Abstract
Carcinoma of gall bladder is the most common malignancy of the biliary tract worldwide and is usually associated with poor prognosis. In this era of laparoscopic cholecystectomy, there has been increase in detection of early stage incidental gall bladder carcinoma in cholecystectomy specimens. A retrospective study was carried out in tertiary care hospital in central India. A total of 2990 patients underwent laparoscopic cholecystectomy during the year 2001–2013. Hospital records and histopathology reports of these patients were studied in detail. Twenty three cases of gall bladder carcinoma were detected incidentally accounting for an incidence of 0.76 %. It was more common in females with an M: F ratio of 1:1.9. Mean age of presentation was 57.8 years. Gall stones were present in 22 cases and one patient presented with features of acute cholecystitis. Three patients had associated xanthogranulomatous inflammation and 10 had associated intestinal metaplasia. It is not uncommon to encounter incidental malignancies of gall bladder in laparoscopic cholecystectomy specimens sent to histopathology for presumably benign disease. Histopathology reports must include comments on extent of infiltration, perineural invasion, tumor differentiation and nodal involvement for oncologist information and subsequent management of patients.
Keywords: Incidental, Carcinoma, Gall bladder
Introduction
Laparoscopic cholecystectomy has now replaced open cholecystectomy procedure and has become the first treatment of choice for removal of gall bladder in cases of gallstones and various forms of cholecystitis. This has resulted in relative increase in incidence of incidental detection of carcinoma gall bladder in comparison to the era of open cholecystectomy [1]. There is marked geographic variability in incidence of gall bladder carcinoma in various parts of India. Higher incidence has been noted in north India and Delhi population as compared to south India [2]. There are limited studies in medical literature documenting incidence and detailed clinic-pathological analysis of incidental gall bladder carcinoma (IGBC) from central India.
This is a single centre study from central India conducted in Bhopal Memorial Hospital and Research Centre (BMHRC) which is a tertiary care multispecialty hospital located in Bhopal, Madhya Pradesh. This hospital caters to the population of victims of gas disaster as well as other patients referred from all over central India. Data from this study will reflect the incidence and clinicopathologic features of incidental carcinoma of gall bladder in central India.
Material and Methods
A retrospective study was done on all laparoscopic cholecystectomy specimens (n = 2990) that were received in Pathology department between Jan 2001 and December 2013 at BMHRC, Bhopal, India. Inclusion criteria for the cases was that there should be no preoperative or intraoperative suspicion of malignancy of gall bladder and the diagnosis of gall bladder carcinoma was made for the first time on histopathology examination. All the selected cases were analyzed for demographics, presenting signs and symptoms, radiology investigations (ultrasonography and computed tomography) and gross pathology details.
Haematoxylin and eosin stained slides were re-examined and studied in detail. Pathologic staging of carcinoma was done according to American Joint Committee recommendations for cancer staging (AJCC) seventh edition, 2010. Re-exploration surgery and its histopathology findings were evaluated. Clinical follow up of the patients was also done. The study was ethically approved by the Institutional Ethical Committee of BMHRC.
Results
The retrospective search showed a total of 2990 cholecystectomies performed during the study period of 13 years. Out of these, 87 cases were proven positive for carcinoma gall bladder by histopathology. Amongst these a total of 23 cases were found incidentally during the study period accounting for an incidence of 26.4 % of all the resectable gall bladder carcinomas. Incidence of incidental carcinoma among all the routine cholecystectomies sent for histopathology examination was thus estimated to be 0.76 %.
