Abstract
Acute kidney injury is an increasingly common complication of hospital admission and is associated with high levels of morbidity and mortality. A hypotensive, septic, or toxic insult can initiate a cascade of events, resulting in impaired microcirculation, activation of inflammatory pathways and tubular cell injury or death. These processes ultimately result in acutely impaired kidney function and initiation of a repair response. This Review explores the various mechanisms responsible for the initiation and propagation of acute kidney injury, the prototypic mechanisms by which a substantially damaged kidney can regenerate its normal architecture, and how the adaptive processes of repair can become maladaptive. These mechanisms, which include G2/M cell-cycle arrest, cell senescence, profibrogenic cytokine production, and activation of pericytes and interstitial myofibroblasts, contribute to the development of progressive fibrotic kidney disease. The end result is a state that mimics accelerated kidney ageing. These mechanisms present important opportunities for the design of targeted therapeutic strategies to promote adaptive renal recovery and minimize progressive fibrosis and chronic kidney disease after acute insults.
Introduction
Despite the advent of dialysis in the second half of the 20th century as a treatment for severe acute kidney injury (AKI), the mortality associated with this condition remains unacceptably high, especially in the intensive care unit population (>50%),1–3 with a paucity of effective therapeutic interventions. The incidence of AKI has been steadily increasing related, in part, to the ageing of the population;4 the increasing prevalence of chronic kidney disease (CKD), which predisposes to AKI;5 and the increasing number of invasive interventions that can result in haemodynamic compromise or septic complications. Furthermore, contrast agents required for imaging studies and an increasing number of therapeutic agents in the pharmacological armamentarium have varying degrees of nephrotoxicity, which can precipitate or worsen AKI.4
In many cases, progression of kidney failure is not due to worsening of primary renal disease, but rather a secondary insult, most commonly associated with transient intrarenal regional or generalized hypoperfusion or sepsis. Ischaemia–reperfusion injury (IRI) and activation of inflammatory pathways initiate diverse processes resulting in acute tubular injury or necrosis, particularly, in the outer stripe of the outer medulla6 where there is baseline hypoxia even under normal conditions.7 Current treatment for AKI is supportive in nature, and trials of agents showing promise in experimental IRI models (for example diuretics and dopamine) have failed to ameliorate clinical AKI in translational studies.8,9
Although the high initial mortality associated with AKI is well recognized,1–3 for many years it was accepted that normal kidney structure and function would return in survivors of AKI. An increasing number of epidemiological studies with both adequate statistical power and length of follow-up10–14 have, however, revealed that survivors of AKI exhibit a persistently increased risk of progressive CKD, proteinuria and an excess risk of cardiovascular mortality. This finding complements results in laboratory animals demonstrating that renal injury produces a senescence-associated profibrotic secretory phenotype and a subsequent inflammatory milieu, which promotes the gradual accumulation of renal fibrosis, vascular rare faction and CKD.15–17 This Review summarizes our emerging knowledge of the factors underlying both adaptive kidney repair and the maladaptive repair linking AKI to CKD, and what therapeutic opportunities they present. Because of length constraints only a portion of the relevant data are included.
Adaptive repair after AKI
An acute renal insult affects the function of several distinct cell populations within the kidney, which contributes to the initiation and amplification of the kidney injury. These various cell types will be discussed along with their potential relevance for the reparative phase of renal recovery. Although clinical AKI is associated with high morbidity and mortality, kidney biopsy is seldom performed. In addition, when a biopsy is available it often does not sample the outer medulla where a considerable component of the pathology may reside. This paucity of outer medullary tissue, together with the fact that the biopsy is often performed during the recovery phase rather than the injury phase, likely explains why the injury to the tubules seen on biopsy may be less than one would expect from the functional impairment of the kidney. The presence of casts, tubular cells and high levels of kidney injury molecule-1 (KIM-1) in the urine confirm the presence of severe proximal tubule injury.
Despite the high level of functional loss often seen in patients with AKI, it is known that in humans the functional loss can be transient. The kidney has the ability to return to normal function following an insult (Figure 1), although there is evidence from experimental models and in humans that complete functional recovery is less likely with ageing.11,18 It must be recognized that functional recovery is usually assessed by measuring levels of serum creatinine, which is an insensitive tool.
Figure 1.
A summary of some of the mechanisms involved in initial tissue injury and subsequent repair of the kidney after acute kidney injury. Maladaptive and incomplete repair leads to the development of fibrosis and, ultimately, chronic kidney disease.
Whether tubular regeneration after injury arises from proliferation of surviving mature cells or from renal stem cells has been debated.19,20 Long-lived ‘label-retaining’ tubular cells have been reported to be found within the renal papilla21 and bear surface markers associated with stem cell properties.22,23 However, a study from our laboratory using genetic fate-mapping of renal tubular epithelial cells provided strong evidence against renal stem cells being the main source of new cells after renalinjury, even after repeated IRI.24 This study confirms prior hypotheses19 that mature surviving cells dedifferentiate and proliferate to replace those tubular epithelial cells that have been lost,25 with no tubular cell subpopulation (for example, a stem cell population), preferentially partaking in the repair process in response to insults that injure the proximal tubule.25,26
Endothelial injury and oxygen delivery
An important approach to facilitating recovery and minimizing long-term sequelae of injury is to minimize the injury itself. After an initial insult there is an ‘extension phase’ during which it might be possible to intervene after recognizing the injury and hence change the long-term outcome by targeting the ‘injury phase’ rather than the ‘recovery phase’. Strategies to improve long-term outcomes after AKI should therefore be based on a thorough understanding of the injury phase as well as the recovery phase. Endothelial cell injury and dysfunction may have an important role in the extension phase.
In the clinical setting, initiating insults include, among a large list, shock, sepsis, surgery and administration of contrast and other nephrotoxic agents. Many of these insults converge on a common ischaemia–reperfusion pathway with localized or generalized renal hypoxia.6,27 The resultant inflammation and ischaemic changes affect tissue oxygenation, the local generation of nitric oxide and a number of profibrotic and proinflammatory cytokines and growth factors, and the subsequent production of deleterious reactive oxygen species. Each of these factors, as well as others, further potentiate tissue damage and dysfunction and initiate a repair process that can be adaptive (Figure 2) or maladaptive (Figure 3).
Figure 2.
The evolution of tissue injury, death and subsequent adaptive repair after AKI. Following an episode of AKI, the kidney is capable of repair back to normal or near-normal structure and function despite apparently severe damage. Initial injury is characterized by endothelial activation, recruitment of myeloid leucocytes and widespread tubular cell injury and death. In ‘adaptive’ repair, over a period of days, debris is cleared by tubular cells and recruited macrophages, and epithelial dedifferentiation occurs followed by proliferation to restore the integrity of the tubular epithelial cell layer. Macrophages support renal growth and recovery by adopting an M2 phenotype before leaving the kidney. Pericytes remain in contact with the capillary network and do not give rise to new myofibroblasts or if they do, this myofibroblast proliferation is reversible. Abbreviations: AKI, acute kidney injury; NO, nitric oxide.
Figure 3.
Maladaptive repair of AKI leads to CKD. Studies have highlighted the importance of G2/M cell-cycle arrest in response to severe, repeated and genotoxic renal insults. In the initial repair phase after injury, cells may become arrested in G2/M phase and release cytokines and growth factors that promote inflammatory cell retention within the kidney and ongoing inflammation. Injury and pro-inflammatory stimuli lead to pericyte dissociation from the endothelium, resulting in microvascular rarefaction and the progressive deposition of collagen I by myofibroblasts arising from activated pericytes. Ageing sensitizes tubular cells to G2/M arrest in response to cell stress and DNA damage, providing a potential explanation for the increased risk of CKD progression after AKI in the elderly. Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease.
