Abstract
Objective
We sought to identify pathways connecting lifecourse socioeconomic status (SES) with chronic, low-grade inflammation, focusing on the explanatory roles of self-control, abdominal adiposity, and health practices.
Methods
Participants were 360 adults aged 15 - 55 who were free of chronic medical conditions. They were roughly equally divided between low and high current SES, with each group further divided between low and high early-life SES. Structural Equation Modeling (SEM) was used to identify direct and indirect pathways linking early-life and current SES with low-grade, chronic inflammation in adulthood, as manifest by serum interleukin-6 and C-reactive protein. Low SES was hypothesized to relate to inflammation by reducing self-control, which in turn was hypothesized to facilitate lifestyle factors that potentiate inflammation (smoking, alcohol use, sedentary behavior, and weight gain).
Results
Analyses revealed that self-control was pivotal in linking both early-life and current SES to inflammation. Low early-life SES was related to a harsher family climate, and in turn lower adult self-control, over and above the effects of current SES. Controlling for early-life SES, low current SES was associated with perceived stress, and in turn diminished self-control. Results showed that lower self-control primarily operated through higher abdominal adiposity to associate with greater inflammation.
Conclusions
The findings suggest a mechanistic scenario wherein low SES in early-life or adulthood depletes self-control and in turn fosters adiposity and inflammation. These pathways should be studied longitudinally to elucidate and potentially ameliorate socioeconomic disparities in health.
Keywords: self-control, socioeconomic status, inflammation, childhood family, stress, adiposity
The socioeconomic gradient in health outcomes across the lifespan is well-recognized, such that low SES–whether measured by income, education, or occupational attainment– is associated with a disproportionately high burden of morbidity and mortality (Braveman, Cubbin, Egerter, Williams, & Pamuk, 2010). These patterns are especially prominent in coronary heart disease (CHD; Galobardes, Smith, & Lynch, 2006; Pollitt, Rose, & Kaufman, 2005). Interestingly, low SES experienced during either childhood or adulthood is associated with a higher incidence and prevalence of CHD, and these relationships are independent of each other. Despite these links, we have a limited understanding of the developmental and mechanistic pathways through which these social gradients emerge (Matthews & Gallo, 2011; Miller, Chen, & Parker, 2011). How does low SES “get under the skin” to influence behavioral and biological processes that underlie CHD risk? To what extent do childhood and adulthood exposures to low SES act through similar versus disparate pathways?
One mechanism thought to underlie the socioeconomic disparities in CHD is chronic, low-grade inflammation (Marmot, Shipley, Hemingway, Head, & Brunner, 2008). CHD is a chronic inflammatory disease that occurs in the walls of the arteries that supply the heart. Inflammation plays a role at each stage of the atherosclerotic process (Libby, 2012) and, similarly to CHD, shows stratification by SES. That is, low SES individuals tend to show higher levels of inflammatory biomarkers, such as C-reactive protein (CRP) and interleukin-6 (IL-6), and this association is present in childhood, adolescence and adulthood (Pietras & Goodman, 2013; Pollitt et al., 2007; Schreier & Chen, 2010; Taylor, Lehman, Kiefe, & Seeman, 2006). Despite theoretical efforts to describe lifespan pathways that may connect the broader socioeconomic context to individual psychosocial characteristics and, in turn, to inflammatory processes (Miller, Chen, & Cole, 2009; Taylor, 2010), there have been few empirical tests of these pathways (see Taylor et al., 2006 for an exception). Guided by the overarching goals of identifying such developmental trajectories, and building on studies that establish links between some of the factors considered here (Evans, Fuller-Rowell, & Doan, 2012; Lee et al., 2013; Matthews, Chang, Thurston, & Bromberger, 2014; Taylor et al., 2006), we proposed a model whereby low lifecourse SES is associated with stressful experiences (in childhood, harsher family climate; in adulthood, the proliferation of demands and stressors in low SES environments), which undermine self-control. The ability to exercise self-control then shapes health practices (smoking, alcohol use, sedentary behavior, weight gain) that potentiate inflammation. Even though this model has been proposed theoretically (Miller, Chen, & Parker, 2011), to date it has never been empirically tested.
Low Self-Control and Health
Self-control, defined as the ability to control one’s impulses and abstain from gratifying immediate needs and desires (Hagger, Wood, Stiff, & Chatzisarantis, 2010), is increasingly recognized as an important contributor to the adoption and maintenance of behaviors associated with morbidity and mortality (Bogg & Roberts, 2004; Moffitt et al., 2011). To the extent that they are low in self-control, individuals tend to have higher rates of tobacco use, excessive alcohol consumption and sedentary behavior or poor adherence to exercise regimens, which promotes weight gain (Bogg & Roberts, 2004; 2012). These behaviors activate inflammatory processes and are also established risk factors for CHD (Marmot et al., 2008).
Pathways from Socioeconomic Disadvantage to Self-Control
Low SES in childhood or adulthood is thought to undermine self-control skills. For instance, one longitudinal population study following children from birth showed that higher chronicity of family poverty was associated with proportionally lower child self-regulation as early as age 4 (Raver, Blair, & Willoughby, 2012). These associations between low SES and reduced performance on self-control tasks continue to be observed across childhood and into adolescence (Evans & English, 2002; C.-T. Lee, McClernon, Kollins, Prybol, & Fuemmeler, 2013; Sarsour et al., 2011). The quality of the family environment is likely an important pathway through which these effects occur. Severe poverty is known to disrupt family systems and increase the risk of harsh or inconsistent parenting, interpersonal conflict, or child abuse in a subset of low SES-families (Blair & Raver, 2012; Conger & Donnellan, 2007). Children growing up in harsh family climates tend to experience dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and of sympathetic-adrenomedullary (SAM) activity (Repetti, Taylor, & Seeman, 2002) and the hormonal products of these systems can alter the function and structure of prefrontal neural circuitry involved in self-regulation, as shown in both human and nonhuman animal studies (Arnsten, 2009). Harsh families can also model impulsive behaviors, demonstrate less effective self-control strategies, and are less likely to invest time in cognitively stimulating activities that exercise children’s inhibitory control (Conger & Donnellan, 2007).
In adults, socioeconomic deprivation can reduce self-control through several pathways. Poverty-related concerns can consume mental resources, which can lead to self-control depletion (Hagger et al., 2010; Mani, Mullainathan, Shafir, & Zhao, 2013). Additionally, it may be adaptive to actively choose short-term rewards over long-term benefits when poverty and scarcity cues are present (Liu, Feng, Suo, Lee, & Li, 2012). Importantly, in low SES environments stressors and challenges are prevalent, and may activate hormonal systems that acutely impair prefrontal systems mediating effective self-regulation (Arnsten, 2009).
