Abstract
There is only marginal improvement in outcome of treating pancreatic cancer in the last two decades. Time to open up and have a fresh look at complementary adjuvant treatment options. Hyperthermia may be one such option. Hyperthermic intraperitoneal chemotherapy (HIPEC) predominantly as a intrasurgical procedure has already proved its justification. Non-invasive loco regional hyperthermia as complement to either chemo or radiation has not yet reached a comparable status of evidence. However the potential to eventually grow into such evidence is already clearly observable. This review presents the various methodologies available for hyperthermia, covers the initial clinical data that has been published and gives an outlook to what can be expected in the next 2–3 years to come. Hyperthermia has the potential to significantly prolong life expectancies and this while maintaining a satisfying quality of life!
Keywords: Hyperthermia, Pancreatic cancer, Adjuvant heat treatment, Palliative pain reduction
Introduction
Pancreatic tumor is still a fatal diagnosis for most patients. Surgery is considered the only cure, provided the disease is in its early phase. However, the majority of newly diagnosed patients are already in advanced stages deemed to be unresectable. These patients have a mean average life expectancy of 6–7 months after the first diagnosis.
Standard therapy currently is chemotherapy using gemcitabine, eventually administered in combination with erlotinib Trials some years ago indicated the superiority of gemcitabine over other cytostatics [1]. But, combination chemotherapy could not improve the results with gemcitabine monotherapy [2]. Recently, however, a multi-agent regimen seems to indicate a superiority (11.1 months) over gemcitabine solo (6.8 months at best) with regard to overall survival expectancy [3]. Since these are recent developments, for the purpose of evaluating the adjuvant use of hyperthermia, most data compare against gemcitabine solo treatment or a gemcitabine/radiation scheme.
Survival expectations, nevertheless, have not been considerably enhanced in the last decade. Pancreatic tumors still remain a rapidly progressing disease with a very limited life expectancy. Thus there remains the need to screen and critically evaluate adjuvant treatment options according to mainly two criteria: 1. Potential to prolong overall survival, and 2. Potential to achieve or maintain a satisfying niveau in quality of life.
Hyperthermia as an adjuvant treatment option has the potential to meet these two criteria with the expectation of an attractive ratio in survival gain/marginal additional side effects. Clinical practice today certainly does not live up to this potential.
About Hyperthermia Techniques and Methodologies
There exist quite a variety of different heating methods and technologies. Those relevant for pancreatic treatments will be categorized and described in due course, and briefly evaluated to their current status in the clinical practice.
A first categorization would be to differentiate between loco-regional heating and whole body heating.
-
I.Whole body heating is achieved by two principles:
- the patient is placed under water-filtered infrared lamps, enwrapped and heated up (classical method)
- the patient is put in hot water bath (up to 44 °C), administered sedation and a cardio/intense care team is designated to supervise the treatment (innovative Russian origin method: Heatheal).
Even though there exist some data [4], whole body hyperthermia for pancreatic cancer treatment is even before its seed phase.
-
II.
Loco-regional hyperthermia is regarded as the main treatment option.
Here again it is useful to differentiate between the varied methods available:- superficial heating (intraoperative)
- interstitial/intraluminal heating
- non invasive deep regional heating
Intraoperative Superficial Heating
Though the idea of a direct heating in the intraoperative phase may sound promising, it is very rarely performed. Criticism 20 years ago was regarding the apparent inability to sufficiently generate surface heat [5]. A Greek group published promising data about unresectable palliative cases with a scheme of multi-schedule chemotherapy combined with radiation (45 Gy) plus a single session of hyperthermia during bypass surgery [6]. Hyperthermia was applied at 433 Mhz frequency which is basically suitable to efficiently heat the surface area. Their finding significantly noted that in the arm including hyperthermia, there was reduction in pain and improvement in the quality of life. Clinical results are summarized in Table 1.
Table 1.
