Abstract
Small-molecule inhibitors of protein kinases have contributed immensely to our understanding of biological signaling pathways and have been exploited therapeutically for the treatment of cancers and other disease states. The pyridinyl imidazole compounds SB 203580 and SB 202190 were identified as ATP competitive antagonists of the p38 stress-activated protein kinases and have been widely used to elucidate p38-dependent cellular processes. Here, we identify SB 203580 and SB 202190 as potent inhibitors of stress-induced CREB phosphorylation on Serine 111 (Ser-111) in intact cells. Unexpectedly, we found that the inhibitory activity of SB 203580 and SB 202190 on CREB phosphorylation was independent of p38, but instead correlated with inhibition of casein kinase 1 (CK1) in vitro. The inhibition of CK1-mediated CREB phosphorylation by concentrations of pyridinyl imidazoles commonly employed to suppress p38, suggests that in some cases conclusions of p38-dependence derived solely from the use of these inhibitors may be invalid.
Keywords: Casein kinase, CK1, CK2, CREB, Cycloheximide, Non-specific, p38, SB202190, SB203580, Stress-activated protein kinase
INTRODUCTION
The closely related pyridinyl imidazole compounds SB 203580 and SB 202190 were originally identified on the basis of their anti-inflammatory inhibition of tumor necrosis factor and interleukin-1 release from stimulated human monocytes (1). Subsequently, both compounds were found to inhibit members of the p38 family of stress-activated protein kinases (SAPKs) in vitro and in vivo (2). In mammals three closely related members of the p38 family, p38α, p38β and p38γ are activated by a wide variety of cellular stressors including hyperosmolarity, protein synthesis inhibition, inflammatory cytokines, and ultraviolet (UV) light (Reviewed in Ref. (3, 4)). SB 203580 and SB 202190 were shown to be potent inhibitors of p38α and p38β but not p38γ (5). p38 is activated upon phosphorylation by the SAP2Ks, MKK3/6 which are in turn activated by several SAP3Ks including MEKK4 and TAK1 (4). Although the downstream consequences of p38 activation are highly context-dependent, this family of kinases is strongly implicated in apoptotic signaling, inflammation, and cell cycle regulation (3). In this regard, the functions of p38 overlap with those of the Jun N-terminal kinases (JNKs), which are activated by many of the same stress signals downstream of overlapping SAP3K and SAP2K pathways (6). The JNKs are inhibited by structurally distinct compounds, including SP 600125, and the differential sensitivities of p38 and JNK SAPKs to inhibitors of SB 202190/SB 203580 and SP 600125 have been widely used to distinguish JNK- from p38-dependent cellular events. Since the original discovery of SB 203580 and SB 202190, second-generation p38 inhibitors belonging to the pyridinyl imidazole family have also been investigated as potential therapeutic agents for autoimmune or inflammatory diseases (7, 8). However, the therapeutic application of pyridinyl imidazoles will require thorough characterization of their biological activities and potential off-target effects.
CK1 and CK2 are two unrelated, constitutively active protein kinase families that participate in a wide variety of cellular processes, including DNA repair, cell cycle control, and circadian rhythm entrainment (9–11). The abilities of CK1 and CK2 to phosphorylate substrates on Ser/Thr residues are strongly enhanced by acidic residues or priming phosphorylation of Ser/Thr residues in the minus three or plus three positions, respectively. Thus, the consensus phosphorylation sites for CK1 and CK2 are D/E/pS-X-X-S and S-X-X-D/E/pS, respectively. As a consequence of the reciprocal requirements for phospho-Ser/Thr residues in the minus three or plus three positions, CK1 and CK2 often cooperate in the processive phosphorylation of protein substrates. We recently observed a role for these kinases, in cooperation with the ataxia telangiectasia-mutated (ATM) kinase, in the co-regulated phosphorylation of the cyclic AMP response element-binding protein (CREB) on multiple sites in response to DNA damage (12, 13). In this study, we used the phosphorylation of CREB on Ser-108, Ser-111, and Ser-114 by CK1/CK2 as a paradigm to demonstrate that SB 203580 and SB 202190 non-specifically inhibit CK1 in intact cells. The ramifications of these findings for studies employing pyridinyl imidazoles are also discussed.