Pathways from Self-Control to Inflammation
There is emerging evidence that low self-control, or related processes such as poor emotion regulation, are associated with low-grade inflammation (Appleton, Buka, Loucks, Gilman, & Kubzansky, 2013; Moffitt et al., 2011). These associations seem to be driven, in part, by variations in health practices, like smoking, alcohol use, and sedentary behavior (Hagger-Johnson, Mõttus, Craig, Starr, & Deary, 2012; Kershaw, Mezuk, Abdou, Rafferty, & Jackson, 2011; Lee et al., 2013), all of which are known to stimulate inflammation. Low self-control might also contribute to inflammation through obesity, brought about by unhealthy eating patterns and a sedentary lifestyle (Vainik, Dagher, Dubé, & Fellows, 2013). Obesity is more prevalent in low SES families in developed countries (McLaren, 2007) and potentiates inflammation (Hotamisligil, 2006). Abdominal fat in particular is a major source of inflammatory mediators such as IL-6 (Hotamisligil, 2006), and across the lifespan, the prevalence of abdominal adiposity socially patterns with low SES (Slopen et al., 2013).
Aims of the Present Study
To our knowledge, this is the first empirical study to test the hypothesis that self-control explains, at least in part, the association between lifecourse SES and low-grade inflammation in adulthood. We proposed and tested a pathway model (upper panel of Figure 1) in a large sample of healthy adults that varied in terms of early-life and current SES. The direct and indirect paths from the two SES measures to self-control and from self-control to inflammation were tested simultaneously in an SEM framework. Lastly, we aimed to examine whether the pattern of associations observed in this study could rule out the possibility of reverse causality, whereby inflammation would influence self-control. This competing explanation was suggested by evidence that pro-inflammatory cytokines exert effects on the central nervous system that can manifest in behavioral and cognitive processes related to self-control (Irwin & Cole, 2011).
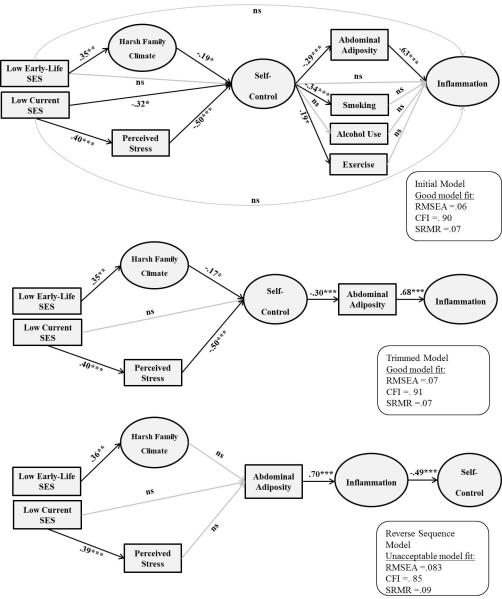
Figure 1.
Structural Equation Models tested. Significant standardized paths are displayed in black, non-significant in gray. Latent variables are represented by circles and observed variables by rectangles. Indicators for each latent factor and paths from age, gender, and ethnicity to the endogenous variables in the structural models were included, but not shown here for visual clarity. ***p < .001; **p < .01; *p < .05; ns = non-significant.
Methods
Participants
The study recruited 360 participants from Vancouver, BC, Canada through postings in local media and public transit. Participants were between the ages of 15 and 55 (M = 36.5 years, SD = 10.8) and fit into one of four groups defined by childhood (low vs. high) and adulthood (low vs. high) SES (see operational definitions under Measures and participant characteristics in Table 1). To minimize confounding by health status, participants had to be (a) free of infectious disease in the two weeks before testing, as evidenced by a normal complete blood count, and (b) without a history of serious and chronic medical illnesses including, but not limited to, cancer, diabetes mellitus, heart disease, stroke, autoimmune disease, HIV/AIDS, hepatitis, chronic obstructive pulmonary disorder, asthma, schizophrenia, bipolar disorder, and dementia. Participants who presented with acute infections were rescheduled after the signs had resolved. Candidates were also screened out if they were not fluent in English, if they were pregnant or had been pregnant in the prior year. The project was approved by the University of British Columbia’s Research Ethics Board.
Table 1.
Characteristics of the sample. Mean (SD) is presented, unless noted otherwise.
| Sample split by early SES | Sample split by current SES | |||||
|---|---|---|---|---|---|---|
|
| ||||||
|
Low SES
(n=192) |
High SES
(n=168) |
p |
Low SES
(n=171) |
High SES
(n=189) |
p | |
| Age | 37.6 (10.3) | 35.2 (11.2) | .03 | 35.4 (10.7) | 37.4 (10.8) | ns |
| Sex, Female, n(%) | 103 (53.6%) | 95 (56.5%) | ns | 91 (53.2%) | 107 (56.6%) | ns |
| Descent, European, n(%) | 126 (65.6%) | 138 (82.1%) | <.001 | 126 (73.7%) | 138 (73%) | ns |
| Mean parental education, 0-7* | 1.72 (1.2) | 3.86 (1.5) | <.001 | 2.5 (1.6) | 2.9 (1.8) | .03 |
| Current education (household maximum), yrs. | 15.1 (2.8) | 15.7 (2.5) | .04 | 14.1 (2.2) | 16.5 (2.5) | <.001 |
| Current annual household income, mean | $35-49.9K | $35-49.9K | ns | $25-34.9K | $50-74.9K | <.001 |
| Waist circumference (cm) | 87.6 (14.4) | 83.7 (12.8) | .007 | 86.9 (14.03) | 84.7 (13.5) | ns |
| BMI (kg/m2) | 26.6 (6.5) | 24.8 (4.8) | .002 | 26.1 (6.1) | 25.4 (5.6) | ns |
| Heavy cigarette smoker, >=10 cigarettes/day, n(%)** | 18 (10%) | 14 (8.6%) | ns | 27 (16.7%) | 5 (2.8%) | <.001 |
| Alcohol user, >=10 drinks/week, n(%)** | 20 (10.9%) | 29 (17.6%) | ns | 22 (13.5%) | 27 (14.6%) | ns |
| Physical activity, hr/week | 2.65 (2.9) | 3.03 (2.9) | ns | 2.9 (3.2) | 2.8 (2.6) | ns |
| Using oral contraceptives, n(%) of females** | 22 (22%) | 28 (37.3%) | ns | 21 (27.6%) | 29 (33.7%) | ns |
Parents’ education when the participant was 0-5 years old was considered; 0=less than high school, 1=some high school, 2=high school, 3=some college, 4=college, 5=university, 6=master’s, and 7=doctoral.