Survey trials on pancreatic cancer treatments including hyperthermia
| Trials on advanced pancreatic cancer | Center | Hyperthermia method | No of patients | Control arm (%) | Arm adding hperth. (%) | Overall Survival (OS) benefit in arm including hyperthermia | Benefit OS odds ratio | Benefit quality of life |
|---|---|---|---|---|---|---|---|---|
| Kouloulias et al. 2002 [6] | Athens, Greece | Intrasurgery surface heating | 65 | 85 % | 15 % | 11 months (SE 2.4 months) | p = 0.029 | Better: p = 0.031 |
| Yamada et al. 1992 [5] | Sendai, Japan | Intrasurgery surface heating | 69 | 80 % | 20 % | 1 year OS:plus 6.9 % | Only marginally better | |
| 2. year plus 4.2 % | ||||||||
| Mi et al. 2013 [10] | Meta-analysis for adv. gastric cancers | Intraoperative HIPEC-thermoenhanced Chemotherapy | 1906 in 16 rand. trials | 1. year OS | 2.99 (95 % Cl) 2.21 to 4.05; p < 0.00001 | No higher risks; but increase incidences of abdominal pain | ||
| Hager et al. 2006 [24] | Bad Bergzabern, Germany | Non-invasive loco-reg. heating | 46 | 100 % | Median OS: 10.8 months | QoL improvement & pain decrease (56 %) | ||
| 1. year OS: 41 % | ||||||||
| Zhang et al. (2008) [30] | China | Non-invasive loco-reg. heating | 75 | 49 % | 51 % | 1. year OS plus 13 % | No serious complicat. | |
| 2. year OS plus 50 % | ||||||||
| Mueller-Huebenthal 2010 [25] | Stuttgart, Germany | Non-invasive loco-reg. heating | 25 | 100 % | Median OS 12.2 months | Pain reduction observed | ||
| 1. year OS: 51 % | ||||||||
| Maluta et al. 2011 [31] | Verona, Italy | Non-invasive loco-reg. heating | 60 | 50 % | 50 % | OS plus 4 months = +36 % | p = 0.025 | No increased toxicity |
| Ishikawa et al. 2012 [32] | Multicentre Japan | Non-invasive loco-reg. heating | 18 | 100 % | Median OS: 8 months; 1 year OS: 33 % | No added toxicity except mild pain & skin rush | ||
| Tschoep-Lechner 2013 [33] | Munich, Germany | Non-invasive loco-reg. heating | 23 | 100 % | Median OS 12.9 months |
Interstitial/Intraluminal Heating/HIPEC
Interstitial heating is, again, a rather rare treatment option even to this day. Nakagawa (1996) reports about successfully applying selective thermocoagulation in unresectable tumors aiming at 50 °C in the region of interest (abt 2 cm3) and 40 °C in the neighboring area [7]. The results, however, are merely case-based and no trial has been performed.
An important role, however, can be attributed to the hyperthermic intraperitoneal chemotherapy (HIPEC) mostly in combination with cytoreductive surgery. Thermo-enhanced cytostatic solutions are not only restricted to large open abdominal surgeries but also performed post-operative as follow up. There is a strong rational for applying HIPAC, intraoperable and postoperative as follow up. Sugerbaker et al. note: “The exposure of peritoneal surfaces to intraperitoneal gemcitabine is approximately 200–500 times the exposure that occurs within the plasma” [8]. While there is wide evidence to show the superiority of HIPEC treatment over conventional methods in the management of peritoneal surface malignancies of colonic origin [9], specifically on pancreatic cancer, no trials have yet been published for this method. Mi et al. [10] conducted a thorough meta analysis of intraoperative HIPEC for advanced gastric cancers, including 16 randomized trials involving 1.906 patients. The authors summarize: “Compared with surgery alone, combination therapy (surgery plus IHIC) was associated with a significant improvement in survival rate at 1 year (hazard ratio (HR) = 2.99; 95 % confidence interval (CI) = 2.21 to 4.05; p < 0.00001), 2 years (HR = 2.43; 95 %CI = 1.81 to 3.26; p < 0.00001), 3 years (HR = 2.63; 95 %CI = 2.17 to 3.20; p < 0.00001), 5 years (HR = 2.49; 95 %CI = 1.97 to 3.14; p < 0.00001), and 9 years (HR = 2.14; 95 %CI = 1.38 to 3.32; p = 0.0007)” [10]. Intraoperable HIPEC, as the authors elaborate further, was not associated with higher risks but increased the incidence of abdominal pain (RR = 21.46; 95 %CI = 5.24 to 87.78; p < 0.00001). Given the extensive meta-analysis of these authors, this method of hyperthermia shall not be evaluated further.
