Abstract
Aims
To describe outcome and examine factors associated with mortality among human immunodeficiency virus (HIV)-infected children in Malaysia after anti-retroviral therapy (ART).
Methods
Retrospective and prospective data collected through March 2009 from children in four different states in Malaysia enrolled in TREAT Asia’s Pediatric HIV Observational Database were analysed.
Results
Of 347 children in the cohort, only 278 (80.1%) were commenced on ART. The median CD4 count and median age at baseline prior to ART was 272 cells/μL and 4.2 years (interquartile range (IQR): 1.4, 7.4 years), respectively. The median duration of follow-up was 3.7 years (IQR: 1.8, 6.0) with 32 deaths giving a crude mortality rate of 2.86 per 100 child-years. The mortality rate highest in the first 6 months of ART was 10.62 per 100 child-years and declined to 1.83 per 100 child-years thereafter. On univariate analyses, only baseline median CD4 percentage, weight for age z score, height for age z score and anaemia were significantly associated with mortality. Upon including all four of these predictors into a single multivariate model, only weight for age z score remained statistically significantly predictive of mortality.
Conclusions
Children commenced on ART had high mortality in the first 6 months especially in those with low CD4 percentage, wasting and anaemia. Poor nutritional status is an important independent predictor of mortality in this study. Besides initiating ART therapy, nutritional support and intervention must receive the utmost attention.
Keywords: anti-retroviral, child, HIV, Malaysia, outcome
Malaysia has been classified by World Health Organization (WHO) as one of the countries in East Asia to have a concentrated human immunodeficiency virus (HIV) epidemic.1 The HIV epidemic in Malaysia, in recent years, was mainly driven by injecting drug use and heterosexual transmission.1
Data from 2010 show that children constitute around 1% of all positive cases in Malaysia; 909 infections have been recorded among children aged ≤13 years of age.2 The low prevalence of HIV in children is due in large part to the prevention of mother-to-child transmission programmes (PMTCT), which have been implemented nationwide since 1998.1 The reported coverage of PMTCT in 2009 was 98.1%, and to date, it has successfully reduced the incidence of maternal to child transmission to 4%.1
Highly active anti-retroviral therapy (ART) has been made available by the Ministry of Health and is provided free to HIV-infected children. As in Western countries, it is anticipated that ART would have a significant impact on reducing mortality in Malaysia. However, data on survival and outcomes of HIV-infected children in Malaysia remain limited.
The aims of this study were to evaluate the treatment outcomes and survival of HIV-infected children in Malaysia after receiving ART and to examine factors associated with mortality among these children.
Methods
The TREAT Asia Pediatric HIV Observational Database (TApHOD) is a multi-centre, observational cohort study of infants and children living with HIV in Asia.3 TApHOD is a member cohort of the US National Institutes of Health’s International Epidemiologic Databases to Evaluate AIDS. Data collection for TApHOD began in 2008.3 Data from children with HIV <18 years of age receiving clinical care at participating sites are submitted biannually by electronic transfer to a central database maintained at the Kirby Institute for Infection and Immunity in Society, Sydney, Australia. Participating sites are largely public referral hospitals based in urban settings. Eligible HIV-infected children receiving care at participating clinical sites are enrolled into the database. Inclusion criteria include the following: (i) age at the first clinic visit of ≤18 years; and (iia) confirmed diagnosis of HIV using age-appropriate tests or (iib) presumptive diagnosis according to WHO criteria until confirmation. At the time of this analysis, the TApHOD cohort included data up to the end of March 2011 from 16 sites in six Asian countries.
Institutional Review Board approval was obtained at participating sites, and the data management and co-ordinating centres (University of New South Wales, Sydney; TREAT Asia, Bangkok). As data are anonymously collected, informed consent was waived, unless locally required.
Recommendation for when to initiate treatment
For this study, we included data from all children under care at the four sites in Malaysia who had commenced ART. The criteria for eligibility of ART were in accordance with the Malaysia Management of HIV in Children Clinical Practice Guidelines 2008, namely, all infants below 12 months of age irrespective of CD4% or viral load and children with significant symptoms (WHO stage 3 or 4) or having a CD4% < 15%.4 Patients were included following the intention-to-treat principle and remained in the study regardless of any subsequent changes to their treatment regimen.