RESULTS AND DISCUSSION
Inhibition of CREB Ser-108/111/114 phosphorylation by SB 203580 and SB 202190
Previous work from our laboratory defined a cluster of phosphorylation sites within CREB (amino acids 108–121) that was phosphorylated in response to DNA-damaging stimuli (12, 13). Within this cluster, the phosphorylation of Ser-111 by ATM triggers the processive phosphorylation of flanking Ser residues (Ser-108, Ser-114, and Ser-117) by CK1 and CK2. Modification of the CK1/CK2 sites is, in turn, required for the DNA damage- and ATM-dependent phosphorylation of Ser-121. Modification of Ser-121 attenuates the affinity of CREB for its transcriptional co-activator, CBP (CREB-binding protein). The DNA damage-induced phosphorylation of CREB on Ser-108/111/114 is highly sensitive to the CK1 inhibitor D4476 and can be conveniently detected using a phospho-specific antibody (13).
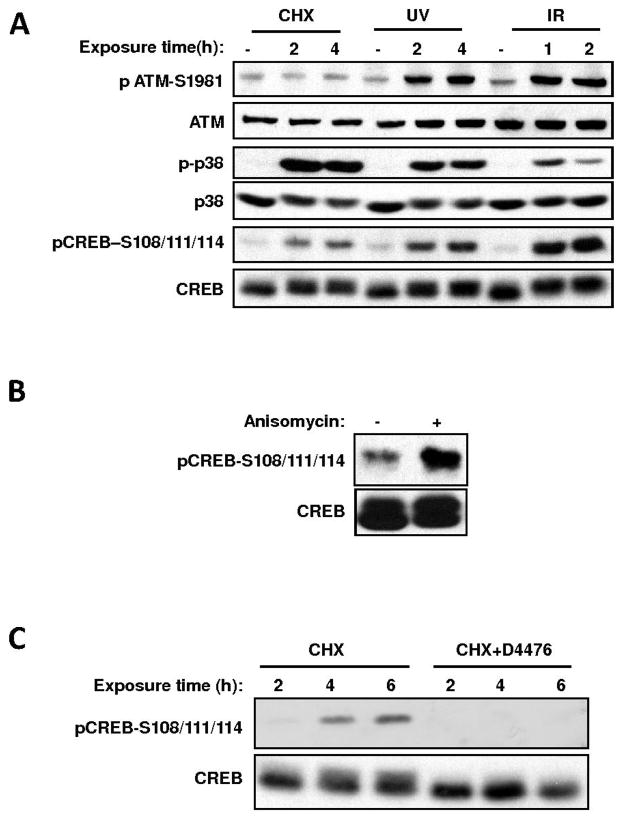
Approximately 10–20% of total cellular CREB is phosphorylated by CK1/CK2 on Ser-108/111/114 in the absence of DNA damage ((13) and Fig. 1). While screening for stimuli that trigger this phosphorylation event, we discovered that the protein synthesis inhibitor cycloheximide (CHX), robustly induced CREB Ser-108/111/114 phosphorylation in HEK 293T cells (Fig. 1A). This induction appeared to be independent of DNA damage as evidenced by a lack of ATM activation (Fig. 1A). A second protein synthesis inhibitor, anisomycin, also robustly induced CREB Ser-108/111/114 phosphorylation (Fig. 1B) suggesting that the observed CHX induced phosphorylation was a general cellular response to protein synthesis inhibition. Interestingly, D4476, an inhibitor of the alpha, delta, and epsilon forms of CK1 blocked CHX-induced-induced phosphorylation of Ser-108/111/114, as well as basal phosphorylation of these sites in the absence of treatment (Fig. 1C). This suggested that CK1 was required for optimal CREB phosphorylation downstream of both the DNA damage-induced and DNA damage-independent pathways.
Fig. 1.
