of locomotor coordination reported in newborn rabbits (Viala, 2006; Viala, Viala & Fayein, 1986). The earliest form of locomotion expressed by rabbit neonates resembles the familiar alternated walking pattern of most adult mammals. During typical development in rabbits, the alternated gait is replaced by synchronized hopping of the hindlegs (a half-bound gait). The alternated gait persists, however, after neonatal spinal cord transection. Moreover, when spinalized rabbits received daily training on a step trainer (with feet attached to pedals that enforced either in-phase or antiphase stepping), their hindlimb coordination could be shifted toward either alternation or hopping. This finding implies that proprioceptive feedback to the developing spinal cord can help shape patterns of locomotor coordination.
A similar training procedure has been reported to effect changes in interlimb coordination in both human infants (Thelen, 1994) and perinatal rats (Robinson & Smotherman, 1994; Robinson, 2005). In this experimental paradigm, an elastic cord (with infants) or length of thread (rats) is attached to both hindlimbs at the ankle, creating a physical linkage that biomechanically constrains independent leg movements. Thelen reported that 10 min of yoke training is sufficient to alter infants’ coordination of kicking of an overhead mobile (Thelen, 1994). We have demonstrated that a 30-min period of exposure to the interlimb yoke is effective in altering interlimb coordination in rat fetuses as young as E19 (Robinson, Kleven & Brumley, 2008). Fetuses that experience yoke training exhibit a 6- to 9-fold increase in conjugate hindlimb movements, in which both limbs initiate movement at the same time and follow parallel paths. Changes in interlimb coordination as a consequence of yoke training imply that both human newborns and fetal rats (a) exhibit functional proprioception, (b) alter the patterning of limb movement to adjust to the constraint imposed by the interlimb yoke, and (c) continue to express altered patterns of coordination after removal of the yoke (Brumley & Robinson, 2010).
Techniques that provide experimental access to active, behaving rat fetuses may help to resolve the question of whether the fetal motor system in general, and spinal mechanisms of motor control in particular, are responsive to sensory feedback. In this paper, I report two experiments designed to evaluate whether the motor plasticity evident in yoke motor learning can be supported by the spinal cord. In the first experiment, fetal rats were prepared by mid-thoracic spinal transection or sham surgery and then exposed to yoke training with the interlimb yoke. In the second experiment, intact fetuses were exposed to an initial session of yoke training, then were expose to a second training session after spinal cord transection or sham treatment. The results suggest that spinal cord circuitry alone is sufficient to support acquisition and retention of learned changes in interlimb coordination in the fetus.
GENERAL METHODS
Subjects
Subjects were the fetal progeny of Sprague-Dawley rats (Harlan) bred in my laboratory. Owing to the length of each experimental session, only a single fetus was used as an experimental subject from each pregnancy. A total of 48 fetuses from as many pregnancies served as subjects in the two experiments of this study. To produce pregnancies of known gestation, female rats were bred in squads of three with a single male during a 4-day breeding period. Vaginal smears were collected daily and examined for presence of sperm; the date of conception (E0) was defined as the first day in which sperm were detected. Rats were housed and bred under a 12-hr light: 12-hr dark photoperiod and provided with food and water ad libitum. All animals were maintained and treated in accordance with guidelines established by the National Institutes of Health (National Research Council, 2011). All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Iowa, where they were performed.
Prenatal Preparation
On day 20 of gestation (E20), pregnant rats were briefly anesthetized by placement in an animal jar suffused with ethyl ether. The rat was removed from the jar at the first sign of muscle relaxation and 100 µ1 of 10% ethanol was injected into the spinal cord between the first and second lumbar vertebrae (L1-L2). This chemomyelotomy procedure is a standard method for producing irreversible spinal blockade, quickly and effectively rendering the pregnant female insensate in the lower half of the body (Smotherman & Robinson, 1991).
Prepared female rats were placed supine in a holding device that elevated the body at a 45° angle, and the uterus was exteriorized through a midline laparotomy into a warm (37.5°C ± 0.5°C) bath containing buffered isotonic saline (Locke’s solution). The female was permitted to acclimate for at least 20 min after spinal preparation and placement in the bath. This period was fully sufficient for her to recover from any residual effects of ether (Devine & Robinson, 2008). At the beginning of the experimental session, a single subject fetus was externalized from the uterus, gently removing the amniotic and chorionic membranes to provide experimental access to the fetus, and taking care to preserve the integrity of the umbilical cord and placental attachment to the uterus. Externalized fetuses lay gently on the surface of the uterus while submerged within the saline bath. The condition of subject fetuses was continuously monitored during the experimental session by noting general coloration of the fetus and umbilical cord (pink indicating good oxygenation), the presence of motor activity, and the absence of stereotypic movements indicative of hypoxia. All fetuses reported in these experiments remained in good physiological condition throughout the experimental session. These methods for preparing fetal subjects for behavioral study permit direct observation and experimental manipulation of fetal subjects and creation of high-quality video recordings for subsequent analysis of fetal motor behavior (Smotherman, Richards & Robinson, 1984; Smotherman & Robinson, 1991).
Fetal Spinal Surgery
Fetal subjects were prepared with a mid-thoracic spinal transection or sham procedure. Spinal transection was performed under visual guidance by inserting a fine stainless steel microknife, fashioned from 0.15 mm diameter wire, into the spinal cord between the 6th and 9th thoracic vertebrae (T6-T9). The microknife was moved from side to side in a transverse plane, taking care to completely sever the cord. The sham procedure involved insertion of the microknife adjacent to the spinal column without contact with the spinal cord or cutting of other tissue. We have previously reported that sham-treated subjects do not differ from unoperated controls (Robertson & Smotherman, 1990; Robinson, et al., 2000; Brumley & Robinson, 2005).
The effectiveness of the spinal transection procedure was immediately assessed by gently pinching the fetus’s tail with forceps. If the fetus showed no response to the pinch, or responded with movement of the forelimbs and head, the transection was considered unsuccessful and another subject was selected for spinal preparation. All fetuses remained submerged in the saline bath during fetal spinal surgery and behavioral assessment. At the end of the experimental session, after the pregnant female was euthanized by carbon dioxide inhalation, spinal fetuses were preserved in a 10% (wt/vol) formalin solution for later histological examination to confirm completion and location of the spinal cord transection.
Procedures for Yoke Training
The motor learning paradigm in this study involved attachment of an interlimb yoke to both hindlimbs to constrain limb movement during a training period. The interlimb yoke consisted of PE-50 polyethylene tubing cut to a specific length (8.0 mm) to accommodate the mean distance between the ankles at rest in E20 fetal subjects. Silk suture (4-0) then was threaded through the tubing, creating two loops at either end of the tubing. The yoke was fitted to the subject by slipping each loop over a hind foot and tightening the loops to fit snugly at the ankle joint. Interlimb yokes of this design resisted forces of tension, compression and torsion.
