Abstract
Some of the most simple, stereotyped, reflexive and spinal-mediated motor behaviors expressed by animals display a level of flexibility and plasticity that is not always recognized. We discuss several examples of how coordinated action patterns have been shown to be flexible and adaptive in response to sensory feedback. We focus on interlimb and intralimb coordination during the expression of two action patterns (stepping and the leg extension response) in newborn rats, as well as interlimb motor learning. We also discuss the idea that the spinal cord is a major mechanism for supporting plasticity in the developing motor system. An implication of this research is that normally occurring sensory stimulation during the perinatal period influences the typical development and expression of action patterns, and that exploiting the developmental plasticity of the motor system may lead to improved strategies for promoting recovery of function in human infants with motor disorders.
Keywords: motor development, motor coordination, sensory feedback, quipazine, treadmill, neonatal rat, stepping, leg extension response, spinal cord, motor learning, experience
Compared to humans, the behavior of other animals often appears more rigid, stereotypical, and “instinctive”. However as we discuss in this review, even some of the most simple, stereotyped, reflexive and spinal-mediated motor behaviors expressed by animals display a level of flexibility and plasticity that is not always recognized. The assumption of simplistic displays and mechanisms of behavior, including a prescribed developmental plan that gradually unfolds, can occlude a deeper understanding of behavioral development and its implications for recovery of function. For example, careful research on the development of what may appear to be a fairly simple human behavior—walking—has revealed that learning to walk is not a uni-linear process, is quite variable, reflects the integration of many endogenous and exogenous influences on behavior, and is heavily influenced by experience (i.e., maternal-infant interactions) (Adolph & Robinson, 2013). When we apply a similar experimental lens through which to investigate the development of behavior in non-human animals, including some of the earliest forms of motor behavior (i.e., spontaneous movements and coordinated action patterns), it becomes apparent that developmental plasticity is more the rule than the exception, including for apparently simple-looking action patterns.
Behavior in all vertebrates, including humans, begins during the prenatal period. Fetal behavior generally may be characterized as spontaneous or nonevoked, and appears to involve an abundance of random or purposeless limb jerks and twitches (Hamburger, 1973). Spontaneous activity continues to be expressed throughout the early postnatal period. Although it appears random and uncoordinated, studies of the rat fetus in vivo have shown spatial and temporal organization during spontaneous motor activity. For example, synchronous limb movements during spontaneous activity at the inception of fetal motility on E16 (embryonic day 16; six days before birth) are not different from chance levels. However, increases in forelimb-forelimb, hindlimb-hindlimb, and intersegmental coupling occur over the next several days (Robinson & Smotherman, 1987; Kleven, Lane, & Robinson, 2004). Research with avian embryos and fetal rats has shown that spontaneous activity is largely generated and modulated by spinal cord mechanisms (Narayanan & Malloy, 1974; Provine, 1972; Robertson & Smotherman, 1990; Robinson, Blumberg, Lane, & Kreber, 2000). Spontaneous activity has been shown to play an organizational role in synapse and motor unit formation and reorganization (Narayanan, Fox, & Hamburger, 1971). Additionally, sensory feedback modulates the expression of spontaneous limb activity (Brumley & Robinson, 2013; Sharp, this issue), promotes interlimb motor learning (Robinson, 2005), and guides the development of nociceptive reflex circuits (Petersson, Waldenström, Fåhraeus, & Schouenborg, 2003). Thus, much of fetal and newborn behavior may not be as random and purposeless as it appears.
In addition to expressing spontaneous activity, fetal rats show many coordinated action patterns that resemble functional postnatal behaviors (Robinson & Brumley, 2005; Robinson & Smotherman, 1992a). For example, a few of days before birth, rat fetuses respond to intraoral infusion of lemon solution with a facial wiping response (Brumley & Robinson, 2004) that resembles grooming and aversion responses of adults (Richmond & Sachs, 1980). The late-gestation fetal rat shows an oral grasp response when presented with an artificial nipple (Robinson, Hoeltzel, Cooke, Murrish, & Smotherman, 1992), and a coordinated stretch response to milk infusion (Robinson & Smotherman, 1992b). The fetal rat also shows episodes of locomotor activity, in the form of alternated stepping behavior, following treatment with the catecholamine precursor L-DOPA (Robinson & Kleven, 2005) or serotonergic receptor agonists (Brumley & Robinson, 2005). However, interlimb coordination during fetal stepping is somewhat variable and exhibits developmental change (Brumley & Robinson, 2010; Robinson & Kleven, 2005). Rat fetuses also show a coordinated leg extension response (LER) to anogenital stimulation (Smotherman & Robinson, 1988) that resembles the response that newborns exhibit during bouts of pup-directed maternal anogenital licking (Moore & Chadwick-Dias, 1986). Both stepping behavior and the LER are not abolished following acute mid-thoracic spinal cord transection (Brumley & Robinson, 2005; Smotherman & Robinson, 1988). Thus, there are several action patterns that have their origins in the prenatal period that appear to anticipate functional behavioral responses of the postnatal animal. Aside from a few studies that mainly examined the biomechanical constraints of facial wiping in the fetal (Robinson & Smotherman, 1991) and newborn rat (Smotherman & Robinson, 1989), the role of sensorimotor experience in shaping these action patterns in mammals during the perinatal period has remained largely unexplored.
To help fill this gap in our understanding of neurobehavioral ontogeny, in the following section we discuss recent research on the sensory modulation of coordinated action patterns during early motor development. We focus on interlimb and intralimb coordination during the expression of two action patterns (stepping behavior and the LER) in newborn rats, which largely includes review of some new empirical findings from our laboratory. We also discuss interlimb yoke training in perinatal rats, which is a form of motor learning. Because stepping behavior, the LER, and interlimb yoke training have been demonstrated to occur in spinal animals, next we discuss the idea that the spinal cord is a major site for supporting plasticity in the developing motor system. An implication of this research is the idea that normally occurring sensory stimulation during the perinatal period influences the typical development and expression of coordinated action patterns, and that exploiting the developmental plasticity of the motor system may lead to improved strategies for promoting recovery of function in human infants with motor disorders.
Sensory Modulation of Action Patterns
Stepping Behavior
Both behavioral (Bekoff & Lau, 1980; Brumley & Robinson, 2005) and neurophysiological studies (i.e., Kudo, Nishimaru, & Nakayama, 2004; Nakayama, Nishimaru, & Kudo, 2001) have provided evidence that the neural mechanisms for locomotion begin developing before birth in the rat. However, weak and unstable postural control and immature neural mechanisms limits the expression of locomotion during the early postnatal period (Gramsbergen, 1998), before the adult pattern of postural control develops by postnatal day 21 (P21) (Geisler, Westerga, & Gramsbergen, 1993). Early forms of spontaneous locomotion by the rat include pivoting and crawling, which appear during the first postnatal week and early during the second postnatal week, respectively (Altman & Sudarshan, 1975). During the first 3–4 postnatal weeks, locomotor behavior gradually improves until the adult form of walking locomotion is expressed (Altman & Sudarshan, 1975).