Last but not least is the novel approach of using ferrobased nanoparticle that are injected into the tumor region and then, at later occasions, heated by applying an alternating magnetic field. There are a few initial explorative cases applied in pancreatic cancer. The method, however, started out with glioma and prostate cancer (Magforce).
Non-invasive Deep Regional Heating
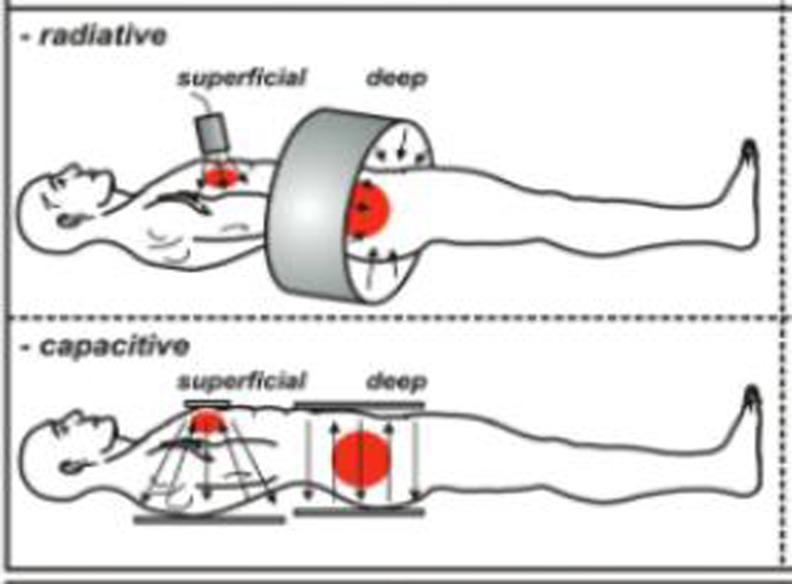
Non-invasive deep region heating is the other clinically relevant heating method. During discussions about hyperthermia in pancreatic cancers, it is this category that is usually referred to. There are basically two technologies available today for heating in the temperature range of 39–44 °C. These are: A. radiative, so called antenna systems, and B. capacitative systems (Fig. 1). In addition, there exists focused ultrasound (HIFU) as a method of non-invasive ablative heating usually combined with an MRI for location control and temperature measurements. Focused ultrasound is a novel treatment and for pancreatic treatment still in nascent stage, and since it is basically a surgical substituting technique and currently not applied for adjuvant heating, it will not be covered in this context (however see [11–13]).
Fig. 1.
Radiative and capacitative techniques for non-invasive loco-regional heating. Reference: Fig drawn from Kadota Fund 2004 clinical group consensus: van der Zee et al. [14]. a In radiative heating, usually an array of transmitting antennas are placed around the body. Each antenna can be controlled separately in power and phase. This in principal enables to maximize the impact to the desired region of interest. Such systems usually work in the range of 100 Mhz with up to 12 radiating antennas in a circular array. While the control over the impact is a main advantage of antenna systems, a disadvantage lies in the complexity of interferences and reflections within a heterogeneous tissue body, eventually resulting in undesirable so-called hot spots. For stretching this technology to reach sufficient temperatures, a real-time temperature monitoring is considered advantageous. Now there are some institutions worldwide which have set up a specifically dedicated MRI to this task of monitoring temperatures real-time in the heating process. b Capacitative systems in contrast are much simpler. There exist two electrodes and the patient’s body is placed in-between, serving as dielectric medium. Heat is generated by movements on the molecular level. Water molecules are dipoles; electrically unbalanced O-molecules bind electrons stronger than the H-molecules. Thus the O-side is electrically negative and turns itself towards the positive electrode. If charge at the electrodes is changed rapidly (in case of 13 Mhz, 13 million changes per second) then this water molecule rotates rapidly. In a connected array with others this causes friction which in turn generates the heat
The frequencies applied range from 8 to 24 Mhz; basically the lower a frequency the better its ability to penetrate into the depth of water-dominant structures such as the human body. Thus 20 Mhz ceteris paribus reach deeper than 100 Mhz; often systems use 13.56 Mhz since this is an open frequency-range which does not require a Faraday cage for shielding, thus cutting down cost.
