Abstract
The discovery of ketamine’s rapid and robust antidepressant effects opened a window into a new generation of antidepressants. Multiple controlled trials and open-label studies have demonstrated these effects across a variety of patient populations known to often achieve little to no response from traditional antidepressants. Ketamine has been generally well tolerated across patient groups, with transient mild to moderate adverse effects during infusion. However, the optimal dosing and route of administration and the safety of chronic treatment is not fully known. This review summarizes the clinical effects of ketamine and its neurobiological underpinnings and mechanisms of action that may provide insight into the neurobiology of depression, relevant biomarkers, and treatment targets. Moreover, we offer suggestions for future research that can continue to advance the field forward and ultimately improve the psychopharmacologic interventions available for those individuals struggling with depressive and trauma-related disorders.
Keywords: ketamine, depression, antidepressant, treatment mechanisms, neurobiology
Introduction
Depressive disorders are a leading cause of disability affecting millions worldwide,1 often causing chronic recurrent symptoms, increased morbidity, heightened risk of suicide, poor functional outcomes, and profound socioeconomic burden.2 However, the past five decades of research focusing on antidepressant development has unfortunately seen little success in creating fundamentally novel psychopharmacologic interventions. Over twenty antidepressant medications are currently available, all targeting the monoaminergic system. However, the efficacy of these medications is limited, with a substantial proportion of patients experiencing residual symptoms, persistent functional impairment, and failure to achieve sustained remission even when symptoms do improve.3 Further, the full clinical benefit of these traditional antidepressants is only achieved following weeks to months of treatment.4 A large study by the National Institute of Mental Health found that regardless of the primary or adjunctive antidepressant medication selected for treatment, less than one-third of depressed patients achieved remission within 12 weeks and 33% of patients did not achieve remission at all despite trials of four antidepressant medications over a 1-year period,5 leaving clinicians with few therapeutic options for treatment-refractory patients. Thus, there is a clear and urgent need for the development of novel, rapid-acting antidepressants with robust efficacy. Mounting evidence suggests that low doses of ketamine may possess both of these properties, acting rapidly and robustly to treat severely treatment-resistant depressed patients.
Ketamine, a glutamate N-methyl-D-aspartate receptor (NMDA-R) antagonist, is a U.S. Food and Drug Administration (FDA)-approved sedative medication originally developed for the induction and maintenance of anesthesia in adults. Ketamine has a short plasma half-life of 4 min and a 2.5-h plasma terminal half-life. It can be administered intravenously (most common), intramuscularly (93% bioavailability (BA)), intranasally (50% BA), intrarectally (25% BA), and orally (20% BA).6 In addition to its role as an anesthetic and as a pharmacological model of the core symptoms of schizophrenia, ketamine has received considerable attention in psychiatric research as a prototype for a new generation of antidepressants after the discovery of its profound and rapid effects on depressive symptoms. This review provides a discussion of clinical trials investigating ketamine’s rapid antidepressant effects, safety, and tolerability. In addition, we summarize some recent research examining the effect of ketamine on symptoms of posttraumatic stress disorder (PTSD) and neurocognitive functioning. We then briefly present mechanisms thought to underlie the rapid antidepressant effects of ketamine, including the neurobiology and potential clinical biomarkers of depression, neurocircuitry critical to affective regulation, and molecular pathways in synaptogenesis. We conclude by discussing the prospect for next-generation rapid-acting antidepressants and the clinical implications of these findings on ketamine.
Robust and rapid antidepressant effects
In the early 1990s, researchers conducting preclinical studies found that NMDA-R antagonists showed promising antidepressant qualities.7, 8 Later in the 1990s, in a pilot study in patients with severely treatment-resistant depression, we discovered that a single subanesthetic dose of ketamine had striking, robust, rapid antidepressant effects within 4 h of intravenous administration.9 This finding has since been well replicated in multiple controlled trials,10, 11 underscoring the rationale of targeting the glutamatergic system and the feasibility of developing truly novel rapid-acting antidepressant agents.12, 13 These noticeable rapid antidepressant effects have also been reported in patient groups known to respond poorly to traditional antidepressant interventions, such as those with bipolar depression, anxious depression, and patients who are treatment resistant to electroconvulsive therapy (ECT).14–17 In addition, ketamine showed efficacy in rapidly reducing suicidal ideation within hours of administration.18–20
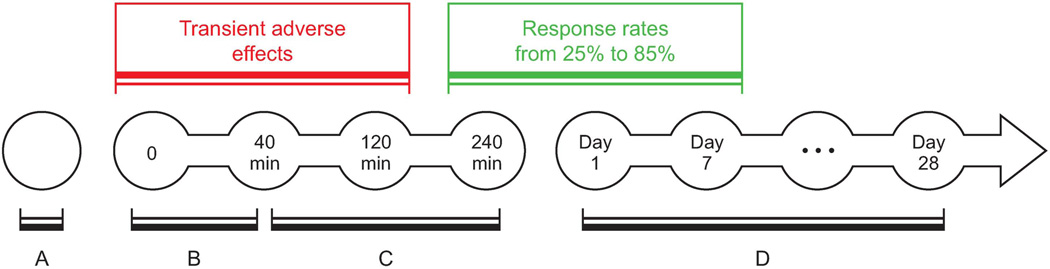
The antidepressant effects of ketamine are evident within 2–4 h of treatment and are sustained for 3–7 days, with a response rate of approximately 25% to 85% within 24 h and 14% to 70% at 72 h post-infusion (see Tables 1 and 2 for selected controlled trials and open-label investigations).21 Early clinical trials primarily administered 0.5 mg/kg of ketamine infused intravenously over approximately 40 min, in a medication-free population (Fig. 1). More recently, several studies reported rapid antidepressant effects following various routes and regimens (Tables 1 and 2). Of note, repeated intravenous doses prolonged treatment response22 and intranasal administration exerted rapid antidepressant effects comparable to intravenous administration but with minimal psychotomimetic and dissociative effects. 23
Table 1.