The purpose of the interlimb yoke was to constrain spontaneous movements of the yoked hindlimbs. With the yoke in place, an independent movement by one limb would be hindered by its connection to the other limb. Conversely, the inactive limb could be moved passively by movement of the active limb. Additional groups of fetuses were Unyoked during training as a control condition. The Unyoked condition consisted of placement of the same interlimb yoke on the hindlimbs, but immediately bisecting the yoke with scissors, so the fetuses experienced no period of limb constraint. Yoked and Unyoked subjects were tested in sessions of 65 min (Experiment 1) or 95 min (Experiment 2).
Histology
After completion of fetal observation, the pregnant rat was euthanized by carbon dioxide inhalation and subject fetuses were delivered immediately by Cesarean section. Fetuses that were treated with a sham spinal surgery were euthanized by decapitation. Fetuses that were treated with a mid-thoracic spinal cord transection were preserved in 10% formalin and later examined for complete bilateral spinal cord separation. Histological examination under low magnification verified the extent of the cut from both the dorsal and ventral sides of the spinal column. Only fetuses that had complete spinal cord transection were included in the spinal treatment groups for purposes of analysis. In Experiments 1 and 3, all 24 spinalized subjects showed complete transection of the spinal cord between thoracic vertebrae T6-T9. (Experiment 1: T6–7 N=4, T7–8 N=5, T8–9 N=3; Experiment 3: T6–7 N=6, T7–8 N=5, T8–9 N=l).
Analysis of Behavioral Data
To facilitate observation of limb movements, fetuses were maintained in a supine posture. Fetal motor activity was monitored continuously during the experimental session, which typically comprised a Baseline period (before attachment of the interlimb yoke), a Training period (when the yoke was attached), and a Test period (after the yoke was bisected and limbs permitted to move independently again). Fetal behavior was quantified by scoring the movement of each limb using real-time event recording software. This data acquisition system preserved information about the limb involved in movement (left vs. right, fore vs. hind), the time the movement occurred (± 0.1 s), and whether the limb movement was independent or conjugate (Robinson, 2005). Conjugate limb movements (CLM) occurred when the two limbs appeared to move as one, with both limbs initiating movement at the same time and following parallel trajectories with similar velocity. CLM were distinctive during Baseline and Testing periods in Yoked subjects, and were readily distinguished from passive dragging of one limb during yoke training, because the passive limb appeared to trail the active limb through a movement trajectory. Further, CLM involved movement of both limbs at an angle orthogonal to the line of the interlimb yoke, whereas passive movement involved one limb moving in the same direction as the line of the yoke. In such cases, the active movement of the leading limb was scored as an individual limb movement, but the passive movement of the trailing limb was not. This method of quantifying fetal movement has been used extensively in previous studies of fetal motor behavior and responsiveness to sensory stimuli (Robinson & Brumley, 2005; Smotherman & Robinson, 1991b). Estimates of reliability have been assessed from repeatedly scoring of the same session recorded on video; real-time scoring of basic categories of limb movement typically results in intra-rater reliability coefficients exceeding 0.95 and inter-rater reliabilities of at least 0.90.
The frequencies of individual limb movements and CLM were summarized in 5-min intervals across the Baseline, Training and Testing periods. Hindlimb activity was computed as the sum of both left and right hindlimb movements. In addition to absolute movement counts, the relative frequency of CLM was calculated as CLM × 2 (to count both limbs), divided by total hindlimb activity. Changes in limb activity were assessed by mixed model Analyses of Variance (ANOVA), with the time factor (5-min intervals) treated as a repeated measure. Following significant interaction effects, one-factor ANOVAs or t-tests were performed to test for simple main effects, and post hoc comparisons of means were conducted by the method of Fisher’s Protected Least Significant Difference (PLSD). The alpha level was set at p<.05 for all tests of statistical significance.
Experiment 1. Yoke training after mid-thoracic spinal transection
My laboratory has previously reported that 30-min exposure to an interlimb yoke that physically links two limbs produces an increase in CLM, even when expressed as a percentage of overall limb activity. The increase in CLM is evident within 15 min of the onset of the yoke training and persists for 25–30 min after the yoke is removed. Further, the effects of yoke training are specific to the limbs that are connected during training: forelimb-forelimb training results in increased CLM of forelimbs, but not hindlimbs, while hindlimb-hindlimb training promotes CLM of hindlimbs, not forelimbs (Robinson, 2005). Thus interlimb yoke training appears to represent a form of motor learning. The aim of Experiment 1 was to determine whether yoke motor learning in the fetal rat could be supported by spinal cord circuitry alone.
Method
Subjects were assigned to one of four experimental groups (N = 6 per group) that resulted from the combination of spinal surgical treatment (Spinal or Sham) and yoke training condition (Yoked or Unyoked). The observation session began 15 min after spinal surgery or sham treatment and consisted of a 5-min Baseline period, a 30-min Training period, and a 30-min Testing period. For subjects in the Yoked group, an interlimb yoke was attached to both hindlimbs during Training, which was divided with scissors at the beginning of Testing. The yoke also was attached to both hindlimbs in the Unyoked group (N=6), but was immediately divided (before Training). Fetal activity was scored to record individual movements of hindlimbs and forelimbs, as well as conjugate limb movements (CLM). Only active movements of a limb were included in activity counts; passive movements resulting from a limb pulled by the interlimb yoke, general movements of the whole body, or swaying within the water bath were not scored as movement events. Summary measures of forelimb and hindlimb activity, CLM, and the relative frequency of CLM were analyzed by ANOVA.
