Abstract
Pancreatic adenocarcinoma is the most common pancreatic malignancy, and it occurs most commonly in the pancreatic head. It has a relatively low incidence; however it is a deadly disease and is the fourth most common cause of cancer deaths for males and females in the United States. Surgical resection in the form of pancreaticoduodenectomy is the mainstay of treatment and can lead to improved overall survival as well as the possibility of a cure, although only 10 % of patients are resectable at presentation. In an attempt to improve outcomes and survival, surgeons over the decades have employed various aggressive resectional strategies to combat this disease. In this paper we review the development of pancreaticoduodenectomy and touch on the role played by the American surgeon Allan Whipple in this development. We review modern data regarding radical pancreaticoduodenectomy and extended lymphadenectomy for pancreatic head cancers, as well as data and controversies regarding arterial and venous resection performed during the course of pancreaticoduodenectomy. The role of extended and vascular resections in the treatment of pancreatic neuroendocrine tumors in contrast to adenocarcinomas is also examined. We summarize the current state of data regarding radical pancreaticoduodenectomy and discuss pushing the boundaries of surgical resection to help improve outcomes for select groups of patients.
Keywords: Pancreatic cancer, Pancreaticoduodenectomy, Extended pancreaticoduodenectomy, Radical pancreaticoduodenectomy, Whipple
Background and Description of Pancreatic Cancer
Malignant lesions arising in the pancreas comprise a heterogeneous group of cancers specific to their cell of origin. The different types of pancreatic cancer also correspond to both endocrine and exocrine functions of the pancreas; thus, a wide variety of tumors ranging from adenocarcinomas to neuroendocrine tumors have been identified. Nevertheless, nearly 95 % of pancreatic cancers arise from the exocrine pancreas and pancreatic duct adenocarcinoma is the most common malignancy [1]. Although pancreatic adenocarcinoma may have a relatively low incidence, this disease has been estimated to be the 9th and 10th most frequent cancer in females and males, respectively. Despite the low incidence, pancreatic adenocarcinoma is quite deadly, ranking as the 4th most-common cause of cancer-related deaths in both males and females in the US [1]. Approximately 75 % of these cancers occur in the pancreatic head and are treated with pancreaticoduodenectomy. The poor outcomes reflect the highly aggressive nature of this cancer and highlight the need to improve upon our current treatment strategies. Since surgical resection is the only treatment to offer potential for cure, we will focus our review on established and promising surgical techniques to eradicate this deadly disease.
Historical Perspectives of Pancreatic Adenocarcinoma
The cornerstone of curative-intent treatment for pancreatic adenocarcinoma is surgical resection and only a mere 10 % of all patients are eligible [2]. Credit for the development of the first successful pancreatic head resection has been bestowed upon Allan Oldfather Whipple. The operation bearing his name (i.e., “Whipple”) was originally performed in two steps: (1) cholecystogastrostomy or other bilio-enteric anastomosis for decompression of the obstructed biliary tract and (2) weeks later, resection of the distal stomach, duodenum, and pancreatic head [3]. AO Whipple subsequently refined the operation as a single-stage procedure [4]. However, the operation as it is performed today is different from the procedure described by AO Whipple. Instead, the current operation as it is performed today involving cholecystectomy, common hepatic duct resection, gastrectomy, duodenectomy and transection at the pancreatic neck, was championed and refined by Waugh and Clagett in 1946 [5]. Modifications to this established series of steps have been attempted to improve the outcomes of our patients with pancreatic adenocarcinoma.
Historical Outcomes for Resection of Pancreatic Head Adenocarcinoma
Despite the low numbers for eligibility, surgical resection can improve the survival of patients with pancreatic adenocarcinoma. Only three to four decades ago, pancreaticoduodenectomy was an operation that was associated with prohibitively high morbidity and with mortality rates reaching 20 %–40 % [6]. Refined surgical techniques and improving surgical critical care have now resulted in operative mortality rates below 2 % [6]. The advances in perioperative outcomes have translated to improvements in long-term outcomes. In one of the largest single-institution series, Cameron et al. reported that the 5-year survival rate for 405 patients who underwent resection for adenocarcinoma of the head of the pancreas was 18 % for the entire cohort and 32 % when the patients had lymph node negative disease [7, 8]. Since surgical resection is the only option to provide durable long-term survival, innovative surgical techniques or strategies have been sought to improve upon current outcomes.