Selected randomized controlled studies examining the rapid antidepressant effect of ketamine
| Study | Subjects | Treatment | ADE 4h-3d |
ADE 7d |
Response rate |
Additional findings |
|---|---|---|---|---|---|---|
| Abdallah et al.79 | N6-MDD, N2-BD, MF | Thiopental 3.5 mg/kg + ECT 3×/week for 2 weeks | NO | — | 13% at 1–3 days | ADE in both groups after 6th ECT; ECT and/or thiopental appears to attenuate the rapid ADE of ketamine. |
| Ketaminea 0.5 mg/kg + Thiopental 3.5 mg/kg + ECT 3×/week for 2 weeks | NO | — | 0% at 1–3 days | |||
| Berman et al.9 | N8-MDD, N1-BD, MF | Placebo | NO | — | 12% at 3 days | |
| Ketamineb0.5 mg/kg | YES | — | 50% at 3 days | |||
| Diazgrandos et al.14 | N18-BD | Placebo | NO | NO | 6% at 1 day | Significant ADE at 40 min |
| Ketamineb0.5 mg/kg | YES | NO | 71% at 1 day | |||
| Ghasemi et al.80 | N9-MDD | Ketamineb0.5 mg/kg 1×/2 days for 6 days | YES | NO | 42% at 1 day; 7% at 7 days | Response rates are following the last infusion. |
| N9-MDD | ECT 1×/2 days for 6 days | NO | YES | 12% at 1 day; 25% at 7 days | ||
| Kudoh et al.81 | N35-MDD | Propofol 1.5 mg/kg + Fentanyl 2 μg/kg | NO | — | — | Lower pain scores among ketamine group |
| Ketaminea 1.0 mg/kg + Propofol 1.5 mg/kg + Fentanyl 2 μg/kg | YES | — | — | |||
| Lapidus et al.23 | N18-MDD | Placebo | NO | — | 6% at 1 day | Significant improvement of anxiety symptoms @ 1 day; dose equivalent to 0.15–0.34 mg/kg intravenous ketamine |
| Ketaminec 50mg | YES | — | 44% at 1 day | |||
| Murrough et al.11 | N47-MDD | Ketamineb0.5 mg/kg | YES | YES | 64% at 1 day; 45% at 7 days |
|
| N25-MDD | Midazolam 0.045 mg/kg | YES | YES | 28% at 1 days 16% at 7 days |
||
| Valentine et al.73 | N10-MDD, MF | Placebo | NO | NO | — | 1H-MRS: Occipital amino acid levels did not correlate with ADE. |
| Ketamineb 0.5 mg/kg | YES | YES | — | |||
| Zarate et al.10 | N15-MDD, MF | Placebo | NO | NO | 0% at 1 day; 0% at 7 days | Depressed mood, guilt, work, interests, and psychic anxiety improved significantly among treatment group. |
| Ketamineb 0.5 mg/kg | YES | YES | 64% at 4 h; 71% at 1 day; 35% at 7 days | |||
| Zarate et al.82 | N15-BD | Placebo | NO | NO | — | Significant ADE at 40 min; decreased suicidal ideations |
| Ketamineb 0.5 mg/kg | YES | NO | 64% at 40 min; 50% at 4 h; 43% at 1 day |
intravenous ketamine bolus.
intravenous ketamine infusion over 40 minutes.
intranasal ketamine.
—, not reported.
Abbreviations: ADE, antidepressant effect; N, number of subjects; MDD, major depressive disorder; BD, bipolar disorder; MF, medication free; 1H-MRS, proton magnetic resonance spectroscopy, ECT: electroconvulsive therapy.
Table 2.
