Abstract
Resistance training (RT) is thought to be effective in preventing muscle depletion, whereas endurance training (ET) is known to improve exercise capacity and health-related quality of life (HRQoL) in chronic obstructive pulmonary disease (COPD). Our objectives were to assess the efficiency of combining RT with ET compared with ET alone. We identified eligible studies through a systematic multi-database search. One author checked titles and abstracts for relevance using broad inclusion criteria, whilst two independent authors checked the full-text copies for eligibility. Two authors independently extracted data, and we assessed the risk of bias and quality of evidence according to the Grading of Recommendations Assessment, Development and Evaluation guidelines. We included 11 randomized controlled trials (331 participants) and 2 previous systematic reviews. The meta-analyses showed equal improvements in HRQoL, walking distance and exercise capacity. However, we found moderate quality evidence of a significant increase in leg muscle strength favouring a combination of RT and ET (standardized mean difference of 0.69 (95% confidence interval: 0.39–0.98). In conclusion, we found significantly increased leg muscle strength favouring a combination of RT with ET compared with ET alone. Therefore, we recommend that RT should be incorporated in rehabilitation of COPD together with ET.
Keywords: Pulmonary disease, chronic obstructive, physical therapy modalities, review, systematic, exercise therapy, muscle depletion, practice guideline
Introduction
Skeletal muscle dysfunction is an important consequence of chronic obstructive pulmonary disease (COPD) and contributes to disease morbidity and possibly also mortality.1 To prevent muscle depletion, international guidelines recommend resistance training (RT) as part of pulmonary rehabilitation (PR) for COPD.2,3 PR in patients with COPD is known to improve exercise capacity, health-related quality of life (HRQoL)4 and reduce the number of days in hospital5 and is today a standard component of COPD treatment. Supervised endurance training (ET) that includes whole body exercise such as cycling and walking has traditionally been the main component of PR.4,6–8 Thus, a combination of RT and ET seems logical. Combined RT and ET (CT) versus ET alone has been reviewed previously where only small differences between these two training strategies were found. However, the two previous reviews did not systematically grade the quality of the evidence of the meta-analyses performed and did not investigate potential harms.9,10 Thus, clinicians may overestimate the potential implications for current practice, as it is generally accepted that meta-analyses are the highest level of evidence. This is also seen in the most recent guidelines, where the evidence regarding CT has achieved very high ratings.2 Further, the two previous reviews of CT were only based on four studies, and in recent years, several new studies have been published.9,10 The present updated systematic review was undertaken to produce a transparent translation of the current evidence for clinical recommendations based on the guidelines from the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group.11,12
Objectives
The aim of this study was to compare the effect of CT versus ET alone on patient-related outcomes (HRQoL, activities of daily living (ADLs), total mortality, adverse events and the degree of dyspnoea) and physiological outcomes (walking distance, muscle strength, lean body mass and exercise capacity) in patients with COPD. The final aim was to formulate evidence-based recommendations on exercise prescription in PR programmes.
Methods
Eligibility criteria
We pre-specified eligibility criteria using the population, intervention, comparison and outcomes (PICO) approach. We considered studies for this review if they compared the effect of CT (intervention) versus ET alone (comparison) as a part of a PR programme in patients with COPD (population), regardless of disease severity. Exercise protocols that used RT high load/low repetitions of both upper and lower extremities were eligible, as was the use of free weights, weight-lifting machines and use of own body weight. ET was also broadly defined but a main component of either moderate- to high-intensity continuous walking and/or ergometer cycling was considered adequate. Our primary outcomes were HRQoL, ADLs, total mortality, adverse events and the degree of dyspnoea measured with the Medical Research Council scale. Secondary outcomes were walking distance, muscle strength, lean body mass and exercise capacity (maximal oxygen uptake and exercise performance in watts). All outcomes were quantified immediately after the intervention and at the longest follow-up. Only randomized controlled trials (RCTs), systematic reviews and guidelines based on RCTs were considered for inclusion in this review.
Information sources
A research librarian performed a systematic literature search including the following databases: Medline, Embase, Physiotherapy Evidence Database, CINAHL, G-I-N international, NICE, National Guideline Clearinghouse, Surgical Implant Generation Network, Cochrane Library and OTseeker.
The search strategy is presented in Appendix 1.
Search strategy
First, we did a comprehensive search in July 2013 for COPD rehabilitation guidelines and systematic reviews, which yielded 2412 records. We then did a more detailed search in November 2013 for RCTs. This second search yielded 872 records. All records were screened for relevant titles or abstracts by one author.
Study selection
Guidelines and systematic reviews relevant to the topic were selected and assessed to justify a performance of a new systematic review. Full-text guidelines selected in the first search were appraised using the Appraisal of Guidelines for Research and Evaluation instrument version II13 (see Appendices 2 and 3). We only used the two relevant guidelines for a screening of reference lists, as no effect estimates were provided, and the methodology quality did not meet the standards proposed by GRADE.2,3,11 Relevant systematic reviews selected from the first search were assessed with A Measurement Tool to Asses Systematic Reviews by three review authors independently (see Appendix 4). Assessments were used for other PICOs as part of a larger Danish guideline.12 We included two systematic reviews.9,10 From the second search (for primary studies), two reviewers independently evaluated the full text of all potentially eligible papers and made a decision whether to include or exclude each study according to our pre-specified criteria following consensus.
