Abstract
For more than 30 years the study of learning and memory in Drosophila melanogaster (fruit fly) has used an olfactory learning paradigm and has resulted in the discovery of many genes involved in memory formation. By varying learning programs, the creation of different memory types can be achieved, from short-term memory formation to long-term. Previous studies in the fruit fly used gene mutation methods to identify genes involved in memory formation. Presumably, memory creation involves a combination of genes, pathways and neural circuits. To examine memory formation at the protein level, a quantitative proteomic analysis was performed using olfactory learning and 15N labeled fruit flies. Differences were observed in protein expression and relevant pathways between different learning programs. Our data showed major protein expression changes occurred between short-term memory (STM) and long-lasting memory, and only minor changes were found between long-term memory (LTM) and anesthesia-resistant memory (ARM).
Keywords: Olfactory learning, Memory, Drosophila melanogaster, Quantitative proteomics, Metabolic labeling, Mass spectrometry
Introduction
‘Learning and memory’ are two of the more fascinating aspects of the neural system. How they are accomplished has been the subject of much inquiry over the last decades. Learning and memory in humans are quite complex, but animal models are available to study the molecular mechanisms involved. A very powerful model for studying learning and memory is Drosophila melanogaster or the fruit fly, which can employ powerful mutagenesis and genetic methods to identify genes involved in physiological processes.
Methods have been developed to train fruit flies using smells. It is believed there are similarities between the olfactory nervous system of insects and mammals and that the mechanisms behind olfactory learning may be conserved. (1) Olfactory memory is created in flies by training them with conditioned stimuli where a specific odor is associated with a mild electric shock. Following the learning procedure, the avoidance of the punitive odor implies the establishment of memory. The established memory can last for hours, termed short-term memory (STM), or up to days or even a lifetime, which is considered a long-term memory (LTM). An intermediate memory between STM and LTM is anesthesia-resistant memory (ARM). LTM and ARM are both considered as long-lasting-memory. Memory persistence is determined by the type of training. Studies have demonstrated LTM can be formed after spaced training trials, in which training is repeated multiple times with longer time intervals (e.g. 5 min). (2) In contrast, memories formed after a single training session or multiple, massed trainings (no interval), diminish more rapidly. The formation of LTM has been shown to involve gene expression and new protein synthesis, (3, 4) and can be disturbed by the protein synthesis inhibitor cyclohexamide. (2) Mutation studies have identified genes involved in learning and memory formation in a one-gene-at-a-time fashion, even though it is very likely that learning and memory involves many genes and proteins within complex networks or pathways. Furthermore, it is believed that explicit networks of neurons in specific sections of a brain may be involved in specific activities such as learning and memory formation (5, 6) and thus changes that are created may not involve all neurons or an entire brain. The study of neural systems at the protein level to uncover changes related to specific perturbations such as learning and memory requires the ability to detect changes in a subset of neurons. In some organisms large neurons allow studies of peptides and proteins in single neurons, which is a powerful approach for study of the neuronal response to stimuli. (7, 8)
Mass spectrometry-based proteomics has emerged as an important tool for biological studies.(9) Quantitative mass spectrometry methods that employ stable isotopes have been developed, including a method developed by, Heck et al to label fruit flies for quantitative proteomic analysis. (10-12) In this study, we used the Heck approach to label fruit flies with 15N and measured protein changes in fruit fly brains trained with multiple spaced or massed sessions at 3 hours and 24 hours after the training procedures (Figure 1). The goal was to determine if gene products known to be involved in learning and memory were changed as a result of different training procedures and memory states and to identify new proteins. A fundamental question in these studies is if mass spectrometry analysis has sufficient dynamic range to measure molecular changes occurring in specific neurons involved in different memory states and if measured differences will be statistically significant.
Figure 1.