Because rats typically do not exhibit spontaneous walking immediately after birth, locomotor behavior is often assessed in these young subjects in vivo by experimentally evoking locomotor-like patterns of limb coordination called “stepping”. Alternated stepping can be reliably evoked in newborn rats using pharmacological agents (i.e, serotonergic or catecholaminergic agonists) (McEwen, van Hartesveldt, & Stehouwer, 1997) or strong sensory stimulation (Fady, Jamon, & Clarac, 1998; Norreel, Pflieger, Pearlstein, Simeoni-Alias, Clarac, & Vinday, 2003). In vitro experiments on the isolated spinal cord have suggested that the mechanisms for generating the alternating locomotor rhythm in the hindlimbs are located in the lower thoracic (T12/13) and lumbar spinal cord (Cazalets, Borde, & Clarac, 1995; Cowley & Schmidt, 1997; Kjaerulff & Kiehn, 1996). Presumably, pharmacological stimulation of neurotransmitter systems and forms of sensory-induced stepping either directly or indirectly activate spinal locomotor mechanisms.
Although sensory modulation of locomotion is well documented in adult animals (e.g., Büschges & Manira, 1998; Pearson, 2000; Rossignol, et al., 2004), far fewer studies have examined the extent to which sensory feedback modulates expression of locomotor behavior before the onset of mature walking. Experiments with humans have shown that young infants demonstrate the ability to adapt locomotor patterns in response to sensory feedback, such as trip-inducing stimuli (Pang, Lam, & Yang, 2003), phase-dependent mechanical stimulation (Lam, Wolstenholme, van der Linden, Pang & Yang, 2003), or increased limb loading (Lam, Wolstenholme, & Yang, 2003). Infants respond in “functionally appropriate” ways to trip-inducing stimuli, as demonstrated by Pang, Lam, and Yang (2003). Infants were presented with trip-inducing stimuli while stepping on a treadmill and responded to stimuli by adapting their stepping patterns to increase the distance between their foot (“high stepping”) and the trip-inducing stimulus after repeated exposure. Similarly, infants also maintained a “high stepping” pattern after increased limb loading with effects persisting after removal of limb weights. Infants demonstrated a capacity to offset their locomotor pattern in response to mechanical stimulation. When infants were exposed to mechanical disturbances, specific to the dorsal surface of the foot, they increased the duration of their step cycle and swing phase. Overall these studies demonstrate locomotor plasticity in young infants, at a functional level, to stimuli that had long-lasting effects on locomotor activity and patterns. Likewise, a study with spinal rabbits showed that pups during the second and third postnatal week developed gait patterns consistent with the form of daily hindlimb motor training (alternated or synchronized) they received (Viala, Viala, & Fayein, 1986). These studies demonstrate the adaptive capacity and plasticity of the developing nervous system. In our lab, we have examined sensory responsiveness during stepping in the newborn rat, which is equivalent to a human fetus during the late second trimester in terms of neurological development (Clancy, Darlington, & Finlay, 2001). Like the research described above, our work shows that the immature nervous system is capable of responding to sensory input during the expression of locomotor behavior, even during this very early period of development.
In a paradigm referred to as “air-stepping”, animals are suspended off the ground in a sling to alleviate gravitational constraints, whereby the limbs are not impeded and stepping behavior may be evoked. In one study, we manipulated the quality of limb proprioceptive and tactile feedback neonatal rats experienced by varying the type of substrate/floor they stepped on following treatment with the 5-HT2 receptor agonist quipazine (3.0 mg/kg; Brumley, Roberto, & Strain, 2012). P1 rats were secured in a sling in the prone posture, and an elastic trampoline-like substrate or a hard, stiff substrate (Plexiglas plate) was placed beneath the paws at a distance of 80% of maximum limb extension (so the substrate could easily be avoided). Pups in the control condition did not have anything placed beneath them. During the peak of stepping (~15 min following treatment with quipazine), pups performed more alternated steps on the elastic substrate and fewer steps on the stiff one, compared to pups in the control condition. Furthermore, during the middle to end of the 45-min test session pups made significantly more contact (i.e., longer durations) with the elastic substrate compared to the stiff substrate, suggesting that their intralimb coordination was different between the two substrates (i.e., their limbs were more flexed in the stiff substrate condition). This is the earliest demonstration of sensory feedback having real-time effects on stepping behavior in rats.
Next we examined real-time and persistent interlimb and intralimb changes during quipazine-induced stepping. In this study, a Plexiglas plate was placed beneath the limbs of newborn rats at 50% of maximum extension immediately following treatment with quipazine (Strain & Brumley, 2014). This manipulation induced a period of range of motion (ROM) restriction while the plate was in place (15 min) because the limbs could not easily avoid it and had to considerably flex or splay out to do so. Stepping significantly decreased during the period of ROM restriction (compared to non-restricted controls). Intralimb adaptations (i.e., more flexed limb postures) occurred during the period of restriction and for a short time after removal of the perturbation. In a follow-up study, persistent effects of ROM restriction were most drastic in pups that received a low thoracic spinal transection compared to sham surgery pups, indicating that the isolated spinal cord can regulate sensory-induced changes in stepping (Strain, Kauer, Kao, & Brumley, 2014).
Finally, a more recent study examined stepping on a treadmill in newborn rats. Stepping on a treadmill is a conventional method used to study and rehabilitate locomotor function in both humans and non-human animals. In the first experiment, we compared stepping behavior in P1 rats when they were on a moving or non-moving treadmill belt (Doherty, Strain, Tappan, & Brumley, 2013). The moving treadmill belt did not induce stepping; pups in the non-moving treadmill condition stepped more than pups in the moving belt condition, however step frequencies remained very low. In a follow-up experiment, we induced stepping with quipazine and then varied the speed of the treadmill belt to evaluate if treadmill speed would influence ongoing stepping. Pups showed significantly more alternated forelimb steps and shorter step cycle durations at the faster treadmill speeds. Interestingly, they also showed significantly shorter stance durations, but no differences in swing durations, among the different treadmill speeds. These effects are shown in Figure 1. The modulation of stance duration in response to faster treadmill speeds is characteristic of adult stepping (Pearson, 2008), and also has been shown to occur in human infants (Thelen, Ulrich, & Niles, 1987). That the perinatal rat has the ability to adapt to changing treadmill speeds in this way suggests that expression of locomotor behavior is modulated by sensory feedback very early in development.
Figure 1.
