Abstract
Excessive ultraviolet radiation (UVR) exposure of the skin is associated with adverse clinical outcomes. Although both exogenous sunscreens and endogenous tissue components (including melanins and tryptophan-derived compounds) reduce UVR penetration, the role of endogenous proteins in absorbing environmental UV wavelengths is poorly defined. Having previously demonstrated that proteins which are rich in UVR-absorbing amino acid residues are readily degraded by broadband UVB-radiation (containing UVA, UVB and UVC wavelengths) here we hypothesised that UV chromophore (Cys, Trp and Tyr) content can predict the susceptibility of structural proteins in skin and the eye to damage by physiologically relevant doses (up to 15.4 J/cm2) of solar UVR (95% UVA, 5% UVB). We show that: i) purified suspensions of UV-chromophore-rich fibronectin dimers, fibrillin microfibrils and β- and γ-lens crystallins undergo solar simulated radiation (SSR)-induced aggregation and/or decomposition and ii) exposure to identical doses of SSR has minimal effect on the size or ultrastructure of UV chromophore-poor tropoelastin, collagen I, collagen VI microfibrils and α-crystallin. If UV chromophore content is a factor in determining protein stability in vivo, we would expect that the tissue distribution of Cys, Trp and Tyr-rich proteins would correlate with regional UVR exposure. From bioinformatic analysis of 244 key structural proteins we identified several biochemically distinct, yet UV chromophore-rich, protein families. The majority of these putative UV-absorbing proteins (including the late cornified envelope proteins, keratin associated proteins, elastic fibre-associated components and β- and γ-crystallins) are localised and/or particularly abundant in tissues that are exposed to the highest doses of environmental UVR, specifically the stratum corneum, hair, papillary dermis and lens. We therefore propose that UV chromophore-rich proteins are localised in regions of high UVR exposure as a consequence of an evolutionary pressure to express sacrificial protein sunscreens which reduce UVR penetration and hence mitigate tissue damage.
Keywords: Photodecomposition, UVA radiation, Photoageing, Sunscreen, Solar simulated radiation, Chromophores
Abbreviations: AFM, atomic force microscopy; ECM, extracellular matrix; HDF, human dermal fibroblast; LTBP, latent transforming growth factor β-binding protein; MED, minimal erythemal dose; MMP, matrix metalloproteinase; ROS, reactive oxygen species; SSR, solar simulated radiation; TGFβ, transforming growth factor β; UVR, ultraviolet radiation.
Graphical abstract
Highlights
Major structural proteins such as collagen I and tropoelastin are UVA-resistant.
In contrast, proteins which are rich in Cys, Trp and Tyr residues are UV-susceptible.
These proteins are concentrated in UV exposed tissues.
UV-chromophore (Cys, Trp, Tyr)-rich proteins may act as endogenous sunscreens.
Introduction
Chronic exposure to ultraviolet radiation (UVR) produces dose-dependent changes in skin structure which have a profound impact on both its mechanical function and clinical appearance (see [1,2] for comprehensive reviews of this photoageing process). In the early stages of photoageing key elastic fibre-associated components such as fibrillin microfibrils and fibulin-5 are lost from the papillary dermis [3,4], whilst the latter stages are characterised by the deposition of disorganised elastotic material (solar elastosis: [5,6], the gain of glycosaminoglycans [7] and the loss of both fibrillar collagens [8,9] and collagen VII anchoring fibrils [10]. Although the potential role of cell-derived extracellular matrix (ECM) proteases in mediating dermal remodelling is well established [11–14] the substrate specificity of the UVR up-regulated matrix metalloproteinases (MMPs-1, −2, −3, −7, −9 and −12) is low [15]. Collectively, these enzymes are capable of degrading many key ECM components including fibrillar collagens, elastic fibre constituents, proteoglycans, adhesive glycoproteins and dermal-epidermal junction components [16–18]. Therefore it is difficult to reconcile the concept of non-specific cell-derived ECM proteases as the sole mediators of matrix degradation with the complex spatial, compositional and temporal ECM remodelling which characterises chronically UV-exposed skin. In common with other groups [19,20] we have therefore suggested that the direct photochemical decomposition of ECM proteins may be an important factor in mediating differential remodelling of the dermis in photoaged skin [15,21,22].
Whilst exposure to UVR can profoundly influence collagen structure and function, such changes have been induced using sources which emit large doses of UVC (<280 nm) radiation (for example [23,24]). In contrast, the wavelengths which comprise sunlight at the Earth’s surface and are capable of penetrating to the dermis (UVA and UVB: Fig. 1) [25] have a minimal effect on collagen structure and function [15,20,26,27] even when the doses employed are more than two orders of magnitude greater than the minimal erythemal doses (MEDs) of the respective wavebands (see Watson et al. [22] for a comprehensive review). Although collagen is largely devoid of sulphur- (cysteine [Cys]) and aromatic ring-containing (Histidine [His], Phenylalanine [Phe], Tryptophan [Trp] and Tyrosine [Tyr]) amino acid residues other ECM components contain a much larger percentage of these UV chromophores [28]. We have previously demonstrated that fibrillin microfibrils (essential elastic fibre components) and the ubiquitous adhesive glycoprotein fibronectin (with UV chromophore contents of 21% and 13% respectively) are susceptible to low doses of broadband UVB radiation (Philips TL-12 source) which have no effect on the electrophoretic mobility of collagen I (UV chromophore content 2%) [15]. However, whilst this broadband UVB source is used extensively in photobiology research, its spectral output is dissimilar to terrestrial solar UVR having a small UVC content and crucially a larger UVB:UVA ratio (Fig. 1a) [29]. Studies on skin optics predict that longer wavelength UVR will penetrate the skin more deeply and there is a consensus that UVA (as opposed to UVB) radiation is the key wave band responsible for photoageing (Fig. 1b) [1,30,31].
Fig. 1.
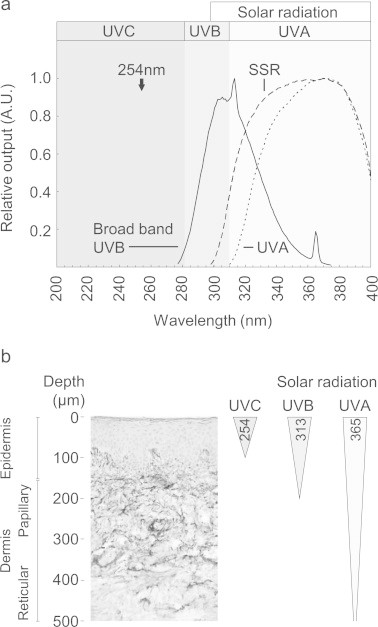
Solar simulated radiation is primarily composed of penetrating, yet low energy, UVA wavelengths. (a) Normalised spectral outputs of broadband UVB, solar simulated radiation (SSR) and filtered SSR (UVA) sources. The spectral output of SSR (WG320 filtered xenon arc lamp) is composed of 5.0% UVB radiation. Further filtration (with WG345) removes the majority of the UVB component (UVA: 99.6%, UVB: 0.4%). In contrast, the spectral output of a broadband UVB source such as the Philips TL-12 [15] contains UVA (44.3%), UVB (55.3%) and UVC (0.4%) components whilst the output of many UVC-rich sources peaks at 254 nm. (b) Although such 254 nm UVC radiation can influence the epidermis, solar UVB and UVA radiations can penetrate the papillary dermis and subcutaneous tissue respectively (adapted from [31]).
In this study we have used a combination of biochemical and ultrastructural analyses to test the hypothesis that environmentally relevant doses of solar simulated radiation (SSR: 95.0% UVA, 5.0% UVB) and UVA radiation (filtered SSR: 99.6% UVA, 0.4% UVB) are capable of differentially degrading key dermal ECM components (collagen I, tropoelastin, collagen VI, fibrillin microfibrils, fibronectin) and lens proteins ((α-, β- and γ-crystallins) according to their terrestrial UV chromophore (Cys, Trp and Tyr) [32–34] content. As a consequence of their high molecular weight, ECM proteins such as fibrillin-1 and fibronectin contain many potential amino acid UV chromophores (fibrillin-1 [Accession number: P3555] for example contains 362 Cys, 13 Trp and 92 Tyr residues). This compositional complexity (which in the case of fibrillin microfibrils is combined with a resistance to dissociation into component monomers), makes it impractical to characterise the effects of UVR exposure on individual amino acid residues. Instead we employed biochemical approaches, including gel electrophoresis which has been used extensively by ourselves, and others, to probe the effects of UVR on the structure of fibronectin, collagen I and tropoelastin and the lens crystallins (see [24] and our comprehensive review of collagen and UVR [22]). For the remaining assemblies, fibrillin and collagen VI microfibrils we characterised the effects of UVR on their ultrastructure. It is clear that the beads-on-a-string morphology of these microfibrils is susceptible to single amino acid substitutions and also to the local micro-environment [15,16,35–37]. Finally we have related UV chromophore content to tissue location (within skin and eyes) and formulated a secondary hypothesis that amino acid chromophore-rich proteins compliment the sunscreen activity of other endogenous chromophores including melanins and tryptophan-derived components such as N′-formylkynurenine and 6-formylindolo[3,2-b]carbazole [38].
Materials and methods
All chemicals were of analytical grade and obtained from Sigma-Aldrich Co. Ltd. (Poole, UK) unless otherwise stated.