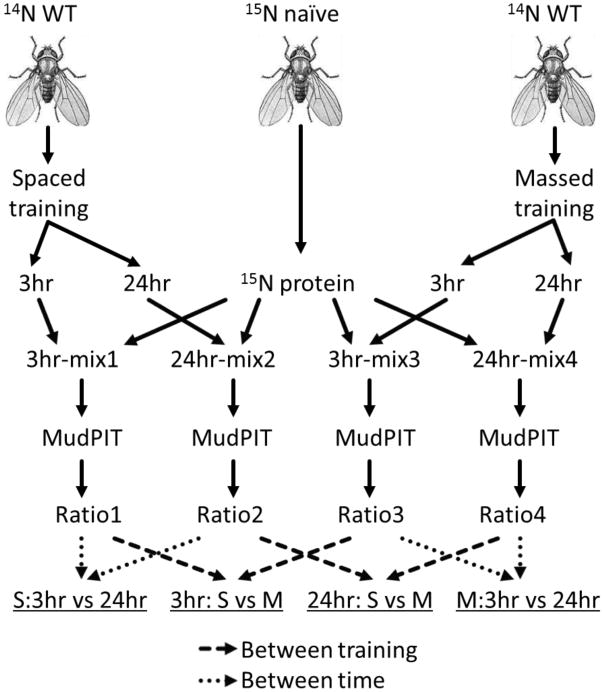
Proteomic experimental scheme for olfactory memory study in Drosophila. Two learning methods were employed: spaced and massed training. 3 hr and 24 hr memories were assessed, for both training separately. Proteome comparisons between different biological statues were executed by using naïve 15N fly brains as internal standard.
In summary, we identified a large number of proteins from the fly brain including many differentially expressed proteins and their pathways related to memory formation from different trainings and/or time points. The proteomes between STM and long-lasting memory (STM vs. LTM, and STM vs. ARM) were significantly different, but only minimal protein expression changes could be observed between LTM and ARM (LTM vs. ARM).
Materials and Methods
Drosophila melanogaster w1118
Flies were raised in accordance with the Cold Spring Harbor Laboratory regulations under the supervision of Dr. Tim Tully. (Dart NeuroScience LLC. CA, USA) The olfactory learning training was performed as described previously. (2, 13) Briefly, the flies were subjected to either multiple massed or spaced training sessions. In each training cycle, the flies were exposed to one odor with electric shock, and the second odor without punishment. The 15N labeling of the flies was completed by feeding them 15N yeast (Silantes GmbH, Munich, Germany) as described previously.(11)
Samples preparation
100 female fly heads were collected, and homogenized in lysis buffer with 10 mM Tris pH 7.4, 300 mM NaCl, 30 mM EDTA, 0.5% Triton X-100, protease inhibitor by using a bead-beating FastPrep homogenizer (MP Biomedicals, LLC. OH, the USA). Protein supernatants were obtained by centrifuging at 14,000 rpm and 4 °C for 30 min, and the protein concentration was determined by using a BCA protein assay. 15N and 14N protein extracts were mixed at 1:1 ratio based on their protein amounts. In total four mixtures were generated for two training types and two test time points respectively (Figure 1).
Thirty micrograms of the protein mixture was precipitated with 5× volume of cold acetone, and then solubilized and reduced with 100 mM Tris-HCl/8M urea/5 mM DTT. Cysteines were then alkylated with 10 mM iodoacetamide. The solution was diluted 1:4 with 100 mM Tris-HCl and digested with 1 μg of trypsin at 37 °C overnight. The digestion was terminated by adding formic acid to 2%.
Shotgun proteomic analysis
The resulting peptides were subjected to 11-step MudPIT LC-MS/MS analysis on a Velos Orbitrap (Thermo Fisher Scientific GmbH, Bremen, Germany) mass spectrometer as described previously. (14) A cycle of one full-scan mass spectrum (300-1600 m/z) at a resolution of 60,000 followed by 20 data dependent MS/MS CID spectra at a 35% normalized collision energy was repeated continuously throughout each step of the multidimensional separation. The maximum ion accumulation times were set to 250 ms for survey MS1 scans and to 25 ms for MS2 scans. Other instrumental parameters include dynamic exclusion with a repeat count 1, duration 30.00S, list size 500, exclusion duration 120.00S, exclusion mass with high/low 1.5/0.51 m/z.
MS data process
Protein identification was performed with the Integrated Proteomics Pipeline - IP2 (Integrated Proteomics Applications, Inc., San Diego, CA. http://www.integratedproteomics.com/) using ProLuCID (15) and DTASelect 2 (16). The tandem mass spectra were searched against UniProt Drosophila melanogaster protein database. Cysteine carboxyamidomethylation was set as a stable modification. In order to accurately estimate peptide probabilities and false discovery rates, we used a target/decoy database containing the reversed sequences of all the proteins appended to the target database. The protein false discovery rates were controlled below 1% for each MudPIT analysis.
Direct peptide/protein quantification was performed with Census software. (17) Isotopic distributions for both unlabeled and labeled peptides were calculated and this information was then used to determine the appropriate m/z ranges from which to extract ion intensities.
