Abstract
Background
Although uncommon, esthesioneuroblastomas may produce clinically significant amounts of catecholamines.
Methods
We report a patient with a catecholamine-secreting esthesioneuroblastoma who developed intraoperative hypertensive crisis.
Results
A patient with history of hypertension was referred to our skull base center for management of a residual esthesioneuroblastoma. A staged endonasal endoscopic approach was planned. At the conclusion of the first stage, a hypertensive crisis occurred. Work-up revealed elevated levels of serum and urinary catecholamines. The patient was treated with alpha adrenoceptor blockade prior to the second stage. Serum catecholamine levels following this second stage were normal. On immunohistochemical analysis, the tumor cells were found to be positive for tyrosine hydroxylase, the rate limiting enzyme in cathecholamine synthesis, and achaete-scute homologue 1, a transcription factor essential in the development of olfactory and sympathetic neurons.
Conclusion
Catecholamine production should be considered in the differential of unexpected extreme hypertension during surgical resection of esthesioneuroblastoma.
Keywords: catecholamine, expanded endonasal endoscopic approach, esthesioneuroblastoma, hypertensive crisis, tyrosine hydroxylase
Introduction
Esthesioneuroblastoma or olfactory neuroblastoma is a rare malignant tumor that occurs in the upper nasal cavity. It was first described by Berger and Luc (1) in 1924 and by 1994, 945 cases had been reported (2). The most common presenting symptoms include epistaxis, nasal congestion, and hyposmia (2–5). These tumors have variable growth, but the average time between the appearance of symptoms and diagnosis is 6 months (3). Although rare, esthesioneuroblastomas can produce hormones including catecholamines (6–10). In this report, we describe a patient with an esthesioneuroblastoma who developed an intraoperative hypertensive crisis and was found to have a catecholamine-secreting tumor.
Case Report
History & Examination
This 56-year-old man was referred to our institution for evaluation and management of a residual esthesioneuroblastoma. His past medical history was significant for hypertension diagnosed at age 51. His preoperative medications included olmesartan and rosuvastatin.
During the previous year, he had developed several episodes of sinusitis and subsequently noted a left nasal mass. The patient presented to a local emergency room where a head CT scan and brain MRI scan demonstrated a large mass involving the left nasal cavity, paranasal sinuses, and skull base. This mass measured 7.4 cm × 2.4 cm × 4.3 cm in the anterior-posterior (AP), transverse, and craniocaudal (CC) dimensions, respectively. He was taken to the operating room for debulking of the mass and placement of a myringotomy tube for Eustachian tube dysfunction. Histopathological analysis of the lesion revealed an esthesioneuroblastoma, with a Hyams' grade of 3 out of 4. Following this diagnosis, the patient was referred to our skull base center.
A high resolution maxillofacial head CT scan revealed a soft tissue mass in the posterior aspect of the left nasal cavity. There was diffuse polypoid mucoperiosteal thickening of the left maxillary sinus with near complete opacification of the left frontal, ethmoid, and sphenoid sinuses. There was mild expansion of the left ethmoid air cells with erosion of the ethmoidal septae. There was also evidence of previous sinus surgery with a left maxillary antrostomy and uncinectomy. The right paranasal sinuses were clear with no evidence of soft tissue mass, mucosal disease, or fluid levels. A high resolution dedicated skull base protocol MRI scan with intravenous contrast was performed through the skull base including the paranasal sinuses and nasal cavity. There was previous paranasal sinus surgery noted with a left maxillary antrostomy, uncinectomy and partial left middle turbinectomy. Within the posterior nasal cavity, there was a 3.4 × 1.7 × 2.1 cm (AP × transverse × CC) enhancing soft tissue mass which protruded through the left choana into the left nasopharynx (Figure 1). There was slight extension of the mass into the anterior aspect of the left sphenoid sinus with post-obstructive changes in the left sphenoid sinus. Within the left anterior ethmoid air cells extending along the margin of the medial orbital wall and to the ethmoidal roof, there was a separate 2.4 × 1.6 × 2.0 cm (AP × transverse × CC) enhancing mass (Figure 1). Enhancement extended to the cribriform plate with a small nodule of tissue protruding through the cribriform plate. There was also prominent dural enhancement along the left anterior cranial fossa floor. There was no abnormal enhancement extending into the brain parenchyma. Extensive post-obstructive secretions were noted in the left anterior ethmoid cells and left frontal sinus as well as moderate mucoperiosteal thickening in the left maxillary sinus. Minimal inflammatory changes in the right ethmoid cells were noted. A PET/CT scan demonstrated intense FDG uptake in the left nasal cavity and ethmoid sinuses. Intense activity was also seen in the left parapharyngeal space just posterior to the left pterygoid muscle which corresponded to a 0.8 cm parapharyngeal lymph node seen on the corresponding IV contrasted CT scan.
Figure 1.
