Abstract
Guillain-Barré syndrome (GBS) is a postinfectious autoimmune neuropathy and anti-ganglioside antibodies (Abs) are strongly associated with this disorder. Several studies have implied that specific anti-ganglioside Abs induce neuropathy in patients with axonal forms of GBS. To study the mechanisms of anti-ganglioside Abs-induced neuropathy, we established a new passive transfer mouse model by L5 spinal nerve transection (L5SNT; modified Chung's model) and systemic administration of anti-ganglioside Abs. L5SNT causes degeneration of a small proportion of fibers that constitute sciatic nerve and its branches, but importantly breaks the blood–nerve barrier, which allows access to circulating Abs and inflammatory cells. Our studies indicate that, in this mouse model, anti-ganglioside Abs induce sequential nodal and axonal injury of intact myelinated nerve fibers, recapitulating pathologic features of human disease. Notably, our results showed that immune complex formation and the activating Fc gamma receptors (FcγRs) were involved in the anti-ganglioside Abs-mediated nodal and axonal injury in this model. These studies provide new evidence that the activating FcγRs-mediated inflammation plays a critical role in anti-ganglioside Abs-induced neuropathy (injury to intact nerve fibers) in GBS.
Keywords: anti-ganglioside antibody, blood–nerve barrier, Fc gamma receptors, Guillain-Barré syndrome, macrophage, nodal and axonal injury
Introduction
Guillain-Barré syndrome (GBS) is an autoimmune neuropathy that is the most common cause of acute flaccid paralysis worldwide. Anti-ganglioside/glycan antibodies (Abs) are the most commonly recognized autoimmune effectors in this disorder and are strongly associated with the axonal forms of GBS (Hughes et al., 1999; Yuki et al., 2001; Willison and Yuki, 2002; Yuki et al., 2004; Hughes and Cornblath, 2005). Experimental and clinical evidence support the pathogenic role of anti-ganglioside Abs in GBS, however, thus far there is lack of reliable passive transfer animal models in which anti-glycan Abs induce injury to the intact myelinated fibers in proximal nerve trunks of experimental animals (Sheikh and Griffin, 2001; Sheikh and Zhang, 2010). Integrity of blood–nerve barrier (BNB) is considered a critical determinant of this failure because it limits the access of circulating Abs and hematogenous inflammatory cells such as macrophages to the nerves to set up inflammation (Pollard et al., 1995; Spies et al., 1995a; Spies et al., 1995b). Availability of a reproducible model would allow dissection of pathogenetic mechanisms involved in nodal and axonal injury seen in axonal forms of GBS.
In axonal GBS, the earliest and mildest change consists of lengthening of the nodes of Ranvier and many nodes have overlying macrophages. Sequentially, macrophages enter the periaxonal space and then advance into the internodal periaxonal space, where they typically lie adjacent to axons. Finally, macrophages extend phagocytic processes around the axon that leads to axonal degeneration (Griffin et al., 1995; Griffin et al., 1996a; Griffin et al., 1996b; Hafer-Macko et al., 1996). It remains unclear whether macrophages interact with immune complexes, formed by anti-glycan Ab binding to gangliosides on nodal and internodal axolemma and, if so, which molecules on macrophages are involved in this interaction.
There are no systematic studies examining the role of Fc-gamma receptors (FcγRs) and macrophages in anti-ganglioside Ab-mediated nodal and axonal injury. The role of complement has been evaluated in previous studies examining the effects of anti-ganglioside Abs on mouse neuromuscular junctions (NMJs) (Plomp et al., 1999; O'Hanlon et al., 2001). These experimental data suggest that motor nerve terminal dysfunction might in part account for motor weakness in axonal forms of GBS. Although the impairment of presynaptic NMJs is experimentally compelling, the clinical involvement of this phenomenon in GBS is less clear because anti-ganglioside Abs can be internalized at the motor nerve terminals to prevent injury at this site (Fewou et al., 2012). Notably, anti-ganglioside Abs cannot be internalized at the nodes of Ranvier (Fewou et al., 2012), which are the primary sites of injury along myelinated fibers in patients with axonal GBS (Griffin et al., 1996b).
Here, we report a new passive transfer model in which BNB is modulated by partial nerve injury and in this model passive transfer of anti-glycan Abs induce sequential injury to nodes of Ranvier and axons, mimicking pathology seen in axonal GBS. Breach of BNB and resultant inflammatory milieu in the injured nerves are prerequisite for anti-glycan Ab-mediated nerve injury in this model. Notably, this experimental neuropathy is dependent on inflammation produced via activating FcγRs and that circulating macrophages are the dominant activating FcγR-expressing cell type that mediates anti-ganglioside Ab-induced nerve injury.
Materials and Methods
Mice.
Adult (8–12 weeks old) female wild-type C57BL/6 and various transgenic/mutant mice (Table 1), including DBA/2J, Fcer1 g-null, Fcgr2b-null, B4galnt1-null, and osteopetrotic (op/op) mice, were used in the study. B4galnt1-null mice were bred in house and all other strains used in the study were from The Jackson Laboratory. All experimental procedures complied with institutional and governmental guidelines for animal research and use of human serum and were approved by the institutional Animal Care and Use Committee and Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston.
Table 1.
Transgenic/mutant mice used in the studies
| Strain name | Strain description |
|---|---|
| Fcer1g-null | Lack all activating but express inhibitory FcγRIIB |
| Fcgr2b-null | Lack inhibitory FcγRIIB but express all activating FcγRs |
| Op/op mice | Devoid of colony stimulating factor 1; are macrophage and microglia deficient |
| DBA mice | Deficient in complement component C5 |
| B4galnt1-null | Lack beta1,4-N-acetylgalactos-aminyltransferase (GM2/GD2 synthase); express only simple gangliosides GM3 and GD3, but not complex gangliosides GM1, GD1a, GD1b, or GT1b |
FcγRs = Fcγ receptors.
Monoclonal antibodies.
Four different anti-ganglioside monoclonal antibodies (mAbs), including GT1b-2b, GM1–2b, GD1a/GT1b-2b, and GD1a-2b, were used in the study. These anti-ganglioside mAbs are designated by their ganglioside specificity and IgG isotype (1, 2a, or 2b); for example, GT1b-2b refers to an mAb with GT1b specificity and IgG2b isotype. GT1b-2b with high affinity/avidity was used extensively in our studies. The generation, specificity, production, and purification of these anti-ganglioside Abs were reported previously (Lunn et al., 2000; Schnaar et al., 2002). Either hollow fiber supernatants or purified anti-ganglioside Abs were used. An irrelevant mouse IgG-2b mAb (Abcam) was used as a negative control.
Human serum.
Serum from one patient with acute motor and sensory axonal neuropathy variant of GBS with high titers of IgG anti-GM1 IgGs (1:10,000; determined by ganglioside ELISA) were collected during the acute phase of the disease (Lopez et al., 2010). Serum was later dialyzed against PBS to remove anticoagulants, filtered, and stored at −80°C until use. Serum from a normal healthy human volunteer without anti-ganglioside reactivity was used as a control.
L5 spinal nerve transection mice model.
The L5 spinal nerve transection (L5SNT) model was generated by transecting the left L5 spinal nerve of wild-type and various mutant mice. Briefly, under anesthetization, the left L5 spinal nerve was exposed and transected with removal of a 1–2 mm segment of nerve to prevent reconnection during the study period. Uninjured right sciatic nerve system served as a control. Some studies were done without Ab administration to characterize the model itself. For passive transfer, mice were administered 3 doses of 1–2 mg of anti-glycan mAbs or control Abs intraperitoneally on days 1, 3, and 7 after surgery. Behavioral, electrophysiological, morphological, and immunohistochemical studies were performed as described previously (Lehmann et al., 2007; Zhang et al., 2014).
In addition to the anti-ganglioside mAbs, a GBS patient serum containing high titers of anti-GM1 antibodies was studied in wild-type mice in this model. The wild-type animals received daily intraperitoneal injection (1 ml/dose) of either GBS or control serum for 5 d/week for 2 weeks. The animals injected with human sera were pretreated with 100 mg/kg cyclophosphamide 2 d before the L5SNT to minimize the immune response (serum sickness) to human proteins, as described previously (Toyka et al., 1977).
Behavioral test.
Mechanical hyperalgesia of the hindpaw was assessed by measuring the paw withdrawal frequency of each mouse with 10 repetitive stimuli using von Frey filaments (VF #3.61, 0.4 g, and VF #3.22, 0.16 g; North Coast Medical) before surgery and every other day after surgery until termination of the experiments, as described previously (Lee et al., 2012).
Electrophysiology.
The electrophysiology studies (sciatic nerve conductions) were performed as described previously (Lehmann et al., 2007). Briefly, mice were anesthetized and placed on a heating pad to maintain body temperature at 37°C. The sciatic nerve was stimulated with needle electrode at the sciatic notch, and compound muscle action potential (CMAP) amplitude was recorded in the tibial innervated muscles (sole/flexor compartment) of the hindpaws at baseline (before administering Abs) and on postsurgical days 1, 3, 7, and 14 with a PowerLab signal acquisition setup (AD Instruments).
Morphometry and immunocytochemistry.
Animal tissues were harvested at the indicated time points after the surgery. Mice were anesthetized and transcardially perfused with 1× PBS. Sciatic and tibial nerves and, in some studies, sural nerves, were collected and fixed in either 3% glutaraldehyde for morphometric studies or 4.0% paraformaldehyde for immunocytochemistry (ICC) studies. Nerve segments used for morphometric analysis were embedded in Epon and 1 μm cross sections were stained with toluidine blue. All myelinated axons in a single whole cross-section of the nerve were counted at light level (40×) by using a motorized stage and stereotactic imaging software (Axiovision; Zeiss), as described previously (Lehmann et al., 2007).
For single- and double-labeling ICC studies, sciatic and tibial nerves were cryoprotected and cryosectioned (10 μm). The nerve samples were incubated with the following primary Abs: rabbit anti-Caspr (1:300; Abcam), rabbit anti-Fcγ common chain (1:1000; US Biological), rat anti-CD68 (1:50; AbD Serotech), and mouse anti-β III tubulin (1:1000; Promega) at 4°C overnight, and then developed with the following flourophore-conjugated secondary Abs: FITC-labeled goat anti-rabbit IgG, Cy3-labeled goat anti-mouse IgG, Cy3-labeled goat anti-rabbit IgG, and Cy2-labeled goat anti-rat IgG (1:200; Jackson ImmunoResearch). These stained nerves were analyzed by fluorescent microscopy (Zeiss); the nodal and paranodal injury was assessed and quantified as described previously (Susuki et al., 2007a).