Patient Evaluation and Selection for Resection
Pancreatic adenocarcinoma is a deadly disease in part because disease-related symptoms are often vague and non-specific. Consequently, patients present for evaluation with advanced stages of disease. Common presenting symptoms include weight loss, jaundice, and abdominal pain and corresponding physical examinations are just as unrevealing. For patients with suspected intra-abdominal malignancy, radiographic imaging is the next step in the evaluation. In fact, proper imaging is a critical and essential component of the preoperative management of patients with suspected pancreatic adenocarcinoma. The extent of disease must be carefully evaluated, especially the spatial relationship of the tumor to the nearby vascular structures, such as the superior mesenteric artery (SMA) and vein (SMV), the splenic vein, and the portal vein (PV). Multidetector computed tomographic (CT) imaging with pancreas protocol is the recommended imaging study for evaluation of pancreatic malignancies exhibiting high positive and low negative predictive values for determining resectability of pancreatic adenocarcinoma [9]. Pancreas protocol CT utilizes thin cuts (1–3 mm) through the pancreas with two phases of intravenous contrast enhancement: first, a pancreatic arterial phase, followed by a portal-venous phase [9]. While multidetector CT imaging has become the preferred modality of imaging diagnosis, MRI can also play a role in diagnosis due to its superior contrast for soft tissues, especially for small tumors less than 2 cm or in cases of fatty infiltration of the pancreatic head [10].
Once imaging has been obtained, pancreatic cancer can be categorized into 3 groups: (1) resectable, (2) borderline resectable, and (3) locally-advanced [11]. Resectable cancers have no extension to the SMA, have a normal fat plane surrounding the SMA, have no extension to the celiac axis or common hepatic artery, and have possible abutment but patency of the SMV and PV. In contrast borderline resectable cancers may have <180° abutment of the SMA, short-segment encasement or abutment of the common hepatic artery, or short-segment segmental occlusion of the SMV/PV with patent proximal and distal vessels. These borderline resectable patients may become eligible for surgical intervention with downsizing of disease, but are not immediate candidates for surgical resection. Finally, locally-advanced pancreatic cancers have >180° encasement of the SMA, encasement of the celiac axis or common hepatic artery, or complete occlusion of the SMV/PV [11]. Patients with locally-advanced cancers are not candidates to undergo surgical resection [12]. Surgeons have pushed the envelope for patients in all three categories of patients and we explore those conditions below.
Extended Pancreaticoduodenectomy With Major Vascular Resection
In the 1970s, the involvement of the major vascular structures (i.e., portal vein, celiac axis, or superior mesenteric artery) was considered a contra-indication for curative-intent surgical resection of pancreatic adenocarcinoma. However, during this period of time Joseph Fortner at Memorial Sloan-Kettering Cancer Center reported on his series of patients who underwent complex resections for cancers involving these vascular structures [8]. Through his technique of regional pancreatectomy, Fortner performed en bloc resection of the pancreatic tumor along with soft tissue margins, regional lymphatics, and a segment of the portal vein immediately adjacent to the pancreas. The portal vein was reconstructed through direct end-to-end anastomosis with the superior mesenteric vein [8]; adequate length for a tension-free anastomosis was obtained by releasing the base of the small bowel mesentery and moving it cephalad [13]. Despite the increased complexity of his procedure, Fortner’s patients had a mortality rate of 8 % and median survival rate of 40 months post-operatively, and Fortner was able to demonstrate that vascular invasion was not a contraindication to surgical resection [13].