Selected open-label studies examining the rapid antidepressant effects of ketamine
| Study | Subjects | Treatment | ADE 4 h-3 days |
ADE 7 days |
Response rate |
Additional findings |
|---|---|---|---|---|---|---|
| Carlson et al.83 | N20-MDD | Ketaminea 0.5 mg/kg | YES | — | 30% at 4 h | Reduced activity in the right habenula |
| Chilukuri et al.55 | N9-MDD | Ketaminea 0.5 mg/kg | YES | — | 59% at 2 h | Improvement sustained at 3 days in all groups; two subjects attempted suicide—both were non-responders to ketamineb. |
| N9-MDD | Ketamineb 0.5 mg/kg | YES | — | 60% at 2 h | ||
| N9-MDD | Ketamineb 0.25 mg/kg | YES | — | 57% at 2 h | ||
| Diazgrandos, et al.19 | N33-MDD, MF | Ketaminea 0.5 mg/kg | YES | — | — | Decreased suicidal ideations |
| Ibrahim et al.15 | N17-MDD-ER, MF | Ketaminea 0.5 mg/kg | YES | — | — | Lower effect size with ECT-resistant (MDD-ER) group |
| N23-MDD, MF | Ketaminea 0.5 mg/kg | YES | — | — | ||
| Ibrahim et al.84 | N42-MDD, MF | Ketaminea 0.5 mg/kg | YES | YES | 62% at 4–6 h | Riluzole following ketamine had no effects; ADE maintained at 28 days |
| Larkin & Beautrais85 | N14-MDD-SI | Ketaminec 0.2 mg/kg | YES | YES | 85% at 10 days | Decreased suicidal ideations |
| Machado et al.74 | N23-MDD, MF | Ketaminea0.5 mg/kg | YES | — | 48% at 4 h | Serum BDNF did not correlate with ADE. |
| Mathew et al.86 | N26-MDD, MF | Ketaminea 0.5 mg/kg | YES | YES | 65% at 1 day; 54% at 3 days | Lamotrigine prior to ketamine and riluzole following ketamine had no effects. |
| Murrough et al.20 | N24-MDD, MF | Ketaminea 0.5 mg/kg 3×/w for 2w | YES | — | 71% at 24 h after last infusion | Significant ADE within 2 h; decreased suicidal ideations in ketamine responders as well as non-responders; median time to relapse was 18 days. |
| Okamoto et al.87 | N11-MDD | Ketaminec 0.86 mg/kg + ECT 2×/w for 4w | — | YES | — | ADE in both groups; ketamine group had lower depression scores at 2nd & 4th ECT sessions and longer seizure at 1st & 6th ECT session. |
| N20-MDD | Propofol 0.94 mg/kg + ECT 2×/w for 4w | — | YES | — | ||
| Phelps et al.88 | N26-MDD, MF | Ketaminea 0.5 mg/kg | YES | — | 43% at 4 h (67% in FH+; 18% in FH−) | Plasma ketamine/norketamine did not correlate with ADE. |
| Rasmussen et al.51 | N10-MDD | Ketaminea 0.5 mg/kg 2×/w for 2w | YES | — | 30% at 1 day; 80% at 4 days | Decreased suicidal ideations |
| Salvadore et al.68 | N11-MDD, N11-HS, MF | Ketaminea 0.5 mg/kg | YES | — | — | Repeated exposure to fearful faces: increased rACC activity in MDD but not HS; rACC activity correlated with ADE; rAG negatively correlated ADE. |
| Salvadore et al.69 | N15-MDD, MF | Ketaminea 0.5 mg/kg | YES | — | — | N-back test (working memory): pgACC activity negatively correlated with ADE. |
| Salvadore et al.89 | N15-MDD, MF | Ketaminea 0.5 mg/kg | YES | — | — | Frontal glx/glutamate negatively correlated with ADE, while glutamate positively correlated with anxiety improvement. |
intravenous ketamine infusion over 40 minutes.
intramuscular ketamine.
intravenous ketamine bolus.
—, not reported.
Abbrevations: ADE: antidepressant effect; N, number of subjects; MDD, major depressive disorder; MDD-SI, major depressive disorder with suicidal ideation; MDD-ER, ECT-resistant major depressive disorder; BD, bipolar disorder; MF, medication free; HS, healthy subjects; FH+/−, family history of alcohol abuse positive/negative; 1H-MRS, proton magnetic resonance spectroscopy; MEG, magnetoencephalography; rACC, rostral anterior cingulate cortex; pgACC, pregenual anterior cingulate cortex; rAG, rostral amygdala; Glx, a 1H-MRS measure reflecting glutamate and glutamine; ECT, electroconvulsive therapy.
Figure 1.
Ketamine administration and time course. (A) Comprehensive screening and evaluation; (B) ketamine 0.5 mg/kg infused over 40 min; (C) immediate antidepressant effect; and (D) rapid antidepressant effects sustained for up to 28 days after a single ketamine infusion.
To date, three meta-analyses have been published that consistently confirmed the efficacy of ketamine’s antidepressant effects, as well as drawing attention to the current limitations and areas for future investigations.24–26 Each study supports the association of ketamine with a superior clinical response and clinical remission relative to comparators (e.g., saline, midazolam) in both unipolar and bipolar depression, in those with treatment-resistant depression and not, and in both medicated and unmedicated study participants.24–26 These meta-analyses also highlight that additional research is needed to evaluate optimal dosing, route of administration, and treatment schedules; to further characterize the duration of effects and the long-term safety, tolerability, and efficacy of ketamine; and to explore the potential effects of other glutamatergic agents that may have fewer or less extreme side effects and lower addiction potential. Other limitations of ketamine trials are the short duration of trials, the short-term efficacy of a single infusion of ketamine, and the lack of complete blinding to treatment, due to the functional unblinding of treatment status by the acute adverse effects of ketamine. This latter limitation was partially addressed in a recent two-site controlled trial that demonstrated the rapid antidepressant effects of ketamine compared to midazolam (an anesthetic benzodiazepine) as an active comparator to optimize blinding to treatment status.11 In addition, the true biological effect of the drug is further supported by the relatively consistent time curve of response to ketamine across studies (i.e., improvement within 4 h, peak response around 24 h, and efficacy for about 1 week, as well as the maintenance of efficacy through repeated treatment (Tables 1 and 2)).