Limitations
There are limitations to a successful sufficient heating. This applies more or less to all non-invasive methods. One restriction is getting the heat impact into the region of interest. Pancreas is located in the center of the body; the task is to avoid neighboring tissues closer to the surface from absorbing too much of the energy which eventually may stress the patient’s tolerance limits to a sufficient energy impact. Thus adipose patients have a disadvantage since much of the energy applied is absorbed in the fat. For pancreatic region, however, this is less relevant than in the lower abdominal area. Bone marrow is a sensitive tissue for electromagnetic field impacts, and pain in the lower rip arches and sternum often act as bottleneck to desirable energy inputs. There are procedural measures though, to overcome such restrictions to a great extent.
Vascular perfusion is the second main restriction. Heat impact is lead away by the blood flow and new blood flowing in actually cools the tissue near by the large vessels. Since perfusion in tumor tissues is highly irregular, so too is this effect, and may yield very different results from one heating session to another. Kandel et al. used dynamic volume CT to track the whole organ perfusion of the pancreas in 30 patients. They reported a selective effect in favor of hyperthermia, observing the perfusion in pancreatic carcinomas to be significantly lower than that of normal pancreatic tissue (P < 0.001) [15].
The problem manifests thus far since direct temperature measurements for quality control are difficult to obtain in this region. So in most cases, heating is performed without temperature feedback as a quality safeguard to efficacy. The justification may be weak but has its argument: against all odds of deep location and perfusion the clinical results demonstrate a clear advantage of applying hyperthermia. Thus, the effect must be something more than negligible. This indicates the potential that is largely unexploited, but needs to be refined in order to optimize the heating procedures and eventually start measuring intraduodenal temperatures. Intratumoral and intraluminal temperatures that were compared in the pelvic area [16] suggest such a surrogate to be fairly accurate.
Adding Hyperthermia as an Adjuvant Treatment Option in Pancreatic Cancer Patients: Rational and Protocols
One biological rational in favor of hyperthermia lies in the observation that malignant tumor cells may have a lower sensitivity to thermal stress than normal cells [17]. Further, cancerous cells in general seem to have higher ionization, thus are more susceptible to electromagnetic heating. It could be shown that an increased energy deposition occurred in a pancreatic tumor tissue compared to a normal pancreatic tissue [18]. Thus there exists a higher selectivity of tumor cells to the heating effect. The fact that pancreatic tumors are comparatively hypovasculated facilitates further heating up effects.
With local heating in the region of interest, blood flow increases, interstitial pressure reduces and vessel permeability increases slightly through warmth expansion. All these effects facilitate a better inflow of the cytostatica into the region of interest.
Heat is stress to cells; they react by synthesizing heat shock proteins. The ones to remain in the cell protect this cell from further stress influences. This is the reason why hyperthermia sessions should not be given in close proximity; there should at least be one alternate day in between. However, the ones exposed at the outer cell membrane act as stimuli to the body’s immune system. Research indicates that a hyperthermia session in a combined regimen with gemcitabine would have best effects in 24 h time gap to the infusion either before or after [19]. On other cytostatica such as cisplatin best effect seems in timely proximity to the infusion [20].
There is no evidence until now to show that moderate hyperthermia (up to 42 °C) has a beneficial effect as a solo treatment. However, research on the immunological side of hyperthermia has become a new field to emerge, with interesting observations and findings such as hyperthermia inducing a protein -calrecitulin—which in the early phase of heating seems to be expressed on cell membrane of tumorous cells acting as eat-me signal to the body’s immune system [21].
Hyperthermia in Combination to Radiation
Many regimens in treating pancreatic tumors rely as well on radiation. Hyperthermia generally is regarded as a potent enhancer to the effects of radiation. There is a good summary available for these preclinical and clinical data [22]. The rational is built on four points: A. the role of hyperthermia to increase oxygenation in the region of interest, B. the role of heat-stress to inhibit the cells DNA repair mechanism, thus fixing radiations DNA damage, C. the complementary effect to prolongates G2 cell cycle arrest which is radiation sensitive. And finally, D. further effects that include enhancing the immune stimulation that radiation induced necrotic cells offer to antigen presenting cells and dendritic cells [23].
Hyperthermia and Pain Reduction in Pancreatic Cancer Patients
In the palliative phase, often a dose reduction of opiates is feasible to last for up to 5 days after a (non-aggressive) hyperthermia session. Hager [24] and Mueller-Huebenthal [25] mention the finding of pain reduction after hyperthermia in their pancreatic trials. Sridhar refers to a small study in applying hyperthermia in gastric tumors where 8 of 10 patients at the end of 6 weeks had more than 80 % pain reduction and were off the analgesics [26].