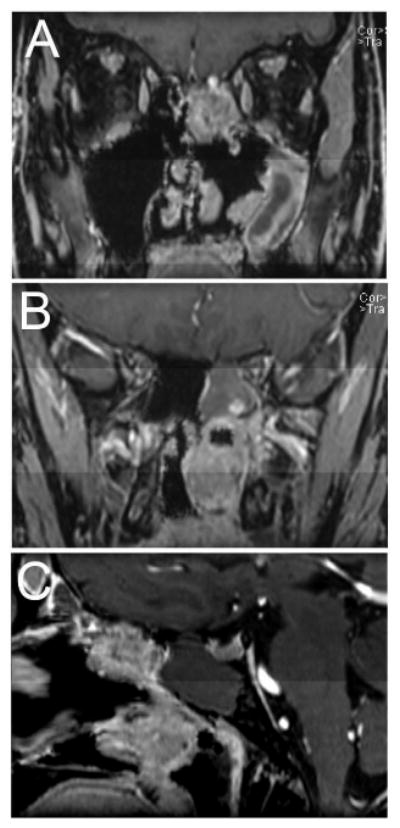
(A and B) Coronal and (C) sagittal preoperative post-contrast T1 weighted fat saturated MRI images of the patient prior to the first stage of surgery demonstrating two tumor masses, one involving the skull base (A and C) and the second in the posterior nasal cavity (B and C).
Various treatment options were discussed with the patient and his family and a staged expanded endonasal endoscopic approach for a cranionasal resection was planned. The first stage included an extended left neck dissection for resection of the FDG avid lymph node and an expanded endonasal endoscopic approach for resection of the nasal and paranasal tumor; the second stage included a formal cribriform drop-out and skull base reconstruction.
Anesthetic, first surgical stage, and post-operative management
Anesthesia was induced for the first stage using propofol, fentanyl, and succinylcholine. The trachea was then intubated. Anesthesia was maintained with isoflurane and continuous infusion of fentanyl and vecuronium. Continuous blood pressure monitoring was achieved with a radial artery catheter.
Surgery began with a left sided selective neck dissection, addressing levels II and III, and a trans-cervical left-sided retropharyngeal dissection. There were small, normal looking nodes in levels II and III. In the retropharynx, however, there was a single small firm node which was positive for esthesioneuroblastoma on frozen histopathological analysis. After the neck dissection, the patient's head was placed in a Mayfield clamp and positioned for an expanded endonasal endoscopic approach to the anterior cranial fossa. The patient's head was then registered to his high resolution preoperative CT and MRI scans. The patient's nose was irrigated with clindamycin solution and his face and abdomen were prepped with betadine. Neuroelectrophysiological monitoring with somatosensory evoked potentials and electroencephalography were utilized during the surgical procedure. Intranasal injections were carried out along the septum and sphenopalatine fossae with 1% lidocaine and 1:100,000 epinephrine and cocaine soaked pledgets were placed for decongestion. These vasoactive agents were used only at the beginning of the endonasal approach. The surgery proceeded in an uncomplicated fashion as described by Gallia et al. (11), until the tumor was pedicled from the horizontal plate of the cribriform and negative circumferential mucosal margins had been achieved. The course of anesthesia was uneventful throughout the majority of surgery, with smooth hemodynamics and systolic blood pressures ranging from 100-120 mmHg. Shortly after amputation of the tumor from the external aspect of the cribriform plate, however, the patient developed severe hypertension with systolic pressures over 240 mmHg. The depth of anesthesia had not changed. This hypertension was refractory to boluses of anesthetic and was only responsive to multiple boluses of sodium nitroprusside. During this time, there were modest heart rate fluctuations with brief periods of bradycardia that did not persist. There were no changes in the intra-operative neuroelectrophysiological monitoring. This was at the end of the planned first stage and several pieces of Gelfoam were placed over the skull base and a piece of Duragen was then placed on top of this layer. The patient was kept intubated. Given concern for possible intracranial hypertension, the patient was taken from the operating room to the CT scanner for a head CT scan. This study did not reveal evidence of intracerebral hemorrhage, hydrocephalus, or other acute intracranial processes. In the intensive care unit, the patient's hypertension persisted and required aggressive management with IV medications for 48 hours postoperatively. The patient was allowed to wake up from anesthesia and was extubated without difficulty. His neurological exam remained nonfocal. Cardiac evaluation and a brain MRI did not reveal any evidence of myocardial event or stroke, respectively.
Given the neuroendocrine features of esthesioneuroblastoma, elevated catecholamines were considered in the differential diagnosis and serum catecholamine and metabolite levels were indeed found to be elevated (Table 1). In addition, 24 hour urine catecholamines were elevated with a total catecholamine level of 170 mcg/24 hours (normal range: 26-121 mcg/24 hours). Twenty four hour urine vanillyl mandelic acid (VMA) was also elevated at 8.4 mg/24 hours (normal range: <6.0 mg/24 hours); total 24 hour urine metanephrines were within normal limits. Due to these findings, the second stage of surgery, which was originally scheduled 2 days after the first procedure, was postponed and the patient was treated and discharged on the alpha-adrenoceptor blocker phenoxybenzamine. Due to orthostatic hypotension he was then transitioned to the alpha/beta blocker labetalol.