Western blotting.
Sciatic and tibial nerves were harvested on postsurgical days 1, 3, 7, and 14; nerve lysates were made in 1% SDS; and the protein concentration was determined by the BCA protein assay according to the manufacturer's instructions (Pierce). The heat-denatured lysates were electrophoresed on 12% SDS polyacrylamide gels, transferred to PVDF membranes, and probed with anti-Fcγ common chain (shared by all activating FcγR) Abs (1:1000, overnight at 4°C) and developed with appropriate HRP-conjugated secondary Abs and chemiluminescent kit as per the manufacturer's instructions (Bio-Rad).
BNB integrity assessment.
Anti-ganglioside Abs (GT1b-2b mAb) was labeled with the DyLight Fluor 594 according to manufacturer's instructions (Thermo Fisher Scientific). The labeled GT1b-2b mAb (500 μg) was injected intraperitoneally to wild-type mice on postsurgical days 1 and 3 and the sciatic and tibial nerves were harvested on day 7. The endoneurial Ab deposition of labeled anti-ganglioside Abs in injured and uninjured side nerves was examined by fluorescent microscopy and quantitative image analysis was conducted.
Statistics.
Data are reported as mean ± SEM. Differences between groups were examined by Student's t test and two-way ANOVA with corrections for multiple comparisons and p < 0.05 was considered statistically significant.
Results
L5SNT induces degeneration of a proportion of nerve fibers and breakdown of BNB in sciatic nerve and its branches
We and others have determined that systemic administration of anti-ganglioside mAbs in wild-type uninjured animals does not induce neuropathic injury (Pollard et al., 1995; Spies et al., 1995b; Westland et al., 1999; Yan et al., 2000; Sheikh et al., 2004). Because breakdown of BNB has been reported to be a critical element in Ab-induced nerve injury (Westland et al., 1999; Yan et al., 2000; Sheikh et al., 2004), we used L5SNT (modified Chung model) to open the BNB in sciatic nerve and its branches (see below). Wild-type and different mutant and transgenic mice used in these studies underwent (left) L5SNT to disrupt BNB in sciatic nerve and its branches to allow increased access to circulating antibodies and inflammatory cells, whereas the uninjured right sciatic nerve and its branches served as controls.
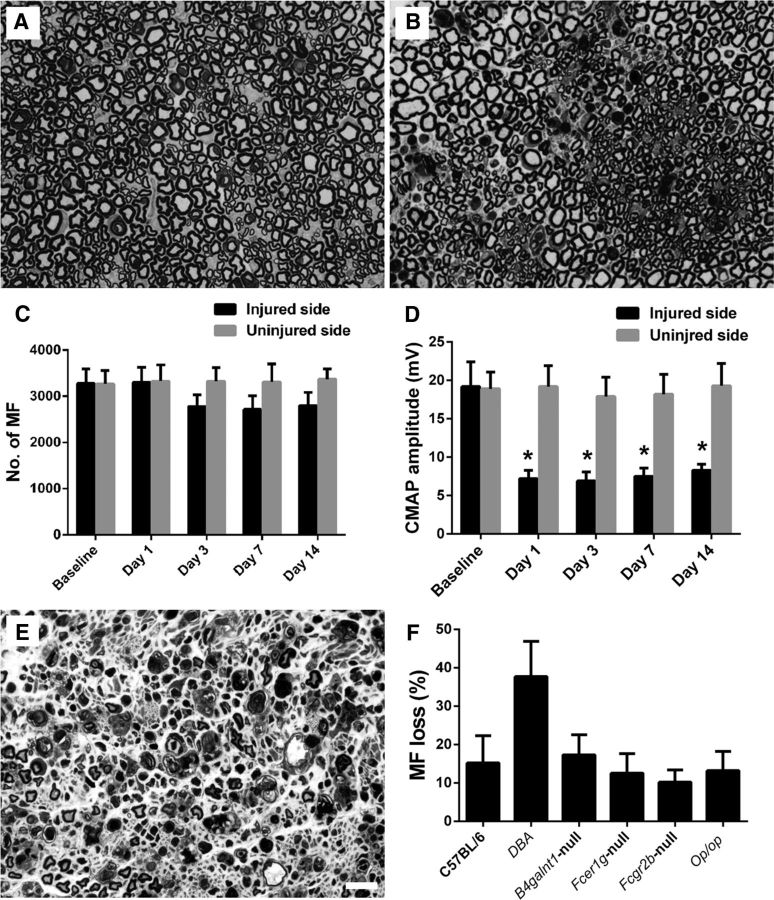
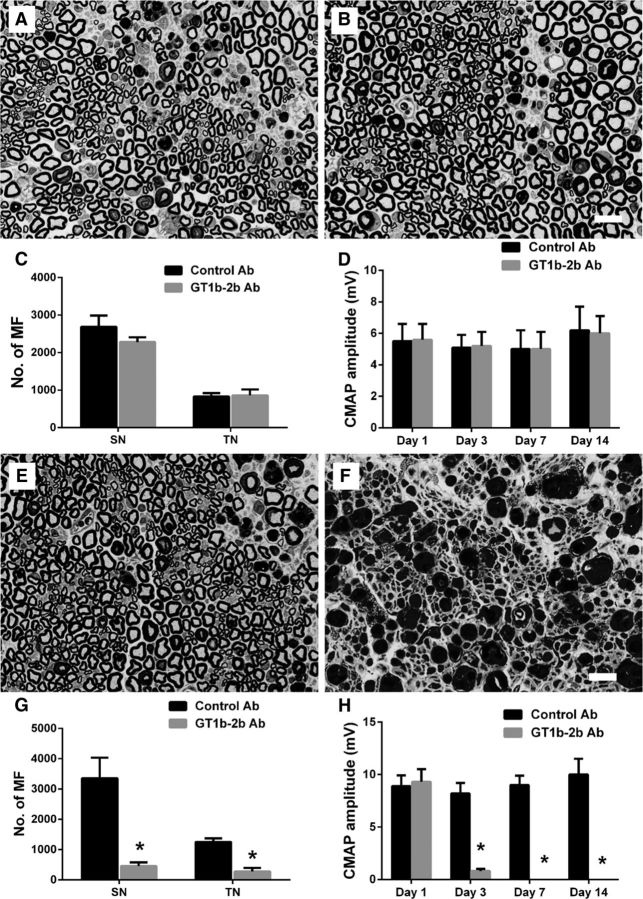
Wild-type animals with L5SNT underwent morphometric and electrophysiological evaluations on days 1, 3, 7, and 14 after surgery. Morphology and morphometry showed that there was Wallerian degeneration indicated by the loss of myelinated nerve fibers (MFs) and degenerating myelin figures on the left/injured side compared with the uninjured side, which was noticeable after 3 d of L5SNT and remained static after that until day 14 (Fig. 1A–C). Electrophysiology showed that there was significant decrease in CMAP amplitudes starting day 1 and persisting until day 14 after the surgery (Fig. 1D). In wild-type animals, the decrease in number of MFs on the injured side was ∼15% compared with the uninjured side. All other mutant/transgenic strains mice had a decrease in the numbers of myelinated fibers with L5SNT that was comparable to wild-type animals except DBA mice, in which L5SNT causes degeneration of ∼40% of MFs (Fig. 1E,F). These studies show that the majority of MFs in sciatic nerves are uninjured despite L5SNT and sciatic nerve conductions show decreased but recordable CMAP amplitudes (6–8 mV), which allows us the opportunity to study the effects of anti-ganglioside Abs on remaining intact nerve fibers by electrophysiology and morphometry in this model.
Figure 1.
L5SNT induces degeneration of a proportion of nerve fibers in sciatic nerve. A, B, Morphology showing that L5SNT does not induce axon degeneration to contralateral sciatic nerve (A), whereas it causes axonal injury in ipsilateral nerve (B). C, Morphometry showing that L5SNT induced MF loss in injured side nerve starting on postsurgical day 3. D, CMAP amplitude of the injured side nerve was significantly reduced after L5SNT. E, Micrograph showing axon degeneration in sciatic nerve of DBA/2J mice after L5SNT. F, L5SNT induced ∼5–20% of MF loss in the injured (left) sciatic nerve in wild-type and all mutant/transgenic mice that we tested except for DBA/2J mouse (∼40% MF loss). n = 6. Scale bar, 20 μm.
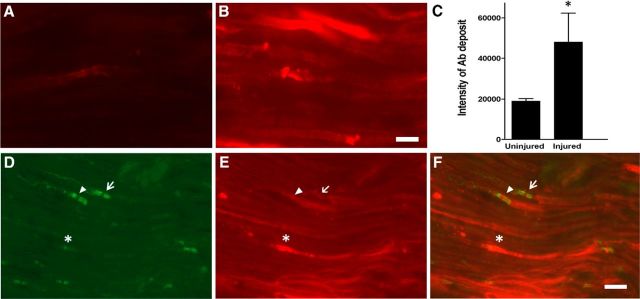
To confirm that degeneration of proportional MFs in the L5SNT model induces breakdown of BNB and increased accumulation of the Abs, a prototypic mAb with specificity for GT1b ganglioside and IgG2b isotype (GT1b-2b) was labeled with a flourophore (DyLight 594) and used in pulse-chase experiments in wild-type mice. Fluorescently labeled GT1b-2b Ab was injected intraperitoneally on days 1 and 3 after L5SNT and the sciatic and tibial nerves were analyzed on postsurgical day 7. Quantitative fluorescent microscopy showed that significantly more labeled GT1b-2b Ab was deposited in the left sciatic and tibial branches with L5SNT compared with the uninjured right side at the time points examined (Fig. 2A–C). The labeled GT1b-2b bound to axons and nodes of Ranvier (Fig. 2D–F). These experiments confirm that there is significantly increased accumulation of circulating Abs in the sciatic nerve and its branches with L5SNT.
Figure 2.