Protein synthesis inhibitor-induced phosphorylation of CREB on Ser-108/111/114 requires CK1. (A), Diverse stress-stimuli induce the phosphorylation of CREB on Ser-108/111/114 in intact cells. HEK 293T cells were either mock-treated or exposed to cycloheximide (CHX, 20 μg/ml), UV light (20 Joules/m2), or ionizing radiation (IR, 5 Gy) and harvested at the indicated time intervals. Cell extracts were resolved by SDS-PAGE and immunoblotted with the indicated antibodies. (B), Anisomycin induces CREB Ser- 108/111/114 phosphorylation. HEK 293T cells were either mock- treated or subjected to 20 μg/ml anisomycin. Cell extracts were analyzed by immunoblotting with α-CREB and α-pCREB– S108/111/114 antibodies. (C), CHX induced CREB Ser-108/111/114 phosphorylation is sensitive to CK1 inhibition. HEK 293T cells were exposed to CHX with or without a 1 h-pretreatment with 75 μM D4476 (a CK1 inhibitor) for the indicated periods of time. The cells were then harvested and processed for immunoblotting with α-CREB and α-pCREB–S108/111/114.
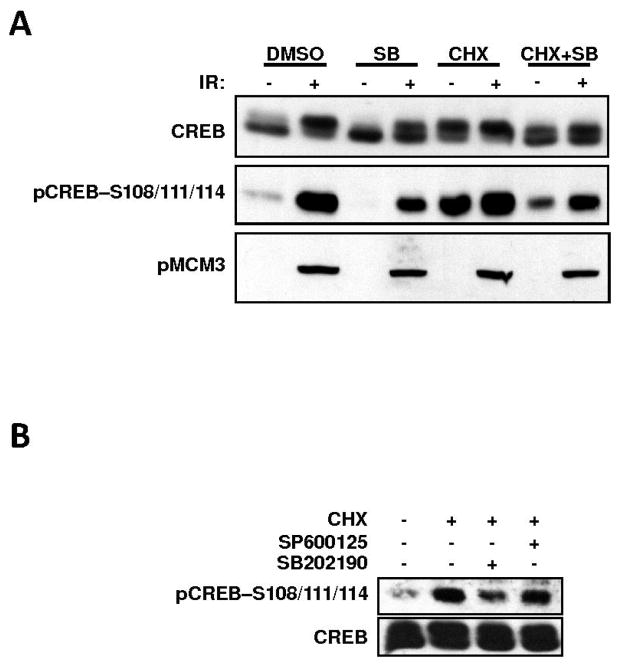
Protein synthesis inhibition, UV light, and ionizing radiation (IR) are well-characterized activators of the mammalian p38 and JNK SAPKs (4, 6). We thus sought to test the involvement of both kinases in CREB Ser-111 phosphorylation, in response to these stimuli. In HEK 293T cells, the phosphorylation of CREB on Ser-108/111/114 in response to CHX, UV light, or IR, correlated with the appearance of the activated form of p38 (Fig. 1A). Furthermore, pre-exposure of HEK 293T cells to the p38 inhibitor SB 203580 attenuated both CHX- and IR-induced CREB Ser-108/111/114 phosphorylation (Fig. 2A). In contrast to this, the JNK inhibitor SP 600125 exerted no effect on CREB Ser-108/111/114 phosphorylation (Fig. 2B). These results suggested a role for the p38 kinase family in stress-mediated CREB phosphorylation. The closely related p38 inhibitor SB 202190 also inhibited CREB phosphorylation in response to CHX and IR with equal potency (data not shown). Inhibition of CREB phosphorylation was not due to cross inhibition of ATM, as ATM kinase activity toward its substrate MCM3 was not suppressed by the p38-inhibitors (Fig. 2A). We also noted that SB 203580 strongly inhibited basal CREB phosphorylation in HEK 293T cells (compare lanes 1 and 3 in Fig. 2A) suggesting that a p38-dependent pathway controls CREB Ser-108/111/114 phosphorylation in the course of a normal cell cycle.
Fig. 2.