Table 1.
Serum levels of catecholamines (dopamine, norepinephrine, and epinephrine) and metabolites (normetanephrine and metanephrine) after the first and second surgical stages.
| Reference Range (pg/ml) |
After 1st stage |
After 2nd stage* |
After 2nd stage* |
|
|---|---|---|---|---|
| Dopamine | <30 | 70 | <30 | <30 |
| Norepinephrine | 112-658 | 1131 | 283 | 223 |
| Epinephrine | <50 | 256 | <20 | <20 |
| Metanephrine | <57 | 32 | 56 | 28 |
| Normetanephrine | <148 | 215 | 57 | 52 |
From two independent lab draws.
Second surgical stage
The second stage was performed four weeks later. Anesthesia for the second stage was induced as described above for the first stage. His systolic blood pressure was well controlled in the 95-110 mmHg range for the majority of the case. The residual tumor, cribriform plate, and dura were then removed in an en-bloc fashion as described previously (11). Negative intra-operative margins, including dural margins, were obtained. The skull base was reconstructed using a multilayered closure. The patient was transported to the neuroscience ICU in stable condition. There was no postoperative hypertension and the patient had an uneventful postoperative course. Serum catecholamine levels following this second stage were within normal limits (Table 1). The patient subsequently was treated with radiotherapy and four cycles of cisplatin (60 mg/m2) and etoposide (120 mg/m2) on days 1, 2, and 3, repeated every three weeks with two cycles given concurrent with adjuvant radiotherapy and two cycles following radiotherapy. The patient is now 36 months out from surgery and has no evidence of disease (Figure 2).
Figure 2.
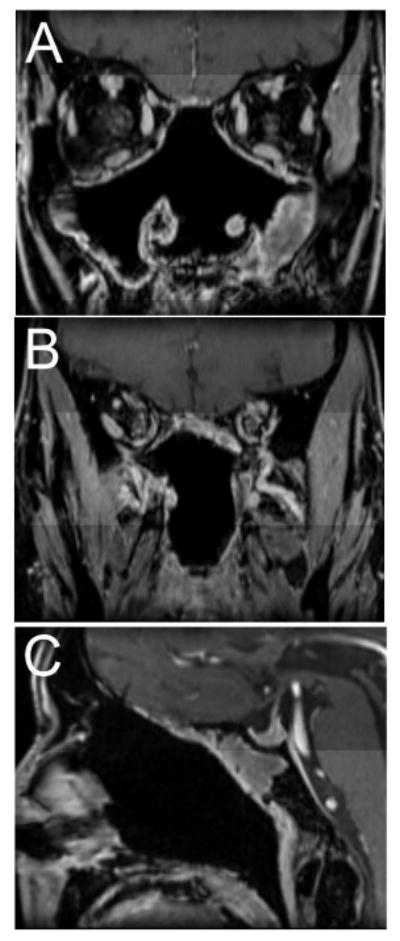
(A and B) Coronal and (C) sagittal post-operative post-contrast T1 weighted fat saturated MRI images obtained 30 months after surgery demonstrating no evidence of recurrent tumor with post-operative changes and minimal inflammatory change in the left maxillary and sphenoid sinuses.
Histopathological analysis and immuno-histochemical findings
Lesional tissue was obtained from both the first and second stages of surgery and demonstrated an infiltrating highly cellular tumor with a sheet-like growth pattern involving the submucosa. There were no rosettes and only minimal fibrillary matrix. The cells had scant cytoplasm and indistinct cell borders. The nuclei were round to oval and had a “salt and pepper” chromatin pattern. The tumor showed prominent mitotic activity and focal necrosis. The tumor cells were immunopositive for chromogranin, synaptophysin, and neurofilament. Perilobular sustentacular cells were immunoreactive with antibodies to S100. The histomorphology and immunohistochemical profile supported the diagnosis of esthesioneuroblatoma, Hyams' grade 3. Of twenty-one lymph nodes evaluated from the neck dissection, only the single firm retropharyngeal lymph node was positive for esthesioneuroblastoma.
Immunohistochemical (IHC) analysis was performed to evaluate the tumor for tyrosine hydroxylase (TH), the rate limiting enzyme in catecholamine synthesis which catalyzes the conversion of tyrosine to 3,4-dihydrophenylalanine (DOPA) . After deparaffinization and hydration of the slides in serial dilutions of ethanol, antigen retrieval was performed with citrate buffer (Biogenex, Fremont, CA), followed by peroxidase and serum blocking. Mouse anti-TH antibody (Clone TH-16, dilution 1:300, Sigma-Aldrich, St. Louis, MO), used as the primary antibody, was incubated overnight in a humid cold chamber. Following washing, horseradish peroxidase–conjugated anti–mouse polymer (EnVision+ System-HRP (DAB), Dako, Carpinteria, CA) was added for detection of antibody binding. Immunostaining was visualized using the chromogen 3′,3′ diaminobenzidine (DAB) (Dako). Control slides did not contain primary antibody. The tumor showed weak cytoplasmic positivity for TH (Figure 3).