Results
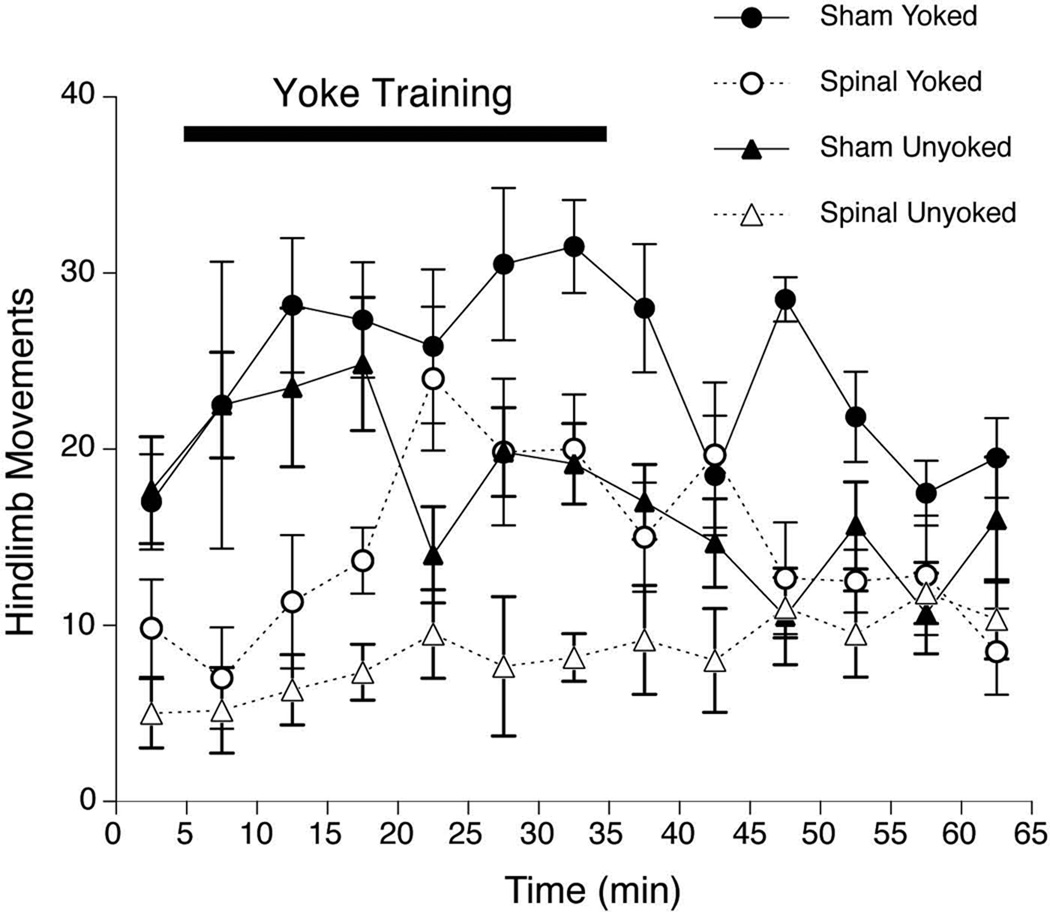
An initial series of three-factor ANOVAs (Surgery × Training Condition × Intervals, with the Intervals factor treated as a repeated measure) was conducted to compare limb activity and CLM across the entire 65-min experimental session. The analysis of hindlimb activity revealed significant main effects of Surgery, F(l,20) = 32.0, p<.001, Training Condition, F(l,20) = 14.9, p=.00l, and Intervals, F(12,240) = 3.1,p<.001. Overall, hindlimb movements occurred at nearly twice the rate among Sham-treated subjects than in fetuses with spinal transections, and were 50% more common in Yoked than Unyoked subjects. Two 2-factor interactions also were significant: Surgery × Intervals, F(12,240) = 3.3,p<.001, and Training Condition × Intervals, F(12,240) = 2.4, p=.006. To simplify this pattern of interactions, a series of one-way ANOVAs was performed to examine the change in hindlimb activity over Intervals in each Surgery × Training Condition group. Each of these analyses were significant: Sham × Unyoked, F( 12,60) = 2.5,p=.01; Sham × Yoked, F(12,60) = 2.0,p=.045; Spinal × Unyoked, F(12,60) = 2.1,p=.031; Spinal × Yoked, F(12,60) = 3.9, p<.001. As shown in Figure 1, hindlimb activity increased only modestly in the Spinal-Unyoked group, but increased sharply during the second half of the Training period in Spinal-Yoked subjects. Hindlimb activity also increased during Training in Sham-Yoked subjects, but was elevated only during the first 15 min of Training in Sham-Unyoked fetuses.
Figure 1.
Overall hindlimb activity of Yoked and Unyoked E20 fetuses prepared by sham surgery (closed symbols) or mid-thoracic spinal transection (open symbols) before interlimb yoke training in Experiment 1. In this and subsequent graphs, the horizontal bar depicts the 30-min period of yoke training. Symbols show the mean number of hindlimb movements in successive 5-min blocks during baseline, training and testing periods; error bars depict SEM.
A parallel three-factor ANOVA of forelimb activity revealed the significant main effects of Training Condition, F(l,20) = 11.7,p=.003, and Intervals, F(12,240) = 3.1,p<.001. In contrast to hindlimb movements, forelimb movements did not differ between Surgery conditions and were 30% more common in Unyoked than Yoked subjects. None of the 2-way or 3-way interactions were significant.
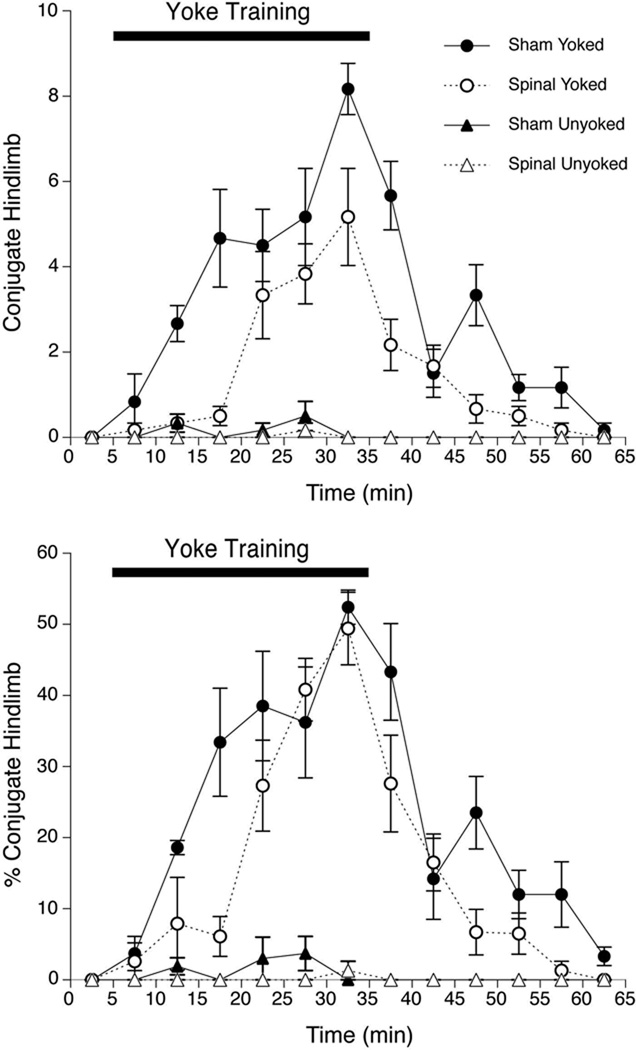
The ANOVA examining CLM of the hindlimbs indicated the significant main effects of Surgery, F(l,20) = 37.1, p<.001, Training Condition, F(l,20) = 258.5, p<.001, and Intervals, F(12,240) = 22.0, p<.001. All interaction effects also were significant, including the 3-way interaction of Surgery × Training Condition × Intervals, F(12,240) = 2.6, p=.003. A series of oneway ANOVAs within each Surgery × Training Condition group indicated the significant effect of Intervals only among Yoked subjects: Sham × Yoked, F(12,60) = 12.7, p<.001; Spinal × Yoked, F(12,60) = 11.3,p<.001. As shown in Figure 2 (top), CLM increased systematically during yoke training in both Sham and Transected subjects, but remained rare in all Unyoked subjects.
Figure 2.
Number of conjugate limb movements (CLM) of hindlimbs (top), and CLM of hindlimbs expressed as a percentage of overall hindlimb activity (bottom) during and after hindlimb yoke training in Experiment 1. Symbols depict mean CLM or CLM% of Yoked and Unyoked fetuses prepared by spinal transection or sham surgery; error bars show SEM.