Data collection process
Two reviewers independently extracted data from the full text of the included studies and recorded details about study design, interventions, patients and outcome measures in a predefined standardized Windows Excel 2010 spread sheet. Disagreements were solved through consensus.
Risk of bias in individual studies
Each included study was assessed using the Cochrane risk of bias tool.14 The risk of bias assessment was done independently by two reviewers following discussion and consensus.
Summary measures
We used mean differences (MDs) to calculate effects for continuous outcome data if the outcome measures were presented on the same scale. When pooling continuous outcome data measured on different scales, we used standardized mean differences (SMDs). We used random effects meta-analyses, as we expected variation in populations, duration of intervention and types of training between the included studies. The Review Manager Version 5.2 software was used for the statistical analyses and to produce forest plots.15
Synthesis of results
If the value (+/−) of the various scales used had different meaning, we inverted the value of one scale. We considered an I 2 score above 50% as indicating significant heterogeneity.
Risk of bias across studies
The quality of the evidence for each pre-specified outcome was assessed across the included studies as proposed by the GRADE Working Group using the GRADE Profiler Version 3.6 software.16 The evidence for each outcome was assessed according to the five GRADE criteria, namely, risk of bias (as assessed with the Cochrane risk of bias tool), inconsistency, indirectness, imprecision and risk of publication bias, by two review authors independently following consensus. If the quality of evidence was downgraded, we mention the reason in a footnote in Table 2.
Table 2.
Summary of findings.a
| Patient or population: Patients with COPD Settings: Inpatient and outpatient Intervention: Combined RT and ET (CT). Control: ET alone | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Illustrative comparative risksb (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | CT versus ET alone for COPD | |||||
| Quality of life-SGRQ Training duration: mean 12 weeks | The mean SGRQ in the intervention groups was 4.23 lower (17.22 lower to 8.75 higher) | 48 (2 Studies) | ⊕⊕⊖⊖ Low1,2,3 | |||
| Quality of life-CRQ Training duration: 8–12 weeks | The mean CRQ in the intervention groups was 0.16 SDs lower (0.35 lower to 0.03 higher) | 90 (3 Studies) | ⊕⊕⊕⊖ Moderate1 | Data from O’Shea et al.9 were re-meta-analysed to get overall result | ||
| Adverse events Training duration: 6–12 weeks | See comment | See comment | – | 101 (4 Studies) | ⊕⊕⊕⊖ Moderate4 | Possible risk of low back pain with intervention. |
| 6MWD Training duration: 3–12 weeks | The mean 6MWD in meters in the intervention groups was 13.29 lower (55.64 lower to 29.07 higher) | 146 (7 Studies) | ⊕⊖⊖⊖ Very low1,5,6 | |||
| VO2max Training duration: 8–12 weeks | The mean VO2max in the intervention groups was 0.07 SDs lower (0.47 lower to 0.33 higher) | 137 (5 Studies) | ⊕⊕⊕⊖ Moderate4 | SMD −0.07 (−0.47 to 0.33) | ||
| Max workload (watts) Training duration: 8–12 weeks | The mean max workload (watts) in the intervention groups was 0.38 higher (13.88 lower to 14.64 higher) | 137 (5 Studies) | ⊕⊖⊖⊖ Very low4,5,7 | |||
| Leg muscle strength Training duration: 8–12 weeks | The mean leg muscle strength in the intervention groups was 0.69 SDs higher (0.39–0.98 higher) | 194 (8 Studies) | ⊕⊕⊕⊖ Moderate1 | SMD 0.69 (0.39 to 0.98). | ||
GRADE Working Group grades of evidence
| ||||||
CRQ: Chronic Respiratory Questionnaire; CT: combined resistance and endurance training; ET: endurance training; GRADE: Grading of Recommendations Assessment, Development and Evaluation; COPD: chronic obstructive pulmonary disease; 6MWT: 6-minute walking test; SGRQ: St. George Respiratory Questionnaire; VO2max: maximal oxygen uptake; CI: confidence interval; RT: resistance training.
aCombined RT and ET versus ET alone for COPD.
bThe basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Additional analyses
Data on HRQoL from O’Shea et al.9 were reanalysed to get an overall result on the Chronic Respiratory Questionnaire (CRQ) and the quality of the evidence was assessed using the GRADE criteria.