Figure 3.
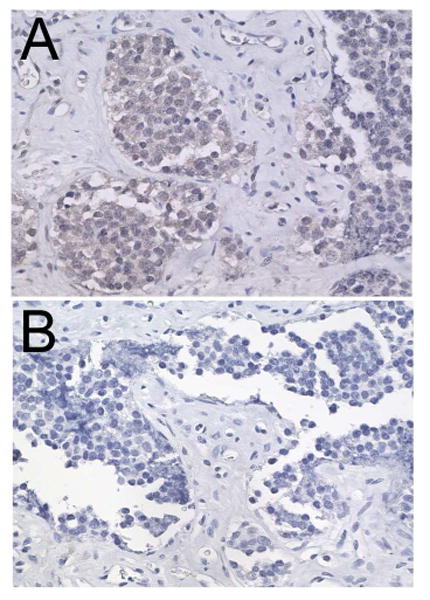
Photomicrograph of immunohistoperoxidase staining for tyrosine hydroxylase showing faint immunoreactivity in the cytoplasm (A) compared to a control section lacking primary antibody (B).
Given the neuroendocrine features of esthesioneuroblastoma, paraffin embedded tissue was also examined by IHC for expression of the human achaete-scute homologue 1 (ASCL1, hASH1), a basic helix-loop-helix transcription factor which is essential for neural differentiation (12, 13). Small cell lung xenograft tumors were used as a positive control tissue. Using SAGE genie (http://cgap.nci.nih.gov/SAGE), medulloblastoma was identified to have undetectable ASCL1 transcript levels and was used as a negative control. Slides were deparafinized in trilogy solution (Cell Marque, Rocklin, CA) followed by incubation with a 3% H2O2 solution to block endogenous peroxide. hASH1 expression was assessed using a monoclonal antibody (Clone 2472D11.1, dilution 1:25; BD, Franklin Lakes, NJ) and slides were incubated overnight in a humid cold chamber. Following washing, a biotin conjugated secondary antibody (115-065-062, dilution 1:50, Jackson ImmunoResearch, West Grove, PA) was added, allowed to incubate for one hour, and then washed twice with PBS. Sections were incubated with ABC reagent (Vector Labs, Burlingame, CA) for thirty minutes. DAB (Vector Labs) was then added for visualization. The esthesioneuroblastoma tumor cells showed strong diffuse staining for ASCL1 (Figure 4A and B). Tumor cells from the small cell lung cancer xenograft tumor were positive (Figure 4C). There was no staining in the medulloblastoma specimen (Figure 4D).
Figure 4.
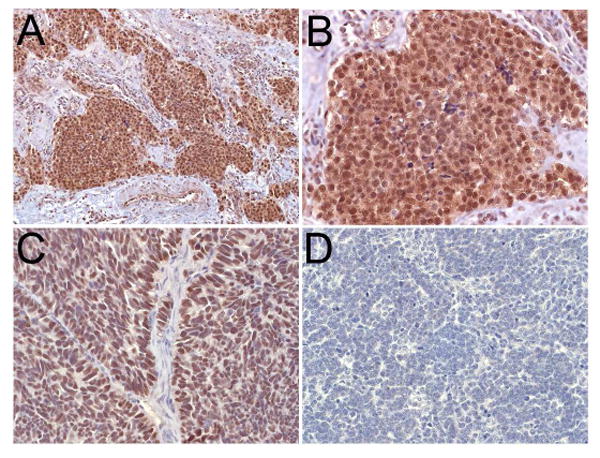
Photomicrograph of immunohistoperoxidase staining for ASCL1 showing strong staining in the tumor cells (A and B). A section from a small cell carcinoma xenograft, used as a positive control, demonstrates strong staining (C). There is no staining seen in a medulloblastoma specimen (D).
Discussion
A hypertensive crisis is defined as a systolic BP greater than 179 mm Hg or a diastolic BP greater than 109 mm Hg (14). Hypertensive crises are further categorized into hypertensive emergencies and hypertensive urgencies. In hypertensive emergencies, there is evidence of acute end-organ injury whereas in hypertensive urgencies, there is no end-organ involvement. In this paper, we describe a patient with an esthesioneuroblastoma with neck positive disease (Kadish stage D) who developed an intra-operative hypertensive crisis, specifically hypertensive urgency (14). The hypertensive episode happened at the end of the first surgical procedure following manipulation of the mass and amputation of the tumor from the external aspect of the cribriform plate. The timing of this event is consistent with other reports in literature which indicate hypertensive episodes are most commonly observed during manipulation of the catecholamine secreting mass (15, 16). Postoperatively, the patient was found to have elevated serum and urine catecholamine levels. The patient's serum catecholamine levels were normal following his second stage of surgery wherein his entire tumor was excised.
