Graphical Abstract
Keywords: Cytokinesis, BKI, MAPK, Toxoplasma gondii
Highlights
-
•
Substitution of Ser191 in TgMAPKL-1 changed T. gondii susceptibility to bumped kinase inhibitors.
-
•
Inhibition of TgMAPKL-1 caused enlarged parasite cells in the host.
-
•
Enlarged parasites replicated DNA but failed to complete cytokinesis.
-
•
Inhibition of TgMAPKL-1 arrested the budding of daughter cells.
Abstract
Toxoplasma gondii is the causative pathogen for Toxoplasmosis. Bumped kinase inhibitor 1NM-PP1 inhibits the growth of T. gondii by targeting TgCDPK1. However, we recently reported that resistance to 1NM-PP1 can be acquired via a mutation in T. gondii mitogen-activated protein kinase like 1 (TgMAPKL-1). Further characterization of how this TgMAPKL-1 mutation restores the inhibitory effect of 1NM-PP1 would shed further light on the function of TgMAPKL-1 in the parasite life cycle. Therefore, we made parasite clones with TgMAPKL-1 mutated at the gatekeeper residue Ser 191, which is critical for 1NM-PP1 susceptibility. Host cell lysis of RH/ku80-/HA-TgMAPKL-1S191A was completely inhibited at 250 nM 1NM-PP1, whereas that of RH/ku80-/HA-TgMAPKL-1S191Y was not. By comparing 1NM-PP1-sensitive (RH/ku80-/HA-TgMAPKL-1S191A) and -resistant (RH/ku80-/HA-TgMAPKL-1S191Y) clones, we observed that inhibition of TgMAPKL-1 blocked cell cycle progression after DNA duplication. Morphological analysis revealed that TgMAPKL-1 inhibition caused enlarged parasite cells with many daughter cell scaffolds and imcomplete cytokinesis. We conclude that the mutation in TgMAPKL-1 restored the cell cycle-arresting effect of 1NM-PP1 on T. gondii endodyogeny. Given that endodyogeny is the primary mechanism of cell division for both the tachyzoite and bradyzoite stages of this parasite, TgMAPKL-1 may be a promising target for drug development. Exploration of the signals that regulate TgMAPKL-1 will provide further insights into the unique mode of T. gondii cell division.
1. Introduction
Toxoplasma gondii is the causative pathogen for Toxoplasmosis. It is a member of the Apicomplexans, which include several important pathogens, such as Plasmodium, Cryptosporidium, and Neospora. Without cell division, parasites cannot increase the parasite burden and cannot effectively disseminate throughout the host. Therefore, the cell division of parasites is essential to their life cycle. Protozoa in the Apicomplexa exhibit various types of cell division (Striepen et al., 2007). Toxoplasma and Neospora replicate via the two cell division process in the asexual stage, whereas Plasmodium species replicate by merogony (Arnot et al., 2011) in the blood stage. How parasites select these cell division types in each infection stage remains largely unknown.
The mitogen-activated protein kinase (MAPK) family functions in cell signaling to regulate cell division, cell differentiation, and stress responses in eukaryotic cells (Zhang and Liu, 2002). Genome analysis suggests that there are three MAPKs in the apicomplexan genome (Lacey et al., 2007). Api-MAPK2 and Api-MAPK3 are conserved among apicomplexans; however, Api-MAPK1 shares no homolog among Plasmodium species (Lacey et al., 2007). T. gondii encodes a single Api-MAPK1, T. gondii mitogen-activated protein kinase like 1 (TgMAPKL1) (TGME49_312570). Studies by Dr. Michael White group referred to TGME49_312570 as TgMAPKL1 and found that its similarity to mammalian MAPK is very low, being limited to the protein kinase domain. We also studied TGME49_312570 and, to avoid confusion, we changed our nomenclature of TgMAPK1 to TgMAPKL1 in agreement with the White group (personal communication).
We recently showed that TgMAPKL-1 appears to function in cell division (Sugi et al., 2013). Brown et al. also demonstrated that the protein kinase inhibitor SB505124, which directly targets TgMAPKL-1, arrests parasite cell division (Brown et al., 2014). Brumlik et al. further reported that parasites that expresses antisense RNA for TgMAPKL-1 have a slow growth rate and altered host cell signaling (Brumlik et al., 2013). Thus, inhibition of TgMAPKL-1 leads to parasite growth arrest, suggesting that TgMAPKL-1 has either a direct or indirect role in parasite replication. Although TgMAPKL-1 seems to function in parasite growth, the predicted genome sequence of T. gondii suggests that it lacks MAPKK and MAPKKK, which are upstream protein kinases for the MAPKs (Miranda-Saavedra et al., 2012).
Bumped kinase inhibitors (BKIs) represent a promising drug lead because they have little effect on mammalian protein kinases (Ojo et al., 2014a) but appear to be a potent inhibitors of parasite growth in vitro (Lourido et al., 2010; Murphy et al., 2010; Ojo et al., 2010; Sugi et al., 2010) and in vivo (Doggett et al., 2014; Lourido et al., 2013; Ojo et al., 2014b; Sugi et al., 2011). The primary targets of the BKIs are CDPK1s that carry a small gatekeeper residue, which makes the protein kinase sensitive to the BKIs. However, we recently showed that TgMAPKL-1 is the secondary target of the BKIs and that mutation of TgMAPKL-1 provides parasites with resistance to BKIs (Sugi et al., 2013). Ojo et al., (2014b) reported that BKI treatment of Neospora caninum inhibited the growth of the parasite in host cells – an effect that could not be explained as the result of CDPK1 inhibition because CDPK1 reportedly works in invasion and egress (Lourido et al., 2010; Sugi et al., 2010). Therefore, it is important to investigate how BKIs inhibit parasites by targeting the secondary target TgMAPKL-1. The investigation of the mode of action of bumped kinase inhibitor will help to reveal the atypical MAPK signaling pathway involved in the parasite life cycle.