Results
Study selection
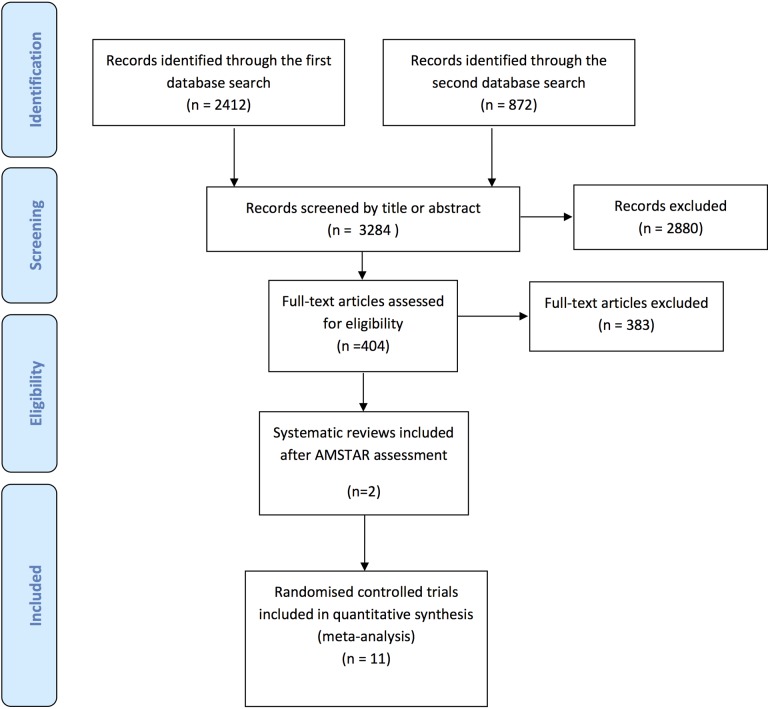
We identified 11 eligible primary studies (RCTs) for our analyses.17–27 These included a total of 331 randomized participants. Four of the 11 studies were included in two previous systematic reviews.9,10 Figure 1 shows the flow diagram for our selection process.
Figure 1.
Flow diagram of the selection process.
Study characteristics
Table 1 shows the characteristics of the included studies. Nine studies were conducted in an outpatient setting18,20–27 and in two studies during admission.17,19 The duration of the different training programmes varied. Although training duration in two of the studies was only 3 weeks19 and 6 weeks,27 respectively, the remaining nine studies were of 8–12 weeks duration.17,18,20–26 Training frequencies varied from two to three times a week. There was no reported difference in baseline characteristics of patients between groups in all but one study, where patients in the CT group were significantly older compared with the ET group.22
Table 1.
Characteristics of included studies.
| Citation | Country | Study design | Setting, duration and frequency | Participants | Intervention | Control | Notes | Outcomes | Scales | Dropouts |
|---|---|---|---|---|---|---|---|---|---|---|
| Ortega et al.24 | Spain | RCT | Setting: outpatient, duration: 12 weeks FU: 12 weeks and frequency: 3 times a week | 54 Patients with COPD (mean age: 64 years, 85% males, FEV1: 70% of predicted) | ET: 20 minutes of cycling 70% of W peak + RT: two series of five weight-lifting procedures 70–85% of 1RM, 6–8 repetitions | ET: 40 minutes of cycle 70% of W peak | 3 Armed study, 36 patients randomized to our intervention and control groups | Adverse events, HRQoL, walking test, muscle strength and C-P exercise test | CRQ, SWT, leg extension 1RM in kilogram, VO2max in L/minute, watts | 6 Dropouts (4 in CT group) |
| Dourado et al.17 | Brazil | RCT | Setting: inpatient, duration: 12 weeks and frequency: 3 times a week | 51 Patients with COPD (mean age: 63 years, 65% males, FEV1: 58% of predicted) | ET: 30 minutes of walking and low intensity strength + RT: 30 minutes of two series of 8 repetitions at 50–80% of 1RM | ET: low intensity general training consisting of 30 minutes walking and 30 minutes of low-intensity general training | 3 Armed study, 33 patients randomized to our intervention and control group | HRQoL, walking test, muscle strength | SGRQ, 6MWT, leg extension and leg press 1RM in kg | 9 Dropouts (3 in CT group) |
| Vonbank et al.18 | Austria | RCT | Setting: outpatient, duration: 12 weeks and frequency: 2 times a week | 43 Patients with COPD (mean age: 60 years, 56% males, FEV1: 56% of predicted) | ET: cycle ergometer training of increasing intensity + RT: two to four series of 8 strength exercises, 8–15 repetitions until severe fatigue. | ET: 1 hour of cycle training of increasing intensity and time, 60% of VO2max | 3 Armed study, 24 patients randomized to our intervention and control group | HRQoL, muscle strength and C-P exercise test | SGRQ, VO2 max in mL/kg/minute, watts | 7 Dropouts (not reported in which group) |
| Bernard et al.21 | Canada | RCT | Setting: outpatient, duration: 12 weeks, and frequency: 3 times a week. | 45 Patients with COPD (mean age: 66 years, 71% males, mean FEV1: 42% of predicted) | ET: 45 minutes on ergometer cycle at 80% of W peak rate+ RT: 45 minutes of 4 weight-lifting exercises, 8–10 repetitions at 60% of 1RM increasing to 80% | ET: 45 minutes on ergometer cycle at 80% of W peak rate + 45 minutes of relaxation and breathing exercises | Adverse events, HRQoL, walking test, muscle strength and C-P exercise test | CRQ, 6MWT, strength of quadriceps in kg, VO2max in L/minute, Wmax in watts | 9 Dropouts (5 in CT group) | |
| Mador et al.22 | United States | RCT | Setting: outpatient, duration: 8 weeks, 24 sessions and frequency: 3 times a week | 32 Patients with COPD (mean age 68 and 74 years, mean FEV1: 42% of predicted) | ET: cycle ergometer training adjusted to level of dyspnoea by increasing intensity + RT: four different strength exercises, increasing from 1 to 3 series of 10 repetitions at 60% of 1RM. | ET: cycle ergometer training adjusted to level of dyspnoea by increasing intensity | Patients in intervention group were significantly older, no information on gender | HRQoL, walking test, C-P exercise test, muscle strength | CRQ, 6MWT, W max in watts, VO2max in L/minute, quadriceps strengths in kilogram | 4 Dropout in each group |