Given the systematic differences in hindlimb activity between Spinal and Sham subjects, changes in CLM also were expressed as a percentage of hindlimb activity. The overall ANOVA showed a similar pattern of results, with significant main effects of Surgery, F(l,20) = 10.8, p=. 004, Training Condition, F(l,20) = 223.4, p<.001, and Intervals, F(12,240) = 26.4,p<.001. All interaction effects also were significant, including the 3-way interaction, Surgery × Training Condition × Intervals, F(12,240) = 2.1, p=.020. As before, a series of one-way ANOVAs indicated the significant effect of Intervals only among Yoked subjects: Sham × Yoked, F(12,60) = 13.2, p<.001; Spinal × Yoked, F(12,60) = 15.2, p<.001. But compensating for different levels of hindlimb activity revealed remarkable similarity in the response to yoke training in the two surgical conditions (see Figure 2, bottom). A series of t-tests to examine the simple main effect of surgical treatment among Yoked subjects in each 5-min interval indicated significant differences in CLM only during the third interval of the Training period and the third and fifth intervals of the Test period, ps<.05.
Experiment 2. Retention of motor learning after spinal transection
The results of Experiment 1 confirmed that fetal rats remain responsive to interlimb yoke training after mid-thoracic spinal cord transection. This implies that acquisition of this form of motor learning can be supported by neural circuitry in the lumbosacral spinal cord alone. In an earlier report, fetal rats were found not only to modify interlimb coordination in response to yoke training, but to retain information from one training session to the next. Fetal rats showed a more rapid increase in CLM during a second yoke training session, suggesting savings in the reacquisition of a previously learned motor pattern (Robinson, 2005). The aim of the second experiment of the present study was to assess whether this savings after prior training is dependent on more rostral sources in the CNS or can be supported by spinal cord circuitry alone.
Method
Subjects were assigned to one of four experimental groups (N = 6 per group) that resulted from the combination of spinal surgical treatment (Spinal or Sham) and yoke training condition (Yoked or Unyoked). The observation session comprised a 5-min Baseline period, a 30-min 1st Training period, a 30-min Testing period, and a 30-min 2nd Training period. For subjects in the Yoked group, an interlimb yoke was attached to both hindlimbs during the 1st Training period, which was divided with scissors at the beginning of Testing. The yoke also was attached to both hindlimbs during the 1st Training period in the Unyoked group (N=6), but was immediately divided (before Training). Midway through the Testing period, 15 min after the end of the 1st Training period, fetuses assigned to Spinal groups were prepared with a mid-thoracic spinal cord transection. The transection procedure typically was completed in less than 30 sec, and subjects remained undisturbed for the remainder of the Testing period. All subjects (both Yoked and Unyoked) were fitted with an intact interlimb yoke during the 2nd Training period. Fetal hindlimb activity and CLM were scored throughout the Baseline, 1st and 2nd Training periods, and during the last 5 min of the Testing period. Summary measures of hindlimb activity, CLM, and the relative frequency of CLM were analyzed by ANOVA.
Results
Analysis of behavioral data from this experiment focused on fetal responses to the two periods of yoke training. The first set of analyses comprised the Baseline and 1st Training periods. The second set comprised the last 5 min of the Testing period, which served as a second baseline, and the 2nd Training period.
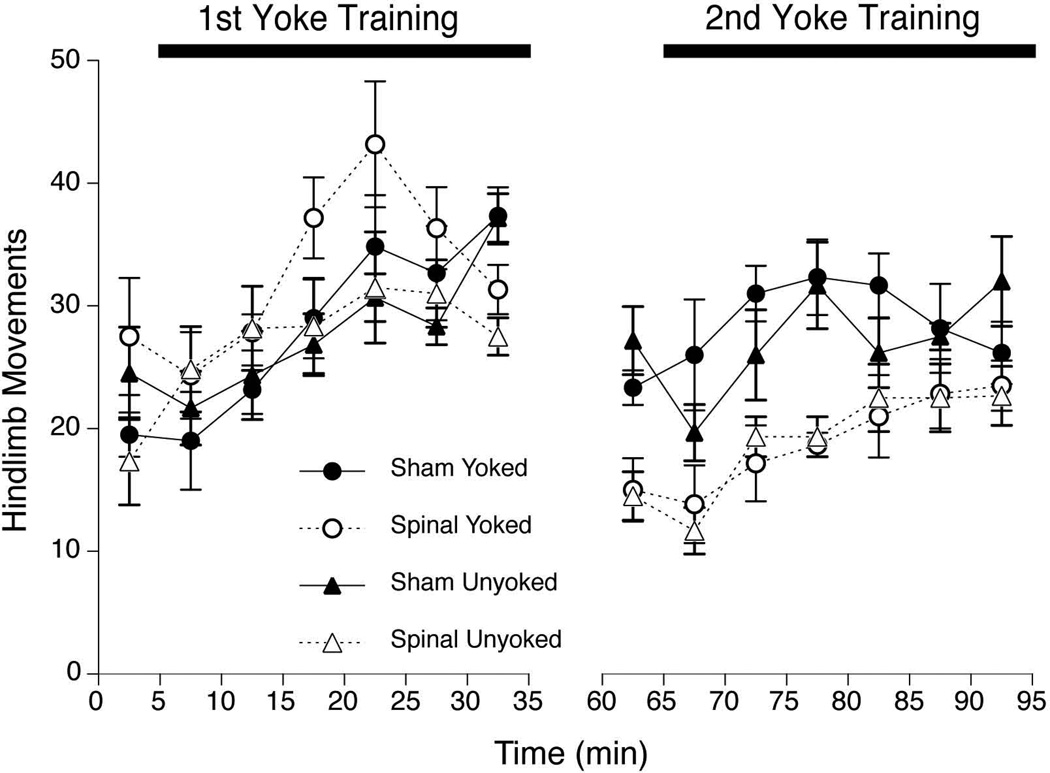
During the first Training period, all fetuses were physically intact and differed only in their assignment to Yoked versus Unyoked training conditions. A preliminary analysis confirmed no main or interaction effects involving the Spinal factor prior to spinal transection. A series of two-factor ANOVAs (Training Condition × Intervals, with the Intervals factor treated as a repeated measure) was conducted to compare limb activity and CLM during Baseline and the 1st Training period. The analysis of hindlimb activity revealed no differences related to Training Condition, but a significant main effects of Intervals, F(6,132) = 12.4, p<.001 (Figure 3, left). Post hoc comparisons (PLSD) indicated that hindlimb movements increased significantly by the third 5-min of the Training period relative to Baseline and remained elevated thereafter (ps<.05).
Figure 3.
Overall hindlimb activity of E20 fetuses in Experiment 2. Subjects were Yoked or Unyoked during the 1st yoke training period, then received mid-thoracic spinal transection or sham surgery 15-min before 2nd training. All subjects were exposed to the hindlimb yoke during the 2nd training period. Symbols depict the mean number of hindlimb movements in successive 5-min blocks during baseline, 1st training, 2nd baseline, and 2nd training periods; error bars depict SEM.