In the present report, we employed chemical genetics to inhibit TgMAPKL-1 in an inducible manner. We used the bumped kinase inhibitor 1NM-PP1 and parasites in which the gatekeeper residue had been genetically mutated such that their susceptibility to this BKI was altered (Bishop et al., 2000). Similar chemical-genetics approaches were previously used to analyze other protein kinases in Toxoplasma (Donald et al., 2006; Lourido et al., 2010; Sugi et al., 2010) and Plasmodium (Ojo et al., 2014a). By using a parasite bearing TgMAPKL-1 with a small gatekeeper amino acid (BKI-susceptible) and a parasite bearing TgMAPKL-1 with a large gatekeeper amino acid (BKI-resistant), we could observe the effect of TgMAPKL-1 inhibition on parasite cell cycle progression. Here, we provide the first evidence that BKI affects parasite cell cycle progression by targeting TgMAPKL-1.
2. Materials and methods
2.1. Chemical reagents and antibodies
The following antibodies were used for Western blotting and immunofluorescence staining: an α-HA epitope tag rat monoclonal antibody (Roche, Basel, Switzerland), α-TgIMC3 rat antisera (Anderson-White et al., 2011), and an α-TgGAP45 rabbit polyclonal antibody (Plattner et al., 2008). 1NM-PP1 (Merck KGaA, Darmstadt, Germany) was dissolved in DMSO; ammonium pyrrolidinedithiocarbamate (PDTC), RNase A, and propidium iodide (Sigma-Aldrich, St. Louis, USA) were dissolved in distilled water.
2.2. Plasmids
To knock-in the gatekeeper mutated TgMAPKL-1 sequence in the native TgMAPKL-1 locus on chromosome XI, we produced a construct containing the 5'UTR from TgMAPKL-1, the HXGPRT selectable marker cassette, and the TgMAPKL-1 cDNA sequence fused with an N-terminal HA-epitope tag under the control of the GRA1 promoter sequence (Fig. 1A). Knock-in constructs for replacing the wild-type TgMAPKL-1 sequence in the chromosome with the gatekeeper-substituted TgMAPKL-1 expression cassette were made as follows: the HA-tag was amplified with primers HA_F and HA_R (Table 1) from pCMV-HA (Takara, Shiga, Japan) and inserted into the EcoT22I and EcoRI sites of pTgMAPK1-WT (Sugi et al., 2013). The resultant plasmid was designated as pHA-TgMAPKL-1, which encodes the TgMAPKL-1 expression cassette fused to the N-terminal HA-epitope. For the knock-in by homologous recombination, the 5'UTR of TgMAPKL-1 was amplified with the primers 5'UTR_F and 5'UTR_R (Table 1) from the genomic DNA of RH/ku80-/hxgprt- and inserted into the HindIII site of pHA-TgMAPKL-1 by using the InFusion cloning system (Takara). The resultant construct was designated as pKnock-In-HA-TgMAPK1. To substitute the gatekeeper residue, pKnock-In-HA-TgMAPK1 was PCR amplified with the primers F_GK_Ala and R_GK_35, or F_GK_Tyr and R_GK_35 (Table 1). The PCR fragments were ligated by using the InFusion cloning system.
Fig. 1.
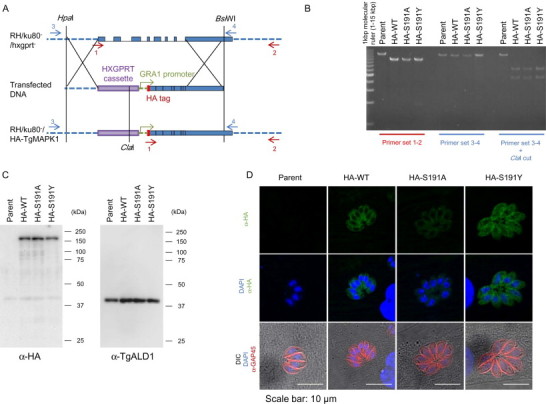
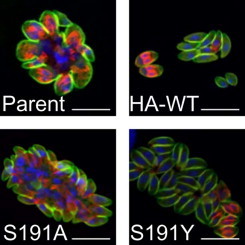
Double homologous recombination of the native TgMAPKL-1 locus in the chromosome to substitute the gatekeeper residue. (A) Schematic depiction of the knock-in construct. The red arrows show the locations of primers 1 and 2 for the PCR analysis. The blue arrows show the locations of primers 3 and 4 for the PCR-RFLP analysis. The graph shows chromosome XI around the TgMAPKL-1 locus. The blue boxes show the coding sequence of TgMAPKL-1. (B) PCR analysis of the recombinant chromosomal sequence in the knock-in clones. The 3'UTR and the coding sequence of TgMAPKL-1 were amplified with primer sets 1 and 2. The 5'UTR and the coding sequence of TgMAPKL-1 were amplified with primer sets 3 and 4. For the PCR-RFLP analysis, the PCR fragments amplified with primers 3 and 4 were digested with the ClaI restriction enzyme. Parent indicates the RH/ku80-/hxgprt- strain. (C) An anti-HA tag antibody (left panel) or an anti-TgALD1 antibody (right) was used to detect protein from the total lysate of each parasite clone. Purified parasites were lysed in 1 × SDS-PAGE sample buffer and 106 parasites/lane were loaded onto the gel. Molecular masses are shown to the right of each panel. (D) Tachyzoites of each clone were cultured in HFF cells on cover slips. Green shows the HA-tagged TgMAPKL-1 detected with the anti-HA antibody and blue shows the nuclei. To observe the parasite shape, TgGAP45 was stained and is shown in red. Scale bars = 10 µm.
Table 1.
