Abstract
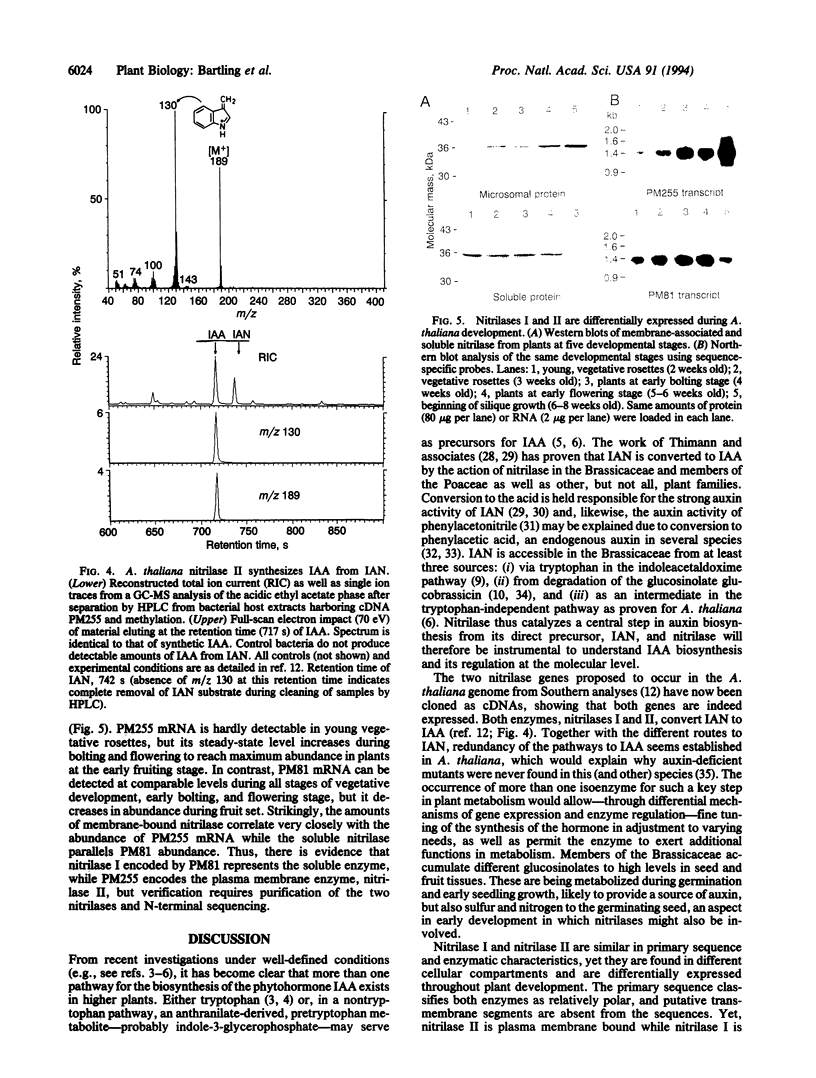
As in maize [Wright, A.D., Sampson, M. B., Neuffer, M. G., Michalczuk, L., Slovin, J. P. & Cohen, J. D. (1991) Science 254, 998-1000], the major auxin of higher plants, indole-3-acetic acid, is synthesized mainly via a nontryptophan pathway in Arabidopsis thaliana [Normanly, J., Cohen, J. D. & Fink, G. R. (1993) Proc. Natl. Acad. Sci. USA 90, 10355-10359]. In the latter species, the hormone may be accessible from the glucosinolate glucobrassicin (indole-3-methyl glucosinolate) and from L-tryptophan via indoleacetaldoxime under special circumstances. In each case, indole-3-acetonitrile is the immediate precursor, which is converted into indole-3-acetic acid through the action of nitrilase (nitrile aminohydrolase, EC 3.5.5.1). The genome of A. thaliana contains two nitrilase genes. Nitrilase I had been cloned earlier in our laboratory. The cDNA for nitrilase II (PM255) was cloned and encodes an enzyme that converts indole-3-acetonitrile to indole-3-acetic acid, the plant hormone. We show that the intracellular location as well as the expression pattern of the two A. thaliana nitrilases are distinctly different. Nitrilase I is soluble and is expressed throughout development, but at a very low level during the fruiting stage, while nitrilase II is tightly associated with the plasma membrane, is barely detectable in young rosettes, but is strongly expressed during bolting, flowering, and especially fruit development. The results indicate that more than one pathway of indole-3-acetic acid biosynthesis via indole-3-acetonitrile exists in A. thaliana and that these pathways are differentially regulated throughout plant development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartling D., Seedorf M., Mithöfer A., Weiler E. W. Cloning and expression of an Arabidopsis nitrilase which can convert indole-3-acetonitrile to the plant hormone, indole-3-acetic acid. Eur J Biochem. 1992 Apr 1;205(1):417–424. doi: 10.1111/j.1432-1033.1992.tb16795.x. [DOI] [PubMed] [Google Scholar]
- Bialek K., Michalczuk L., Cohen J. D. Auxin Biosynthesis during Seed Germination in Phaseolus vulgaris. Plant Physiol. 1992 Sep;100(1):509–517. doi: 10.1104/pp.100.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brent R., Ptashne M. Mechanism of action of the lexA gene product. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4204–4208. doi: 10.1073/pnas.78.7.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M. C., Stowe B. B. Distribution and Variation of Indole Glucosinolates in Woad (Isatis tinctoria L.). Plant Physiol. 1971 Oct;48(4):498–503. doi: 10.1104/pp.48.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Höhfeld J., Veenhuis M., Kunau W. H. PAS3, a Saccharomyces cerevisiae gene encoding a peroxisomal integral membrane protein essential for peroxisome biogenesis. J Cell Biol. 1991 Sep;114(6):1167–1178. doi: 10.1083/jcb.114.6.