Posttraumatic stress disorder
Preliminary evidence supports the utility and safety of ketamine in treating PTSD symptoms, which are often highly comorbid and sometimes overlapping with depressive disorders. In an early case report, a young military veteran with highly treatment-resistant PTSD27 showed rapid improvement following a single infusion of a subanesthetic dose of ketamine treatment, with a 56% reduction in his PTSD symptoms, and this treatment response was maintained for 15 days.27 Ketamine was well tolerated and no adverse effects were reported.27 Another case series reported a marked reduction in flashbacks in three women with PTSD treated with ifenprodil—an NMDA-R antagonist that selectively binds to the GluN2B subunit.28 The safety of subanesthetic doses of ketamine was reviewed in patients with PTSD or trauma history who were treated with ketamine for comorbid depression. Ketamine was well tolerated with no evidence of worsening in PTSD symptoms.29
More recently, Feder and colleagues provided promising pilot evidence supporting the utility and safety of ketamine in treating PTSD symptoms.30 This study randomized a cohort of PTSD patients to a single infusion of ketamine (0.5 mg/kg infused over 40 min) or midazolam in a double-blind crossover design. Compared to midazolam, ketamine showed a significant improvement in PTSD symptoms 24 h post-infusion. Similar to depression studies, dissociative symptoms occurred during infusion, peaked at 40 min, were generally well tolerated, and subsided within 80 min following ketamine administration.30 Also, analogous to ketamine studies in depressed and healthy subjects, physical adverse effects were transient.30
Neurocognitive functioning
Cognitive deficits cardinal to depressive disorders contribute significantly to disability, risk for suicide, treatment noncompliance, and refractory treatment response.31–37 As further described below, ketamine’s antagonism of the glutamatergic NMDA-R appears to be the first step in a cascade of events that converges to produce enhanced activity in excitatory networks and marked changes in synaptic plasticity and strength.38–44 This enhanced synaptic plasticity occurs approximately 24 h after ketamine administration, the peak time of antidepressant response. Considering the critical role of synaptic plasticity in cognitive function, the question arises as to whether the demonstrated ketamine-induced synaptogenesis would translate into enhanced cognition 24 h post-treatment.
Although ketamine studies have consistently demonstrated transient cognitive deficits during infusion, the hypothesized pro-cognitive effects of ketamine 24 h post-treatment, and the relationship between cognitive functions and treatment response are not fully studied. In a controlled trial in which participants were randomized to ketamine or midazolam, there were no differential effects of treatment on cognitive performance and no correlation with antidepressant response. However, low baseline cognitive processing speed uniquely predicted depression improvement at 24 h post-ketamine.45 Another recent study of ketamine’s effect on the neurocognitive performance of patients with bipolar depression revealed pro-cognitive effects approximately 72 h post-infusion.46 These results appeared to be independent of depression severity or improvement, suggesting ketamine may possess pro-cognitive effects in addition to its influence on mood symptoms.46 These two investigations provide promising preliminary evidence. However, both studies have a relatively small sample size and did not include any long-term evaluation of cognitive changes. Future studies with larger sample sizes and longer-term follow-up are required to further characterize the cognitive effects of ketamine.
Safety and tolerability
Ketamine is generally well tolerated across both patient groups and healthy controls, with mild to moderate transient adverse side effects observed within the first 2 h of treatment. Ketamine administration produces transient perceptual disturbances, dissociation, dysphoria, euphoria, and/or anxiety during infusion. Physical adverse effects include dizziness, nausea, and mild increases in blood pressure and heart rate. There is also potential risk of ulcerative cystitis,47 although this is typically associated with chronic use at higher doses than what is generally administered in psychiatric clinical investigations. Given the short half-life of the drug, these adverse effects abate within a few minutes of stopping ketamine infusion and generally fully remit within 2 hours.
Open-label and placebo-controlled trials have supported the safety and tolerability of a single infusion of ketamine.21 However, the optimal dose, route of administration, and the safety of repeated or chronic treatment are still not fully known. This is particularly important given the addiction and excitotoxicity potential of ketamine. Case reports suggest that repeated ketamine infusions may safely and effectively extend the antidepressant effects of the drug for several months.48, 49 Up to six infusions of repeated ketamine administered once, twice, or three times per week were found to be safe and efficacious in maintaining treatment response.22, 50–53 Other studies reported safety and efficacy using a single administration of various routes and doses of ketamine, including 0.2 mg/kg intravenous bolus,54 50 mg intranasal,23 and 0.5 mg/kg or 0.25 mg/kg intramuscular injection.55
Mechanism of action
Convergent evidence implicates synaptic plasticity in the pathophysiology of depression.56, 57 Sustained stress and depression precipitates neuronal atrophy and overall synaptic depression, particularly in the prefrontal cortex (PFC).58–60 These synaptic deficits are the result of reduced neurotrophin function (e.g., brain-derived neurotrophic factor or BDNF)61 and of the inhibition of the mammalian target of rapamycin (mTOR) pathway.62 Reduction of BDNF or inhibition of mTOR signaling leads to depressive-like behavior and blocks the effects of antidepressants in rodents.61,62 Conversely, increasing BDNF or enhancing mTOR signaling produces antidepressant-like effects.61,62 Therefore, it is proposed that activation of BDNF and mTOR signaling is a required step for efficacious antidepressant treatment. Ketamine is believed to exert its rapid antidepressant effect by increasing BDNF function and activating mTOR signaling.