Percentage is reported based on participants who provided health behavior information.
Procedure
All participants completed laboratory sessions between 8:00 a.m. and 10:00 a.m. following an overnight fasting period. After informing participants about the study and obtaining written consent, they completed a battery of self-report measures and experimental tasks, described below. Participants had their height measured on a balance beam scale and their weight measured on an electronic scale; waist circumference was read at the midpoint between the upper iliac crest and lower costal margin at the mid-axillary line. Waist circumference was used as a measure of adiposity, because abdominal fat in particular is a source of inflammation (Hotamisligil, 2006). Peripheral blood was then collected by antecubital venipuncture to measure inflammatory endpoints.
Measures
Socioeconomic status
Participants were recruited based on their early-life and current SES, as defined by occupational status ratings derived from the United Kingdom’s National Statistics Socioeconomic Classification (NSSC). This system is used widely in epidemiology and has been updated regularly since 1911, allowing for comparability to previous research. It is also well-suited to the Canadian social structure. Occupational status is often a more visible aspect of SES than educational attainment and is a more stable measure of SES than income, which can fluctuate markedly. Furthermore, reports of parental occupation are less likely to be prone to recall problems compared to information on parents’ income during childhood or their exact educational level. To classify the early-life SES of prospective subjects, we coded parental occupational status during their first 5 years of life, using the higher of mother’s vs. father’s ratings. To classify the current SES of prospective subjects, we coded their occupational status over the past 5 years, as well as that of their spouse or partner (the higher of the two ratings was used). A small minority of the subjects in our study were ages 15-23 (10.6%) and were full-time students, financially supported by their families. For these cases, we used parental occupation to categorize current SES, unless the subject was financially independent. Candidates whose lifecourse SES fell into one of four categories, as defined by early-life and current circumstances, were enrolled in the study. The categories were low early/low current; low early/high current; high early/low current; and high early/high current SES. In this sample, low SES occupations included clerical and manual positions, such as cleaners, laborers, and transportation operatives. High SES included higher managerial and professional occupations, such as architects, engineers, and medical practitioners.
Childhood family climate
Participants were prompted to think about their family relationships between the ages of 0 to 161 and complete four well-validated questionnaires. The Risky Families (RF) questionnaire (Taylor, Lerner, Sage, Lehman, & Seeman, 2004) includes 13 Likert-type items using 5-point scales (from 1=“Not at all” to 5= “Very often”) to answer questions such as “How often would you say that a parent or other adult in the household behaved violently toward a family member or visitor in your home?” The scale had high reliability in our sample (Cronbach’s alpha = .89) and is known to have high validity, being correlated with clinical interview information (Taylor et al., 2004). Participants also completed the short form (28-item version) of the Childhood Trauma Questionnaire (CTQ, Bernstein et al., 2003), a self-report measure of physical, emotional or sexual abuse, and emotional or physical neglect caused by a family member. The scale had high reliability in this sample (Cronbach’s alpha = .86) and is known to have high convergent validity with clinical interviews (Bernstein et al., 2003). Lastly, participants completed the Indifference Scale of the Measure of Parental Style (MPS, Parker et al., 1997) to retrospectively report on the parenting style of their Mothers (6 items) and Fathers (6 items). Each item used a 4-level Likert-type scale (from 0 = “not true at all” to 3 = “extremely true”) for rating how characteristic the behaviors were for their mother or father (e.g., “ignored me”, “was uninterested in me”). The two scales had high reliability (Cronbach’s alpha = .87 and .93, respectively) and have been shown to have high external validity (Parker et al., 1997).
Perceived stress
Participants completed the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983) to rate their stressful experiences in the previous month by using 10 Likert-type items on a 5-point scale, from 0 = “never” to 4 = “very often” (e.g., “In the last month, how often have you felt difficulties were piling up so high that you could not overcome them?”). The scale had high reliability in this sample (Cronbach’s alpha = .90) and has high convergent validity with life-events scores and physical or mental health symptoms (Cohen et al., 1983).
Self-control
A multi-method battery was used to assess self-control. It included the 13-item Brief Self-Control Scale (BSCS, Tangney, Baumeister, & Boone, 2004), which had high reliability (Cronbach’s alpha = .85) and has had high validity in prior research (i.e., predicts academic and social adjustment, less binge eating and alcohol abuse, Tangney et al., 2004). Participants completed the 13 Likert-type items using 1-5 scales (1= “Not at all” to 5 = “Very much”) to endorse statements such as “I am good at resisting temptation” or “I often act without thinking through all the alternatives.” We also administered a computerized Delayed Discounting Task (DDT) in which participants chose between immediate and postponed monetary rewards (De Wit, Flory, Acheson, McCloskey, & Manuck, 2007). Participants chose between hypothetical amounts of money available that day ($0.10 to $105) and $100 available after a delay of 0, 7, 30, 90, 180, 365, or 1825 days. All combinations of immediate reward and delay interval were presented on a computer screen in randomized order. A nonlinear curve-fitting program was used to estimate the steepness of the discount function k, a hyperbolic function fitted to model the relation between the value of the delayed outcome and the length of the delay. This measure (k) is known to have high test-retest reliability (.81), is stable across different types of rewards, and has high validity, being correlated with real-world impulsive behaviors such as drug abuse (Odum, 2011). The last indicator of self-control was a widely-used laboratory task targeting eating Self-Control (e.g., Tice, Bratslavsky, & Baumeister, 2001) which asked participants to taste three types of candy from different bowls and rate their taste, texture, and freshness. They were told to eat as many pieces as they need to for having accurate ratings. The total quantity of candy (in grams) consumed during this task, and for the rest of the session, was recorded surreptitiously to index self-control of eating behavior.