SB 203580 and SB 202190 are potent inhibitors of CREB Ser-108/111/114 phosphorylation. (A), SB 203580 inhibits CREB Ser-108/111/114 phosphorylation. HEK 293T cells were either mock-treated or pretreated with the p38 inhibitor SB 203580 (10 μM). They were then exposed to CHX (20 μg/ml) or 5 Gy IR or both for a period of 2 h. Cell extracts were immunoblotted with α-CREB, α-pCREB–S108/111/114 and α-pMCM3 antibodies. Note: The α-pMCM3 blot was probed with the α–pCREB-S121 antibody, which crossreacts with the ATM-phosphorylated form of MCM3 (22). Only the 100-kDa region of the membrane containing MCM3 is shown. (B), The JNK inhibitor SP 600125 does not affect CREB Ser108/111/114 phosphorylation. HEK 293T cells were either left untreated or pretreated with the JNK inhibitor SP 600125 (10 μM) or p38 inhibitor SB 202190 (10 μM). The cells were then exposed to CHX (20 μg/ml) for a period of 2 h. Cell extracts were analyzed by immunoblotting with α-CREB and α-pCREB–S108/111/114 antibodies.
Genetic suppression of p38 did not inhibit stress-induced CREB Ser-108/111/114 phosphorylation
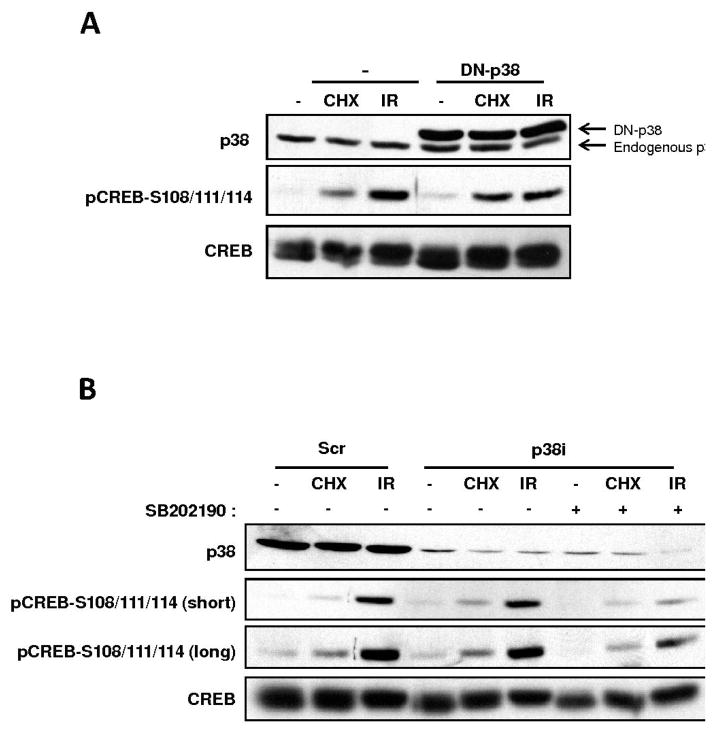
Given that multiple p38 activators induced CREB Ser-108/111/114 phosphorylation and that small molecule p38 inhibitors attenuated this phosphorylation, we hypothesized a critical role for p38 in CREB phosphorylation in response to various cellular stressors. The CREB Ser-111 site does not conform to the minimal Ser/Thr-Pro consensus site for p38 or its downstream effector kinase MAPKAP K2, suggesting that CREB is not directly phosphorylated by either kinase (14). However, a number of recent reports have suggested that p38 functions upstream of CK2, which plausibly links p38 to the CREB Ser-108/111/114/117 cluster that is processively phosphorylated by CK1 and CK2 (15, 16). To further probe the p38-dependence of CREB Ser-108/111/114 phosphorylation, we tested if over-expression of a catalytically-inactive, dominant negative, p38 construct inhibited CREB phosphorylation in HEK 293T cells exposed to CHX or IR. This dominant-negative construct has been extensively used in previous literature to test the p38-dependence of various cellular events (17, 18). However, robust overexpression of the DN-p38 construct failed to attenuate CREB Ser-108/111/114 phosphorylation in CHX or IR-treated cells (Fig. 3A).