Both arterial pressure and segmental vascular resistance will influence kidney oxygen delivery and it is increasingly recognized that alterations in tissue hypoxia can vary substantially in different regions of the injured kidney28 Factors such as sodium reabsorption, nitric oxide release, angiotensin II generation, tissue haemoglobin concentration and oxygen saturation have been shown in humans or experimental models to influence the balance between oxygen delivery and consumption in the injured organ27 with the potential to modulate hypoxic injury. In addition to these factors, altered production of prostaglandins and reactive oxygen species due to injury of neighbouring tubules27, 29 further impair tissue oxygen delivery, leading to a local ‘no-reflow’ phenomenon where the occluded microvasculature further amplifies initial injury. Administration or generation of agents that act on the vasculature, such as adenosine, in experimental animals have shown promise in promoting early vascular patency and hence minimizing resultant hypoxia in experimental IRI.30,31
Studies in rats demonstrate that following acute IRI, vascular function remains impaired for several days.32 During this period there is impaired autoregulation of blood flow, potentially reflecting in part the failure of endothelial nitric oxide synthase to generate nitric oxide.33 Injured endothelial cells and changes to the integrity of the glycocalyx of the peritubular capillaries can result in exposure of a range of surface markers that promote the recruitment and adhesion of leucocytes34, 35 and platelets, which can interfere with perfusion and contribute to additional endothelial cell injury and inflammation. Vascular permeability is increased, leading to interstitial tissue swelling, further compromising microvascular perfusion.29 The availability of intravital microscopy has enabled the visualization of the post-ischaemic tissue, revealing that blood flow in cortical peritubular capillaries becomes sluggish and may even reverse in the first 30 min after injury36, 37
Following apparently recovered IRI in the rat, there is a long-term reduction in peritubular capillary density,17 which precedes the development of visible fibrosis. Renal hypoxia is a common feature of diverse chronic renal injuries, and is likely to contribute to the progression of renal failure.37 Early tubular regeneration after IRI is associated with high levels of transforming growth factor β (TGF-β), but reduced vascular endothelial growth factor (VEGF), suggesting that factors involved in proliferation of the tubular compartment impede repair or contribute to ongoing loss in the microvascular compartment, in which the endothelial cells show little early proliferation after injury38 Studies examining the response to kidney injury have implicated the pericyte, which is an important mediator of vascular stability in embryogenesis, as a key contributor to vascular homeostasis.38, 39 Animals with defective pericytes that lack the matrix metalloproteinase inhibitor TIMP-3 spontan eously develop a microvascular phenotype in the kidney40 with both vascular rarefaction and increased fibrosis.
The cellular immune response
IRI results in a rapid immune response and the recruitment of leucocytes to the injured kidney. These cells may have dual roles: potentiating early injury, but also being necessary for the subsequent successful resolution of damage.41 Kidneys contain an extensive network of resident mononuclear phagocytes that mount an early response to injury by releasing a systemic burst of tumour necrosis factor.42 The combination of chemotac-tic signals from resident immune cells, injured tubular cells and the altered characteristics of injured endothelial cells promotes vascular adhesion and transmigration of neutrophils within the first 24 h after injury.43
Neutrophils
The presence and accumulation of interstitial and marginating neutrophils in very early experimental IRI is well established,44,45 with one study demonstrating that cytokines, such as IL-17, that are released from neutrophils contribute to downstream immune activation.46 Whether their presence within the acutely injured kidney actively promotes injury remains controversial, with various approaches to inhibit or deplete neutrophil function demonstrating either protection44,47,48 or lack of protection49–51 depending on the technique employed and the species studied.
Monocytes and macrophages
The early influx of neutrophils is followed by monocyte recruitment, predominantly of the Ly6Chi subset.43 Macrophages exhibit phagocytic properties for both dying tubular cells and neutrophils.52 Studies where macro-phages were depleted before AKI have demonstrated protection from injury or a net neutral effect depending on the approach used.53–55 It is generally agreed that recruited macrophages amplify initial injury. While initially of the Ly6Chi M1 inflammatory subset, macrophages persist in the kidney but the population progressively loses Ly6C and adopts M2 polarization over the course of the first 5 days,41 after which their numbers decline.43 Macrophages of the Ly6Clo M2 subtype are believed to be beneficial in the repair of the kidney after injury.
Macrophages play key roles in supporting kidney growth during nephrogenesis,56 and undertake the vital removal of cellular debris and dying neutrophils after renal injury.57 After adopting an M2 phenotype in the recovery phase of AKI,41 macrophages secrete compounds, including growth factors, fibronectin, Wnt-7b and IL-1 receptor antagonist, all of which support epithelial proliferation and repair.57,58 While pre-injury depletion of macrophages is often protective, depletion of macrophages from mice with established AKI results in the absence of M2-secreted products and prolongation of renal injury.59 Furthermore, polarization towards a proinflammatory M1 phenotype resulted in failure to resolve the injury. This finding provides further evidence for the importance of macrophages in facilitating eventual renal repair.41,58,60
Lymphocytes
Neither B lymphocytes nor T lymphocytes are present in large numbers in the kidney before or after AKI;43 however, studies of mouse models do support their influence on the evolution of renal injury.61 B-cell-deficient (µMT) and CD4/CD8-deficient mice are both protected from AKI, whereas Rag1-knockout mice demonstrate no alteration in susceptibility to injury.62–64 In another study using Rag1-deficient mice, protection from injury was restored by adoptive transfer of either B cells or T cells alone, but not by both,49 suggesting that complex and poorly understood interactions between lymphocyte populations contribute to the injury phenotype. Regulatory T cells positive for FoxP3 and CD25 have been reported to limit tissue injury.65 Mice with a depleted regulatory T cell population and those lacking regulatory T cells exhibit heightened levels of immune activation and tissue damage after IRI.65
The role of lymphocytes in mediating tissue repair remains incompletely understood. Work using µMT mice demonstrated that chimeric animals had a more rapid recovery than did control mice after experimental IRI, suggesting that B cells themselves inhibit tissue repair.66 Other work reveals that lymphocytes of animals previously exposed to severe IRI can induce albuminuria after transfer to naive recipients, indicating that immuno logical memory may contribute to the development of proteinuria after AKI67 and this proteinuria may have secondary effects to facilitate the onset of CKD.
Circulating and endogenous compounds
A number of circulating and endogenous compounds have been implicated in both tissue protection and exacerbation of AKI. Many immune cells express the A2A receptor for circulating adenosine, which down-regulates inflammatory activation and tissue injury in multiple organs, including the kidney.68 In addition to beneficial effects of local adenosine production on the survival and function of the endothelium,69 macrophages and dendritic cells have been shown to be sensitive to local adenosine production,55,70 with regulatory T cells implicated as a local producer of adenosine, exerting anti-inflammatory effects.71
The endogenous anti-inflammatory compound haem oxygenase 1 (HO-1) is induced in response to hypoxia and implicated as a cellular protector against AKI. Several groups have demonstrated the protective effects of HO-1 induction before experimental AKI,54,55,72 which seemed to be mediated via expression in macrophages rather than tubular epithelial cells,72 where it promotes an anti-inflammatory M2 phenotype.73
Resolvins and protectin D1 are natural anti-inflammatory compounds capable of reducing experimental AKI and subsequent fibrosis,74 and exhibit protective effects with both pre-injury and early post-injury dosing. Administration of these agents resulted in reduced leucocyte accumulation and blocked Toll-like-receptor-mediated macrophage activation. Administration of a lipoxin A4 analogue in vivo downregulated inflammatory cytokine expression while upregulating anti-inflammatory cytokine expression, potentially via an effect on interactions between resident cells and recruited leucocytes.75 Hepatocyte growth factor (HGF) has also been evaluated for potential beneficial effects to protect against injury. Overexpression of HGF in kidneys was associated with reduced injury, leucocyte recruitment and TGF-β1 expression after ischaemic injury, and treatment of epithelial cells in vitro with HGF was associated with reduced production of C-C motif chemokine 2, supporting a key role for the epithelium in the recruitment of myeloid inflammatory cells.75–77
Serum complement is an evolutionarily conserved activator of the innate immune response. Widespread tubular deposition of complement has been documented in the first few hours following IRI,78 and, in the rodent, is associated with a loss of the complement inhibitor Crry from the tubular basolateral surface.79 In situ activation of the complement system may also be important, where production of C3 by renal dendritic cells promotes their subsequent inflammatory activation and ability to activate T lymphocytes.80 Unlike other solid organs, complement deposition in the kidney is documented to occur via the alternative pathway,78 activating in response to hydrolysis of C3 rather than the presence of antigen– antibody complexes. Selective inhibition of this alternative pathway protects against experimental ischaemic injury.81 Evidence also exists for the importance of the terminal phase of complement deposition (formation of the C5b–9 complex) in mediating the severity of ischaemic AKI.82
Tubular cell injury and death
Tubular cells in the S3 segment of the proximal tubule are characterized by high metabolic activity with low baseline ambient oxygen delivery from the capillary network.7 In the context of AKI they can experience profound hypoxia and subsequent rebound reoxygenation, followed by further fluctuations owing to capillary dysfunction and the recruitment of activated and cytotoxic leucocytes. These multiple insults result in a loss of physical cell–cell interactions, and tubular cell death with apoptosis and prominent programmed and unprogrammed necrosis.83,84 Cell death, combined with the detachment and loss of viable epithelial cells into the tubular lumen, leads to the denudation of areas of the S3 segment. Cell death can extend to earlier segments of the proximal tubule also, and results in a failure of tubular function, structural obstruction of flow, and the distal delivery of a solute-rich fluid activating tubuloglomerular feedback, which further inhibits glomerular filtration.