Systemic inflammation
We assessed the extent of low-grade, chronic inflammation using serum levels of C-reactive protein (CRP) and interleukin-6 (IL-6). These are the two most widely used biomarkers of systemic inflammation, and they have been shown to forecast the development of CHD, above and beyond traditional risk factors (Libby, 2012; Yeh & Willerson, 2003). Blood was collected into Serum-Separator Tubes (Becton-Dickinson, Oakville, Ontario) and following the manufacturer’s guidelines, left to clot for 60 min, followed by centrifugation for 10 min at 1000 x g. The serum was harvested and frozen at −80° C until analysis. CRP was measured using a high-sensitivity, chemiluminescent technique on an IMMULITE 2000 (Diagnostic Products Corporation). The CRP assay had an average inter-assay coefficient of variation (CV) of 2.2% and a detection threshold of .20 mg/L. IL-6 was assayed using commercially available high-sensitivity ELISA kits from R&D Systems, which had a detection threshold of 0.04 pg/ml and an average intra-assay CV of 6.19%.
Demographics and health behaviors
Subjects completed questionnaires about basic demographic information (age, gender, and ethnicity) and about their health habits including smoking, drinking, and exercise. Given the ethnic distribution of the sample (73.3% European descent, 16.8% Asian descent, <5% any other ethnic group), the variable was recoded (0= European descent, 1=non-European) to maximize statistical power for ethnicity effects. Using a previously validated instrument (Miller, Cohen, Rabin, Doyle, & Skoner, 1999), we collected information on daily smoking and alcohol use. The distributions of both variables were highly skewed, so they were converted into ordinal scales. For smoking, the new variable was coded as 0 = non-smoker, 1 = less than 10 daily cigarettes, and 2 = 10 or more cigarettes per day. For alcohol, it was 0 = zero drinks per week, 1 = less than 10 drinks per week, and 2 = 10 or more drinks per week. Lastly, regular physical activity was measured with the well-validated Paffenbarger Activity Scale (Paffenbarger, Blair, Lee, & Hyde, 1993), which estimates typical weekly energy expenditure. The total number of hours of brisk physical activity per week was used in analyses.
Data Analysis
Data preparation
Variables were examined for outliers and for their approximation of the normal distribution before analyses. CRP values greater than 10 mg/L are indicative of infection, trauma, or disease (e.g., Yeh & Willerson, 2003), and thus they were excluded from analyses (n = 7). For variables without clear prior guidelines on meaningful cut-offs, we excluded values exceeding 4 standard deviations from the mean (IL-6: n = 5; Waist circumference: n = 2; Paffenbarger total: n = 2; DDT k: n = 2). Next, a logarithmic transformation was applied to normalize the distributions of skewed variables (CRP, IL-6, DDT k, quantity of candy, and scores on the CTQ, MPS-Mother, MPS-Father, and Paffenbarger; all had a right skew before log transformation).
Missing data
Less than 5% missing data was observed for each of the variables used in analyses, making imputation unnecessary. Given that SEM uses the correlation matrix of observed variables for analyses and that at least some pair-wise correlations were drawn from n = 360, participant information is presented for the full sample.
Statistical analyses
The proposed model was tested using SEM, implemented with Mplus Software (version 6.12, Muthén & Muthén, 2011). SEM enables users to examine the validity of models that specify relations amongst multiple constructs of interest. With SEM, one can simultaneously estimate the strength of a direct pathway between two constructs (e.g., early-life SES to low-grade inflammation), and indirect pathways that involve proposed mediators (e.g., from self-control to cigarette smoking to low-grade inflammation). SEM can also make use of latent constructs, which are formed by extracting the common variance among several observed variables, in the process excluding the measurement error associated with each indicator. Dummy-coding (0 = “high”, 1 = “low”) was used for both early-life and current SES, given that Mplus allows both ordinal and continuous variables. Because a dummy-coded interaction term of early-life SES x current SES had no significant paths to any of the outcome variables (p’s >.13), only the main effects of early-life and current SES were kept in all models to preserve parsimony.
Data analyses proceeded in four phases. In the first step, we constructed latent variables using the multiple observed indices of Harsh Family Climate, Self-Control, and Inflammation, and then evaluated the fit of this measurement model. The three latent factors were allowed to co-vary and the strengths of their associations were estimated in Mplus. We did not construct a latent factor of Health Behaviors because the correlations amongst smoking, alcohol use, and physical activity were low (Table 2), suggesting they would not aggregate on a single factor. Next, we estimated an initial model, depicted in the upper panel of Figure 1. It posited that (a) early-life SES would relate to inflammation via family climate, self-control, and health practices and that (b) current SES would relate to inflammation via perceived stress, self-control, and health practices. The model also included direct pathways from SES indicators to self-control (to examine the incremental value of having family climate and perceived stress in the model) and from self-control to low-grade inflammation (to examine the incremental value of having abdominal fat and health practices in the model). We also included direct pathways from the SES indicators to Inflammation, to examine whether self-control and health practices added incremental value to the models. In the third step of the analysis, we dropped non-significant paths from the Initial Model, and examined whether the data still continued to fit the resulting Trimmed Model. In the last step we tested a competing model, wherein the ordering of constructs was switched, so that SES related to Inflammation, which in turn affected Self-Control. As noted, this model was suggested by evidence that pro-inflammatory cytokines have neurobehavioral and neurocognitive effects on processes related to the self (Irwin & Cole, 2011). All models controlled for age, gender, and ethnicity by including paths from these covariates to each endogenous variable in the structural model.
Table 2.
Zero-order correlations amongst observed variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Low early-life SES | - | 0.08 | .20** | .23** | 0.09 | .13* | 0.06 | −0.01 | 0.04 | 0.02 | .14** | −0.08 | 0.00 | −0.09Δ | 0.04 | 0.10Δ |
| 2. Low current SES | - | .21** | .19** | .16** | .13* | −.16** | −.23** | 0.06 | .19** | 0.08 | −0.01 | .24** | −0.04 | 0.01 | .16** | |
| 3. Ln CTQ total | - | .86** | .69** | .58** | −.17** | −0.08 | 0.01 | .28** | 0.08 | −0.03 | .23** | −0.03 | 0.08 | .14* | ||
| 4. Risky Families total | - | .64** | .58** | −.15** | −.14* | 0.03 | .31** | 0.06 | 0.01 | .18** | −0.06 | 0.05 | 0.10Δ | |||
| 5. Ln MPS Maternal Indifference | - | .57** | −.15** | −0.03 | 0.06 | .24** | 0.01 | −0.02 | .12* | 0.02 | 0.05 | 0.07 | ||||
| 6. Ln MPS Paternal Indifference | - | −.18** | −0.03 | 0.07 | .30** | 0.04 | −0.03 | 0.11Δ | −0.05 | 0.05 | 0.05 | |||||
| 7. Self-Control Scale total | - | .22** | .12* | −.44** | −.22** | −.17** | −.15** | 0.03 | −.14* | −.17** | ||||||
| 8. Ln Delay Discounting k (rev) | - | .16** | −.13* | −.12* | −0.07 | −.21** | 0.06 | −0.08 | −0.07 | |||||||
| 9. Ln Candy Visit total (rev) | - | −0.04 | −0.04 | 0.00 | −0.07 | −0.06 | −0.06 | 0.00 | ||||||||
| 10. Perceived Stress Scale total | - | 0.09Δ | −0.02 | .15** | −.17** | 0.01 | 0.10* | |||||||||
| 11. Waist circumference | - | 0.01 | 0.10Δ | −.15** | .39** | .37** | ||||||||||
| 12. Alcohol use | - | 0.06 | .16** | 0.04 | −0.03 | |||||||||||
| 13. Cigarette smoking | - | −0.07 | 0.06 | .16** | ||||||||||||
| 14. Ln Paffenbarger total | - | −.14* | −.12* | |||||||||||||
| 15. Ln C-reactive protein | - | .44** | ||||||||||||||
| 16. Ln interleukin-6 | - |
p < .01;
p < .05;
p < .10.