There are previous reports describing secreting esthesioneuroblastomas, but few reports describing catecholamine producing esthesioneuroblastomas (6–10). Other studies have reported secretion of adrenocorticotropic hormone (ACTH) from esthesioneuroblastomas (17–24). Syndrome of inappropriate antidiuretic hormone (SIADH) has also been reported in patients with esthesioneuroblastoma (9, 25–36).
With respect to catecholamine-secreting esthesioneuroblastoma, there is, to the best of our knowledge, only a single report describing a clinically significant case (9). In that study, Nabili et al. (9) described a patient whose original surgical resection of a right sided intranasal mass was aborted due to severe intraoperative hypertension. A biopsy was performed which demonstrated an esthesioneuroblastoma. Additional evaluation of the severe hypertension demonstrated elevated 24 hour urinary catecholamine levels and mild uptake in the right nasal region of iobenguane 131I. The patient was treated with phenoxybenzamine and phentolamine and then underwent an uneventful surgical resection of the tumor. The patient's urine catecholamine levels decreased after surgery and normalized following postoperative radiation therapy. This patient also had an elevated serum arginine vasopressin hormone level.
There are several additional studies reporting the catecholamine producing ability of esthesioneuroblastoma. Castaneda et al. (6) reported a case of elevated urine catecholamines, VMA and HVA, in a 16 year old boy with an esthesioneuroblastoma at recurrence. Interestingly, the urinary VMA and HVA were normal at initial workup. Takahashi et al. (10) reported immunopositivity for TH in an esthesioneuroblastoma tumor resected from a 16 year old patient. Prior to this, Micheau et al. (7, 8) evaluated 6 cases for dopamine beta hydroxylase (DBH), the enzyme that converts dopamine to norepinephrine. In these studies, DBH activity was demonstrated in all 6 of cases using an immunohistochemical method. Epinephrine and norepinephrine were also found to be strongly positive in 2 cases and faintly positive in the others. Interestingly, using a biochemical assay, DBH was evaluated in 3 tumors and found to be low. Also, DBH activity in the blood of 3 patients was not elevated and urinary excretion of catecholamines was found to be within normal limits in the 3 cases examined. Other authors were unable to find detectable catecholamines in a tumor sample (37) and urinary VMA was reported as normal in another case of esthesioneuroblastoma (38). There are also numerous studies describing neuro-secretory granules (10, 31, 37), which could possibly contain catecholamines among many other agents; however, these studies do not establish the identity of the granules.
Several medications have been reported to falsely elevate catecholamine levels including beta blockers (39). In our case, the only preoperative medications were olmesartan and rosuvastatin, neither of which is associated with falsely elevated catecholamines. The patient did receive some labetolol intraoperatively and some boluses in the ICU. We do not feel, however, that this is the cause of his elevated catecholamine levels. Following his first surgery and the detection of the elevated catecholamines, he was pretreated with phenoxybenzamine. Due to orthostatic hypotension, however, this was changed to labetolol. He also received boluses of labetolol following his second surgery. Thus, he had received labetolol prior to his second surgery and received postoperative labetolol as needed following both surgical procedures, but his catecholamine levels were elevated only after the first surgery. This finding, coupled with the staining of the tumor for tyrosine hydroxylase, supports the fact that this was a clinically significant catecholamine secreting esthesioneuroblastoma.
The histopathogenesis of esthesioneuroblastoma has been and remains a subject of debate. There have been numerous proposed sites of origin including the sympathetic fibers of anterior nasal cavity, the neuro-ectodermal cells of the spheno-palatine ganglion, the organ of Jacobson (vomeronasal organ), olfactory placode, and olfactory neuroepithelium (2, 4, 7, 37, 38, 40-43). It has also been hypothesized that these tumors are similar to primitive neuro-ectodermal tumors (PNETs) because they share some genetic similarities with Ewing sarcomas (44). Further studies, however, showed that these tumors are genetically distinct from PNETs (45, 46). Other studies suggested these tumors originated from the amine precursor uptake and decarboxylation (APUD) system (47) and another hypothesis is that these tumors are of neuronal origin (41, 48). Currently, esthesioneuroblastomas are thought to arise from the olfactory neuroepithelium (41, 49). This is based on the location of these tumors and the expression of the neural developmental transcription factor ASCL1/hASH1 (50, 51), which is also expressed in some other tumors with neuroendocrine phenotype like small cell lung cancer and medullary thyroid cancer (51–53). ASCL1 is essential for early development of neuronal progenitors in the olfactory epithelium and autonomic nervous system (12). A few studies have investigated hASH1 in esthesioneuroblastoma (50, 51, 54). In two of these studies, hASH1 transcripts were detected in 7 and 4 esthesioneuroblastoma tumors (50, 51); in a third study, expression was seen by immunofluorescense staining (54).