Ratio/ratio quantification and statistical analysis between biological replicates were computed by the module ‘quantification compare’ in IP2. P-values and adjusted BH P-values (18) were calculated for each protein.
GO and KEGG pathway analysis
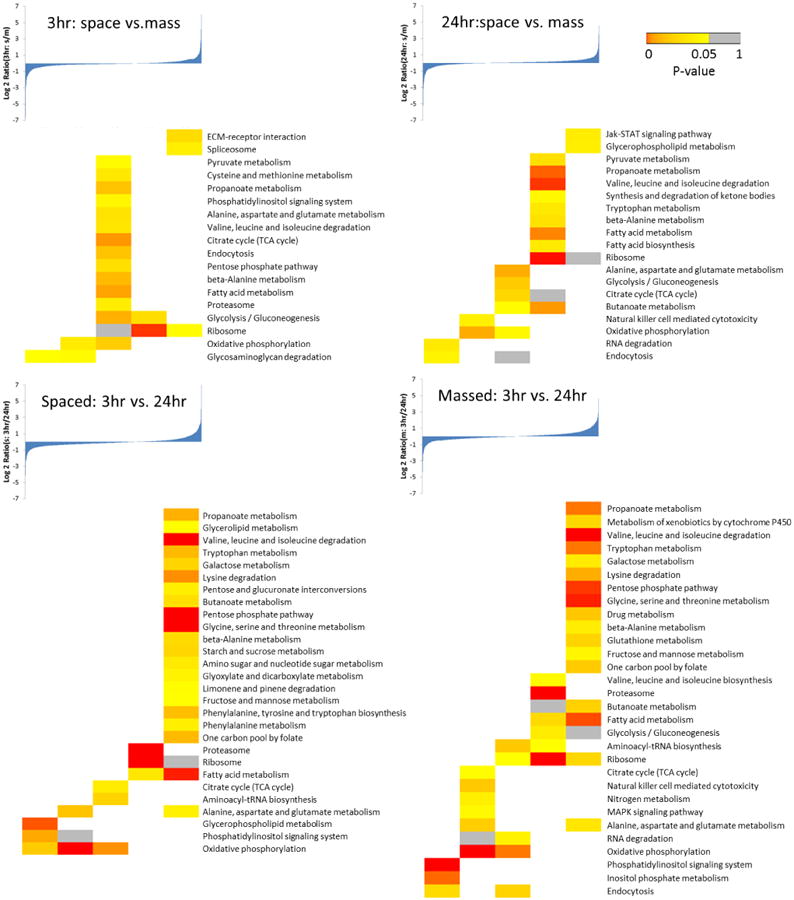
For each experimental comparison, the log2 ratios of protein expression changes were sorted in ascending order, and divided into five bins evenly (see the protein ratio distributions above the heat maps in Figure 3). Each protein bin was subjected to Gene Ontology (GO) (19) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (20) analysis performed using DAVID (21) (http://david.abcc.ncifcrf.gov). The enriched GO FAT categories and KEGG pathways were exported using default filtering parameters. The P-values or BH-adjusted P-values calculated by DAVID were displayed in heat maps with the Gitools. (22) For fly and bee comparison, the whole identified brain proteomes were used as the input in the DAVID analysis.
Figure 3. KEGG pathway analysis for four proteome comparisons.
Results and discussions
Fly brain proteome profile
Four sample sets were analyzed using a shotgun quantitative proteomic approach (Figure 1). Each sample set comprised three biological replicates. For each proteomic analysis, approximately 4,000 protein groups (grouping all the ambiguous protein IDs) and 30,000 peptides were identified at a protein FDR of 1%. A total of 13,271 protein accession IDs and 58,976 peptides were identified overall, which is the largest fly brain proteome obtained to our knowledge.
Proteome quantification
14N-15N peak quantifications were performed using the mass spectrometry data analysis algorithm Census. In this study, the 15N signals served as internal standards, and four indirect comparisons between either different training types or different test time points were generated (Figure 1). The ratio/ratio strategy enabled us to minimize any potential effects caused by the 15N diet. All the quantitative results can be found in the supplementary tables.