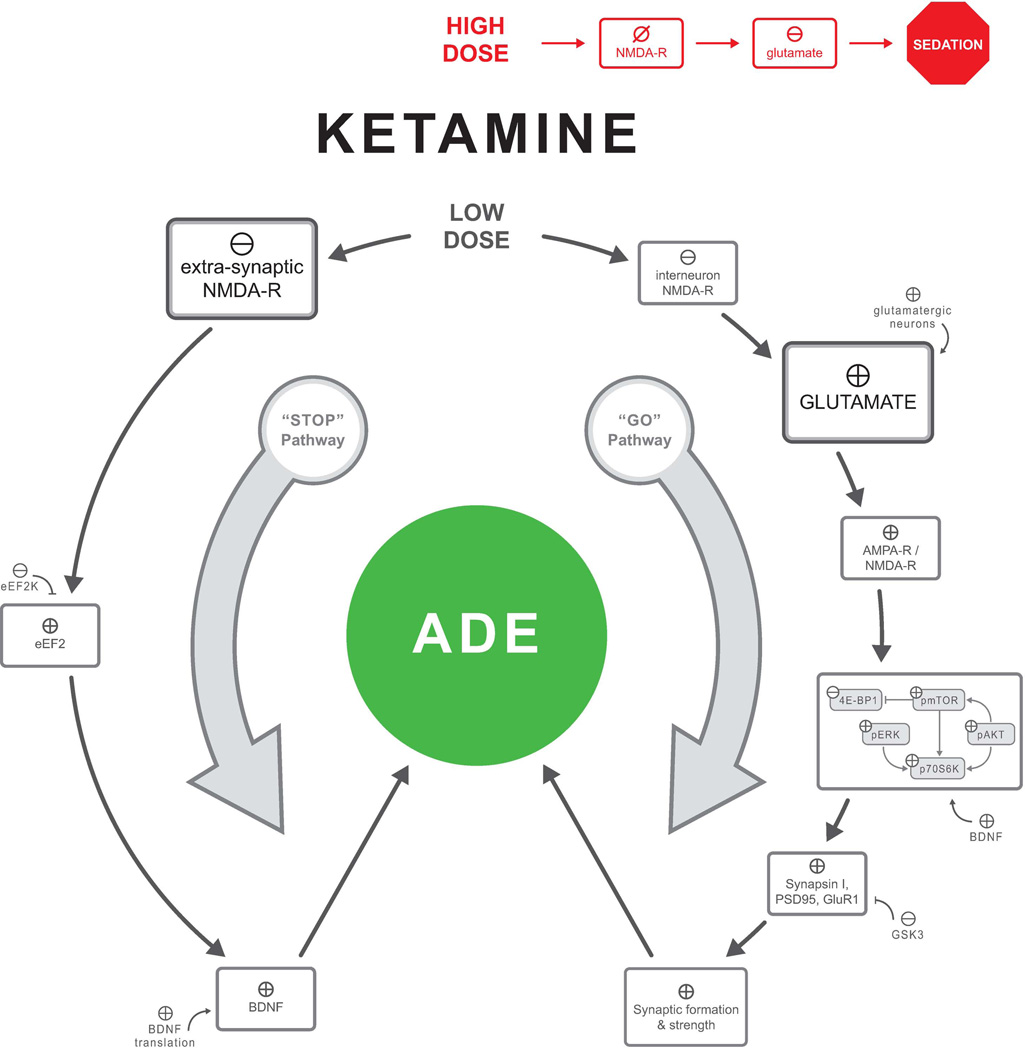
High doses of ketamine inhibit overall NMDA-R activities, leading to sedation and to reduced glutamate signaling, as evident by reduced extracellular glutamate.39 In contrast, low doses of ketamine have a paradoxical effect on glutamatergic activity, leading to an increase of glutamate release.39,63 This paradoxical effect is hypothesized to be the consequence of a preferential blockade of the NMDA-R on a subpopulation of gamma-aminobutyric acid (GABA)ergic interneurons.64,65 Inhibition of these interneurons disinhibits prefrontal glutamatergic cells, leading to a glutamate surge, which appears to play a critical role in the antidepressant effects of ketamine.13
Briefly, mounting evidence suggests that ketamine exerts its antidepressant effect through two pathways (Fig. 2).38,66 The “stop pathway” involves at-rest inhibition of the NMDA-R,66 presumably extrasynaptic NMDA-R, which is known to promote synaptic atrophy and neuronal death.67 At-rest inhibition of the NMDA-R culminates in increasing BDNF function by disinhibiting eEF2.66 The “go pathway” engages a cascade of events that includes: (1) disinhibition of glutamatergic neurons, leading to a glutamate surge,39, 63 (2) stimulation of synaptic glutamate receptors, particularly α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors,38 and (3) activation of mTOR signaling and synaptic formation.38 The increase in BDNF function and mTOR signaling converges to promote synaptogenesis and reversal of the synaptic deficits induced by prolonged stress and depression.13
Figure 2.