| Nakamura et al.23 | Japan | RCT | Setting: outpatient, duration: 12 weeks And frequency: 3 times a week | 42 Patients with COPD (mean age: 68–69 years, mean FEV1: 53% of predicted) | ET: 20 minutes of walking at 3–5 on Borg scale + RT: 30 minutes of seven strength exercises using self-weight or elastic bands, 3 sets of 10 repetitions, no progression | ET: 20 minutes of walking at Borg 3–5 + 30 minutes of recreational activities of balance, agility and coordination | 3 Armed study, 28 patients randomized to our intervention and control group | HRQoL, walking test and muscle strength | SF 36, 6MWT, VO2 max in mL/kg/minute, Wmax in watts, grip strength in kilogram | 5 Dropouts (4 in CT group) |
| Panton et al.25 | United States | RCT | Setting: outpatient, duration: 12 weeks And frequency: 2 times a week | 18 Patients with COPD (age between 50 and 72 years, mean FEV1: 40% of predicted) | ET: 60 minutes of chair aerobic, cycling and walking at intensity of 50–70% + RT: 45–60 minutes of 12 strength exercises, 3 sets of 12 repetitions, progressive increasing weight | ET: 60 minutes of chair aerobic, cycling and walking at intensity of 50–70% | Adverse events, ADLs, walking test, muscle strength and lean body mass | Time per ADLs 12MWT, Leg extension in NM, repetition, BMI | 1 Dropout in ET group | |
| Phillips et al.26 | United States | RCT | Setting: outpatient, duration: 8 weeks and frequency: 2 times a week | 22 Patients with COPD (mean age: 70 years, mean FEV1: 32% (CT) and 42% (ET) of predicted) | ET: 20–40 minutes of cycling (arms and legs) and walking exercises at METS 3 and low-intensity to high-repetition RT + RT: five strength exercises at 50% of 1RM, progressive increasing weight | ET: 20–40 minutes of cycling (arms and legs) and walking exercises at METS 3 and low-intensity to high-repetition RT | More males (6) in CT group than control group (1) | Adverse events, walking test, muscle strength | 6MWT, leg press in lb | 3 Dropouts (unclear in which group) |
| Ries et al.27 | United States | RCT | Setting: outpatient, duration: 6 weeks, frequency: unclear | 45 Patients with COPD (no baseline characteristics) | ET: PR programme activities including walking training and 15–30 minutes of arm cycling + RT: four strength exercises, 3 sets of 4–10 repetitions, progressive increasing weight | ET: PR programme activities including walking training | 3 Armed study, 20 patients completed 9 in CT group and 11 in ET group | Adverse events, ADLs, C-P exercise test | ADLs test in seconds, W max in watts, (VO2max investigated but data not reported) | 17 Dropouts (unclear in which group) |
| Würtemberger and Bastian19 | Germany | RCT | Setting: inpatient, duration: 3 weeks and frequency: 3 times per week | 69 Patients with COPD (mean age: 61–65 years, FEV1 range: 30–62% of predicted, male: 64%) 10 patients in CT group and 12 in ET group with supplemental oxygen | ET: 20 minutes sessions on a calibrated ergo cycle, intensity of 70% of W max + RT: include 5 strength exercises of muscle major groups, 2–4 series of 20–25 repetitions, intensity of 40% of W max | ET: 20 minutes sessions on a calibrated ergo cycle, intensity of 70% of W max | 3 Armed study, 46 patients randomized to our intervention and control groups | Walking test and ADLs | 6MWT and ADLs in time | No Dropouts reported |
| Alexander et al.20 | United States | RCT | Setting: outpatient, duration: 8–10 weeks and frequency: 16 sessions total | 27 Patients with COPD (mean age: 65–73 years, mean FEV1: 30–39% of predicted) | ET: 20–40 minutes of cycling (arms and legs) and treadmill walking intensity adjusted individually + RT: 5 strength exercises of major muscle groups, 1 set of 12 repetitions at 50% of 1RM and progressive increasing weight | ET: 20–40 minutes of cycling (arms and legs) and treadmill walking, intensity adjusted individually + low-intensity upper extremity strength training | ADLs, muscle strength and walking test | 6MWT, senior fitness test and seated leg press in lb | 7 Dropouts (5 in CT group) |
ADLs: activities of daily living; C-P: cardio-pulmonal; COPD: chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Questionnaire; CT: combined resistance and endurance training; ET: endurance training; FEV1: forced expiratory volume in 1 second; FU: follow-up; HRQoL: health-related quality of life; RCT: randomized controlled trial; 1RM: one repetition maximum; RT: resistance training; SGRQ: St George Respiratory Questionnaire; SWT: shuttle walk test; VO2max: maximal oxygen uptake; W peak: peak workload in watts; W max: maximal workload in watts; 6MWT: 6-minute walking test; 12MWT: 12-minute walking test; PR: pulmonary rehabilitation.