Subsequent studies have also attempted to better define the role of vascular resection in patients with pancreatic adenocarcinoma. Hartel et al., examined outcomes between their cohort of patients (n = 271) who underwent pancreaticoduodenectomy +/− major vein resection. Their analysis revealed no difference in the rates of perioperative morbidity, mortality, and 5-year survival between the two groups [14]. Similar results were observed by Tseng et al., at MD Anderson Cancer Center for their patients who underwent pancreaticoduodenectomy with major vascular resection. The rates of overall survival were similar among patients regardless whether major vein resection was performed [15]. Other series have further verified the safety and efficacy of including major vein resection in select patients with pancreatic head adenocarcinoma [16]. Thus, it is clear that although major venous resection may not improve survival beyond pancreaticoduodenectomy alone, it nevertheless allows patients who would otherwise be deemed to be unresectable to undergo curative-intent surgical resection (Fig. 1).
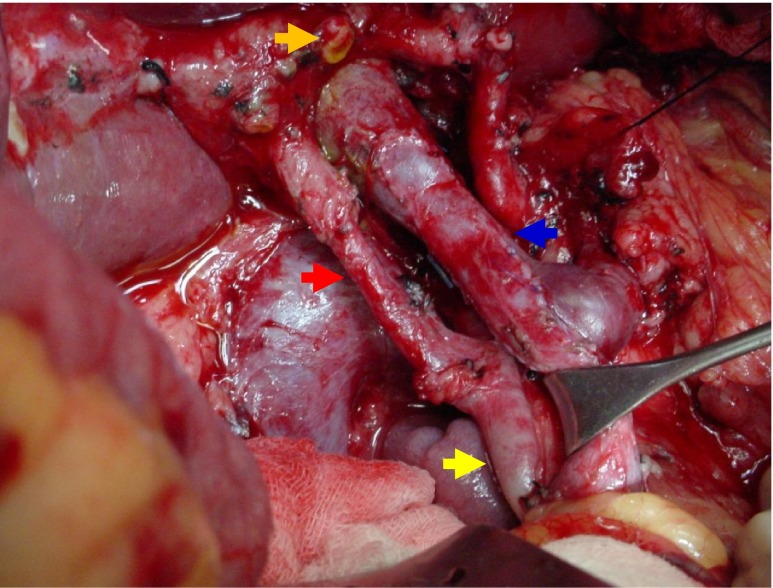
Fig. 1.
Completed radical resection of the head of the pancreas with reconstruction of the portomesenteric complex with a saphenous vein patch. Orange Arrow: Common Hepatic Duct, Red Arrow: Replaced Right Hepatic Artery arising from the Superior Mesenteric Artery (Yellow Arrow). Blue Arrow: reconstructed Portal Vein at the junction of the Superior Mesenteric Vein and Splenic Vein
Extended Lymphadenectomy
In standard pancreaticoduodenectomy the local lymph nodes near the head of the pancreas are resected in the routine course of the procedure. Surgeons have questioned whether more extensive or extended lymphadenectomy may improve outcomes in patients with pancreatic adenocarcinoma. This clinical question is based on the rationale that complete resection of regional lymph nodes may increase disease control and consequently increase patient survival [8]. Randomized trials have been completed and provide a clear answer to this question. The first of these trials was reported by Pedrazzoli et al. from the Lymphadenectomy Study Group [17]. In this multi-institutional, randomized trial, patients were prospectively accrued to undergo either standard pancreaticoduodenectomy or pancreaticoduodenectomy with extended lymphadenectomy. Standard lymphadenectomy involved resection of the anterior and posterior pancreaticoduodenal, superior and inferior pancreatic, pancreatic body, and pyloric and bile duct lymph nodes. In contrast, extended lymphadenectomy also involved resection of lymph nodes at the hepatic hilum, along the aorta from the diaphragmatic crus to the inferior mesenteric artery, at the bilateral renal hila, and at the origin of both the celiac axis and superior mesenteric artery. In the final analysis, although a greater number of lymph nodes were harvested and identified in the extended lymphadenectomy group (18.8 vs. 13.3, respectively), there was no difference in overall survival between the two groups. Yeo and colleagues from The Johns Hopkins Hospital conducted a single-institution randomized trial of standard versus extended lymphadenectomy [18]. The authors also noted greater lymph node yields in the extended lymphadenectomy group (27 vs. 16, respectively), but again no difference in overall survival between the two groups. Long-term follow-up verified the lack of difference in overall survival between the two groups [19, 20]. Similar outcomes were observed in a separate single-institution study at the Mayo Clinic [21].