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Izui H., Nagasawa T., Yamada H. Nitrilase in biosynthesis of the plant hormone indole-3-acetic acid from indole-3-acetonitrile: cloning of the Alcaligenes gene and site-directed mutagenesis of cysteine residues. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):247–251. doi: 10.1073/pnas.90.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last R. L., Bissinger P. H., Mahoney D. J., Radwanski E. R., Fink G. R. Tryptophan mutants in Arabidopsis: the consequences of duplicated tryptophan synthase beta genes. Plant Cell. 1991 Apr;3(4):345–358. doi: 10.1105/tpc.3.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last R. L., Fink G. R. Tryptophan-Requiring Mutants of the Plant Arabidopsis thaliana. Science. 1988 Apr 15;240(4850):305–310. doi: 10.1126/science.240.4850.305. [DOI] [PubMed] [Google Scholar]
- Meyer C., Feyerabend M., Weiler E. W. Fusicoccin-Binding Proteins in Arabidopsis thaliana (L.) Heynh. : Characterization, Solubilization, and Photoaffinity Labeling. Plant Physiol. 1989 Feb;89(2):692–699. doi: 10.1104/pp.89.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczuk L., Ribnicky D. M., Cooke T. J., Cohen J. D. Regulation of indole-3-acetic Acid biosynthetic pathways in carrot cell cultures. Plant Physiol. 1992 Nov;100(3):1346–1353. doi: 10.1104/pp.100.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J., Cohen J. D., Fink G. R. Arabidopsis thaliana auxotrophs reveal a tryptophan-independent biosynthetic pathway for indole-3-acetic acid. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10355–10359. doi: 10.1073/pnas.90.21.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C. K., O'Hara P. J., Eichinger G., Rothman J. H., Stevens T. H. Molecular analysis of the yeast VPS3 gene and the role of its product in vacuolar protein sorting and vacuolar segregation during the cell cycle. J Cell Biol. 1990 Sep;111(3):877–892. doi: 10.1083/jcb.111.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker D. M., Malyj L. D., McBride K. E. Purification and properties of a nitrilase specific for the herbicide bromoxynil and corresponding nucleotide sequence analysis of the bxn gene. J Biol Chem. 1988 May 5;263(13):6310–6314. [PubMed] [Google Scholar]
- THIMANN K. V., MAHADEVAN S. Enzymatic hydrolysis of indoleacetonitrile. Nature. 1958 May 24;181(4621):1466–1467. doi: 10.1038/1811466a0. [DOI] [PubMed] [Google Scholar]
- THIMANN K. V., MAHADEVAN S. NITRILASE. I. OCCURRENCE, PREPARATION, AND GENERAL PROPERTIES OF THE ENZYME. Arch Biochem Biophys. 1964 Apr;105:133–141. doi: 10.1016/0003-9861(64)90244-9. [DOI] [PubMed] [Google Scholar]
- Wright A. D., Sampson M. B., Neuffer M. G., Michalczuk L., Slovin J. P., Cohen J. D. Indole-3-Acetic Acid Biosynthesis in the Mutant Maize orange pericarp, a Tryptophan Auxotroph. Science. 1991 Nov 15;254(5034):998–1000. doi: 10.1126/science.254.5034.998. [DOI] [PubMed] [Google Scholar]