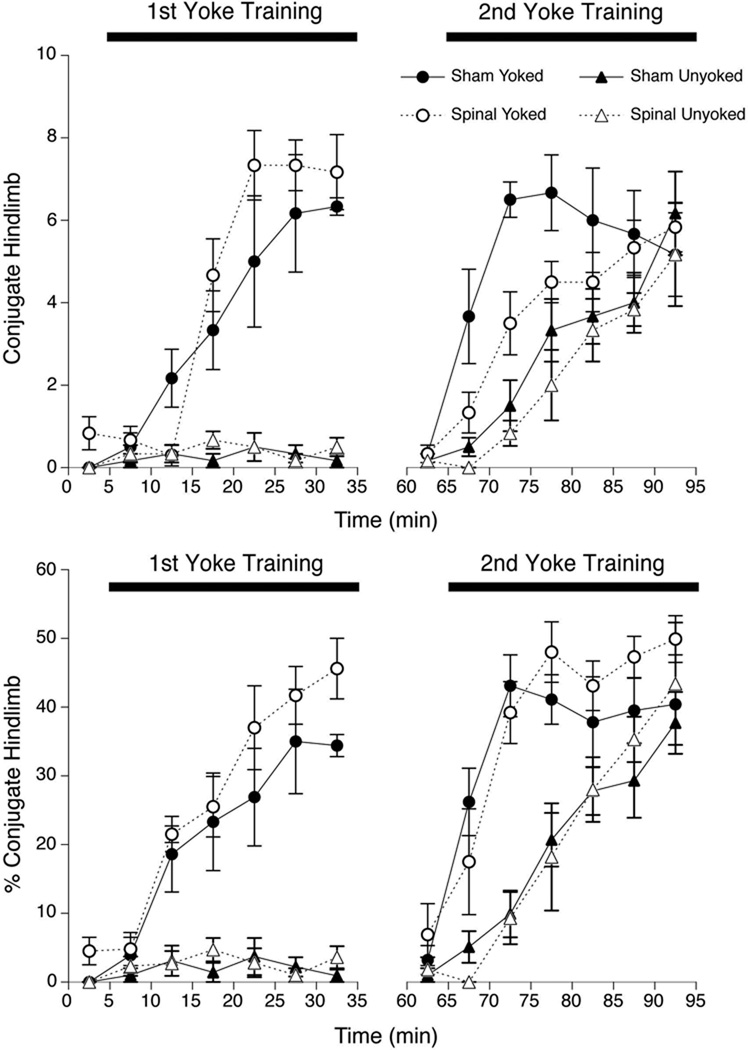
However, CLM was markedly affected by yoke training, as evidenced by significant main effects of Training Condition, F(l,22) = 161.7, p<.001, and Intervals, F(6,132) = 25.9, p<.001, and the interaction of Training Condition × Intervals, F(6,132) = 22.6, p<.001 (Figure 4, top left). To simplify the interaction, a series of t-tests was performed to examine the simple effect of Training Condition in each 5-min interval. There was no difference between Yoked and Unyoked subjects during Baseline or the first 5-min interval of the Training period, but Yoked fetuses expressed significantly more CLM during all subsequent Training intervals (ps<.001). A similar pattern of results was found when CLM was expressed as a percentage of hindlimb activity. The two-way ANOVA indicated significant main effects of Training Condition, F(l,22) = 132. l, p<. 001, and Intervals, F(6,132) = 24.8, p<.001, and the interaction of Training Condition × Intervals, F(6,132) = 21.1,p<.001 (Figure 4, bottom left). Post hoc comparison of Training Condition in each 5-min interval showed significantly higher rates of CLM in Yoked subjects in the second interval of the Training period and thereafter (ps<.001).
Figure 4.
Number of conjugate limb movements (CLM) of hindlimbs (top), and CLM of hindlimbs expressed as a percentage of overall hindlimb activity (bottom) during two consecutive training periods in Experiment 2. Fetuses received spinal transection or sham surgery midway between training periods; all subjects were yoked during 2nd training. Symbols depict mean CLM or CLM% of Yoked and Unyoked fetuses prepared by spinal transection or sham surgery between the 1st and 2nd training periods; error bars show SEM.
The second set of analyses examined responses of fetal subjects to a second session of yoke training. During the 2nd Training, half of the subjects were spinalized and half exposed to a sham surgery. The 3-way ANOVA (Surgery × Training Condition × Intervals) examining hindlimb activity indicated the significant main effects of Surgery, F(l,20) = 32.8, p<.001 and Intervals, F(6,120) = 7.0, p<.001. None of the interactions among the three factors was significant (ps>. 20). As in Experiment 1, Sham-treated fetuses showed nearly 50% more hindlimb movements than Spinal subjects, and hindlimb activity gradually increased during the Training period (Figure 3, right).
The analysis of CLM during the 2nd Training period revealed main effects of Surgery, F(l,20) = 7.9,p=.01, Training Condition, F(l,20) = 29.9, p<.001, and Intervals, F(6,120) = 29.8, p<.001, as well as the interaction of Training Condition × Intervals, F(6,120) = 4. l,p<.001 (Figure 4, top right). The main effect of Surgery was straightforward, with Sham-treated fetuses expressing about 30% more CLM than Spinal subjects. To simplify the interaction, a series of t-tests compared Training Conditions within each Interval. These post hoc comparisons indicated no difference between Yoked and Unyoked subjects during the 2nd Baseline (the last 5 min of the Testing period), significantly more CLM expressed by Yoked than Unyoked subjects in the first three intervals of the 2nd Training period (ps<.01), and no differences during the last three intervals of Training (ps>.05).
To compensate for differences in hindlimb movements in Sham and Spinal subjects, CLM also was expressed as a percentage of hindlimb activity. In contrast to the comparison of raw frequency, the 3-way ANOVA of relative CLM in the 2nd Training period revealed the significant main effect of Training Condition, F(l,20) = 42.4, p<.001. It also found a main effect of Intervals, F(6,120) = 42.8,p<.001, and the interaction of Training Condition × Intervals, F(6,120) = 5.7, p<.001. The effect of Training Condition was substantial, with Yoked subjects expressing an average of 34.5% CLM compared to just 19.1% among Unyoked subjects. (Recall that all fetuses received training with an intact interlimb yoke during the 2nd Training period.) A series of t-tests confirmed significant differences between Yoked and Unyoked subjects during all but the last interval of yoke training (Figure 4, bottom right). Most notably, there was no evidence of main or interaction effects involving surgical treatment after compensating for differences in overall hindlimb activity (ps>.25).