L5SNT induces breakdown of BNB. A, B, Immunofluorescent micrographs showing that significantly more labeled GT1b–2b mAb (DyLight 594; red) deposits in the injured side sciatic nerve (B) compared with control side nerve (A) on postsurgical day 7. Scale bar, 10 μm. C, Quantitative analysis of labeled GT1b–2b deposition in uninjured vs injured nerve. n = 3. *p < 0.05. D–F, The injured sciatic nerve was immunostained for paranodal marker Caspr (green) and labeled GT1b–2b (red) binds to axon (*), node of Ranvier (arrowhead), and paranodal axon (arrow). Scale bar, 20 μm.
Anti-ganglioside Abs induce stepwise nodal and axonal degeneration in nerves with L5SNT
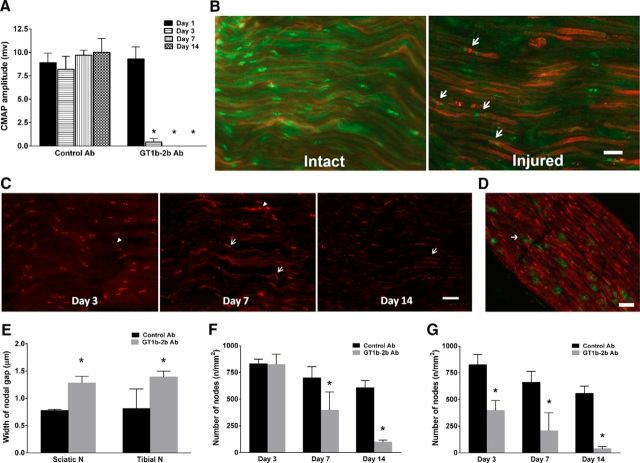
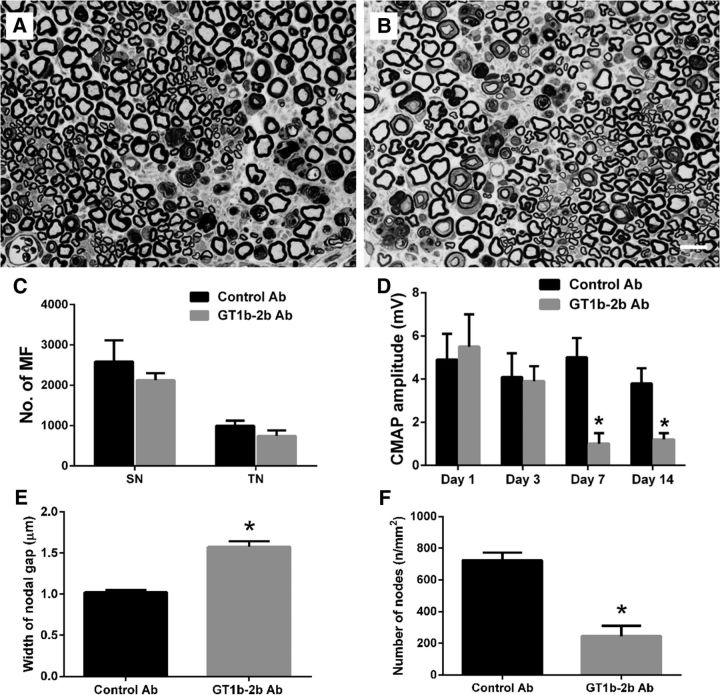
Whether anti-ganglioside Abs can induce nodal and axonal injury was examined in an L5SNT model. Wild-type mice with unilateral L5SNT were administered GT1b-2b or control Abs by intraperitoneal injections on days 1, 3, and 7 after surgery. Behavioral (mechanical allodynia in hindpaws), electrophysiology, and morphological studies were performed at various time points (1–14 d) after surgeries. Clinical and experimental data suggest that, in axonal GBS, nodal damage precedes axon degeneration (Griffin et al., 1996b; Hafer-Macko et al., 1996); therefore, we initially examined this issue by evaluating the nodal and axonal integrity by serial electrophysiological and morphological studies of sciatic nerves and its branches. Electrical studies showed that animals in the GT1b-2b and control Ab arms had similar CMAP amplitudes on postsurgical day 1 before treatment with Abs. Animals treated with GT1b-2b mAb had a significant decrease in CMAP amplitudes on postsurgical day 3 and completely inexcitable nerves on days 7 and 14, whereas CMAP amplitudes in animals treated with control Abs remained unchanged over time (Fig. 3A). Uninjured sciatic nerves of both GT1b-2b and control Ab groups did not show decrease in CMAP amplitudes (data not shown). The decrease in CMAP amplitudes correlated with nodal disruption, which was assessed by caspr immunolabeling of nerve sections to stain paranodes and to measure nodal gap and number of nodes/unit area. In some studies, binding of DyLight 594-conjugated-GT1b-2b to nodes and axons was correlated with caspr immunostaining. Our results show that significantly more labeled Ab bound to the nodes (Fig. 3B) and correlated with widened nodes in the injured nerves at sciatic and tibial levels starting at day 3 and all later time points examined in the GT1b-2b-treated group compared with the control Ab-treated group (Fig. 3B,C,E). Notably, many macrophage/microglia can be found in injured nerve and some are adjacent to the widened nodes (Fig. 3D). The numbers of nodes of Ranvier (defined by caspr labeling of two hemi-nodes) per unit area was similar in GT1b-2b and control Ab-treated groups on postsurgical day 3 at the sciatic level (Fig. 3F). However, the number of nodes was significantly decreased at the tibial level on postsurgical day 3 (Fig. 3G) and at both the sciatic and tibial levels on postsurgical days 7 and 14 in the GT1b-2b-treated group compared with controls (Fig. 3F,G). These electrical and morphological findings show that GT1b-2b mAbs induce a stepwise nodal injury that starts with nodal widening and subsequent nodal loss and CMAP decrease correlates with nodal widening on postsurgical day 3 and nodal widening and loss at subsequent time points. CMAP amplitudes and nodal architecture were preserved in the right/uninjured nerves in both GT1b-2b- and control Ab-treated groups (data not shown).
Figure 3.
Anti-ganglioside Abs induce nodal injury in animal with L5SNT. A, GT1b–2b mAb significantly decreased CMAP amplitudes on postsurgical day 3 and induced complete loss of CMAP amplitudes on postsurgical day 7 and 14. n = 6. *p < 0.05. B, Significantly more deposition of GT1b–2b (red) can be found in injured sciatic nerve compared with intact control nerve. Injured nerve has widened nodes (arrow) as assessed by Caspr staining (green). C, GT1b–2b treatment widened the nodal gap (arrowheads) in the injured nerves at sciatic level on postsurgical days 3–14. Spread of caspr staining along paranodal axons can be seen on postsurgical days 7 and 14 in GT1b–2b-treated nerves (arrows). D, Macrophages, labeled by anti-CD68 Ab (green), can be seen adjacent to widened nodes (arrow) as assessed by Caspr staining (red) on the injured side. E, Quantitative analysis of nodal length defined by the distance between paranodes (Caspr) showing that the GT1b–2b significantly increased the nodal gap. F, The numbers of nodes of Ranvier /unit area in GT1b–2b treated-sciatic nerves was significantly decreased on postsurgical days 7 and 14 compared with control Ab. G, GT1b–2b treatment significantly reduced the nodal numbers in tibial nerves on postsurgical days 3, 7, and 14. n = 6. *p < 0.05. Scale bar, 20 μm.
Although the changes in nerve conductivity and associated nodal disruption occurred by day 3 after the L5SNT, no significant change in the number of MFs was found (as determined by morphometry on epon-embedded sections) at this time point in the GT1b-2b mAb-treated group compared with the control Ab-treated animals (Fig. 4C,D). Morphological and morphometric studies at later time points showed that there was axon degeneration and that the number of MFs in both sciatic and tibial nerves was significantly reduced in GT1b-2b-treated mice on postsurgical days 7 and 14 (Fig. 4A–D) compared with the control Ab-treated animals. There was no injury to MFs in the right/uninjured nerves in either the GT1b-2b-treated or the control Ab-treated groups. Overall, our studies indicate sequential nodal and then axonal injury in animals treated with GT1b-2b mAb.
Figure 4.
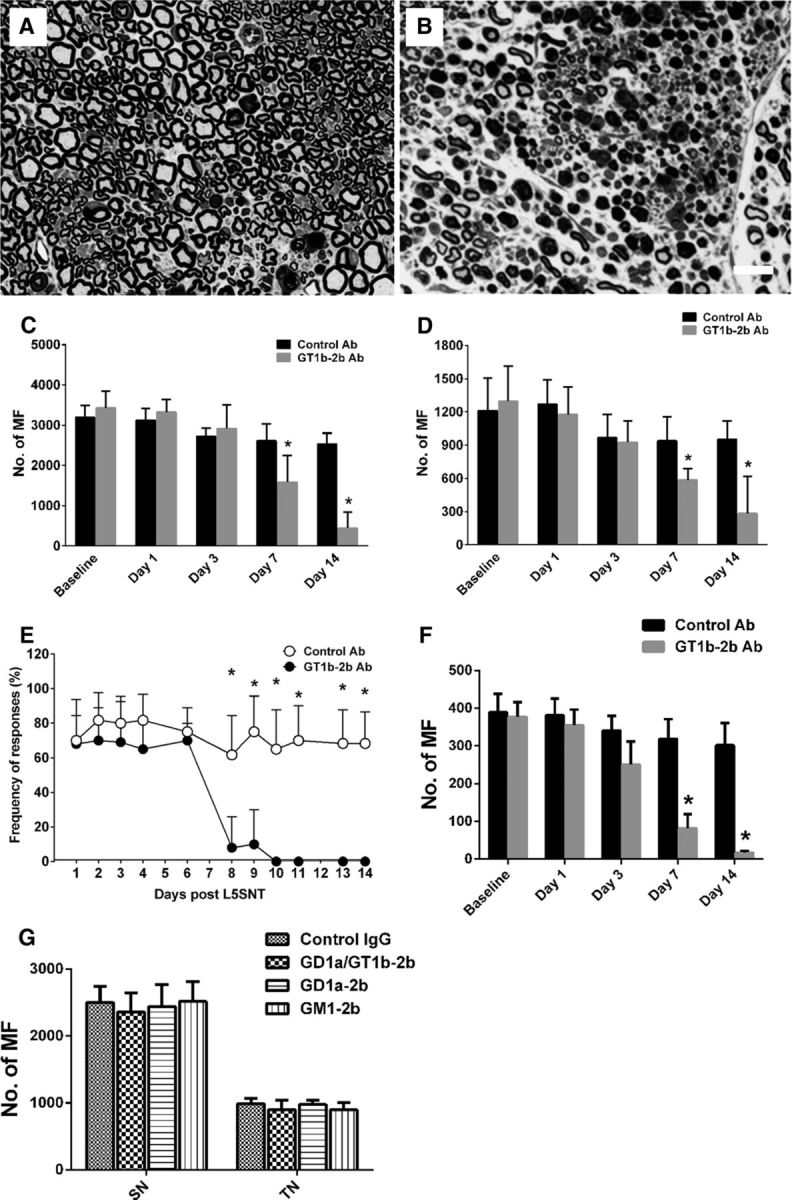
Anti-ganglioside Abs induce axonal degeneration in sciatic nerves with L5SNT. A, B, Micrographs showing significantly more axonal degeneration in GT1b–2b treated-nerve (B) compared with control-Ab treated-sciatic nerve (A). Scale bar, 20 μm. C, D, Morphometry showing that there was a significant decrease in the number of MFs at both the sciatic (C) and tibial (D) levels in GT1b–2b treated-animals on postsurgical days 7 and 14. E, Behavioral studies show that mice treated with GT1b–2b or control Abs developed mechanical allodynia 1 d after L5SNT and the allodynia in control Ab-treated mice persisted throughout the study, whereas there was significant reversal of allodynia in mice treated with GT1b–2b beginning at postsurgical day 8. F, Significant MF loss in sural nerves was found in GT1b–2b treated-animals on postsurgical days 7 and 14 compared with the control Ab treated-group. G, Morphometry showing that no significant MF loss in sciatic nerve was found in animals treated with GD1a/GT1b–2b, GD1a–2b, and GM1–2b mAbs on postsurgical day 14. n = 12. *p < 0.05. Scale bar, 20 μm. SN, Sciatic nerve; TN, tibial nerve.