We first compared the brain proteomes derived from two training types. Both spaced and massed trained flies had STM and long-lasting memory at 3 hr and 24 hr time points, respectively. However, the 24 hr long-lasting memories for spaced and massed trained flies are different; spaced trained flies had LTM, whereas massed trained flies had ARM. At 3 hr time points, 8,435 protein IDs were quantified using 15N as the reference. Over 95% of proteins showed average fold changes smaller than 2. No protein expression changes greater than 2 fold between spaced and massed training procedures had a p-value less than 0.05 for all three replicates. By using alternate criteria (2 out of 3 replicates; and average change >1.5 fold, or >1.2 fold change and a P-value <0.05), 218 proteins were down regulated and 149 proteins were up regulated in spaced trained flies. (Figure 2) These results were in line with the behavioral results, in which STM was observed in both training types, and the memory performance indices (PI) of spaced and massed trained flies were quite similar at this time. (2) In addition, the overall unchanged proteomes at 3 hr actually provided evidence that our experimental data had very high quantification accuracy.
Figure 2.
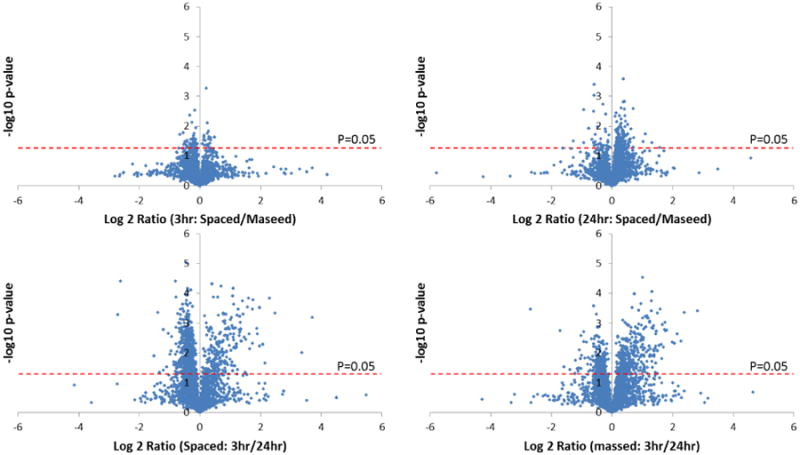
Volcano plots for four proteome comparisons. In each plot, Log2 fold changes were plotted against−long10 (p-values). The dashed red line indicates a significant p-value of 0.05. Briefly, the differentially expressed proteins in 3 hr vs. 24 hr comparisons were much more than those derived from spaced vs. massed comparisons.
By applying the same criteria (2 out of 3 replicates and average change >1.5 fold, or >1.2 fold change and a P-value <0.05) to the comparison of spaced and massed trainings at the 24 hr time point, 224 proteins out of 7,352 total proteins had lower expression levels in the spaced trained flies, and 284 proteins had increased expression levels in the spaced trained flies (Figure 2). 24 proteins had >1.5 fold changes with P-values better than 0.05. (Table 1) This result was again consistent with the behavioral tests, as the memory performance indices were discriminated clearly at the 24 hr time point, (2) and more changed proteins were found at the 24 hr time point as well. These 24 changed proteins represent differences between LTM and STM, therefore are of great importance. Two proteins associated with immune function, hemolectin and immune-induced peptide 4, were found to be up-regulated in spaced trained flies. Hemolectin and immune-induced peptide 4 themselves have not been linked with memory previously, but there is increasing evidence the immune systems plays an important role in learning and memory. (23, 24) In addition, several ion binding related proteins (Q9VTB0_DROME, HGD_DROME, RENT1_DROME, Q0E9F9_DROME) were found to be changed at 24 hr, which indicate ion modulation may be involved in memory formation as well.
Table 1. Proteins significantly altered between spaced and massed trained flies at 24 hr.