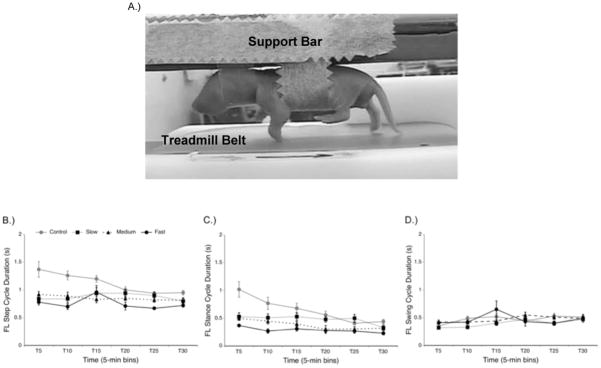
Treadmill stepping in the newborn rat. (A) Photograph of a P1 rat pup stepping on a treadmill. The pup is secured to a support bar in the prone posture, with the treadmill placed just below. (B) Following treatment with quipazine (3.0 mg/kg intraperitoneal injection, 50 microliters), pups stepping on a non-moving (control), slow (1.8 cm/s), medium (2.5 cm/s), or fast (3.2 cm/s) treadmill belt showed differences in step cycle duration. Pups generally showed shorter step cycle durations at the faster treadmill speeds, particularly in the first half of the test session. Step cycle duration was calculated for 4–5 forelimb steps per 5-min time bin. (C) Stance cycle duration was generally shorter for pups stepping on faster moving treadmill belts. (D) Swing cycle duration was not different among the different treadmill speeds.
Taken together, these studies suggest that locomotor mechanisms in the perinatal rat, while not yet fully mature, are remarkably responsive to sensory input. Because spinal and supraspinal mechanisms for motor production and sensory processing are undergoing rapid development during this period (i.e., Fizgerald & Jennings, 1999; Vinay, Brocard, Clarac, Norreel, Pearlstein, & Pflieger, 2002), the immature rat provides a valuable experimental model to explore the dynamic interaction among peripheral and central processes that occurs during neurobehavioral ontogeny.
Leg Extension Response
Newborn rats show a LER, which is an action pattern characterized by bilateral hyperextension of both hindlimbs. It is normally expressed in a reflexive manner in the context of pup-directed maternal anogenital (AG) licking (Moore & Chadwick-Dias, 1986). During AG licking, the dam rhythmically strokes the perineum of the pup with her tongue to stimulate urine release. Newborn rats are not able to urinate on their own, and AG stimulation activates a bladder spinal reflex pathway (de Groat, 2002). The dam ingests the pup urine, replacing electrolytes she has lost through nursing (Friedman, Bruno, & Alberts, 1981; Gubernick & Alberts, 1983). However, before the infant rat releases urine it expresses the LER. Thus, this species-typical action pattern is functionally related to behavior that is critical for pup survival—removal of waste products from the body.
Rat dams are more attracted to and prefer the pheromones excreted by male pups, and thus lick them more frequently (Brouette-Lahlou, Godinot, & Vernet-Maury, 1999; Moore, 1985). Moore and Chadwick-Dias (1986) reported that duration of the LER was different between infant male and female rats, and that duration of the LER generally declined with age. Given that males and females experience AG licking at different rates, this sex-difference suggests that maternal-infant interactions and related sensorimotor feedback may shape the development of this action pattern. Extra AG licking from the dam during the newborn period contributes to the development of masculine behavior (Moore, 1992) and anatomical differences in the spinal cord (Lenz & Sengelaub, 2006, 2009; Moore, Dou, & Juraska, 1992). Additionally, variations in maternal behavior (specifically maternal licking/grooming patterns) have been shown to be related to later emotional, physiological, and social responses (Champagne & Curley, 2005; Liu, et al., 1997), and regional brain differences (Bredy, Grant, Champagne, & Meaney, 2003; Caldji, Diorio, and Meaney, 2003; Champagne, et al., 2008).
Does sensory stimulation influence the development of the LER, a relatively simple action pattern? Securing the neonatal rat to a bar in a sling apparatus as previously described, so that the limbs hang pendant, we can experimentally evoke the LER. Application of a vibrating strip of latex (120 Hz) to the AG region evokes expression of the LER in perinatal rats (Roberto & Brumley, 2014). Using this form of AG stimulation, we experimentally evoked the LER 15 times during a 60-min test session (stimulation occurred every 4 min). During this period of LER training, half of the subjects received ROM restriction. Thus, this experiment provided pups with concurrent proprioceptive (from 15 LER expressions) and cutaneous feedback (from their paws touching the ROM restriction plate) during the test session. Following training, ROM restriction was removed and a final LER test occurred. P1 rats that experienced ROM restriction showed significantly shorter bilateral LER durations and smaller hip and ankle angles during the LER test. That is, pups that experienced 60-min of ROM restriction showed a more flexed LER, demonstrating persistent effects of sensory feedback on this action pattern (Belnap, Allmond, Boomhower, Roberto, & Brumley, in press). In another experiment, pups received LER training with a weight (equaling 100% of leg mass) attached to one hindlimb. For this experiment pups were tested in the supine posture. While the weight was attached to the leg, pups did not extend the weighted limb as far as the nonweighted limb during AG stimulation. However, after 15 trials of LER training (LER evoked every 4 min over a period of 60 min) and removal of the unilateral limb weight, the previously weighted limb extended significantly further than the nonweighted limb, suggesting that compensation for the weight occurred during LER training (Mendez-Gallardo, Bean, & Brumley, 2013).
Because manipulation of sensory feedback affected LER expression during acute experimental sessions, next we examined if more naturalistic forms of sensory stimulation would influence LER expression and development. In one study, pups were delivered prematurely, one day before birth. These pups spent one less day in a uterine environment and one day more in a postnatal nest environment, compared to both vaginal-delivered and cesarean-delivered control pups. To achieve this, premature pups were delivered by cesarean section on E21 and then fostered to surrogate dams. Subjects in the control groups were delivered on E22 by either cesarean section (and then were fostered to a surrogate dam for a few hours before testing) or normal vaginal birth (no fostering). Wholesale fostering occurred; there were no mixed litters in this experiment. More time in the postnatal environment should result in more maternal-infant interactions, including more AG licking and thus more LER practice. At test, all pups were age-matched (post-conception day 22; the typical day of birth in the rat) but differed in amount of time they spent in a prenatal vs. postnatal environment (approximately 4 hrs for control pups but 1 day for premature pups). Pups with the most postnatal experience showed significantly more bilateral coordination during LER expression and significantly longer LER durations during AG stimulation (Roberto & Brumley, 2014). This suggests that additional LER practice in the nest environment influenced development of the LER, but the experiment did not directly test this notion.