Conclusion
This case highlights a patient with a catecholamine-secreting esthesioneuroblastoma that was found during evaluation of an intraoperative hypertensive crisis. The patient's catecholamine levels returned to normal following a gross total resection of the tumor. The possibility of a catecholamine-secreting tumor should be considered in patients with esthesioneuroblastoma; however, routine screening for this complication does not seem to be warranted considering its rarity
Footnotes
Portions of this work were presented at the 22nd North American Skull Base Society Annual Meeting in Las Vegas, Nevada, February 2012
References
- 1.Berger, Luc Richard. L'esthesioneuroepitheliome Olfactif. Bulletin de l'Association française pour l'étude du cancer. 1924;13:410–420. [Google Scholar]
- 2.Broich G, Pagliari A, Ottaviani F. Esthesioneuroblastoma: a general review of the cases published since the discovery of the tumour in 1924. Anticancer Res. 1997;17(4A):2683–706. [PubMed] [Google Scholar]
- 3.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970-1990. Laryngoscope. 1992;102(8):843–9. doi: 10.1288/00005537-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Oskouian RJ, Jr, Jane JA, Sr, Dumont AS, Sheehan JM, Laurent JJ, Levine PA. Esthesioneuroblastoma: clinical presentation, radiological, and pathological features, treatment, review of the literature, and the University of Virginia experience. Neurosurg Focus. 2002;12(5):e4. doi: 10.3171/foc.2002.12.5.5. [DOI] [PubMed] [Google Scholar]
- 5.Resto VA, Eisele DW, Forastiere A, Zahurak M, Lee DJ, Westra WH. Esthesioneuroblastoma: the Johns Hopkins experience. Head Neck. 2000;22(6):550–8. doi: 10.1002/1097-0347(200009)22:6<550::aid-hed2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Castaneda VL, Cheah MS, Saldivar VA, Richmond CM, Parmley RT. Cytogenetic and molecular evaluation of clinically aggressive esthesioneuroblastoma. Am J Pediatr Hematol Oncol. 1991;13(1):62–70. doi: 10.1097/00043426-199121000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Micheau C. A new histochemical approach to olfactory esthesioneuroma. A nasal tumor of neural crest origin. Cancer. 1977;40(1):314–8. doi: 10.1002/1097-0142(197707)40:1<314::aid-cncr2820400144>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Micheau C, Guerinot F, Bohuon C, Brugere J. Dopamine-B-hydroxylase and catecholamines in an olfactory esthesioneuroma. Cancer. 1975;35(5):1309–12. doi: 10.1002/1097-0142(197505)35:5<1309::aid-cncr2820350509>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Nabili V, St John M, Cohan P, Lufkin R, Bhuta S, Abemayor E. Radiology quiz case 1. Catecholamine-secreting olfactory neuroblastoma (OFN) Arch Otolaryngol Head Neck Surg. 2006;132(3):342, 344–5. doi: 10.1001/archotol.132.3.342. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi H, Wakabayashi K, Ikuta F, Tanimura K. Esthesioneuroblastoma: a nasal catecholamine-producing tumor of neural crest origin. Demonstration of tyrosine hydroxylase-immunoreactive tumor cells. Acta Neuropathol. 1988;76(5):522–7. doi: 10.1007/BF00686393. [DOI] [PubMed] [Google Scholar]
- 11.Gallia GL, Reh DD, Salmasi V, Blitz AM, Koch W, Ishii M. Endonasal endoscopic resection of esthesioneuroblastoma: the Johns Hopkins Hospital experience and review of the literature. Neurosurg Rev. 34(4):465–75. doi: 10.1007/s10143-011-0329-2. [DOI] [PubMed] [Google Scholar]
- 12.Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75(3):463–76. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- 13.Lo LC, Johnson JE, Wuenschell CW, Saito T, Anderson DJ. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991;5(9):1524–37. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- 14.Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949–62. doi: 10.1378/chest.06-2490. [DOI] [PubMed] [Google Scholar]
- 15.Hariskov S, Schumann R. Intraoperative management of patients with incidental catecholamine producing tumors: A literature review and analysis. J Anaesthesiol Clin Pharmacol. 2013;29(1):41–6. doi: 10.4103/0970-9185.105793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domi R, Laho H. Management of pheochromocytoma: old ideas and new drugs. Niger J Clin Pract. 2012;15(3):253–7. doi: 10.4103/1119-3077.100616. [DOI] [PubMed] [Google Scholar]