| Locus | P-value | Log2 R (Spaced/Massed) | Description |
|---|---|---|---|
| Q9VK03_DROME | 0.0326 | -1.28 | CG15639, isoform A |
| Q9VNY2_DROME | 0.0360 | -0.60 | IP02644p |
| Q9GT69_DROME | 0.0360 | -0.60 | Alpha/beta-syntrophin-like protein SYN1 |
| Q8IPW2_DROME | 0.0122 | -0.76 | Dreadlocks, isoform A |
| Q9VTB0_DROME | 0.0029 | -0.92 | CG8003 |
| Q9VYU7_DROME | 0.0282 | -0.67 | Dusky |
| Q9VPU1_DROME | 0.0122 | -0.76 | Dreadlocks, isoform B |
| Q9VNY4_DROME | 0.0360 | -0.60 | Syntrophin-like 1, isoform A |
| O16050_DROME | 0.0447 | -0.59 | Anon2A5 (Fragment) |
| Q24218_DROME | 0.0122 | -0.76 | SH2/SH3 adaptor protein |
| Q24328_DROME | 0.0282 | -0.67 | Transmembrane protein |
| RENT1_DROME | 0.0032 | -0.60 | Regulator of nonsense transcripts 1 |
| Q9VRX1_DROME | 0.0370 | -0.92 | CG8270 |
| Q9U5D0_DROME | 0.0126 | 0.63 | Hemolectin |
| Q9W366_DROME | 0.0027 | 0.71 | CG12121 |
| Q95SU2_DROME | 0.0355 | 0.60 | SD07888p |
| O46085_DROME | 0.0185 | 1.09 | CG14815, isoform A |
| IM04_DROME | 0.0276 | 0.62 | Immune-induced peptide 4 |
| HGD_DROME | 0.0360 | 1.32 | Homogentisate 1,2-dioxygenase |
| Q86P95_DROME | 0.0330 | 1.04 | GM01959p |
| Q7KW08_DROME | 0.0185 | 1.09 | CG14815, isoform B |
| Q0E9F9_DROME | 0.0330 | 1.04 | CG2915, isoform A |
| Q9VU94_DROME | 0.0248 | 0.73 | Hemolectin |
| O96692_DROME | 0.0355 | 0.60 | RE63021p |
Although the spaced trained flies showed much higher memory retention compared with those from the massed training, the massed trained flies still demonstrated a 24 hour memory, which was ARM. (2) The ARM can decay completely in a day 4, (2). ARM and LTM are two parallel but distinct long-lasting memories, and both exist at the 24-hour time point. Spaced training produces LTM together with ARM 24 hours after training. Therefore at 24 hour, both ARM- and LTM-specific proteins should be expressed in spaced trained flies. In contrast, for the massed trained flies, only ARM related proteins should be present. The largely overlapping proteomes between spaced and massed trained flies indicated that there were not many proteins expressed specifically for either LTM or ARM.
Next, we investigated the proteome changes between two time points from the same training method. In figure 2 volcano plots show that many proteins had significant expression changes (Figure 2). By using stringent filters (2 out of 3 replicates; and average change >1.5 fold; and P-value <0.05), 112 and 49 proteins were found to have lower expression levels at 3 hr versus 24 hr in spaced and massed trainings, respectively; and 191 and 233 proteins had increased expression levels at 3 hr, respectively.
Functional analysis
GO and KEGG pathway analyses were performed using the DAVID platform. (21) Based on expression changes, proteins were placed into 5 bins and subjected to enrichment analysis within each bin. As the majority of the proteome showed unchanged protein expression levels, the central three bins contained proteins with no or very small changes between the two samples. The first and fifth bins contained proteins with significant alterations (See the protein ratio distributions above the heat maps in Figure 3).
As with the protein quantification data, the significantly enriched GO or KEGG categories derived from different training comparisons were small, especially for the 3 hr data set, which implied that the proteomes did not change significantly between training types, therefore few functional groups were altered. As shown in the Figure 3, almost all of the enriched KEGG categories are localized in the 3rd bin representing unchanged proteomes between spaced and massed training at 3 hr. As for the 24 hr time point, although the number of enriched categories was still small, more proteins were enriched in the 4th bin, indicating the spaced trained flies had slightly higher protein expression levels in some pathways, especially metabolism-related pathways, including pyruvate, propanoate, valine, leucine/isoleucine, tryptophan, beta-alanine, and fatty acid metabolism, etc. The correlation between metabolism and memory formation is still unclear. A recent study has shown that glycogen could impair LTM formation and learning-dependent synaptic plasticity in mice, and in our data several proteins involved glycogen metabolism were found to be changed. (25) Another study discovered that a key component of fatty acid catabolism, peroxisome proliferator-activated receptor α (PPARα), plays an essential role in memory. (26) All these results implied that the metabolism may influence memory formation and synaptic plasticity directly or indirectly, possibly through transcriptional regulation. (27) Notably, the pathway ‘Jak-STAT signaling’ was highly expressed in the spaced trained flies after 24 hr (Figure 3). The Jak-STAT signaling pathway is involved in many cellular processes, including several neuronal functions. Recent studies have demonstrated the roles of the Jak-STAT signaling pathway in spatial working memory in mammals (28) and long-term memory in Drosophila. (29). Moreover, synaptic plasticity, (30) a process widely believed to underlie learning and memory, (31) was also found to be regulated by Jak-STAT pathway. (32) For comparisons between different time points, KEGG pathway analysis demonstrated that proteins involved in metabolism were highly expressed at 3 hr in both training types. ‘Phosphoinositol signaling system’ showed a higher enrichment level at the 24 hr time point. GO analysis again confirmed STMs or long-lasting memories generated by two different training types were quite comparable. However, the altered GO categories between STM and long-lasting memory were much more significant (Figure 4).