Several indices of model fit were considered jointly. Given that the chi-square test of model fit is almost always significant in larger samples (Bentler & Bonett, 1980), we used the following commonly accepted criteria to assess model fit: the root mean square error of approximation (RMSEA) being <.05 for good fit and <.08 for acceptable fit, a comparative fit index (CFI) of at least .90 and the standardized root mean square residual (SRMR) <.08 (McDonald & Ho, 2002). Additionally, the AIC and sample size-adjusted BIC indices (which increase as information is lost, indicating worse model fit) were used to compare models. Maximum likelihood estimation with robust standard errors was employed in all models.
Results
Descriptive Statistics
Table 1 displays sample characteristics on major constructs and the extent to which they differed by early-life and current SES. As expected, low and high early SES groups differed significantly on parental education during their first 5 years of life. They also differed by age, ethnicity, current years of education, waist circumference, and BMI, but were similar on other major constructs. For these reasons, all analyses controlled for age, ethnicity, and paths originating from current SES, whereas adiposity (waist circumference) was included in all models as one of the constructs of interest. As expected, low and high current SES groups differed significantly in current educational level and current annual income. Additionally, they differed in mean parental education, which is why analyses considering current SES parsed out variance due to paths originating from early-life SES; they also differed in the prevalence of heavy smoking, which was included as a mediator in the pathways estimated by our models.
Evaluating the Measurement Model
Table 2 presents zero-order correlations amongst the measured variables. As noted, we formed three latent factors based on having multiple indicators of each construct. They reflected Harsh Family Climate, Self-Control, and Inflammation. As Table 2 shows, the indicators of each construct were consistently inter-correlated. Moreover, the confirmatory factor analysis revealed excellent model fit for this measurement model (RMSEA = .048, CFI = .98, SRMR = .04). The latent Harsh Family Climate factor was well defined by the RF total, CTQ total, MPS-Mother and MPS-Father Indifference scores as the four indicators (the standardized paths, i.e., factor loadings, were all significant with p < .001: .91, .94, .73, and .64, respectively). Similarly, the Self-Control factor was well defined by the BSCS scale, the DDT k measure, and the quantity of candy consumed (with the latter two reverse coded; factor loadings of .63, .38, and .20, respectively, with p < .001 for the first two and p = .01 for the latter). Lastly, the Inflammation factor was well defined by serum CRP and IL-6 (loadings of .63 and .72, p < .001 for each).
The measurement model was specified to allow inter-correlations among the three latent factors. As estimated from the SEM measurement model, the Harsh Family Climate and Self-Control factors were inversely correlated (standardized β = −.28, p = .001). Both of these factors were associated with the Inflammation factor. As predicted, Inflammation was inversely associated with Self-Control (standardized β = −.35, p = .001) and positively associated with Harsh Family Climate (standardized β = .15, p = .03).
Testing the Structural Models
Initial Model
We started by testing the model depicted in the upper panel of Figure 1. Results indicated the data were a good overall fit to this model (RMSEA = .06, CFI = .90, SRMR = .07). As predicted, low early-life SES was associated with a more Harsh Family Climate, which in turn was related to low Self-Control (for the indirect path: β = −.07, p = .049). The direct pathway from early-life SES to Self-Control was non-significant (β = .05, p = .73). As predicted, low Current SES was related to greater Perceived Stress, which in turn was associated with low Self-Control (for the indirect path: β = −.20, p = .001). There also was a significant direct path from Current SES to Self-Control (β = −.32, p = .03), indicating these constructs were associated via routes other than Perceived Stress. As predicted, low Self-Control was associated with more Abdominal Adiposity, which in turn was related to higher Inflammation (for the indirect path: β = −.18, p < .001). The direct pathway from Self-Control to Inflammation was non-significant (β = −.10, p = .26), indicating this association was principally accounted for by Abdominal Adiposity. Consistent with this result, none of the health behaviors (Smoking, Alcohol Use, and Physical Exercise) were significantly associated with the Inflammation factor (β = .09, p = .20; β = .02, p = .78; and β = −.07, p = .25, respectively). Moreover, there were no significant indirect pathways linking Self-Control and Inflammation via Health Behaviors (β = −.03, p = .21; β = −.001, p = .79; and β = −.01, p = .30). Self-Control was inversely associated with Smoking (β = −.34, p < .001) and positively associated with Physical Activity (β = .19, p = .02).
Trimmed Model
In the next step, we removed all non-significant paths from the initial model (but we retained all paths involving age, gender, and ethnicity to prevent confounding). The Trimmed Model is depicted in the middle panel of Figure 1. As is evident, most of the paths from the previous model remained statistically significant, with the exception of the direct link between Current SES and Self-Control (β = −.18, p = .16). The observed data fit the Trimmed Model well (RMSEA = .07, CFI = .91, SRMR = .07). In fact, in head-to-head comparisons the Trimmed Model emerged as superior to the Initial Model. Not only was the Trimmed Model more parsimonious, but it had better relative fit (lower AIC = 15296.1 and lower sample size-adjusted BIC = 15333.9) compared to the initial model (AIC = 17396.6, sample-size adjusted BIC =17452.2). As a result, the Trimmed Model was retained. It explained a substantial amount of variance (42%) in the Inflammation construct.