Fig. 3.
Genetic interference of p38 does not impact stress-induced CREB Ser-108/111/114 phosphorylation. (A), A p38 dominant-negative (DN) construct does not impact CREB Ser-108/111/114 phosphorylation. HEK 293T cells were either transfected with vector DNA (−) or a plasmid encoding a dominant negative form of p38 (DN-p38) harboring T180A/Y182F mutations (17, 18). The cells were then either left untreated or exposed to CHX (20 μg/ml) or 5 Gy IR. Cell extracts were prepared and analyzed by immunoblotting with α-CREB, α-p38, and α-pCREB–S108/111/114 antibodies. (B), RNAi mediated knockdown of p38α does not inhibit CREB Ser-108/111/114 phosphorylation. HEK293T cells were transfected with scramble control (Scr) siRNA or siRNAs specific for p38α (p38i). The cells were then exposed to CHX (20 μg/ml) or 5 Gy IR or mock-treated. Additionally, one set of samples transfected with siRNAs specific for p38 (p38i) were pretreated with SB 202190 for a period of 1 h before exposure to CHX and IR. Cell extracts were prepared and immunoblotted with α-CREB, α-p38 and α-pCREB–S108/111/114 antibodies.
The incongruity between the inhibitor and dominant-negative approaches prompted us to use RNAi studies to definitively establish a role for p38 as an upstream CREB kinase. For these experiments, we targeted the p38α isoform, as it was found to be the principal isoform in 293T cells. HEK 293T cells were transfected with a p38α siRNA mixture and then exposed to DMSO, CHX, or IR 48 h later. Although total p38 protein levels were strongly suppressed after siRNA transfection, neither CHX- nor IR-induced phosphorylation of CREB Ser-108/111/114 was affected, even though CREB phosphorylation retained sensitivity to SB 202190 (Fig. 3B). Thus, the apparent participation of p38 in stress-induced CREB phosphorylation in intact cells, inferred from SB inhibitor sensitivity, was not supported by more rigorous genetic interrogation. Instead, these data suggested a potential off-target effect of the p38 inhibitor on a CREB Ser-108/111/114 kinase. Additional experiments also argued against a role for p38 in CREB phosphorylation. Osmotic shock with sorbitol, a potent activator of p38, failed to induce CREB phosphorylation (Sup. Fig. S1). Moreover, the over-expression of MKK6, a SAP2K that is known to activate p38, did not affect CREB Ser-108/111/114 phosphorylation (Sup. Fig. S2).
SB 203580 inhibited CREB phosphorylation through an off-target effect on CK1
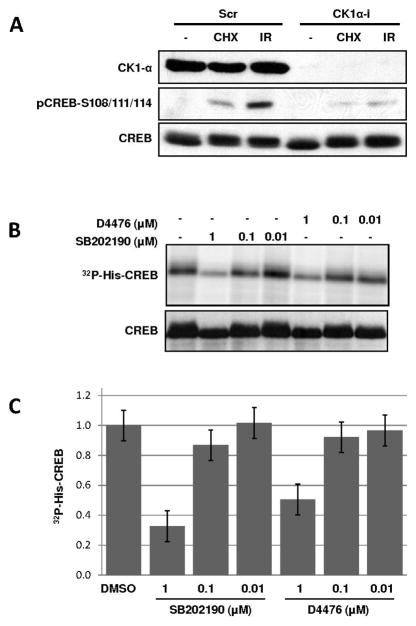
The above findings suggested that SB inhibited CHX–induced CREB Ser-108/111/114 phosphorylation non-specifically. Combined with earlier results showing that the CK1 inhibitor D4476 strongly suppressed CHX-induced Ser-108/111/114 phosphorylation, these findings suggested that non-specific inhibition of CK1 might account for the effect of the inhibitors on CREB phosphorylation. To test this hypothesis, we first used RNAi studies to prove that CK1 was required for Ser-108/111/114 phosphorylation. Transfection of HEK 293T cells with siRNA targeting CK1α led to the attenuation of CREB Ser-108/111/114 phosphorylation in response to both IR and CHX exposure (Fig. 4A). This result, combined with our previously published findings, strongly suggests that CK1 directly phosphorylates the Ser-108/111/114 cluster. We next tested whether SB 202190 inhibited CK1-dependent phosphorylation of CREB in vitro. SB 202190 inhibited CK1-mediated phosphorylation of a 6X histidine-tagged CREB fusion protein in a dose dependent manner (Fig. 4B). The estimated IC50 of 0.6 μM for SB 202190 was comparable to the IC50 of CK1 inhibition by D4476. On the other hand, SB 202190 showed no effect on CK2 kinase activity at all concentrations tested (Sup. Fig. S3). Thus, the inhibition of CK1 by SB in vitro correlates with the SB-dependent suppression of CREB Ser-108/111/114 phosphorylation in intact cells, strongly suggesting that the antagonistic effects of this compound on CREB phosphorylation are a consequence of CK1 inhibition.