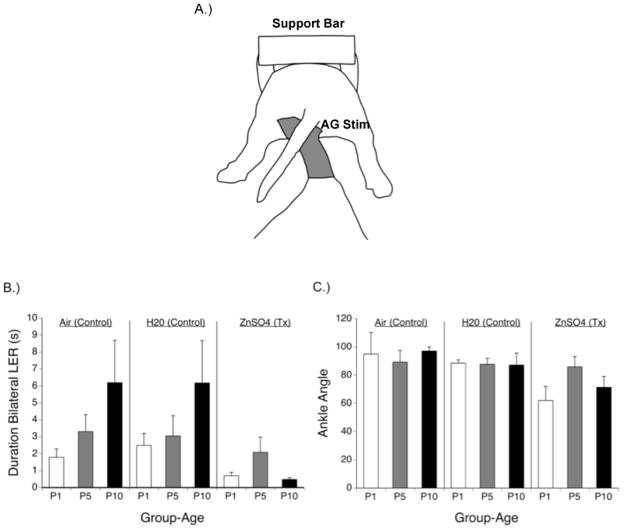
In the next study, maternal behavior was directly addressed. Dams received regular intranasal application of ZnSO4 to render them hyponosmic during the post-partum period. Dams treated with ZnSO4 show a specific reduction—but not elimination—of AG licking directed towards their offspring, but no change in other maternal behaviors (Mayer & Rosenblatt, 1993). Pups reared by hyponosmic dams showed shorter bilateral LER durations and smaller ankle angles during the course of the first 10 days after birth (Figure 2). Their LERs were very brief and their limbs did not extend as far (because of the smaller ankle angles, the limbs were relatively more flexed) as pups reared by control dams. Compared to control dams, hyponosmic dams showed less anogential licking directed toward their pups, but no differences in nursing overall. Thus this apparently simple reflex behavior that is present at birth and is expressed following a mid-thoracic spinal cord transection (Smotherman & Robinson, 1988) is quite responsive to sensory input, including naturalistic forms of stimulation which occur in the natal environment.
Figure 2.
Leg extension response (LER) in the newborn rat. (A) Illustration of a newborn rat expressing the LER during vibrotactile stimulation of the anogenital area (AG Stim). The pup is secured to a support bar in the prone posture, allowing unimpeded movement of the limbs. The vibrotactile stimulus has a latex strip that is applied to the anogenital area from underneath the pup. (B) Rat pups were born to dams treated intranasally with air (control), H20 (control), or ZnSO4 (experimental treatment). Dams treated with ZnSO4 exhibit hyponosmia and reduced AG licking of their pups. LER expression was tested at P1, P5, and P10. Pups born to ZnSO4-treated dams showed shorter LER durations. (C) Pups born to ZnSO4-treated dams showed smaller ankle angles, and thus more flexed limb postures, during the LER test.
The LER, like stepping, is not immune to sensory stimulation and is modulated by sensory feedback during early motor development, suggesting that sensory feedback likely shapes the development of even simple motor responses. Therefore, what often appears to be a simple or sometimes stage-like maturation of motor behavior during early development may instead be a dynamic process of ongoing sensorimotor integration, as animals learn to move and adaptively respond to features of their environment and a dynamically changing body (Adolph & Berger, 2006; Robinson & Kleven, 2005). Perinatal animals are not passive, reflex machines—they use sensory feedback to respond to their environment in real-time, and often show lasting effects of that experience.
Interlimb Yoke Training
Stepping behavior and the LER are two species-typical, coordinated action patterns that have their developmental roots in the prenatal period. But can perinatal rats learn a novel pattern of limb coordination by manipulating sensory feedback? Robinson (2005) developed an interlimb yoke training paradigm whereby two limbs are physically linked by a small piece of polyethylene tubing that is secured around the ankles with thread. When one of the yoked limbs moves, the other limb moves with it, thus altering proprioceptive feedback. When the interlimb yoke is in place, E20 rat fetuses show a specific and gradual increase in conjugate limb movements (CLMs: limb movements that are initiated at the same time and follow the same spatial trajectories) for the yoked limbs, during a 30-min training period. Only the limb pairs (hindlimb-hindlimb, forelimb-forelimb, forelimb-hindlimb) that experience the interlimb yoke show an increase in CLM (Robinson, 2005). Once the yoke is cut, CLM continues to be expressed above control levels for a period of time after training (see Robinson, this issue). Interlimb yoke training develops on E19 in fetal rats (Robinson et al., 2008), and also occurs in newborn rats (Brumley & Robinson, 2010).
Fetal rats learn and retain hindlimb CLM following a mid-thoracic spinal transection, indicating that the spinal cord has the capacity to support this form of motor learning (Robinson, this issue). During interlimb yoke training there is no explicit reinforcement or paired stimulus presentation. The yoke is simply placed between two limbs and left there for a 30-min period, when it is then bisected. Thus the perinatal rat appears to learn the CLM pattern through its own spontaneous limb activity, and movement-related sensory feedback. Therefore, sensory feedback is not only used to modulate species-typical action patterns during perinatal development, but it can be used to adaptively respond to environmental constraints to learn novel action patterns.
Developmental Mechanisms of Motor Plasticity
During early motor development, the spinal cord displays amazing plasticity. It is capable of generating and organizing spontaneous limb activity and coordinated action patterns, and mediating sensory-induced changes in spontaneous activity, action patterns, and interlimb motor learning. Neonatal rats with a complete spinal cord transection recover significantly more motor function, including locomotion, than animals with the same transection after P15 (Weber & Stelzner, 1977). This developmental difference in recovery is largely due to increased synaptogenesis and decreased denervation and spinal shock in the immature cord, as opposed to regrowth across the lesion site (Stelzner, Weber, & BryzGornia, 1986; Tillakaratne et al., 2010; Weber & Stelzner, 1977, 1980). For example, rats that underwent a mid-thoracic spinal transection shortly after birth showed the same developmental changes in motorneuron morphology seen during normal development, whereas rats that received the transection as weanlings or adults showed regressive changes in motorneuron morphology (Cummings & Stelzner, 1988). Although sparing of function is greatest in immature animals, the adult spinal cord retains some level of plasticity, as both classical and operant conditioning has been shown to occur in the adult cord (Crown, Ferguson, Joynes, & Grau, 2002; Grau, 2014; Wolpaw, 2006).
It is clear that the spinal cord is a major regulator of perinatal behavior. Yet the nervous mechanisms controlling sensorimotor behavior undergo rapid change during the perinatal period. In the spinal cord, the size, complexity, synaptic organization, and firing properties of motorneurons change during the pre- and early postnatal period (Gramsbergen, 1998; Vinay et al., 2002). Likewise, changes occur in spinal sensory processing. Modification of cutaneous and reflex thresholds, the growth and integration of primary afferent terminals in the dorsal horn, receptive field properties, and neurotransmitter regulation of sensorimotor circuits happens during this time (e.g., Baccei & Fitzgerald, 2004; Fitzgerald & Jennings, 1999). In terms of brain regulation of motor behavior, the first descending pathways including the reticulospinal, vestibulospinal, and interstitiospinal tracts, begin to reach the lumbar spinal cord at E15 (Clarac et al., 1998; de Boer-vanHuizen & ten Donkelaar, 1999). Raphespinal and coereleospinal, rubrospinal, and corticospinal tracts reach the lumbar cord at E15, E18, and P6, respectively (Lakke, 1997; Vinay et al., 2002). Additional development (increase in fiber number, myelinization, function) is required before these pathways attain adult form and function. Thus while perinatal rats are able to use sensory information to modulate their motor behavior, the exact mechanisms involved may be different from one day to the next. Also, although the isolated spinal cord is capable of generating stepping behavior, the LER, and interlimb motor learning, under normal circumstances (i.e., in intact and not spinal transected subjects) multiple levels of the neuraxis may be involved in sensory regulation of these action patterns.