Synthesis and purification of ECM proteins
Placental bovine collagen I and human plasma fibronectin were purchased from Sigma-Aldrich Co. Ltd and recombinant human tropoelastin (SHELΔ26A [Synthetic Human Elastin without domain 26A] which contains residues 27–724 and corresponds to the 60 kDa mature protein following signal peptide removal) was expressed in bacteria and purified as previously described [39]. Fibrillin and collagen VI microfibrils were extracted and co-purified from post-confluent cultures of human dermal fibroblasts (HDFs) by bacterial collagenase digestion and size exclusion chromatography [15,40]. The HDFs (a gift from Dr Stuart Cain, University of Manchester UK and originally sourced from Cascade Biologics, Invitrogen, Paisley, UK) were maintained to 6 weeks post-confluency at 37 °C and 5% CO2 in DMEM + GlutaMAX (supplemented with 10% foetal calf serum and 50 µg/mL penicillin/streptomycin; Gibco, Paisley, UK). Cell cultures were digested for 2 h with 0.5 mg/mL bacterial collagenase type IA (suspended in 0.4 M NaCl, 0.05 M Tris–HCl, 0.01 M CaCl2 at pH 7.4, and supplemented with protease inhibitors: 2 mM phenylmethanesulfonyl fluoride and 5 mM N-Ethylmaleimide) and subsequently purified by low pressure chromatography on an AKTA prime plus system coupled to a Sepharose CL-2B column (GE Healthcare, Little Chalfont, UK) which was equilibrated in high salt buffer (0.4 M NaCl, 0.05 M Tris–HCl at pH 7.4). Irradiation experiments were conducted on two pooled microfibril-rich fractions from the centre of the excluded volume (V0) peak.
Whilst the susceptibility of isolated proteins to UVR exposure is commonly investigated using molecules extracted from non-human sources including rat, chick and cow [22], it is important to establish the inter-species homology and in particular the relative chromophore content for each of the target proteins. The primary amino acid sequences of both the alpha 1 and alpha 2 chains of bovine collagen I are highly homologous to their human counterparts (col1a1=98%; col1a2=93%) and as a consequence the concentration and relative proportion of UV chromophore and oxidation sensitive amino acids is identical for the alpha 1 chains and nearly identical for the alpha 2 chain (human: 0 Cys, 5 Met, 0 Trp, 2 Tyr; bovine: 0 Cys, 4 Met, 0 Trp, 1 Tyr). The situation is more complicated for plasma and tissue fibronectin. Whilst both molecular species function as dimers, in plasma fibronectin only one sub-unit contains a V domain and both sub-units are missing the EIIIA and EIIIB modules. However it is also the case that tissue fibronectin is a mixture of alternatively spliced isoforms which vary in a tissue-, cell-type- and temporal-manor [41]. Therefore given the heterogeneity of tissue fibronectin isoforms, and common use of plasma fibronectin as a functional homologue for tissue fibronectin (in experiments designed to promote cell-adhesion and ECM synthesis [42]) we felt that conclusions concerning the UVR susceptibility of plasma fibronectin could reasonably be extrapolated to tissue fibronectin. The lens crystallins are a complex family of related proteins but the amino acid sequence homology between human and lens crystallins is very high as is the identity of the putative UVR and ROs-susceptible amino acid residues (e.g. human and bovine beta B2 crystallins share 97% homology and identical numbers of Cys, Met, Trp and Tyr residues). In addition to these proteins we also test the effects of UVR on recombinant human tropoelastin and fibrillin and collagen VI microfibrils which were synthesised by human dermal fibroblasts. Despite the high sequence homology which is evident between these structural proteins, it is also clear that additional post-translational modifications (occurring either during synthesis or as a consequence of ageing) are likely to alter the photochemistry of these proteins [43].
UV irradiation of isolated proteins and ECM assemblies
Prior to irradiation experiments collagen I was digested with pepsin in acetic acid and neutralised with 1 M Tris, fibronectin was solubilised in a neutral high salt buffer (0.4 M NaCl, 0.05 M Tris–HCl, 0.01 M CaCl2 at pH 7.4: [15]) and recombinant tropoelastin was reconstituted in phosphate buffered saline. Subsequently, protein suspensions were irradiated using a Solar Simulator (Applied Photophysics, Cambridge, UK) consisting of a xenon arc lamp filtered with Schott (Stafford, UK) WG320 and WG345 filters for SSR and UVA respectively. The suspensions (diluted to a final protein concentration of 0.5;mg/mL) and the pooled microfibril-rich fractions were exposed in triplicate to radiation doses of 0.8, 7.7 and 15.4 J/cm2 (SSR, exposure times: 25 s, 4 min 16 s and 8 min 32 s) and 1, 10 and 20 J/cm2 (UVA, exposure times: 20 s, 3 min 20 s and 6 min 40 s) in 2×10 mm (height×diameter) polyethylene lids (total volume 250 µL). These environmentally relevant doses equated to 0.21–4.16 times the SSR MED and 0.01–0.20 times the UVA MED respectively (as calculated from skin phototest data described in 22). All suspensions were incubated at room temperature for the duration of the UVR exposure. Spectral outputs of the solar simulator were measured using a double grating spectroradiometer (Bentham Instruments Ltd., Reading, UK) calibrated to National Physical Laboratory (Teddington, UK) standards. Routine irradiance measurements were made using a UVX radiometer and UVX-31 and UVX-36 detectors (UVR Products; Upland, CA, USA) calibrated against the spectroradiometer measurements for the SSR (irradiance=54.3 mW/cm2) and UVA (irradiance=51.4 mW/cm2) spectral outputs. The structural effects of UV irradiation on protein structure were characterised by gel electrophoresis and atomic force microscopy (AFM).
Gel electrophoresis of isolated proteins
Gel electrophoresis approaches have frequently been used to identify UVR induced changes in collagen [15,24,44,45] and H2O2 induced changes in fibronectin [46]. In this study we assessed the effects of exposure to both SSR and UVA radiation on suspensions of collagen I, tropoelastin, fibronectin and lens crystallins by reducing SDS-PAGE. Boiled protein suspensions (in Laemmli sample buffer containing 1.43 M β-mecaptoethanol) were electrophoresed on either 6% resolving / 4% stacking gels (for 4 h at 150 V) or pre-cast 4–12% gradient NuPAGE®Novex® Bis-Tris Mini Gels with MOPS buffer (Invitrogen, Paisley, UK; for 50 min at 200V). The low Mw lens crystallins were electrophoresed on pre-cast 4–12% gradient gels (NuPAGE®Novex® Bis-Tris Mini Gels with MES SDS running buffer (Invitrogen, Paisley, UK) for 35 min at 200 V. Protein bands were visualised by Instant Blue™ staining (Expedeon Ltd., Cambridge, UK) prior to drying onto blotting paper (Gel Master dryer, Gardner Denver Ltd., Alton, UK). Gel images were digitised on a high resolution flat-bed scanner (Epson expression 1600; Seiko Epson Corp., Hemel Hempstead, UK) at 800 dpi prior to analysis using ImageJ software [47] with the inbuilt gel analysis tools to measure the optical density (O.D.: normalised against the optical density of non-UVR exposed α1(I) and α2(I) collagen and tropoelastin bands or total stained protein [fibronectin]) and position of protein bands.
Ultrastructural characterisation of fibrillin and collagen VI microfibrils
In contrast to monomeric and polymeric suspensions of collagen I, tropoelastin, fibronectin and the lens crystallins, which are readily resolved by gel electrophoresis, fibrillin and collagen VI microfibrils were extracted from HDF cultures as ultrastructurally distinct macro-molecular assemblies. As a consequence we employed intermittent contact mode AFM (Nanoscope IIIa Multimode AFM: Bruker, Corp., Santa Bárbara, CA, USA) to characterise the effects of UVR exposure on the morphology of microfibril-rich suspensions as previously described [15,48]. Height images (2×2 µm, sampled at 512×512 pixels) of fibrillin and collagen VI microfibrils were captured using OTESPA high aspect ratio etched silicon probes at scan rates of 1.97 Hz and software determined cantilever oscillation frequencies and drive amplitudes (Bruker, Corp.). For each experimental group, fibrillin microfibril periodicity (bead to bead distance; n=500) and flexion angle (a measure of microfibril flexibility; n=500) were determined by WSxM scanning probe microscopy software and bespoke routines written in Microsoft Visual Basic [15,49,50]. The periodicity of collagen VI microfibrils was also measured using WSxM software to generate axial height profiles (n=150 repeats) [48]. Although we have previously demonstrated that these techniques (electrophoretic mobility and ultrastructural analysis) are capable of identifying UVR-induced changes in protein structure [15] it is also possible that exposure to SSR and UVA may induce functional changes (including increased susceptibility to proteases) which cannot be detected by these techniques. In addition the effects of the buffer constituents (phosphate based for re-suspended, lyophilised proteins and Tris-based for isolated microfibrils) on protein-radical transfer reactions remain undefined.
Bioinformatic analyses
The ProtParam tool (Swiss Institute of Bioinformatics: http://expasy.org/) was used to calculate the UVA chromophore (Cys, Trp and Tyr) content of 244 human proteins located in the dermis, epidermis, hair, cornea, lens and vitreous humour. The protein and gene names, UVA chromophore contents and individual Cys, Trp and Tyr contents of these proteins are detailed in the Supplementary Table S1A–F.
Statistical analysis
Data are expressed as mean ± SEM and statistical comparisons were made by Students t-test (p<0.05 considered significant) Q–Q normality plots. Box plots depict the population median and the 1st, 25th, 75th and 99th percentiles. All statistical analyses were conducted using SPSSv20 (IBM, Portsmouth, UK).