RT was performed on weight-lifting machines, with free weights, or by calisthenics and included both the upper and lower body musculature. In most of the included studies, the work load was increased over time, either by adjusting the work load to the one repetition maximum load or by a predetermined increment.17–22,24–27 In one study, no progression in work load was reported.23 ET was performed as ergometer cycling, as treadmill walking or a combination, and in one study with low-intensity upper extremity strength training. Intensity of ET was determined as the percentage of maximum exercise capacity, as self-determined intensity or by adjustment according to the level of dyspnoea/heart rate during exercise.
Risk of bias within studies
Figure 2 shows risk of bias of the included studies. None of the 11 included randomized studies were double-blinded, as the participants were impossible to blind to the training intervention. In one study, the outcome assessor was blinded.22 Allocation concealment and the randomization method were not described in eight studies.17–20,23,24,26,27 One study used block randomization for allocation concealment but used toss of a coin for the randomization procedure.21 Mador et al. used opaque, sealed envelopes for allocation concealment but the sequence generation was not described.22 Panton et al. ascribed patients to the control group due to time restraints.25 Four studies were assessed as having a high risk of incomplete outcome data reporting due to a large or uneven dropout.17,20,23,27 In only one study, we detected selective reporting of outcome measures, as the investigators measured maximal oxygen uptake but did not report the results.27 Three studies had other bias, as differences in baseline characteristics19,20 or patient cross over after randomization.26 Thus, the quality of evidence from all studies included was downgraded due to risk of bias (Table 2).
Figure 2.
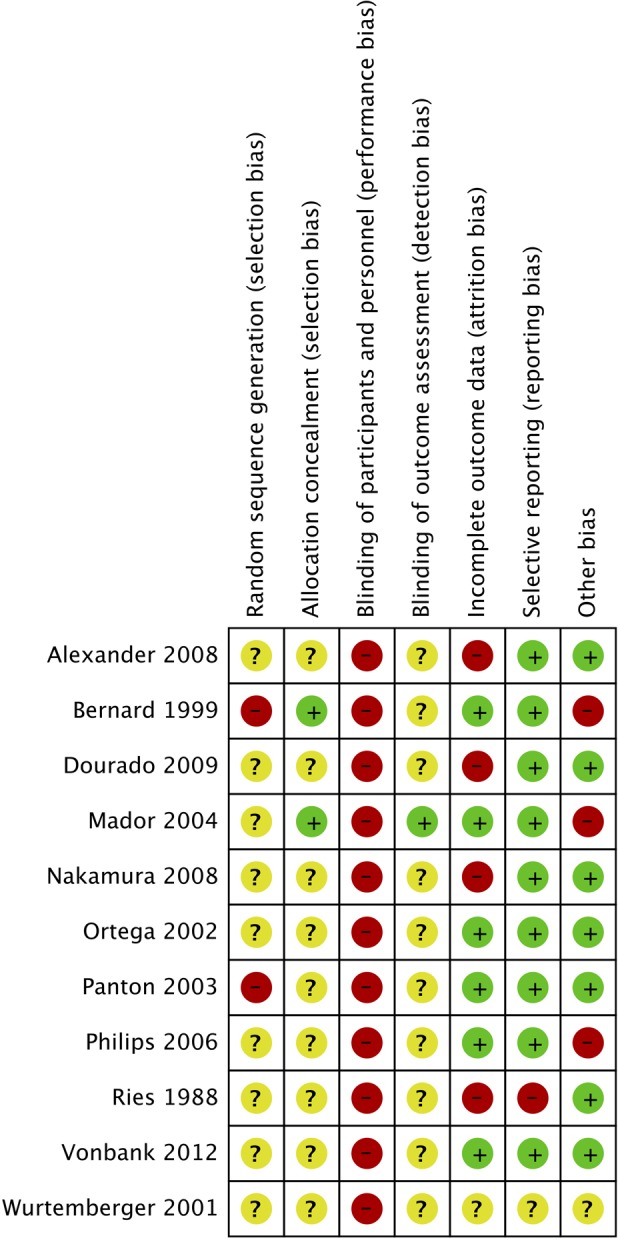
Risk of bias.
Effects of the intervention
Only results recorded immediately after the intervention were analysed, as only one trial presented results after extended follow-up.24 Thus, we could not assess the long-term effect of CT compared to ET.