Fig. 4.
CK1 inhibition is an off-target effect of SB 203580 and SB 202190. (A), CK1α knockdown inhibits CREB Ser-108/111/114 phosphorylation. HEK293T cells were transfected with scrambled control (Scr) siRNA or siRNAs specific for CK1α (CK1α-i). The cells were then exposed to CHX (20 μg/ml), 5 Gy IR, or mock- treated. Cell extracts were immunoblotted with α-CREB, α-pCREB–S108/111/114 and α-CK1α antibodies. (B, C) SB 202190 inhibits CK1 in vitro. Purified His-CREB protein was incubated with CK1δ in vitro in the presence of [32P-γ]—ATP and the indicated inhibitors as described in the Materials and Methods. The reaction products were visualized by SDS-PAGE, autoradiography, and immunoblotting with α-CREB (B) or subjected to quantification using a phosphorimager (C). The error bars in panel (C) reflect the standard deviation of three independent experiments.
In this study, we have shown that the pyridinyl imidazole p38-inhibitors suppressed CK1α-mediated phosphorylation events in intact cells. Specifically, SB 203580 and SB 202190 inhibited CK1-dependent phosphorylation of CREB on Ser-108/111/114 in response to protein synthesis inhibition and DNA damage. The relevant upstream pathways governing this phosphorylation event are currently under investigation in our laboratory. However, in vitro and cell-based assays strongly suggest that CK1 is a direct CREB Ser-111 and/or Ser-114 kinase, since CK1-specific inhibitors and RNAi ablated immunoreactivity with α-pCREB Ser-108/111/114 antibodies (13). Thus, in all likelihood, the inhibitory effects of the SB compounds on CHX- and anisomycin-induced CREB phosphorylation on Ser-108/111/114 stem from non-specific inhibition of CK1.
Our findings support recent in vitro studies that have questioned the specificity of pryidnyl imidazole inhibitors (19, 20). Since the CK1 family of kinases has widespread effects on cellular signaling, with hundreds of identified substrates (9), it is a virtual certainty that its non-specific inhibition would cause unintended effects on cell signaling that may be erroneously attributed to p38. Furthermore, because CK1-mediated phosphorylation can create consensus CK2 sites, CK2-dependent phosphorylation events may also be indirectly suppressed by SB 203580 and SB 202190. Thus, the cross inhibition of CK1 by SB 203580 and SB 202190 is expected to pose a particularly difficult challenge in those situations where p38 may act upstream of CK1 and/or CK2 in a linear signaling pathway. In this scenario, it would be difficult to determine whether the compound is inhibiting CK1/CK2-mediated phosphorylation indirectly through suppression of p38, or directly through antagonism of CK1. In light of our findings, we recommend the confirmatory use of structurally unrelated p38-inhibitors such as BIRB 0796 (20, 21) as well as genetic approaches to accurately assess the role of p38 in cellular processes.