Primers used in the present study.
| ID | Sequence (5'- > 3') |
|---|---|
| HA_F | GAAATGCATACCATGTACCCATACGATGT |
| HA_R | TGTGAATTCGCGTAATCTGGAACATCGT |
| 5'UTR_F | CGGTATCGATAAGCTCAAGAAAAGCAACGAGAGAT |
| 5'UTR_R | CGTGCTGATCAAGCTATGGTGGCGAAGAGTTGAG |
| R_GK_35 | GACGAGGTAAATGTCCTCGAAATCTGGAGTCAG |
| F_GK_Ala | GACATTTACCTCGTCgcTGACCTGATGGACAC |
| F_GK_Tyr | GACATTTACCTCGTCTaTGACCTGATGGACAC |
| PCR_screen_1 | TGAGTCAGAGCACGAGTGCCGGAGGCAGCCAAGCT |
| PCR_screen_2 | CCGACAGAAGTCAAAAGGGAATGAGATGCCAGGTAT |
| PCR_screen_3 | TATTTCTTCTGACCGCACGACCTTTCGCAGTTCAG |
| PCR_screen_4 | AGCTGTTGCTGGCCATGCTGCAGCTGTTGTTGCCT |
Boldface type shows the restriction enzyme sites. Underlined sequences are homologous sequences for the In-Fusion cloning system. Lower case italicized characters represent the mutated sequences.
2.3. Host cell culture
Human foreskin fibroblast (HFF) cells were maintained in the Dulbecco's modified Eagle medium (DMEM) (Life Technologies) supplemented with 25 mM HEPES, 50 U/ml penicillin, 50 µg/ml streptomycin, and 10% fetal calf serum (FCS). Vero cells were maintained in the DMEM (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 50 U/ml penicillin, 50 µg/ml streptomycin, and 5% FCS. When the parasites were inoculated, DMEM (Nissui Pharmaceutical) with 1% FCS was used.
2.4. Transgenic parasites
The parasite strain RH/ku80-/hxgprt- (Huynh and Carruthers, 2009) (ATCC: PRA319) was used as the parental strain of the transgenic parasites throughout this study. Parasites were maintained and serially passaged to new host Vero cells as described elsewhere (Roos et al., 1994). For transfection, plasmids were linearized with HpaI and BsiWI. Then, 2.5 µg of linearized DNA was introduced into 106 parasites by using Nucleofection with Nucleofector II (Lonza, Basel, Switzerland) as described elsewhere (Sugi et al., 2013). After selection with 25 µg/mL mycophenolic acid and 25 µg/mL xanthine, parasites were cloned by limiting dilution, and the resultant clones were subjected to PCR and PCR-RFLP analysis to screen for clones with the replaced chromosome.
2.5. Host cell monolayer disruption assay
Host Vero cells were seeded in 96-well plates at a density of 104/well and incubated for 24 h before parasite inoculation. Parasites were filter-purified, counted, and inoculated at a density of 3 × 103/well following incubation with the test concentration of 1NM-PP1 at room temperature for 10 min. Cells were incubated for 5 days with medium changes every 2 days. After this incubation, the infected host cells were fixed with methanol, stained with crystal violet, and then measured at OD600. Average values from non-treated and mock-infected (control) wells were estimated to be 100%.
2.6. Cell cycle analysis
Cell cycle synchronization was performed by use of PDTC treatment as described elsewhere (Conde de Felipe et al., 2008). Briefly, parasites were inoculated into Vero cells at a multiple of infection 1.0 and incubated for 12 h before PDTC treatment. Infected cells were then treated with 80 µM PDTC for 8 h and then washed three times at 37 °C with warm medium to wash out the PDTC. After the washout, medium with or without 250 nM 1NM-PP1 was added and incubated extensively for 7 hours. Purified samples were prepared for flow cytometric analysis as described elsewhere (Conde de Felipe et al., 2008). Briefly, infected cells were washed with ice-cold phosphate buffered saline (PBS) three times to remove the extracellular parasites and were then harvested with scrapers. Host cells were ruptured by serial passage through a 25G needle three times. Parasites were filter-purified with a φ5-µm pore filter unit (Millipore, Billerica, USA), and then centrifuged at 2,000 × g for 5 min at 4 °C. To fix and permeabilize the parasites, the pellets were resuspended in 300 µl of ice-cold 2% FCS PBS to which 700 µl of ice-cold ethanol was added drop-wise with vigorous pipetting; they were then incubated at –30 °C for more than 1 day. Fixed cells were then washed once with 2% FCS PBS and the pellet was resuspended in PI solution (40 µg/ml propidium iodide, 360 µg/ml RNase A, and 2% bovine serum albumin (BSA) in PBS) and filtered with a φ5-µm pore filter just after resuspension. The filtered parasites in PI solution were incubated at 37 °C for 30 min and then used for flow cytometric analysis with Epics XL (Beckman Coulter).
2.7. Immunofluorescence staining
Parasites were inoculated into host HFF cells cultured on cover slips and incubated for 24 or 48 h to observe the effect of treatment with 250 nM 1NM-PP1. 1NM-PP1 treatments were started from 2 h post-parasite inoculation. To observe the normal tachyzoite, inoculated parasites were incubated for 24 h. Staining was performed as described elsewhere (Tomita et al., 2013); briefly, cells were fixed with 4% paraformaldehyde for 20 min, and were then permeabilized with 0.2 M glycine, 0.2% TrintonX-100 in PBS for 15 min at room temperature and blocked with 2% BSA in 0.1% Tween-20 PBS (blocking buffer) for 30 min. The primary antibody reaction was performed in blocking buffer at a dilution of 1:500. After the primary antibody reaction, the cells were washed three times with 0.1% Tween-20 PBS and then incubated with secondary antibodies conjugated to ALEXA (Life Technologies) and DAPI (Life Technologies) in blocking buffer at dilutions of 1:1000. Antibody reactions were performed overnight at 4 °C. After the secondary antibody reaction, the cells were washed five times with PBS and mounted with fluorescence mounting medium (DAKO, Glostrup, Denmark). Stained cells were observed by using a confocal laser scanning microscope TCS SP5 (Leica Microsystems, Wetzlar, Germany).