Studies using surface markers have lent weight to the importance of modification of the epithelial cell pheno-type to assume more mesenchymal characteristics during both acute and chronic kidney injury and repair.85 A number of groups have demonstrated that surviving tubular cells may transiently upregulate markers such as vimentin, α-smooth muscle actin and S100A4, which are often associated with the fibroblast lineage but not indicative of transformation to fibroblasts.86,87 The coexpression of these markers with proliferating cell nuclear antigen suggests that the ‘dedifferentiated’ cells are undergoing active replication.87,88
One pathway of documented importance in the repair process after injury is Wnt/β-catenin signalling. Transient IRI has been shown to induce a rapid induction of Wnt4 that returns to baseline by 24 h.89 Factors present in AKI, including disruption of cell–cell junctions, lead to nuclear translocation of β-catenin.89 This finding echoes embryogenesis, where expression of Wnt contributes to maintenance of the undifferentiated embryonic state.90 The dedifferentiated cells respond to various proliferative cues, including HGF, epidermal growth factor, TGF-β and VEGF,85 with a restricted burst of TGF-β expression, potentially maximizing a therapeutic rather than profibrotic outcome. With subsequent increases in cell number and resultant increased cell–cell contacts, the Wnt signalling pathway is suppressed. Studies of tubulogenesis in vitro suggest that sequential Wnt downregulation and expression of matrix metalloproteinase is necessary for the recovery of a differentiated phenotype,91,92 hinting at the importance of cell–matrix interactions in achieving and maintaining the mature state.
Mechanisms of CKD development post AKI
As stated previously, it is now widely accepted that episodes of AKI predispose to the development of CKD even in those patients judged to have normal pre-injury renal function based on measurement of serum creatin-ine levels.10 Several emerging theories to explain the difference between adaptive (without scarring) and maladaptive repair leading to CKD are discussed below.
Recurrent tubular injury promotes scarring
Advances in molecular biology have enabled the generation of transgenic animals susceptible to the selective injury of particular cell types such as the renal tubular epithelial cell.16 The generation of transgenic mice expressing the simian diphtheria toxin receptor on the tubular epithelia has enabled interrogation of the role of specific injury to this cell type without associated concurrent injury to surrounding capillaries, pericytes and other nearby cells. Interestingly, a single dose of diphtheria toxin was followed by a vigorous inflammatory response and subsequent complete repair. By contrast, three doses of diphtheria toxin over 3 weeks resulted in persistent inflammation, microvascular rarefaction, increased expression of TGF-β1, collagen α-1(I) and fibronectin, progressive fibrogenesis, and secondary glomerulosclerosis.16
Multiple sublethal injuries could lead to premature cell senescence and a failure to respond to subsequent injury with adaptive proliferation. In addition, repeated insults may lead to tubular cells remaining arrested in a dedifferentiated state with ongoing production of profibrotic factors,93,94 which contribute to microvasular loss after solated tubular epithelial injury.16 It has been shown that c-Jun N-terminal kinase (JNK) remains active for weeks after injury to tubular epithelial cells.95 Our laboratory demonstrated that, in multiple models of AKI, arrest of tubular cells in G2/M resulted in JNK activation, with subsequent fibrosis which was reduced by JNK inhib-ition.15 These findings are compatible with the hypothesis that progressive nephron loss and failed redifferentiation with ageing may facilitate maladaptive repair after AKI.96
Epigenetic changes after AKI
The role of epigenetic changes in the kidney following AKI is a new and rapidly developing field. There are diverse mechanisms by which modifications to the epigenome can alter the subsequent expression of pro-inflammatory and anti-inflammatory genes.97,98 Studies of patients with CKD have already implicated DNA methylation and histone modifications as contributors to the progression of the fibrotic process.98 AKI has been associated with alterations in DNA methylation and histone modification,99,100 leading to altered transcription of genes implicated in renal injury, including TNF and C-C motif chemokine 2. Advances in epigenetic assessment technology in the kidney are aiding the search for changes after AKI.101 These approaches have the potential to reveal mechanisms by which ‘adaptively repaired’ kidneys may undergo long-lasting transcriptional changes that predispose to CKD.
KIM-1 expression and kidney fibrosis
KIM-1 is an important biomarker for renal injury, with multiple log-order increases in expression and shedding from injured tubular cells after renal insults in rodents and humans.102,103 KIM-1 promotes the clearance of apoptotic cells by tubular epithelia,104 a finding of importance for endogenous kidney repair after injury. With prolonged epithelial cell expression of KIM-1, however, our recent work has demonstrated a profibrotic phenotype associated with increased leucocyte recruitment to the injured kidney. Thus KIM-1 may provide a mechanistic link between injury and fibrosis.105 While acute expression of KIM-1 is adaptive and facilitates repair,106 chronic expression is maladaptive and promotes fibrosis.104,105 Ta k e n together, these data support the hypothesis that a focused and repeated tubular injury can be sufficient to initiate progressive scarring.16
Origins of myofibroblasts in renal fibrosis
A key feature of maladaptive repair after AKI is the appearance of increased numbers of myofibroblasts which contribute to the deposition of collagen and other components of the fibrotic matrix in the kidney.107 An understanding of the origin of these cells is therefore an important step towards the development of antifibrotic therapies. Although it is accepted that stellate cells in the liver act as pericytes and mediate collagen deposition after injury,108 in the kidney the source of the interstitial fibroblasts responsible for the deposition of scar tissue is a topic of ongoing debate. Candidates include circulating progenitors (fibrocytes), epithelial–mesenchymal transdifferentiation (EMT) and, more recently, renal pericytes or perivascular fibroblasts as the cells giving rise to pathogenic myofibroblasts in injury.
Fibrocytes
Fibrocytes are circulating cells of myeloid lineage that are proposed to be precursors for the fibroblasts responsible for tissue scarring in fibrotic disease of various organs including the kidney.109–111 The contribution of these cells to the direct deposition of matrix within scarred tissues has been challenged, however, both historically by the lack of bone-marrow-derived cells in a parabiotic rat model of skin scarring,112 and more recently by fate-mapping techniques.113,114 Although bone- marrow-derived cells do not seem to be major contributors to the direct transdifferentiation to myofibroblasts within the kidney, similar to macrophages, these cells may function in a paracrine fashion to support or promote renal scarring by interacting with other kidney cells or by producing factors that other cells respond to, thereby contributing to the fibrotic response.
EMT
The role of EMT as the source of myofibroblasts and subsequent fibrosis after renal injury has generated much interest.115 While there is abundant documentation of expression of mesenchymal markers when epithelial cells are injured or exposed to certain cytokines in vitro, genetic lineage tracing studies supporting the existence of EMT in vivo in the kidney have been much more limited.116 Most studies have challenged the conclusion that epithelial cells transdifferentiate to myofibroblasts. Fluorescence labelling of cells expressing collagen α-1(I)117 or use of genetic crosses in renal epithelia involving Six2-cre (to mark cells that are descendents of the metanephric mesenchyme), HoxB7-cre (to mark cells that descend from the ureteric bud), or FoxD1-cre-positive mesenchymal cells (which are destined to primarily form the kidney interstitial fibroblasts, vascular smooth muscle cells, pericytes of peritubular capillaries and mesangial cells) has enabled fate tracing of cells after renal injury24, 39 These studies demonstrated no evidence for transdifferentiation of epithelial cells to fibroblasts and pointed to the pericyte as an important precursor of myofibroblasts.39 Importantly, while renal epithelia can be readily induced to express mesenchymal markers in vitro, expression of these markers by epithelial cells in vivo should be interpreted as a reflection of dedif-ferentiation rather than conversion to fibroblasts.19, 87 Conversion of epithelial cells to fibroblasts may therefore be primarily an in vitro phenomenon.