Figure 4. GO molecular function analysis for four proteome comparisons.
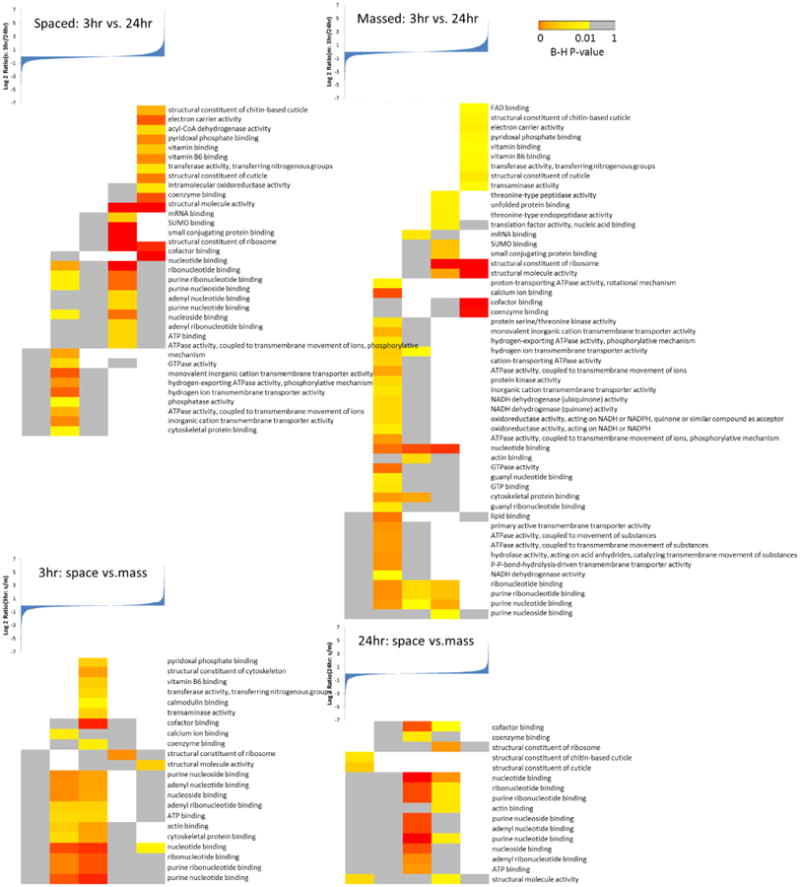
A number of genes have been reported to be associated with olfactory memory and learning in Drosophila, (33) and many of their protein products have been quantified in our proteomic experiments (Table 2). In general, the majority of the memory related proteins had small expression changes. Octopamine, a key neurotransmitter, has been linked to memory formation in fly. (34, 35) The Octopamine receptor in the mushroom body (oamb) plays an important role in mediating the octopamine signaling pathway. (36, 37) Previous studies have showed that different memory formation required different catecholamines even with the same odor. (34) Our data for the spaced trained flies at the 24 hr time point had decreased oamb expression level, suggesting that the LTM did not greatly rely on Octopamine modulatory pathway.
Table 2. Genes involved in Drosophila melanogaster memory and theirs expressions in this study.