3. Results
3.1. Construction of gatekeeper amino acid-substituted TgMAPKL-1 by use of double homologous recombination
First, we introduced the mutation into TgMAPKL-1 in the parasite chromosome by using double homologous recombination. The knock-in construct (Fig. 1A) contained the 5'UTR from TgMAPKL-1, a HXGPRT selectable marker cassette, and the coding sequence of TgMAPKL-1 with or without the mutation in the gatekeeper residue Ser 191 fused to an N-terminal HA-epitope tag and driven by the GRA1 promoter. Linearized construct was introduced into the RH/ku80-/hxgprt- parental strain to obtain recombinant clones effectively. We established clones with the wild-type TgMAPKL-1 sequence (RH/HA-WT) and the mutated TgMAPKL-1 sequence (RH/HA-S191A and RH/HA-S191Y). Double homologous recombination by the knock-in construct was confirmed by PCR and a PCR-RFLP assay. Primer pairs 1 and 2 amplified about 14 kbp of DNA from parental RH/ku80-/hxgprt-, whereas about 9 kbp of DNA was amplified from the recombinant clones (Fig. 1B). Fragments amplified with primer pairs 3 and 4 and digested with the ClaI restriction enzyme showed a single band for the parental RH/ku80-/hxgprt-, whereas two bands were detected after ClaI digestion for the knock-in clones (Fig. 1B).
Expression of the HA-tagged TgMAPKL-1 was confirmed with Western blotting and immunofluorescence staining. An anti-HA-epitope tag antibody detected a single band with a molecular weight of approximately 150 kDa from the protein lysate of RH/HA-WT, RH/HA-S191A, and RH/HA-S191Y, whereas no specific band was detected from the parental RH/ku80-/hxgprt- (Fig. 1C). HA-TgMAPKL-1 was detected in the parasite cytosol of RH/HA-WT, RH/HA-S191A and RH/HA-S191Y, but no signal was detected from RH/ku80-/hxgprt- (Fig. 1D).
3.2. Gatekeeper amino acid substitution of TgMAPKL-1 subverts the 1NM-PP1 effect on parasite growth
Next, we determined the influence of substituting the gatekeeper residue from wild-type Ser 191 to Ala (RH/HA-S191A) or Tyr (RH/HA-S191Y) on parasite susceptibility to the bumped kinase inhibitor 1NM-PP1. We used a host monolayer disruption assay to evaluate the 1NM-PP1-induced inhibition of parasite growth. Treatment with 250 nM 1NM-PP1 inhibited RH/HA-S191A and parental RH/ku80-/hxgprt- parasite growth and host cells were not lysed (Fig. 2), whereas host cells were lysed when RH/HA-WT and RH/HA-S191Y were treated with 250 nM 1NM-PP1 (Fig. 2). RH/HA-WT grew more slowly than the other strain in the absence of 1NM-PP1 (Fig. 2).
Fig. 2.

Gatekeeper substitution alters the concentration at which host monolayer disruption is inhibited. Host monolayer disruption was analyzed with various concentrations of 1NM-PP1 and the knock-in parasite clones. At 5 days after parasite inoculation, cells were fixed and stained with crystal violet and OD600 values were measured. The decrease in the OD600 value relative to the mock was calculated as the parasite growth rate, and the average value for the non-treated (DMSO) group was estimated to be 100%. Average values ± s.d. from three independent experiments are shown. Statistical analysis was performed by using the Student's t-test to compare the parent for each condition with the same drug treatment. * indicates p < 0.05 and ** indicates p < 0.01.
3.3. Inhibition of TgMAPKL-1 by 1NM-PP1 causes cell cycle arrest
Next, by comparing the 1NM-PP1-sensitive RH/HA-S191A and the 1NM-PP1-resistant RH/HA-S191Y following 1NM-PP1 treatment, we analyzed the effect of TgMAPKL-1 on cell cycle progression. We used flow cytometry to measure the DNA contents in the single parasite cells to analyze the cell cycle populations of G1 (1N) and S, M/C ( over 1N). All strains, parental RH/ku80-/hxgprt-, RH/HA-WT, RH/HA-S191A, and RH/HA-S191Y, were synchronized by using PDTC treatment and showed G1 accumulated peaks in the DNA content analysis by flow cytometry (Fig. 4A and 4B upper panels). After PDTC release, all of the strains progressed to the next G1 cycle during a 7-h incubation in the absence of 1NM-PP1 (Fig. 3A and 3B, middle panels). However, in the presence of 250 nM 1NM-PP1 during the 7-h incubation after PDTC release, parental RH/ku80-/hxgprt- and RH/HA-S191A showed accumulation of parasites with 2N DNA content (M/C), whereas RH-WT and RH/HA-S191Y showed G1 peaks (Fig. 3B bottom panels). Accumulation of the over 1N peak in the flow cytometric analysis suggested that the parasite cell cycle did not progress normally, such that the cell cycle was delayed or arrested after DNA duplication.
Fig. 4.
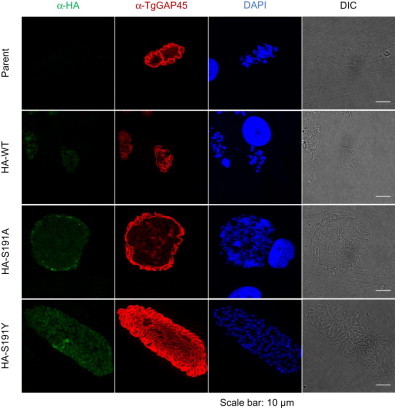
Inhibition of TgMAPKL-1 causes enlarged parasite cells. Parasites were treated with 250 nM 1NM-PP1 for 2 days. To observe the expression of HA-tagged TgMAPKL-1, an anti-HA antibody was used (shown in green). To observe the inner membrane complex underneath the parasite cell membrane, an anti-TgGAP45 antibody was used (shown in red). DNA was detected by use of DAPI (blue). Scale bars = 10 µm.