Results and discussion
UV chromophore-poor ECM proteins are resistant to environmentally relevant doses of solar simulated and UVA radiation
Whilst there is evidence that fibrillar collagen I is UVR labile, UV-mediated changes in collagen structure and function are only induced by exposure to non-physiologically relevant wavelengths (UVC) and/or doses up to three orders of magnitude greater than the MED [22]. Having previously shown that exposure to 10 MEDs of broadband UVB had no observable effect on the quaternary structure of monomeric collagen I or the electrophoretic mobility of individual collagen I α chains [15] we now show that UVA-chromophore-poor collagen I monomers (Table S1: 0.32% Cys, Trp and Tyr) are similarly unaffected by exposure to environmentally relevant doses of both SSR (up to 15.4 J/cm2; 4.2×MED) and UVA radiation (20 J/cm2; 0.2×MED) (Fig. 2a and b). These wavebands failed to induce aggregation or decomposition in a suspension of monomeric collagen I and had no effect on electrophoretic mobility or normalised optical density of either the α1(I) and α2(I) bands (SSR: α1[I]: r2=0.016, slope=−6.0×10− 5, α2(I): r2=0.558, slope= 2.1×10−3, UVA radiation: α1[I]: r2=0.445, slope=−1.6×10−3, α2(I): r2=0.310, slope=−4.0×10−4). As a consequence, atrophy of dermal fibrillar collagens characteristic of photoaged skin is unlikely to arise from either direct absorption of terrestrial solar UVA or UVB radiation or from interaction with the reactive oxygen species (ROS) that may generated via photosensitisation [51–53]. Instead, UV-upregulation of MMPs-1, −2, −3, and −9 [11,12] may be the main mediators of fibrillar collagen (I, III and V) degradation [17].
Fig. 2.
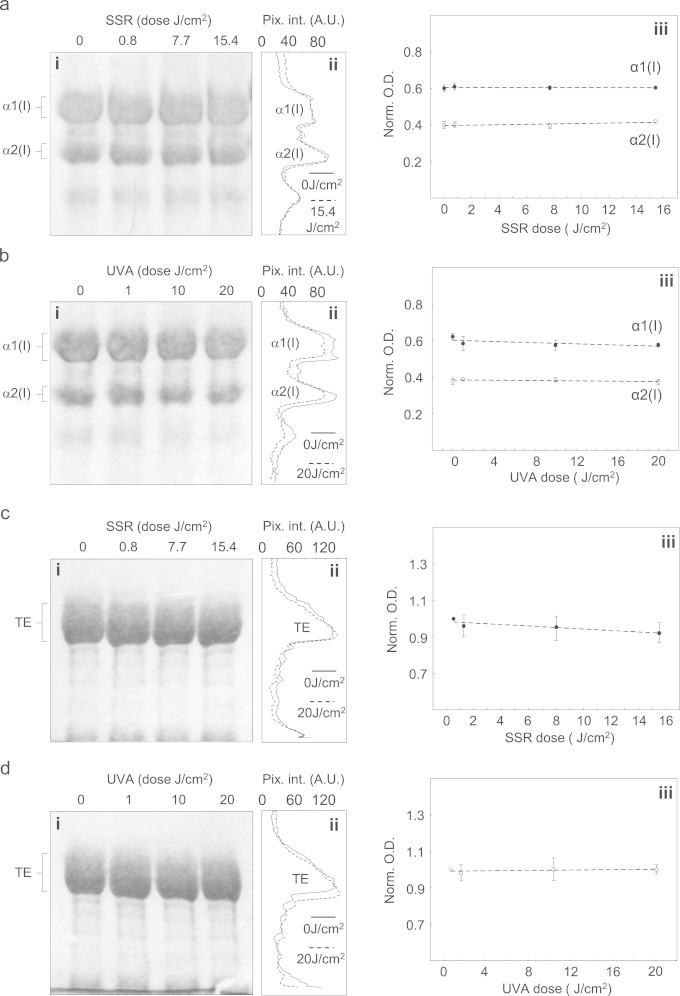
Key UV chromophore-poor, dermal structural proteins are unaffected by exposure to either solar simulated or UVA radiation. (a) and (b) Following exposure to SSR radiation suspensions of monomeric collagen I were size fractionated by reducing SDS-PAGE and the constituent α chains identified according to their molecular weights (α1:130 kDa, α2:107 kD). Even at the highest doses there was no evidence of protein aggregation or decomposition on the gel (i) or derived densitometric line profiles (ii), whilst the normalised optical density of both α chains remained invariant (iii). (c) and (d) The electrophoretic mobility and optical density of tropoelastin (TE) was similarly unaffected by exposure to both SSR (c) and UVA (d) radiation (SDS-PAGE [i], densitometric line profile [ii] and normalised optical density [iii]).
In addition to the tensile strength conferred by collagen I and III fibres, skin relies on the mechanical properties of elastic fibres to impart elastic recoil. Whilst these fibres are complex, macro-molecular assemblies [54], the most abundant component, elastin, and its precursor tropoelastin, are also largely devoid of UV chromophores (Table S1: elastin and tropoelastin: 2.34% and 2.16% respectively). Compared with the extensive literature on UVR/collagen interactions, the effects of UVR on the structure of elastin are poorly defined. Elastin-derived peptides as simple model systems undergo structural modification following exposure to high dose UVC radiation (300 × MED) [55,56]. In this study, we exposed human tropoelastin, to environmentally relevant UVR doses and wavelengths. As as with collagen I, tropoelastin was remarkably stable. Neither SSR nor UVA radiation induced any observable change in the electrophoretic profile of purified tropoelastin bands visualised by SDS-PAGE (Fig. 2c and 2d: [iii] normalised optical density of the tropoelastin monomer following exposure to SSR: r2=0.784, slope=−3.1×10−3 or UVA radiation: r2=0.264, slope=0.5×10−3). It therefore appears likely that likely that secondary enzymatic mechanisms and/or cell signalling pathways, are likely to drive the profound remodelling of the elastic fibre architecture (termed solar elastosis) which characterises chronically photoaged skin enzymatic [1,57].
Although collagen I and elastin are the most abundant cutaneous ECM proteins, the dermal matrix is a complex structure which also relies on glycoproteins, proteoglycans and minor collagens in order to function. One such minor (with regards to abundance) yet ubiquitous collagen is collagen VI. Three alpha chains of collagen VI form extracellular tetramers which in turn assemble into characteristic double-beaded microfibrils which play important roles in cell-matrix interactions [58,59]. Crucially the characteristic double-beaded morphology and native periodicity (~109 nm) of these microfibrils means that environmentally induced changes in their structure (by surface charge or [Ca2+]) can be quantified by AFM [48,50]. Whilst collagen VI monomers are slightly enriched in UVA chromophores (3.66%) compared with collagen I (0.32%) and tropoelastin (2.16%), exposure to either SSR or UVA, at doses of 15.4 and 20 J/cm2 respectively, had no detectable effect on the morphology or periodicity of isolated collagen VI microfibrils (Fig. 3: SSR: 0 J/cm2=107.9±0.6 nm, 15.4 J/cm2=107.2±0.9 nm, p=0.080; UVA radiation: 0 J/cm2=108.2±0.1 nm, 20 J/cm2=108.3±0.3 nm, p=0.744). In contrast to the fibrillar collagens, the distribution and expression of network forming collagen VI is unaffected in photoaged skin [60]. Whilst there is evidence of MMP-2- and −9-mediated collagen VI degradation [61,62] it is unknown if the assembled microfibril (as opposed to isolated α3[VI] chains or pepsin/acid extracted full length collagen VI) is susceptible to these proteases. Therefore the persistence of collagen VI in photoaged skin may be attributable to the resistance of the microfibrillar form to the action of both MMPs and exposure to direct SSR or UVA radiation (Fig. 3). Collectively these data support a model where UV chromophore-poor dermal ECM proteins, elastin and collagen (Table S1) are resistant to damage following direct exposure to solar radiation.
Fig. 3.
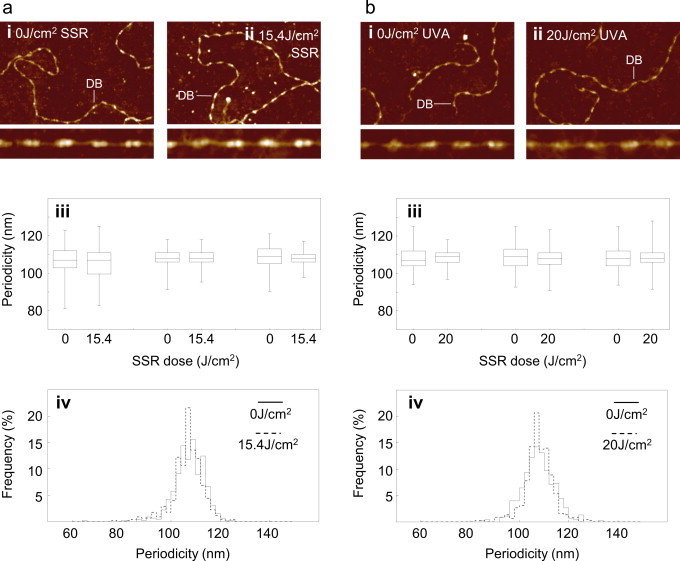
Network forming collagen VI microfibrils are resistant to direct UV exposure. Collagen VI microfibrils were exposed to SSR (a) and UVA (b) radiation doses of 15.4 and 20 J/cm2 respectively. The influence of UVR on microfibril periodicity was assessed by AFM. (a) In AFM height images (1.5×1 µm, 4 nm height) collagen VI microfibrils exposed to 15.4 J/cm2 of SSR (ii) retained their characteristic double bead (DB) structure (i). Microfibril periodicity (iii) was not significantly different in exposed and unexposed populations (performed in triplicate) and the global periodicity frequency distribution remained unimodal and parametric (assessed from Q–Q plots) following SSR exposure (iv). (b) Similarly, exposure to 20 J/cm2 of UVA radiation had no discernible effect on microfibril structure (i) and (ii), periodicity (iii) or global periodicity frequency distribution (iv).
Both SSR and UVA radiation readily degrade UV chromophore-rich fibrillin microfibrils.