Consider development of the nociceptive withdrawal reflex in rats. This reflex is where the paw or tail moves away from a noxious stimulus, and it develops over the course of the first few postnatal weeks. The neural circuitry controlling this reflex has been localized to the spinal cord (Schouenborg, 2002; 2010). Sensory feedback from spontaneous movements expressed during the newborn period has been shown to be necessary for the correct development of this reflex (Petersson et al., 2003 Waldenström, Thelin, Thimansson, Levinsson, & Schouenborg, 2003). Although the spinal cord can generate spontaneous twitches and eventually houses the withdrawal reflex circuitry, a neonatal spinal transection leads to aberrant responses and the reflex circuit does not develop properly (Levinsson, Luo, Holmberg, & Schouenborg, 1999). Therefore, proper development and fine-tuning of the nociceptive withdrawal reflex is the product of spinal activity, sensory feedback from spontaneous movements, and supraspinal regulation of spinal mechanisms. This example nicely highlights the dynamic interplay between experience (i.e., sensory feedback) and neural mechanisms in the development of an adaptive reflex.
Implications
From this perspective, two major implications may be drawn. The first implication is that normally occurring sensory stimulation during the perinatal period influences development and expression of coordinated action patterns. Although the action patterns discussed may appear very stereotyped and reflexive, it is clear that sensory feedback—whether from a treadmill, maternal stimulation, or spontaneous activity—influences them. The idea that experience influences development is not new. Gottlieb (1976) outlined multiple roles that experience can have on development, including maintenance, facilitation, and induction. Gottlieb’s view came out of his research on perceptual development, and in fact much of the research on neural plasticity has focused on sensory systems (largely inspired by Hubel’s and Wiesel’s classic experiments on information processing in the visual cortex (e.g., Hubel & Wiesel, 1970; Wiesel & Hubel, 1963)). However, this idea also may be applied to motor systems. For example, human infants that received daily stepping practice showed longer durations of stepping and an earlier onset of independent walking, compared to infants that did not receive stepping practice (Zelazo, Zelazo & Kolb, 1972). Additionally, cultural differences in infant rearing practices has been shown to produce differences in the onset of infant postural and locomotor behaviors (e.g., Adolph, Karasik, & Tamis-Lemonda, 2010; Adolph & Robinson, 2013; Bornstein, 2010; Hopkins & Westra, 1988, 1989), suggesting that the facilitation of locomotion is not merely a laboratory phenomenon.
The second implication pertains to therapeutic interventions. Given the developmental plasticity of the motor system, and the responsiveness of the system to sensory feedback, understanding how sensory feedback influences neuromotor development should factor into interventions for children that suffer from motor disabilities. Infants with motor disorders are likely not receiving typical forms of sensory stimulation, as they are likely not moving in typical ways either. By taking an empirically-based approach, perhaps developmental outcomes in the pediatric population can be optimized (Ulrich, 2010). For example, in adults research on spinalized animals has had important implications for humans with a spinal cord injury (SCI). Robotic training significantly improves the consistency of locomotor behavior in spinal adult mice, more so than in subjects that were manually trained or given pharmacological treatments without training (Fong et al., 2005). Such findings have led to implementation of similar training devices in physical therapy treatments in adult humans with SCI. The use of robotic training devices in physical therapy permits standardization and optimization of parameters that influence recovery in these individuals (Reinkensmeyer et al., 2006). In some cases, the use of robotic training has been successful in allowing patients with incomplete SCI to recover independent walking and improved gait and stamina (Hornby, Zemon, & Campbell, 2005).
In the developmental literature, infants with myelomeningocele (MMC; a severe form of spina bifida) given stepping practice on a treadmill showed an increase in stepping and motor activity off of the treadmill compared to MMC infants that were supported over a nonmoving treadmill (Teulier et al., 2009). Thus by introducing additional sensorimotor feedback through the moving treadmill belt, which moves the limbs and allows the joints and muscles to gain experience with those movements as well, there was an improvement in motor function in these infants. Similarly, early experience with alternated stepping on a moving treadmill in Down syndrome infants showed that these infants demonstrate walking in response to the treadmill well before they showed independent walking (Ulrich, Ulrich, Collier, & Cole, 1995). Early intervention has recently been shown to be beneficial to children with stroke-induced hemiplegia as well. Yang and colleagues (2013) implemented an early, intensive intervention in a pilot study of children with focal stroke-induced injuries under the age of 2 years, before the critical period for maturation of descending cortical tracts occurs in humans. They demonstrated improvement in motor function in these children, highlighting the importance of taking mechanisms of developmental motor plasticity into consideration.
Finally, what do stepping, LER-expressing newborn rats have to do with all of this? It is our view that research examining the development of coordinated action patterns in animals provides insight into general issues of neurobehavioral development and plasticity. Understanding how sensory feedback shapes reflex circuits and how maternal stimulation influences development of the LER demonstrates general principles of sensorimotor development, such as the flexibility of coordinated action during ontogeny. Although the behavior of human babies and their environment may be more complex than that of immature rats, the information we gain from experiments with perinatal rats (i.e., testing at earlier ages, neural manipulations, treatments, etc.) should allow us to better help infants with motor disabilities.
Acknowledgments
This research was supported by NIH grant Nos. 1R15HD062980-01, P20 RR016454, and P20 GM103408.