Renal pericytes
Pericytes are specialized cells present in close proximity to the endothelial cells of multiple organs.118 Although they were first noted in electron microscopic studies of the kidney in the early 1980s, over the past decade there has been a rapid increase in research interest in the role of the pericyte in embryogenesis and tissue homeostasis, and its potential role in tissue fibrosis and vascular rarefaction after renal injury38 Pericytes maintain vascular stability via cell-cell communication and released factors including PDGF,119 angiopoietin,120 TGF-β,121 VEGF122 and sphingosine-1-phosphate.123
It has been proposed that the renal pericyte has a central role in the development of fibrosis after AKI39, 117,124 by migrating from its perivascular location and acquiring the markers and phenotype of the myofibroblast. The propensity of the injury-associated activated pericyte to break contact with endothelial cells and migrate may deliver a ‘double hit’ of vascular instability and collagen deposition, which are both hallmarks of progressive CKD (Figure 3). Studies demonstrate reduced vascular stability with loss of TIMP-3 and ADAM-TS 1, which is associated with worsened injury40 As such, the pericyte is now the subject of active research aimed at targeting these cells to inhibit fibrosis and preserving the vascular network. Blockade of the PDGF receptor and VEGF receptor 2 protects against fibrosis and vascular rarefaction.117 Recently Kramann et al. identified a perivascular Gli-1(+) population of cells as a major cellular origin of fibrosis.125
Ageing and kidney repair after injury
The incidence of AKI increases with each decade of life.4, 126 Patients with in-hospital AKI are often elderly; in one study of Medicare recipients the mean age was approximately 75 years.4 The increased comorbidities and associated polypharmacy might account for some of the increased risk of AKI associated with increasing age; however, there are likely to be underlying poorly understood biological changes that contribute to susceptibility. Recent work has sought to define the underlying processes and cellular responses that represent the ‘hallmarks of ageing’,127 implicating factors such as genomic instability, loss of proteostasis and increasing cellular senescence as key contributors to the aged phenotype. Disease-induced cellular senescence has been shown within the kidney and other organs.128–130 Increased levels of cellular senescence have been reported in patients with hypertension,131 in tubular cells of patients with IgA nephropathy132 and in type 2 diabetes,133 where they correlate with severity of renal disease. Our own data demonstrate growth-arrested tubular cells to be predictive of the development of CKD subsequent to AKI.15 Registry data demonstrating the susceptibility of the CKD population and healthy elderly individuals to AKI and progressive kidney disease10,13,14 further point to shared mechanisms between ageing and susceptibility to progressive kidney disease that is associated with maladaptive repair post AKI (Figure 4).
Figure 4.
Common mechanisms in kidney ageing and progressive kidney injury. Features of kidney ageing include tubular loss, glomerulosclerosis, microvascular rarefaction and deposition of interstitial collagen. The number of senescent and G2/M arrested cells present in the ageing kidney also progressively increases. All the above cellular changes are also seen in progressive renal disease in younger patients following acute renal insults. These shared features suggest that progressive chronic kidney disease is functionally equivalent to accelerated ageing of the kidney. Abbreviation: AKI, acute kidney injury.
The ageing immune system
Immunosenescence refers to alterations in the function of the ageing immune system. With ageing there is low-grade immune system activation, and a skewing towards increased myeloid cell responses together with reduced function of T lymphocytes.134 An aged human kidney attracts an increased quantity of inflammatory infiltrates when transplanted into a young or old recipient.135 While it has been suggested that this finding represents the effects of increased expression of PECAM-1 in the donor kidney,136 it remains plausible that this result is related to age-related increased cellular senescence. Epithelial cell senescence in the aged kidney allograft may limit the ability of the kidney to mount an adequate adaptive repair response to the long-term immunological attack. As discussed previously, macrophage phenotypic switching facilitates renal repair after AKI.41 This phenotypic change is impaired in macrophages in other organs of the ageing rodent,137 but remains less well explored in the kidney.
Senescence of epithelial cells with ageing
With increasing age evidence exists for a reduced proliferative capacity within the kidney in the aftermath of injury in vivo and in vitro.138 Microarray studies of ageing rat kidneys identified age-related alterations in multiple gene networks at baseline.139 Multiple-fold induction of injury biomarkers, such as KIM-1, occurs in ageing animals, which is reflective of ongoing age-related kidney injury at baseline. As discussed above, persistent KIM-1 expression may be maladaptive and facilitate chronic scarring, which can be exacerbated after de novo renal insults.
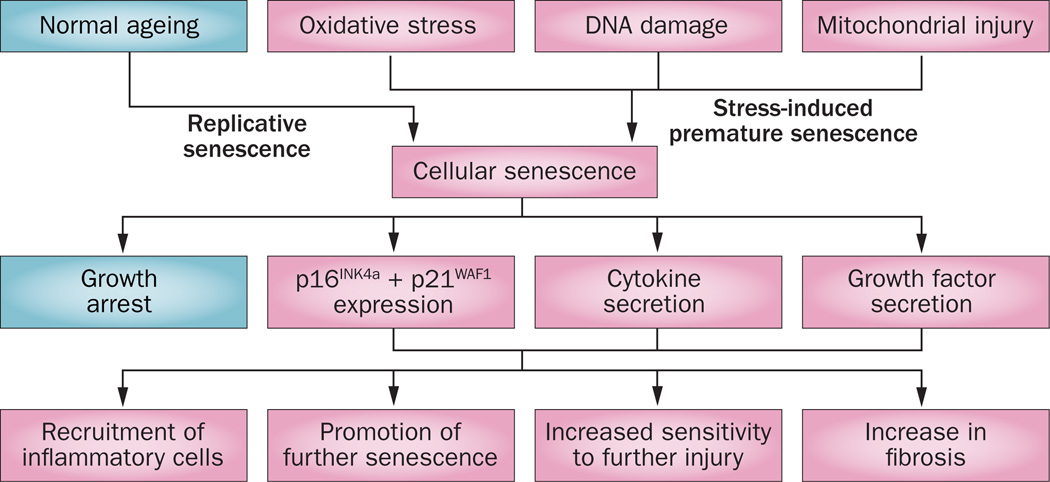
Increased rates of cellular senescence with ageing are associated with a state of growth arrest that can be triggered by a critical shortening in telomere length or by stressors such as hypoxia or genotoxic insults.139 Senescent cells are resistant to apoptosis and, rather than being inert, they can secrete numerous cytokines and chemokines, including (C-X-C motif) ligand 1 (CXCL1), IL-6 and IL-8, which all promote maintenance of a chronic inflammatory state (Figure 5).140 Senescence-associated β-galactosidase has been used as a marker for senescence.141 Senescence is associated with increased expression of p16INK4a and p21WAF1, which contribute to growth arrest. Aged animals have increased levels of p21WAF1 at baseline and levels are further increased in stress-induced premature senescence, leading to proliferative arrest and apoptosis.142 Surprisingly, studies of p21-deficient mice exposed to IRI showed a worsened injury phenotype.143 Increased levels of p16INK4a inhibit CDK4 and CDK6, leading to stabilization of retinoblastoma-associated protein in its phosphorylated form, causing growth arrest. Pharmacological blockade of both CDK4 and CDK6 in young animals protected against AKI, despite a transient reduction in cellular proliferation.144 Thus, particularly in young animals, there may potentially be deleterious effects of excessive proliferation in the immediate aftermath of IRI; however, a persistent decrease in proliferative capacity may lead to a chronic profibrotic phenotype.
Figure 5.
Replicative and stress-induced premature senescence in kidney injury. Renal cells become senescent either through ageing and telomere shortening, or via genotoxic insults resulting in stress-induced premature senescence. While in terminal growth arrest, these senescent cells remain metabolically active and secrete a range of factors that contribute to the chronic inflammatory state, the progression of renal fibrosis and increased susceptibility of other cells to subsequent insults and senescence. Modified with permission of American Society of Nephrology, from Yang, H. & Fogo, A. B. J. Am. Soc. Nephrol. 21, 1436–2439 (2010); permission conveyed through Copyright Clearance Center, Inc.
In contrast to what is seen in younger mice, reduced proliferation was noted in the recovery phase after IRI in older mice, together with upregulation of zinc-α-2-glycoprotein (Azgp1); however, attempts to increase cell-cycle turnover by knocking down Azgp1 resulted in markedly worse fibrosis in recovery.138 Hence, while increasing levels of senescent epithelial cells with either ageing or injury promotes sensitivity to further injury and progressive fibro-sis, it is important to select therapeutic targets to increase proliferation without a similar increase in fibrosis. It is possible, for example, that interventions that increase movement through the G0/G1 phase of the cell cycle without increasing movement through G2/M may then increase the number of cells trapped in G2/M and enhance the generation of profibrotic cytokines.
Whether the above factors are fixed and intrinsic to the aged kidney itself or are influenced and hence modifiable by circulating factors remains unknown. Elegant studies in other organs, such as the aged brain, skeletal muscle and heart, using heterochronic parabiosis to create a shared circulation, have demonstrated the modification of aged injury phenotypes when mice are exposed to a young circulation,145–147 or by systemic depletion of senescent cells.148 A study examining the effect of transplanting young and old bone marrow into aged mice showed reduced levels of fibrosis and markers of senescence in the resident renal cells of the recipients of young bone marrow. 149 This observation raises the possibility of modulating the levels of epithelial cell senescence by altering the systemic milieu to delay or prevent age-facilitated renal disease.