Mechanisms underlying the rapid andidepressant effects of ketamine. Changes in GluR1 appear to be region specific, with evidence for increased GluR1 in the medial prefrontal cortex but not the hippocampus. Abbreviations: ADE, antidepressant-like effects; NMDA-R, N-methyl-D-aspartate glutamate receptor; AMPA-R, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GluR1, R1 subunit of AMPA-R; eEF2k, eukaryotic elongation factor 2 kinase; BDNF, brain-derived neurotrophic factor; pmTOR, mammalian target of rapamycin; GSK3, glycogen synthase kinase 3.
⊕, enhance/stimulate; ⊖, inhibit/reduce; ⊘, blockade.
Clinical biomarkers
The rapid and robust antidepressant effects of ketamine offer a unique opportunity for clinical biomarker development to better understand the mechanism underlying rapid-acting antidepressants and to gain insight into the neurobiology of depression.13 To date, studies on biomarkers can largely be grouped into two categories: studies examining prefrontal excitability and synaptic strength or studies that investigated the role of neurotrophins, primarily BDNF.
An enhanced response to ketamine was associated with (1) pre-treatment high medial PFC (mPFC) activity in response to fearful faces,68 (2) pre-treatment low mPFC activity during a working memory task,69 (3) pre-treatment low connectivity between the mPFC and amygdala,69 (4) post-treatment increases in synaptic strength,70, 71 and (5) low pre-treatment mPFC (glutamate + glutamine)/glutamate ratio,72 although not without inconsistencies.73
In addition, while early studies failed to demonstrate a significant correlation between peripheral BDNF and response to ketamine,74, 75 more recent studies confirmed a relationship between increased BDNF and enhanced treatment following ketamine administration.71, 76 These findings were supported by a study showing a positive relationship between the functional variant of BDNF (Val66Met) and response to ketamine treatment.77
Conclusions
The discovery of the rapid antidepressant effects of ketamine opened a window into a new generation of antidepressants and a better understanding of the biological underpinnings of depression. Ketamine studies showed us that rapid improvement in complex mood states such as depression, PTSD, and suicidality are possible with therapeutic interventions. It also highlighted the utility of targeting the glutamatergic system, a truly novel antidepressant mechanism. However, it is important to emphasize that ketamine remains primarily an investigational drug at this stage, with abuse liability and unknown safety and efficacy following chronic treatment.
Excitement in the field has led to concerted preclinical and clinical effort over the past decade by many research groups across the nation and throughout the world. Significant discoveries and major pathways have been implicated in the pathology and treatment of depression. Novel NMDA-R modulators are being tested to mimic the rapid antidepressant effects of ketamine while minimizing adverse effects (for a review, see Ref. 78). However, several questions remain unanswered and require further investigation: (1) the optimal dose and preferable route of administration of ketamine, (2) the frequency and dose at which ketamine administration becomes harmful instead of beneficial, (3) the regimen to maintain treatment response, (4) the relationship between cognitive function and ketamine treatment, (5) the sustainability of anti-suicidal properties and generalizability to the general population, and (6) the role of combined ketamine treatment and cognitive behavioral therapy.
Acknowledgments
The authors gratefully acknowledge Christopher Averill for his contribution to the preparation of the figures; the State of Connecticut Department of Mental Health and Addiction Services for its support of the Abraham Ribicoff Research Facilities of the Connecticut Mental Health Center; the U.S. Department of Veterans Affairs, via its funding of the VA National Center for PTSD and the Office of Academic Affairs Advanced Fellowship in Mental Illness and Research; the National Institute of Mental Health (MH101498); the NARSAD Young Investigator Award—Vital Projects Fund Inc., from the Brain and Behavior Research Foundation; and the National Center for Advancing Translational Science (CTSA Grant Number UL1 RR024139).
Dr. Abdallah has received research funding or consultation fees from the Brain and Behavior Research Foundation (NARSAD), the American Psychiatric Foundation, and Genentech. Dr. Krystal has served as a scientific consultant to the following companies (the Individual Consultant Agreements listed are less than $10,000/year): Aisling Capital, Astellas Pharma Global Development, AstraZeneca Pharmaceuticals, Biocortech, Brintnall & Nicolini, Easton Associates, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Lundbeck Research USA, Medivation, Merz Pharmaceuticals, MK Medical Communications, Hoffmann–La Roche, SK Holdings, Sunovion Pharmaceuticals, Takeda Industries, and Teva Pharmaceutical Industries. He is on the Scientific Advisory Board for the following companies: Abbott Laboratories, Bristol-Myers-Squibb, Eisai, Eli Lilly, Forest Laboratories, Lohocla Research Corporation, Mnemosyne Pharmaceuticals, Naurex, Pfizer Pharmaceuticals, and Shire Pharmaceuticals. He holds less than $150 in exercisable warrant options with Tetragenex Pharmaceuticals. He is also on the Board of Directors of the Coalition for Translational Research in Alcohol and Substance Use Disorders and was the principal investigator of a multicenter study in which Janssen Research Foundation provided drug and some support to the Department of Veterans Affairs. He is Editor of Biological Psychiatry. In addition, he has a patent on dopamine and noradrenergic reuptake inhibitors in the treatment of schizophrenia (patent number 5447948) and is a coinventor with Dr. Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1). He has a patent pending on intranasal administration of ketamine to treat depression.