| Gene | Log2 ratios | |||
|---|---|---|---|---|
|
| ||||
| Spaced: 3hr/24hr | Massed: 3hr/24hr | 3hr: space/mass | 24hr: space/mass | |
| Adf1 | -0.41908 | -0.05344 | -0.25219 | 0.11346 |
| aPKC | -0.29956 | 0.30218 | 0.03368 | 0.63543 |
| cre | -0.42809 | -0.1078 | -0.07662 | 0.24367 |
| dnc | -0.59646 | -0.3112 | 0.18442 | |
| DopR | 0.02697 | |||
| fas2 | -0.20282 | -0.16506 | 0.09856 | 0.13632 |
| NF1 | -0.45066 | -0.33082 | -0.10806 | 0.09163 |
| Nmdar1 | -0.51903 | 0.03882 | -0.22027 | 0.33758 |
| Nmdar2 | -0.67084 | -0.20282 | 0.23786 | 0.58134 |
| Notch | 0.09622 | |||
| oamb | 1.73697 | -0.53703 | -0.29546 | -2.30256 |
| PKA-r1 | -0.52607 | -0.23816 | -0.1635 | 0.0614 |
| pum | 0 | 0.09856 | -0.17333 | -0.07477 |
| rut | -0.69773 | 0.19704 | ||
| S6KII | -0.26546 | -0.5801 | 0 | -0.31464 |
| syn | -0.44137 | -0.32473 | -0.11664 | 0 |
| tbh | -1.09433 | -0.82103 | 0.30256 | 0.57586 |
| teq | 0.07215 | -0.15948 | 0.09077 | -0.14086 |
The importance of these genes (Table 2) to memory formation was discovered by a one by one gene mutation approach, in which the corresponding proteins did not express or did not function perturbing memory formation. In contrast, the flies employed in our study were wild type animals exhibiting normal gene expression and function. The small expression changes observed in our training regimens may be due to a role for post translational control in memory formation or that protein expression changes are occurring in a few neurons or sections of the brain and are being diluted by our analysis of whole fly heads. For example, mushroom bodies have been strongly associated with olfactory learning and memory in flies and many memory related genes have only showed protein expression difference in mushroom bodies, not other brain regions. (33) Nevertheless, we did observe changes associated with memory formation suggesting improvements to methodology or technology could improve signals further.
Conclusions
Olfactory learning and the resulting memory in Drosophila are of great value in helping us understand the mechanism of the memory formation. Our study used wild type flies and quantitative proteomics to investigate changes in the proteome during memory development, and found most of the previously known memory related gene had mild protein expression level changes in fly brains.
On the proteome scale, only minor protein changes were detected between spaced and massed trained flies, indicating that global protein regulation is not necessary for forming LTM. This finding suggests there may be a role for post translational modifications in the formation of memories. The differentially expressed proteins between LTM and STM are found to be related to immune function and ion binding. In contrast, a significant number of proteins showed altered expression levels after 24 hr of training versus 3 hr showing larger proteome changes to form long-lasting memories versus STM. Future studies should exam the role of post translational modification in memory formation. In particular, how much of a role does phosphorylation have in the formation of memory? To test our hypothesis, we have performed a database search targeting phosphorylation and ubiquitination on our current datasets (data not shown). But only a few modified peptides could be identified from unenriched samples, and the statistics for the data were poor because the modified peptides were not always found in each replicate. These studies also demonstrate it is feasible to study memory formation in whole fly heads. These methods will only improve with advances in mass spectrometry technology to increase the number of proteins that can be identified and thus the dynamic range of measurements.
Supplementary Material
Acknowledgments
The authors would like to thank Chang Liu and Claire M. Delahuny for constructive comments and discussions. The Yates laboratory is supported by R01 MH067880, P41 GM103533, and HHSN268201000035C. Dart Neuroscience provided a grant to fund Yaoyang Zhang for this work.
References
- 1.Davis RL. Olfactory learning. Neuron. 2004;44(1):31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79(1):35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 3.Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96(3):518–59. [PubMed] [Google Scholar]
- 4.Hinz FI, Aizenberg M, Tushev G, Schuman EM. Protein synthesis-dependent associative long-term memory in larval zebrafish. J Neurosci. 2013;33(39):15382–7. doi: 10.1523/JNEUROSCI.0560-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Squire LR, van der Horst AS, McDuff SG, Frascino JC, Hopkins RO, Mauldin KN. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci U S A. 2010;107(44):19044–8. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17(1):5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan Y, Rubakhin SS, Sweedler JV. Collection of peptides released from single neurons with particle-embedded monolithic capillaries followed by detection with matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 2011;83(24):9557–63. doi: 10.1021/ac202338e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micheva KD, Busse B, Weiler NC, O'Rourke N, Smith SJ. Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron. 2010;68(4):639–53. doi: 10.1016/j.neuron.