Electrophysiology and morphology were also correlated with behavioral testing. We focused on the sensory function because electrophysiology assessed motor fiber function and the majority of nerve fibers in sciatic nerve are sensory in nature (Schmalbruch, 1986). L5SNT is widely used as a pain model to assess the mechanical allodynia (Tanga et al., 2005), so we evaluated the mechanical allodynia with Von Fery hair in our model. Mice treated with GT1b-2b or control Abs developed mechanical allodynia 1 d after the surgery and the allodynia in control Ab-treated mice persisted for the entire 14 d of observation. In contrast, there was significant reversal of allodynia in mice treated with GT1b-2b from postsurgical days 8–14 (Fig. 4E), implicating the sensory nerve fiber loss in these animals, which is confirmed by the morphometric analysis of the sural nerve (Fig. 4F). The right/uninjured side in both GT1b-2b- and control Ab-treated groups did not develop pathologic pain behavior or loss of sensations (data not shown).
Three other anti-ganglioside mAbs, GD1a/GT1b-2b, GM1–2b, and GD1a-2b, were compared with GT1b-2b in wild-type animals. We found that these mAbs did not induce nerve injury in mice with L5SNT (Fig. 4G). Notably, our previous studies have shown that GD1a/GT1b-2b mAb induces severe inhibition of axon regeneration in a nerve crush model, but it does not produce injury to intact nerve fibers.
In summary, our findings indicate that breakdown of BNB is necessary to induce anti-ganglioside Ab-mediated nerve fiber injury because the uninjured side does not develop any behavioral, electrophysiological, or morphological deficits. Further, all anti-ganglioside Abs do not induce injury to intact nerve fibers in this model.
Anti-ganglioside Abs did not cause nerve injury in transgenic mice lacking corresponding ganglioside
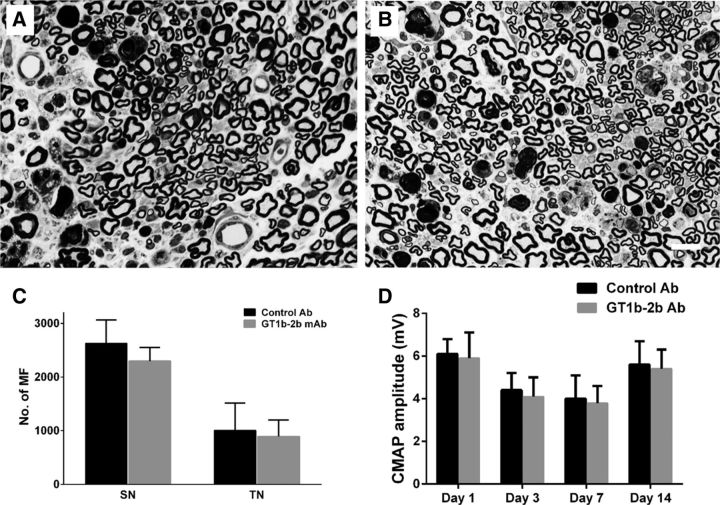
Mice with altered ganglioside expression were used to verify whether the anti-ganglioside Abs-induced axonal degeneration in our animal model depends on the expression of specific ganglioside in the nerves. The effects of GT1b-2b were examined in B4galnt1-null mutant mice that lack the key enzyme β1, 4-N-acetylgalatosaminylatransferase required for the synthesis of complex gangliosides, including GM1, GD1a, GD1b, and GT1b (Liu et al., 1999). These mice underwent L5SNT and GT1b-2b mAb was administered as described above. Our electrophysiological and morphometric data show that GT1b-2b-mediated nerve injury found in wild-type mice was abolished in these mutant animals that lack GT1b ganglioside (Fig. 5, Table 2). These findings suggest that the nodal and axonal injury induced by anti-ganglioside Abs depends on the expression of specific corresponding ganglioside, which support the notion that the formation of immune complexes (ICs) by anti-ganglioside Abs and its target antigen, ganglioside, is required for anti-ganglioside Abs to induce axon degeneration in our animal model.
Figure 5.
Anti-ganglioside Abs-induced nerve injury requires the formation of ICs. A, B, Micrographs showing MF in sciatic nerves of B4galnt1-null mice treated with control (A) and GT1b–2b (B) mAb. Nerve fiber degeneration because of L5SNT can be appreciated in the nerves of both treatment groups. C, D, Morphometric (C) and electrophysiological (D) analyses indicate that neither the numbers of MF at the sciatic nerve (SN) and tibial nerve (TN) levels nor CMAP amplitudes were altered by GT1b–2b treatment. n = 6. Scale bar, 20 μm.
Table 2.
Summary of pathophysiologic effects of GT1b-2b Ab in different mouse strains/mutants
| Mouse strain | No. of MF (SN) |
No. of MF (TN) |
CMAP amplitude (mV) |
|||
|---|---|---|---|---|---|---|
| Control Ab | GT1b-2b | Control Ab | GT1b-2b | Control Ab | GT1b-2b | |
| WT | 2135 ± 268 | 446 ± 300* | 955 ± 166 | 284 ± 233* | 9.2 ± 1.8 | 0 ± 0* |
| B4galnt1-null | 2630 ± 436 | 2296 ± 508 | 1007 ± 254 | 892 ± 309 | 5.6 ± 1.1 | 5.4 ± 0.9 |
| Fcer1g-null | 2689 ± 298 | 2280 ± 129 | 833 ± 87 | 862 ± 156 | 6.2 ± 1.5 | 6 ± 1.1 |
| Fcgr2b-null | 3354 ± 682 | 455 ± 121* | 1253 ± 120 | 276 ± 119* | 10 ± 1.5 | 0 ± 0* |
| DBA mice | 1617 ± 279 | 221 ± 72* | 519 ± 117 | 52 ± 37* | 5.7 ± 0.8 | 0 ± 0* |
| Op/op mice | 2584 ± 526 | 2125 ± 174 | 997 ± 124 | 745 ± 138 | 3.8 ± 0.7 | 1.2 ± 0.3* |
Comparisons of control and anti-ganglioside mAb-treated animals.
*p < 0.05 was considered as significant. WT, Wild type; SN, sciatic nerve; TN, tibial nerve.
Innate immune effectors are involved in anti-ganglioside Abs-mediated nerve injury
Innate immune effectors are composed of the complement system and FcγR, which provide an important link between the humoral and cellular immune systems. These effectors mediate immune-complex-initiated inflammation and tissue injury in autoimmune disorders (Su et al., 2004; Nimmerjahn and Ravetch, 2011). We examined the role of FcγR and complement system in transgenic/mutant mice lacking or deficient in activating or inhibitory FcγR and C5 component of complement pathway. These animals underwent L5SNT and anti-ganglioside Ab administration as described above.
Activating Fcγ receptors are involved in anti-ganglioside Abs-induced axonal degeneration
Initially, we measured the expression of activating FcγRs in wild-type mice in sciatic nerves of animals that underwent L5SNT on postsurgical days 1, 3, 7, and 14 by immunoblotting and ICC studies using an Ab specific for gamma common chain shared by all activating FcγRs (Zhang et al., 2014). Compared with intact/uninjured nerve, there was a significant increase in activating FcγRs in the injured nerves on postsurgical day 1 and later time points examined (Fig. 6A,B). This finding was confirmed by ICC studies showing that the upregulated activating FcγRs were initially found on endoneurial glia (including microglia and Schwann cells as identified by cellular morphology) at early time points and later on, predominantly by infiltrating circulating macrophages as identified by double labeling ICC in sciatic nerves of animals with L5SNT (Fig. 6C–I).
Figure 6.