In contrast to the low UV chromophore content of collagens and tropoelastin, many important dermal ECM proteins are rich in UV-absorbing amino acid residues [15,22]. In particular, over 16% of the amino acid residues in fibrillin-1, the main component of fibrillin microfibrils, are UVA chromophores (Table S1). When visualised by AFM, fibrillin microfibrils appear as discrete, uniform and semi-rigid beads on a string (Fig. 4ai and bi) with a mean bead to bead periodicity of 55–60 nm (Figs. 4aiii and biii) [63,64]. Exposure to SSR or UVA induced a dose dependent increase in microfibril flexibility (as quantified by a significant decrease in flexion angle: data not shown) and promoted aggregation at higher doses (Figs. 4aii and bii). Both UVR radiation wavebands significantly reduced microfibril periodicity (SSR: 0 J/cm2=56.7±0.2 nm, 15.4 J/cm2=53.3±0.3 nm, p<0.001; UVA: 0 J/cm2 = 58.0±0.3nm, 20 J/cm2=55.7±0.4 nm, p<0.001) and were similarly effective at inducing changes in microfibril ultrastructure (Fig. 4aiii, 4biii and 4c: relative slopes of the periodicity dose response curves: SSR = −0.23 and UVA = −0.14).
Our experiments demonstrate that cystine-rich fibrillin microfibrils are susceptible to UVA radiation which has no effect on UV chromophore-poor tropoelastin (Fig. 2c, 2 d and Fig. 4) and hence supports a selective multi-hit model of photoageing[22]. In this model the susceptibility of these disulphide bonded microfibrils to environmentally relevant doses of SSR or UVA radiation provides a selective mechanism for: (i) the early photochemical decomposition of fibrillin microfibril oxytalan fibres in the papillary dermis by the UVA and UVB components of solar radiation and (ii) the subsequent remodelling of the elastic fibre system in the reticular dermis as a consequence of microfibril exposure to penetrating UVA radiation. In this latter case, photochemical decomposition of fibrillin microfibrils appears to be the triggering event which leads to aberrant TGFβ signalling, the up-regulation of both MMP and tropoelastin synthesis, the subsequent dysregulation of elastogenesis and hence the deposition of elastotic material (solar elastosis)[65–69].
Fig. 4.
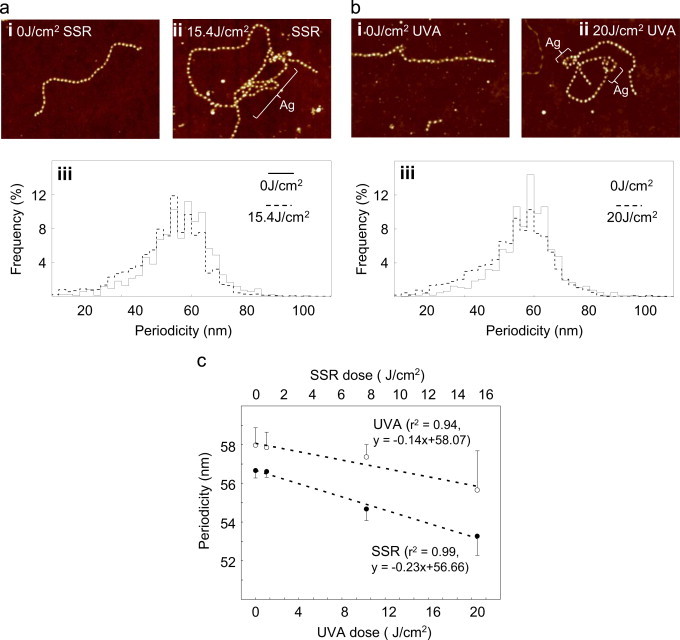
UV chromophore-rich fibrillin microfibrils undergo dose-dependent structural re-arrangements in response to both solar simulated and UVA radiation. (a) Whilst the characteristic beaded structure of fibrillin microfibrils was evident in AFM height images (2×1.7 µm, 10 nm height) of both SSR exposed (ii) and non-exposed (i) populations, following UV irradiation, these macro-molecular assemblies appeared more flexible and prone to aggregation (Ag) and their mean periodicity was significantly reduced. (b)The changes in microfibril structure, as effected by SSR, were replicated by exposure to UVA radiation. Mean microfibril periodicity was reduced by exposure to both SSR and UVA (aiii and biii) and negatively correlated with UV dose (SSR: r2 = 0.99, UVA: r2 = 0.94) (c).
Fibronectin is preferentially degraded by the UVB component of solar simulated radiation.
Fibronectin is a ubiquitous adhesive glycoprotein which undergoes aggregation in response to broadband UVB irradiation [15]. Such structural remodelling (with its attendant risk of cytotoxic amyloid formation) is a common response of polypeptide chains to oxidation and/or UV irradiation [70] both in vitro [71] and in tissues such as the lens [72]. Here, we show that fibronectin aggregation is also induced by exposure to physiological doses of SSR and UVA radiation (Fig. 5a). Crucially however, the structure of fibronectin was markedly more sensitive to SSR, which contains 5% UVB radiation, than to UVA radiation (WG345-filtered SSR: ~0.4% UVB) (Fig. 5b: relative slopes of the fibronectin aggregation dose response curves: SSR = 5.6×10−3 and UVA=0.8×10−3 [normalised optical density of the aggregate band]). Compared with human fibrillin-1, human fibronectin is rich in Trp but contains fewer Cys residues. Fibrillin-1 contains 12.7% Cys, 0.5% Trp, 3.3% Tyr; fibronectin: 2.7% Cys, 1.7% Trp, 4.3% Tyr although, as in fibrillin-1, the majority of these Cys residues, 60 out of 63 in fibronectin, are predicted to be disulphide bonded (forming Cystines where tail absorption stretches into the UVA range). Therefore, whilst both fibrillin-1 and fibronectin are relatively Trp and Cystine-rich (in contrast to collagen I which is devoid of cysteines and hence cystines, tropoelastin which contains only one cysteine and collagen VI which contains two cystines and 16 free cysteines) these moieties are likely to be the key mediators of photochemical damage to proteins [32–34] and hence the differential amino acid content of these proteins may be the primary cause of their differential sensitivity to UVA radiation. As the penetration of UVB radiation into the dermis is limited (Fig. 1) it follows that any biological effects of UVR/fibronectin interaction (which may include, but are unlikely to be limited to, the promotion of proteolysis [73]), will be maximal in the papillary dermis where the early effects of photoageing are observed [4,74]. In common with many dermal matrix proteins, proteins within the eye are also exposed to UVR. As lens crystallins are proposed to accumulate damage with prolonged UVR exposure [75] we also characterised the effects of SSR on the electrophoretic mobility of α-, β-and γ-crystallins.
Fig. 5.
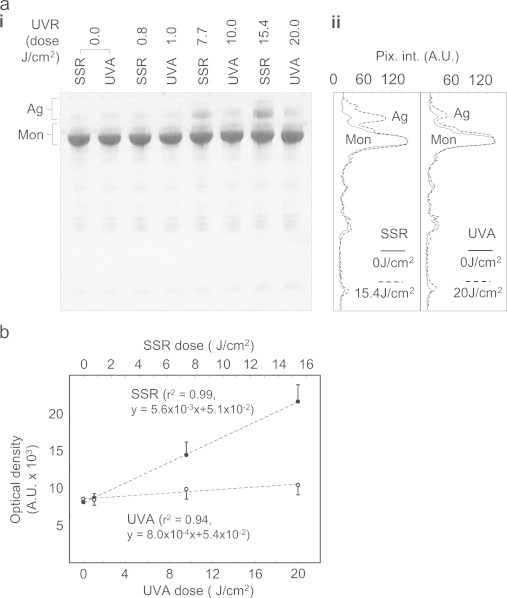
Fibronectin is differentially degraded by solar simulated and UVA radiation. (a) The 220 kDa fibronectin monomer (Mon: identified by reference to a 217 kDa Mw. Marker), was clearly evident by SDS-PAGE (i) regardless of UVR exposure and there was no evidence of UVR induced fragmentation. However the normalised optical intensity of higher Mw. fibronectin species (aggregates: Ag), was increased at higher UVR doses (SSR 7.7 and 15.4 J/cm2 and UVA 10 and 20 J/cm2) and evident in densitometric line profiles extracted from non-UV exposed and SSR and UVA exposed suspensions (ii). (b) Although both UVR sources induced fibronectin aggregation in a dose-dependent manner (SSR: r2 = 0.99, UVA: r2 = 0.94), UVB-containing SSR was seven times as effective in inducing aggregation compared with UVA alone.
SSR induces dimerisation and fragmentation of UV chromophore-rich β- and γ-crystallins.
The formation of age-related nuclear cataracts in the human lens is associated with a loss of optical transparency [76]. Crystallins, which are the major protein components of the lens, are organised into families of molecular chaperones (α-crystallins) and structural proteins (β- and γ-crystallins) [75]. These proteins undergo extensive age-related oxidation [77] and have been shown to be susceptible to UVC (γ), broad-spectrum UVA, B and C (β) and UVA (α) damage [78–81]. As the amino acid composition of the crystallins is largely conserved between species [82] we exposed purified suspensions of bovine lens-derived α-, β- and γ-crystallins to 15.4 J/cm2 SSR (Fig. 6). Alpha-crystallin underwent slight dimerisation following SSR exposure. Krivandin and colleagues have suggested that the structure of the α-crystallins may allow these proteins to accumulate UVA induced damage without undergoing major conformational changes [81]. Whilst our bioinformatic analyses demonstrate that both αA- and αB-crystallins are relatively rich in UVA chromophores (Table S1: 4.6% and 2.3% respectively) compared with collagen I, both proteins are chromophore-poor compared with the fibrillin-1, fibronectin and crucially the β- and γ-crystallins.
Fig. 6.