Growth factor production with ageing
In response to tubular injury, epithelial cells produce a number of growth factors, including epidermal growth factor, HGF, and insulin-like growth factor 1.150 There is evidence that levels of renal epidermal growth factor decrease with increasing age,151 with similar reductions seen in VEGF expression, coupled with increased levels of thrombospondin-1.152 An age-associated increase in levels of TGF-β has also been shown.153 TGF-β is potently profibrotic with sustained release. These changes in growth-factor expression could contribute to the increased susceptibility to tissue injury, fibrosis and vascular rarefaction seen in the elderly.154
Circulating progenitor cells in ageing
The role of circulating stem cells, progenitor cells or marrow stromal cells (MSCs) in mediating repair after AKI is controversial. An initial report suggesting that MSCs were incorporated into the tubules of the repairing kidney155 was followed by a study from our laboratory that demonstrated no evidence of incorporation of non-renal cells into the tubules of the repaired kidney.156 Several studies have, however, reported partial protection against kidney injury with the administration of MSCs.157–159 These effects have been attributed to paracrine actions of bone-marrow-derived cells, either acting systemically or locally, as some of these cells enter the kidney interstitium after injury.160 Importantly, although the number of MSCs in the blood of aged animals remain constant, it is reported that their secretion of TGF-β, bone morphogenetic protein 2 and bone morphogenetic protein 4 are reduced, while secretion of proinflammatory IL-6 is increased. Numbers of circulating endothelial progenitor cells are reduced with advancing age, as is their mobilization and incorporation into the injured endothelium.161 Ageing is also associated with the progressive accumulation of genomic damage, which impacts negatively on stem cell function and likely dedifferentiated epithelial cell function.162 These changes contribute to a less reparative phenotype,163 indeed one that may actually impair wound healing.164
Maladaptive repair and G2/M arrest
Maladaptive repair leading to CKD is characterized by persistent parenchymal inflammation, with increased numbers of myofibroblasts and accumulation of extracellular matrix.16 Risk factors for the maladaptive repair response include the type and duration of injury, the pre-injury glomerular filtration rate and the age of the patient.14 With recent studies failing to demonstrate convincing evidence for epithelial-to-fibroblast transdifferentiation to account for progressive fibrosis,24,39,117 increasing interest has focused on alterations in the injured tubular epithelium as the potential cause of ongoing activation and proliferation of pericytes, which may represent the important precursor population for myofibroblasts.15 As discussed above, contrary to their name, senescent cells can remain metabolically active and adopt a senescence-associated secretory phenotype, which is associated with the release of connective tissue growth factor and TGF-β, both contributors to chronic inflammation, collagen deposition and vascular rarefaction.165,166 Senescent cells are also documented to produce IL-8, which may potentiate cells entering G2/M arrest.167
Checkpoints in the cell cycle involving cyclins, cyclin-dependent kinases and cyclin-dependent kinase inhibitors ensure the arrest of cell division in the presence of inadequate cell size, DNA damage or cell stress (Figure 6).142 Recent work in our laboratory supports G2/M arrest in tubular cells as an important driver of maladaptive repair and progressive CKD after AKI (Figure 6).15,168,169 We examined multiple experimental models of murine kidney injury, including severe bilateral IRI, unilateral IRI, aristo-lochic acid nephropathy and unilateral ureteral obstruction, and identified the accumulation of cells in G2/M growth arrest as the common feature predicting progressive fibrotic kidney disease.15 G2/M arrest in tubular epithelial cells in response to injury results in the acquisition of a pathogenic phenotype characterized by the sustained expression of profibrotic growth factors. This hypothesis is supported by reports from other laboratories168–170 and by pharmacological inhibition of G2/M-arrested cells, which reduced fibrosis, whereas increases in the proportion of G2/M-arrested cells in the cell cycle exacerbated fibrosis.2,168–170 Notably, humans with the FAN1 mutation and defects in DNA repair develop karyomegalic interstitial nephritis, with evidence of increased levels of DNA damage and cell-cycle arrest in the late G2 phase.171 As ageing is associated with increased levels of DNA damage and cell senescence,172 predisposition to G2/M arrest provides another plausible mechanism to explain the heightened risk of fibrotic kidney disease complicating AKI in the elderly, which is likely to be related to a combination of factors as described in this Review.
Figure 6.
Eukaryotic cell-cycle checkpoints. Four phases of cell-cycle progression are seen in eukaryotic cells. Cellular DNA is replicated in the S phase, and cell mitosis occurs in M phase, separated by two gap phases, G1 and G2. Critical checkpoints exist at G1/S and G2/M, where the actions of cyclins, CDKs and their inhibitors determine whether appropriate cell size, DNA replication and integrity exist to allow the initiation of DNA synthesis or the completion of cell division, respectively. Increased numbers of G2/M-arrested cells have been identified as a common feature in models of progressive chronic kidney disease. Abbreviations: CDK, cyclin-dependent kinase; CTGF, connective tissue growth factor; TGF-β, transforming growth factor β.
Conclusions
Work published over the past few years has revealed new targets for antifibrotic therapies such as G2/M arrested cells, immunosenescence, and modulation of growth factors and inflammatory mediators. The role of EMT versus pericytes (or other interstitial cell populations) in driving renal fibrosis has been re-evaluated. Targeting perivascular cells may address both the deposition of fibrosis and microvascular rarefaction characteristic of progressive CKD after AKI.173 Various compounds that modulate pericyte function are being investigated to assess their ability to promote kidney recovery.174
With recognition of the importance of abnormalities in the epithelial cell cycle in determining the renal outcome after injury comes the potential to target this process using novel therapeutic strategies. Experiments in rodents suggest the therapeutic potential of blocking the initiation of the G2/M checkpoint, or encouraging cells to transit through G2/M to complete mitosis, using agents such as histone deacetylase inhibitors170 or p53 inhibition.15,175 Clinical application in humans will, however, need careful assessment of the safety of the drugs and pathways under investigation given the function of G2/M in preventing the perpetuation of dangerous DNA mutations. Recent work in rhabdomyosarcoma indicates that bypassing cell-cycle checkpoints promotes tumourigenesis.176 Other strategies could focus on either blocking the profibrotic signalling of G2/M-arrested cells (as has been performed successfully in rodents using JNK-pathway inhibition15), or promoting their apoptosis or pharmacological depletion. Notably, in mice with a progeroid background of accelerated ageing, sustained depletion of senescent cells expressing p16INK4a significantly delayed the onset of age-related disorders in multiple tissues.148 This finding suggests that senescent cells actively promote the disease phenotype rather than simply representing a marker of an ongoing process. Another approach would be to target perivascular gli1(+) progenitors.125 Translation of these strategies to humans will require the identification of novel markers permitting the specific targeting of these cells.
The contribution of ageing to immuosenescence and to the pool of senescent tubular cells within the kidney may explain the increased susceptibility to AKI displayed by the elderly as well as the age-influenced increased rates of CKD progression. Progressive CKD may be mechanistically synonymous with accelerated ageing of the kidney. Given the ageing population and increasing burden of age-related progressive CKD in both developed and developing nations, novel therapies for this pervasive disease deserve the highest priority.
Key points.
-
▪
Acute injury to the kidney is often associated with maladaptive repair and incomplete resolution, leading to residual abnormalities in kidney structure and function
-
▪
Increasing age, and chronic low-grade insults to the tubular epithelium increase epithelial cell sensitivity to episodes of acute kidney injury, leading to maladaptive repair and progression of chronic kidney disease
-
▪
Maladaptive repair is characterized by fibrosis, vascular rarefaction, tubular loss, glomerulosclerosis and the presence of a chronic inflammatory infiltrate within the kidney
-
▪
Injured renal tubular epithelial cells become arrested at G2/M and adopt a profibrotic phenotype, which affects other epithelial cells, pericytes and the immune system
-
▪
Myofibroblasts that likely arise from renal pericytes proliferate and contribute to the deposition of extracellular matrix and resulting fibrosis within the injured kidney
-
▪
Maladaptive repair after acute kidney insults shares many common features with kidney ageing and can be thought of as a state of accelerated kidney ageing
Acknowledgments
J.V.B. is co-inventor on KIM-1 patents that are assigned to Partners HealthCare and licensed to Johnson & Johnson, Sekisui, Novartis, Biogen Idec., R & D, and Astute. He is a consultant for Astellas, Novartis, Roche and Sekisui regarding the safety and efficacy of therapeutics or diagnostics for acute kidney injury. He holds equity in MediBeacon, Sentien and Thrasos, and has grant support from Novo Nordisk and Roche.
Footnotes
Competing interests
D.A.F. declares no competing interests.
Author contributions
The authors contributed equally to researching data for the article, discussion of its content, writing and reviewing and/or editing of the manuscript before submission.