- 17.Arnesen MA, Scheithauer BW, Freeman S. Cushing's syndrome secondary to olfactory neuroblastoma. Ultrastruct Pathol. 1994;18(1-2):61–8. doi: 10.3109/01913129409016275. [DOI] [PubMed] [Google Scholar]
- 18.Galioto S, Di Petrillo A, Pastori M, Arecchi A. Metastatic esthesioneuroblastoma secreting adrenocorticotropic hormone in pediatric patients. J Craniofac Surg. 2011;22(5):1924–9. doi: 10.1097/SCS.0b013e318210bce4. [DOI] [PubMed] [Google Scholar]
- 19.Josephs L, Jones L, Marenette L, McKeever P. Cushing's Syndrome: An Unusual Presentation of Olfactory Neuroblastoma. Skull Base. 2008;18(1):73–6. doi: 10.1055/s-2007-994291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanno K, Morokuma Y, Tateno T, et al. Olfactory neuroblastoma causing ectopic ACTH syndrome. Endocr J. 2005;52(6):675–81. doi: 10.1507/endocrj.52.675. [DOI] [PubMed] [Google Scholar]
- 21.Koo BK, An JH, Jeon KH, et al. Two cases of ectopic adrenocorticotropic hormone syndrome with olfactory neuroblastoma and literature review. Endocr J. 2008;55(3):469–75. doi: 10.1507/endocrj.k07e-005. [DOI] [PubMed] [Google Scholar]
- 22.Mintzer DM, Zheng S, Nagamine M, Newman J, Benito M. Esthesioneuroblastoma (Olfactory Neuroblastoma) with Ectopic ACTH Syndrome: A Multidisciplinary Case Presentation from the Joan Karnell Cancer Center of Pennsylvania Hospital. Oncologist. 2010 doi: 10.1634/theoncologist.2009-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reznik M, Melon J, Lambricht M, Kaschten B, Beckers A. [Neuroendocrine tumor of the nasal cavity (esthesioneuroblastoma). Apropos of a case with paraneoplastic Cushing's syndrome] Ann Pathol. 1987;7(2):137–42. [PubMed] [Google Scholar]
- 24.Yu J, Koch CA, Patsalides A, et al. Ectopic Cushing's syndrome caused by an esthesioneuroblastoma. Endocr Pract. 2004;10(2):119–24. doi: 10.4158/EP.10.2.119. [DOI] [PubMed] [Google Scholar]
- 25.al Ahwal M, Jha N, Nabholtz JM, Hugh J, Birchall I, Nguyen GK. Olfactory neuroblastoma: report of a case associated with inappropriate antidiuretic hormone secretion. J Otolaryngol. 1994;23(6):437–9. [PubMed] [Google Scholar]
- 26.Bernard P, Vitrey D, Boursier C, Brunot J, Flechaire A. [Olfactory esthesioneuroma manifesting as Schwartz-Bartter syndrome] Rev Med Interne. 2000;21(3):278–81. doi: 10.1016/s0248-8663(00)80047-8. [DOI] [PubMed] [Google Scholar]
- 27.Cullen MJ, Cusack DA, O'Briain DS, Devlin JB, Kehely A, Lyons TA. Neurosecretion of arginine vasopressin by an olfactory neuroblastoma causing reversible syndrome of antidiuresis. Am J Med. 1986;81(5):911–6. doi: 10.1016/0002-9343(86)90368-2. [DOI] [PubMed] [Google Scholar]
- 28.Miura K, Mineta H, Yokota N, Tsutsui Y. Olfactory neuroblastoma with epithelial and endocrine differentiation transformed into ganglioneuroma after chemoradiotherapy. Pathol Int. 2001;51(12):942–7. doi: 10.1046/j.1440-1827.2001.01300.x. [DOI] [PubMed] [Google Scholar]
- 29.Muller MB, Landgraf R, Keck ME. Vasopressin, major depression, and hypothalamic-pituitary-adrenocortical desensitization. Biol Psychiatry. 2000;48(4):330–3. doi: 10.1016/s0006-3223(00)00886-6. [DOI] [PubMed] [Google Scholar]
- 30.Myers SL, Hardy DA, Wiebe CB, Shiftman J. Olfactory neuroblastoma invading the oral cavity in a patient with inappropriate antidiuretic hormone secretion. Oral Surg Oral Med Oral Pathol. 1994;77(6):645–50. doi: 10.1016/0030-4220(94)90328-x. [DOI] [PubMed] [Google Scholar]
- 31.Osterman J, Calhoun A, Dunham M, et al. Chronic syndrome of inappropriate antidiuretic hormone secretion and hypertension in a patient with olfactory neuroblastoma. Evidence of ectopic production of arginine vasopressin by the tumor. Arch Intern Med. 1986;146(9):1731–5. [PubMed] [Google Scholar]
- 32.Plasencia YL, Cortes MB, Arencibia DM, et al. Esthesioneuroblastoma recurrence presenting as a syndrome of inappropriate antidiuretic hormone secretion. Head Neck. 2006;28(12):1142–6. doi: 10.1002/hed.20352. [DOI] [PubMed] [Google Scholar]
- 33.Singh W, Ramage C, Best P, Angus B. Nasal neuroblastoma secreting vasopressin. A case report. Cancer. 1980;45(5):961–6. doi: 10.1002/1097-0142(19800301)45:5<961::aid-cncr2820450521>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 34.Srigley JR, Dayal VS, Gregor RT, Love R, van Nostrand AW. Hyponatremia secondary to olfactory neuroblastoma. Arch Otolaryngol. 1983;109(8):559–62. doi: 10.1001/archotol.1983.00800220065016. [DOI] [PubMed] [Google Scholar]