Data collected included demographic information, clinical and laboratory monitoring data, and treatment outcomes collected during clinic visits as part of routine care by paediatricians’ trained in HIV care (Table 1). The frequency of visits and monitoring is determined by standard patient care indications and availability. Compiled data are subject to extensive, routine quality checks. For this study, we included data up to end of March 2011 from four sites in Malaysia.
Table 1.
Baseline characteristics of Malaysia children at initiation of ART
| Characteristics | N |
|---|---|
| All patients | 278 |
| Female, n (%) | 133 (48%) |
| Age, median (IQR) years | 4.2 (1.4–7.4) |
| Baseline WHO stage, n (%) | |
| Stage 1 | 91 (33%) |
| Stage 2 | 39 (14%) |
| Stage 3 | 90 (32%) |
| Stage 4 | 58 (21%) |
| ART regimen | |
| ART with PI | 51 (18%) |
| ART without PI | 227 (82%) |
| CD4 percentage | |
| <5% | 41 (15%) |
| 5 to <15% | 45 (16%) |
| 15 to <25% | 44 (16%) |
| ≥25% | 27 (10%) |
| Unknown | 121 (44%) |
| Median (IQR) | 13 (4–21) |
| CD4 cell count (×106 cells/L) | |
| <50 | |
| 50 to <250 | 53 (19%) |
| 250 to <500 | 54 (19%) |
| 500 to <1000 | 42 (15%) |
| ≥1000 | 42 (15%) |
| Unknown | 34 (12%) |
| Median (IQR) | 53 (19%) |
| 272 (60–725) | |
| Median HIV RNA log 10 copies/ml (n = 162) | 5.4 (4.9–5.9) |
| Baseline weight for age z score, median (n = 244) | −2.6 (−3.9 to −1.3) |
| Baseline height for age z score, Median (n = 241) | −2.1 (−2.8 to −1.1) |
| Severe anaemia n (%) (n = 192) | 66 (24%) |
| Family status up to 1 year after baseline (n = 173) | 61 (22%) |
| Both parents involved | 54 (19%) |
| Single parent involved | 58 (21%) |
| Orphan | 105 (38%) |
| Unknown | |
ART, anti-retroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; WHO, World Health Organization.
The baseline measurement was taken to be the closest observation to the date of ART commencement within the interval from 6 months prior to 14 days after. Exceptions were WHO staging,5 where all observations prior to and none after commencement of ART were included, and family status, where observations prior to and up to 1 year after commencement of ART were included. CD4 cell counts were measured by flow cytometry at each centre, and viral load was performed using plasma HIV-1 RNA assays by Roche Amplicor HIV-1 Monitor System (version 1.5) and the Roche COBAS Taqman HIV-1 (version 2.0 by Roche Molecular Systems Inc., Pleasanton, CA, USA).
For height-for-age z score, the WHO 2006/2007 Child Growth Standards were used.6 WHO 1977 Standards were used for weight-for-age z score in order to allow for scoring children >10 years of age.7 Previous assessments have confirmed the applicability of the 1977 growth references in this cohort by comparing them to the WHO 2006/2007 reference curves in children < 10 years of age.8
Anaemia was defined as haemoglobin <10 g/dL for children ≤21 days of age, <8 g/dL for children 22–35 days of age, <7 g/dL for children 36–56 days of age and <7.5 g/dL for children >56 days of age.9
ART was defined as a treatment regimen containing at least three different anti-retroviral drugs. Children were initiated on first line anti-retroviral drug combinations as recommended by the Malaysia Management of HIV in Children Clinical Practice Guidelines 2008 which consist of two nucleoside reverse transcriptase inhibitor (NRTIs) and one non-NRTI (NNRTI).4
Adherence data were not systematically collected in the TApHOD, but some adherence support was provided by counsellor during the clinic visits.
Descriptive results are presented as proportions for categorical variables and medians and interquartile range (IQR) for quantitative variables. Hazard ratios describing relative risk of death with associated 95% confidence intervals and P values are computed by fitting Cox proportion hazard models. All analyses are conducted using the SAS v9.2 software package.
Results
As of March 2011, there were a total of 347 children from Malaysia in the cohort; 278 (80.1%) children who had commenced ART were included in this analysis (Table 1). The median age at ART initiation was 4.2 years (IQR: 1.4, 7.4 years). NNRTI-based regimens (82%) were more commonly prescribed than protease inhibitor (PI) based regimens. A total of 26 children had prior monotherapy or dual therapy before ART initiation.
The median duration of follow-up was 3.7 years (IQR: 1.8, 6.0 years), and 189 (67.9%) children were followed-up for more than 2 years after initiation of ART. A total of 20 (7.2%) children were lost to follow-up.
Immune recovery defined as CD4% greater than 25% at 12 months was seen in 51% of children and was 58% at 2 years after ART. Age at commencement of ART (P < 0.001), baseline CD4% (P < 0.001), baseline viral load (P < 0.042) and baseline weight for age z score (P < 0.015) was associated with immune recovery at 12 months after initiation of ART. Family status, baseline WHO staging and presence of anaemia were not significant predictors of immune recovery in this cohort.
Viral load results were available for 148 of the 278 children on ART at 12 months, and 91 (61%) of these 148 had HIV RNA < 200 copies/mL. At 24 months, 78 (57%) of 137 had HIV RNA < 200 copies/mL.
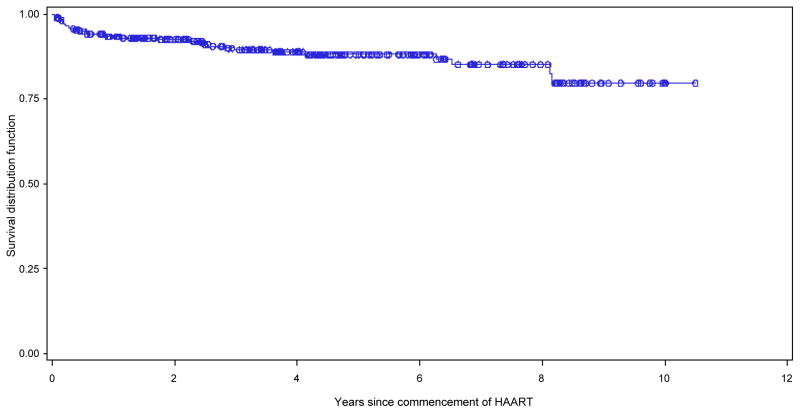
Deaths were recorded for 32 (11.5%) of the 278 children who had initiated ART. The median age at death was 6.3 years (IQR: 2.9, 8.7). The crude mortality rate was 2.86 per 100 child-years; 31% of deaths occurred during the first 3 months of ART. The mortality rate at 3 months of ART was 14.8 per 100 child-years, and this progressively fell to 6.2 per 100 child-years during the fourth to sixth months. The mortality after 12 months of ART was 1.6 per 100 child-years. The probability of survival to 5 years was 88% (Fig. 1).
Fig. 1.
Kaplan–Meier estimates of survival time for Malaysian children receiving anti-retroviral therapy (ART) for human immunodeficiency virus (HIV) infection. ——, product-limit estimate curve;, ○○○ censored observations.
The primary causes of death recorded for the 32 children who died were mostly related to infection: septicaemia (n = 12), pneumonia of any cause (n = 7), encephalitis (n = 3), tuberculosis (n = 2), diarrhoea (n = 2) and one each from malnutrition, cardiomyopathy, Mycobacterium avium, rhabdomyosarcoma, lymphoma and progressive multi-focal leukoencephalopathy. Ten (31.2%) and 18 (56.2%) children died within 3 months and 1 year of ART initiation, respectively. All these 18 children, except for one, died of infection-related causes with septicaemia and Pneumocystic jirovecii pneumonia contributing to the greatest number of death. The median baseline CD4 count (P < 0.027), baseline weight for age z score (P < 0.007) and baseline height for age z score (P < 0.01) for children who died within the first year of ART initiation were much lower compared with those who did not die in the first year of ART.
On univariate analyses, baseline median CD4 percentage, weight for age z score, height for age z score and anaemia were statistically significantly associated with mortality (Table 2). Upon including all four of these predictors into a single multivariate model, only weight for age z score remained statistically significantly predictive of mortality (Table 3).
Table 2.
Correlates of mortality among children with ART initiation
| Univariate
| ||||
|---|---|---|---|---|
| Characteristic | HR | 95% CI | P | Number available for analysis |
| Age at ART initiation | 1.03 | 0.93–1.14 | 0.553 | 278 |
| Sex, female | 1.46 | 0.72–2.96 | 0.297 | 278 |
| CD4% | 0.93 | 0.88–1.0 | 0.037 | 157 |
| CD4 | 0.999 | 0.998–1.000 | 0.072 | 225 |
| HIV RNA, log10 | 0.82 | 0.50–1.53 | 0.427 | 162 |
| WHO stages | ||||
| Stage 2 | 1.24 | 0.37–4.12 | 0.723 | 39 out of 278 |
| Stage 3 | 1.68 | 0.70–4.07 | 0.246 | 90 out of 278 |
| Stage 4 | 1.47 | 0.53–4.07 | 0.455 | 58 out of 278 |
| Weight for age z score | 0.77 | 0.66–0.90 | <0.001 | 243 |
| Height for age z score | 0.80 | 0.66–0.96 | 0.015 | 241 |
| Anaemia | 4.04 | 1.68–9.79 | 0.002 | 192 |
Bold text indicates significant value. ART, anti-retroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; WHO, World Health Organization.
Table 3.
Multivariate model analysis on mortality at 12 months of ART
| Variable | Available sample size | Odds ratio | P value |
|---|---|---|---|
| Baseline weight for age z score | 220/253 | 1.47 (1.16, 1.87) | 0.001 |
| Baseline weight for age z score and baseline height for age z score | 217/253 | 1.32 (1.01, 1.74) | 0.043 |
| 1.23 (0.89, 1.63) | 0.164 | ||
| Baseline weight for age z score and anaemia | 167/253 | 1.74 (1.26, 2.51) | 0.014 |
| 1.91 (0.48, 8.23) | 0.358 | ||
| Baseline weight for age z score and baseline CD4% | 127/253 | 2.06 (1.41, 3.24) | <0.001 |
| 0.96 (0.89, 1.04) | 0.316 |
ART, anti-retroviral therapy.
Discussion
In our study, the overall mortality rate was 2.86 per 100 child-years, and the probability of 5-year survival from the time of ART initiation was 88%. The survival rate is comparable with rates in Thailand and other Asian countries but much higher than those reported in African countries.8,10–12 The higher survival could be due to earlier diagnosis from the successful implementation of PMTCT programme across the country and better access to treatment in Malaysia compared with Africa. The mortality rate was highest in the first 3 months after ART initiation and progressively fell over time on ART, a trend observed in other cohorts.11,13,14
Deaths were mainly related to infectious conditions; specifically, septicaemia contributed to the greatest number of deaths followed by pneumonia. Most of the children who died early had advanced immunosuppression and growth failure with significantly lower baseline CD4 count, and lower weight and height for z score prior to the ART initiation. Although we do not have data on immune reconstitution inflammatory syndrome (IRIS), we believe it may have played a role in the observed higher early mortality. Severe immunosuppression and low weight for age are two well-known risk factors for IRIS.15
Multiple factors were initially associated with mortality, but only weight for z score was significantly predictive of mortality in the final multivariate model. A low weight for age z score usually indicates protein energy malnutrition. Severe malnutrition has significant deleterious effect on both humoural and cell-mediated immunity. Impaired immune function could then predispose these children to life-threatening infections.12,16–18 The loss of statistical significance of the other predictors could have resulted from low statistical power associated with the relatively small number of deaths.
Our findings showed that although ART has reduced the overall mortality of children with HIV, there remains a consistent pattern of high mortality in the first 3 months of ART initiation. These children usually have advanced HIV disease with immunosuppression and malnutrition. They remained susceptible to infections despite of ART therapy as the benefits of ART can only be fully realised after several months. It is therefore crucial to initiate early ART therapy in HIV-infected children. Besides early initiation, early identification of these infants and children is of utmost importance to halt disease progression and early mortality. Efforts to prevent transmission through PMTCT must also be intensified to achieve further reduction of vertical transmission.
There were several limitations in this study. As the data are observational and collected according to local clinical management practices and monitoring resources, some data on outcome such as CD4 and viral load were not available. Subjects lost to follow-up may be unreported deaths, resulting in underestimation of mortality. Adherence data and information on prior anti-retroviral experience were also not systematically collected. This study is limited to children in four centres which constitute close to 40% of the HIV-infected children in Malaysia. Caution is needed if we were to generalise the results to the rest of Malaysia.
Conclusions
Malaysian children on ART have a high overall survival rate. The highest mortality rate is in the first 3 months after ART initiation, and infections remain the most important cause of mortality. Poor nutritional status is an important independent predictor of mortality. Besides initiating ART therapy, nutritional support and interventions should receive careful attention.
What is already known on this topic
Anti-retroviral therapy (ART) reduces mortality and improves outcome in children.