Fig. 3.
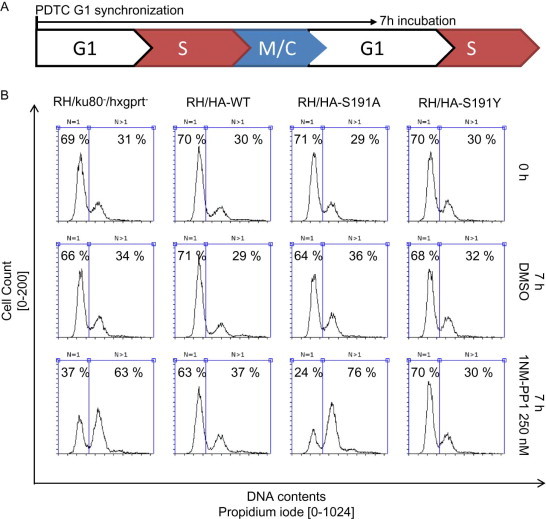
Inhibition of TgMAPKL-1 causes cell cycle arrest after S phase. (A) Schematic depiction of the cell cycle analysis experiments. PDTC treatment synchronizes the parasites in G1 phase; 7 h after PDTC washout, the parasites progress to the next G1 phase. (B) Flow cytometric analysis of the knock-in clones at the time point just after PDTC treatment (upper panels), 7 h after PDTC treatment without drug treatment (middle panels), and 7 h after PDTC with 250 nM 1NM-PP1 treatment (bottom panels). The DNA contents of the parasites were measured by use of PI staining. Total cell counts of 8,000 are shown in the histogram. Parasite having 1N and over 1N DNA contents were gated as shown in the panels; the parasite population was calculated and is shown in each panel.
3.4. Morphological analysis of cell cycle-arrested parasites
To find out whether the cell cycle progression was arrested or progression was merely slowed by the inhibition of TgMAPKL-1, we examined the morphology of 1NM-PP1-treated parasites. We stained TgGAP45 to observe the parasite inner membrane complex beneath the single parasite cell cytoplasmic membrane. Parasite nuclei were stained with DAPI to determine whether a single nucleus or multiple nuclei were located in individual parasite cells. When parasites were treated with 250 nM 1NM-PP1 for 2 days, parental RH/ku80-/hxgprt- and RH/HA-S191A grew abnormally, and enlarged cells were observed in the parasitophorous vacuole (Fig. 4). In the enlarged cells, TgGAP45 was distributed beneath the large cell membrane and a protrusion from the large cell body was observed (Fig. 4). 1NM-PP1-resistant RH/HA-WT and RH/HA-S191Y, however, showed the normal tachyzoite shape after 250 nM 1NM-PP1 treatment (Fig. 4). The expression of HA-TgMAPKL-1 was not diminished by 1NM-PP1 treatment; however, the expression of HA-TgMAPKL-1S191A in the midportion of the enlarged cells was faint (Fig. 4 left panels). RH/HA-WT grew normally in the tachyzoite form but undivided parasites were also observed in the parasitophorous vacuole (Fig. 4). DNA staining revealed that the enlarged cells of RH/ku80-/hxgprt- and RH/HA-S191A had dispersed nuclei (Fig. 4). These data suggest that inhibition of TgMAPKL-1 led to incomplete cell division, in addition to a possible delay in cell cycle progression, but did not completely arrest DNA replication.
3.5. Inhibition of TgMAPKL-1 affects daughter cell formation
Next, we checked daughter cells formation in the cytokinesis-defect parasites. To distinguish between the mother cell inner membrane and the daughter cell scaffold, we used TgGAP45 as a mother cell inner membrane complex marker (Gaskins et al., 2004) and TgIMC3 as a daughter cell inner membrane complex marker. When the parasites were incubated with the DMSO solvent control, the dividing mother parasites stained with TgGAP45 contained two daughter cells stained with TgIMC3 (Fig. 5A), suggesting that knock-in of the HA-tagged TgMAPKL-1 did not alter the “endodyogeny-type cell division”.
Fig. 5.
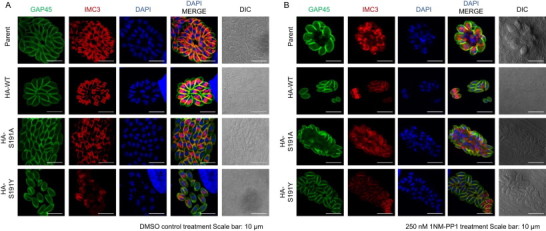
Effects of TgMAPK1 inhibition on daughter cell formation. HFF host cells were infected with each parasite clone. After a 2-h incubation, the medium was changed and the cells were incubated with DMSO (A) or 250 nM 1NM-PP1 (B) for 24 h before fixing. To observe daughter cell formation, cells were stained with anti-TgGAP45 (green) for the mother cell inner membrane complex, anti-TgIMC3 (red) for the daughter cell inner membrane complex, and DAPI (blue) for the nuclei. Scale bars = 10 um.
When we treated the infected cells with 250 nM 1NM-PP1, the dividing mother cells of RH/HA-WT and RH/HA-S191Y contained two daughter cells, which were similar to the non-treated group (Fig. 5B). However, 1NM-PP1 treatment caused enlarged mother cells in the parental RH/ku80-/hxgprt- and RH/HA-S191A clones. In these enlarged cells, protrusions of the TgGAP45-positive inner membrane complex and of the TgIMC3-positive daughter cell inner membrane complex were observed (Fig. 5B).
Segregated nuclei were observed in the daughter cells of the enlarged mother cells (Fig. 5B); at the same time, large nuclei which were not enveloped by the daughter cell inner membrane complex were also observed (Fig. 5B). Collectively, these data suggest that TgMAPKL-1 inhibition caused a cytokinesis defect in the enlarged parasite cells, but did not completely arrest nucleus duplication and daughter cell formation in the cytokinesis-disrupted enlarged cells.