Outlook
Promising research currently involves nanoparticle with controlled release and thermosensitive lipides with encapsulated cytostatica. While doxorubicin was among the first drugs to be tested in this way, others like cisplatin and gemcitabine follow [27–29]. Lipidal strcutures can be designed in a thermosensitive manner that release only at predesigned thresholds (e.g. 38.5 °C). The rational is that a systemic infusion can be targeted to a region of interest in locally heating up the specific tissue environment. For treatment of pancreatic tumors, this however has not yet reached clinical relevance.
Review of Clinical Data
The main methodologies of pancreatic-related hyperthermia nowadays are hyperthermic intraperitoneal chemotherapy (HIPEC) and the non-invasive loco-regional deep region heating. As mentioned earlier, for HIPEC, the best reference is to the meta-analysis of advanced gastric tumors by Mi et al. [10]. This section now concentrates on non-invasive loco-regional deep region heating.
The trials available show a wide variety of regimens with different cytostatica, different dosages, including and excluding radiation and naturally the same variety in the way hyperthermia was applied. This is typical of a field that has not yet reached a consensus and still is in an experimental phase. However, the clinical benefit is quite evident despite these variations. For trials, we have screened the Pubmed and Cochraine database, and the presentations in oncological hyperthermia conferences for the last 5 years that we are aware of. The trials found on non-invasive regional heating shall be briefly characterized.
The German Study I (Hager et al. 2006) [24]
The authors present a single arm prospective study on advanced surgically not totally resectable pancreatic cancer with a complex treatment regimen involving enzyme-therapy, anti-hormonal therapy (tamoxifen, flutamid, LH-RH-Analoga), immune-modulating agents, differentiating agents (high-dose vitamin A-E-palmitate, alpha-hydroxycalciferol, coumarines), endogenous hyperpyrexia with a mixed bacterial vaccine and hyperthermia. Those patients in progress under this regimen were treated with 5-FU; Mitomycin C and alpha-Interferon, again in combination with hyperthermia.
Median overall survival was 10.8 months with a 5-year survival rate of 9 % (usually lesser than 2 %). Especially notable, as the authors add: “Most patients experienced partially excellent improvement in quality of life (gain of appetite and weight, pain relief, improvement in general condition)”. For hyperthermia, the study is of limited value since, owing to the rather exotic regimen, its results are not causally traceable to any single treatment component.
The Chinese Trial (Zhang et al. 2008) [30]
This trial in advanced pancreatic cancers compared radiation administered by gamma knife (3–4.5 Gy; 8–11 fractions) as solo treatment versus an adjuvant thermo-chemotherapy administrating tegafur (0.5–1.0 g) and calcium folinate (CF, 0.2 g) for 4–6 times or—and this is unfortunately not specified in more detail—gemcitabine (0.6–1.0 g/m2) on days 1 and 8 and cisplatin (DDP) (20–30 mg/m2) on days 1–3, repeated every 28 days for 3–6 cycles. Hyperthermia was given twice a week for 1 h in six fractions seemingly on the day of infusion.
One-year overall survival (OS) in thermo-chemo combination group was 51.2 % compared to 45.2 % for radiotherapy solo group and 2-year OS was 26.5 % in combination group versus 17.6 % for radiation solo group. The authors report that no serious complications such as perforation, bleeding or high fever has been observed neither in treatment phase nor in the follow up. It is obvious to critically annotate that the treatment arm included two added procedures (chemotherapy plus hyperthermia) instead of one.
German Study II (Mueller-Huebenthal 2010) [25]
This retrospective study included 25 patients with locally advanced (5 pat.) or metastatic (20 pat.) adenocarcinoma of the pancreas. All patients received gemcitabine-based standard chemotherapy combined with a loco-regional session of hyperthermia of 1 h duration twice a week. The ECOG performance status varied between 0 and 1.
The median overall survival showed 12.2 months (versus expected 6–7 months). Three patients showed complete response (CR), seven patients partial response (PR), one patient is still alive (updated 2014). Thus cancer control (CR + PR + SD) was 65 % and 1 year survival 51 % (versus expected 25 %). Negative side effects due to adding hyperthermia have not been found. Though not systematically documented, there was the observation of pain reduction in some patients.