Footnotes
Conflicts of interest
Dr. Averill declares no conflicts of interest.
References
- 1.Collins PY, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA : the journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Katz MM, et al. Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:566–579. doi: 10.1038/sj.npp.1300341. [DOI] [PubMed] [Google Scholar]
- 5.Gaynes BN, et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatric services (Washington, D.C.) 2009;60:1439–1445. doi: 10.1176/ps.2009.60.11.1439. [DOI] [PubMed] [Google Scholar]
- 6.Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings) CNS neuroscience & therapeutics. 2013;19:370–380. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skolnick P, et al. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 8.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 9.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 10.Zarate CA, Jr, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of general psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 11.Murrough JW, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. The American journal of psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niciu MJ, et al. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annual review of pharmacology and toxicology. 2014;54:119–139. doi: 10.1146/annurev-pharmtox-011613-135950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdallah CG, et al. Ketamine and Rapid-Acting Antidepressants: A Window into a New Neurobiology for Mood Disorder Therapeutics. Annual review of medicine. 2014 doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diazgranados N, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Archives of general psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ibrahim L, et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1155–1159. doi: 10.1016/j.pnpbp.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ionescu DF, et al. Effect of baseline anxious depression on initial and sustained antidepressant response to ketamine. The Journal of clinical psychiatry. 2014;75:e932–e938. doi: 10.4088/JCP.14m09049. [DOI] [PubMed] [Google Scholar]
- 17.Ionescu DF, et al. A single infusion of ketamine improves depression scores in patients with anxious bipolar depression. Bipolar disorders. 2014 doi: 10.1111/bdi.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price RB, et al. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiazGranados N, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. The Journal of clinical psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballard ED, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161–166. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aan Het Rot M, et al. Ketamine for Depression: Where Do We Go from Here? Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murrough JW, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapidus KA, et al. A Randomized Controlled Trial of Intranasal Ketamine in Major Depressive Disorder. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caddy C, et al. Ketamine as the prototype glutamatergic antidepressant: pharmacodynamic actions, and a systematic review and meta-analysis of efficacy. Therapeutic advances in psychopharmacology. 2014;4:75–99. doi: 10.1177/2045125313507739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fond G, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology. 2014;231:3663–3676. doi: 10.1007/s00213-014-3664-5. [DOI] [PubMed] [Google Scholar]
- 26.McGirr A, et al. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychological medicine. 2014:1–12. doi: 10.1017/S0033291714001603. [DOI] [PubMed] [Google Scholar]
- 27.D'Andrea D, Andrew Sewell R. Transient Resolution of Treatment-Resistant Posttraumatic Stress Disorder Following Ketamine Infusion. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto A, et al. Ifenprodil for the treatment of flashbacks in female posttraumatic stress disorder patients with a history of childhood sexual abuse. Biol Psychiatry. 2012;71:e7–e8. doi: 10.1016/j.biopsych.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Zeng MC, et al. Acute stress symptoms do not worsen in posttraumatic stress disorder and abuse with a single subanesthetic dose of ketamine. Biol Psychiatry. 2013;73:e37–e38. doi: 10.1016/j.biopsych.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Feder A, et al. Efficacy of Intravenous Ketamine for Treatment of Chronic Posttraumatic Stress Disorder: A Randomized Clinical Trial. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- 31.Herrera-Guzman I, et al. Major Depressive Disorder in recovery and neuropsychological functioning: effects of selective serotonin reuptake inhibitor and dual inhibitor depression treatments on residual cognitive deficits in patients with Major Depressive Disorder in recovery. Journal of affective disorders. 2010;123:341–350. doi: 10.1016/j.jad.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Baune B, et al. The role of cognitive impairment in general functioning in major depression. Psychiatry research. 2010;176:183–189. doi: 10.1016/j.psychres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Simon GE, et al. Recovery from depression, work productivity, and health care costs among primary care patients. General Hospital Psychiatry. 2000;22:153–162. doi: 10.1016/s0163-8343(00)00072-4. [DOI] [PubMed] [Google Scholar]
- 34.Zajecka JM. Treating Depression to Remission. Journal of Clinical Psychiatry. 2003;64:7–12. [PubMed] [Google Scholar]
- 35.Bora E, et al. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychological medicine. 2013;43:2017–2026. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- 36.Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. Journal of affective disorders. 2011;134:20–31. doi: 10.1016/j.jad.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Hasselbalch BJ, et al. Cognitive deficits in the remitted state of unipolar depressive disorder. Neuropsychology. 2012;26:642–651. doi: 10.1037/a0029301. [DOI] [PubMed] [Google Scholar]
- 38.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moghaddam B, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends in neurosciences. 2011 doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anand A, et al. Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Archives of general psychiatry. 2000;57:270–276. doi: 10.1001/archpsyc.57.3.270. [DOI] [PubMed] [Google Scholar]