4. Discussion
In this report, we characterized the function of TgMAPKL-1 in parasite growth by using a chemical genetics approach. When the gatekeeper residue of TgMAPKL-1 is mutated, the inhibitory effect of 1NM-PP1 drastically changes especially with respect to cell cycle arrest after DNA duplication. 1NM-PP1-sensitive transgenic parasites containing TgMAPKL-1S191A produced many daughter cell scaffolds within the enlarged maternal cells. This finding indicates that the mutation in TgMAPKL-1 changed the cell division style of the T. gondii tachyzoite, which usually divides in the endodyogeny style.
The previously reported phenotype of TgMAPKL-1 inhibition causing slow parasite growth (Brumlik et al., 2013; Sugi et al., 2013) is consistent with our findings in this study. The previous report by Brumlik et al. showed that downregulation of TgMAPKL-1 causes changes in host manipulation (Brumlik et al., 2013); however, our HA-tagged TgMAPKL-1 localized in the parasite cytosol. Therefore, the concept of TgMAPKL-1 directly manipulating host signals remains to be confirmed. We only tested the N-terminal HA-tagged full-length mRNA isoform of TgMAPKL-1 because the HA-TgMAPKL-1 successfully replaced the native TgMAPKL-1 locus, suggesting that there was no loss of function in the parasite. However, the tagging and the mRNA splicing of the isoform might have affected the localization of TgMAPKL-1. Overexpression of the wild-type TgMAPKL-1 locus confers resistance to 1NM-PP1. Therefore, the overexpression of TgMAPKL-1 itself must have an effect on resistance. However, all of the overexpressed TgMAPKL-1 protein (i.e., WT, S191A, and S191Y) were expressed to the same level, according to our Western blotting analysis. Nevertheless, we cannot rule out the possibility that mutation of the TgMAPKL-1 gatekeeper residue changes the kinase activity, which in turn could alter the underlying BKI sensitivity of the parasites. TgMAPKL-1 variant expression conferred a different level of resistance to the parasites. This resistance appeared to result from the TgMAPKL-1 function. However to determine whether 1NM-PP1 directly targets TgMAPKL-1, in vitro protein kinase assays with TgMAPKL-1 should be performed in future studies. We have tried to express the full-length form of TgMAPKL-1, but so far we have been unsuccessful.
Notably, we observed that the nucleus was somewhat segregated in the enlarged cells, that daughter cell scaffold stained by TgIMC3 was constructed, but that the inner membrane complex stained by TgGAP45 was not divided. TgGAP45 is located reportedly in the inner membrane complex of mature (or completely budded) cells (Gaskins et al., 2004); therefore, our findings suggest that the normal function of TgMAPKL-1 is essential for the completion of budding. Recent work by Brown et al. showed that the ALK4, 5, and 7 inhibitor SB505124 inhibits TgMAPKL-1 directly (Brown et al., 2014). They also found that the nucleus number in the parasite body was abnormal and that SB505124-treated parasites produced swollen cells (Brown et al., 2014). Another study by Dr. Michael White's group using a temperature-sensitive allele also demonstrated that TgMAPKL-1 is needed for the coordinated progression of cytokinesis (personal communication).
Ojo et al. recently reported similar enlarged parasite cells in Neospora caninum treated with the bumped kinase inhibitor 1294 (Ojo et al., 2014b). N. caninum treated with 1294 also showed segregated nuclei, and large nuclei within the undivided cells were observed by use of TEM analysis (Ojo et al., 2014b). Ojo et al. did not determine the specific target molecule responsible for the enlargement, however they described the TgMAPKL-1 homolog in the Neospora genome (NcMAPKL-1) (NCLIV_056080) (Ojo et al., 2014b) as being of high similarity (72% positive amino acid by Blast analysis) (ToxoDB ver. 11.0). NcMAPKL-1 has a gatekeeper residue of Ser, which is the same as that of T. gondii. Residues 162 Leu and 171 Ile of TgMAPKL-1, which are the mutation sites that influence BKI susceptibility (Sugi et al., 2013), are conserved in NcMAPKL-1 (159 Leu and 168 Ile). Therefore, the bumped kinase inhibitor may target MAPK1 and cause the enlarged parasite cells in both species. In the present report, we obtained resistant parasites by substituting the gatekeeper residue S191 of TgMAPKL-1. However, the mutation site in the TgMAPKL-1 of the resistant parasites in the previous report (Brown et al., 2014; Sugi et al., 2013) was located at L162 and I171. It will be important to compare the level of resistant to the BKI induced by the gatekeeper substitution to that induced by the previously found mutation to predict the occurrence of such BKI-resistant mutations in parasites.
Abnormal undivided parasite cells and upregulation of bradyzoite-specific gene expression following protein kinase inhibitor treatment have been reported (Brown et al., 2014; Wei et al., 2002). We recently showed that bumped kinase inhibitors increase bradyzoite-specific BAG1 gene promoter activity by using reporter parasites (Sugi et al., 2014). Bradyzoite-inducible stressors such as high pH and CO2 depletion cause a cell division defect, which results in the asynchronous cell division of parasites in the parasitophorous vacuoles (Dzierszinski et al., 2004). However, enlarged parasites, such as those seen with the 2-day TgMAPKL-1 inhibition, are not seen in the native cyst. Therefore, future studies should examine whether inhibiting TgMAPKL-1 leads to bradyzoite-inducing effects.