References
- 1.Metcalfe W, et al. Acute renal failure requiring renal replacement therapy: incidence and outcome. QJM. 2002;95:579–583. doi: 10.1093/qjmed/95.9.579. [DOI] [PubMed] [Google Scholar]
- 2.Ali T, et al. Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J. Am. Soc. Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 3.Noble JS, Simpson K, Allison ME. Long-term quality of life and hospital mortality in patients treated with intermittent or continuous hemodialysis for acute renal and respiratory failure. Ren. Fail. 2006;28:323–330. doi: 10.1080/08860220600591487. [DOI] [PubMed] [Google Scholar]
- 4.Xue JL, et al. Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 5.Wonnacott A, Meran S, Amphlett B, Talabani B, Phillips A. Epidemiology and outcomes in community-acquired versus hospital-acquired aki. Clin. J. Am. Soc. Nephrol. 2014;9:1007–1014. doi: 10.2215/CJN.07920713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N. Engl. J. Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 7.Brezis M, Rosen S. Hypoxia of the renal medulla—its implications for disease. N. Engl. J. Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich JO, Adhikari N, Herridge MS, Beyene J. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann. Intern. Med. 2005;142:510–524. doi: 10.7326/0003-4819-142-7-200504050-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ. 2006;333:420. doi: 10.1136/bmj.38902.605347.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishani A, et al. Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimoi T, et al. The significant impact of acute kidney injury on CKD in patients who survived over 10 years after myeloablative allogeneic SCT. Bone Marrow Transplant. 2013;48:80–84. doi: 10.1038/bmt.2012.85. [DOI] [PubMed] [Google Scholar]
- 13.Belayev LY, Palevsky PM. The link between acute kidney injury and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013 doi: 10.1097/01.mnh.0000441051.36783.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grgic I, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Renal Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt R, Cantley LG. The impact of aging on kidney repair. Am. J. Physiol. Renal Physiol. 2008;294:F1265–F1272. doi: 10.1152/ajprenal.00543.2007. [DOI] [PubMed] [Google Scholar]
- 19.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J. Am. Soc. Nephrol. 2003;14(Suppl. 1):S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 20.Cantley LG. Adult stem cells in the repair of the injured renal tubule. Nat. Clin. Pract. Nephrol. 2005;1:22–32. doi: 10.1038/ncpneph0021. [DOI] [PubMed] [Google Scholar]
- 21.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J. Clin. Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bussolati B, et al. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dekel B, et al. Isolation and characterization of nontubular sca-1+lin multipotent stem/ progenitor cells from adult mouse kidney. J. Am. Soc. Nephrol. 2006;17:3300–3314. doi: 10.1681/ASN.2005020195. [DOI] [PubMed] [Google Scholar]
- 24.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Humphreys BD, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc. Natl Acad. Sci. USA. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger K, Moeller MJ. Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin. Nephrol. 2014;34:394–403. doi: 10.1016/j.semnephrol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Evans RG, et al. Haemodynamic influences on kidney oxygenation: clinical implications of integrative physiology. Clin. Exp. Pharmacol. Physiol. 2013;40:106–122. doi: 10.1111/1440-1681.12031. [DOI] [PubMed] [Google Scholar]
- 28.Pallone TL, Robertson CR, Jamison RL. Renal medullary microcirculation. Physiol. Rev. 1990;70:885–920. doi: 10.1152/physrev.1990.70.3.885. [DOI] [PubMed] [Google Scholar]
- 29.Basile DP. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 30.Kim M, et al. The volatile anesthetic isoflurane induces ecto-5’-nucleotidase (cd73) to protect against renal ischemia and reperfusion injury. Kidney Int. 2013;84:90–103. doi: 10.1038/ki.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkatachalam MA, Weinberg JM. The conundrum of protection from AKI by adenosine in rodent clamp ischemia models. Kidney Int. 2013;84:16–19. doi: 10.1038/ki.2013.101. [DOI] [PubMed] [Google Scholar]
- 32.Conger JD, Robinette JB, Hammond WS. Differences in vascular reactivity in models of ischemic acute renal failure. Kidney Int. 1991;39:1087–1097. doi: 10.1038/ki.1991.138. [DOI] [PubMed] [Google Scholar]
- 33.Noiri E, et al. Oxidative and nitrosative stress in acute renal ischemia. Am. J. Physiol. Renal Physiol. 2001;281:F948–F957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 34.De Greef KE, Ysebaert DK, Persy V, Vercauteren SR, De Broe ME. ICAM-1 expression and leukocyte accumulation in inner stripe of outer medulla in early phase of ischemic compared to hgCl2-induced ARF. Kidney Int. 2003;63:1697–1707. doi: 10.1046/j.1523-1755.2003.00909.x. [DOI] [PubMed] [Google Scholar]
- 35.Kelly KJ, et al. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am. J. Physiol. Renal Physiol. 2004;287:F760–F766. doi: 10.1152/ajprenal.00050.2004. [DOI] [PubMed] [Google Scholar]
- 36.Brodsky SV, et al. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am. J. Physiol. Renal Physiol. 2002;282:F1140–F1149. doi: 10.1152/ajprenal.00329.2001. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto T, et al. Intravital videomicroscopy of peritubular capillaries in renal ischemia. Am. J. Physiol. Renal Physiol. 2002;282:F1150–F1155. doi: 10.1152/ajprenal.00310.2001. [DOI] [PubMed] [Google Scholar]
- 38.Schrimpf C, Duffield JS. Mechanisms of fibrosis: the role of the pericyte. Curr. Opin. Nephrol. Hypertens. 2011;20:297–305. doi: 10.1097/MNH.0b013e328344c3d4. [DOI] [PubMed] [Google Scholar]
- 39.Humphreys BD, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schrimpf C, et al. Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J. Am. Soc. Nephrol. 2012;23:868–883. doi: 10.1681/ASN.2011080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong X, et al. Resident dendritic cells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007;71:619–628. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]
- 43.Ysebaert DK, et al. Identification and kinetics of leukocytes after severe ischaemia/ reperfusion renal injury. Nephrol. Dial. Transplant. 2000;15:1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- 44.Kelly KJ, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J. Clin. Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bolisetty S, Agarwal A. Neutrophils in acute kidney injury: not neutral any more. Kidney Int. 2009;75:674–676. doi: 10.1038/ki.2008.689. [DOI] [PubMed] [Google Scholar]
- 46.Li L, et al. IL-17 produced by neutrophils regulates IFN-γ-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaturvedi S, et al. Slit2 prevents neutrophil recruitment and renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2013;24:1274–1287. doi: 10.1681/ASN.2012090890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Awad AS, et al. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009;75:689–698. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burne-Taney MJ, Yokota-Ikeda N, Rabb H. Effects of combined T- and B-cell deficiency on murine ischemia reperfusion injury. Am. J. Transplant. 2005;5:1186–1193. doi: 10.1111/j.1600-6143.2005.00815.x. [DOI] [PubMed] [Google Scholar]
- 50.Melnikov VY, et al. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J. Clin. Invest. 2002;110:1083–1091. doi: 10.1172/JCI15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thornton MA, Winn R, Alpers CE, Zager RA. An evaluation of the neutrophil as a mediator of in vivo renal ischemic-reperfusion injury. Am. J. Pathol. 1989;135:509–515. [PMC free article] [PubMed] [Google Scholar]
- 52.Savill J, Smith J, Sarraf C, Ren Y, Abbott F, Rees A. Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis. Kidney Int. 1992;42:924–936. doi: 10.1038/ki.1992.369. [DOI] [PubMed] [Google Scholar]
- 53.Ferenbach DA, et al. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:928–933. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- 54.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol. Dial. Transplant. 2006;21:1231–1239. doi: 10.1093/ndt/gfk047. [DOI] [PubMed] [Google Scholar]
- 55.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2a receptor-mediated tissue protection: Role of macrophages. Am. J. Physiol. Renal Physiol. 2005;288:F722–F731. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- 56.Rae F, et al. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a csf1r–egfp transgene reporter. Dev. Biol. 2007;308:232–246. doi: 10.1016/j.ydbio.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 57.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J. Clin. Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin SL, et al. Macrophage wnt7b is critical for kidney repair and regeneration. Proc. Natl Acad. Sci. USA. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jang HS, Kim J, Park YK, Park KM. Infiltrated macrophages contribute to recovery after ischemic injury but not to ischemic preconditioning in kidneys. Transplantation. 2008;85:447–455. doi: 10.1097/TP.0b013e318160f0d1. [DOI] [PubMed] [Google Scholar]
- 60.Lech M, et al. Macrophage phenotype controls long-term AKI outcomes—kidney regeneration versus atrophy. J. Am. Soc. Nephrol. 2013;25:292–304. doi: 10.1681/ASN.2013020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant. Rev. 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burne-Taney MJ, et al. B cell deficiency confers protection from renal ischemia reperfusion injury. J. Immunol. 2003;171:3210–3215. doi: 10.4049/jimmunol.171.6.3210. [DOI] [PubMed] [Google Scholar]