MATERIALS AND METHODS
Cell culture, antibodies and inhibitors
HEK 293T cells were maintained in Eagle’s minimum essential medium (MEM) containing 5% fetal bovine serum (FBS). The generation and use of the α-pCREB-S108/111/114 antibody was previously described (13). Antibody suppliers included Rockland (α-pATM-S1981), Novus Biologicals (α-pCREB-S121), Genetex (α-ATM), Cell Signaling (α-CREB, α-p38 and α-p-p38), and Santa Cruz Biotechnology (α-CK1α). The CK1 inhibitor D4476 (4-(4-(2,3-Dihydrobenzo[1,4]dioxin-6-yl)-5-pyridin-2-yl-1H-imidazol-2-yl)benzamide) and the CK2 inhibitor TBB (4,5,6, 7-Tetrabromo-benzotriazole) were used at 75 μM and 50 μM, respectively. The JNK inhibitor SP 600125 (10 μM) and p38-inhibitors SB203580 and SB202190 were used at a concentration of 10 μM. All inhibitors were purchased from EMD Biosciences and added 1 h prior to cell manipulation.
Transfections and immunoblotting
siRNA and plasmid transfections were performed using the calcium phosphate DNA precipitation procedure as previously described (13). The dominant-negative p38 plasmid harboring T180A/Y182F mutations was a kind gift from Dr. Zigang Dong (Hormel Institute, Austin, MN, USA). siRNA mixtures against p38α and CK1α were obtained from Dharmacon Inc. Cells were harvested 48 h after transfection and extracts prepared as described previously (13). Seventy-five micrograms of total protein was separated by 10% SDS-PAGE (sodium dodecyl sulphate-polyacrylamide gel electrophoresis) and transferred to Immobilon PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blocked in Tris- buffered saline (TBS) containing 0.2% Tween-20 (TBS-T) and 5% dried milk and incubated overnight at 4°C with the indicated primary antibodies diluted in blocking solution. After washing, the blots were incubated with HRP-conjugated sheep anti-mouse or goat anti-rabbit secondary antibodies (Jackson Lab, Bar Harbor, ME, USA) and developed using SuperSignal chemilluminescent substrate (Pierce, Rockford, IL, USA). Where indicated, band pixel intensities were determined using the Gelplot2 function of ScionImage (Scion Corp., Frederick, MD, USA).
Kinase assays
CK1 kinase assays were performed using purified, recombinant CK1δ enzyme (New England Biolabs, Beverly, MA, USA) according to the manufacturer’s conditions. Two micrograms of purified, 6X-histidine-tagged CREB (His-CREB) were used for each assay as described [12]. His-CREB was incubated with 0.2 μl (200 units) of CK1δ enzyme in the presence of 5 μCi [γ-32P]-ATP and 50 μM unlabeled ATP in a total reaction volume of 40 μl. Reactions were performed in the presence or absence of D4476 and SB 202190 inhibitors (prepared as 100X stock solutions in DMSO) for 30 min at 30°C and terminated by the addition of 4X SDS sample loading buffer as previously described (13). The reaction products were analyzed by SDS/PAGE and autoradiography. 32P-incorporation into substrate was measured using a Molecular Dynamics STORM phosphorimager.
Acknowledgments
This work was supported by grants from the American Cancer Society, the NIH (GM067868-02), and a Shaw Scientist Award to R.S.T from the Greater Milwaukee Foundation. NS was supported by an American Heart Association Predoctoral fellowship.