- 42.Deakin JF, et al. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Archives of general psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- 43.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krystal JH, et al. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology. 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- 45.Murrough JW, et al. Neurocognitive Effects of Ketamine and Association with Antidepressant Response in Individuals with Treatment-Resistant Depression: A Randomized Controlled Trial. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Permoda-Osip A, et al. Single Ketamine Infusion and Neurocognitive Performance in Bipolar Depression. Pharmacopsychiatry. 2014 doi: 10.1055/s-0034-1394399. [DOI] [PubMed] [Google Scholar]
- 47.Shahani R, et al. Ketamine-associated ulcerative cystitis: a new clinical entity. Urology. 2007;69:810–812. doi: 10.1016/j.urology.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 48.Blier P, Zigman D, Blier J. On the safety and benefits of repeated intravenous injections of ketamine for depression. Biol Psychiatry. 2012;72:e11–e12. doi: 10.1016/j.biopsych.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 49.Szymkowicz SM, Finnegan N, Dale RM. A 12-month naturalistic observation of three patients receiving repeat intravenous ketamine infusions for their treatment-resistant depression. Journal of affective disorders. 2013;147:416–420. doi: 10.1016/j.jad.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diamond PR, et al. Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol. 2014;28:536–544. doi: 10.1177/0269881114527361. [DOI] [PubMed] [Google Scholar]
- 51.Rasmussen KG, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27:444–450. doi: 10.1177/0269881113478283. [DOI] [PubMed] [Google Scholar]
- 52.Shiroma PR, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. Journal of affective disorders. 2014;155:123–129. doi: 10.1016/j.jad.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 53.Segmiller F, et al. Repeated S-ketamine infusions in therapy resistant depression: a case series. Journal of clinical pharmacology. 2013;53:996–998. doi: 10.1002/jcph.122. [DOI] [PubMed] [Google Scholar]
- 54.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 55.Chilukuri H, et al. Acute antidepressant effects of intramuscular versus intravenous ketamine. Indian journal of psychological medicine. 2014;36:71–76. doi: 10.4103/0253-7176.127258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Popoli M, et al. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature reviews. Neuroscience. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang HJ, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nature medicine. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bessa JM, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Molecular psychiatry. 2009;14:764–773. 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 60.Yuen EY, et al. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Ota KT, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nature medicine. 2014 doi: 10.1038/nm.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chowdhury GM, et al. (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine's effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71:1022–1025. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neymotin SA, et al. Ketamine disrupts theta modulation of gamma in a computer model of hippocampus. J Neurosci. 2011;31:11733–11743. doi: 10.1523/JNEUROSCI.0501-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. LID - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nature reviews. Neuroscience. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salvadore G, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salvadore G, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cornwell BR, et al. Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psychiatry. 2012;72:555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duncan WC, et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2013;16:301–311. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salvadore G, et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011:1–10. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valentine GW, et al. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Machado-Vieira R, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. The Journal of clinical psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rybakowski JK, et al. Single ketamine infusion in bipolar depression resistant to antidepressants: are neurotrophins involved? Human psychopharmacology. 2013;28:87–90. doi: 10.1002/hup.2271. [DOI] [PubMed] [Google Scholar]
- 76.Haile CN, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17:331–336. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laje G, et al. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72:e27–e28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdallah CG, et al. Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting. The journal of ECT. 2012;28:157–161. doi: 10.1097/YCT.0b013e31824f8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghasemi M, et al. Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res. 2014;215:355–361. doi: 10.1016/j.psychres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Kudoh A, et al. Small-dose ketamine improves the postoperative state of depressed patients. Anesthesia and analgesia. 2002;95:114–118. doi: 10.1097/00000539-200207000-00020. table of contents. [DOI] [PubMed] [Google Scholar]
- 82.Zarate CA, Jr, et al. Replication of Ketamine's Antidepressant Efficacy in Bipolar Depression: A Randomized Controlled Add-On Trial. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carlson PJ, et al. Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol Psychiatry. 2013;73:1213–1221. doi: 10.1016/j.biopsych.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ibrahim L, et al. Course of Improvement in Depressive Symptoms to a Single Intravenous Infusion of Ketamine vs Add-on Riluzole: Results from a 4-Week, Double-Blind, Placebo-Controlled Study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012 doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011:1–5. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 86.Mathew SJ, et al. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okamoto N, et al. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. The journal of ECT. 2010;26:223–227. doi: 10.1097/YCT.0b013e3181c3b0aa. [DOI] [PubMed] [Google Scholar]
- 88.Phelps LE, et al. Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salvadore G, et al. An investigation of amino-acid neurotransmitters as potential predictors of clinical improvement to ketamine in depression. Int J Neuropsychopharmacol. 2012;15:1063–1072. doi: 10.1017/S1461145711001593. [DOI] [PMC free article] [PubMed] [Google Scholar]