- 63.Rabb H, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am. J. Physiol. Renal Physiol. 2000;27:F525–F531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 64.Park P, et al. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T lymphocytes. Am. J. Physiol. Renal Physiol. 2002;282:F352–F357. doi: 10.1152/ajprenal.00160.2001. [DOI] [PubMed] [Google Scholar]
- 65.Kinsey GR, et al. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jang HR, et al. B cells limit repair after ischemic acute kidney injury. J. Am. Soc. Nephrol. 2010;21:654–665. doi: 10.1681/ASN.2009020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burne-Taney MJ, et al. Transfer of lymphocytes from mice with renal ischemia can induce albuminuria in naive mice: A possible mechanism linking early injury and progressive renal disease? Am. J. Physiol. Renal Physiol. 2006;291:F981–F986. doi: 10.1152/ajprenal.00229.2005. [DOI] [PubMed] [Google Scholar]
- 68.Day YJ, et al. Renal protection from ischemia mediated by a2a adenosine receptors on bone marrow-derived cells. J. Clin. Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basile DP, et al. Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am. J. Physiol. Renal Physiol. 2011;300:F721–F733. doi: 10.1152/ajprenal.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L, et al. Dendritic cells tolerized with adenosine A2 AR agonist attenuate acute kidney njury. J. Clin. Invest. 2012;122:3931–3942. doi: 10.1172/JCI63170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kinsey GR, et al. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J. Am. Soc. Nephrol. 2012;23:1528–1537. doi: 10.1681/ASN.2012010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferenbach DA, et al. The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int. 2011;79:966–976. doi: 10.1038/ki.2010.535. [DOI] [PubMed] [Google Scholar]
- 73.Ferenbach DA, et al. Macrophages expressing heme oxygenase-1 improve renal function in ischemia/reperfusion injury. Mol. Ther. 2010;18:1706–1713. doi: 10.1038/mt.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duffield JS, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J. Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 75.Leonard MO, et al. 15-epi-16-(para-fluorophenoxy)-lipoxin a(4)-methyl ester, a synthetic analogue of 15-epi-lipoxin A(4), is protective in experimental ischemic acute renal failure. J. Am. Soc. Nephrol. 2002;13:1657–1662. doi: 10.1097/01.asn.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- 76.Franquesa M, et al. Tubular epithelial cells transfected with hHGF counteracts monocyte chemotactic protein-1 up-regulation after hypoxia/reoxygenation insult. Transplant. Proc. 2009;41:2069–2072. doi: 10.1016/j.transproceed.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 77.Franquesa M, et al. Direct electrotransfer of hHGF gene into kidney ameliorates ischemic acute renal failure. Gene Ther. 2005;12:1551–1558. doi: 10.1038/sj.gt.3302569. [DOI] [PubMed] [Google Scholar]
- 78.Thurman JM, Lucia MS, Ljubanovic D, Holers VM. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int. 2005;67:524–530. doi: 10.1111/j.1523-1755.2005.67109.x. [DOI] [PubMed] [Google Scholar]
- 79.Thurman JM, et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/ reperfusion. J. Clin. Invest. 2006;116:357–368. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peng Q, Li K, Patel H, Sacks SH, Zhou W. Dendritic cell synthesis of C3 is required for full T cell activation and development of a th1 phenotype. J. Immunol. 2006;176:3330–3341. doi: 10.4049/jimmunol.176.6.3330. [DOI] [PubMed] [Google Scholar]
- 81.Thurman JM, et al. Treatment with an inhibitory monoclonal antibody to mouse factor b protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J. Am. Soc. Nephrol. 2006;17:707–715. doi: 10.1681/ASN.2005070698. [DOI] [PubMed] [Google Scholar]
- 82.Renner B, et al. The complement inhibitors Crry and factor H are critical for preventing autologous complement activation on renal tubular epithelial cells. J. Immunol. 2010;185:3086–3094. doi: 10.4049/jimmunol.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Linkermann A, et al. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Linkermann A, De Zen F, Weinberg J, Kunzendorf U, Krautwald S. Programmed necrosis in acute kidney injury. Nephrol. Dial. Transplant. 2012;27:3412–3419. doi: 10.1093/ndt/gfs373. [DOI] [PubMed] [Google Scholar]
- 85.Ishibe S, Cantley LG. Epithelial- mesenchymal-epithelial cycling in kidney repair. Curr. Opin. Nephrol. Hypertens. 2008;17:379–385. doi: 10.1097/MNH.0b013e3283046507. [DOI] [PubMed] [Google Scholar]
- 86.Villanueva S, Cespedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 87.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J. Clin. Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Witzgall R, O’Leary E, Gessner R, Ouellette AJ, Bonventre JV. Kid-1, a putative renal transcription factor: Regulation during ontogeny and in response to ischemia and toxic injury. Mol. Cell. Biol. 1993;13:1933–1942. doi: 10.1128/mcb.13.3.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Price VR, Reed CA, Lieberthal W, Schwartz JH. ATP depletion of tubular cells causes dissociation of the zonula adherens and nuclear translocation of β-catenin and LEF-1. J. Am. Soc. Nephrol. 2002;13:1152–1161. doi: 10.1097/01.asn.0000012609.22035.44. [DOI] [PubMed] [Google Scholar]
- 90.Kuure S, Popsueva A, Jakobson M, Sainio K, Sariola H. Glycogen synthase kinase-3 inactivation and stabilization of β-catenin induce nephron differentiation in isolated mouse and rat kidney mesenchymes. J. Am. Soc. Nephrol. 2007;18:1130–1139. doi: 10.1681/ASN.2006111206. [DOI] [PubMed] [Google Scholar]
- 91.Ishibe S, Haydu JE, Togawa A, Marlier A, Cantley LG. Cell confluence regulates hepatocyte growth factor-stimulated cell morphogenesis in a β-catenin-dependent manner. Mol. Cell. Biol. 2006;26:9232–9243. doi: 10.1128/MCB.01312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Brien LE, et al. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Develop. Cell. 2004;7:21–32. doi: 10.1016/j.devcel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Kimura M, et al. Role of atrophic changes in proximal tubular cells in the peritubular deposition of type IV collagen in a rat renal ablation model. Nephrol. Dial. Transplant. 2005;20:1559–1565. doi: 10.1093/ndt/gfh872. [DOI] [PubMed] [Google Scholar]
- 94.Suzuki T, Kimura M, Asano M, Fujigaki Y, Hishida A. Role of atrophic tubules in development of interstitial fibrosis in microembolism-induced renal failure in rat. Am. J. Pathol. 2001;158:75–85. doi: 10.1016/S0002-9440(10)63946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Borst MH, et al. C-Jun NH2-terminal kinase is crucially involved in renal tubulo-interstitial inflammation. J. Pharmacol. Exp. Ther. 2009;331:896–905. doi: 10.1124/jpet.109.154179. [DOI] [PubMed] [Google Scholar]
- 96.Venkatachalam MA, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am. J. Physiol. Renal Physiol. 2010;298:F1078–F1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bomsztyk K, Denisenko O. Epigenetic alterations in acute kidney injury. Semin. Nephrol. 2013;33:327–340. doi: 10.1016/j.semnephrol.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wing MR, Ramezani A, Gill HS, Devaney JM, Raj DS. Epigenetics of progression of chronic kidney disease: fact or fantasy? Semin. Nephrol. 2013;33:363–374. doi: 10.1016/j.semnephrol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naito M, Zager RA, Bomsztyk K. BRG1 increases transcription of proinflammatory genes in renal ischemia. J. Am. Soc. Nephrol. 2009;20:1787–1796. doi: 10.1681/ASN.2009010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zager RA, Johnson AC. Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes. Am. J. Physiol. Renal Physiol. 2009;296:F1032–F1041. doi: 10.1152/ajprenal.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu J, Feng Q, Ruan Y, Komers R, Kiviat N, Bomsztyk K. Microplate-based platform for combined chromatin and DNA methylation immunoprecipitation assays. BMC Mol. Biol. 2011;12:49. doi: 10.1186/1471-2199-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonventre JV. Kidney injury molecule-1 (Kim-1): a urinary biomarker and much more. Nephrol. Dial. Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 103.Ichimura T, et al. Kidney injury molecule-1 (Kim-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 104.Ichimura T, et al. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Humphreys BD, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J. Clin. Invest. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang L, et al. KIM-1/TIM-1 mediated-phagocytosis reduces acute injury to the kidney. J. Clin. Invest. in press doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mederacke I, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nature Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williams SM, et al. Class I HDACs regulate angiotensin ii-dependent cardiac fibrosis via fibroblasts and circulating fibrocytes. J. Mol. Cell. Cardiol. 2014;67:112–125. doi: 10.1016/j.yjmcc.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sahebally SM, et al. Circulating fibrocytes and Crohn’s disease. Br. J. Surg. 2013;100:1549–1556. doi: 10.1002/bjs.9302. [DOI] [PubMed] [Google Scholar]
- 111.Wada T, et al. Involvement of bone-marrow-derived cells in kidney fibrosis. Clin. Exp. Nephrology. 2011;15:8–13. doi: 10.1007/s10157-010-0372-2. [DOI] [PubMed] [Google Scholar]