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Fonslow BR, Shan B, Baek MC, Yates JR., 3rd Protein analysis by shotgun/bottom-up proteomics. Chem Rev. 2013;113(4):2343–94. doi: 10.1021/cr3003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Filiou MD, Reckow S, Gormanns P, Maccarrone G, Kessler MS, Frank E, Hambsch B, Holsboer F, Landgraf R, Turck CW. Proteomic and metabolomic profiling of a trait anxiety mouse model implicate affected pathways. Molecular & Cellular Proteomics. 2011;10(12) doi: 10.1074/mcp.M111.008110. M111 008110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krijgsveld J, Ketting RF, Mahmoudi T, Johansen J, Artal-Sanz M, Verrijzer CP, Plasterk RH, Heck AJ. Metabolic labeling of C. elegans and D. melanogaster for quantitative proteomics. Nature Biotechnology. 2003;21(8):927–31. doi: 10.1038/nbt848. [DOI] [PubMed] [Google Scholar]
- 12.Wu CC, MacCoss MJ, Howell KE, Matthews DE, Yates JR., 3rd Metabolic labeling of mammalian organisms with stable isotopes for quantitative proteomic analysis. Analytical Chemistry. 2004;76(17):4951–9. doi: 10.1021/ac049208j. [DOI] [PubMed] [Google Scholar]
- 13.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157(2):263–77. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 14.Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nature Biotechnology. 2001;19(3):242–7. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 15.T X, D VJ, D C, B L, L L, J W, J H, Y JR., 3rd ProLuCID, a fast and sensitive tandem mass spectra-based protein identification program. Mol Cell Proteomics. 2006;5(S174) [Google Scholar]
- 16.Tabb DL, McDonald WH, Yates JR., 3rd DTASelect and Contrast: tools for assembling and comparing protein identifications from shotgun proteomics. Journal of Proteome Research. 2002;1(1):21–6. doi: 10.1021/pr015504q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SK, Venable JD, Xu T, Yates JR., 3rd A quantitative analysis software tool for mass spectrometry-based proteomics. Nat Methods. 2008;5(4):319–22. doi: 10.1038/nmeth.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57(1):289–300. [Google Scholar]
- 19.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature Genetics. 2000;25(1):25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Research. 2004;32(Database issue):D277–80. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Research. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Llamas C, Lopez-Bigas N. Gitools: Analysis and Visualisation of Genomic Data Using Interactive Heat-Maps. Plos One. 2011;6(5) doi: 10.1371/journal.pone.0019541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Marin I, Kipnis J. Learning and memory … and the immune system. Learn Mem. 2013;20(10):601–6. doi: 10.1101/lm.028357.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duran J, Saez I, Gruart A, Guinovart JJ, Delgado-Garcia JM. Impairment in long-term memory formation and learning-dependent synaptic plasticity in mice lacking glycogen synthase in the brain. J Cereb Blood Flow Metab. 2013;33(4):550–6. doi: 10.1038/jcbfm.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy A, Jana M, Corbett GT, Ramaswamy S, Kordower JH, Gonzalez FJ, Pahan K. Regulation of cyclic AMP response element binding and hippocampal plasticity-related genes by peroxisome proliferator-activated receptor alpha. Cell Rep. 2013;4(4):724–37. doi: 10.1016/j.celrep.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86(2):465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 28.Chiba T, Yamada M, Sasabe J, Terashita K, Shimoda M, Matsuoka M, Aiso S. Amyloid-beta causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Molecular Psychiatry. 2009;14(2):206–22. doi: 10.1038/mp.2008.105. [DOI] [PubMed] [Google Scholar]
- 29.Copf T, Goguel V, Lampin-Saint-Amaux A, Scaplehorn N, Preat T. Cytokine signaling through the JAK/STAT pathway is required for long-term memory in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(19):8059–64. doi: 10.1073/pnas.1012919108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127(1):49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Ho VM, Lee JA, Martin KC. The cell biology of synaptic plasticity. Science. 2011;334(6056):623–8. doi: 10.1126/science.1209236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolas CS, Peineau S, Amici M, Csaba Z, Fafouri A, Javalet C, Collett VJ, Hildebrandt L, Seaton G, Choi SL, Sim SE, Bradley C, Lee K, Zhuo M, Kaang BK, Gressens P, Dournaud P, Fitzjohn SM, Bortolotto ZA, Cho K, Collingridge GL. The Jak/STAT pathway is involved in synaptic plasticity. Neuron. 2012;73(2):374–90. doi: 10.1016/j.neuron.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nature Reviews Neuroscience. 2007;8(5):341–54. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- 34.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. Journal of Neuroscience. 2003;23(33):10495–502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492(7429):433–7. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YC, Lee HG, Lim J, Han KA. Appetitive learning requires the alpha1-like octopamine receptor OAMB in the Drosophila mushroom body neurons. Journal of Neuroscience. 2013;33(4):1672–7. doi: 10.1523/JNEUROSCI.3042-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. Journal of Neuroscience. 1998;18(10):3650–8. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