- 35.Gray ST, Holbrook EH, Najm MH, Sadow PM, Curry WT, Lin DT. Syndrome of inappropriate antidiuretic hormone secretion in patients with olfactory neuroblastoma. Otolaryngol Head Neck Surg. 2012;147(1):147–51. doi: 10.1177/0194599812438842. [DOI] [PubMed] [Google Scholar]
- 36.Senchak A, Freeman J, Ruhl D, Senchak J, Klem C. Low-Grade Esthesioneuroblastoma Presenting as SIADH: A Review of Atypical Manifestations. Case Rep Otolaryngol. 2012;2012 doi: 10.1155/2012/582180. 582180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taxy JB, Hidvegi DF. Olfactory neuroblastoma: an ultrastructural study. Cancer. 1977;39(1):131–8. doi: 10.1002/1097-0142(197701)39:1<131::aid-cncr2820390123>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 38.Kolze V. Olfactory esthesioneuroma. Acta Otolaryngol. 1968;65(4):397–402. doi: 10.3109/00016486809120981. [DOI] [PubMed] [Google Scholar]
- 39.Eisenhofer G, Goldstein DS, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results. J Clin Endocrinol Metab. 2003;88(6):2656–66. doi: 10.1210/jc.2002-030005. [DOI] [PubMed] [Google Scholar]
- 40.Djalilian M, Zujko RD, Weiland LH, Devine KD. Olfactory neuroblastoma. Surg Clin North Am. 1977;57(4):751–62. doi: 10.1016/s0039-6109(16)41285-5. [DOI] [PubMed] [Google Scholar]
- 41.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001;2(11):683–90. doi: 10.1016/S1470-2045(01)00558-7. [DOI] [PubMed] [Google Scholar]
- 42.Oberman HA, Rice DH. Olfactory neuroblastomas: a clinicopathologic study. Cancer. 1976;38(6):2494–502. doi: 10.1002/1097-0142(197612)38:6<2494::aid-cncr2820380639>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 43.Obert GJ, Devine KD, McDonald JR. Olfactory neuroblastomas. Cancer. 1960;13:205–15. doi: 10.1002/1097-0142(196001/02)13:1<205::aid-cncr2820130132>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 44.Sorensen PH, Wu JK, Berean KW, et al. Olfactory neuroblastoma is a peripheral primitive neuroectodermal tumor related to Ewing sarcoma. Proc Natl Acad Sci U S A. 1996;93(3):1038–43. doi: 10.1073/pnas.93.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Argani P, Perez-Ordonez B, Xiao H, Caruana SM, Huvos AG, Ladanyi M. Olfactory neuroblastoma is not related to the Ewing family of tumors: absence of EWS/FLI1 gene fusion and MIC2 expression. Am J Surg Pathol. 1998;22(4):391–8. doi: 10.1097/00000478-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Perlman E, Pack S, et al. Absence of EWS/FLI1 fusion in olfactory neuroblastomas indicates these tumors do not belong to the Ewing's sarcoma family. Hum Pathol. 1999;30(11):1356–60. doi: 10.1016/s0046-8177(99)90068-0. [DOI] [PubMed] [Google Scholar]
- 47.Judge DM, McGavran MH, Trapukdi S. Fume-induced fluorescence in diagnosis of nasal neuroblastoma. Arch Otolaryngol. 1976;102(2):97–8. doi: 10.1001/archotol.1976.00780070075009. [DOI] [PubMed] [Google Scholar]
- 48.Trojanowski JQ, Lee V, Pillsbury N, Lee S. Neuronal origin of human esthesioneuroblastoma demonstrated with anti-neurofilament monoclonal antibodies. N Engl J Med. 1982;307(3):159–61. doi: 10.1056/NEJM198207153070305. [DOI] [PubMed] [Google Scholar]
- 49.Wenig B, Dulguerov P, Kapadia S, Prasad M, Fanburg-Smith J, Thompson L. Neuroectodermal Tumors: World Health Organization. 2005:430. [Google Scholar]
- 50.Carney ME, O'Reilly RC, Sholevar B, et al. Expression of the human Achaete-scute 1 gene in olfactory neuroblastoma (esthesioneuroblastoma) J Neurooncol. 1995;26(1):35–43. doi: 10.1007/BF01054767. [DOI] [PubMed] [Google Scholar]
- 51.Mhawech P, Berczy M, Assaly M, et al. Human achaete-scute homologue (hASHl) mRNA level as a diagnostic marker to distinguish esthesioneuroblastoma from poorly differentiated tumors arising in the sinonasal tract. Am J Clin Pathol. 2004;122(1):100–5. doi: 10.1309/QD0K-9Q1J-BH6B-5GQQ. [DOI] [PubMed] [Google Scholar]
- 52.Jiang T, Collins BJ, Jin N, et al. Achaete-scute complex homologue 1 regulates tumor-initiating capacity in human small cell lung cancer. Cancer Res. 2009;69(3):845–54. doi: 10.1158/0008-5472.CAN-08-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ball DW, Azzoli CG, Baylin SB, et al. Identification of a human achaete-scute homolog highly expressed in neuroendocrine tumors. Proc Natl Acad Sci U S A. 1993;90(12):5648–52. doi: 10.1073/pnas.90.12.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121(8):1687–701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]