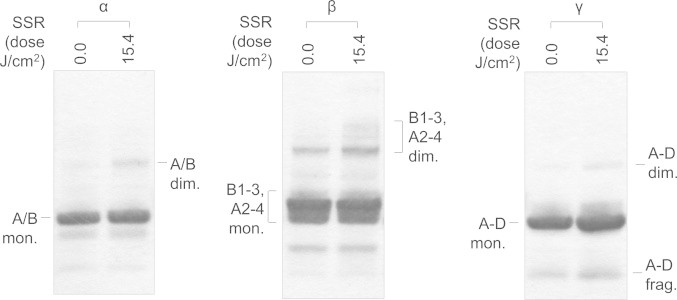
The susceptibility of lens crystallins to solar simulated radiation is predicted by their UV chromophore-content. Bovine lens crystallins (α, β and γ) were exposed to an SSR dose of 15.4 J/cm2, reduced and electrophoresed on 4–12% gradient gels in MOPS buffer. The mixture of 19.9 kDa αA- and 20.2 kDa αB-lens crystallin monomers (A/B mon.) migrated as a distinct 20 kDa band. SSR exposure induced detectable, yet minimal, dimerisation (A/B dim.). The multiple β-crystallin proteins present in the commercial preparation[83] migrated as a monomeric doublet (likely to be comprised of β-crystallin B1 28.1 kDa, B2 23.3 kDa, B3, 24.3 kDa, A2 22.2 kDa, A3 25.1 kDa and A4 23.8 kDa) and a ~40 kDa dimer. SSR exposure induced extensive dimerisation, increasing the intensity of the 40 kDa band and higher Mw bands (up to ~60 kDa). The four γ-crystallins (A)–(D) range in Mw from 20.9–21.1 kDa. Exposure to SSR induced both dimerisation (~40 kDa A−D dim.) and fragmentation (~10 kDa A−D frag.) of the γ-crystallin monomers (A−D mon.).
The β-crystallins co-purify as a complex mixture of B1, B2, B3, A2, A3 and A4 forms [83]. Collectively these proteins contain between 7.8% (B2) and 13.0% (A3) Cys, Trp and Tyr residues (Table S1). In contrast to the α-crystallins, the β-crystallins undergo more extensive structural re-organisation as a consequence of SSR exposure (Fig. 6) and the increased staining intensity of multiple bands > 40 kDa in Mw indicates that most, if not all, β-crystallins undergo SSR-mediated dimerisation. Although we cannot rule out the potential for SSR to induce larger oligomeric aggregates, the similar staining intensity of the monomeric bands makes this unlikely. Also the suspension of γ-crystallins (potentially containing all four UVA chromophore-rich forms: A−[14.3%], B−[14.8%], C−[16.7%] and D−[14.4%]) underwent both dimerisation (40 kDa band) and fragmentation (10 kDa band). Therefore, in common with dermal ECM proteins, the susceptibility of lens crystallins to SSR correlated with Cys, Trp and Tyr content, although cysteine mediated absorption may not be a factor (bovine β2 lens crystallin, for example, contains nine un-disulphide bonded cysteines). The β- and γ-crystallins appear to be well placed to act, alongside other lens components, as photosensitisers, antioxidants or benign filters [84].
Previous studies have concentrated primarily on characterising the susceptibility of a few cutaneous proteins to UVR exposure as a consequence of either their tryptophan residues (in the case of keratin [85]) or the prevalence of age-related post-translational modifications (which principally affect collagen and elastin) (see Wondrak et al. for a comprehensive review [38]). Crucially however, tissues such as human skin are composed of hundreds of proteins [86,87] many of which have the potential to absorb UVR as a consequence of their amino acid composition alone. Therefore, we propose that the content of amino acid UV chromophores can predict the relative susceptibility of proteins to UVA exposure in vitro and that UV exposed human tissues (skin and the eye) contain both UV-stable and UV labile proteins. If the relative UV-stability of proteins impacts on biological function then evolution might be expected to exert a selective pressure on the distribution of UV-susceptible proteins in these organs.
UV exposed tissues are rich in UV- and potentially oxidation-susceptible proteins
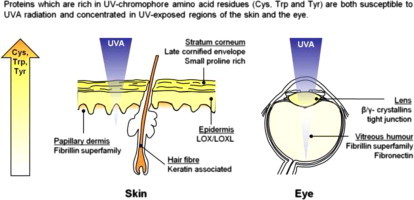
Having initially identified 244 proteins characteristic of human epidermis [88–92], dermis [15], hair shaft [93–96], lens [97,98], lens capsule [99–103], cornea [99,104,105] and vitreous humour [106,107] (Table S1), we then used the UniProt protein database (http://www.uniprot.org) to calculate the relative composition of UV chromophores (Fig. 7a). We found that UV-exposed organs contain proteins that are highly enriched in UV chromophores and other proteins which are largely devoid of Cys, Trp and Tyr. In skin, UVA chromophore-rich proteins are concentrated in the stratum corneum of the epidermis (the Cys-rich late cornified envelope proteins and small proline-rich proteins), the papillary dermis (the structurally related and Cys-rich fibrillins, LTBPS and fibulins) and the hair shaft (both Cys and Tyr-rich keratin associated proteins) (Fig. 7a and Table S1). Furthermore, the epidermis also contains abundant collagen and elastin-cross-linking enzymes (lysyl oxidase and lysyl oxidase-like proteins) which have no known function in this ECM-lacking tissue [108] yet are rich in Tyr residues. The significant up-regulation of Cys-rich late cornified envelope proteins in response to UVB radiation exposure [91] provides further support for this hypothesis, it remains to be determined if other potential proteins rich in UV chromophores are similarly UV inducible. We propose that, in skin, evolution rather than exerting an adaptive pressure to locate UV susceptible proteins in UV-shielded anatomical sites, has favoured the expression of said proteins in tissues and tissue regions which not only receive maximal UV exposure but will also be exposed to oxidative stress as a consequence of photodynamic processes (although our current experiments cannot establish the relative contribution of direct and ROS mediated pathways [52,53,109]). Crucially whilst UV-stable proteins such as collagen I and elastin are distributed throughout the dermis, fibrillin microfibril bundles are concentrated in the outmost (and therefore most UV exposed) layer of the dermis whilst the late cornified envelope proteins (which are also chromophore rich) are abundant in the outer stratum corneum.
Fig. 7.
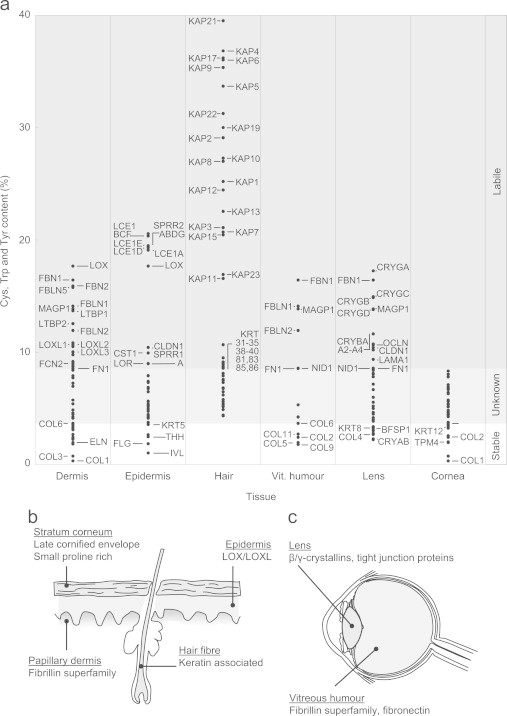
The distribution of UV chromophore-rich proteins within tissues is correlated with UV exposure. (a) Cumulative UV chromophore (Cys, Trp and Tyr) content of characteristic cutaneous (dermal, epidmeral and hair) and ocular (vitreous humour, lens and corneal) proteins. Selected proteins are highlighted by their gene name. From the experimental data presented in this study we would predict that proteins with a Cys, Trp and Tyr content equal to or less than 3.7% (the UVA chromophore content of collagen VI) will be stable under physiological UVA doses. Whilst proteins composed of more than 8.5% UV chromophore (such as fibronectin) will be UVA labile. (b) and (c) UV chromophore-rich proteins (grey shading) are unevenly distributed in the skin and eye.
This differential localisation of UV chromophore-rich proteins is also a feature of the eye, where the collagenous cornea is largely devoid of Cys, Trp and Tyr containing proteins but the lens, which undergoes profound age-related remodelling, is rich in non-proteinaceous chromophores [77] and in β- and γ-crystallins (Fig. 7c). The vitreous humour also contains free ECM molecules, such as fibrillin-1, fibulin-1, MAGP-1 and fibronectin [106]. It is currently unknown whether these matrix components perform any structural function in the vitreous humour but their chromophore content may help to act as an additional sunscreen for retinal cells.
Conclusion
On the basis of these data we propose that proteins rich in UV absorbing and oxidation-sensitive amino acid residues may perform additional functions (to their structural roles) as sacrificial sunscreens in UV exposed tissues.
Conflicts of interest
This study was funded in part by an Alliance Boots programme Grant awarded to CEMG, MJS, REBW and NKG. Alliance Boots was involved in the decision to submit this work for publication but played no role in the study design or subsequent data collection, analysis and interpretation. We have also carried out independent commercial studies on topical skin treatments funded by: biominerals NV; Croda Chemicals Europe Limited; Degussa AG; Kao Corporation; L’Oréal Recherche; Oriflame GTC Limited; Proctor and Gamble Technical Centers and Unilever R&D Colworth.
Acknowledgements
This work was supported by a programme Grant from Alliance Boots, Nottingham, UK and by a Senior Age, UK Fellowship awarded to MJS. The authors are grateful to Dr Nigel Hodson of the University of Manchester BioAFM Facility for his expert advice.