References
- Adolph KE, Berger SA. Motor development. In: Damon W, Lerner R, Kuhn D, Siegler RS, editors. Handbook of Child Psychology: Vol. 2: Cognition, Perception, and Language. 6. New York: Wiley; 2006. pp. 161–216. [Google Scholar]
- Adolph KE, Karasik LB, Tamis-LeMonda S. Moving between cultures: cross-cultural research on motor development. In: Bornstein MH, editor. Handbook of Cultural Developmental Science. New York: Taylor & Francis Group; 2010. pp. 61–88. [Google Scholar]
- Adolph KE, Robinson SR. The road to walking: what learning to walk tells us about development. In: Zelazo P, editor. Oxford Handbook of Developmental Psychology. Vol. 1. New York: Oxford University Press; 2013. pp. 403–443. [Google Scholar]
- Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Animal Behavior. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Baccei ML, Fitzgerald M. Development of GABAergic and glycinergic transmission in the neonatal rat dorsal horn. The Journal of Neuroscience. 2004;24:4749–4757. doi: 10.1523/JNEUROSCI.5211-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A, Lau B. Interlimb coordination in 20-day old rat fetuses. Journal of Experimental Zoology. 1980;214:173–175. doi: 10.1002/jez.1402140207. [DOI] [PubMed] [Google Scholar]
- Belnap SC, Allmond J, Boomhower SR, Roberto ME, Brumley MR. Sensorimotor training during expression of the leg extension response (LER) in one-day-old rats. Developmental Psychobiology. doi: 10.1002/dev.21250. in press. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Cazalets J-R. The respective contribution of lumbar segments to the generation of locomotion in the isolated spinal cord of the newborn rat. European Journal of Neuroscience. 2002;16:1741–1750. doi: 10.1046/j.1460-9568.2002.02233.x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH, editor. Handbook of cultural developmental science. New York: Taylor & Francis Group; 2010. [Google Scholar]
- Bredy TW, Grant RJ, Champagne DL, Meaney MJ. Maternal care influences neuronal survival in the hippocampus of the rat. European Journal of Neuroscience. 2003;18:2903–2909. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- Brouette-Lahlou I, Godinot F, Vernet-Maury E. The mother rats’s vomeronasal organ is involved in detection of dodecyl propionate, the pup’s preputial gland pheromone. Physiology & Behavior. 1999;66:427–436. doi: 10.1016/s0031-9384(98)00334-5. [DOI] [PubMed] [Google Scholar]
- Brumley MR, Roberto ME, Strain MM. Sensory feedback modulates quipazine-induced stepping behavior in the newborn rat. Behavioural Brain Research. 2012;229:257–264. doi: 10.1016/j.bbr.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumley MR, Robinson SR. Facial wiping in the rat fetus: variation of chemosensory stimulus parameters. Developmental Psychobiology. 2004;44:219–229. doi: 10.1002/dev.20005. [DOI] [PubMed] [Google Scholar]
- Brumley MR, Robinson SR. The serotonergic agonists quipazine, CGS-12066A, and alpha-methylserotonin alter motor activity and induce hindlimb stepping in the intact and spinal rat fetus. Behavioral Neuroscience. 2005;119:821–833. doi: 10.1037/0735-7044.119.3.821. [DOI] [PubMed] [Google Scholar]
- Brumley MR, Robinson SR. Experience in the perinatal development of action systems. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 181–209. [Google Scholar]
- Brumley MR, Robinson SR. Sensory feedback alters spontaneous limb movements in newborn rats: effects of unilateral forelimb weighting. Developmental Psychobiology. 2013;55:323–333. doi: 10.1002/dev.21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büschges A, Manira AE. Sensory pathways and their modulation in the control of locomotion. Current Opinion in Neurobiology. 1998;8:733–739. doi: 10.1016/s0959-4388(98)80115-3. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. The Journal of Neuroscience. 1995;15:4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. How social experiences influence the brain. Current Opinion in Neurobiology. 2005;15:704–709. doi: 10.1016/j.conb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. The Journal of Neuroscience. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Clarac F, Vinay L, Cazalets JR, Fady JC, Jamon M. Role of gravity in the development of posture and locomotion in the neonatal rat. Brain Research Reviews. 1998;28:35–43. doi: 10.1016/s0165-0173(98)00024-1. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. Journal of Neurophysiology. 1997;77:247–259. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord. II. Evidence for central mediation. Physiology & Behavior. 2002;77:259–267. doi: 10.1016/s0031-9384(02)00859-4. [DOI] [PubMed] [Google Scholar]
- de Boer-van Huizen RT, Ten Donkelaar HJ. Early development of descending supraspinal pathways: A tracing study in fixed and isolated rat embryos. Anatomy and Embryology. 1999;199:539–547. doi: 10.1007/s004290050251. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Plasticity of bladder reflex pathways during postnatal development. Physiology & Behavior. 2002;77:689–692. doi: 10.1016/s0031-9384(02)00919-8. [DOI] [PubMed] [Google Scholar]
- Doherty TS, Strain MM, Tappan D, Brumley MR. Stepping behavior increases on a non-moving, but not a moving, treadmill belt in weight-supported neonatal rats. Developmental Psychobiology. 2013;55:770. Abstract. [Google Scholar]
- Fady JC, Jamon M, Clarac F. Early olfactory-induced rhythmic limb activity in the newborn rat. Developmental Brain Research. 1998;108:111–123. doi: 10.1016/s0165-3806(98)00040-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Jennings E. The postnatal development of spinal sensory processing. Proceedings of the National Academy of Sciences. 1999;96:7719–7722. doi: 10.1073/pnas.96.14.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. The Journal of Neuroscience. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ, Bruno JP, Alberts JR. Physiological and behavioral consequences in rats of water recycling during lactation. Journal of Comparative & Physiological Psychology. 1981;95:26–35. doi: 10.1037/h0077753. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. The roles of experience in the development of behavior and the nervous system. In: Gottlieb G, editor. Neural and Behavioral Specificity: Vol. 3: Studies on the Development of Behavior and the Nervous System. New York: Academic Press; 1976. pp. 25–54. [Google Scholar]
- Geisler HC, Westerga J, Gramsbergen A. Development of posture in the rat. Acta Neurobiologiae Experimentalis. 1993;53:517–523. [PubMed] [Google Scholar]
- Gramsbergen A. Posture and locomotion in the rat: Independent or interdependent development? Neuroscience and Biobehavioral Reviews. 1998;22:547–553. [PubMed] [Google Scholar]
- Grau JW. Learning from the spinal cord: how the study of spinal cord plasticity informs our view of learning. Neurobiology of Learning and Memory. 2014;108:155–171. doi: 10.1016/j.nlm.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubernick DJ, Alberts JR. Maternal licking of young: Resource exchange and proximate controls. Physiology & Behavior. 1983;31:593–601. [PubMed] [Google Scholar]
- Hamburger V. Anatomical and physiological basis of embryonic motility in birds and mammals. In: Gottlieb G, editor. Behavioral Embryology. Vol. 1. New York: Academic Press; 1973. pp. 51–76. [Google Scholar]
- Hopkins B, Westra T. Maternal handling and motor development: an intracultural study. Genetic, Social, and General Psychology Monographs. 1988;114:377–408. [PubMed] [Google Scholar]