Reverse Sequence Model
Lastly, we tested a competing explanation for the observed pattern of associations. It posited that inflammatory processes have neurobehavioral effects that manifest as low self-control. As depicted in the lower panel of Figure 1, the model was structured in a manner similar to the Trimmed Model, except that Self-Control was hypothesized to be a consequence of Adiposity and Inflammation, rather than a cause. The observed data were not a good fit to this Reverse Sequence model; none of the three overall fit indices were in the acceptable range (RMSEA = .083, CFI = .85, SRMR = .09). Furthermore, this model had worse fit (i.e., higher AIC = 15370.4 and higher sample size-adjusted BIC = 15408.2) compared to the Trimmed Model (AIC = 15296.1 and sample size-adjusted BIC = 15333.9).
Discussion
Despite well-documented gradients in CHD risk by lifecourse SES, little is known about the mechanisms through which low SES “gets under the skin” to influence behavioral and biological processes that underlie CHD risk. The present study focused on self-control as one such mechanism that may connect these different levels of analysis and help to delineate the scenarios linking lifecourse SES with low-grade, chronic inflammation in adulthood. The model identified as best capturing the data supported the pivotal role of self-control in explaining associations of both early-life and current SES with inflammation. Additionally, we identified significant indirect pathways that further explained how SES may relate to self-control and self-control might potentiate inflammation, bringing us closer to understanding how social and economic contexts are transduced into signals that are relevant for individual behavior and biological processes. Low early-life SES was linked to a harsher family climate, lower self-control, more abdominal adiposity, and low-grade, chronic inflammation (in this ordered sequence). These associations were independent of subjects’ current SES, age, gender, and ethnicity. Low current SES was associated with higher perceived stress, which in turn related to lower self-control, more abdominal adiposity, and low-grade, chronic inflammation. Again, these associations were independent of age, gender, ethnicity, and early-life SES. These findings echo previous research that highlights low early-life SES, harsh family climates, self-control, and adiposity as correlates of inflammation (Brody et al., 2013; Danese et al., 2007; Hagger-Johnson et al., 2012; Matthews et al., 2014; Moffitt et al., 2011; Taylor et al., 2006). Here, we build upon these contributions and demonstrate how these constructs are inter-related.
Our findings are consistent with a developmental scenario, wherein low-SES youth tend to be reared in harsher family climates, which impede the maturation and/or expression of self-regulatory behaviors. Indeed, research indicates that harsh family climates are associated with patterns of HPA and SAM activation (Repetti et al., 2002), that can alter the structural and functional development of regions of the prefrontal cortex (Arnsten, 2009), some of which support self-regulatory processes (Arnsten, 2009). Harsh families are also less likely to engage their children in play that promotes inhibitory control and may directly model impulsive behaviors (Conger & Donnellan, 2007). Previous research had linked harsh childhood family climates to inflammation in adolescence or adulthood (e.g., Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Matthews et al., 2014; Miller & Chen, 2010; Taylor et al., 2006) and the present study makes a novel contribution in suggesting that self-control depletion may be an important intermediary link in the indirect pathway between early-life SES and later inflammation.
Our findings are also consistent with a scenario wherein low-SES environments continue to deplete self-control across the lifespan. According to the Trimmed Model, perceived stress is a principal statistical mediator of this association. This finding makes sense conceptually. Lower-SES individuals tend to experience more stress and, as noted above, stress can deplete self-control through numerous behavioral, cognitive, and biological mechanisms. Surprisingly, we did not observe a significant residual association between Current SES and Self-Control after the mediating role of Perceived Stress was considered. We expected that other processes might connect these constructs. For example, low-SES individuals might voluntarily shift away from future-oriented behaviors when scarcity cues are present (Liu et al., 2012). Deprivation might also enhance the perceived value of immediate rewards to levels where it becomes very difficult to exercise self-control (i.e., the task of self-control requires much more of individuals in low SES). However, it is possible –and indeed consistent with our results-that stress might foster these processes. In future research, it will be important to directly measure these additional constructs (future orientation, reward sensitivity) and perhaps test their role as mediators between Perceived Stress and Self-Control, so that we can more fully characterize the pathways linking Current SES with Self-Control.
The results were also consistent with a scenario wherein low Self-Control, acting through abdominal fat accumulation, promotes the development of chronic, low-grade inflammation. A long-term prospective study from the Dunedin Cohort has previously shown that children with low self-control tend to have more inflammation when they reach early adulthood (Moffitt et al. 2011). Our study builds on these results by identifying abdominal adiposity as a mediator of this association, and ruling out other candidate health practices. Moreover, our models showed that adiposity and inflammation were related even with physical activity controlled. This result suggests that self-control is likely to promote inflammation via regulation of food intake rather than physical activity. However, given the cross-sectional design of this study, the effects of prior physical activity on current adiposity cannot be ascertained. Future studies with multi-wave assessments of these constructs are needed to clarify the sequencing and patterning of these associations. With respect to nutritional pathways, it is well known that low-SES neighborhoods have fewer full-service grocery stores that stock healthy foods and more fast-food outlets that serve calorically dense meals (Richardson, Boone-Heinonen, Popkin, & Gordon-Larsen, 2012). When superimposed upon high perceived stress and low self-control, these neighborhood circumstances may facilitate the consumption of calorically dense, obesity-promoting “comfort foods.” In turn, expanded adipose tissue would release pro-inflammatory cytokines like IL-6. These cytokines recruit macrophages to the abdomen, where they attempt to clear necrotic adipocytes, and in doing so further potentiate inflammation (Hotamisligil, 2006).
Previous research has identified health behaviors as mediators of the association between SES and inflammation (Hagger-Johnson et al., 2012; Kershaw et al., 2011). However, we did not find evidence that smoking, alcohol use, or physical activity played substantial explanatory roles in this sample. We do not believe these inconsistencies were principally a result of measurement error (e.g., distorted by self-report biases such as social desirability), as there were significant bivariate correlations between health practice variables and indicators of Self-Control that were in the expected direction, suggesting that the measures did capture some meaningful variance. However, our project did have very stringent eligibility criteria with regard to health, and that may have resulted in a limited range of unhealthy behaviors in the sample. Future studies with a less-restricted sample are needed to more fully evaluate the relative contribution of health behavior to these processes.