Appendix A. Supplementary materials
Supplementary materials
References
- 1.Yaar M., Gilchrest B.A. Photoageing: mechanism, prevention and therapy. British Journal of Dermatology. 2007;157(5):874–887. doi: 10.1111/j.1365-2133.2007.08108.x. 17711532 [DOI] [PubMed] [Google Scholar]
- 2.Naylor E.C., Watson R.E., Sherratt M.J. Molecular aspects of skin ageing. Maturitas. 2011;69(3):249–256. doi: 10.1016/j.maturitas.2011.04.011. 21612880 [DOI] [PubMed] [Google Scholar]
- 3.Watson R.E., Griffiths C.E., Craven N.M., Shuttleworth C.A., Kielty C.M. Fibrillin-rich microfibrils are reduced in photoaged skin. Distribution at the dermal-epidermal junction. Journal of Investigative Dermatology. 1999;112(5):782–787. doi: 10.1046/j.1523-1747.1999.00562.x. 10233772 [DOI] [PubMed] [Google Scholar]
- 4.Kadoya K. Fibulin-5 deposition in human skin: decrease with ageing and ultraviolet B exposure and increase in solar elastosis. British Journal of Dermatology. 2005;153(3):607–612. doi: 10.1111/j.1365-2133.2005.06716.x. 16120151 [DOI] [PubMed] [Google Scholar]
- 5.Dahlbäck K. Fibrillin immunoreactive fibers constitute a unique network in the human dermis: immunohistochemical comparison of the distributions of fibrillin, vitronectin, amyloid P component, and orcein stainable structures in normal skin and elastosis. Journal of Investigative Dermatology. 1990;94(3):284–291. doi: 10.1111/1523-1747.ep12874430. 1689758 [DOI] [PubMed] [Google Scholar]
- 6.Hunzelmann N., Nischt R., Brenneisen P., Eickert A., Krieg T. Increased deposition of fibulin-2 in solar elastosis and its colocalization with elastic fibres. British Journal of Dermatology. 2001;145(2):217–222. doi: 10.1046/j.1365-2133.2001.04337.x. 11531782 [DOI] [PubMed] [Google Scholar]
- 7.Bernstein E.F., Underhill C.B., Hahn P.J., Brown D.B., Uitto J. Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. British Journal of Dermatology. 1996;135(2):255–262. doi: 10.1111/j.1365-2133.1996.tb01156.x. 8881669 [DOI] [PubMed] [Google Scholar]
- 8.Talwar H.S., Griffiths C.E., Fisher G.J., Hamilton T.A., Voorhees J.J. Reduced type I and type III procollagens in photodamaged adult human skin. Journal of Investigative Dermatology. 1995;105(2):285–290. doi: 10.1111/1523-1747.ep12318471. 7543550 [DOI] [PubMed] [Google Scholar]
- 9.El-Domyati M. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Experimental Dermatology. 2002;11(5):398–405. doi: 10.1034/j.1600-0625.2002.110502.x. 12366692 [DOI] [PubMed] [Google Scholar]
- 10.Craven N.M. Clinical features of photodamaged human skin are associated with a reduction in collagen VII. British Journal of Dermatology. 1997;137(3):344–350. 9349327 [PubMed] [Google Scholar]
- 11.Fisher G.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0. 8552187 [DOI] [PubMed] [Google Scholar]
- 12.Fisher G.J., Voorhees J.J. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal Transduction cascades that induce AP-1-regulated Matrix metalloproteinases that degrade human skin in vivo. Journal of Investigative Dermatology Symposium Proceedings. 1998;3(1):61–68. 9732061 [PubMed] [Google Scholar]
- 13.Saarialho-Kere U. Accumulation of matrilysin (MMP-7) and macrophage metalloelastase (MMP-12) in actinic damage. Journal of Investigative Dermatology. 1999;113(4):664–672. doi: 10.1046/j.1523-1747.1999.00731.x. 10504457 [DOI] [PubMed] [Google Scholar]
- 14.Chung J.H. Ultraviolet modulation of human macrophage metalloelastase in human skin in vivo. Journal of Investigative Dermatology. 2002;119(2):507–512. doi: 10.1046/j.1523-1747.2002.01844.x. 12190877 [DOI] [PubMed] [Google Scholar]
- 15.Sherratt M.J. Low-dose ultraviolet radiation selectively degrades chromophore-rich extracellular matrix components. The Journal of Pathology. 2010;222(1):32–40. doi: 10.1002/path.2730. 20552716 [DOI] [PubMed] [Google Scholar]
- 16.Ashworth J.L. Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodeling. Biochemical Journal. 1999;340:171–181. [PMC free article] [PubMed] [Google Scholar]
- 17.Chakraborti S., Mandal M., Das S., Mandal A., Chakraborti T. Regulation of matrix metalloproteinases: an overview. Molecular and Cellular Biochemistry. 2003;253(1–2):269–285. doi: 10.1023/a:1026028303196. 14619979 [DOI] [PubMed] [Google Scholar]
- 18.Pasternak B., Aspenberg P. Metalloproteinases and their inhibitors-diagnostic and therapeutic opportunities in orthopedics. Acta Orthopaedica. 2009;80(6):693–703. doi: 10.3109/17453670903448257. 19968600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson R.J., Cooper D.R. The effect of ultraviolet irradiation on acid-soluble collagen. Biochemical Journal. 1967;105(3):965–969. doi: 10.1042/bj1050965. 16742572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menter J.M. Effect of UV on the susceptibility of acid-soluble Skh-1 hairless mouse collagen to collagenase. Photodermatology, Photoimmunology, and Photomedicine. 2003;19(1):28–34. doi: 10.1034/j.1600-0781.2003.00004.x. 12713552 [DOI] [PubMed] [Google Scholar]
- 21.Thurstan S.A. Chemical consequences of cutaneous photoageing. Chemistry Central Journal. 2012;6(1):34. doi: 10.1186/1752-153X-6-34. 22534143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson R.E., Gibbs N.K., Griffiths C.E., Sherratt M.J. Damage to skin extracellular matrix induced by UV exposure. Antioxidants and Redox Signaling. 2014;21(7):1063–1077. doi: 10.1089/ars.2013.5653. 24124905 [DOI] [PubMed] [Google Scholar]
- 23.Miyata T., Sode T., Rubin A.L., Stenzel K.H. Effects of ultraviolet irradiation on native and telopeptide-poor collagen. Biochimica et Biophysica Acta. 1971;229(3):672–680. doi: 10.1016/0005-2795(71)90283-2. 5103026 [DOI] [PubMed] [Google Scholar]
- 24.Jariashvili K. UV damage of collagen: insights from model collagen peptides. Biopolymers. 2012;97(3):189–198. doi: 10.1002/bip.21725. 22002434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grether-Beck S., Buettner R., Krutmann J. Ultraviolet a radiation-induced expression of human genes: molecular and photobiological mechanisms. Journal of Biological Chemistry. 1997;378(11):1231–1236. 9426182 [PubMed] [Google Scholar]
- 26.Kato Y., Uchida K., Kawakishi S. Oxidative degradation of collagen and its model peptide by ultraviolet irradiation. Journal of Agricultural and Food Chemistry. 1992;40(3):373–379. [Google Scholar]
- 27.Menter J.M., Patta A.M., Sayre R.M., Dowdy J., Willis I. Effect of UV irradiation on type I collagen fibril formation in neutral collagen solutions. Photodermatology, Photoimmunology, and Photomedicine. 2001;17(3):114–120. doi: 10.1034/j.1600-0781.2001.170302.x. 11419538 [DOI] [PubMed] [Google Scholar]
- 28.Bensasson R.V., Land E.J., Truscott T.G. Excited States and Free Radicals in Biology and Medicine Contributions from Flash Photolysis and Pulse Radiolysis. Oxford Univeristy Press Inc.; 1993. pp. Oxford, 24–63. [Google Scholar]
- 29.Diffey B.L. Sources and measurement of ultraviolet radiation. Methods. 2002;28(1):4–13. doi: 10.1016/s1046-2023(02)00204-9. 12231182 [DOI] [PubMed] [Google Scholar]
- 30.Kligman L.H., Agin P.P., Sayre R.M. Broad-spectrum sunscreens with UVA I and UVA II absorbers provide increased protection against solar-simulating radiation-induced dermal damage in hairless mice. Journal of the Society of Cosmetic Chemists. 1996;47:129–155. [Google Scholar]
- 31.Askew E.W. Work at high altitude and oxidative stress: antioxidant nutrients. Toxicology. 2002;180(2):107–119. doi: 10.1016/s0300-483x(02)00385-2. 12324188 [DOI] [PubMed] [Google Scholar]
- 32.Creed D. The photophysics and photochemistry of the near-UV absoring amino-acids. 1.Tryptophan and its simple derivatives. Photochemistry and Photobiology. 1984;39:537–562. [Google Scholar]
- 33.Creed D. The photophysics and photochemistry of the near-UV absoring amino-acids. 2.Tyrosineand its simple derivatives. Photochemistry and Photobiology. 1984;39:563–575. [Google Scholar]
- 34.Creed D. The photophysics and photochemistry of the near-UV absoring amino-acids. 3.Cysteine and its simple derivatives. Photochemistry and Photobiology. 1984;39:577–583. [Google Scholar]
- 35.Kielty C.M. Marfan syndrome: fibrillin expression and microfibrillar abnormalities in a family with predominant ocular defects. Journal of Medical Genetics. 1995;32(1):1–6. doi: 10.1136/jmg.32.1.1. 7897619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson P.N., Booms P. The molecular pathogenesis of the Marfan syndrome. Cellular and Molecular Life Sciences. 2001;58(11):1698–1707. doi: 10.1007/PL00000807. 11706995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wess T.J. Calcium determines the supramolecular organization of fibrillin-rich microfibrils. The Journal of Cell Biology. 1998;141(3):829–837. doi: 10.1083/jcb.141.3.829. 9566980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wondrak G.T., Jacobson M.K., Jacobson E.L. Endogenous UVA-photosensitizers: mediators of skin photodamage and novel targets for skin photoprotection. Photochemical and Photobiological Sciences. 2006;5(2):215–237. doi: 10.1039/b504573h. 16465308 [DOI] [PubMed] [Google Scholar]