- Hornby TG, Zemon DH, Campbell D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Physical Therapy. 2005;114:52–66. [PubMed] [Google Scholar]
- Hopkins B, Westra T. Maternal expectations of their infants’ development: some cultural differences. Developmental Medicine and Child Neurology. 1989;31:384–390. doi: 10.1111/j.1469-8749.1989.tb04008.x. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. The Journal of Physiology. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamon M, Clarac F. Early walking in the neonatal rat: A kinematic study. Behavioral Neuroscience. 1998;112(5):1218–1228. doi: 10.1037//0735-7044.112.5.1218. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. The Journal of Neuroscience. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven GA, Lane MS, Robinson SR. Development of interlimb movement synchrony in the rat fetus. Behavioral Neuroscience. 2004;118:835–44. doi: 10.1037/0735-7044.118.4.835. [DOI] [PubMed] [Google Scholar]
- Kudo N, Nishimaru H, Nakayama K. Developmental changes in rhythmic spinal neuronal activity in the rat fetus. Progress in Brain Research. 2004;143:49–55. doi: 10.1016/s0079-6123(03)43005-7. [DOI] [PubMed] [Google Scholar]
- Lakke EAJF. The projections to the spinal cord of the rat during development; a time-table of descent. In: Beck F, Christ B, et al., editors. Advances in Anatomy Embryology and Cell Biology. Vol. 135. Berlin, Germany: Springer-Verlag; 1997. [DOI] [PubMed] [Google Scholar]
- Lam T, Wolstenholme C, van der Linden M, Pang MY, Yang JF. Stumbling corrective responses during treadmill-elicited stepping in human infants. Journal of Physiology. 2003;553:319–331. doi: 10.1113/jphysiol.2003.043984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T, Wolstenholme C, Yang JF. How do infants adapt to loading of the limb during the swing phase of stepping? Journal of Neurophysiology. 2003;89:1920–1928. doi: 10.1152/jn.01030.2002. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Sengelaub DR. Maternal licking influences dendritic development of motoneurons in a sexually dimorphic neuromuscular system. Brain Research. 2006;1092:87–99. doi: 10.1016/j.brainres.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Sengelaub DR. Maternal care effects in SNB motoneuron development: the mediating role of sensory afferent distribution and activity. Developmental Neurobiology. 2009;69:603–615. doi: 10.1002/dneu.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinsson A, Luo X-L, Holmberg H, Schouenborg J. Developmental tuning in a spinal nociceptive system: effects of neonatal spinalization. The Journal of Neuroscience. 1999;19:10397–10403. doi: 10.1523/JNEUROSCI.19-23-10397.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Peripheral olfactory deafferentation of the primary olfactory system in rats using ZnSO4 nasal spray with special reference to maternal behavior. Physiology & Behavior. 1993;53:587–592. doi: 10.1016/0031-9384(93)90157-b. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Van Hartesveldt C, Stehouwer DJ. L-DOPA and quipazine elicit air-stepping in neonatal rats with spinal cord transections. Behavioral Neuroscience. 1997;111(4):825–833. doi: 10.1037//0735-7044.111.4.825. [DOI] [PubMed] [Google Scholar]
- Mendez-Gallardo V, Bean RC, Brumley MR. Persistent effects of a proprioceptive perturbation on expression of a reflex action pattern in neonatal rats. Developmental Psychobiology. 2013;55:780. Abstract. [Google Scholar]
- Moore CL. Sex differences in urinary odors produced by young laboratory rats (Rattus norvegicus) Journal of Comparative Psychology. 1985;99:336–341. [PubMed] [Google Scholar]
- Moore CL. The role of maternal stimulation in the development of sexual behavior and its neural basis. Annals of the New York Academy of Sciences. 1992;662:160–177. doi: 10.1111/j.1749-6632.1992.tb22859.x. [DOI] [PubMed] [Google Scholar]
- Moore CL, Chadwick-Dias A. Behavioral responses of infant rats to maternal licking: Variations with age and sex. Developmental Psychobiology. 1986;19:427–438. doi: 10.1002/dev.420190504. [DOI] [PubMed] [Google Scholar]
- Moore CL, Dou H, Juraska JM. Maternal stimulation affects the number of motor neurons in a sexually dimorphic nucleus of the lumbar spinal cord. Brain Research. 1992;572:52–56. doi: 10.1016/0006-8993(92)90449-j. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nishimaru H, Kudo N. Developmental changes in 5-hydroxytryptamine-induced rhythmic activity in the spinal cord of rat fetuses in vitro. Neuroscience Letters. 2001;307:1–4. doi: 10.1016/s0304-3940(01)01913-9. [DOI] [PubMed] [Google Scholar]
- Narayanan CH, Fox MW, Hamburger V. Prenatal development of spontaneous and evoked activity in the rat (rattus norvegigus albinus) Behaviour. 1971;40(1):100–133. doi: 10.1163/156853971x00357. [DOI] [PubMed] [Google Scholar]
- Narayanan CH, Malloy RB. Deafferentation studies on motor activity in the chick. I. Activity pattern of hindlimbs. Journal of Experimental Zoology. 1974;189:163–176. doi: 10.1002/jez.1401890204. [DOI] [PubMed] [Google Scholar]
- Norreel JC, Pflieger JF, Pearlstein E, Simeoni-Alias J, Clarac F, Vinay L. Reversible disorganization of the locomotor pattern after neonatal spinal cord transection in the rat. The Journal of Neuroscience. 2003;23:1924–1932. doi: 10.1523/JNEUROSCI.23-05-01924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang MY, Lam T, Yang JF. Infants adapt their stepping to repeated trip-inducing stimuli. Journal of Neurophysiology. 2003;90:2731–2740. doi: 10.1152/jn.00407.2003. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Plasticity of neuronal networks in the spinal cord: modifications in response to altered sensory input. Progress in Brain Research. 2000;128:61–70. doi: 10.1016/S0079-6123(00)28007-2. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Role of sensory feedback in the control of stance duration in walking cats. Brain Research Review. 2008;57:222–227. doi: 10.1016/j.brainresrev.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Petersson P, Waldenström A, Fåhreus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424:72–75. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- Provine RR. Ontogeny of bioelectric activity in the spinal cord of the chick embryo and its behavioral implications. Brain Research. 1972;41:365–378. doi: 10.1016/0006-8993(72)90508-2. [DOI] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Aoyagi D, Emken JL, Galvez JA, Ichinose W, Kerdanyan G, Edgerton VR. Tools for understanding and optimizing robotic gait training. Journal of Rehabilitation Research and Development. 2006;43:657–670. doi: 10.1682/jrrd.2005.04.0073. [DOI] [PubMed] [Google Scholar]
- Richmond G, Sachs BD. Grooming in norway rats: the development and adult expression of a complex motor pattern. Behaviour. 1980;75:82–96. [Google Scholar]
- Roberto ME, Brumley MR. Prematurely delivered rats how improved motor coordination during sensory-evoked motor responses compared to age-matched controls. Physiology & Behavior. 2014;130:75–84. doi: 10.1016/j.physbeh.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SS, Smotherman WP. The neural control of cyclic motor activity in the fetal rat (rattus nrovegicus) Physiology & Behavior. 1990;47:121–126. doi: 10.1016/0031-9384(90)90049-a. [DOI] [PubMed] [Google Scholar]
- Robinson SR. Conjugate limb coordination after experience with an interlimb yoke: Evidence for motor learning in the rat fetus. Developmental Psychobiology. 2005;47:328–344. doi: 10.1002/dev.20103. [DOI] [PubMed] [Google Scholar]