Health-related quality of life
HRQoL was investigated in six (198 participants) of the included studies.17–18,21–24 Three studies included in an earlier review9 showed a small trend favouring ET alone but found no statistically significant difference (Table 2). In two trials, HRQoL was measured on the St George Respiratory Questionnaire (SGRQ) scale.17,18 We found no significant difference in HRQoL in the trials using the SGRQ scale, MD of −4.23 (95% confidence interval (CI): −17.22 to 8.75). The quality of the evidence was downgraded due to imprecision, as indicated by a wide CI and the small number of participants (Table 2). Nakamura et al. showed HRQoL improvements in both groups but no significant difference between the two groups. These results could not be pooled in the SGRQ or the CRQ meta-analyses, as a different questionnaire was used.23
Activities of daily living
Time to finish a test set of ADLs after the intervention was investigated in two trials (73 participants) but no difference between groups was found.19,20 As different ADLs tests were used, data was deemed incomparable and, thus, not meta-analysed.
Adverse events
Four studies reported on adverse events (105 participants).21,25–27 In two trials, the authors stated that there were no adverse events related to the training programme,19,25 whereas two trials reported a total of two cases with back pain possible due to CT and one case of hip pain possible due to ET.26,27 Results were not meta-analysed.
Total mortality and dyspnoea
None of the included studies had investigated the effect on neither the level of dyspnoea nor the mortality.
Walking distance
Walking distance was evaluated in nine of the included studies (287 participants).17,19–26 Data from seven trials were pooled, as all these trials used the 6-minute walk test (6MWT). Our meta-analysis did not show statistically or clinically significant difference between CT and ET (Figure 3), MD of −7.77 meters (95% CI: −43.93 to 28.40). Ortega et al. found no significant difference between groups using the shuttle walk test.24 Panton et al. used the 12-minute walk test and found a statistically significant difference (p < 0.05) between groups favouring CT.25 However, these two trials were not included in the meta-analysis because we assessed that data could not be directly compared with the 6MWT. The quality of the evidence from the meta-analysis of this outcome was downgraded due to imprecision and inconsistency (Table 2).
Figure 3.
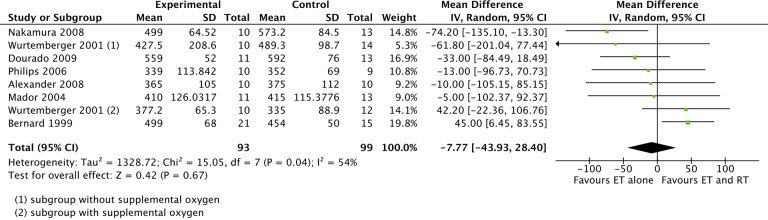
The effect of RT and ET compared with ET alone. Outcome: walking distance using the 6-minute walking test. RT: resistance training; ET: endurance training; 6MWT: 6-minute walk test.
Maximal oxygen uptake
The change in the maximal oxygen uptake was measured in five trials (165 participants).18,21–24 All tests were described as cardiopulmonary exercise tests and used analyses of exhaled gas. We included all results of peak oxygen uptake and maximal oxygen uptake and pooled data using SMD. We found no difference between the two groups, SMD −0.07 (95% CI: −0.47 to 0.33) (Table 2).
Maximal work load
Five trials (165 participants) measured the maximal work load in watts.18,21–24 The pooled results showed no difference between the compared training modalities, SMD 0.38 (95% CI: −13.88 to 14.64). There was some heterogeneity between the study results (I 2 of 61%), and the point estimates showed effects in opposite directions. Thus, we downgraded the quality of the evidence due to both inconsistency and imprecision (Table 2).
Muscle strength
Nine trials (265 participants) performed muscle strength measurements of various muscle groups.17–18,20–26 Eight trials presented results of one or more measurements of muscle strength in the lower extremities.17–18,20–22,24–26 We found test results of leg press and leg extension comparable and pooled these data in a common leg strength category using SMD. This leg strength category represented our pre-specified secondary outcome of muscle strength. The pooled analysis showed a statistically significant increased muscle strength in the CT group compared with ET (SMD of 0.69 (95% CI: 0.39–0.98); Figure 4). Results were consistent across trials and no heterogeneity was seen (I 2 = 0%; Table 2). Nakamura et al. found an increase in muscle strength after CT compared with ET, although not statistically significant.23 However, results were not included in our meta-analysis, as this trial used handgrip strength and the remaining trials used leg strength.
Figure 4.
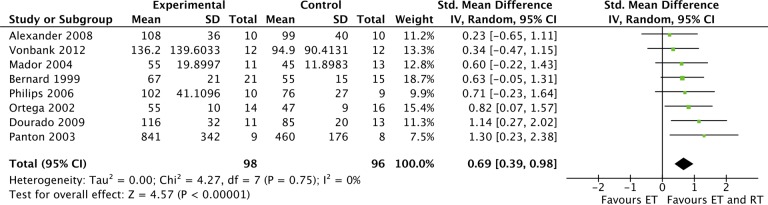
The effect of RT and ET compared to ET alone. Outcome: leg muscle strength. RT: resistance training; ET: endurance training.
Discussion
Summary of main findings
Based on the eligible 11 randomized trials (331 participants), we found no significant differences in our primary outcome measures between CT compared with ET but observed moderate quality evidence supporting a significant improvement in leg muscle strength favouring CT.
Primary outcomes
QoL is probably increased by physical activity and is not likely to be influenced by the type of exercise training. Based on the results from the included review,9 where we reanalysed data and assessed the quality of evidence according to the GRADE criteria and by pooling of recorded data from the newer trials, we found no difference in HRQoL between CT and ET. However, this was an uncertain finding due to risk of bias and imprecision and future research could change the effect estimate.