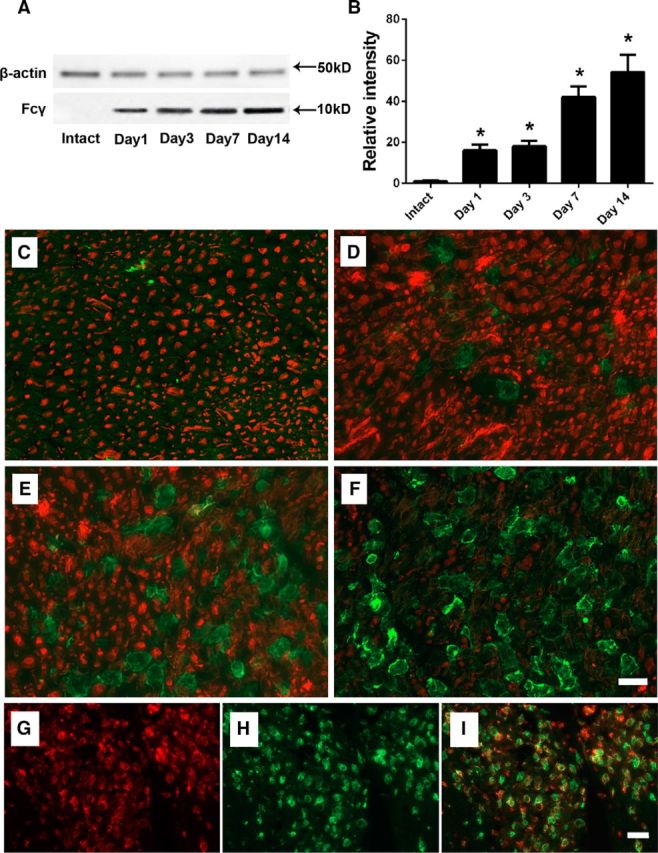
L5SNT upregulates the expression of activating FcγRs in the injured nerves. A, B, Immunoblotting (A) and corresponding densitometry (B) study showing significant upregulation of activating FcγRs on injured side after L5SNT. C–F, Double-labeling ICC studies using antibodies against Fcγ common chain (green) and β-III tubulin (red) showing upregulation of activating FcγRs expression in injured sciatic nerves on postsurgical days 1 (C), 3 (D), 7 (E), and 14 (F). Scale bar, 10 μm. G–I, The double-labeling ICC study showing colocalization (I) of upregulated activating FcγRs (red; Fcγ common chain; G) and macrophage/microglia (green; CD68; H) on postsurgical day 14 after L5SNT. n = 2. *p < 0.05. Scale bar, 20 μm.
Next, we investigated whether GT1b-2b-mediated nodal and axonal injury depends on immune complex interaction with activating FcγRs. For this issue, two transgenic mice strains, Fcer1 g-null (lack all activating FcγRs but express inhibitory FcγRII) and Fcgr2b-null (express all activating FcγRs but lack inhibitory FcγRII), were studied. Our results show that Fcer1 g-null mice were completely resistant to GT1b-2b-mediated nodal and axonal injury (Fig. 7A–D, Table 2), whereas Fcgr2b-null mice were highly susceptible to GT1b-2b-mediated nerve injury (Fig. 7E–H, Table 2). Notably, in the first set of experiments with Fcgr2b-null mice, administration of 2/3 doses of GT1b-2b mAb led to fatality of majority of animals (3/4 mice). Results shown in Figure 7 are based on subsequent studies in which Fcgr2b-null mice were administered only a single Ab dose on day 1. These findings demonstrate that activating FcγRs are involved in the anti-ganglioside Abs-mediated axonal degeneration. Moreover, inhibitory FcγRII receptors attenuate this injury and this assertion is supported by enhanced injury in Fcgr2b-null mice. Interestingly, the right/uninjured side in Fcgr2b-null mice treated with GT1b-2b Abs did not develop pathological changes, emphasizing the importance of L5SNT-associated breakdown of BNB and upregulation of activating FcγRs (data not shown).
Figure 7.
Anti-ganglioside Abs-induced nerve injury depends on the expression of activating FcγRs. A, B, Micrographs showing MF in sciatic nerves of Fcer1 g-null mice treated with control (A) and GT1b–2b mAb (B). C, D, Morphometric (C) and electrophysiological (D) analyses indicate that Fcer1 g-null mice are not susceptible to GT1b–2b mAb-mediated nerve injury. n = 6. Scale bar, 20 μm. E, F, Micrographs of sciatic nerves from Fcgr2b-null mice showing that there is significantly more axonal degeneration in GT1b–2b-treated sciatic nerve (F) than control Ab-treated nerve (E). G, Morphometry shows that MFs at the sciatic and tibial nerve levels were significantly reduced in GT1b–2b-treated Fcgr2b-null mice. H, Nerve conduct studies show that GT1b–2b mAb significantly decreased CMAP amplitudes in Fcgr2b-null mice on postsurgical day 3, 7, and 14 compared with control Ab (D). n = 6. *p < 0.05. Scale bar, 20 μm.
In summary, these set of studies strongly suggest that activating FcγR-mediated inflammation participates in anti-ganglioside Abs-induced axonal/nerve injury.
Complement involvement in anti-ganglioside Abs-induced axonal degeneration
The role of the complement arm of innate immunity in GT1b-2b-mediated axonal injury was assessed in mutant mice deficient in C5 (DBA/2J). We found that DBA/2J mice (which have exaggerated axonal degeneration with L5SNT) were extremely susceptible to GT1b-2b-mediated nodal and axonal injury. GT1b-2b Ab-mediated axon degeneration in DBA mice was relatively more severe than that in C5-sufficient wild-type animals (Fig. 8A–D, Table 2). In addition, we examined the expression of activating FcγRs in DBA mice with L5SNT and found significantly increased FcγR expression in these animals compared with wild-type mice with L5SNT (Fig. 8E–G). Our results show that C5 component of complement and associated downstream components are not necessary for nodal and axonal injury in this model.
Figure 8.
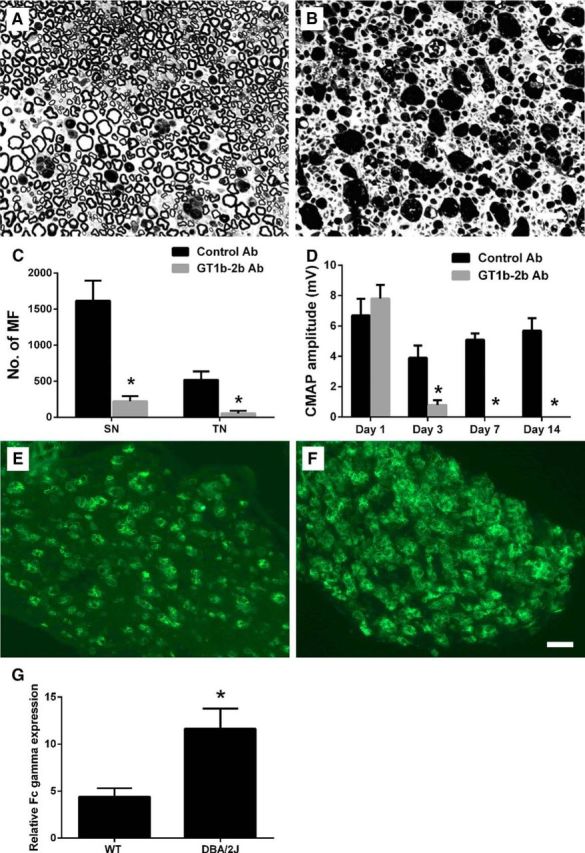
Anti-ganglioside Abs induced nerve injury is not dependent on the terminal complement complex. A, B, Micrographs showing severe axonal degeneration in GT1b–2b-treated DBA/2J mice (B) compared with control Ab-treated animals (A). C, GT1b–2b Ab induces significant reduction in MF count in sciatic and tibial nerves of DBA/2J mice compared with the control Ab-treated group. D, Electrophysiological studies show that GT1b–2b significantly decreased the CMAP amplitudes of DBA/2J mice on postsurgical days 3, 7, and 14 compared with control Ab. E, F, ICC images showing the activating FcγRs expression in injured sciatic nerves in wild-type (E) and DBA/2J (F) mice on postsurgical day 14. G, Quantitative ICC analysis showing that there is significant upregulation of activating FcγRs in sciatic nerve of DBA mice compared with wild-type mice. n = 6. *p < 0.05. Scale bar, 20 μm. WT, Wild-type.
Macrophages/microglia lineage cells are involved in the anti-ganglioside Abs induced axonal degeneration
Our ICC studies showed that macrophages are the dominant cell type that expresses activating FcγRs in L5SNT model (Fig. 6G–I). The macrophage staining is present in injured nerves as early as day 3 after surgery in this model, increases over time, and peaks ∼14 d after the surgery. Further, pathological studies indicate that macrophages play an important role in the pathogenesis of axonal GBS (Griffin et al., 1990; McKhann et al., 1993; Griffin et al., 1995; Kiefer et al., 2001; Susuki et al., 2003). Moreover, circulating macrophages have been shown to participate in nerve fiber injury in experimental models of immune neuropathies (Zhang et al., 2014). Therefore, we investigated the role of circulating macrophages in GT1b-2b-mediated axonal injury. For this purpose, L5SNT and passive GT1b-2b transfer was performed in mutant osteopetrotic (op/op) mice with macrophage deficiency. Compared with wild-type mice, op/op mice are much less susceptible to GT1b-2b-mediated axonal injury (Table 2). Electrical studies showed that CMAP amplitudes were significantly reduced on postsurgical days 7 and 14 (Fig. 9D), whereas in wild-type animals, CMAP amplitudes are significantly decrease on postsurgical day 3 and nerves become virtually inexcitable on postsurgical days 7 and 14 (Fig. 3A). Morphological studies showed that the number of MFs in sciatic and tibial nerves was minimally decreased in op/op mice treated with GT1b-2b compared with the control Ab-treated group; however, this decrease did not reach statistical significance (Fig. 9A–C). We further examined the nodal size and numbers in op/op mice with L5SNT. Our data demonstrate that there was significant nodal injury (widened nodal gap and decreased numbers of nodes of Ranvier) in these mice (Fig. 9E,F). These observations support the notion that circulating macrophages are the dominant activating FcγR-carrying cell type that mediates anti-ganglioside Ab-induced axonal injury.
Figure 9.
Macrophages are involved in anti-ganglioside Abs-induced nerve injury. A, B, Micrographs showing MFs in sciatic nerves of mutant osteopetrotic (op/op) mice treated with control (A) and GT1b–2b mAb (B). C, Morphometry shows that GT1b–2b treatment does not significantly alter the number of MF in sciatic and tibial nerves of op/op mice compared with control Ab-treated group. D, Electrophysiological studies show that evoked CMAP amplitudes in GT1b–2b-treated mice were significantly decreased on postsurgical days 7 and 14 compared with the control Ab-treated animals. E, GT1b–2b significantly increased the nodal gap in sciatic nerves of op/op mice. F, Numbers of nodes of Ranvier in GT1b–2b treated-sciatic nerves was significantly decreased on postsurgical day 14. n = 6. *p < 0.05. Scale bar, 20 μm.