- Robinson SR. Spinal mediation of motor learning and memory in the rat fetus. Developmental Psychobiology. doi: 10.1002/dev.21277. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SR, Blumberg MS, Lane MS, Kreber LA. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behavioral Neuroscience. 2000;114:328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Brumley MR. Prenatal behavior. In: Whishaw IQ, Kolb B, editors. The Behavior of the laboratory rat: A handbook with tests. New York: Oxford University Press; 2005. pp. 257–265. [Google Scholar]
- Robinson SR, Hoeltzel TC, Cooke KM, Umphress SM, Smotherman WP, Murrish DE. Oral capture and grasping of an artificial nipple by rat fetuses. Developmental Psychobiology. 1992;25:543–555. doi: 10.1002/dev.420250802. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Kleven GA. Learning to move before birth. In: Hopkins B, Johnson SP, editors. Prenatal Development of Postnatal Functions (Advances in Infancy Research series) Westport, CT: Praeger Publishers; 2005. pp. 131–175. [Google Scholar]
- Robinson SR, Kleven GA, Brumley MR. Prenatal development of interlimb motor learning in the rat fetus. Infancy. 2008;13:204–228. doi: 10.1080/15250000802004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Environmental determinants of behaviour in the rat fetus. II. The emergence of synchronous movement. Animal Behaviour. 1987;35:1652–1662. [Google Scholar]
- Robinson SR, Smotherman WP. The amniotic sac as scaffolding: prenatal ontogeny of an action pattern. Developmental Psychobiology. 1991;24:463–485. doi: 10.1002/dev.420240703. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Fundamental motor patterns of the mammalian fetus. Journal of Neurobiology. 1992a;23:1574–1600. doi: 10.1002/neu.480231013. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Smotherman WP. Organization of the stretch response to milk in the rat fetus. Developmental Psychobiology. 1992b;25:33–49. doi: 10.1002/dev.420250104. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Bouyer L, Langlet C, Barthelemy D, Chau C, Giroux N, Reader TA. Determinants of locomotor recovery after spinal injury in the cat. Progress in Brain Research. 2004;143:163–172. doi: 10.1016/S0079-6123(03)43016-1. [DOI] [PubMed] [Google Scholar]
- Schouenborg J. Modular organization and spinal somatosensory imprinting. Brain Research Reviews. 2002;40:80–91. doi: 10.1016/s0165-0173(02)00191-1. [DOI] [PubMed] [Google Scholar]
- Schouenborg J. Role of spontaneous movements in imprinting an action-based body representation in the spinal cord. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. New York: Oxford University Press; 2010. pp. 254–261. [Google Scholar]
- Sharp A. Sensory regulation of spontaneous limb movements in the embryonic chick. Developmental Psycholobiology. doi: 10.1002/dev.21292. this issue. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Fetal expression of the leg extension response to anogenital stimulation. Physiology & Behavior. 1988;43:243–244. doi: 10.1016/0031-9384(88)90246-6. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Cryptopsychobiology: the appearance, disappearance, and reappearance of a species-typical action pattern during early development. Behavioral Neuroscience. 1989;103:246–253. doi: 10.1037//0735-7044.103.2.246. [DOI] [PubMed] [Google Scholar]
- Stelzner DJ, Weber ED, BryzGornia WF. Sparing of function in developing spinal cord: Anatomical substrate. In: Goldstein ME, Gorio A, Murray M, editors. Development and Plasticity of the Mammalian Spinal Cord. Vol. 3. Padova: Liviana Press; 1986. pp. 81–99. [Google Scholar]
- Strain MM, Brumley MR. Range of motion (ROM) restriction influences quipazine-induced stepping behavior in postnatal day one and day ten rats. Behavioural Brain Research. 2014;274:365–381. doi: 10.1016/j.bbr.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain MM, Kauer SD, Kao T, Brumley MR. Inter- and intralimb adaptations to a sensory perturbation during activation of the serotonin system after a low spinal cord transection in neonatal rats. Frontiers in Neural Circuits. 2014;8:1–13. doi: 10.3389/fncir.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teulier C, Smith BA, Kubo M, Chang CL, Moerchen V, Murazko K, Ulrich BD. Stepping responses of infants with myelomeningocele when supported on a motorized treadmill. Physical Therapy. 2009;89:60–72. doi: 10.2522/ptj.20080120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E, Ulrich BD, Niles D. Bilateral coordination in human infants: stepping on a split-belt treadmill. Journal of Experimental Psychology Human Perception and Performance. 1987;13:405–140. doi: 10.1037//0096-1523.13.3.405. [DOI] [PubMed] [Google Scholar]
- Tillakaratne NJK, Guu JJ, De Leon RD, Bigbee AJ, London NJ, Zhong H, Edgerton VR. Functional recovery of stepping in rats after a complete neonatal spinal cord transection is not due to regrowth across the lesion site. Neuroscience. 2010;166:23–33. doi: 10.1016/j.neuroscience.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich BD. Opportunities for early intervention based on theory, basic neuroscience, and clinical science. Physical Therapy. 2010;90:1868–1880. doi: 10.2522/ptj.20100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich BD, Ulrich DA, Collier DH, Cole EL. Developmental shifts in the ability of infants with Down syndrome to produce treadmill steps. Physical Therapy. 1995;75:14–23. doi: 10.1093/ptj/75.1.14. [DOI] [PubMed] [Google Scholar]
- Viala D, Viala G, Fayein N. Plasticity of locomotor organization in infant rabbits spinalized shortly after birth. In: Goldberger ME, Gorio A, Murray M, editors. Development and Plasticity of the Mammalian Spinal Cord. Padova, Italy: Liviana Press; 1986. pp. 301–10. [Google Scholar]
- Vinay L, Brocard F, Clarac F, Norreel JC, Pearlstein E, Pflieger JF. Development of posture and locomotion: An interplay of endogenously generated activities and neurotrophic action by descending pathways. Brain Research Reviews. 2002;40:118–129. doi: 10.1016/s0165-0173(02)00195-9. [DOI] [PubMed] [Google Scholar]
- Waldenström A, Thelin J, Thimansson E, Levinsson A, Schouenborg J. Developmental learning in a pain-related system: evidence for a cross-modality mechanism. The Journal of Neuroscience. 2003;23:7719–7725. doi: 10.1523/JNEUROSCI.23-20-07719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber ED, Stelzner DJ. Behavioral effects of spinal cord transection in the developing rat. Brain Research. 1977;125:241–255. doi: 10.1016/0006-8993(77)90618-7. [DOI] [PubMed] [Google Scholar]
- Weber ED, Stelzner DJ. Synaptogenesis in the intermediate gray region of the lumbar spinal cord in the postnatal rat. Brain Research. 1980;185(1):17–37. doi: 10.1016/0006-8993(80)90667-8. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. Journal of Neurophysiology. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. The education and re-education of the spinal cord. In: Miller AR, editor. Progression in Brain Research. Vol. 157. New York: Elsevier; 2006. pp. 261–280. [DOI] [PubMed] [Google Scholar]
- Yang JF, Livingstone D, Brunton K, Kim D, Lopetinsky B, Roy F, Gorassini M. Training to enhance walking in children with cerebral palsy: are we missing the window of opportunity? Seminars in Pediatric Neurology. 2013;20:106–115. doi: 10.1016/j.spen.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Zelazo PR, Zelazo NA, Kolb S. “Walking” in the newborn. Science. 1972;176:314–315. doi: 10.1126/science.176.4032.314. [DOI] [PubMed] [Google Scholar]