If discomfort is experienced as a direct consequence of the exercise training, adherence to a PR programme may be reduced and high-intensity resistance or ET may not be tolerated by all patients. However, training intensities should be high enough to achieve the beneficial physiological adaptations.28 In many of the included studies, we observed high dropout rate that allows for unknown harms of the different training modalities. In two trials, participants in the CT group reported lower back pain, possibly due to the training programme, but the majority of studies did not report any adverse events. We would however not expect major adverse events as a consequence of exercise training if the type of training and level intensity is adjusted individually.
Secondary outcomes
We found equal improvements in walking distance, maximal work load and maximal oxygen uptake but no differences between the two groups. However, we found moderate quality evidence showing a significantly increased leg muscle strength favouring CT compared with ET. The leg muscle dysfunction contributes to COPD morbidity, and reduced quadriceps strength has been shown to be a significant predictor of mortality in COPD, independent of lung function impairment. Exercise training is thought to be an effective countermeasure of muscle dysfunction.1,29 Our results show that prescribing exercise training with a component of both RT and ET seems beneficial.
Exploring the optimal amount of training and the dose–response relation of RT was not a part of the scope in this study. Table 1 describes the quite heterogeneous exercise protocols used but, overall, the RT in the included studies was in alignment with current recommendations.1,20,30 As the dosage of ET could have been higher in the CT group, and as some patients in the control group did RT at low intensities, we have downgraded the evidence accordingly. A previous review suggested that the longer duration of rehabilitation has a greater effect on improvement in walking distance31 and this could explain the heterogeneity in our results.
The recommendations regarding the intensity and the duration of RT for patients with COPD are mainly based on consensus statements and findings from studies including healthy elderly,1,28,30 and the minimal clinically important difference for muscle strength is, to our knowledge, not established. Therefore, we suggest that muscle strength should therefore routinely be assessed in COPD patients enrolled in PR in order to ensure efficient individualized RT.
Limitations
An important limitation was that the quality of evidence from all studies included in the present review was downgraded due to risk of bias according to the GRADE guidelines. This was done mainly because of unknown randomization and sequence generation methods but also due to the nature of training, as the participants were impossible to blind. An additional limitation was the high dropout rate in many of the included studies, which allows for unknown effects and harms.
Conclusions
The results of this updated systematic review of 11 randomized trials show that CT compared with ET is equally effective in improving QoL and exercise capacity in COPD. However, our results show that the addition of RT to ET is superior with regard to improving leg muscle strength. Skeletal muscle dysfunction influences the clinical outcome of COPD, and it is likely that RT plays an important role in the prevention and treatment of this co-morbid condition. Therefore, we make a weak recommendation of routine prescription of a combination of resistance and ET in COPD rehabilitation. Although we recommend that health-care providers include patient preferences and clinical assessments of muscle dysfunction in the clinical decision-making when offering physical training, future prospective studies should allow for a better understanding of the long-term effects of the different training modalities in COPD and we call upon future research that explore the mechanisms involved in the beneficial effects of exercise training.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The Centre of Inflammation and Metabolism (CIM) is supported by a grant from the Danish National Research Foundation (DNRF55). The Centre for Physical Activity Research (CFAS) is supported by a grant from TrygFonden. This study was further supported by the Danish Health and Medicine Authority, the Axel Muusfeldts Foundation, the Capital Region of Denmark and the Novo Nordisk Foundation. CIM is a member of DD2 – the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant no. 09-067009 and 09-075724).
Footnotes
Authors’ Note: UWI, KJJ, TR, HH, CS and PL developed the study design. CS conducted the multi-base search. UWI screened titles and abstracts. UWI and KJJ assessed eligible full-text articles and performed the data extraction, the risk of bias assessment and the meta-analysis. UWI, KJJ, TR, HH and PL contributed to the interpretation of data. UWI drafted the manuscript. KJJ, TR, HH, CS and PL revised the manuscript critically and approved the final version. All authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest: UWI, CS and KJJ declared no conflicts of interest. TR received fee for speaking from AstraZeneca, Boehringer Ingelheim and Novartis and received fee for consulting from Boehringer Ingelheim, GlaxoSmithCline and Pfizer. PL received research grants from Almirall, Boehringer Ingelheim, GlaxoSmithKline, Novartis and Pfizer; received fee for speaking from Almirall, AstraZeneca, Boehringer Ingelheim, GlaxoSmithCline, Norpharma, Novartis, Nycomed, Pfizer and Sandoz; and received fee for consulting from Almirall, AstraZeneca, Boehringer Ingelheim, GlaxoSmithCline, Mundipharma, Novartis, Nycomed, Sandoz and Pfizer.
Funding: This study was funded by the Danish Health and Medicine Authority and by Centre of Inflammation and Metabolism/Centre of Physical Activity Research, University of Copenhagen, Rigshospitalet.
Supplemental material: The supplementary material is available at http://crd.sagepub.com/supplemental.