The present study had a number of limitations that need to be acknowledged. First, its cross-sectional design precludes any definitive inferences about causality or the temporal ordering of phenomena. Multi-wave, prospective research is needed to substantiate these findings and shed light on how they unfold developmentally. Second, the retrospective assessment of early-life SES and family climate raises concerns about veridicality and directionality. Reverse causality is an unlikely explanation for some of the associations we observed; e.g., it is difficult to imagine how levels of adult inflammation could affect parental occupational status many years prior. Nonetheless, cytokines released in the periphery can trigger parallel inflammatory cascades in the brain, with implications for a variety of cognitive, emotional, and behavioral processes (Irwin & Cole, 2011). The analyses suggested that a scenario in which our distal measures influenced inflammation and inflammation impacted self-control did not adequately fit the data, but the possibility of bidirectional causal arrows cannot be definitively ruled out given that self-control, adiposity, and inflammation were measured at the same time point in this study. That being said, prospective longitudinal studies support the directionality presumed in our models: e.g., prediction from low early-life SES to adult inflammation (Hagger-Johnson et al., 2012), low SES to later self-regulation (Raver et al., 2012), childhood adversity to self-control and to later BMI (Evans et al., 2012), child self-control to adult inflammation (Moffitt et al., 2011), and adiposity to later inflammation (Hotamisligil, 2006). A third limitation was that we lacked a measure of childhood self-control, making it impossible to disentangle how early-life versus current SES affected this competency. Previous research shows moderate stability of self-control across childhood (Moffitt et al., 2011) and the lifecourse more generally (Bogg & Roberts, 2012). These reports of moderate stability support the view that participants’ self-control reflects both early experiences and recent circumstances. Fourth, another limitation is that our models presumed and tested linear associations among constructs. Future studies should examine the possibility of nonlinear patterns (e.g., threshold functions for risk conferred by low SES or harsh family climate). Additionally, the present study aimed to disentangle the effects of early-life versus current SES through a design that rendered these two dimensions approximately orthogonal. That feature precluded us from accounting for their tendency to be associated in the population at large. Although this approach brings us closer to understanding how current and early-life SES may independently contribute to adult health, the necessary trade-off was that our models could not account for the temporal features of and the pathways between early-life SES or family climate and current perceived stress or current SES. Furthermore, the study was not designed to capture SES conditions experienced across the entire lifespan, but future research designed with these goals in mind should pursue these important questions. Finally, we were somewhat surprised to find that an interaction term of early-life SES and current SES did not significantly predict any outcomes. This may also have been a result of study design constraints, or the large statistical power needed to detect such interactions. Future studies with larger samples and continuous indicators of early and current SES are needed to explore this issue more thoroughly.
Even though the best-fitting model explained a substantial proportion of variability in the inflammation construct (42%), the residual variance suggests that other mediators (dietary habits, physical environment) and moderators (genetic liabilities, buffering characteristics) must be considered. To thoroughly understand CHD risk, future studies should also strive to examine more proximal indicators of disease progression, for instance carotid atherosclerosis or coronary artery calcification.
Despite these limitations, the present study yielded innovative findings that shed light on the developmental and mechanistic pathways that connect SES with health. Of particular interest are the pivotal roles identified for Self-Control and Abdominal Adiposity. These observations have important implications for public health promotion, especially since self-control skills are malleable and can be improved with training (Diamond et al., 2007). Furthermore, policy-level interventions which transform healthy choices into the default option and reduce the self-control burden required to achieve health goals could also be effective (e.g., restrictions on high-sugar beverage sales). The present results lay the foundation for testing these pathways longitudinally in order to better understand and minimize socioeconomic health disparities.
Acknowledgements
We thank the participants for their contribution to this project. This research was supported by National Institutes of Health Grant HD058502 to G. E. Miller and F32HD078048 to C. E. Hostinar. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
All results were identical when excluding participants between the ages of 15-17 (2.2% of sample), for whom there was some temporal overlap for the periods captured by childhood family climate ratings and current perceived stress levels. Given that results did not change when excluding them, analyses are reported for the full sample.
References
- Appleton AA, Buka SL, Loucks EB, Gilman SE, Kubzansky LD. Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychology. 2013;32(7):748–56. doi: 10.1037/a0030068. doi:10.1037/a0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. doi:10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychological Bulletin. 1980;88(3):588–606. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Blair C, Raver CC. Child development in the context of adversity: Experiential canalization of brain and behavior. American Psychologist. 2012;67(4):309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin. 2004;130:887–919. doi: 10.1037/0033-2909.130.6.887. doi: 10.1037/0033-2909.130.6.887. [DOI] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. The case for conscientiousness: Evidence and implications for a personality trait marker of health and longevity. Annals of Behavioral Medicine. 2012;45(3):278–88. doi: 10.1007/s12160-012-9454-6. doi: 10.1007/s12160-012-9454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P. A, Cubbin, C., Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: What the patterns tell us. American Journal of Public Health. 2010;100(Suppl):S186–96. doi: 10.2105/AJPH.2009.166082. doi:10.2105/AJPH.2009.166082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Beach SRH, Kogan SM, Windle M, Philibert RA. Harsh parenting and adolescent health: A longitudinal analysis with genetic moderation. Health Psychology. 2013 doi: 10.1037/a0032686. [Epub]. doi:10.1037/a0032686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck TW, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Conger RD, Donnellan MB. An interactionist perspective on the socioeconomic context of human development. Annual Review of Psychology. 2007;58:175–99. doi: 10.1146/annurev.psych.58.110405.085551. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit H, Flory JD, Acheson A, McCloskey M, Manuck SB. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personality and Individual Differences. 2007;42(1):111–121. [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318(5855):1387–1388. doi: 10.1126/science.1151148. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, English K. The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development. 2002;73(4):1238–48. doi: 10.1111/1467-8624.00469. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12146745. [DOI] [PubMed] [Google Scholar]