Based on the strength of data from several randomized trials, extended lymphadenectomy for pancreatic adenocarcinoma does not confer a survival advantage. Therefore, pancreaticoduodenectomy with extended lymphadenectomy remains investigational and should be avoided in the routine surgical management of patients with pancreatic adenocarcinoma.
Oncologic Controversy: Pancreaticoduodenectomy and Arterial Resection
While it may be clear that major venous resection can be performed in curative-intent pancreaticoduodenectomy, the resection of major arterial structures (celiac axis and superior mesenteric artery) for pancreatic adenocarcinoma is controversial and not well studied. Indeed, it is a commonly accepted contraindication to curative surgery. One early concern with arterial resection is simply the risk of devascularization of distal organs with resulting malperfusion and possible organ necrosis. An early report of major arterial resection for intra-abdominal malignancies came in 1953 from Appleby, who reported on 13 cases of total gastrectomy in conjunction with resection of the celiac axis and distal pancreatectomy for locally-advanced gastric cancers with pancreatic invasion [22]. In that series of patients collateral blood flow from the SMA through the gastroduodenal artery was the key in organ perfusion. None of the patients had evidence of organ ischemia, and there were no postoperative deaths. More recently, Makary et al., described a case of en bloc pancreatectomy, total gastrectomy, splenectomy, and resection of the celiac axis for a pancreatic adenocarcinoma that invaded the posterior stomach and the celiac axis [23]. In their patient, flow from the superior mesenteric artery through the gastroduodenal artery was first seen on CT imaging, and later confirmed intraoperatively by vascular testing prior to resection. The patient’s post-operative course was unremarkable, and the paper also demonstrated that resection of the celiac axis is possible.
Singh and colleagues at the University of Southern California University Hospital also reported their experience with major arterial resection. In a modification of the Appleby procedure [24], three patients with central pancreatic adenocarcinomas and malignant involvement of the celiac trunk were reviewed. In their series, all patients underwent CT imaging pre-operatively, and one also underwent mesenteric angiography to determine circulation from the superior mesenteric artery. All patients underwent extended pancreatectomy with resection of the celiac axis, and subsequently had a mean hospital stay of 8.3 ± 1.1 days. At the time of the report, all three patients were alive and free of disease at 34, 14, and 14 months of follow-up, respectively.
A larger series examining arterial resections for pancreatic adenocarcinoma was reported by Stitzenberg et al. [25]. In their series, 12 patients underwent pancreaticoduodenectomy with arterial resection (celiac axis resection, n = 10; and hepatic artery resection, n = 2). All patients suffered at least one complication, and median survival after resection was not significantly different when compared to their group of 252 patients undergoing pancreaticoduodenectomy without arterial resection (20 vs 21 months). The authors concluded that while arterial resections are technically feasible, major artery involvement by tumor should remain a contraindication to resection.
Amano and colleagues from Tokyo recently published their experience with arterial resections and pancreaticoduodenectomy [26]. In this series, 23 patients with pancreatic adenocarcinoma who underwent arterial resections with pancreatectomies were retrospectively reviewed. Operative mortality was 4.3 %. Based on the pathologic results of the resected specimens, fifteen patients who were found to have UICC M0 disease were compared to 8 patients who were found to have M1 disease. Patients with M0 disease had a one-year survival rate of 62 % and a median survival time of 16 months, versus M1 patients who were found to have a median survival time of only 10 months. Eighteen patients with R0 resections had a one-year survival rate of 67 % and 13 months median survival, as opposed to R1/R2 patients who had a 6 month survival rate. The paper concluded that arterial resection is useful only when an R0 resection can be done in a patient with M0 disease [26]. Median operating time in this series was 686 min, and median blood loss was 2,830 mL, clearly illustrating that pancreatectomies with arterial resections are technically demanding cases.