A final planned analysis was conducted to compare responses to yoke training during the 1st and 2nd Training periods. Because hindlimb activity was strongly affected by spinal transection, this analysis only examined effects on CLM expressed as a percentage of hindlimb activity. A series of 2-way repeated measures ANOVAs (Surgery × Intervals) compared responses of Yoked subjects in corresponding intervals of the 1st and 2nd Training periods. None of these tests indicated main or interaction effects of the Surgery factor, indicating no evident difference between Sham and Spinal subjects. Post hoc comparisons of corresponding Intervals in the 1st and 2nd Training periods indicated no difference in relative CLM rates in the 1st and 2nd Baselines or in the last 15 min of training in each period (ps>. 10). However, in the first half of each period (comprising the first three 5-min Intervals), Yoked subjects showed higher rates of CLM in the 2nd Training compared to the 1st (all ps<.01). In other words, fetuses that experienced yoke training in the 1st Training period showed a more rapid increase in CLM during the 2nd Training period (see Figure 4).
Discussion
We have previously reported that interlimb yoke training is effective in altering the coordination of two hindlimbs, two forelimbs, or a forelimb-hindlimb pair in rat fetuses as early as E19 of gestation (Robinson, 2005; Robinson, Kleven & Brumley, 2008). The ability of the fetal rat to adaptively alter the coordination of its limbs in response to yoke training implies that the fetus can (a) detect the presence of the interlimb yoke, (b) generate kinesthetic signals that distinguish between independent and yoked movements, (c) discriminate between these different kinesthetic signals, and (d) modify motor output to selectively favor paths and velocities that result in coordinated movement of both legs in 3-dimensional space. It is possible that the interlimb yoke causes a mismatch between corollary discharge (which preserves the commands sent out to muscles) and proprioceptive feedback (which reports what the muscles actually did). It is also possible that the yoke elicits a strong cutaneous sensation when the fetus attempts to move the legs independently. Evidence for both functional proprioception from muscle spindles and responsiveness to cutaneous stimulation has been reported for rat fetuses at this age (Fitzgerald, 1987; Kucera, Walro & Reichler, 1989; Robinson & Smotherman 1994; Smotherman & Robinson, 1988b). But even if the yoke elicits a cutaneous sensation that the fetus seeks to avoid, it is difficult to imagine how the 3-dimensional positions of the hindlegs could be synchronized without proprioceptive feedback. Thus, interlimb yoke training is an exceedingly simple task that reveals surprising sophistication in motor control before birth.
The two experiments of the present study confirm that yoke motor learning can be supported by spinal cord circuitry alone. In the first experiment, CLM increased in subjects exposed to hindlimb yoke training, but not in unyoked subjects. Although overall levels of hindlimb activity were depressed in subjects prepared by mid-thoracic spinal transection, the rate of acquisition of CLM during training was not substantially different between intact and spinal subjects, especially when CLM was adjusted for rates of hindlimb movement (see Figure 3). This result suggests that spinal cord transection did not impair the ability of the fetal rat to respond to biomechanical constraint of limb movement.
In the second experiment, CLM increased at a more rapid rate during a second yoke training session in both intact and spinal subjects (see Figure 4). This improvement in acquisition is consistent with savings: a reduction in the time required to relearn a task after a prior learning experience. Savings has been cited in both human and nonhuman infants as evidence of memory retention (Cornell, 1979; Monk, Gunderson, Grant & Mechling, 1996). In the present study, savings during the second training period implies a form of motor memory, albeit brief, in the rat fetus. Because the transection was performed after an initial training session, but before the second training, the savings reported in Experiment 2 suggests that information acquired during normal yoke training is stored in the spinal cord, and that fetuses continue to have access to that information after connections with the brain have been severed. Taken together, these experimental results add to a growing number of examples illustrating the plasticity and learning capacity of the isolated spinal cord (Grau 2014; Grau et al., 2006; Wolpaw 2006, 2007).
The implications of spinally-mediated motor learning in the rat fetus are manifold. First, the ability of the fetal rat to respond to biomechanical constraint of limb movement suggests that experience and feedback from motor performance may play a more substantial role in the early development of the motor system than is typically assumed. Perinatal rats have been shown to alter motor behavior in response to tiny weights attached to the limbs (Brumley & Robinson, 2013), reduction of the range of limb motion (Brumley, Roberto & Strain, 2012; see also Brumley, Kauer & Swann, this issue), and interlimb yoke training (Brumley & Robinson, 2010). Moreover, experimentally altered feedback from tail movements has been related to the development of aberrant reflexive responses in the neonatal rat (Petersson, Waldenström, Fåhraeus & Schouenborg, 2003; Schouenborg, 2010). This evidence argues that sensory feedback from spontaneously produced movement contributes to the organization and development of coordinated motor behavior at a stage of neural development approximately equivalent to the early second trimester (~15 weeks) in human fetuses (Clancy, Finlay, Darlington & Anand, 2007; Workman, Charvet, Clancy, Darlington & Finlay, 2013). Although direct evidence of experiential changes in fetal motor behavior in humans is lacking, 4D ultrasonic imaging of human fetuses has revealed an increase in the frequency of hand-to-mouth movement and anticipatory opening of the mouth before hand contact between 24–36 weeks (Reissland, Francis, Aydin, Mason & Schaal, 2014). These behavioral changes suggest both the opportunity for frequent motor practice and improvement in motor performance in utero.
The finding that fetal behavior may be responsive to feedback from motor performance stands in stark contrast to classic studies in behavioral embryology and developmental neuroscience. Experiments in which amphibian larvae were pharmacologically immobilized during a portion of development, with no evident impairment or delay in later swimming behavior, have been widely cited as evidence that early motor behavior is inconsequential for behavioral development (Haverkamp & Oppenheim, 1986; Matthews & Detwiler, 1926; but see Fromme, 1941, for a contrasting view). Avian embryos were once described as relatively unresponsive to proprioceptive cues (e.g., Hamburger, Wenger & Oppenheim, 1966; Oppenheim, 1972). But more recent research has provided support for the opposite conclusion, that embryonic behavior is strongly influenced by proprioception (Bekoff & Kauer, 1984; Bradley, 1997, 2001; Bradley & Sebelski, 2000; also see Sharp, this issue). It should be scarcely surprising that original conceptions of central pattern generators as rigid, dedicated circuits in the spinal cord (Grillner, 1985) should be replaced by recognition that spinal circuits are flexible, multifunctional, and modulated by sensory feedback and experience (Bekoff, 1992; Briggman & Kristan, 2008; Vinay, Pearlstein & Clarac, 2010; see also Yang, Mitton, Musselman, Patrick & Tajino, this issue).
Conventional thinking is naturally disposed toward dichotomies, and research on early behavioral development is replete with examples: nature vs. nurture, instinct vs. learning, hard-wired vs. plastic, brain vs. spinal cord, prenatal vs. postnatal. As this study illustrates, reality often resides somewhere in the middle. Just because a pattern of behavior is present at birth does not mean it lacks a developmental history. Organized neural activity can be generated by the isolated spinal cord, but this fact does not preclude sensory feedback in its development, or in modulating its expression in the whole animal. Early learning does not necessitate adult-like brain organization, or even a brain. What is required to supersede conventional thinking is greater appreciation of the potential for the soft assembly of hard-wiring.
Acknowlegement
This research was supported by NIH grant HD 33862 to SRR. Preliminary versions of these data have been reported at annual meetings of the International Society for Developmental Psychobiology and the Society for Neuroscience. I thank A. M. Misfeldt and A. J. Marcano-Reik for their assistance in conducting these fetal experiments.