- 39.Martin S.L., Vrhovski B., Weiss A.S. Total synthesis and expression in Escherichia coli of a gene encoding human tropoelastin. Gene. 1995;154(2):159–166. doi: 10.1016/0378-1119(94)00848-m. 7890158 [DOI] [PubMed] [Google Scholar]
- 40.Kielty C.M., Cummings C., Whittaker S.P., Shuttleworth C.A., Grant M.E. Isolation and ultrastructural analysis of microfibrillar structures from foetal bovine elastic tissues. Relative abundance and supramolecular architecture of type VI collagen assemblies and fibrillin. Journal of Cell Science. 1991;99(4):797–807. doi: 10.1242/jcs.99.4.797. 1770007 [DOI] [PubMed] [Google Scholar]
- 41.To W.S., Midwood K.S. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair. 2011;4:21. doi: 10.1186/1755-1536-4-21. 21923916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang G. Fibronectin binds and enhances the activity of bone morphogenetic protein 1. The Journal of Biological Chemistry. 2009;284(38):25879–25888. doi: 10.1074/jbc.M109.024125. 19617627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wondrak G.T., Roberts M.J., Cervantes-Laurean D., Jacobson M.K., Jacobson E.L. Proteins of the extracellular matrix are sensitizers of photo-oxidative stress in human skin cells. Journal of Investigative Dermatology. 2003;121(3):578–586. doi: 10.1046/j.1523-1747.2003.12414.x. 12925218 [DOI] [PubMed] [Google Scholar]
- 44.Rabotyagova O.S., Cebe P., Kaplan D.L. Collagen structural hierarchy and susceptibility to degradation by ultraviolet radiation. Material Science and Engineering C: Materials for Biological Applications. 2008;28(8):1420–1429. doi: 10.1016/j.msec.2008.03.012. 22199459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato Y., Uchida K., Kawakishi S. Aggregation of collagen exposed to UVA in the presence of riboflavin: a plausible role of tyrosine modification. Photochemistry and Photobiology. 1994;59(3):343–349. doi: 10.1111/j.1751-1097.1994.tb05045.x. 8016214 [DOI] [PubMed] [Google Scholar]
- 46.Vartio T. Regular fragmentation of hydrogen peroxide-treated fibronectin. The Journal of Biological Chemistry. 1989;264(8):4471–4475. 2538445 [PubMed] [Google Scholar]
- 47.Abramoff M.D., Magelhaes P.J., Ram S.J. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 48.Sherratt M.J. Substrate chemistry influences the morphology and biological function of adsorbed extracellular matrix assemblies. Biomaterials. 2005;26(34):7192–7206. doi: 10.1016/j.biomaterials.2005.05.010. 15967495 [DOI] [PubMed] [Google Scholar]
- 49.Horcas I. WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Review of Scientific Instruments. 2007;78(1):013705. doi: 10.1063/1.2432410. 17503926 [DOI] [PubMed] [Google Scholar]
- 50.Sherratt M.J., Baldock C., Morgan A., Kielty C.M. The morphology of adsorbed extracellular matrix assemblies is critically dependent on solution calcium concentration. Matrix Biology. 2007;26(3):156–166. doi: 10.1016/j.matbio.2006.10.015. 17166706 [DOI] [PubMed] [Google Scholar]
- 51.Foote C.S. Definition of type I and type II photosensitized oxidation. Photochemistry and Photobiology. 1991;54(5):659. doi: 10.1111/j.1751-1097.1991.tb02071.x. 1798741 [DOI] [PubMed] [Google Scholar]
- 52.Davies M.J. Reactive species formed on proteins exposed to singlet oxygen. Photochem. Photobiol. Sci. 2004;3(1):17–25. doi: 10.1039/b307576c. 14743273 [DOI] [PubMed] [Google Scholar]
- 53.Pattison D.I., Rahmanto A.S., Davies M.J. Photo-oxidation of proteins. Photochemical and Photobiological Sciences. 2012;11(1):38–53. doi: 10.1039/c1pp05164d. 21858349 [DOI] [PubMed] [Google Scholar]
- 54.Kielty C.M., Sherratt M.J., Shuttleworth C.A. Elastic fibres. Journal of Cell Science. 2002;115(14):2817–2828. doi: 10.1242/jcs.115.14.2817. 12082143 [DOI] [PubMed] [Google Scholar]
- 55.Sionkowska A., Skopinska J., Wisniewski M., Leznicki A., Fisz J. Spectroscopic studies into the influence of UV radiation on elastin hydrolysates in water solution. Journal of Photochemistry and Photobiology B: Biology. 2006;85(1):79–84. doi: 10.1016/j.jphotobiol.2006.05.005. 16829118 [DOI] [PubMed] [Google Scholar]
- 56.Sionkowska A., Skopinska J., Wisniewski M., Leznicki A. Spectroscopic studies into the influence of UV radiation on elastin in the presence of collagen. Journal of Photochemistry and Photobiology B: Biology. 2007;86(2):186–191. doi: 10.1016/j.jphotobiol.2006.09.004. 17055284 [DOI] [PubMed] [Google Scholar]
- 57.Schwartz E., Kligman L.H. Topical tretinoin increases the tropoelastin and fibronectin content of photoaged hairless mouse skin. Journal of Investigative Dermatology. 1995;104(4):518–522. doi: 10.1111/1523-1747.ep12606007. 7706770 [DOI] [PubMed] [Google Scholar]
- 58.Baldock C., Sherratt M.J., Shuttleworth C.A., Kielty C.M. The supramolecular organization of collagen VI microfibrils. Journal of Molecular Biology. 2003;330(2):297–307. doi: 10.1016/s0022-2836(03)00585-0. 12823969 [DOI] [PubMed] [Google Scholar]
- 59.Bönnemann C.G. The collagen VI-related myopathies: muscle meets its matrix. Nature Reviews Neurology. 2011;7(7):379–390. doi: 10.1038/nrneurol.2011.81. 21691338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watson R.E. Distribution and expression of type VI collagen in photoaged skin. British Journal of Dermatology. 2001;144(4):751–759. doi: 10.1046/j.1365-2133.2001.04012.x. 11298533 [DOI] [PubMed] [Google Scholar]
- 61.Myint E., Brown D.J., Ljubimov A.V., Kyaw M., Kenney M.C. Cleavage of human corneal type VI collagen alpha 3 chain by matrix metalloproteinase-2. Cornea. 1996;15:490–496. 8862926 [PubMed] [Google Scholar]
- 62.Veidal S.S. MMP mediated degradation of Type VI collagen is highly associated with liver fibrosis—identification and validation of a novel biochemical marker assay. PLOS One. 2011;6(9):e24753. doi: 10.1371/journal.pone.0024753. 21935455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanssen E., Franc S., Garrone R. Atomic force microscopy and modeling of natural elastic fibrillin polymers. Biology of the Cell. 1998;90(3):223–228. doi: 10.1016/s0248-4900(98)80018-x. 9726120 [DOI] [PubMed] [Google Scholar]
- 64.Sherratt M.J. Fibrillin microfibrils are stiff reinforcing fibres in compliant tissues. Journal of Molecular Biology. 2003;332(1):183–193. doi: 10.1016/s0022-2836(03)00829-5. 12946356 [DOI] [PubMed] [Google Scholar]
- 65.Booms P. A fibrillin-1-fragment containing the elastin-binding-protein GxxPG consensus sequence upregulates matrix metalloproteinase-1: biochemical and computational analysis. Journal of Molecular and Cellular Cardiology. 2006;40(2):234–246. doi: 10.1016/j.yjmcc.2005.11.009. 16442122 [DOI] [PubMed] [Google Scholar]
- 66.Neptune E.R. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nature Genetics. 2003;33(3):407–411. doi: 10.1038/ng1116. 12598898 [DOI] [PubMed] [Google Scholar]
- 67.Neumann C., Yu A., Welge-Lüssen U., Lütjen-Drecoll E., Birke M. The effect of TGF-beta 2 on elastin, type VI collagen, and components of the proteolytic degradation system in human optic nerve astrocytes. Investigative Ophthalmology and Visual Science. 2008;49(4):1464–1472. doi: 10.1167/iovs.07-1053. 18385064 [DOI] [PubMed] [Google Scholar]
- 68.Yang S., Nugent M.A., Panchenko M.P. EGF antagonizes TGF-beta-induced tropoelastin expression in lung fibroblasts via stabilization of Smad corepressor TGIF. American Journal of Physiology—Lung, Cellular, and Molecular Physiology. 2008;295(1):L143–L151. doi: 10.1152/ajplung.00289.2007. 18441095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doyle J.J., Gerber E.E., Dietz H.C. Matrix-dependent perturbation of TGF beta signaling and disease. FEBS Letters. 2012;586(14):2003–2015. doi: 10.1016/j.febslet.2012.05.027. 22641039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stefani M., Dobson C.M. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. Journal of Molecular Medicine. 2003;81(11):678–699. doi: 10.1007/s00109-003-0464-5. 12942175 [DOI] [PubMed] [Google Scholar]
- 71.Voss P. Irradiation of GAPDH: a model for environmentally induced protein damage. The Journal of Biological Chemistry. 2007;388(6):583–592. doi: 10.1515/BC.2007.068. 17552905 [DOI] [PubMed] [Google Scholar]