Radical Resections for Pancreatic Neuroendocrine Tumors
Pancreatic adenocarcinoma and the role of various types of vascular resections has been focused on in this review, however an aspect of pancreatic surgery for which the role of vascular resection differs is in the treatment of pancreatic neuroendocrine tumors; we will briefly touch on this topic. In general, pancreatic neuroendocrine tumors (PNET) are a rare group of tumors with an incidence of 1 to 10 per million [27, 28]. These tumors include insulinoma, glucagonoma, gastrinoma, VIPoma, and somatostatinoma, and are generally classified as functioning or nonfunctioning tumors. As with pancreatic adenocarcinoma, patients with PNET may present for medical evaluation late, and locally-advanced and metastatic disease are commonly seen. Hellman and colleagues from Sweden have described their series of 31 patients, all with large or malignant PNET [27]. They described 8 patients with suspected preoperative vascular involvement, either the superior mesenteric vein (SMV) or the portal vein (PV), and an additional 15 with adjacent organ involvement. At surgery, 12 of the patients were found to have involvement of the SMV, PV, or splenic vein. In this report, the operation performed depended on the location of the tumor within the pancreas, and included pylorus-preserving pancreaticoduodenectomy, classic Whipple pancreaticoduodenectomy, total pancreatectomy, distal pancreatectomy, and subtotal pancreatectomy. Traditionally, as with pancreatic adenocarcinoma, PNET vascular involvement was considered a contraindication to resection, however based on the experience previously reviewed regarding adenocarcinoma [14, 15, 17], resection of these locally advanced, and even metastatic tumors was undertaken. Of the patients described above, 4 had PV or SMV involvement and all received complete resection of their tumor with vascular reconstruction by vein patch, vein graft, or synthetic graft. The group also reported a low rate of complications as well as mortality.
While Hellman’s group did not describe survival in these cases of advanced disease, a larger prospective trial enrolling 273 patients was conducted by Norton and colleagues from Stanford University [29]. They found that 46 of the patients had radiographic evidence of vascular involvement preoperatively; however at operation, 29 of the patients had tumors encroaching, abutting, or distorting the major vasculature, while only 15 of these patients were found to have actual vascular invasion. Vascular reconstructions after resections of the PV, SMV, or SMA were performed using venous grafts. Forty-one percent of the patients in the series were also found to have liver metastasis and underwent successful metastasectomy either by wedge or anatomic resection. The groups’ surgical outcomes revealed no operative mortalities. Ten-year actuarial overall survival was 60 %, with a disease-free survival of 30 %. Disease-free survival was unchanged by vascular reconstruction, though was negatively affected by hepatic resection. Despite 40 % of the patients undergoing liver resection and 20 % undergoing vascular reconstruction, the overall survival was far superior to previously-reported historical reports of 40 % 5-year survival [30].
While PNET are rare and research in this area has such been limited, radical resections for locally advanced and even metastatic disease have been shown to be safe and feasible. Research has shown that preoperative vascular evaluation can be incorrect in up to 50 % of cases. There has now been shown to be a significant survival advantage in undergoing a radical resection, including arterial resections (Fig. 2), in comparison to historical survival data. The biology of neuroendocrine tumors is obviously different than that of adenocarcinomas, however in the case of PNET, surgery even in the presence of vascular invasion or liver metastasis is indicated.
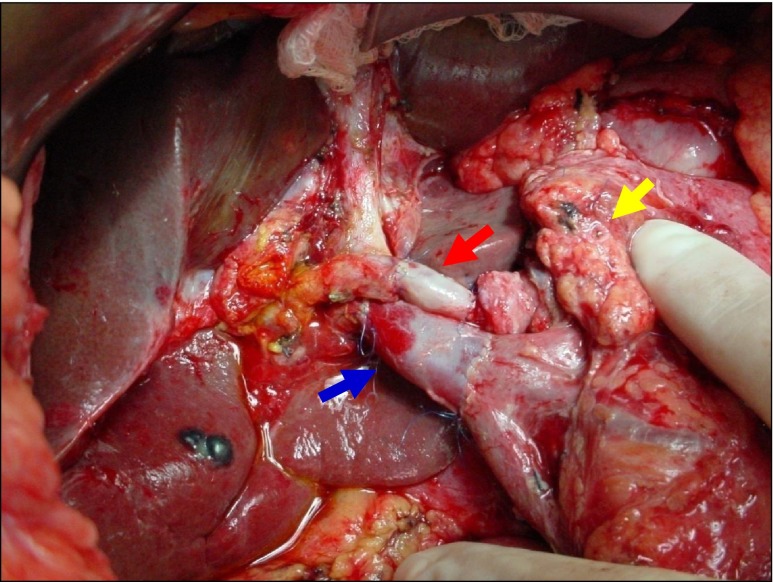
Fig. 2.