The Italian Trial (Maluta et al. 2011) [31]
Underlying is a combination treatment of radiation (from 30 Gy/10 fractions to 66 Gy/33 fractions) with chemotherapy (CT). CT consisted of gemcitabine alone or in association with either oxaliplatin, cisplatin or 5-FU. Hyperthermia was delivered twice a week on days of radiation. Median OS was 15 months compared to 11 months in control group (p = 0.025). Hyperthermia, the authors add, did not increase toxicity of chemo/radiation therapy. Criticism to this trial refers to a heterogeneous regimen of various chemotherapeutica.
The Japanese Trial (Ishikawa et al. 2012) [32]
Patients were administered gemcitabine 1,000 mg/m2 on days 1, 8 and 15 every 4 weeks. Loco-regional hyperthermia (capacitative system) was delivered once per week, a day preceding the gemcitabine infusion. Median Overall Survical (OS) was 8 months, but for locally advanced pancreatic case patients, it was 17.7 months and for pancreatic metastatic cases, 5.2 months. The response rate (partial response (PR)) plus stable disease (SD) was reported to be 61 %. Thus the results were clearly superior to what would have been expected in a gemcitabine chemotherapy.
German Study II (Tschoep-Lechner et al. 2013) [33]
This retrospective study starts out as a second line therapy after gemcitabine failure. Patients received gemcitabine 1,000 mg/m2 on day 1 combined with cisplatin 25 mg/m2 combined with non invasive loco-regional radiative heating on day 2 and 4, biweekly for 4 months. Median time to second progression was 4.3 months (95 %CI: 1.2–7.4) and median OS 12.9 months (95 %CI: 9.9–15.9). The disease control rate of patients with available CT scans was 50 %.
The ESHO HEAT Phase III Trial (Hyperthermia European Adjuvant Trial)—2014 Open Recruitment
Even though this is an ongoing trial in an early stage, thus no results available, it still should be mentioned as this would be the first large-scale, randomized trial for hyperthermia in pancreatic cancer treatment. Control arm assumes gemcitabine solo treatment versus intensified therapy including gemcitabine (1,000 mg/m2 on days 1 and 15 q4w), cisplatin (25 mg/m2 on days 2, 16 q4w) plus loco-regional hyperthermia (1 h on days 2, 16 q4w) with R0/R1 resected pancreatic patients.
The variety of the trial designs summarized in Table 1 is quite diverse. And still, across 2,287 patients, there is clear evidence of the survival benefit of adding hyperthermia to whatever regimen it was compared with! Even leaving the intrasurgical methods out of consideration, we have consistent evidence over 6 trials including 247 patients. To observe the potential of pain reduction in the studies mentioned was mostly not the prime objective; future trials should keep an open perspective to this issue as it has the potential to yield promising evidence.
Conclusion
Even though the currently existing data is heterogeneous and in parts methodologically criticizable, still it indicates a clear advantage of adjuvant hyperthermia in prolonging overall survival. Apart from incidences of abdominal pain in the HIPEC method, quality of life is even improved by adding hyperthermia.
Expectations are that the ongoing European prospective randomized phase III HEAT trial will yield valuable insight. Though this treatment option is still rather novel and the trials mentioned use different treatment schema, despite all the variations, they all show clear beneficial results indicating the potential that lies in hyperthermia as an additional adjuvant and palliative treatment option.
References
- 1.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 2.Riess H, Helm A, Niedergethmann M et al (2005) A randomised, prospective, multicenter, phase III trial of gemcitabine, 5-fluorouracil (5-FU), folinic acid vs. gemcitabine alone in patients with advanced pancreatic cancer. Proc. ASCO; Abstr No: 4009
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Bakshandeh-Bath A, Stoltz AS, Homann N, Wagner T, Stölting S, Peters SO. Preclinical and clinical aspects of carboplatin and gemcitabine combined with whole-body hyperthermia for pancreatic adenocarcinoma. Anticancer Res. 2009;29(8):3069–3077. [PubMed] [Google Scholar]
- 5.Yamada S, Takai Y, Nemoto K, Ogawa Y, Kakuto Y, Hoshi A, Sakamoto K, Kimura Y, Kobari M. Intraoperative radiation therapy combined with hyperthermia against pancreatic carcinoma. Int J Oncol. 1992;1(7):795–798. [PubMed] [Google Scholar]
- 6.Kouloulias VE, Kouvaris JR, Nikita KS, Golematis BC, Uzunoglu NK, Mystakidou K, Papavasiliou C, Vlahos L. Intraoperative hyperthermia in conjunction with multi-schedule chemotherapy (pre-, intra- and post-operative), by-pass surgery, and post-operative radiotherapy for the management of unresectable pancreatic adenocarcinoma. Int J Hyperth. 2002;18(3):233–252. doi: 10.1080/02656730110108794. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa A, Kamiyama Y, Matsui Y, Okuno M, Imamura A, Tu W, Nakagawa M, Kanemaki T, Takai S, Uetsuji S, Noro C, Kubo N, Nakase Y. Selective thermocoagulation of unresectable malignant tumors using radiofrequency [Article in Japanese] RiGan To Kagaku Ryoho. 1996;23(12):1651–1653. [PubMed] [Google Scholar]
- 8.Sugarbaker PH, Stuart OA, Bijelic L (2011) Intraperitoneal gemcitabine chemotherapy treatment for patients with resected pancreatic cancer: rationale and report of early data. Int J Surg Oncol 2011:185092. doi:10.1155/2011/185092 [DOI] [PMC free article] [PubMed]
- 9.Esquivel J, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Ann Surg Oncol. 2006 doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 10.Mi D-H, Li Z, Yang K-H, Cao N, Lethaby A, Tian J-H, Santesso N, Ma B, Chen Y-L, Liu Y-L. Surgery combined with intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) for gastric cancer: a systematic review and meta-analysis of randomised controlled trials. Int J Hyperth. 2013;29(2):156–167. doi: 10.3109/02656736.2013.768359. [DOI] [PubMed] [Google Scholar]
- 11.Xiaoping L, Leizhen Z. Advances of high intensity focused ultrasound (HIFU) for pancreatic cancer. Int J Hyperth. 2013;29(7):678–682. doi: 10.3109/02656736.2013.837199. [DOI] [PubMed] [Google Scholar]
- 12.Wang K, Chen L, Meng Z, Lin J, Zhou Z, Wang P, Chen Z. High intensity focused ultrasound treatment for patients with advanced pancreatic cancer: a preliminary dosimetric analysis. Int J Hyperth. 2012;28(7):645–652. doi: 10.3109/02656736.2012.713541. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Yang G, Wang D, Yu X, Zhang Y, Zhu J, Ji Y, Zhong B, Zhao W, Yang Z, Aziz F. Concurrent gemticatbine and high-intensity fucused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010;21(4):447–452. doi: 10.1097/CAD.0b013e32833641a7. [DOI] [PubMed] [Google Scholar]
- 14.Van der Zee J, Vujaskovic Z, Kondo M, Sugarhara T. Part I. Clinical hyperthermia. The Kadota Fund International Forum 2004 – clinical group consensus. Int J Hyperth. 2008;24(2):111–122. doi: 10.1080/02656730801895058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandel S, Kloeters C, Meyer H, Hein P, Hilbig A, Rogalla P. Whole-organ perfusion of the pancreas using dynamic volume CT in patients with primary pancreas carcinoma: acquisition technique, post-processing and initial results. Eur Radiol. 2009;19(11):2641–2646. doi: 10.1007/s00330-009-1453-z. [DOI] [PubMed] [Google Scholar]
- 16.Fatehi D, van der Zee J, Notenboom A, van Rhoon GC. Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther Onkol. 2007;183:479–486. doi: 10.1007/s00066-007-1768-0. [DOI] [PubMed] [Google Scholar]
- 17.Hahn GM, Braun J, Har-Kedar I. Thermochemotherapy: synergism between hyperthermia 42–43 degrees) and Adriamycin (of bleomycin) in mammalian cell inactivation. Proc Natl Acad Sci U S A. 1975;72:937–940. doi: 10.1073/pnas.72.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raoof M, Cisneros BT, Corr SJ, Palalon F, Curley SA, Koshkina NV. Tumor selective hyperthermia induced by short-wave capacitively-coupled RF electric-fields. PLoS One. 2013;4:8(7). doi: 10.1371/journal.pone.0068506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adachi S, Kokura S, Okayama T, Ishikawa T, Takagi T, Handa O, Naito Y, Yoshikawa T. Effect of hyperthermia combined with gemcitabine on apoptotic cell death in cultured human pancreatic cancer cell lines. Int J Hyperth. 2009;25(3):210–219. doi: 10.1080/02656730802657036. [DOI] [PubMed] [Google Scholar]
- 20.Bull JM, Scott GL, Strebel FR, Nagle VL, Oliver D, Redwine M, Rowe RW, Ahn CW, Koch SM. Fever-range whole-body thermal therapy combined with cisplatin, gemcitabine, and daily interferon-alpha: a description of a phase I-II protocol. Int J Hyperth. 2008;24(8):649–662. doi: 10.1080/02656730802104740. [DOI] [PubMed] [Google Scholar]
- 21.Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68(11):4026–4030. doi: 10.1158/0008-5472.CAN-08-0427. [DOI] [PubMed] [Google Scholar]
- 22.Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol. 2007;19:418–426. doi: 10.1016/j.clon.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 23.Rubner Y, Wunderlich R, Rühle PF, Kulzer L, Werthmöller N, Frey B, Weiss EM, Keilholz L, Fietkau R, Gaip US. How does ionizing irradiation contribute to the induction of anti-tumor immunity? Front Oncol. 2012;2:75. doi: 10.3389/fonc.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hager ED, Migeod F, Koomagi R, Schrittwieser G, Krautgartner I. Multimodal complementary therapy for advanced pancreas carcinoma; translation of publication of Deutsche. Zeitschrift für Onkologie. 2006;38:100–107. doi: 10.1055/s-2006-952051. [DOI] [Google Scholar]
- 25.Mueller-Huebenthal B (2010) Hyperthermia and gemcitabine for the treatment of advanced inoperable pancreatic cancer: a retrospective analysis of 25 cases. Presentation at the 6th binneal conference of Indian Association of Hyperthermia Oncology IAHOM; Jan 2010 Bangalore. (Updated data to be published 2014)
- 26.Sridhar PS (2012) Pain reduction in hyperthermia: 10 cases. Presentation at the 7th biennal conference of IAHOM, Febr. 2012 Indore
- 27.Lee GY, Qian WP, Wang L, Wang YA, Staley CA, Satpathy M, Nie S, Mao H, Yang L. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 2013;7(3):2078–2089. doi: 10.1021/nn3043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DH, Guo Y, Zhang Z, Procissi D, Nicolai J, Omary RA, Larson AC (2013) Temperature-sensitive magnetic drug carriers for concurrent gemcitabine chemohyperthermia. Adv Healthc Mater 3(5):714–24. doi:10.1002/adhm.201300209 [DOI] [PMC free article] [PubMed]
- 29.McDaniel JR, Dewhirst MW, Chilkoti A. Actively targeting solid tumours with thermoresponsive drug delivery systems that respond to mild hyperthermia. Int J Hyperth. 2013;29(6):501–510. doi: 10.3109/02656736.2013.819999. [DOI] [PubMed] [Google Scholar]
- 30.Zhang LP, Nie Q, Kang JB, Wang B, Cai CL, Li JG, Qi WJ. Efficacy of whole body gamma-knife radiotherapy combined with thermochemotherapy on locally advanced pancreatic cancer. Ai Zheng. 2008;27(11):1204–1207. [PubMed] [Google Scholar]
- 31.Maluta S, Schaffer M, Pioli F, Dall’oglio S, Pasetto S, Schaffer PM, Weber B, Giri MG. Regional hyperthermia combined with chemoradiotherapy in primary or recurrent locally advanced pancreatic cancer: an open-label comparative cohort trial. Strahlenther Onkol. 2011;187(10):619–625. doi: 10.1007/s00066-011-2226-6. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa T, Kokura S, Sakamoto N, et al. Phase II trial of combined regional hyperthermia and gemcitabine for locally advanced or metastatic pancreatic cancer. Int J Hyperth. 2012;28(7):597–604. doi: 10.3109/02656736.2012.695428. [DOI] [PubMed] [Google Scholar]
- 33.Tschoep-Lechner KE, Milani V, Berger F, Dieterle N, Abdel-Rahman S, Salat C, Issels RD. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperth. 2013;29(1):8–16. doi: 10.3109/02656736.2012.740764. [DOI] [PubMed] [Google Scholar]