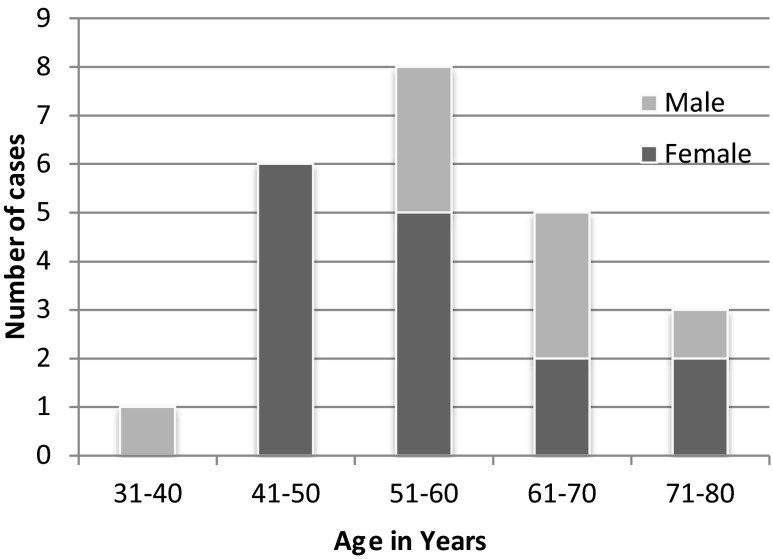
The age of patients at diagnosis ranged from 32 to 80 years (mean age 57.78 years) with a female predominance (15 women and 8 men). The Male: Female ratio was 1:1.9. Majority of the patients were in fifth and sixth decade, each accounting for seven cases, followed by seventh decade in which there were five cases (Fig. 1). Thirteen patients of these 23 selected cases were gas victims and ten were non gas victims. The most common clinical complaints were pain in right hypochondria, nausea, vomiting and fever. A preoperative abdominal ultrasound was done in all the cases and 22 patients had symptomatic gallstones for which laparoscopic cholecystectomy were done. One of the patients had empyema gall bladder with acute cholecystitis.
Fig. 1.
Age and sex wise distribution of cases with incidental detection of gall bladder carcinoma
On gross examination, the commonest site of involvement was gall bladder fundus along with body (10 cases), followed by fundus alone (07 cases). Body ± neck were involved in 4 cases and total gall bladder involvement was seen in 2 cases. The tumor size grossly varied in maximum dimension from 6 cms to the smallest tumor measuring 1.3 cms. Wall thickness > 3 mm was noted in 21 of the 23 cases and it varied in thickness from 0.5 to 1.7 cms. One of the cases showed tiny small papillary projections in fundus and body of gall bladder that were spread over an area of 4 × 2 cms. In one of the cases gross examination showed small polypoidal mass that measured 2.5 × 1.5 cms. In rest of the cases the growth was ulceroinfiltrative with thickened wall.
Three of the patients had nodal involvement, one of the case was that of a 49 years old female who had regional cystic lymph node involvement that measured 1 cm and in another case patient was HBsAg positive and had cholelithaisis with mesenteric lymphadenopathy. With a clinical impression of abdominal tuberculosis, laparoscopic cholecystectomy and mesenteric lymph node biopsy (1 cm) was done. Gross examination showed that gall bladder was only mildly thickened to 0.7 cms in fundus and body