Completed radical resection of the head of the pancreas with reconstruction of the Portal Vein with an Internal Jugular Vein (IJV) Graft. Red Arrow: Reconstructed Hepatic Artery using a saphenous vein interposition graft. Blue Arrow: reconstructed Portal Vein with an IJV graft extending up to the confluence of the Superior Mesenteric Vein and Splenic Vein. Yellow Arrow: Pancreatic duct
Conclusion
Great advances in operative technique, anesthesia, and critical care have been made since Allen O. Whipple first published his report on pancreaticoduodenectomy; however, pancreatic cancer still remains a disease that is challenging and frustrating for patients and physicians alike. Rates of recurrence can be high, ranging from 50 % to 87 % of patients, even after resection with curative intent [31–33]. Adjuvant chemotherapy with gemcitabine has been shown to significantly increase overall survival and disease-specific survival in pancreatic cancer patients after resection compared to observation alone, although this increase in overall survival is seen to be only 2 months [34]. The care of patients with pancreatic cancer will continue to be multi-disciplinary in the future; however surgery remains the cornerstone in improving outcomes for patients, and by pushing the boundaries of resection in appropriately-selected patients we may see improved outcomes and survival in the future.
Acknowledgments
Financial Disclosures
None.
References
- 1.American Cancer Society . Cancer facts & figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 3.Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg. 1935;102(4):763–779. doi: 10.1097/00000658-193510000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whipple AO. The rationale of radical surgery for cancer of the pancreas and ampullary region. Ann Surg. 1941;114(4):612–615. doi: 10.1097/00000658-194111440-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waugh JM, Clagett OT. Resection of the duodenum and head of the pancreas for carcinoma; an analysis of thirty cases. Surgery. 1946;20:224–232. [PubMed] [Google Scholar]
- 6.Crist DW, Sitzmann JV, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg. 1987;206(3):358–365. doi: 10.1097/00000658-198709000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortner JG. Regional resection of cancer of the pancreas: a new surgical approach. Surgery. 1973;73(2):307–320. [PubMed] [Google Scholar]
- 9.Faria SC, Tamm EP, Loyer EM, Szklaruk J, Choi H, Charnsangavej C. Diagnosis and staging of pancreatic tumors. Semin Roentgenol. 2004;39(3):397–411. doi: 10.1016/j.ro.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012;18(6):511–522. doi: 10.1097/PPO.0b013e318274a461. [DOI] [PubMed] [Google Scholar]
- 11.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13(8):1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. Pancreatic adenocarcinoma. http://www.nccn.org/professionals/physician_gls/default.asp. Accessed February 24, 2013.