- Evans GW, Fuller-Rowell TE, Doan SN. Childhood cumulative risk and obesity: The mediating role of self-regulatory ability. Pediatrics. 2012;129(1):e68–73. doi: 10.1542/peds.2010-3647. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16(2):91–104. doi: 10.1016/j.annepidem.2005.06.053. doi:10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NLD. Ego depletion and the strength model of self-control: A meta-analysis. Psychological Bulletin. 2010;136(4):495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Hagger-Johnson G, Møttus R, Craig LCA, Starr JM, Deary IJ. Pathways from childhood intelligence and socioeconomic status to late-life cardiovascular disease risk. Health Psychology. 2012;31(4):403–12. doi: 10.1037/a0026775. doi:10.1037/a0026775. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature Reviews Immunology. 2011;11(9):625–632. doi: 10.1038/nri3042. doi:10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw KN, Mezuk B, Abdou CM, Rafferty JA, Jackson JS. Socioeconomic position, health behaviors, and C-reactive protein: A moderated-mediation analysis. Health Psychology. 2011;29(3):307–316. doi: 10.1037/a0019286. doi:10.1037/a0019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-T, McClernon FJ, Kollins SH, Prybol K, Fuemmeler BF. Childhood economic strains in predicting substance use in emerging adulthood: Mediation effects of youth self-control and parenting practices. Journal of Pediatric Psychology. 2013;38:1130–43. doi: 10.1093/jpepsy/jst056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(9):2045–51. doi: 10.1161/ATVBAHA.108.179705. doi:10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Feng T, Suo T, Lee K, Li H. Adapting to the destitute situations: Poverty cues lead to short-term choice. PloS one. 2012;7(4):e33950. doi: 10.1371/journal.pone.0033950. doi:10.1371/journal.pone.0033950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013;341(6149):976–80. doi: 10.1126/science.1238041. doi:10.1126/science.1238041. [DOI] [PubMed] [Google Scholar]
- Marmot MG, Shipley MJ, Hemingway H, Head J, Brunner EJ. Biological and behavioural explanations of social inequalities in coronary heart disease: The Whitehall II study. Diabetologia. 2008;51(11):1980–8. doi: 10.1007/s00125-008-1144-3. doi:10.1007/s00125-008-1144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Chang Y-F, Thurston RC, Bromberger JT. Child abuse is related to inflammation in mid-life women: Role of obesity. Brain, Behavior, and Immunity. 2014;26:29–34. doi: 10.1016/j.bbi.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annual Review of Psychology. 2011;62:501–30. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RP, Ho M-HR. Principles and practice in reporting structural equation analyses. Psychological Methods. 2002;7(1):64–82. doi: 10.1037/1082-989x.7.1.64. doi:10.1037//1082-989X.7.1.64. [DOI] [PubMed] [Google Scholar]
- McLaren L. Socioeconomic status and obesity. Epidemiologic Reviews. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21(6):848–56. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–24. doi: 10.1146/annurev.psych.60.110707.163551. doi:10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137(6):959–97. doi: 10.1037/a0024768. doi:10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Rabin BS, Doyle WJ, Skoner D. Personality and tonic cardiovascular, neuroendocrine, and immune parameters. Brain, Behavior, and Immunity. 1999;13:109–123. doi: 10.1006/brbi.1998.0545. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):2693–8. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Sixth Muthén & Muthén; Los Angeles, CA: 2011. [Google Scholar]
- Odum AL. Delay discounting: I’m a k, you're a k. Journal of the Experimental Analysis of Behavior. 2011;96(3):427–39. doi: 10.1901/jeab.2011.96-423. doi:10.1901/jeab.2011.96-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenbarger RS, Blair SN, Lee I, Hyde RT. Measurement of physical activity to assess health effects in a free-living population. Medicine and Science in Sports and Exercise. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Parker G, Roussos J, Hadzi-Pavlovic D, Mitchell P, Wilhelm K, Austin M-P. The development of a refined measure of dysfunctional parenting and assessment of its relevance in patients with affective disorders. Psychological Medicine. 1997;27:1193–1203. doi: 10.1017/s003329179700545x. [DOI] [PubMed] [Google Scholar]
- Pietras SA, Goodman E. Socioeconomic status gradients in inflammation in adolescence. Psychosomatic Medicine. 2013;75:442–448. doi: 10.1097/PSY.0b013e31828b871a. doi: 10.1097/PSY.0b013e31828b871a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt R. A, Kaufman, J. S., Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. European Journal of Epidemiology. 2007;22(1):55–66. doi: 10.1007/s10654-006-9082-1. doi:10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: A systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. doi:10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Blair C, Willoughby M. Poverty as a predictor of 4-year-olds’ executive function: New perspectives on models of differential susceptibility. Developmental Psychology. 2012;49(2):292–304. doi: 10.1037/a0028343. doi:10.1037/a0028343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128(2):330–366. [PubMed] [Google Scholar]
- Richardson AS, Boone-Heinonen J, Popkin BM, Gordon-Larsen P. Are neighbourhood food resources distributed inequitably by income and race in the USA? Epidemiological findings across the urban spectrum. BMJ Open. 2012;2(2):e000698. doi: 10.1136/bmjopen-2011-000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, Boyce WT. Family socioeconomic status and child executive functions: The roles of language, home environment, and single parenthood. Journal of the International Neuropsychological Society. 2011;17(1):120–32. doi: 10.1017/S1355617710001335. doi:10.1017/S1355617710001335. [DOI] [PubMed] [Google Scholar]
- Schreier HM, Chen E. Socioeconomic status in one’s childhood predicts offspring cardiovascular risk. Brain, Behavior, and Immunity. 2010;24:1324–1331. doi: 10.1016/j.bbi.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Slopen N, Goodman E, Koenen KC, Kubzansky LD. Socioeconomic and other social stressors and biomarkers of cardiometabolic risk in youth: A systematic review of less studied risk factors. PloS one. 2013;8(5):e64418. doi: 10.1371/journal.pone.0064418. doi:10.1371/journal.pone.0064418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. Journal of Personality. 2004;72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15016066. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8507–12. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults study. Biological Psychiatry. 2006;60(8):819–24. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lerner JS, Sage RM, Lehman BJ, Seeman TE. Early environment, emotions, responses to stress, and health. Journal of Personality. 2004;72(6):1365–1394. doi: 10.1111/j.1467-6494.2004.00300.x. doi: 10.1111/j.1467-6494.2004.00300.x. [DOI] [PubMed] [Google Scholar]
- Tice DM, Bratslavsky E, Baumeister RF. Emotional distress regulation takes precedence over impulse control: If you feel bad, do it! Journal of Personality and Social Psychology. 2001;80(1):53–67. doi:10.1037//0022-3514.80.1.53. [PubMed] [Google Scholar]
- Vainik U, Dagher A, Dubé L, Fellows LK. Neurobehavioural correlates of body mass index and eating behaviours in adults: A systematic review. Neuroscience and Biobehavioral Reviews. 2013;37(3):279–99. doi: 10.1016/j.neubiorev.2012.11.008. doi:10.1016/j.neubiorev.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh ETH, Willerson JT. Coming of age of C-reactive protein: Using inflammation markers in cardiology. Circulation. 2003;107(3):370–371. doi: 10.1161/01.cir.0000053731.05365.5a. [DOI] [PubMed] [Google Scholar]