region and there was no proliferative lesion. Histopathology examination showed adenocarcinoma in gall bladder that was also metastatic to mesenteric lymph node. The third case of nodal involvement was identified after re-exploration surgery in which small mesenteric lymph node measuring 0.7 cms in diameter showed metastatic adenocarcinoma.
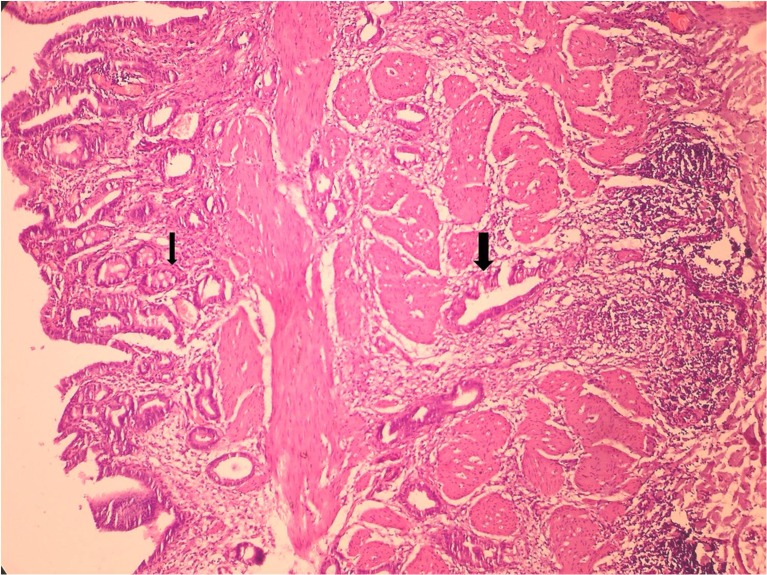
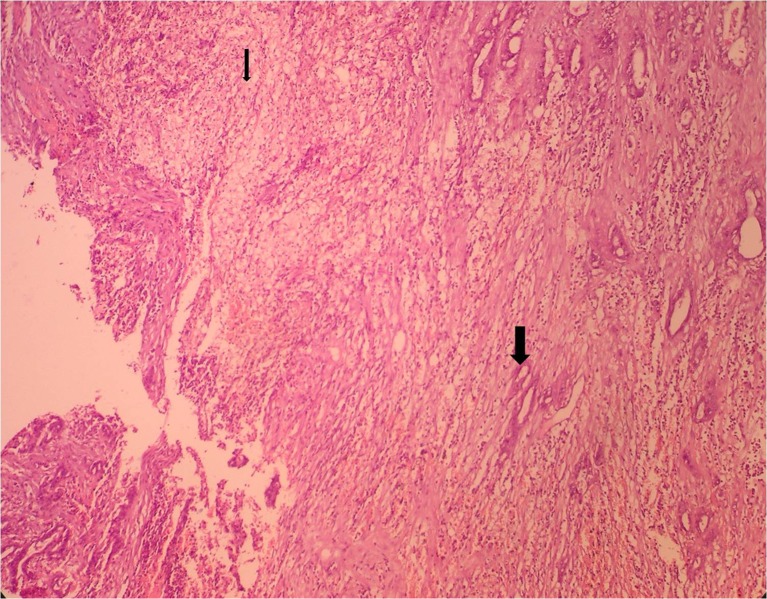
Microscopic examination revealed that all the 23 cases were adenocarcinoma. The tumor was well differentiated in 06 cases, moderately differentiated in 16 cases and showed poor differentiation in one case. Papillary arrangement of tumor cells was noted in six cases. Intestinal metaplasia was noted in 10 cases and pyloric gland metaplasia was present in 7 cases (Fig. 2). Xanthogranulomatous inflammation was noted in three cases wherein sheets of foamy histiocytes were seen in between the tumor cells (Fig. 3).
Fig. 2.
H&E stained section (200× magnification) showing intestinal metaplasia (thin arrow) and tumor glands infiltrating the gall bladder wall (thick arrow)
Fig. 3.
H&E stained section (200× magnification) showing xanthogranulomatous reaction with presence of sheets of foamy histiocytes (thin arrow) and gall bladder wall infiltration by moderately differentiated adenocarcinoma (thick arrow)
Of the total 23 cases, 5 patients were in stage T1b, 14 patients in stage T2 and four patients were in stage T3. Resected cystic duct margin was involved in four cases and one case showed dysplasia. Perineural invasion was present in eight cases. Re-exploration surgery was performed in 10 cases and histopathology showed presence of residual tumor in two cases wherein tumor infiltrates were present in resected liver wedge. In one of the above mentioned cases mesenteric lymph node also showed metastatic adenocarcinoma. Additional histological findings included presence of caseating granulomatous inflammation in two cases.
Patient’s demographics, clinical details, gross and microscopic findings, re-exploration status and follow up of the patients have been summarised in Table 1.
Table 1.
Patient characteristics: demographic data, clinical & morphologic findings, stage, re-exploration and follow up
| SNo | Age /Sex | Clinical Features | Site of Tumor | Macroscopic/Max Tr size | Stone | Type of tumor /Differentiation | Cystic margin | PN | Stage | Re-exploration | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50/F | Pain,Nausea, vomiting | Fundus | Polypoidal/ 2.5 cms | Yes | Adenoca, Mod Papillary | Free | − | Stage II (T2Nx) | Not Done | Died (8 years) |
| 2 | 58/F | Nausea, vomiting | Fundus | Ulceroinfil/1.5 cms | Yes | Adenoca, Mod papillary | Dysplasia | − | Stage II (T2No) | Done/Free | Died (12 months) |
| 3 | 61/M | Pain, Fever, vomiting | Fundus | Ulceroinfil/1.3 cms | Yes | Adenoca, Mod papillary | Free | − | Stage II (T2No) | Done/Free | Alive |
| 4 | 55/M | Pain, Jaundice | Body, Neck | Infiltrative/1.5 cms | Yes | Adenoca, Mod | Invclved | + | Stage II (T2No) | Not Done | Lost to f/up |
| 5 | 70/F | Pain, Fever | Body | Infiltrative/1.3 cms | Yes | Adenoca, Mod | Free | + | Stage II (T2No) | Not Done | Died (15 months) Abdominal lump + |
| 6 | 32/M | Pain, Jaundice | Fundus, Body | Infiltrative/2 cms | Yes | Adenoca, Mod | Free | − | Stage IIIa (T3No) | Done/Liver Pos | Lost to f/up |
| 7 | 55/M | Pain, Vomiting | Fundus, Body | Ulceroinfil/1.8 cms | Yes | Adenoca, well | Free | − | Stage I (T1No) | Done/Free of Tr | Alive |
| 8 | 58/M | Pain, Jaundice | Fundus | Ulceroprol/2 cms | Yes | Adenoca, Mod | Free | − | Stage IIIb (T3N1) | Done Positive Liver,LN) | Lost to f/up |
| 9 | 54/F | Pain,oral ulcers, weakness, Old TB | Fundus | Infiltrative/1.5 cms | Yes | Adenoca, Mod papillary | Free | − | Stage I (T1Nx) | Not done | Alive |
| 10 | 45/F | Pain, Vomiting | Fundus, Body | Infiltrative/3 cms | Yes | Adenoca, Mod | Free | − | Stage I (T1No) | Done/Free of Tr | Alive |
| 11 | 80/F | Pain,Vomiting NFK | Fundus, Body | Ulceroinfil/3 cms | Yes | Adenoca, Mod | Free | − | Stage II (T2No) | Not done | Died (aspiration Pneumonia 1 month) |
| 12 | 75/M | Pain, Vomiting | Fundus, Body | Infiltrative/2.5 cms | Yes | Adenoca, Mod | Involved | − | Stage IIIa (T3Nx) | Not done | Lost to f/up |
| 13 | 49/F | Nausea, Vomiting | Fundus, Body | Infiltrative/1.5 cms | Yes | Adenoca, Mod papillary | Free | − | StageIIIb (T2N1) | Done/Free of Tr | Lost to f/up |
| 14 | 80/F | Fever,Pain, vomiting | Fundus | Ulceroinfil/1.7 cms | No | Adenoca, Mod | Involved | + | Stage II (T2Nx) | Not Done | Lost to f/up |
| 15 | 50/F | Pain | Fundus | Ulcerinfil/1.7 cms | Yes | Adenoca, Mod | Free | − | Stage II (T2Nx) | Done/Free of Tr /TB present | Lost to f/up |
| 16 | 42/F | Pain, Fever, Vomiting | Fundus, Body, Neck | Ulceroinfil/6 cms | Yes | Adenoca, Poor | Involved | + | Stage II (T2Nx) | Not Done | Died (15 months) |
| 17 | 45/F | Pain, Vomiting | Body, Neck | Infiltrative/4 cms | Yes | Adenoca, well | Free | − | Stage IIIb (T3N1) | Not Done | Died (2 months) |
| 18 | 66/M | Intermittent pain, renal calculi | Fundus, Body | Ulceroprol/2.4 cms | Yes | Adenoca, Mod | Free | + | Stage II (T2Nx) | Not Done | Lost to f/up |
| 19 | 60/F | Pain, Vomiting | Fundus, Body, Neck | Infiltrative/4 cms | Yes | Adenoca, Mod | Free | + | Stage II (T2Nx) | Not Done | Lost to f/up |
| 20 | 61/M | Intermittent pain 1mth | Fundus, Body | Infiltrative/5 cms | Yes | Adenoca, well | Free | − | Stage I (T1bNx) | Not Done | Alive |
| 21 | 65/F | Pain, Fever, Vomiting | Body | Infiltrative/2.5 cms | Yes | Adenoca, well | Free | + | Stage II (T2Nx) | Not Done | Alive |
| 22 | 60/F | Pain | Fundus, Body | Infiltrative/4 cms | Yes | Adenoca, well papillary | Free | + | Stage I (T1Nx) | Done/Free,TB present | Alive |
| 23 | 58/F | Internittent pain | Fundus, Body | Ulceroinfil/4.2 cms | Yes | Adenoca, well | Free | + | Stage II (T2Nx) | Done/Free of tr | Alive |
Discussion
Carcinoma of gall bladder is the most common malignancy of the biliary tract worldwide and is regarded as dreadful malignancy with ominous prognosis. [3] Inapparent (Incidental) gall bladder carcinoma is defined as carcinoma of gall bladder that was unrecognised before or at operation and detected for the first time on histopathology examination of gall bladder. These account for approximately 0.25 to 3 % of all cholecystectomy procedures performed for presumably benign disease of gall bladder [1]. Incidence of incidental carcinoma gall bladder at our institute in central India was 0.76 % and was slightly less than other studies from north India (Table 2). Studies from Nepal and Italy have reported a higher incidence of 1.28 and 1.38 % respectively [9, 11]. Low incidence of incidental gall bladder malignancy in our patients could be attributed to exclusion of cases diagnosed intraoperatively and performance of CT imaging in patients with doubtful findings on Ultrasonography (USG).
Table 2.
List of various studies worldwide showing incidence of incidental detection of carcinoma gall bladder
| Studies | Year | Place of study | Sample Size | M:F | Mean Age (Yrs) | Incidence |
|---|---|---|---|---|---|---|
| Daphna et al. [4] | – | Israel | 1697 | 1:5 | 70 | 0.3 % |
| Khoo JJ & Nurul AM [5] | 2000–2005 | Malaysia | 1122 | 1:2 | 56.7 | 0.62 % |
| Mittal R et al. [6] | 1998–2007 | India | 1305 | 1.5:5 | 56.2 | 0.99 % |
| Zhang WJ et al. [7] | 1999–2007 | China | 10,466 | 1:4 | 65.7 | 0.19 % |
| Amanullah et al. [8] | 2000–2002 | India | 428 | 1:7 | 47 | 1.87 % |
| Ghimire P et al. [9] | 1998–2009 | Nepal | 783 | 1:2.3 | 63.8 | 1.28 % |
| Siddiqui et al. [10] | 2010–2012 | Pakistan | 220 | 1:7 | – | 2.8 % |
| Ferrarese [11] | 2008–2012 | Italy | 508 | 4:7 | 67.8 | 1.38 % |
| Sujata et al. [12] | 2007–2012 | North India | 622 | All females | 53 | 0.96 % |
| Kwon et al. [13] | 2008 | Japan | 1793 | 1:1.2 | 66 | 2.12 |
| Ghnnam et al. [14] | 2007–2012 | Saudi | 1982 | 1:4 | 73.6 | 0.5 % |
| Present Study | 2001–2013 | India (Bhopal) | 2990 | 1:1.9 | 57.8 | 0.76 % |
According to literature, 15–40 % of gall bladder malignancies remain undetected even at surgery; this corresponds with our present study in which 26.4 % of gall bladder carcinomas were diagnosed on histopathology [15]. Gallstones remain the major risk factor for incidental malignancies also and were detected by USG in 22 cases. Only one patient manifested with empyema gall bladder and presented with symptoms of acute cholecystitis. Despite being the gold standard and investigation of choice for benign gall bladder diseases, USG tends to miss the infiltrative malignancies of gall bladder. In the present study predominantly infiltrative growth pattern with mild thickening of the wall was noted in 21 cases.
In the present study, although the disease was more commonly detected in females, however the male: female ratio was comparatively higher (0.52) than other Indian studies by Sujata and Amanullah et al. [8, 12]. The mean age of incidental carcinoma detection in our study was 57.8 years, however, in other studies from various parts of world including Saudi, Japan, China and Italy the mean age of presentation is higher than 65 years [7, 11, 13, 14]. The youngest male patient affected in our study group was 32 years and on re-exploration surgery he had liver involvement.
In three of the cases the gall bladder cancer could have been suspected radiologically and intraoperative frozen section should have been submitted in such suspicious cases. One of these cases had fine papillary projections spread over an area of 4 × 2 cms on gross examination. The other case had a polypoidal mass in fundal region that measured 2.5 × 1.5 cms and the third case showed enlarged mesenteric lymph nodes that were ignored due to possibility of associated abdominal tuberculosis.
In a country like India, where incidence of gall bladder cancer is high all the gall bladders with suspicious radiological findings on ultrasonography or peroperatively, gall bladder specimens should be submitted for intraoperative frozen sectioning to reduce the chances of missed gall bladder cancer. In a clinical study by Shukla et al. conducted at Tata Memorial Centre two new terms were introduced: “Potential” gall bladder carcinoma and “suspicious” gall bladder carcinoma to highlight these clinical categories so that correct surgical procedure may be performed. The correct strategy for managing gall bladder cancer more than stage T1 is to convert to open radical cholecystectomy [16].
Most of the patients were in stage T1 (5 cases) or T2 (14 cases), only four patients were in stage T3. Perineural invasion was present in stages T2 or T3. None of the pts in T1 stage carcinoma had perineural invasion. Similar results were obtained in studies by Siddiqui and Ghnnam et al. [10, 14].
According to a cumulative study by Hale et al. xanthogranulomatous inflammation is a global disease and associated with gall bladder carcinoma. In the present study three patients with IGBC had associated xanthogranulomatous inflammation with presence of sheets of foamy histiocytes and occasional multinucleated giant cells [17]. In an immunohistochemical study of maspin expression aberrant reactivity was noted in both intestinal metaplasia as well as gall bladder malignancies supporting the assumption that intestinal metaplasia predisposes to carcinoma [18]. In the present study 10 patients with IGBC had intestinal metaplasia.
Although incidental malignancies are diagnosed at earlier stage, patient’s prognosis depends on several individual parameters. Individual risk factors associated with higher stage of the disease and poor survival in the present study were older age at presentation, tumor involving neck of gall bladder, resected cystic duct margin involvement, maximum dimension of tumor > 3 cms, poor histologic differentiation of tumor, muscle wall invasion, perineural invasion and nodal involvement. In a large study by Roa et al. in Chile of 1366 gall bladder carcinomas, the independent prognostic factors were infiltration of gall bladder wall, tumor differentiation and lymphatic involvement [19]. In a recent report of French survey pathology reports on gall bladder carcinoma frequently lacked important information on key prognostic histological factors [20]. It is therefore important that histopathologist must comment on all these parameters in patient’s reports.
There are few limitations of the study that include the retrospective nature of study and nine of the patients were lost to follow up.
Conclusion
The incidence of incidental detection of gall bladder carcinoma in present study was 0.76 % and accounted for 26.4 % of all resected gall bladder malignancies. The disease is not uncommon in males and also detected in younger individuals. Gallstones are the major risk factor and association with intestinal metaplasia and xanthogranulomatous inflammation is observed even in IGBC. Incidental detection of gall bladder carcinoma will continue to surprise the surgeon as well as histopathologist till more sensitive and cost effective imaging techniques are introduced for routine use. Intraoperative frozen sectioning should be done in all suspicious cases.
Contributor Information
Hanni V Gulwani, Email: hannigulwani@yahoo.com.
Suneeta Gupta, Email: sunita1feb@yahoo.co.in.
Sukhpreet Kaur, Email: drsukh_kaur@yahoo.co.in.
References
- 1.Cavallaro A, Piccolo G, Panebianco V, Menzo EL, Berrettta M, Zanghi A, Vita MD, Cappellani A. Incidental gallbladder cancer during laparoscopic cholecystectomy: managing an unexpected finding. World J Gastroenterol. 2012;18(30):4019–4027. doi: 10.3748/wjg.v18.i30.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sachidananda S, Krishnan AK, Venkataraman J. Characteristics of gallbladder cancer in South India. Indian J Surg Oncol. 2012;3(3):228–230. doi: 10.1007/s13193-012-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalita D, Pant L, Singh S, Jain G, Kudesia M, Gupta K, Kaur C. Impact of routine histopathological examination of gall bladder specimens on early detection of malignancy – study of 4,115 cholecystectomy specimens. Asian Pan J Cancer Prev. 2013;14(5):3315–8. doi: 10.7314/APJCP.2013.14.5.3315. [DOI] [PubMed] [Google Scholar]
- 4.Daphna W, Mehrdad H, Noa BJ, Sandbanand AH. Incidental finding of gall bladder carcinoma. Israel Med Assoc J. 2002;4:334–6. [Google Scholar]