References
- 1. Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society Statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2014; 189(9): e15–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bolton CE, Bevan-Smith EF, Blakey JD, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013; 68(Suppl 2): ii1–30. [DOI] [PubMed] [Google Scholar]
- 3. Marciniuk DD, Brooks D, Butcher S, et al. Optimizing pulmonary rehabilitation in chronic obstructive pulmonary disease – practical issues: a Canadian thoracic society clinical practice guideline. Can Respir J 2010; 17(4): 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; (4): CD003793. [DOI] [PubMed] [Google Scholar]
- 5. Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000; 355(9201): 362–368. [DOI] [PubMed] [Google Scholar]
- 6. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of COPD, http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html (2014, accessed 2 September 2014).
- 7. National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update), http://www.nice.org.uk/nicemedia/live/13029/49397/49397.pdf (2010, accessed 2 September 2014).
- 8. COPD Working Group. Pulmonary rehabilitation for patients with chronic pulmonary disease (COPD): an evidence based analysis. Ont Health Technol Assess Ser [Internet] 2012; 12(6): 1–75, www.hqontario.ca/en/mas/tech/pdfs/2012/rev_Pulmomary_Rehab_March.pdf (2012, accessed 2 November 2014). [PMC free article] [PubMed] [Google Scholar]
- 9. O’Shea SD, Taylor NF, Paratz JD. Progressive resistance exercise improves muscle strength and may improve elements of performance of daily activities for people with COPD: a systematic review. Chest 2009; 136(5): 1269–1283. [DOI] [PubMed] [Google Scholar]
- 10. Puhan MA, Schünemann HJ, Frey M, et al. How should COPD patients exercise during respiratory rehabilitation? Comparison of exercise modalities and intensities to treat skeletal muscle dysfunction. Thorax 2005; 60(5): 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The grading of recommendations assessment, development and evaluation (GRADE) Working Group. Available at: http://www.gradeworkinggroup.org (accessed 2 April 2014).
- 12. The Danish Health and Medicine Authority. National Clinical Guidelines. Available at: https://sundhedsstyrelsen.dk/da/sundhed/kvalitet-og-retningslinjer/nationale-kliniske-retningslinjer (accessed 1 April 2014).
- 13. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010; 14: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JPT, Green S. (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The cochrane collaboration, www.cochrane-handbook.org (2011, accessed 1 April 2014).
- 15. Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012. [Google Scholar]
- 16. GRADEpro [Computer program]. Version 3.2 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann 2008.
- 17. Dourado VZ, Tanni SE, Antunes LC, et al. Effect of three exercise programs on patients with chronic obstructive pulmonary disease. Braz J Med Biol Res 2009; 42(3): 263–271. [DOI] [PubMed] [Google Scholar]
- 18. Vonbank K, Strasser B, Mondrzyk J, et al. Strength training increases maximum working capacity in patients with COPD – randomized clinical trial comparing three training modalities. Respir Med 2012; 106(4): 557–563. [DOI] [PubMed] [Google Scholar]
- 19. Würtemberger G, Bastian K. Functional effects of different training in patients with COPD. Pneumologie 2001; 55(12): 553–562. [DOI] [PubMed] [Google Scholar]
- 20. Alexander JL, Phillips WT, Wagner CL. The effect of strength training on functional fitness in older patients with chronic lung disease enrolled in pulmonary rehabilitation. Rehabil Nurs 2008; 33(3): 91–97. [DOI] [PubMed] [Google Scholar]
- 21. Bernard S, Whittom F, Leblanc P, et al. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159(3): 896–901. [DOI] [PubMed] [Google Scholar]
- 22. Mador MJ, Bozkanat E, Aggarwal A, et al. Endurance and strength training in patients with COPD. Chest 2004; 125(6): 2036–2045. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura Y, Tanaka K, Shigematsu R, et al. Effects of aerobic training and recreational activities in patients with chronic obstructive pulmonary disease. Int J Rehabil Res 2008; 31(4): 275–283. [DOI] [PubMed] [Google Scholar]
- 24. Ortega F, Toral J, Cejudo P, et al. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 166(5): 669–674. [DOI] [PubMed] [Google Scholar]
- 25. Panton LB, Golden J, Broeder CE, et al. The effects of resistance training on functional outcomes in patients with chronic obstructive pulmonary disease. Eur J Appl Physiol 2004; 91(4): 443–449. [DOI] [PubMed] [Google Scholar]
- 26. Phillips WT, Benton MJ, Wagner CL, et al. The effect of single set resistance training on strength and functional fitness in pulmonary rehabilitation patients. J Cardiopulm Rehabil 2006; 26(5): 330–337. [DOI] [PubMed] [Google Scholar]
- 27. Ries AL, Ellis B, Hawkins RW. Upper extremity exercise training in chronic obstructive pulmonary disease. Chest 1988; 93(4): 688–692. [DOI] [PubMed] [Google Scholar]
- 28. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of sports medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009; 41(7): 1510–1530. [DOI] [PubMed] [Google Scholar]
- 29. Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007; 62(2): 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188(8): e13–e64. [DOI] [PubMed] [Google Scholar]
- 31. Troosters T, Casaburi R, Gosselink R, et al. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172(1): 19–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.