Even if TgMAPKL-1 plays a role in the regulation of the cell cycle, the Toxoplasma genome lacks the typical upstream activator MAPKK class protein kinases (Miranda-Saavedra et al., 2012). Other protein kinases in T. gondii, TgNEK1 (Chen and Gubbels, 2013) and TgCDPK7 (Morlon-Guyot et al., 2014), reportedly play roles in mitosis, particularly in normal centrosome segregation. The enlarged parasite cells in our study showed segregated nuclei, but some of the nuclei seemed to be larger than the single nucleus of a normal tachyzoite. Interestingly, activation of ApiMAPKs by NEK has been reported for pfmap2 by pfnek1 in vitro (Dorin et al., 2001). Even if TgMAPKL-1 has no homolog in Plasmodium species, the activation mechanisms of MAPKs might be common among the Apicomplexan MAPKs. TgNEK1 and TgCDPK7 may work as upstream regulators of TgMAPKL-1 instead of MAPKK, which is absent in T. gondii. The budding defect in the enlarged cells is the same as that seen with the MORN1 knockout parasite (Lorestani et al., 2010). Moreover, Gubbels et al. reported on temperature-sensitive clones that have a defect in late budding and cell division (Gubbels et al., 2008). A better understanding of the relationship between TgMAPKL-1 and these other reported proteins that are essential to cytokinesis will help to reveal the cell cycle regulating signals. In order to discover the signaling pathway through which TgMAPKL-1 participates in cell cycle regulation, further quantitative analysis using other cell cycle markers, such as TgMORN1 and centrosomes, will be needed to determine the exact step(s) that involve TgMAPKL-1.
Our data suggest that TgMAPKL-1 is involved in the control of normal cytokinesis following single DNA duplication in enlarged parasite cells. Hu et al. reported that some populations of T. gondii can go through multiple daughter cell divisions rather than diploid cell division spontaneously (Hu et al., 2004). Therefore, there may be an adjustable signal for selecting the type of cell division. The characterization of the upstream and downstream effectors of TgMAPKL-1, as well as studies of how TgMAPKL-1 is regulated without a typical upstream MAPKK, may provide insights into the mechanisms that regulate the Toxoplasma cell cycle. Given that cell division is essential for the growth of parasites, our data suggest that TgMAPKL-1 is a promising drug target for the Toxoplasma parasite.
Acknowledgements
α-TgIMC3 antibody was a kind gift from Dr. Marc-Jan Gubbels. α-TgGAP45 antibody was a kind gift from Dr. Dominique Soldati. TS is supported by a JSPS Research Fellowship for Young Scientists. KK is supported by grants-in-aid for Young Scientists, Exploratory Research, Scientific Research on Innovative Areas from the Ministry of Education, Culture, Science, Sports, and Technology (MEXT) of Japan, the Program for the Promotion of Basic and Applied Research for Innovations in Bio-oriented Industry (BRAIN), the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry, and the Program to Disseminate Tenure Tracking System from the Japan Science and Technology Agency (JST). TS and KK designed the study, TS performed the experiments, TS, SK, TH, and KK analyzed the data, and TS and KK wrote the paper.
References
- Anderson-White B.R., Ivey F.D., Cheng K., Szatanek T., Lorestani A., Beckers C.J. A family of intermediate filament-like proteins is sequentially assembled into the cytoskeleton of Toxoplasma gondii. Cell. Microbiol. 2011;13:18–31. doi: 10.1111/j.1462-5822.2010.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnot D.E., Ronander E., Bengtsson D.C. The progression of the intra-erythrocytic cell cycle of Plasmodium falciparum and the role of the centriolar plaques in asynchronous mitotic division during schizogony. Int. J. Parasitol. 2011;41:71–80. doi: 10.1016/j.ijpara.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Bishop A.C., Ubersax J.A., Petsch D.T., Matheos D.P., Gray N.S., Blethrow J. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- Brown K.M., Suvorova E., Farrell A., McLain A., Dittmar A., Wiley G.B. Forward genetic screening identifies a small molecule that blocks Toxoplasma gondii growth by inhibiting both host- and parasite-encoded kinases. PLoS Pathog. 2014;10:e1004180. doi: 10.1371/journal.ppat.1004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumlik M.J., Pandeswara S., Ludwig S.M., Jeansonne D.P., Lacey M.R., Murthy K. TgMAPK1 is a Toxoplasma gondii MAP kinase that hijacks host MKK3 signals to regulate virulence and interferon-γ-mediated nitric oxide production. Exp. Parasitol. 2013;134:389–399. doi: 10.1016/j.exppara.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.T., Gubbels M.J. The Toxoplasma gondii centrosome is the platform for internal daughter budding as revealed by a Nek1 kinase mutant. J. Cell Sci. 2013;126:3344–3355. doi: 10.1242/jcs.123364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde de Felipe M.M., Lehmann M.M., Jerome M.E., White M.W. Inhibition of Toxoplasma gondii growth by pyrrolidine dithiocarbamate is cell cycle specific and leads to population synchronization. Mol. Biochem. Parasitol. 2008;157:22–31. doi: 10.1016/j.molbiopara.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett J.S., Ojo K.K., Fan E., Maly D.J., Van Voorhis W.C. Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob. Agents Chemother. 2014;58:3547–3549. doi: 10.1128/AAC.01823-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald R.G., Zhong T., Wiersma H., Nare B., Yao D., Lee A. Anticoccidial kinase inhibitors: identification of protein kinase targets secondary to cGMP-dependent protein kinase. Mol. Biochem. Parasitol. 2006;149:86–98. doi: 10.1016/j.molbiopara.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Dorin D., Le Roch K., Sallicandro P., Alano P., Parzy D., Poullet P. Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum Biochemical properties and possible involvement in MAPK regulation. Eur. J. Biochem. 2001;268:2600–2608. doi: 10.1046/j.1432-1327.2001.02151.x. [DOI] [PubMed] [Google Scholar]