- 13.Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla, and other related sites. Tumor staging and results. Ann Surg. 1984;199(4):418–425. doi: 10.1097/00000658-198404000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartel M, Niedergethmann M, Farag-Soliman M, Sturm JW, Richter A, Trede M, Post S. Benefit of venous resection for ductal adenocarcinoma of the pancreatic head. Eur J Surg. 2002;168(12):707–712. doi: 10.1080/00000000000000007. [DOI] [PubMed] [Google Scholar]
- 15.Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, Gomez HF, Sun CC, Crane CH, Wolff RA, Evans DB. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8(8):935–949. doi: 10.1016/j.gassur.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Wu H, Xiong J, Zhou F, Tao J, Liu T, Zhao G, Gou S. Pancreaticoduodenectomy with vascular resection for local advanced pancreatic head cancer: a single center retrospective study. J Gastrointest Surg. 2008;12(12):2183–2190. doi: 10.1007/s11605-008-0621-9. [DOI] [PubMed] [Google Scholar]
- 17.Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, Kloppel G, Dhaene K, Michelassi F. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228(4):508–517. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeo CJ, Cameron JL, Sohn TA, Coleman J, Sauter PK, Hruban RH, Pitt HA, Lillemoe KD. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg. 1999;229(5):613–622. doi: 10.1097/00000658-199905000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, Abrams RA, Laheru D, Hruban RH, Yeo CJ. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma–part 3: update on 5-year survival. J Gastrointest Surg. 2005;9(9):1191–1204. doi: 10.1016/j.gassur.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 20.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236(3):355–366. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, Foster N, Sargent DJ. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138(4):618–628. doi: 10.1016/j.surg.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 22.Appleby LH. The coeliac axis in the expansion of the operation for gastric carcinoma. Cancer. 1953;6(4):704–707. doi: 10.1002/1097-0142(195307)6:4<704::AID-CNCR2820060410>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Makary MA, Fishman EK, Cameron JL. Resection of the celiac axis for invasive pancreatic cancer. J Gastrointest Surg. 2005;9(4):503–507. doi: 10.1016/j.gassur.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Gagandeep S, Artinyan A, Jabbour N, Mateo R, Matsuoka L, Sher L, Genyk Y, Selby R. Extended pancreatectomy with resection of the celiac axis: the modified Appleby operation. Am J Surg. 2006;192(3):330–335. doi: 10.1016/j.amjsurg.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Stitzenberg KB, Watson JC, Roberts A, Kagan SA, Cohen SJ, Konski AA, Hoffman JP. Survival after pancreatectomy with major arterial resection and reconstruction. Ann Surg Oncol. 2008;15(5):1399–1406. doi: 10.1245/s10434-008-9844-y. [DOI] [PubMed] [Google Scholar]
- 26.Amano H, Miura F, Toyota N, Wada K, Katoh K, Hayano K, Kadowaki S, Shibuya M, Maeno S, Eguchi T, Takada T, Asano T. Is pancreatectomy with arterial reconstruction a safe and useful procedure for locally advanced pancreatic cancer? J Hepatobiliary Pancreat Surg. 2009;16(6):850–857. doi: 10.1007/s00534-009-0190-7. [DOI] [PubMed] [Google Scholar]
- 27.Hellman P, Andersson M, Rastad J, Juhlin C, Karacagil S, Eriksson B, Skogseid B, Akerstrom G. Surgical strategy for large or malignant endocrine pancreatic tumors. World J Surg. 2000;24(11):1353–1360. doi: 10.1007/s002680010224. [DOI] [PubMed] [Google Scholar]
- 28.Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier RF, Yao JC, Jensen RT. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39(6):735–752. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norton JA, Harris EJ, Chen Y, Visser BC, Poultsides GA, Kunz PC, Fisher GA, Jensen RT. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch Surg. 2011;146(6):724–732. doi: 10.1001/archsurg.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson GB, van Heerden JA, Grant CS, Carney JA, Ilstrup DM. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery. 1988;104(6):1011–1017. [PubMed] [Google Scholar]
- 31.Gnerlich JL, Luka SR, Deshpande AD, Dubray BJ, Weir JS, Carpenter DH, Brunt EM, Strasberg SM, Hawkins WG, Linehan DC (2012) Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg 147(8):753–760. doi:10.1001/archsurg.2012.1126 [DOI] [PubMed]
- 32.Hattangadi JA, Hong TS, Yeap BY, Mamon HJ. Results and patterns of failure in patients treated with adjuvant combined chemoradiation therapy for resected pancreatic adenocarcinoma. Cancer. 2009;115(16):3640–3650. doi: 10.1002/cncr.24410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez JM, Morton CA, Al-Saadi S, Villadolid D, Cooper J, Bowers C, Rosemurgy AS (2010) The natural history of resected pancreatic cancer without adjuvant chemotherapy. Am Surg 76(5):480–485 [PubMed]
- 34.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C, Gutberlet K, Kettner E, Schmalenberg H, Weigang-Koehler K, Bechstein WO, Niedergethmann M, Schmidt-Wolf I, Roll L, Doerken B, Riess H. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]