Footnotes
There is no conflict of interest to declare.
REFERENCES
- Angulo y Gonzalez AW. The prenatal growth of the albino rat. Anatomical Record. 1932;52:117–138. [Google Scholar]
- Bateson P, Mameli M. The innate and the acquired: useful clusters or a residual distinction from folk biology? Developmental psychobiology. 2007;49:818–831. doi: 10.1002/dev.20277. [DOI] [PubMed] [Google Scholar]
- Bekoff A. Neuroethological approaches to the study of motor development in chicks: achievements and challenges. Journal of Neurobiology. 1992;23:1486–1505. doi: 10.1002/neu.480231009. [DOI] [PubMed] [Google Scholar]
- Bekoff A, Kauer JA. Neural control of hatching: fate of the pattern generator for the leg movements of hatching in posthatching chicks. Journal of Neuro science. 1984;4:2659–2666. doi: 10.1523/JNEUROSCI.04-11-02659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. [Google Scholar]
- Bradley NS. Reduction in buoyancy alters parameters of motility in E9 chick embryos. Physiology & Behavior. 1997;62:591–595. doi: 10.1016/s0031-9384(97)00168-6. [DOI] [PubMed] [Google Scholar]
- Bradley NS. Age-related changes and condition-dependent modifications in distribution of limb movements during embryonic motility. Journal of Neurophysiology. 2001;86:1511–1522. doi: 10.1152/jn.2001.86.4.1511. [DOI] [PubMed] [Google Scholar]
- Bradley NS, Sebelski C. Ankle restraint modifies motility at E12 in chick embryos. Journal of Neurophysiology. 2000;83:431–440. doi: 10.1152/jn.2000.83.1.431. [DOI] [PubMed] [Google Scholar]
- Briggman KL, Kristan WB., Jr. Multifunctional pattern-generating circuits. Annual Review of Neuroscience. 2008;31:271–294. doi: 10.1146/annurev.neuro.31.060407.125552. [DOI] [PubMed] [Google Scholar]
- Brumley MR, Kauer SD, Swann HE. Developmental plasticity of coordinated action patterns in the perinatal rat. Developmental Psychobiology. doi: 10.1002/dev.21280. (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumley MR, Roberto ME, Strain MM. Sensory feedback modulates quipazine-induced stepping behavior in the newborn rat. Behavioural Brain Research. 2012;229:257–264. doi: 10.1016/j.bbr.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumley MR, Robinson SR. The serotonergic agonists quipazine, CGS-12066A and α-methylserotonin alter motor activity and induce hindlimb stepping in the intact and spinal rat fetus. Behavioral Neuroscience. 2005;119:821–833. doi: 10.1037/0735-7044.119.3.821. [DOI] [PubMed] [Google Scholar]
- Brumley MR, Robinson SR. Experience in the perinatal development of action systems. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 181–209. [Google Scholar]
- Brumley MR, Robinson SR. Sensory feedback alters spontaneous limb movements in newborn rats: Effects of unilateral forelimb weighting. Developmental Psychobiology. 2013;55:323–333. doi: 10.1002/dev.21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets J-R, Grillner P, Menard I, Crémieux J, Clarac F. Two types of motor rhythm induced by NMDA and amines in an in vitro spinal cord preparation of neonatal rat. Neuro science Letters. 1990;111:116–122. doi: 10.1016/0304-3940(90)90354-c. [DOI] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJS. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell EH. Infants' recognition memory, forgetting, and savings. Journal of Experimental Child Psychology. 1979;28:359–374. doi: 10.1016/0022-0965(79)90095-x. [DOI] [PubMed] [Google Scholar]
- Devine MK, Robinson SR. Maternal anesthesia via isoflurane, ether or CO2/O2 does not differentially affect spontaneous movement or sensory responsiveness in the fetal rat (Abstract) Developmental Psychobiology. 2008;50:725. [Google Scholar]
- Drachman DB, Sokoloff L. The role of movement in embryonic joint development. Developmental Biology. 1966;14:401–420. [Google Scholar]
- Edgerton VR, Roy RR. Robotic training and spinal cord plasticity. Brain Research Bulletin. 2009;78:4–12. doi: 10.1016/j.brainresbull.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. Spontaneous and evoked activity of primary afferents in vivo. Nature. 1987;326:603–605. doi: 10.1038/326603a0. [DOI] [PubMed] [Google Scholar]
- Fromme A. An experimental study of the factors of maturation and practice in the behavioral development of the embryo of the frog Rana pipiens . Genetic Psychological Monographs. 1941;24:219–256. [Google Scholar]
- Gottlieb G. Synthesizing nature-nurture: Prenatal roots of instinctive behavior. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- Grau JW. Learning from the spinal cord: how the study of spinal cord plasticity informs our view of learning. Neurobiology of Learning and Memory. 2014;108:155–171. doi: 10.1016/j.nlm.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behavioral and Cognitive Neuroscience Reviews. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Grillner S. Neurobiological bases of rhythmic motor acts in vertebrates. Science. 1985;228:143–149. doi: 10.1126/science.3975635. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Experimental Brain Research. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Anatomical and physiological basis of embryonic motility in birds and mammals. In: Gottlieb G, editor. Behavioral embryology. Vol. 1. New York: Academic Press; 1973. pp. 51–76. [Google Scholar]
- Hamburger V, Wenger E, Oppenheim RW. Motility in the chick embryo in the absence of sensory input. Journal of Experimental Zoology. 1966;162:133–160. [Google Scholar]
- Haverkamp LJ, Oppenheim RW. Behavioral development in the absence of neural activity: Effects of chronic immobilization on amphibian embryos. Journal of Neuroscience. 1986;6:1332–1331. doi: 10.1523/JNEUROSCI.06-05-01332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess E. Imprinting. New York: Van Nostrand Reinhold; 1973. [Google Scholar]
- James DK. Fetal learning: A critical review. Infant and Child Development. 2010;19:45–54. [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kisilevsky BS, Low JA. Human fetal behavior: 100 years of study. Developmental Review. 1998;18:1–29. [Google Scholar]
- Kleven G, Bellinger SA. Developmental pathways of motor dysfunction. Developmental Psychobiology. doi: 10.1002/dev.21304. (this issue). [DOI] [PubMed] [Google Scholar]
- Kucera J, Walro JM, Reichler J. Role of nerve and muscle factors in the development of rat muscle spindles. American Journal of Anatomy. 1989;186:144–160. doi: 10.1002/aja.1001860205. [DOI] [PubMed] [Google Scholar]
- Kudo N, Yamada T. N-Methyl-D, L-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neuroscience Letters. 1987;75:43–48. doi: 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- Kuo Z-Y. The dynamics of behavior development. New York: Random House; 1967. [Google Scholar]
- Lecanuet J-R, Fifer WP, Krasnegor NA, Smotherman WP. Fetal Development: A Psychobiological Perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. [Google Scholar]
- Lorenz KZ. Der Kumpan in der Umwelt des Vogels. Journal für Ornithologie. 1935;83:137–213. 289–413. (Translated: 1937. The companion in the bird's world. Auk, 54, 245–273). [Google Scholar]