Patient serum with anti-ganglioside Abs induces axonal degeneration
In addition to experimental anti-ganglioside mAbs, we investigated whether anti-ganglioside Abs in GBS patient serum can also induce nodal and axonal injury in our model. An axonal GBS patient serum containing high titers of IgG anti-GM1 antibodies was studied in wild-type mice with L5SNT. GBS serum significantly decreased CMAP amplitudes on postsurgical days 7 and 14, but not at earlier time points, compared with control-serum-treated animals (Fig. 10D). This decrease in evoked motor amplitudes correlated with reduced numbers of nodes of Ranvier in GBS serum-treated animals on postsurgical day 14 (Fig. 10A–C), the only time point at which morphological studies were performed. In the GBS-serum-treated group, nodal disruption was also manifested as a spread of Caspr staining along the paranodes and paranodal axons (Fig. 10E). This pathological spread of Caspr staining has been noted in pathological materials from patients with immune neuropathies (Cifuentes-Diaz et al., 2011). Morphometry showed that nerves treated with GBS serum did not induce significant axonal degeneration compared with the control-serum-treated group on postsurgical day 14 (Fig. 10F).
Figure 10.
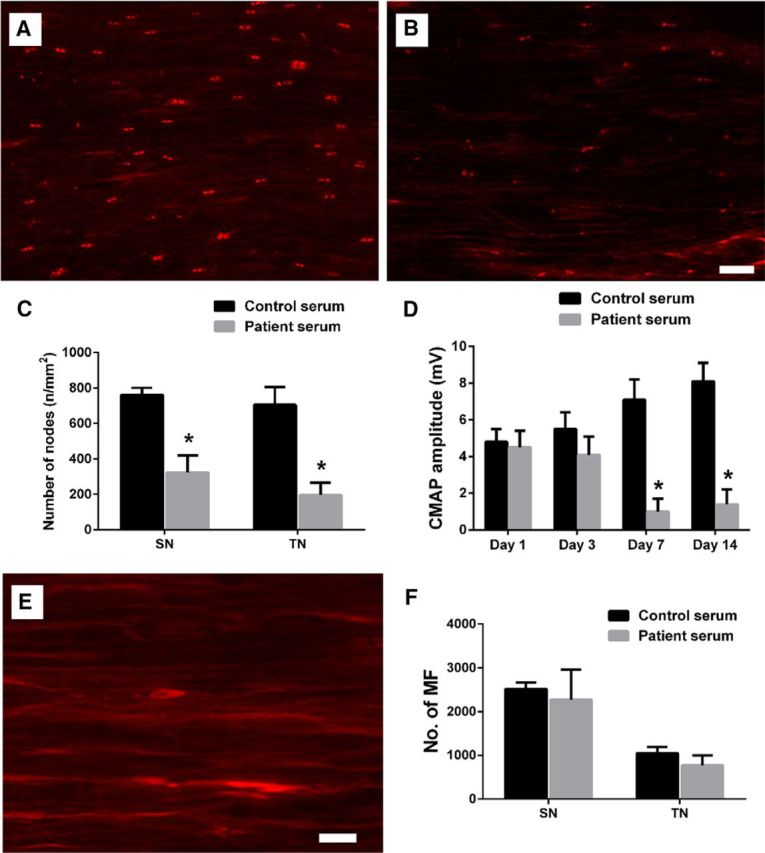
GBS patient serum-derived anti-ganglioside Abs induce nodal but not axonal injury in wild-type animals with L5SNT. A, B, Immunofluorescent micrographs showing the paranodes stained by anti-Caspr Ab in sciatic nerves of control-serum-treated (A) and GBS-serum-treated animals (B) on postsurgical day 14. Scale bar, 20 μm. C, Quantitative ICC analysis shows that GBS serum-treated mice have significantly less number of nodes of Raniver at sciatic and tibial nerve levels than the control serum-treated nerves on postsurgical day 14. D, The significant reduction of CMAP amplitudes was observed in mice treated with GBS patient serum on postsurgical days 7 and 14 compared with the controls. E, The spread of Caspr staining along the paranodal axons can be found in GBS-serum-treated nerves. Scale bar, 10 μm. F, Morphometry shows that there is no significant difference in MF count in sciatic and tibial nerves between the control-serum-treated and GBS-serum-treated animals on postsurgical day 14. n = 6. *p < 0.05.
Discussion
This study demonstrates that anti-ganglioside Abs, including experimental mAbs and GBS patient serum, induce sequential nodal and/or axonal injury in a new passive transfer mouse model that recapitulates the salient pathologic features found in axonal GBS (Griffin et al., 1996b). We found that the breakdown of BNB induced by L5SNT was essential for Ab-mediated nerve injury. Furthermore, this anti-ganglioside Abs-mediated neuropathy (injury to intact nerve fibers) depends on activating FcγRs bearing macrophages/microglia-mediated inflammation triggered by ICs formed by anti-ganglioside Abs and their target antigens on the nerves. Notably, we found that the terminal complement complex was not involved in the anti-ganglioside Abs-mediated axonal degeneration in this animal model. Overall, our study supports the notion that cellular elements of innate immunity are required for Ab-mediated nerve injury and involved in the pathogenesis of GBS. The identification of activating FcγRs in anti-ganglioside Abs-mediated nodal and axonal degeneration could allow the development of new therapeutic strategies for the treatment of autoantibody-induced immune neuropathies including GBS. These findings add to the complexity of axon degeneration in neuroimmunological disorders.
We found that the formation of ICs was required for the anti-ganglioside Abs-mediated axonal degeneration, which is supported by our findings that transgenic mice lacking complex gangliosides including GT1b do not develop nodal and axonal injury. These findings are consistent with our previous study showing that IC formation played a critical role in the anti-ganglioside Abs-mediated pathological effect on axonal regeneration (Zhang et al., 2014). IgG deposition in GBS patient nerves also supports the idea that the formation of ICs is part of the pathogenic cascade (Hughes et al., 1999; Willison and Yuki, 2002). The formation of ICs allows them to interact with innate immune effectors, in this case, activating FcγRs.
This is first set of experimental data that link autoantibody-mediated peripheral neuropathy to activating FcγRs. Moreover, the significantly enhanced nerve injury found in Fcgr2b-null mice supports the notion that inhibitory FcγRII participates in amelioration of this inflammation and limits nerve injury induced by anti-ganglioside Abs. Classical FcγRs consist of three activating (FcγRI, III, IV in mice; FcγRIA, IIA, IIIA in humans) and one inhibitory (FcγRIIB in mice and humans) member. IgG and FcγR link Ab specificity and effector cells. Cross-linking of activating FcγRs induce a sustained calcium influx that participates in inflammation and cytotoxicity via degranulation, phagocytosis, Ab-dependent cellular cytotoxicity (ADCC), and release of cytokines and other proinflammatory mediators (Nimmerjahn and Ravetch, 2007; Nimmerjahn and Ravetch, 2008). In contrast, cross-linking of inhibitory FcγRs results in the arrest of these effector responses (Smith and Clatworthy, 2010). Three isoforms of leukocyte FcγR—FcγRIIA, FcγRIIIA, and FcγRIIIB—display biallelic functional polymorphisms that significantly influence the receptor affinity for IgG subclasses and consequently the efficacy of IgG-mediated effector functions including ADCC and phagocytosis (Deo et al., 1997). Some previous studies suggest that specific FcγR polymorphisms are associated with disease susceptibility and severity, thereby implying that FcγRs play a role in the pathogenesis of GBS (Vedeler et al., 2000; van Sorge et al., 2005). In addition, anti-GM1 IgG antibodies from GBS patients can induce leukocyte degranulation and phagocytosis, functions that can be completely abrogated in the presence of FcγR-blocking antibodies (van Sorge et al., 2003). These clinical and experimental findings support a role of FcγRs in GBS pathogenesis. Consistent with these findings, we previously showed the upregulation of FcγRs on macrophage/microglia lineage cells in the nerves from GBS patients (Zhang et al., 2014). Furthermore, we demonstrated that specific activating FcγRs carried by macrophages induce inflammation that was detrimental to axon regeneration/nerve repair (Zhang et al., 2014).
Our studies reproduce a cardinal pathologic feature seen in axonal GBS, the presence of macrophages adjacent to widened/disrupted nodes of Ranvier (Griffin et al., 1995; Griffin et al., 1996a; Griffin et al., 1996b). The experimental studies in op/op mice also establish the role of macrophage/microglia lineage cells in anti-ganglioside Ab- and FcγR-mediated inflammation in the nerves and associated nodal and axonal injury to intact nerve fibers. Macrophages are an essential component of innate immunity and a key regulator of inflammation. A growing body of research indicates that inflammation orchestrated by macrophages is a critical component in autoimmune neuropathies (Griffin et al., 1990; Kiefer et al., 2001). A large number of clinicopathological studies in GBS have implied a central pathogenic role of these cells in producing inflammation and nerve fiber injury (Griffin et al., 1990; McKhann et al., 1993; Griffin et al., 1995; Kiefer et al., 2001). Our recent study demonstrates that macrophages rapidly infiltrate the injured nerves and directly links these macrophages to anti-ganglioside Ab-mediated inhibition of axon regeneration (Zhang et al., 2014).