- 112.Ross R, Everett NB, Tyler R. Wound healing and collagen formation. VI. The origin of the wound fibroblast studied in parabiosis. J. Cell Biol. 1970;44:645–654. doi: 10.1083/jcb.44.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of adam12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat. Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 114.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J. Am. Soc. Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 116.Iwano M, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lin SL, et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am. J. Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith SW, Chand S, Savage CO. Biology of the renal pericyte. Nephrol. Dial. Transplant. 2012;27:2149–2155. doi: 10.1093/ndt/gfs134. [DOI] [PubMed] [Google Scholar]
- 119.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 120.Sundberg C, Kowanetz M, Brown LF, Detmar M, Dvorak HF. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo . Lab. Invest. 2002;82:387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 121.Carvalho RL, et al. Defective paracrine signalling by TGFβ in yolk sac vasculature of endoglin mutant mice: A paradigm for hereditary haemorrhagic telangiectasia. Development. 2004;131:6237–6247. doi: 10.1242/dev.01529. [DOI] [PubMed] [Google Scholar]
- 122.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-b and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 123.Chae SS, Paik JH, Allende ML, Proia RL, Hla T. Regulation of limb development by the sphingosine 1-phosphate receptor S1p1/EDG-1 occurs via the hypoxia/ VEGF axis. Dev. Biol. 2004;268:441–447. doi: 10.1016/j.ydbio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 124.Chen YT, et al. Platelet-derived growth factor receptor signaling activates pericyte- myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011;80:1170–1181. doi: 10.1038/ki.2011.208. [DOI] [PubMed] [Google Scholar]
- 125.Kramann R, et al. Perivascular gli1(+) progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat. Rev. Nephrol. 2013;9:77–85. doi: 10.1038/nrneph.2012.280. [DOI] [PubMed] [Google Scholar]
- 127.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clements ME, Chaber CJ, Ledbetter SR, Zuk A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS ONE. 2013;8:e70464. doi: 10.1371/journal.pone.0070464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, Larusso NF. Cholangiocyte senescence via N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–2275. doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marongiu F, Serra MP, Sini M, Angius F, Laconi E. Clearance of senescent hepatocytes in a neoplastic-prone microenvironment delays the emergence of hepatocellular carcinoma. Aging. 2014;6:26–34. doi: 10.18632/aging.100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Westhoff JH, et al. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16ink4a. Hypertension. 2008;52:123–129. doi: 10.1161/HYPERTENSIONAHA.107.099432. [DOI] [PubMed] [Google Scholar]
- 132.Liu J, et al. Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Transl. Res. 2012;159:454–463. doi: 10.1016/j.trsl.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 133.Verzola D, et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2008;295:F1563–F1573. doi: 10.1152/ajprenal.90302.2008. [DOI] [PubMed] [Google Scholar]
- 134.Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 135.Oien CM, et al. Living donor kidney transplantation: the effects of donor age and gender on short- and long-term outcomes. Transplantation. 2007;83:600–606. doi: 10.1097/01.tp.0000255583.34329.dd. [DOI] [PubMed] [Google Scholar]
- 136.Reutzel-Selke A, et al. Donor age intensifies the early immune response after transplantation. Kidney Int. 2007;71:629–636. doi: 10.1038/sj.ki.5002098. [DOI] [PubMed] [Google Scholar]
- 137.Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J. Clin. Invest. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schmitt R, Marlier A, Cantley LG. Zag expression during aging suppresses proliferation after kidney injury. J. Am. Soc. Nephrol. 2008;19:2375–2383. doi: 10.1681/ASN.2008010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kwekel JC, Desai VG, Moland CL, Vijay V, Fuscoe JC. Life cycle analysis of kidney gene expression in male f344 rats. PLoS ONE. 2013;8:e75305. doi: 10.1371/journal.pone.0075305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated β-galactosidase assay. Methods Mol. Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
- 142.Yang H, Fogo AB. Cell senescence in the aging kidney. J. Am. Soc. Nephrol. 2010;21:1436–1439. doi: 10.1681/ASN.2010020205. [DOI] [PubMed] [Google Scholar]
- 143.Megyesi J, Andrade L, Vieira JM, Jr, Safirstein RL, Price PM. Positive effect of the induction of p21WAF1/CIP1 on the course of ischemic acute renal failure. Kidney Int. 2001;60:2164–2172. doi: 10.1046/j.1523-1755.2001.00044.x. [DOI] [PubMed] [Google Scholar]
- 144.Dirocco D, et al. CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am. J. Physiol. Renal Physiol. 2013;306:F379–F388. doi: 10.1152/ajprenal.00475.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 146.Loffredo FS, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baker DJ, et al. Clearance of p16(Ink4a)-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang HC, et al. Cells derived from young bone marrow alleviate renal aging. J. Am. Soc. Nephrol. 2011;22:2028–2036. doi: 10.1681/ASN.2010090982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J. Am. Soc. Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 151.Shurin GV, et al. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine. 2007;39:123–129. doi: 10.1016/j.cyto.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 152.Kang DH, et al. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am. J. Kidney Dis. 2001;37:601–611. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- 153.Ruiz-Torres MP, et al. Age-related increase in expression of TGF-β1 in the rat kidney: relationship to morphologic changes. J. Am. Soc. Nephrol. 1998;9:782–791. doi: 10.1681/ASN.V95782. [DOI] [PubMed] [Google Scholar]
- 154.Karam Z, Tuazon J. Anatomic and physiologic changes of the aging kidney. Clin. Geriatr. Med. 2013;29:555–564. doi: 10.1016/j.cger.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 155.Poulsom R, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J. Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 156.Duffield JS, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J. Clin. Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morigi M, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 158.Semedo P, et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int. Immunopharmacol. 2009;9:677–682. doi: 10.1016/j.intimp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 159.Hagiwara M, Shen B, Chao L, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum. Gene Ther. 2008;19:807–819. doi: 10.1089/hum.2008.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu. Rev. Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 161.Thum T, et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ. Res. 2007;100:434–443. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 162.Behrens A, van Deursen JM, Rudolph KL, Schumacher B. Impact of genomic damage and ageing on stem cell function. Nat. Cell Biol. 2014;16:201–207. doi: 10.1038/ncb2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stolzing A, Scutt A. Age-related impairment of mesenchymal progenitor cell function. Aging Cell. 2006;5:213–224. doi: 10.1111/j.1474-9726.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 164.Schatteman GC, Ma N. Old bone marrow cells inhibit skin wound vascularization. Stem Cells. 2006;24:717–721. doi: 10.1634/stemcells.2005-0214. [DOI] [PubMed] [Google Scholar]
- 165.Gewin L, Zent R. How does TGF-β mediate tubulointerstitial fibrosis? Semin. Nephrol. 2012;32:228–235. doi: 10.1016/j.semnephrol.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qi W, Chen X, Poronnik P, Pollock CA. Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int. J. Biochem. Cell Biol. 2008;40:9–13. doi: 10.1016/j.biocel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 167.Acosta JC, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 168.Wu CF, et al. Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am. J. Pathol. 2013;182:118–131. doi: 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tang J, et al. Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am. J. Pathol. 2013;183:160–172. doi: 10.1016/j.ajpath.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cianciolo Cosentino C, et al. Histone deacetylase inhibitor enhances recovery after AKI. J. Am. Soc. Nephrol. 2013;24:943–953. doi: 10.1681/ASN.2012111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou W, et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat. Genet. 2012;44:910–915. doi: 10.1038/ng.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang HC, et al. Cells derived from young bone marrow alleviate renal aging. J. Am. Soc. Nephrol. 2011;22:2028–2036. doi: 10.1681/ASN.2010090982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kramann R, Humphreys BD. Kidney pericytes: roles in regeneration and repair. Semin. Nephrol. 2014;34:374–383. doi: 10.1016/j.semnephrol.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Duffield JS, Lupher ML., Jr PRM-151 (recombinant human serum amyloid p/pentraxin 2) for the treatment of fibrosis. Drug News Perspect. 2010;23:305–315. doi: 10.1358/dnp.2010.23.5.1444206. [DOI] [PubMed] [Google Scholar]
- 175.Zhou L, et al. Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. J. Am. Soc. Nephrol. 2010;21:31–41. doi: 10.1681/ASN.2008111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Crose LE, et al. Alveolar rhabdomyosarcoma-associated PAX3-FOXO1 promotes tumorigenesis via hippo pathway suppression. J. Clin. Invest. 2014;124:285–296. doi: 10.1172/JCI67087. [DOI] [PMC free article] [PubMed] [Google Scholar]