- Matthews SA, Detwiler SR. The reaction of Amblystoma embryos following prolonged treatment with chloretone. Journal of Experimental Zoology. 1926;45:279–292. [Google Scholar]
- Moessinger AC. Fetal akinesia deformation sequence: an animal model. Pediatrics. 1983;72:857–863. [PubMed] [Google Scholar]
- Monk CS, Gunderson VM, Grant KS, Mechling JL. A demonstration of the memory savings effect in infant monkeys. Developmental Psychology. 1996;32:1051. [Google Scholar]
- Müller GB. Embryonic motility: environmental influences and evolutionary innovation. Evolution & Development. 2003;5:56–60. doi: 10.1046/j.1525-142x.2003.03009.x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- Narayanan CH, Malloy RB. Deafferentation studies on motor activity in the chick: I. Activity pattern of hindlimbs. Journal of Experimental Zoology. 1974;189:163–176. doi: 10.1002/jez.1401890204. [DOI] [PubMed] [Google Scholar]
- Navarrete R, Vrbova G. Activity-dependent interactions between motoneurones and muscles: Their role in the development of the motor unit. Progress in Neurobiology. 1993;41:93–124. doi: 10.1016/0301-0082(93)90041-p. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. An experimental investigation of the possible role of tactile and proprioceptive stimulation in certain aspects of embryonic behavior in the chick. Developmental Psychobiology. 1972;5:71–91. doi: 10.1002/dev.420050109. [DOI] [PubMed] [Google Scholar]
- Petersson P, Waldenstrom A, Fåhraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nature Reviews Neuroscience. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Reissland N, Francis B, Aydin E, Mason J, Schaal B. The development of anticipation in the fetus: A longitudinal account of human fetal mouth movements in reaction to and anticipation of touch. Developmental Psychobiology. 2014;56:955–963. doi: 10.1002/dev.21172. [DOI] [PubMed] [Google Scholar]
- Robertson GA, Mortin LI, Keifer J, Stein PS. Three forms of the scratch reflex in the spinal turtle: central generation of motor patterns. Journal of Neurophysioly. 1985;53:1517–1534. doi: 10.1152/jn.1985.53.6.1517. [DOI] [PubMed] [Google Scholar]
- Robertson SS, Smotherman WP. The neural control of cyclic motor activity in the fetal rat (Rattus norvegicus) Physiology & Behavior. 1990;47:121–126. doi: 10.1016/0031-9384(90)90049-a. [DOI] [PubMed] [Google Scholar]
- Robinson SR. A comparative study of prenatal behavioral ontogeny in altricial and precocial murid rodents. Unpublished doctoral dissertation. Corvallis: Oregon State University; 1989. [Google Scholar]
- Robinson SR. Conjugate limb coordination after experience with an interlimb yoke: evidence for motor learning in the rat fetus. Developmental Psychobiology. 2005;47:328–344. doi: 10.1002/dev.20103. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Blumberg MS, Lane MS, Kreber LA. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behavioral Neuroscience. 2000;114:328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Kleven GA. Learning to move before birth. In: Hopkins B, Johnson SP, editors. Prenatal development of postnatal functions. (Advances in Infancy Research series) Westport, CT: Praeger Publishers; 2005. pp. 131–175. [Google Scholar]
- Robinson SR, Kleven GA, Brumley MR. Prenatal development of interlimb motor learning in the rat fetus. Infancy. 2008;13:204–228. doi: 10.1080/15250000802004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Fundamental motor patterns of the mammalian fetus. Journal of Neurobiology. 1992;23(10):1574–1600. doi: 10.1002/neu.480231013. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Behavioral effects of milk in the rat fetus. Behavioral Neuroscience. 1994;108:1139–1149. [PubMed] [Google Scholar]
- Schouenborg J. Role of spontaneous movements in imprinting an action-based body representation in the spinal cord. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 254–261. [Google Scholar]
- Sharp A. Sensory regulation of spontaneous limb movements in the embryonic chick. Developmental Psycholobiology. doi: 10.1002/dev.21292. (this issue). [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Richards LS, Robinson SR. Techniques for observing fetal behavior in utero: A comparison of chemomyelotomy and spinal transection. Developmental Psychobiology. 1984;17:661–674. doi: 10.1002/dev.420170608. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR, editors. Behavior of the Fetus. Caldwell, NJ: Telford Press; 1988a. [Google Scholar]
- Smotherman WP, Robinson SR. Fetal expression of the leg extension response to anogenital stimulation. Physiology & Behavior. 1988b;43:243–244. doi: 10.1016/0031-9384(88)90246-6. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Accessibility of the rat fetus for psychobiological investigation. In: Shair H, Barr GA, Hofer MA, editors. Developmental Psychobiology: New Methods and Changing Concepts. New York: Oxford University Press; 1991. pp. 148–166. [Google Scholar]
- Strain MM, Kauer SD, Kao T, Brumley MR. Inter- and intralimb adaptations to a sensory perturbation during activation of the serotonin system after a low spinal cord transection in neonatal rats. Frontiers in Neural Circuits. 2014;8:1–13. doi: 10.3389/fncir.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teulier C, Lee DK, Ulrich BD. Early gait development: Plasticity and clinical applications. Developmental Psychobiology. doi: 10.1002/dev.21291. (this issue). [DOI] [PubMed] [Google Scholar]
- Thelen E. Three-month-old infants can learn task-specific patterns of interlimb coordination. Psychological Science. 1994;5:280–285. [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Thompson W. Synapse elimination in neonatal rat muscle is sensitive to pattern of muscle use. Nature. 1983;302:614–616. doi: 10.1038/302614a0. [DOI] [PubMed] [Google Scholar]
- Viala D. Evolution and behavioral adaptation of locomotor pattern generators in vertebrates. Comptes Rendus Palevol. 2006;5:661–614. [Google Scholar]
- Viala D, Viala G, Fayein N. Plasticity of locomotor organization in infant rabbits spinalized shortly after birth. In: Goldberger ME, Gorio A, Murray A, editors. Development and plasticity of the mammalian spinal cord. New York: Springer-Verlag; 1986. pp. 301–310. [Google Scholar]
- Vinay L, Pearlstein Edouard, Clarac F. Development of spinal cord locomotor networks controlling limb movements. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 210–239. [Google Scholar]
- Wolpaw JW. The education and re-education of the spinal cord. Progress in Brain Research. 2006;157:261–281. doi: 10.1016/s0079-6123(06)57017-7. [DOI] [PubMed] [Google Scholar]
- Wolpaw JW. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiologica. 2007;189:155–169. doi: 10.1111/j.1748-1716.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. Journal of Neuroscience. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JE, Mitton M, Musselman K, Patrick S, Tajino J. Characteristics of the developing human locomotor system: Similarities to other mammals. Developmental Psychobiology. doi: 10.1002/dev.21289. (this issue). [DOI] [PubMed] [Google Scholar]