The studies in DBA/2J (C5-deficient) mice suggest that terminal complement complex does not participate in anti-ganglioside Abs-mediated nodal and axonal injury in our mouse model. This is similar to our previous findings showing that complement activation through the classic pathway is not required for anti-ganglioside Ab-mediated inhibition of axon regeneration (Lehmann et al., 2007; Zhang et al., 2014). However, complement involvement has been shown in other animal models of anti-ganglioside Ab-mediated nerve injury (Plomp et al., 1999; Goodfellow et al., 2005; Susuki et al., 2007b; Susuki et al., 2012). Whether one or both arms of innate immunity contribute to anti-ganglioside Ab-mediated nerve injury is a very complex issue and is beyond the scope of this study. First, all previous studies with complement were performed on FcγR-competent animals and the role of FcγRs was not evaluated in these studies. Second, almost all studies required the use of exogenous heterologous source of complement, which is partly attributable to the low lytic capacity of mouse complement as reported by us and others (Zhang et al., 2004; Willison et al., 2008). Third, exogenous/heterologous complement is foreign protein for the host animal and can enhance the inflammatory response, including macrophage infiltration, which might participate in nerve injury in these models. Fourth, degenerating myelin (independent of antibodies) in injured nerves can itself activate complement as reported by a series of studies form Koski and colleagues (Vanguri et al., 1982; Koski et al., 1985). Fifth, macrophages have the capacity to synthesize and secrete complement in the local milieu (Zimmer et al., 1982; Nathan, 1987). Sixth, complement and FcγRs likely regulate each other in complicated ways that are incompletely defined. Our current study showed that the C5-deficient DBA mice with nerve injury have even more robust upregulation of FcγRs compared with wild-type mice. Overall, previous work (Plomp et al., 1999; Goodfellow et al., 2005; Susuki et al., 2007b; Susuki et al., 2012) and our current study suggest that, in the human/GBS settings, it is likely that both arms of innate immunity, complement and FcγRs, participate in Ab-mediated inflammation and nerve injury.
Our studies with human serum show nodal but not axonal injury. Because animal studies require large amount of antibodies, sera instead of IgG fractions or affinity-purified anti-ganglioside antibodies were used in the study. The milder nerve injury with human autoAbs is likely multifactorial. For example, the half-life of human/heterologous IgG in mouse is much shorter compared with the endogenous mouse IgG. Moreover, the binding of human IgG with mouse FcγRs is much lower (Nimmerjahn and Ravetch, 2010), and the relative affinity of IgG Fcs for respective FcγRs dictates the ensuing inflammatory response and inflammation-mediated injury (Nimmerjahn and Ravetch, 2007; Nimmerjahn and Ravetch, 2010; Nimmerjahn and Ravetch, 2011). We suspect that these factors attenuate inflammatory response produced by human autoAbs in the mouse nerves. This attenuated inflammation was sufficient to disrupt nodal integrity but insufficient to cause axon degeneration. These experimental findings suggest that nodal and axonal injury can be dissociated; whether axon degeneration follows nodal injury may depend upon the severity of autoAb-induced inflammation. This postulate is consistent with the notion that axonal GBS patients with rapid recovery have nodal injury without significant axonal degeneration (Ho et al., 1997; Kokubun et al., 2010; McGonigal et al., 2010).
The current study tested four experimental monoclonal anti-ganglioside antibodies and found that all anti-ganglioside Abs do not induce injury to intact nerve fibers in this model. Our finding that GD1a-2b did not induce nerve injury in wild-type animals is consistent with previous studies showing that higher nerve GD1a density is necessary to produce anti-GD1a Ab-mediated axonal injury in mice and wild-type animals are resistant to anti-GD1a Ab-mediated nerve injury (Goodfellow et al., 2005; Zhang et al., 2014). Further, failure of GM1–2b to produce nerve injury reflects the lower affinity of this mAb (Lopez et al., 2010) and cryptic nature of GM1 gangliosides in the peripheral nerves, as reported previously (Greenshields et al., 2009). Interestingly, GD1a/GT1b-2b mAb caused severe inhibition of axon regeneration in a nerve crush model (Lehmann et al., 2007), but failed to produce injury in the L5SNT model. This discrepancy could be due to differences in the fine specificity of these mAbs and the extent of breakdown of BNB and inflammatory responses in L5SNT and nerve crush models. The current study suggests that multiple factors, such as Ab fine specificity, antigen-binding affinity, antigen density in the target nerve fibers, Ab affinity to FcγRs, breakdown of BNB, and extent of inflammation, are all relevant in producing autoAb-mediated nerve fiber injury.
Footnotes
This work was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health (Grant R01 NS42888, R01 NS54962, R21NS087467), the GBS/CIDP Foundation International, and Frank Gruen and family.
The authors declare no competing financial interests.
References
- Cifuentes-Diaz C, Dubourg O, Irinopoulou T, Vigny M, Lachkar S, Decker L, Charnay P, Denisenko N, Maisonobe T, Léger JM, Viala K, Hauw JJ, Girault JA. Nodes of Ranvier and paranodes in chronic acquired neuropathies. PLoS One. 2011;6:e14533. doi: 10.1371/journal.pone.0014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo YM, Graziano RF, Repp R, van de Winkel JG. Clinical significance of IgG Fc receptors and Fc gamma R-directed immunotherapies. Immunol Today. 1997;18:127–135. doi: 10.1016/S0167-5699(97)01007-4. [DOI] [PubMed] [Google Scholar]
- Fewou SN, Rupp A, Nickolay LE, Carrick K, Greenshields KN, Pediani J, Plomp JJ, Willison HJ. Anti-ganglioside antibody internalization attenuates motor nerve terminal injury in a mouse model of acute motor axonal neuropathy. J Clin Invest. 2012;122:1037–1051. doi: 10.1172/JCI59110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow JA, Bowes T, Sheikh K, Odaka M, Halstead SK, Humphreys PD, Wagner ER, Yuki N, Furukawa K, Furukawa K, Plomp JJ, Willison HJ. Overexpression of GD1a ganglioside sensitizes motor nerve terminals to anti-GD1a antibody-mediated injury in a model of acute motor axonal neuropathy. J Neurosci. 2005;25:1620–1628. doi: 10.1523/JNEUROSCI.4279-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenshields KN, Halstead SK, Zitman FM, Rinaldi S, Brennan KM, O'Leary C, Chamberlain LH, Easton A, Roxburgh J, Pediani J, Furukawa K, Furukawa K, Goodyear CS, Plomp JJ, Willison HJ. The neuropathic potential of anti-GM1 autoantibodies is regulated by the local glycolipid environment in mice. J Clin Invest. 2009;119:595–610. doi: 10.1172/JCI37338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, Stoll G, Li CY, Tyor W, Cornblath DR. Macrophage responses in inflammatory demyelinating neuropathies. Ann Neurol. 1990;27:S64–S68. doi: 10.1002/ana.410270717. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Li CY, Ho TW, Xue P, Macko C, Gao CY, Yang C, Tian M, Mishu B, Cornblath DR. Guillain-Barre syndrome in northern China: the spectrum of neuropathological changes in clinically defined cases. Brain. 1995;118:577–595. doi: 10.1093/brain/118.3.577. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Li CY, Ho TW, Tian M, Gao CY, Xue P, Mishu B, Cornblath DR, Macko C, McKhann GM, Asbury AK. Pathology of the motor-sensory axonal Guillain-Barre syndrome. Ann Neurol. 1996a;39:17–28. doi: 10.1002/ana.410390105. [DOI] [PubMed] [Google Scholar]
- Griffin JW, Li CY, Macko C, Ho TW, Hsieh ST, Xue P, Wang FA, Cornblath DR, McKhann GM, Asbury AK. Early nodal changes in the acute motor axonal neuropathy pattern of the Guillain-Barre syndrome. J Neurocytol. 1996b;25:33–51. doi: 10.1007/BF02284784. [DOI] [PubMed] [Google Scholar]
- Hafer-Macko C, Hsieh ST, Li CY, Ho TW, Sheikh K, Cornblath DR, McKhann GM, Asbury AK, Griffin JW. Acute motor axonal neuropathy: an antibody-mediated attack on axolemma. Ann Neurol. 1996;40:635–644. doi: 10.1002/ana.410400414. [DOI] [PubMed] [Google Scholar]
- Ho TW, Hsieh ST, Nachamkin I, Willison HJ, Sheikh K, Kiehlbauch J, Flanigan K, McArthur JC, Cornblath DR, McKhann GM, Griffin JW. Motor nerve terminal degeneration provides a potential mechanism for rapid recovery in acute motor axonal neuropathy after Campylobacter infection [see comments] Neurology. 1997;48:717–724. doi: 10.1212/WNL.48.3.717. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Cornblath DR. Guillain-Barre syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Hadden RD, Gregson NA, Smith KJ. Pathogenesis of Guillain-Barre syndrome. J Neuroimmunol. 1999;100:74–97. doi: 10.1016/S0165-5728(99)00195-2. [DOI] [PubMed] [Google Scholar]
- Kiefer R, Kieseier BC, Stoll G, Hartung HP. The role of macrophages in immune-mediated damage to the peripheral nervous system. Prog Neurobiol. 2001;64:109–127. doi: 10.1016/S0301-0082(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Kokubun N, Nishibayashi M, Uncini A, Odaka M, Hirata K, Yuki N. Conduction block in acute motor axonal neuropathy. Brain. 2010;133:2897–2908. doi: 10.1093/brain/awq260. [DOI] [PubMed] [Google Scholar]
- Koski CL, Vanguri P, Shin ML. Activation of the alternative pathway of complement by human peripheral nerve myelin. J Immunol. 1985;134:1810–1814. [PubMed] [Google Scholar]