References
- 1.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 2.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 3.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Mittelstadt PR, Salvador JM, Fornace AJ, Jr, Ashwell JD. Activating p38 MAPK: new tricks for an old kinase. Cell Cycle. 2005;4:1189–1192. doi: 10.4161/cc.4.9.2043. [DOI] [PubMed] [Google Scholar]
- 5.Young PR, McLaughlin MM, Kumar S, Kassis S, Doyle ML, McNulty D, Gallagher TF, Fisher S, McDonnell PC, Carr SA, Huddleston MJ, Seibel G, Porter TG, Livi GP, Adams JL, Lee JC. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem. 1997;272:12116–12121. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]
- 6.Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta. 2007;1773:1341–1348. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, White RF, McVey MJ, Legos JJ, Erhardt JA, Nelson AH, Ohlstein EH, Hunter AJ, Ward K, Smith BR, Adams JL, Parsons AA. SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J Pharmacol Exp Ther. 2001;296:312–321. [PubMed] [Google Scholar]
- 8.Ward KW, Proksch JW, Gorycki PD, Yu CP, Ho MY, Bush BD, Levy MA, Smith BR. SB-242235, a selective inhibitor of p38 mitogen-activated protein kinase. II: in vitro and in vivo metabolism studies and pharmacokinetic extrapolation to man. Xenobiotica. 2002;32:235–250. doi: 10.1080/00498250110100711. [DOI] [PubMed] [Google Scholar]
- 9.Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stoter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 11.Loizou JI, El-Khamisy SF, Zlatanou A, Moore DJ, Chan DW, Qin J, Sarno S, Meggio F, Pinna LA, Caldecott KW. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell. 2004;117:17–28. doi: 10.1016/s0092-8674(04)00206-5. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Venkataraman SL, Dodson GE, Mabb AM, LeBlanc S, Tibbetts RS. Direct regulation of CREB transcriptional activity by ATM in response to genotoxic stress. Proc Natl Acad Sci USA. 2004;101:5898–5903. doi: 10.1073/pnas.0307718101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanware NP, Trinh AT, Williams LM, Tibbetts RS. Coregulated ataxia telangiectasia-mutated and casein kinase sites modulate cAMP-response element-binding protein-coactivator interactions in response to DNA damage. J Biol Chem. 2007;282:6283–6291. doi: 10.1074/jbc.M610674200. [DOI] [PubMed] [Google Scholar]
- 14.Stokoe D, Caudwell B, Cohen PT, Cohen P. The substrate specificity and structure of mitogen-activated protein (MAP) kinase-activated protein kinase-2. Biochem J. 1993;296:843–849. doi: 10.1042/bj2960843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato T, Jr, Delhase M, Hoffmann A, Karin M. CK2 Is a C-Terminal IkappaB Kinase Responsible for NF-kappaB Activation during the UV Response. Mol Cell. 2003;12:829–839. doi: 10.1016/s1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 16.Sayed M, Kim SO, Salh BS, Issinger OG, Pelech SL. Stress-induced activation of protein kinase CK2 by direct interaction with p38 mitogen-activated protein kinase. J Biol Chem. 2000;275:16569–16573. doi: 10.1074/jbc.M000312200. [DOI] [PubMed] [Google Scholar]
- 17.He Z, Cho YY, Liu G, Ma WY, Bode AM, Dong Z. p38 Mitogen-activated protein kinase regulation of JB6 Cl41 cell transformation promoted by epidermal growth factor. J Biol Chem. 2003;278:26435–26442. doi: 10.1074/jbc.M303859200. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Zhang Y, Bode AM, Ma WY, Dong Z. Phosphorylation of 4E-BP1 is mediated by the p38/MSK1 pathway in response to UVB irradiation. J Biol Chem. 2002;277:8810–8816. doi: 10.1074/jbc.M110477200. [DOI] [PubMed] [Google Scholar]
- 19.Godl K, Wissing J, Kurtenbach A, Habenberger P, Blencke S, Gutbrod H, Salassidis K, Stein-Gerlach M, Missio A, Cotten M, Daub H. An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc Natl Acad Sci USA. 2003;100:15434–15439. doi: 10.1073/pnas.2535024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MR, Dominguez C. MAP kinase p38 inhibitors: clinical results and an intimate look at their interactions with p38alpha protein. Curr Med Chem. 2005;12:2979–2994. doi: 10.2174/092986705774462914. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Dodson GE, Mukhopadhyay PS, Shanware NP, Trinh AT, Tibbetts RS. Identification of carboxyl-terminal MCM3 phosphorylation sites using polyreactive phosphospecific antibodies. J Biol Chem. 2007;282:9236–9243. doi: 10.1074/jbc.M609256200. [DOI] [PubMed] [Google Scholar]