Mortality is usually highest in first 3 months after initiation of ART and various factors such as viral load, CD4 percentage and anaemia are associated with mortality.
Early identification and early initiation of ART is of utmost importance to halt diesase progression and early mortality.
What this paper adds
Outcome and survival data on human immunodeficiency virus-infected children after initiation of ART in Malaysia.
Mortality is comparable with other Asian countries.
Nutritional status is an important determinant of survival and should receive careful attention.
Acknowledgments
The TApHOD is an initiative of TREAT Asia, a programme of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907) and the AIDS Life Association. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales.
Footnotes
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Conflict of interest: All authors declare no conflicts of interest and did not receive any financial support associated with the study.
References
- 1. [accessed March 2013];2010 UNGASS Country Progress Report – Malaysia. Available from http://www.data.unaids.org/pub/Report/2010/malaysia_201_country_progress_report_en.pdf.
- 2.Ministry of Health Malaysia. HIV and AIDS Statistics as of 31 December 2010. 2010 [Google Scholar]
- 3.Kariminia A, Chokephaibulkit K, Pang J, et al. Cohort profile: the TREAT Asia Pediatric HIV observational database. Int J Epidemiol. 2011;40:15–24. doi: 10.1093/ije/dyp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministry of Health Malaysia. CPG Management of HIV in Children. 2008 [Google Scholar]
- 5.World Health Organization (WHO) WHO case Definition of HIN for surveillance and Revised clinical Staging and Immunological Classification of HIV- Related Disease in Adults and Children. Geneva, Switzerland: World Health organization; 2006. [Google Scholar]
- 6.World Health Organization (WHO) [accessed September 2010];WHO growth chart. 2006 Available from: http://www.who.int/childgrowth/en/
- 7.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002:1–190. [PubMed] [Google Scholar]
- 8.Hansudewechakul R, Sirisanthana V, Kurniati N, et al. Antiretroviral therapy outcomes of HIV-infected children in the TREAT Asia pediatric HIV observational database. J Acquir Immune Defic Syndr. 2010;55:503–9. doi: 10.1097/QAI.0b013e3181f5379a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Division of AIDS. [accessed October 2011];Table for grading the severity of adult and pediatric adverse events. Version 1.0. 2004 Dec; clarification August 2009. Available from: http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table for Grading Severity of Adult Pediatric Adverse Events.
- 10.Lumbiganon P, Kariminia A, Aurpibul L, et al. Survival of HIV-infected children: a cohort from the Asia-Pacific region. J Acquir Immune Defic Syndr. 2011;56:365–71. doi: 10.1097/QAI.0b013e318207a55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wamalwa D, Obimbo E, Farquhar C, et al. Predictors of mortality in HIV-infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr. 2010;10:1471–2431. doi: 10.1186/1471-2431-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peacock-Villada E, Richardson B, John-Stewart G. Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics. 2011;127:423–41. doi: 10.1542/peds.2009-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins IJ, Jourdain G, Hansudewechakul R, et al. Long-term survival of HIV-infected children receiving antiretroviral therapy in Thailand: a 5-year observational cohort study. Clin Infect Dis. 2010;51:1449–58. doi: 10.1086/657401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marazzi MC, Liotta G, Germano P, et al. Excessive early mortality in the first year of treatment in HIV type1 infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retroviruses. 2008;24:555–60. doi: 10.1089/aid.2007.0217. [DOI] [PubMed] [Google Scholar]
- 15.Smith K, Kuhn L, Coovadia A, et al. Immune reconstitution inflammatory syndrome among HIV-infected South African infants initiating antiretroviral therapy. AIDS. 2009;23:1097–107. doi: 10.1097/QAD.0b013e32832afefc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMurray DN. Cell-mediated immunity in nutritional deficiency. Prog Food Nutr Sci. 1984;8:193–228. [PubMed] [Google Scholar]
- 17.Nájera O, González C, Cortés E, Toledo G, Ortiz R. Effector T lymphocytes in malnourished and well-nourished infected children. Clin Exp Immunol. 2007;148:501–6. doi: 10.1111/j.1365-2249.2007.03369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nájera O, González C, Toledo G, López L, Ortiz R. Flow cytometry study of lymphocytes in malnourished and well-nourished children with bacterial infection. Clin Diagn Lab Immunol. 2004;11:577–80. doi: 10.1128/CDLI.11.3.577-580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]