- Dzierszinski F., Nishi M., Ouko L., Roos D.S. Dynamics of Toxoplasma gondii differentiation. Eukaryot. Cell. 2004;3:992–1003. doi: 10.1128/EC.3.4.992-1003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins E., Gilk S., DeVore N., Mann T., Ward G., Beckers C. Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J. Cell Biol. 2004;165:383–393. doi: 10.1083/jcb.200311137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbels M.J., Lehmann M., Muthalagi M., Jerome M.E., Brooks C.F., Szatanek T. Forward genetic analysis of the apicomplexan cell division cycle in Toxoplasma gondii. PLoS Pathog. 2008;4:e36. doi: 10.1371/journal.ppat.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K., Roos D.S., Angel S.O., Murray J.M. Variability and heritability of cell division pathways in Toxoplasma gondii. J. Cell Sci. 2004;117:5697–5705. doi: 10.1242/jcs.01494. [DOI] [PubMed] [Google Scholar]
- Huynh M.H., Carruthers V.B. Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot. Cell. 2009;8:530–539. doi: 10.1128/EC.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M.R., Brumlik M.J., Yenni R.E., Burow M.E., Curiel T.J. Toxoplasma gondii expresses two mitogen-activated protein kinase genes that represent distinct protozoan subfamilies. J. Mol. Evol. 2007;64:4–14. doi: 10.1007/s00239-005-0197-x. [DOI] [PubMed] [Google Scholar]
- Lorestani A., Sheiner L., Yang K., Robertson S.D., Sahoo N., Brooks C.F. A Toxoplasma MORN1 null mutant undergoes repeated divisions but is defective in basal assembly, apicoplast division and cytokinesis. PLoS ONE. 2010;5:e12302. doi: 10.1371/journal.pone.0012302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido S., Shuman J., Zhang C., Shokat K.M., Hui R., Sibley L.D. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature. 2010;465:359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido S., Zhang C., Lopez M.S., Tang K., Barks J., Wang Q. Optimizing small molecule inhibitors of calcium-dependent protein kinase 1 to prevent infection by Toxoplasma gondii. J. Med. Chem. 2013;56:3068–3077. doi: 10.1021/jm4001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Saavedra D., Gabaldón T., Barton G.J., Langsley G., Doerig C. The kinomes of apicomplexan parasites. Microbes Infect. 2012;14:796–810. doi: 10.1016/j.micinf.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Morlon-Guyot J., Berry L., Chen C.T., Gubbels M.J., Lebrun M., Daher W. The Toxoplasma gondii calcium-dependent protein kinase 7 is involved in early steps of parasite division and is crucial for parasite survival. Cell. Microbiol. 2014;16:95–114. doi: 10.1111/cmi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R.C., Ojo K.K., Larson E.T., Castellanos-Gonzalez A., Perera B.G., Keyloun K.R. Discovery of potent and selective inhibitors of calcium-dependent protein kinase 1 (CDPK1) from C. parvum and T. gondii. ACS Med Chem Lett. 2010;1:331–335. doi: 10.1021/ml100096t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo K.K., Larson E.T., Keyloun K.R., Castaneda L.J., DeRocher A.E., Inampudi K.K. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat. Struct. Mol. Biol. 2010;17:602–607. doi: 10.1038/nsmb.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo K.K., Eastman R.T., Vidadala R., Zhang Z., Rivas K.L., Choi R. A specific inhibitor of PfCDPK4 blocks malaria transmission: chemical-genetic validation. J. Infect. Dis. 2014;209:275–284. doi: 10.1093/infdis/jit522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo K.K., Reid M.C., Kallur Siddaramaiah L., Müller J., Winzer P., Zhang Z. Neospora caninum calcium-dependent protein kinase 1 is an effective drug target for neosporosis therapy. PLoS ONE. 2014;9:e92929. doi: 10.1371/journal.pone.0092929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner F., Yarovinsky F., Romero S., Didry D., Carlier M.F., Sher A. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Roos D.S., Donald R.G., Morrissette N.S., Moulton A.L. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- Striepen B., Jordan C.N., Reiff S., van Dooren G.G. Building the perfect parasite: cell division in apicomplexa. PLoS Pathog. 2007;3:e78. doi: 10.1371/journal.ppat.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi T., Kato K., Kobayashi K., Watanabe S., Kurokawa H., Gong H. Use of the kinase inhibitor analog 1NM-PP1 reveals a role for Toxoplasma gondii CDPK1 in the invasion step. Eukaryot. Cell. 2010;9:667–670. doi: 10.1128/EC.00351-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi T., Kato K., Kobayashi K., Kurokawa H., Takemae H., Gong H. 1NM-PP1 treatment of mice infected with Toxoplasma gondii. J. Vet. Med. Sci. 2011;73:1377–1379. doi: 10.1292/jvms.11-0085. [DOI] [PubMed] [Google Scholar]
- Sugi T., Kobayashi K., Takemae H., Gong H., Ishiwa A., Murakoshi F. Identification of mutations in TgMAPK1 of Toxoplasma gondii conferring resistance to 1NM-PP1. Int. J. Parasitol Drugs Drug Resist. 2013;3:93–101. doi: 10.1016/j.ijpddr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi T., Masatani T., Murakoshi F., Kawazu S.-I., Kato K. Microplate assay for screening Toxoplasma gondii bradyzoite differentiation with DUAL luciferase assay. Anal. Biochem. 2014 doi: 10.1016/j.ab.2014.06.018. accepted. [DOI] [PubMed] [Google Scholar]
- Tomita T., Bzik D.J., Ma Y.F., Fox B.A., Markillie L.M., Taylor R.C. The Toxoplasma gondii cyst wall protein CST1 is critical for cyst wall integrity and promotes bradyzoite persistence. PLoS Pathog. 2013;9:e1003823. doi: 10.1371/journal.ppat.1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Marches F., Daniel B., Sonda S., Heidenreich K., Curiel T. Pyridinylimidazole p38 mitogen-activated protein kinase inhibitors block intracellular Toxoplasma gondii replication. Int. J. Parasitol. 2002;32:969–977. doi: 10.1016/s0020-7519(02)00061-9. [DOI] [PubMed] [Google Scholar]
- Zhang W., Liu H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]