- Lee DZ, Chung JM, Chung K, Kang MG. Reactive oxygen species (ROS) modulate AMPA receptor phosphorylation and cell-surface localization in concert with pain-related behavior. Pain. 2012;153:1905–1915. doi: 10.1016/j.pain.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann HC, Lopez PH, Zhang G, Ngyuen T, Zhang J, Kieseier BC, Mori S, Sheikh KA. Passive immunization with anti-ganglioside antibodies directly inhibits axon regeneration in an animal model. J Neurosci. 2007;27:27–34. doi: 10.1523/JNEUROSCI.4017-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wada R, Kawai H, Sango K, Deng C, Tai T, McDonald MP, Araujo K, Crawley JN, Bierfreund U, Sandhoff K, Suzuki K, Proia RL. A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder [see comments] J Clin Invest. 1999;103:497–505. doi: 10.1172/JCI5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez PH, Zhang G, Zhang J, Lehmann HC, Griffin JW, Schnaar RL, Sheikh KA. Passive transfer of IgG Anti-GM1 antibodies impairs peripheral nerve repair. J Neurosci. 2010;30:9533–9541. doi: 10.1523/JNEUROSCI.2281-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn MP, Johnson LA, Fromholt SE, Itonori S, Huang J, Vyas AA, Hildreth JE, Griffin JW, Schnaar RL, Sheikh KA. High-affinity anti-ganglioside IgG antibodies raised in complex ganglioside knockout mice: reexamination of GD1a immunolocalization. J Neurochem. 2000;75:404–412. doi: 10.1046/j.1471-4159.2000.0750404.x. [DOI] [PubMed] [Google Scholar]
- McGonigal R, Rowan EG, Greenshields KN, Halstead SK, Humphreys PD, Rother RP, Furukawa K, Willison HJ. Anti-GD1a antibodies activate complement and calpain to injure distal motor nodes of Ranvier in mice. Brain. 2010;133:1944–1960. doi: 10.1093/brain/awq119. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Cornblath DR, Griffin JW, Ho TW, Li CY, Jiang Z, Wu HS, Zhaori G, Liu Y, Jou LP. Acute motor axonal neuropathy: a frequent cause of acute flaccid paralysis in China. Ann Neurol. 1993;33:333–342. doi: 10.1002/ana.410330402. [DOI] [PubMed] [Google Scholar]
- Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev. 2010;236:265–275. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. FcgammaRs in health and disease. Curr Top Microbiol Immunol. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- O'Hanlon GM, Plomp JJ, Chakrabarti M, Morrison I, Wagner ER, Goodyear CS, Yin X, Trapp BD, Conner J, Molenaar PC, Stewart S, Rowan EG, Willison HJ. Anti-GQ1b ganglioside antibodies mediate complement-dependent destruction of the motor nerve terminal. Brain. 2001;124:893–906. doi: 10.1093/brain/124.5.893. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, Molenaar PC, O'Hanlon GM, Jacobs BC, Veitch J, Daha MR, van Doorn PA, van der Meché FG, Vincent A, Morgan BP, Willison HJ. Miller Fisher anti-GQ1b antibodies: alpha-latrotoxin-like effects on motor end plates. Ann Neurol. 1999;45:189–199. doi: 10.1002/1531-8249(199902)45:2<189::AID-ANA9>3.0.CO%3B2-T. [DOI] [PubMed] [Google Scholar]
- Pollard JD, Westland KW, Harvey GK, Jung S, Bonner J, Spies JM, Toyka KV, Hartung HP. Activated T cells of nonneural specificity open the blood–nerve barrier to circulating antibody. Ann Neurol. 1995;37:467–475. doi: 10.1002/ana.410370409. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. Fiber composition of the rat sciatic nerve. Anat Rec. 1986;215:71–81. doi: 10.1002/ar.1092150111. [DOI] [PubMed] [Google Scholar]
- Schnaar RL, Fromholt SE, Gong Y, Vyas AA, Laroy W, Wayman DM, Heffer-Lauc M, Ito H, Ishida H, Kiso M, Griffin JW, Shiekh KA. IgG-class mouse monoclonal antibodies to major brain gangliosides. Anal Biochem. 2002;302:276–284. doi: 10.1006/abio.2001.5540. [DOI] [PubMed] [Google Scholar]
- Sheikh KA, Griffin JW. Variants of the Guillain Barre syndrome: progress toward fulfilling “Koch's postulates.”. Ann Neurol. 2001;49:694–696. doi: 10.1002/ana.1057. [DOI] [PubMed] [Google Scholar]
- Sheikh KA, Zhang G. An update on pathobiologic roles of anti-glycan antibodies in Guillain-Barré syndrome. F1000 Biol Rep. 2010;2 doi: 10.3410/B2-21. pii:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh KA, Zhang G, Gong Y, Schnaar RL, Griffin JW. An anti-ganglioside antibody-secreting hybridoma induces neuropathy in mice. Ann Neurol. 2004;56:228–239. doi: 10.1002/ana.20173. [DOI] [PubMed] [Google Scholar]
- Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies JM, Pollard JD, Bonner JG, Westland KW, McLeod JG. Synergy between antibody and P2-reactive T cells in experimental allergic neuritis. J Neuroimmunol. 1995a;57:77–84. doi: 10.1016/0165-5728(94)00164-J. [DOI] [PubMed] [Google Scholar]
- Spies JM, Westland KW, Bonner JG, Pollard JD. Intraneural activated T cells cause focal breakdown of the blood–nerve barrier. Brain. 1995b;118:857–868. doi: 10.1093/brain/118.4.857. [DOI] [PubMed] [Google Scholar]
- Su K, Wu J, Edberg JC, Li X, Ferguson P, Cooper GS, Langefeld CD, Kimberly RP. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcgammaRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J Immunol. 2004;172:7186–7191. doi: 10.4049/jimmunol.172.11.7186. [DOI] [PubMed] [Google Scholar]
- Susuki K, Nishimoto Y, Yamada M, Baba M, Ueda S, Hirata K, Yuki N. Acute motor axonal neuropathy rabbit model: immune attack on nerve root axons. Ann Neurol. 2003;54:383–388. doi: 10.1002/ana.33333. [DOI] [PubMed] [Google Scholar]
- Susuki K, Baba H, Tohyama K, Kanai K, Kuwabara S, Hirata K, Furukawa K, Furukawa K, Rasband MN, Yuki N. Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers. Glia. 2007a;55:746–757. doi: 10.1002/glia.20503. [DOI] [PubMed] [Google Scholar]
- Susuki K, Rasband MN, Tohyama K, Koibuchi K, Okamoto S, Funakoshi K, Hirata K, Baba H, Yuki N. Anti-GM1 antibodies cause complement-mediated disruption of sodium channel clusters in peripheral motor nerve fibers. J Neurosci. 2007b;27:3956–3967. doi: 10.1523/JNEUROSCI.4401-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susuki K, Yuki N, Schafer DP, Hirata K, Zhang G, Funakoshi K, Rasband MN. Dysfunction of nodes of Ranvier: a mechanism for anti-ganglioside antibody-mediated neuropathies. Exp Neurol. 2012;233:534–542. doi: 10.1016/j.expneurol.2011.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyka KV, Drachman DB, Griffin DE, Pestronk A, Winkelstein JA, Fishbeck KH, Kao I. Myasthenia gravis: study of humoral immune mechanisms by passive transfer to mice. N Engl J Med. 1977;296:125–131. doi: 10.1056/NEJM197701202960301. [DOI] [PubMed] [Google Scholar]
- van Sorge NM, van den Berg LH, Geleijns K, van Strijp JA, Jacobs BC, van Doorn PA, Wokke JH, van de Winkel JG, Leusen JH, van der Pol WL. Anti-GM1 IgG antibodies induce leukocyte effector functions via Fcgamma receptors. Ann Neurol. 2003;53:570–579. doi: 10.1002/ana.10503. [DOI] [PubMed] [Google Scholar]
- van Sorge NM, van der Pol WL, Jansen MD, Geleijns KP, Kalmijn S, Hughes RA, Rees JH, Pritchard J, Vedeler CA, Myhr KM, Shaw C, van Schaik IN, Wokke JH, van Doorn PA, Jacobs BC, van de Winkel JG, van den Berg LH. Severity of Guillain-Barre syndrome is associated with Fc gamma Receptor III polymorphisms. J Neuroimmunol. 2005;162:157–164. doi: 10.1016/j.jneuroim.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Vanguri P, Koski CL, Silverman B, Shin ML. Complement activation by isolated myelin: activation of the classical pathway in the absence of myelin-specific antibodies. Proc Natl Acad Sci U S A. 1982;79:3290–3294. doi: 10.1073/pnas.79.10.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedeler CA, Raknes G, Myhr KM, Nyland H. IgG Fc-receptor polymorphisms in Guillain-Barre syndrome. Neurology. 2000;55:705–707. doi: 10.1212/WNL.55.5.705. [DOI] [PubMed] [Google Scholar]
- Westland KW, Pollard JD, Sander S, Bonner JG, Linington C, McLeod JG. Activated non-neural specific T cells open the blood-brain barrier to circulating antibodies. Brain. 1999;122:1283–1291. doi: 10.1093/brain/122.7.1283. [DOI] [PubMed] [Google Scholar]
- Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. 2002;125:2591–2625. doi: 10.1093/brain/awf272. [DOI] [PubMed] [Google Scholar]
- Willison HJ, Halstead SK, Beveridge E, Zitman FM, Greenshields KN, Morgan BP, Plomp JJ. The role of complement and complement regulators in mediating motor nerve terminal injury in murine models of Guillain-Barre syndrome. J Neuroimmunol. 2008;201:172–182. doi: 10.1016/j.jneuroim.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Yan WX, Taylor J, Andrias-Kauba S, Pollard JD. Passive transfer of demyelination by serum or IgG from chronic inflammatory demyelinating polyneuropathy patients. Ann Neurol. 2000;47:765–775. [PubMed] [Google Scholar]
- Yuki N, Yamada M, Koga M, Odaka M, Susuki K, Tagawa Y, Ueda S, Kasama T, Ohnishi A, Hayashi S, Takahashi H, Kamijo M, Hirata K. Animal model of axonal Guillain-Barre syndrome induced by sensitization with GM1 ganglioside. Ann Neurol. 2001;49:712–720. doi: 10.1002/ana.1012. [DOI] [PubMed] [Google Scholar]
- Yuki N, Susuki K, Koga M, Nishimoto Y, Odaka M, Hirata K, Taguchi K, Miyatake T, Furukawa K, Kobata T, Yamada M. Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barre syndrome. Proc Natl Acad Sci U S A. 2004;101:11404–11409. doi: 10.1073/pnas.0402391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Lopez PH, Li CY, Mehta NR, Griffin JW, Schnaar RL, Sheikh KA. Anti-ganglioside antibody-mediated neuronal cytotoxicity and its protection by intravenous immunoglobulin: implications for immune neuropathies. Brain. 2004;127:1085–1100. doi: 10.1093/brain/awh127. [DOI] [PubMed] [Google Scholar]
- Zhang G, Bogdanova N, Gao T, Song JJ, Cragg MS, Glennie MJ, Sheikh KA. Fcgamma receptor-mediated inflammation inhibits axon regeneration. PLoS One. 2014;9:e88703. doi: 10.1371/journal.pone.0088703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B, Hartung HP, Scharfenberger G, Bitter-Suermann D, Hadding U. Quantitative studies of the secretion of complement component C3 by resident, elicited and activated macrophages: comparison with C2, C4 and lysosomal enzyme release. Eur J Immunol. 1982;12:426–430. doi: 10.1002/eji.1830120513. [DOI] [PubMed] [Google Scholar]