- 5.Khoo JJ, Nurul AM. A clinicopathological study of nine cases of gallbladder carcinoma in 1122 cholecystectomies in Johor Malaysia. Malays J Pathol. 2008;30(1):21–6. [PubMed] [Google Scholar]
- 6.Mittal R, Jesudason MR, Nayak S. Selective histopathology in cholecystectomy for gallstone disease. Indian J Gastroenterol. 2010;29:26–30. doi: 10.1007/s12664-010-0056-6. [DOI] [PubMed] [Google Scholar]
- 7.Zhang WJ, Xu GF, Petal ZX. Incidental gallbladder carcinoma diagnosed during or after laparoscopic cholecystectomy. World J Surg. 2009;33:2651–6. doi: 10.1007/s00268-009-0218-9. [DOI] [PubMed] [Google Scholar]
- 8.Amanullah MK, Rizwn AK, Shahid S, Veena M. Occult carcinoma of gallbladder: Incidence and role of simple cholecystectomy. JK-Practitioner. 2007;14:22–3. [Google Scholar]
- 9.Ghimire P, Yogi N, Shrestha BB. Incidence of incidental carcinoma gall bladder in cases of routine cholecystectomy. Kathmandu Univ Med J (KUMJ) 2011;9(34):3–6. doi: 10.3126/kumj.v9i2.6278. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui F G, Memon A A, Abro A H, Sasoli N A, Ahmad L (2013) Routine Histopathology of gallbladder after elective cholecystectomy for gallstones. BMC Surg 13:26 [DOI] [PMC free article] [PubMed]
- 11.Ferrarese AG, Solej M, Enrico S, Falcone A, Catalano S, Pozzi G, Marola S, Martino V. Diagnosis of incidental gallbladder cancer after laparoscopic cholecystectomy: our experience. BMC Surg. 2013;13(2):S20. doi: 10.1186/1471-2482-13-S2-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sujata JSR, Sabina K, Mi H, Jairaipuri ZS. Incidental gall bladder carcinoma in laparoscopuc cholecystectomy: a report of 6 cases and review of the literature. J Clin Diagn Res. 2013;7:85–8. doi: 10.7860/JCDR/2012/5001.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon AH, Imamura A, Kitade H, Kamiyama Y. Unsuspected gallbladder cancer diagnosed during or after laparoscopic cholecystectomy. J Surg Oncol. 2008;97:241–5. doi: 10.1002/jso.20944. [DOI] [PubMed] [Google Scholar]
- 14.Ghnnam WM, Elbeshry TMAS, Malek JR, Emarra ES, Alzahrany ME, Alqarni AA, Khattab AA. Incidental gallbladder carcinoma in laparoscopic cholecystectomy: Five years local experience. El Med J. 2014;2(1):47–51. doi: 10.18035/emj.v2i2.67. [DOI] [Google Scholar]
- 15.Romano F, Franciosi C, Caprotti R, et al. Laparocsopic cholecystectomy and unsuspected gall bladder carcinoma. Eur J Surg Oncol. 2001;27:225–8. doi: 10.1053/ejso.2000.1036. [DOI] [PubMed] [Google Scholar]
- 16.Shukla PJ, Barreto G, Neve R, Mohandas KM. Shrikhande SV Can we do better than ‘incidental’ gallbladder cancer? Hepatogastroenterology. 2007;54(80):2184–5. [PubMed] [Google Scholar]
- 17.Hale MD, Roberts KJ, Hodson J, Scott N, Sheridan M, Toogood GJ. Xanthogranulomatous cholecystitis: a European and global perspective. HPB (Oxford) 2014;16(5):448–58. doi: 10.1111/hpb.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maesawa C, Ogasawara S, Yashima-Abo A, Kimura T, Kotani K, Masuda S, Nagata Y, Iwaya T, Suzuki K, Oyake T, Akiyama Y, Kawamura H, Masuda T. Aberrant maspin expression in gallbladder epithelium is associated with intestinal metaplasia in patients with cholelithiasis. J Clin Pathol. 2006;59(3):328–30. doi: 10.1136/jcp.2005.027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roa I, Ibacache G, Munoz S, de Aretxabala X. Gallbladder cancer in Chile: pathologic characteristics of survival and prognostic factors: analysis of 1,366 cases. Am J Clin Pathol. 2014;141(5):675–82. doi: 10.1309/AJCPQT3ELN2BBCKA. [DOI] [PubMed] [Google Scholar]
- 20.Chatelain D, Fuks D, Farges O, Attencourt C, Pruvot FR, Regimbeau JM. Pathology report assessment of incidental gallbladder carcinoma diagnosed from cholecystectomy specimens: results of a French multicentre survey. Dig Liver Dis. 2013;45(12):1056–60. doi: 10.1016/j.dld.2013.07.004. [DOI] [PubMed] [Google Scholar]