- 72.Moreau K.L., King J.A. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends in Molecular Medicine. 2012;18(5):273–282. doi: 10.1016/j.molmed.2012.03.005. 22520268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Labat-Robert J. Cell–matrix interactions, the role of fibronectin and integrins. A survey. Pathologie Biologie. 2012;60(1):15–19. doi: 10.1016/j.patbio.2011.10.003. 22265966 [DOI] [PubMed] [Google Scholar]
- 74.Watson R.E. A short-term screening protocol, using fibrillin-1 as a reporter molecule, for photoaging repair agents. Journal of Investigative Dermatology. 2001;116(5):672–678. doi: 10.1046/j.1523-1747.2001.01322.x. 11348454 [DOI] [PubMed] [Google Scholar]
- 75.Stewart D.N. Carbon turnover in the water-soluble protein of the adult human lens. Molecular Vision. 2013;19:463–475. 23441119 [PMC free article] [PubMed] [Google Scholar]
- 76.Bloemendal H. Ageing and vision: structure, stability and function of lens crystallins. Progress in Biophysics and Molecular Biology. 2004;86(3):407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. 15302206 [DOI] [PubMed] [Google Scholar]
- 77.Truscott R.J. Age-related nuclear cataract—oxidation is the key. Experimental Eye Research. 2005;80(5):709–725. doi: 10.1016/j.exer.2004.12.007. 15862178 [DOI] [PubMed] [Google Scholar]
- 78.Fujii N., Uchida H., Saito T. The damaging effect of UV-C irradiation on lens alpha-crystallin. Mol. Vis. 2004;10:814–820. 15534584 [PubMed] [Google Scholar]
- 79.Wang S.S., Wen W.S. Examining the influence of ultraviolet C irradiation on recombinant human gamma d-crystallin. Molecular Vision. 2010;16:2777–2790. 21197112 [PMC free article] [PubMed] [Google Scholar]
- 80.Muranov K.O. Mechanism of aggregation of UV-irradiated beta(L)-crystallin. Experimental Eye Research. 2011;92(1):76–86. doi: 10.1016/j.exer.2010.11.005. 21093434 [DOI] [PubMed] [Google Scholar]
- 81.Krivandin A.V. Resistance of alpha-crystallin quaternary structure to UV irradiation. Biochemistry (Moscow) 2009;74(6):633–642. doi: 10.1134/s0006297909060078. 19645668 [DOI] [PubMed] [Google Scholar]
- 82.Avivi A., Joel A., Nevo E. The lens protein alpha-B-crystallin of the blind subterranean mole-rat: high homology with sighted mammals. Gene. 2001;264(1):45–49. doi: 10.1016/s0378-1119(00)00603-x. 11245977 [DOI] [PubMed] [Google Scholar]
- 83.Berbers G.A. Homology between the primary structures of the major bovine beta-crystallin chains. European Journal of Biochemistry. 1984;139(3):467–479. doi: 10.1111/j.1432-1033.1984.tb08029.x. 6698025 [DOI] [PubMed] [Google Scholar]
- 84.Balasubramanian D. Photodynamics of cataract: an update on endogenous chromophores and antioxidants. Photochemistry and Photobiology. 2005;81(3):498–501. doi: 10.1562/2004-11-01-RA-354. 15623354 [DOI] [PubMed] [Google Scholar]
- 85.Kollias N., Gillies R., Moran M., Kochevar I.E., Anderson R.R. Endogenous skin fluorescence includes bands that may serve as quantitative markers of aging and photoaging. Journal of Investigative Dermatology. 1998;111(5):776–780. doi: 10.1046/j.1523-1747.1998.00377.x. 9804337 [DOI] [PubMed] [Google Scholar]
- 86.Mikesh L.M. Proteomic anatomy of human skin. Journal of Proteomics. 2013;84:190–200. doi: 10.1016/j.jprot.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 87.Pontén F., Jirström K., Uhlen M. The human protein Atlas—a tool for pathology. The Journal of Pathology. 2008;216(4):387–393. doi: 10.1002/path.2440. 18853439 [DOI] [PubMed] [Google Scholar]
- 88.Manabe M., Mizoguchi M., Suto H., Ogawa H. Epidermal structural proteins in skin disorders. Journal of Dermatological Science. 1997;15(3):143–165. doi: 10.1016/s0923-1811(97)00618-x. 9302643 [DOI] [PubMed] [Google Scholar]
- 89.Chu P.G., Weiss L.M. Keratin expression in human tissues and neoplasms. Histopathology. 2002;40(5):403–439. doi: 10.1046/j.1365-2559.2002.01387.x. 12010363 [DOI] [PubMed] [Google Scholar]
- 90.Uitto J., Richard G., McGrath J.A. Diseases of epidermal keratins and their linker proteins. Experimental Cell Research. 2007;313(10):1995–2009. doi: 10.1016/j.yexcr.2007.03.029. 17531221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jackson B. Late cornified envelope family in differentiating epithelia—response to calcium and ultraviolet irradiation. Journal of Investigative Dermatology. 2005;124(5):1062–1070. doi: 10.1111/j.0022-202X.2005.23699.x. 15854049 [DOI] [PubMed] [Google Scholar]
- 92.Kypriotou M., Huber M., Hohl D. The human epidermal differentiation complex: cornified envelope precursors, S100 proteins and the ’fused genes’ family. Experimental Dermatology. 2012;21(9):643–649. doi: 10.1111/j.1600-0625.2012.01472.x. 22507538 [DOI] [PubMed] [Google Scholar]
- 93.Shimomura, Y., Christiano, A.M., 2010. In: Annual Review of Genomics and Human Genetics, vol 11, pp. 109–132 (Annual reviews). [DOI] [PubMed]
- 94.Langbein, L., Schweizer, J., 2005. In: International Review of Cytology—A Survey of Cell Biology. Jeon, K.W. (Ed.), vol. 243. Elsevier Academic Press Inc., p. 1±.
- 95.Langbein L. Novel type I hair keratins K39 and K40 are the last to be expressed in differentiation of the hair: completion of the human hair keratin catalog. Journal of Investigative Dermatology. 2007;127(6):1532–1535. doi: 10.1038/sj.jid.5700734. 17301834 [DOI] [PubMed] [Google Scholar]
- 96.Rogers, M.A., Langbein, L., Praetzel-Wunder, S., Winter, H., Schweizer, J., 2006. In: International Review of Cytology—A Survey of Cell Biology. Jeon, K.W. (Ed.), vol. 251. Elsevier Academic Press Inc., p. 209±. [DOI] [PubMed]
- 97.Hoehenwarter W., Klose J., Jungblut P.R. Eye lens proteomics. Amino Acids. 2006;30(4):369–389. doi: 10.1007/s00726-005-0283-9. 16583312 [DOI] [PubMed] [Google Scholar]
- 98.Nagaraj R.H., Linetsky M., Stitt A.W. The pathogenic role of Maillard reaction in the aging eye. Amino Acids. 2012;42(4):1205–1220. doi: 10.1007/s00726-010-0778-x. 20963455 [DOI] [PubMed] [Google Scholar]
- 99.Ashworth J.L., Kielty C.M., McLeod D. Fibrillin and the eye. British Journal of Ophthalmology. 2000;84(11):1312–1317. doi: 10.1136/bjo.84.11.1312. 11049961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cain S.A. Proteomic analysis of fibrillin-rich microfibrils. Proteomics. 2006;6(1):111–122. doi: 10.1002/pmic.200401340. 16302274 [DOI] [PubMed] [Google Scholar]
- 101.Mir S., Wheatley H.M., Hussels I.E., Whittum-Hudson J.A., Traboulsi E.I. A comparative histologic study of the fibrillin microfibrillar system in the lens capsule of normal subjects and subjects with Marfan syndrome. Investigative Ophthalmology and Visual Science. 1998;39(1):84–93. 9430549 [PubMed] [Google Scholar]
- 102.Martinez G., de Iongh R.U. The lens epithelium in ocular health and disease. The International Journal of Biochemistry and Cell Biology. 2010;42(12):1945–1963. doi: 10.1016/j.biocel.2010.09.012. 20883819 [DOI] [PubMed] [Google Scholar]
- 103.Wederell E.D., de Iongh R.U. Extracellular matrix and integrin signaling in lens development and cataract. Seminars in Cell and Developmental Biology. 2006;17(6):759–776. doi: 10.1016/j.semcdb.2006.10.006. 17134921 [DOI] [PubMed] [Google Scholar]
- 104.Karring H., Thøgersen I.B., Klintworth G.K., Møller-Pedersen T., Enghild J.J. The human cornea proteome: bioinformatic analyses indicate import of plasma proteins into the cornea. Molecular Vision. 2006;12:451–460. 16710169 [PubMed] [Google Scholar]
- 105.Jester J.V. Corneal crystallins and the development of cellular transparency. Seminars in Cell and Developmental Biology. 2008;19(2):82–93. doi: 10.1016/j.semcdb.2007.09.015. 17997336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bishop P.N. Structural macromolecules and supramolecular organisation of the vitreous gel. Progress in Retinal and Eye Research. 2000;19(3):323–344. doi: 10.1016/s1350-9462(99)00016-6. 10749380 [DOI] [PubMed] [Google Scholar]
- 107.Yamane K. Proteome analysis of human vitreous proteins. Molecular and Cellular Proteomics. 2003;2(11):1177–1187. doi: 10.1074/mcp.M300038-MCP200. 12975481 [DOI] [PubMed] [Google Scholar]
- 108.Langton A.K., Sherratt M.J., Griffiths C.E., Watson R.E. Differential expression of elastic fibre components in intrinsically aged skin. Biogerontology. 2012;13(1):37–48. doi: 10.1007/s10522-011-9332-9. 21461665 [DOI] [PubMed] [Google Scholar]
- 109.Foote C.S. Definition of type I and type II photosensitized oxidation. Photochemistry and Photobiology. 1991;54(5):659. doi: 10.1111/j.1751-1097.1991.tb02071.x. 1798741 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials


